Abstract
Viral vector-mediated gene transfer utilizing adeno-associated viral vectors has recently entered clinical testing as a novel tool for delivery of therapeutic agents to the brain. Clinical trials in Parkinson’s disease using adeno-associated viral vector-based gene therapy have shown the safety of the approach. Further efforts in this area will show if gene-based approaches can rival the therapeutic efficacy achieved with the best pharmacological therapy or other, already established, surgical interventions. One of the strategies under development for clinical application is continuous 3,4-dihydroxyphenylalanine delivery. This approach has been shown to be efficient in restoring motor function and reducing established dyskinesias in rats with a partial lesion of the nigrostriatal dopamine projection. Here we utilized high purity recombinant adeno-associated viral vectors serotype 5 coding for tyrosine hydroxylase and its co-factor synthesizing enzyme guanosine-5'-triphosphate cyclohydrolase-1, delivered at an optimal ratio of 5 : 1, to show that the enhanced 3,4-dihydroxyphenylalanine production obtained with this optimized delivery system results in robust recovery of function in spontaneous motor tests after complete dopamine denervation. We found that the therapeutic efficacy was substantial and could be maintained for at least 6 months. The tyrosine hydroxylase plus guanosine-5'-triphosphate cyclohydrolase-1 treated animals were resistant to developing dyskinesias upon peripheral l-3,4-dihydroxyphenylalanine drug challenge, which is consistent with the interpretation that continuous dopamine stimulation resulted in a normalization of the post-synaptic response. Interestingly, recovery of forelimb use in the stepping test observed here was maintained even after a second lesion depleting the serotonin input to the forebrain, suggesting that the therapeutic efficacy was not solely dependent on dopamine synthesis and release from striatal serotonergic terminals. Taken together these results show that vector-mediated continuous 3,4-dihydroxyphenylalanine delivery has the potential to provide significant symptomatic relief even in advanced stages of Parkinson’s disease.
Keywords: gene therapy, enzyme replacement, dopamine, tyrosine hydroxylase, GTP cyclohydrolase-1
Introduction
Gene therapy based on enzyme delivery for dopamine replacement has reached the clinic as two alternative approaches for the treatment of Parkinson’s disease (Eberling et al., 2008; Oxford Biomedica, 2009; reviewed in Björklund and Kirik, 2009). Both strategies aim to reconstitute the striatal dopaminergic tone by reinstating one or a number of the enzymes required for the synthesis of this neurotransmitter. Whereas the first approach only delivers the aromatic l-amino acid decarboxylase (AADC) enzyme responsible for the conversion of 3,4-dihydroxyphenylalanine (DOPA) to dopamine, in addition the latter utilizes a tricistronic lentiviral vector to over-express, the DOPA synthesizing enzyme tyrosine hydroxylase (TH) together with the co-factor synthesizing enzyme GTP cyclohydrolase-1 (GCH1) in the striatum (Bencsics et al., 1996). Both strategies will result in ectopic dopamine synthesis in the transduced striatal neurons, although the first only upon peripheral l-3,4-dihydroxyphenylalanine (l-DOPA) administration.
The rationale for enzyme replacement is straightforward, as the cardinal pathology in patients with Parkinson’s disease is the degeneration of the dopaminergic neurons projecting to the striatum (recently reviewed in Hornykiewicz, 2001). However, the synthesis of dopamine in a cellular compartment without storage and release mechanisms, i.e. in cells that lack the vesicular monoamine transporter, could result in multiple detrimental effects. In contrast to l-DOPA, dopamine is not efficiently transported over the cellular membrane by the L-type large amino acid transporter (Kageyama et al., 2000), and thus the release could be very inefficient. Furthermore, an uncontrolled increase in cytosolic catecholamine levels in striatal neurons could inhibit dopamine precursor synthesis (Kumer and Vrana, 1996; Wachtel et al., 1998), or might even be toxic (Chen et al., 2008). An alternative therapeutic approach is recombinant adeno-associated virus (rAAV)-mediated continuous DOPA delivery. By omitting the AADC enzyme from the genes delivered by the viral vector, the aforementioned complications can be avoided. Viral vector delivery of DOPA, however, builds on the assumption that the remaining endogenous AADC activity in the parkinsonian brain is sufficient for synthesis of dopamine at therapeutic levels. This should not be a limiting factor in early stages of the disease, when oral l-DOPA medication is effective, but could be a concern at more advanced stages when dopamine denervation in some areas of the forebrain is close to complete (Lloyd and Hornykiewicz, 1970; Nagatsu et al., 1979).
The rationale for continuous DOPA delivery stems from clinical observations in Parkinson’s disease patients that severe abnormal involuntary movements (i.e. dyskinesias), induced by oral l-DOPA medication, can be alleviated by l-DOPA or dopamine agonists delivered as a continuous infusion via the intravenous or duodenal route (Mouradian et al., 1987; Nyholm et al., 2003). Thus, there is considerable evidence that dyskinesias develop, at least in part, owing to intermittent, pulsatile stimulation of striatal dopamine receptors produced by intermittent oral l-DOPA administration. Moreover, these patients benefit from continuous dopamine stimulation by a dramatic reduction in total time spent in the ‘OFF’ state.
Continuous DOPA delivery obtained by a combined TH plus GCH1 gene transfer is highly efficient in promoting behavioural recovery of motor function and reversal of l-DOPA-induced dyskinesias, accompanied by a normalization of dopamine neurotransmission, as demonstrated in rodent models of Parkinson’s disease (Kirik et al., 2002; Carlsson et al., 2005; Leriche et al., 2009). However, for successful translation of rAAV-mediated continuous DOPA delivery strategy to the clinic, a number of outstanding issues need to be addressed. First, the extent of the host striatal dopamine denervation is known to limit the magnitude of recovery after dopamine replacement therapy. In cell transplantation studies, animals and patients with extensive dopamine denervation and significant loss of extra-striatal dopamine input to the forebrain fail to show the expected recovery (Piccini et al., 2005; Breysse et al., 2007). These observations point to the importance of spared host dopamine projections for optimal efficacy of this treatment. Whether this is the case in enzyme replacement therapy needs to be clarified. Second, in cases where striatal dopamine innervation is severely reduced, forebrain serotonin (5-hydroxytryptamine) innervation has been shown to play an important role in production of dopamine from peripherally administered l-DOPA (Ng et al., 1970; Hollister et al., 1979; Carlsson et al., 2007; Carta et al., 2007). Therefore, it is possible that the loss of serotonin terminals, known to occur in some patients with advanced Parkinson’s disease (Bernheimer et al., 1961; Chase, 1972; Scatton et al., 1983; Kish et al., 2008), may compromise the therapeutic benefits of viral vector-mediated DOPA delivery. This is a point of critical importance that must be addressed in order to establish which patients are more likely to benefit from the present approach. Third, continuous dopamine stimulation using infusion of l-DOPA or dopamine agonists has been shown to result in gradual induction of tolerance in patients, which could be avoided by dose level switches between wake and sleep hours (Mouradian et al., 1990; Nutt et al., 2000; Olanow et al., 2006). Since it is not possible to regulate the level of DOPA synthesis dynamically with the vector systems currently considered for clinical use, it is important to demonstrate that possible development of tolerance does not compromise the long-term therapeutic benefits of the treatment.
This study was designed to address these issues in a model of advanced Parkinson’s disease using intrastriatal delivery of two rAAV vectors encoding for either TH or GCH1 transgenes, mixed at a ratio of 5 : 1 for optimal functionality (Björklund et al., 2009). The results show that long-lasting therapeutic efficacy, superior to earlier studies, could be obtained in rats with complete lesions of the dopamine projection system. Furthermore, we found that host serotonin neurons are involved in mediating this effect, but that part of the therapeutic efficacy of TH plus GCH1 gene delivery is preserved in animals with combined forebrain dopamine and serotonin denervation.
Materials and methods
Subjects
Sixty-five adult female Sprague-Dawley rats weighing 225–250 g were purchased from Charles River (Germany) and housed, two to three per cage, with free access to food (except when restricted during behavioural testing in staircase test) and water under a 12 h light/12 h dark cycle. All experimental procedures performed in this study were approved by the Ethical Committee for Use of Laboratory Animals in the Lund–Malmö region.
Experimental design
Sixty rats received a unilateral injection of 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle, to achieve a complete lesion of the nigrostriatal pathway. Starting 3 weeks post-lesion, the animals were tested using a battery of behavioural tests. Their forelimb performance was monitored using the stepping, staircase and cylinder tests, as well as apomorphine- and d-amphetamine-induced rotations. Motor asymmetry scores in the d-amphetamine rotation were used as an indicator for lesion severity. Only animals exhibiting more than six full-body turns per minute towards the dopamine-depleted side were included in the study.
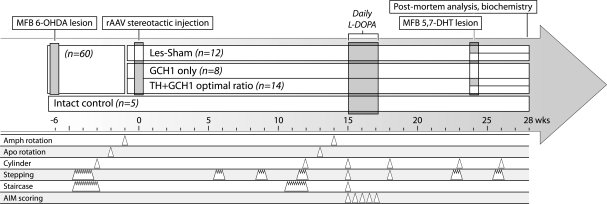
Thirty-four rats with a confirmed 6-OHDA lesion were then allocated into the three treatment groups (Les-Sham, GCH1-only and TH + GCH1) that were balanced on the basis of performance in stepping, cylinder as well as apomorphine- and amphetamine-induced rotation tests (Fig. 2). At Week 6 post-lesion, the animals in the Les-Sham group (n = 12) underwent a sham surgery, which involved opening the scalp and drilling a hole at the correct site but with the dura not penetrated. The animals in the GCH1-only group (n = 8) received a stereotactic injection of 3.0E10 genome copies of rAAV-GCH1, and those in the TH + GCH1 group (n = 14) received 2.9E10 genome copies of rAAV-TH and 5.8E9 genome copies of rAAV-GCH1, resulting in a ratio of 5 : 1. We have earlier shown that this ratio yields optimal TH functionality (Björklund et al., 2009). At Weeks 6 and 9 post-rAAV injection, the animals were tested in the stepping test, and at Week 12 the complete test battery was repeated (see Fig. 1 for details). At Week 15, daily peripheral l-DOPA administration was initiated, and abnormal involuntary movements were scored on Days 1, 4, 8, 12 and 16 of the treatment according to previously published protocols (Winkler et al., 2002; Carlsson et al., 2007). On Days 2 and 3 of l-DOPA treatment, the animals were tested ‘ON’ l-DOPA for assessment of l-DOPA response in stepping, staircase and cylinder tests. A fourth group of animals without a dopamine lesion (intact control, n = 5) was included as a control group for abnormal involuntary movement scoring and post-mortem analysis. Finally, to elucidate the role of the serotonergic system in the functional recovery, sub-groups of animals in the TH + GCH1 (n = 5) and the Les-Sham (n = 5) groups were tested in the stepping and cylinder tests at Week 23, before they received a unilateral injection of 5,7-dihydroxytryptamine (5,7-DHT) into the medial forebrain bundle. At Week 26, the same animals were re-tested in the two tests for assessment of the serotonergic lesion on motor performance.
Figure 2.
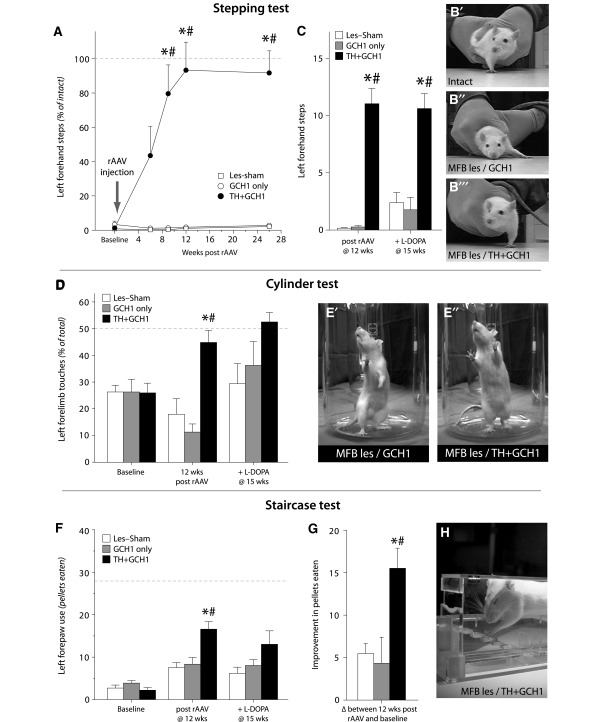
Recovery of motor function in tests of forelimb use. Near complete lesion of the ascending dopamine innervation by injection of 6-OHDA in the medial forebrain bundle (MFB) unilaterally caused a severe impairment in sidestepping using the contralateral limb in all animals (A). After striatal injection of rAAV vectors, the affected-limb use in the TH + GCH1 treated animals gradually improved over the first 9 weeks, to reach the level of use on the intact side at 12 weeks post-injection (illustrated by the dashed line in A, also shown in photographs in panels B’–B’’’). The therapeutic efficacy of continuous DOPA delivery was superior to the effect of peripheral l-DOPA injection, which improved the stepping performance in the two control groups (Les-Sham and GCH1-injected animals) only partially and did not have any detrimental effect in the TH + GCH1 group (C). Similar results were obtained in the cylinder test, where the animals in the TH + GCH1 group performed significantly better than the lesion and vector control groups at 12 weeks post-rAAV injection (D, see also photographs in E′ and E′′). This difference was diminished after peripheral administration of l-DOPA. In the staircase test (H), during baseline testing prior to vector treatment, all animals displayed severe impairment on the contralateral side in the number of sugar pellets eaten as compared with the ipsilateral side (dashed line in F). At 12 weeks post-rAAV, the animals in the TH + GCH1 group improved to a significantly greater extent than the lesion and vector control groups (F, G). In contrast to the other tests, peripheral l-DOPA administration had no impact on number of pellets eaten in the control groups (F). Statistics: (A). Repeated measures ANOVA time F(4,88) = 22.8, P < 0.001; time × group F(8,88) = 24.13, P < 0.001; one-way ANOVA for each time point baseline: F(2,22) = 1.01, P = 0.35; 6 weeks: F(2,22) = 5.70, P = 0.01; 9 weeks: F(2,22) = 10.04, P < 0.001; 12 weeks: F(2,22) = 27.48, P < 0.001; 26 weeks: F(2,22) = 45.74, P < 0.001, followed by Bonferroni-corrected Tukey’s HSD post hoc test. (C) Repeated measures ANOVA time F(1,22) = 5.52, P < 0.05; time × group F(2,22) = 2.95, P = 0.073; one-way ANOVA 12 weeks: F(2,22) = 58.28, P < 0.001, + l-DOPA at 15 weeks: F(2,22) = 19.92, P < 0.001, followed by Bonferroni-corrected Tukey’s HSD post hoc test and paired t-tests for within-group comparisons, P = 0.20 and P = 0.036 for GCH1-only and Les-Sham groups, respectively. (D) Repeated measures ANOVA time, F(1,22) = 0.28, P = 0.6; time × group, F(2,22) = 13.96, P < 0.001; group, F(2,22) = 6.94, P = 0.005; pre-test one-way ANOVA F(2,22) = 0.3, P = 0.997; 12 weeks post-AAV one-way ANOVA F(2,22) = 15.57, P < 0.001, followed by Bonferroni-corrected Tukey’s HSD post hoc test, + l-DOPA at 15 weeks one-way ANOVA F(2,21) = 2.85, P = 0.08. (F) Repeated measures ANOVA time F(1,21) = 33.41, P < 0.001; time × group F(2,21) = 5.65, P = 0.011; pre-score two-way ANOVA side F(1,138) = 717.20, P < 0.05; group F(2,144) = 2.25, P = 0.11; side × group F(2,138) = 2.10, P = 0.13; 12 weeks post-AAV two-way ANOVA side F(1,138) = 346.01, P < 0.05; group F(2,144) = 10.33, P < 0.05; side × group F(2,138) = 0.082, P = 0.082, followed by one-way ANOVA on the lesioned side, F(2,138) = 6.75, P < 0.05 and Tukey’s HSD post hoc test. After l-DOPA one-way ANOVA F(2,21) = 2.72, P = 0.09. (G) One-way ANOVA F(2,21) = 6.92, P < 0.05 and Tukey’s HSD post hoc test. Asterisk indicates different from Les-Sham group, hash indicates different from GCH1-only group.
Figure 1.
Experimental design. At the onset of the experiment, 60 animals received a unilateral 6-OHDA lesion of the medial forebrain bundle (MFB) in order to obtain a complete lesion of the nigrostriatal dopamine pathway. Thirty-four animals that displayed more than six full-body turns per minute, towards the lesioned side, in the d-amphetamine (Amph)-induced rotation test were selected for further analysis in a battery of motor tests. At 6 weeks post-lesion, the animals were balanced into three treatment groups on the basis of performance in stepping, cylinder and the apomorphine (Apo)- and amphetamine-induced rotation tests to receive either the two vector mix (TH + GCH1 group, n = 14) or only the control vector (GCH1 only group, n = 8), or be treated as non-vector-injected lesion controls (Les-Sham group, n = 12). A fourth group with intact animals (n = 5) was included in the experiment as a non-lesioned control group in the l-DOPA-induced dyskinesia test. Over the 6 months that followed, the animals were tested repeatedly using the different motor tests. At Week 15 post-transduction, daily peripheral l-DOPA administration was initiated, and abnormal involuntary movement (AIM) scoring was conducted on treatment Days 1, 4, 8, 12 and 16. At Week 23, sub-groups of the TH + GCH1 group (n = 5) and the Les-Sham group (n = 5) were tested in the stepping and cylinder tests, whereafter they received a unilateral injection of 5,7-DHT into the medial forebrain bundle. At week 26, the same animals were re-tested in the two tests. All animals were killed for biochemical analysis at 28 weeks after vector delivery.
At Week 28, all animals were decapitated and the brains were quickly removed. The forebrain was dissected into two sub-fractions. The first fraction contained the dorsal striatum, and the second, the ventral striatum (nucleus accumbens, ventral pallidum and the olfactory tubercle). All tissue fractions were dissected out quickly, weighed, and then snap-frozen for biochemical analysis. The midbrain was post-fixed in 4% paraformaldehyde and processed for histological analysis for validation of the 6-OHDA lesion.
Surgical procedures
All surgical procedures were conducted under anaesthesia induced by a 20 : 1 mixture of fentanyl and medetomidine (Dormitor, Apoteksbolaget, Sweden), injected i.p. at a total volume of ∼6 ml/kg. The injections were conducted using a 10 µl Hamilton syringe, with the animal mounted in a stereotactic frame (Stoelting, Wood Dale, IL, USA). The anteroposterior and mediolateral coordinates were calculated from bregma and the dorsoventral coordinates from the dural surface (Watson and Paxinos, 1986).
Neurotoxic lesions
The animals received injections of the 6-OHDA neurotoxin (Sigma-Aldrich AB, Sweden) unilaterally into the right medial forebrain bundle to lesion the ascending dopamine projections (Ungerstedt and Arbuthnott, 1970). Fourteen micrograms free base of the neurotoxin was administered in ascorbate-saline (0.02%) at a concentration of 3.5 µg/µl into one position along the fibre bundle in order to ensure specific lesion of the dopamine fibres, sparing the serotonin projection system (Carta et al., 2007). The injection was conducted at the following coordinate: anteroposterior –4.4 mm; mediolateral –1.2 mm; and dorsoventral –7.8 mm, with the tooth bar set to –0.0 mm using a 26 gauge needle attached to the Hamilton syringe. For the serotonergic lesion, 5,7-DHT creatine sulphate (10 µg free base in 2 µl 0.02% ascorbate-saline, Sigma-Aldrich AB) was infused unilaterally at the following coordinates: anteroposterior –3.0 mm; mediolateral –1.6 mm; dorsoventral –7.8 mm; the tooth bar was set to 3.3 mm. A total of 10 µg toxin was injected into a single deposit as previously described (Muñoz et al., 2009). The toxins were injected at a speed of 1 µl/min and the needle kept in place for an additional 3 min before it was slowly retracted from the brain parenchyma.
rAAV vector injections
The animals in the vector treatment groups received a stereotactic injection of 4 µl rAAV5 vectors in ringer lactate suspension. The injections were targeted to the striatum. They were performed as two 1 µl deposits delivered along each of two needle tracts using a pulled glass capillary (outer diameter 60–80 µm) mounted on a 22 gauge needle attached to the Hamilton syringe. The injection coordinates were as follows: (i) anteroposterior +1.0 mm; mediolateral −2.8 mm; and dorsoventral −4.5, −3.5 mm and (ii) anteroposterior 0 mm; mediolateral −4.0 mm; and dorsoventral −5.0, −4.0 mm with the tooth bar set at −2.4 mm. The injection rate was 0.4 µl/min, and the needle was kept in place for 1 min after the first deposit and 3 min after the second deposit before it was slowly retracted from the brain parenchyma.
rAAV vectors
The two viral vectors used in this experiment were constructed, produced and titered at the University of Florida, Gene Therapy Centre Vector Core. In these pseudotyped rAAV2/5 vectors, the transgene of interest is flanked inverted terminal repeats of the AAV2 and packaged in an AAV5 capsid (for reasons of simplicity, the vectors will be referred to as rAAV5 hereafter in the paper). The vector plasmids contained the gene coding either for the human TH or the GCH1 gene under the control of the synthetic chicken β-actin promoter containing a rabbit gamma-globulin intron, preceded with an enhancer element from the cytomegalovirus promoter (termed as the chicken β-actin, CBA, promoter), and the human bovine growth hormone polyadenylation sequence, flanked by AAV2 inverted terminal repeats. The rAAV vectors were produced using a double transfection method, with the appropriate transfer plasmid and helper plasmid containing the essential adenoviral packaging genes as described previously (Grimm et al., 1998; Hauswirth et al., 2000). They were further purified by iodixanol step gradients and Sepharose Q column chromatography, and then titered using a dot-blot assay, as described in detail elsewhere (Zolotukhin et al., 1999, 2002). The vector batches had final stock titers of 8.6E12 and 1.3E13 genome copies/ml for rAAV5-TH and rAAV5-GCH1, respectively.
Behavioural tests
Drug-induced rotation
This was assessed by measuring left and right full-body turns using automated rotometer bowls (AccuScan Instruments Inc., Columbus, OH, USA) after injection of either d-amphetamine sulphate (2.5 mg/kg, i.p.; Apoteksbolaget, Sweden) or apomorphine-HCl (0.05 mg/kg, s.c.; Sigma-Aldrich AB) and monitored continuously for 90 and 40 min, respectively. Rotational asymmetry scores are expressed as net 360° turns per minute and ipsilateral rotations (i.e. towards the injected side) were assigned a positive value.
Cylinder test
This test, assessing forelimb use asymmetry, was conducted essentially as described by Kirik et al. (2001), with minor modifications (Schallert and Tillerson, 2000). The rats were placed individually in a 20 cm Ø glass cylinder and allowed to move freely, while being recorded with a digital video camera. Two perpendicular mirrors were placed behind the glass cylinder, which allowed the complete surface of the cylinder to be clearly visible on the screen. The animals were left in the cylinder until at least 20 touches on the cylinder wall were observed. Forelimb placements on the cylinder wall during this exploratory phase were then scored off-line using frame-by-frame analysis by an observer blinded to the group identity of the animals. The paw use during each of the 20 contacts with the cylinder walls during rearing was scored as left forepaw touches and presented as a percentage of the total number of touches. In this test, normal animals would score, on average, 50%.
Stepping (forelimb akinesia) test
Forelimb akinesia was assessed by an investigator blinded to the group identity of the individual rats using the bracing test initially described by Schallert et al. (1979), modified to a side-stepping test by Olsson et al. (1995) and deployed as previously described (Kirik et al., 1998). Briefly, the investigator firmly held the rat, using both hands, lifting the hind paws and one forepaw, enabling only the unrestrained paw to contact the table surface. The animal was then moved over a defined distance of 90 cm across the table surface at a slow pace over 4–5 s. The investigator scored the numbers of adjusting side steps in both forehand and backhand direction twice, and the average was calculated. The animals were trained for 4 days during the third week post-6-OHDA lesion. The average score of Days 5–7, when the animals had reached a stable baseline performance, formed the final dependent variable. Thus, the average of the three last days was used as a pre-treatment performance score. At the three following test sessions, 6, 9 and 12 weeks post-rAAV injection, the animals were first habituated to the test for 1 or 2 days, and then assessed for three consecutive days.
Staircase test
This test was used to assess the skilled forelimb reaching and grasping abilities by employing a modified version of the original test design described by Montoya et al. (1991) according to Winkler et al. (1999). Briefly, sugar pellets (TestDiet, Richmond, VA, USA) were placed on steps of a double staircase divided by a wide central platform (35 mm), all enclosed in a small Plexiglas box (285 × 60 × 90 mm). The animals were food deprived 48 h before the first testing day and kept on a restricted food intake (6–8 g/day) throughout the test period, with food administrated only after the daily test session. Starting the second week post-lesion, the animals were trained in the staircase test for 15 min/day, with 10 sugar pellets placed on each of steps 2–5 on both sides (total 40 pellets/side), where Day 1 was defined as the first day the rats started retrieving sugar pellets, which is typically within the first 2–3 trials. The number of pellets successfully retrieved and the errors made were scored for 10 consecutive test sessions. The average of the three last days was used as the pre-treatment performance score. At 12 weeks post-rAAV injections, the animals were re-tested using the same test paradigm. Skilled forelimb test results are expressed as the number of pellets eaten (taken minus missed) on the parkinsonian (left) side.
Behavioural tests after peripheral l-DOPA administration
A second set of behavioural tests were employed to determine the magnitude of motor behavioural improvement that can be obtained in the complete lesion model after peripheral l-DOPA at 15 weeks post-rAAV injection. The dose was adjusted to 6 mg/kg l-DOPA methyl ester combined with 15 mg/kg benserazide-HCl s.c., which corresponds to a therapeutic dose level in patients. Because of the rapid onset of l-DOPA-induced dyskinesias in the medial forebrain bundle lesioned rat model, the temporal profile of the involuntary movements was monitored on Day 1 of l-DOPA injection. At 140–160 min post-injection, single animals showed signs of mild dyskinesia, but at sufficiently low amplitude not to interfere with behavioural tests. These observations indicated that the l-DOPA levels still remained over the therapeutic threshold at this time interval; the tests were performed on the next 2 days during this interval to avoid the influence of the l-DOPA-induced dyskinesias on the animals’ performance. The animals were injected at 5 min intervals to enable the tests to be conducted at the same time point in all cases following the same testing procedures as described above. On Day 2 of the l-DOPA treatment, the animals were scored using the stepping test at 140 min, and then evaluated in the staircase test for 15 min, starting at 145 min. On the following day (Day 3 of treatment), the animals were scored in the cylinder test at 140 min. One animal in the rAAV-TH/GCH1 group had to be excluded from the cylinder test owing to remaining dyskinesias at 140 min.
Abnormal involuntary movements
Abnormal involuntary movements were monitored over a 16 day treatment period of daily l-DOPA injections initiated at Week 13 post-rAAV injection. l-DOPA methyl ester (6 mg/kg; Research organics, Cleveland, OH, USA) was injected daily s.c. together with a peripheral DOPA-decarboxylase inhibitor, benserazide (HCl salt 15 mg/kg, Sigma-Aldrich, Sweden) dissolved in physiological saline. The rats were scored on Days 1, 4, 8, 12 and 16 of the l-DOPA treatment by an observer blinded to the identity of the animals using the rat dyskinesia scale, described in detail previously (Cenci et al., 1998; Winkler et al., 2002). Briefly, the animals were placed individually in plastic cages with a filter top without bedding material and scored every 20 min for 140 min (or as long as abnormal involuntary movements were observed). The abnormal involuntary movements were divided into four subtypes, classified by their topographic distribution: locomotor, axial, forelimb and orolingual. Enhanced manifestations of otherwise normal behaviours such as rearing, sniffing, grooming and gnawing are not included in the rating scale. However, some animals exhibited excessive chewing or licking in a stereotypic manner and this behaviour was included in the orolingual score. The severity was scored as: 0 = absent; 1 = occasional, i.e. present during less than 50% of the observation time; 1.5 = present 50% of the observation time; 2 = frequent, i.e. present during more than 50% of the observation time; 2.5 = present most of the time, with very short duration of non-dyskinetic episodes; 3 = continuous, but interruptible by repeated sensory stimuli, e.g. sudden noise, opening of the cage lid; 3.5 = continuous, but inconsistently interrupted by repeated sensory stimuli; 4 = continuous, not interrupted by repeated sensory stimuli. The abnormal involuntary movement scores are presented, both as total time-integrated score (the sum of the individual scores for each assessed time point multiplied by the assessment interval in minutes, i.e. 20 min) of axial + forelimb + orolingual abnormal involuntary movements and as time-integrated scores of individual abnormal involuntary movement subtypes.
Biochemical analyses
All animals were killed by decapitation, after which the brain was removed and sliced in the coronal axis into two parts using a brain mould. The striatal tissue from each hemisphere of the anterior part was then rapidly dissected and frozen individually on dry ice and stored at −80°C until further analysis. The caudal part containing the midbrain–hindbrain regions was post-fixed in 4% paraformaldehyde for 24 h at 4°C, and then kept in 25% sucrose for at least 24 h. The dissected brain tissue was homogenized and prepared using a modified version of a previously described protocol, which enables detection of monoamines and 5,6,7,8-tetrahydro-l-biopterin (BH4) by high performance liquid chromatography (HPLC), and in vitro activity assays from the same sample (Romero-Ramos et al., 2000). Briefly, the tissue was homogenized on ice in tris–acetate buffer (5 µl/mg, 20 mM, pH 6.1) using an ultrasonic disintegrator. Eighty microlitres of the homogenate was then pipetted into ice-cold 0.8 mM perchloric acid for HPLC measurements. The remaining homogenate was centrifuged (15 min at 4600g at 4°C); thereafter the supernatant was frozen until analysed in the AADC enzyme activity assay.
HPLC analysis of monoamines and BH4
The tissue homogenate in perchloric acid was incubated on ice for at least 20 min before centrifugation for 15 min at 4600g at 4°C. The supernatant was filtered through 96 well 0.45 µm polyvinylidene difluoride filter plate by centrifugation for an additional 5 min at 4600g. Thereafter the sample was diluted 1 : 4 in Mili-Q-filtered de-ionized water and stored in −80°C until analysis. The tissue extracts were then analysed by HPLC with electrochemical detection in three separate measurements for (i) dopamine and serotonin, (ii) DOPA and (iii) BH4. For each measurement, 25 µl of each sample was injected by a cooled autosampler (Spark Holland Midas) into an electrochemical detector (ESA Coulochem III) coupled to a guard cell (ESA 5020) and a glass-carbon electrode analytical cell (ESA 5011). For dopamine/serotonin and DOPA detection, a reverse-phase C18 column (3 µm ReproSil-pur, 4.6 mm Ø, 150 mm length, Chrompack) was used for compound separation, whereas for BH4 detection, this was replaced with another reverse-phase C18 column (5 µm ReproSil-pur, 4.6 mm Ø, 250 mm length, Chrompack) preceded by a C8 column (5 µm ReproSil 80, 4.6 mm Ø, 33 mm length, Chrompack).
The mobile phase for dopamine/serotonin detection contained 60 mM sodium acetate, 90 µM Na2-EDTA and 460 µM 1-octanesulphonic acid in 9% methanol, where pH was adjusted to 4.2. For DOPA detection it contained 100 mM NaH2PO4 adjusted to pH 3.0 with H3PO4, 90 µM Na2-EDTA and 2.8 mM 1-octanesulfonic acid in 10% methanol. The mobile phase used for BH4 detection, on the other hand, was modified from a previously described method BH4 detection protocol (Howells and Hyland, 1987) and composed of 50 mM sodium acetate, 5 mM citric acid, 48 µM EDTA and 160 µM dithioerythritol in 5% methanol, pH 5.2. The mobile phases were delivered at a flow rate of 500 µl/min for catecholamines and 1 ml/min for BH4. Peak identification and quantification were conducted using the Clarity Chromatographic software package (DataApex, Prague, Czech Republic).
AADC enzyme activity assay
The enzyme activity protocol is modified from Nagatsu et al. (1979). Briefly, 5 µl of tissue supernatant was mixed with 95 µl of assay buffer solution (0.01 mM pyridoxal phosphate, 0.17 mM ascorbic acid, 0.1 mM pargyline, 1 mM 2β-mercaptoethanol and 0.1 mM EDTA in 50 mM sodium phosphate buffer, adjusted to pH 7.2). The samples were run in triplicates, where two received assay buffer containing 0.25mM l-DOPA and the third acted as blank reference. The samples were incubated at 37°C for 20 min before the reaction was halted by addition of 150 µl of ice-cold 0.1M perchloric acid. After centrifugation (15 min at 4600g at 4°C), 200 µl supernatant was transferred to a 96 well 0.45 µm polyvinylidene difluoride filter plate (Whatman, Florham Park, NJ, USA) containing 100 µl ice-cold 0.1 M perchloric acid, and the filtrate was diluted in 300 µl ice-cold 0.1M perchloric acid before analysis of dopamine content using HPLC, as described below. Thereafter, the blank values were subtracted from the l-DOPA-containing samples. The average of the two background-subtracted values constituted the final dependent variable for this assay.
Histological analysis
The post-fixed midbrain regions were cut into 40 µm coronal sections on a semi-automated freezing microtome (Microm HM 450) and collected into six series and stored in anti-freeze solution (0.5 M sodium phosphate buffer, 30% glycerol and 30% ethylene glycol) at −20°C, until further processing. Immunohistochemistry was performed using antibodies raised against TH (rabbit IgG 1 : 10 000 Pel-Freez, Rogers, AR, USA), and GCH1 (custom made rabbit IgG, 1: 5000). The staining was visualized using biotinylated secondary antibodies (goat anti-rabbit BA1000, Vector Laboratories, Burlingame, CA, USA) followed by a 1 h incubation with avidin-biotin peroxidase solution (ABC Elite, Vector Laboratories) developed by 3, 3′-diaminobenzidine in 0.01% H2O2 colour reaction.
Statistical analysis
All statistics were conducted using the Statistical Package for the Social Sciences 17 statistical package (SPSS Inc., Chicago, IL, USA). Repeated measures ANOVA for data in Fig. 2A, C, D F was conducted using the general linear model. This was followed by a one-way ANOVA at each time point with Tukey’s HSD post hoc comparison. Two-way ANOVA tests performed on data presented in Figs. 4–7 were analysed using the univariate general linear model. Post hoc side comparisons were conducted using Bonferroni-corrected t-tests and group comparisons on the treated side using one-way ANOVA followed by Tukey’s HSD. In cases where the Levene test for unequal variance was significant, Tukey’s HSD was replaced with Dunnett’s T3 test. Because of the non-parametric nature of the abnormal involuntary movement scoring paradigm, all statistics in Fig. 3 were conducted using non-parametric statistics. A Friedman test of non-parametric repeated measures was conducted (Fig. 4A), followed by a Kruskal–Wallis analysis at each time point. The Kolmogorov–Smirnoff post hoc test was applied for each comparison. All significances were corrected for multiple comparisons using false discovery rate compensation. Data in Fig. 4B–E were analysed using Kruskal–Wallis, followed by false discovery rate corrected Kolmogorov–Smirnoff post hoc test.
Figure 4.
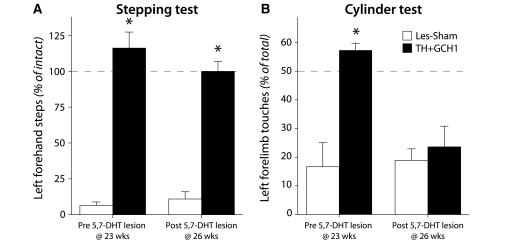
Impact of serotonin innervation in stepping and cylinder tests. Intact forebrain serotonin innervation is critical for recovery in some of the behavioural test paradigms. At 23 weeks post-rAAV injection, all animals in the Les-Sham and TH + GCH1 groups were re-tested in the cylinder and stepping tests. Thereafter, a sub-group of the animals received a unilateral injection of 5,7-DHT into the medial forebrain bundle and were re-assessed behaviourally 2 weeks later. In the cylinder test, the improvement caused by the rAAV-TH + GCH1 delivery was abolished (A), whereas the recovery in stepping performance was maintained (B). Statistics: Repeated measures ANOVA (A) Time, P = 0.027, F(1,6) = 8.51; time × group, P = 0.016, F(1,6) = 11.08; group, P = 0.015, F(1,6) = 11.27. (B) Time, P = 0.64, F(1,6) = 0.25; time × group, P = 0.42, F(1,6) = 0.76; group, P < 0.001, F(1,6) = 420. Post hoc paired t-test (A) TH + GCH1 pre-post P = 0.009; Bonferroni-corrected P = 0.018 (B) TH + GCH1 pre-post P = 0.48. Asterisk indicates significantly different from pre-5,7-DHT lesion.
Figure 5.
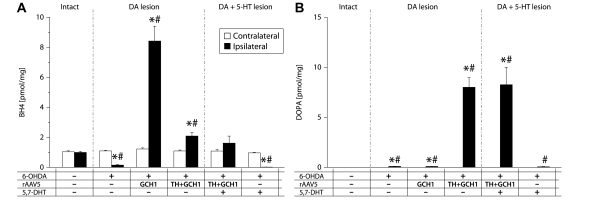
Measurement of tetrahydrobiopterin (BH4) (A) and DOPA (B) levels in striatal tissue using HPLC with electrochemical detection after rAAV-mediated TH + GCH1 gene transfer. In the intact striatum, BH4 levels averaged ∼1 pmol/mg in the intact striatum (open bars in A). Depletion of the dopamine afferents to the striatum alone reduced BH4 levels by 85%, while the combination with a 5,7-DHT lesion caused a >98% reduction. TH + GCH1 delivery resulted in complete recovery to normal levels (solid bars in A). Striatal DOPA levels were below detection limit (<0005 pmol) in the intact rat striatum and just at the detection range in the lesioned striatum (open bars in B). The rAAV TH + GCH1 gene transfer induced an accumulation of ∼8 pmol/mg, which was not affected by the 5,7-DHT lesion. Statistics: Two-way ANOVA, (A) Side F(1,68) = 23.37, P < 0.001; group F(5, 68) = 39.3, P < 0.001; side × group F(5,68) = 36.15, P < 0.001. (B) Side F(1,68) = 57.54, P < 0.001; group F(5,68) = 26.29, P < 0.001; side × group F(5,68) = 26.29, P < 0.001. One-way ANOVA contralateral side not significant. Ipsilateral side: (A) F(5,34) = 38.340, P < 0.001; (B) F(5,34) = 26.292, P < 0.001, followed by Dunnett’s T3 post hoc test and Bonferroni-corrected paired t-tests for within-group comparisons Asterisk indicates different from contralateral side, hash indicates different from ipsilateral side of intact control group. 5-HT = serotonin; DA = dopamine.
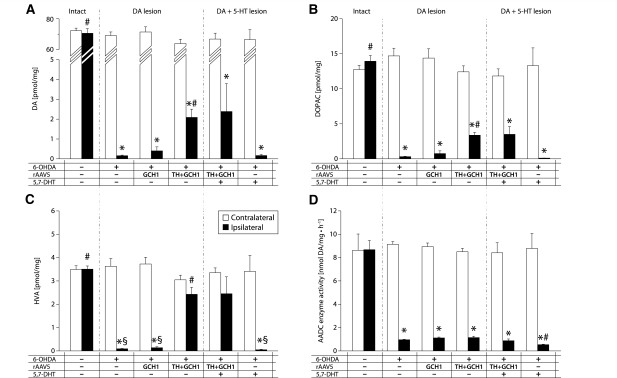
Figure 6.
Measurement of dopamine (A), 3,4-dihydroxyphenylacetic acid (DOPAC) (B) and homovanillic acid (HVA) (C) levels in striatal tissue and assay of aromatic L-amino acid decarboxylase (AADC) enzyme activity (D), quantified using HPLC-EC detection. The 6-OHDA medial forebrain bundle lesion removes >99.7% of dopamine input to the striatum and consequently results in 97% reduction in its metabolites. Following TH + GCH1 gene transfer, there is a small but clearly detectable increase in tissue dopamine levels (A). The reinstitution of 3,4-dihydroxyphenylacetic acid (B) and homovanillic acid (C) levels, in the latter case to normal level, better reflects the dopamine synthesis capacity after gene therapy. Lesioning of the serotonin system has no detectable impact on any of the measures (right-most section in A–C). The vast majority of striatal AADC activity is located in the dopamine terminals, while the remaining fraction around half appears to be located in serotonin terminals. Statistics: Two-way ANOVA, (A) Side F(1,68) = 1405.17, P < 0.001; group F(5,68) = 72.75, P < 0.001; side × group F(5,68) = 56.76, P < 0.001. (B) Side F(1,68) = 291.84, P < 0.001; group F(5,68) = 12.55, P < 0.001; side × group F(5,68) = 19.37, P < 0.001. (C) Side F(1,68) = 114.64, P < 0.001; group F(5,68) = 9.87, P < 0.001; side × group F(5,68) = 15.29, P < 0.001. (D) Side F(1,68) = 400.73, P < 0.001; group F(5,68) = 15.86, P < 0.001; side × group F(5,68) = 17.04, P < 0.001. One-way ANOVA contralateral side not significant. Ipsilateral side: (A) F(5,34) = 493.00, P < 0.001; (B) F(5,34) = 73.97, P < 0.001; (C) F(5,34) = 22.12, P < 0.001; (D), F(5,34) = 99.35, P < 0.001, followed by Dunnett’s T3 post hoc test and Bonferroni-corrected paired t-tests for within-group comparisons. Asterisk indicates different from contralateral side, hash indicates different from ipsilateral side of Les-Sham group. 5-HT = serotonin; DA = dopamine.
Figure 7.
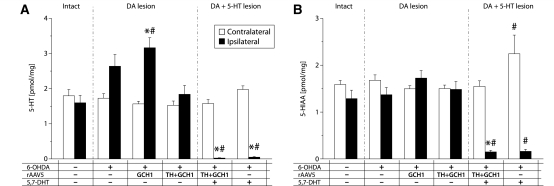
Measurement of serotonin (5HT) (A) and 5-hydroxyindolacetic acid (5-HIAA) (B) levels in striatal tissue quantified using HPLC-EC detection. The 6-OHDA lesion paradigm used here spared the serotonin projections to the striatum, and the TH + GCH1 delivery did not alter the tissue 5-hydroxytryptamine levels, whereas the 5,7-DHT lesion efficiently depleted both serotonin and 5-hydroxyindolacetic acid in the striatum in both vector-treated and lesion control animals. Statistics: two-way ANOVA, (A) Side F(1,68) = 1.18, P = 0.282; group F(5,68) = 15.44, P < 0.001; side × group F(5,68) = 17.46, P < 0.001. (B) Side F(1,68) = 55.83, P < 0.001; group F(5,68) = 7.64, P < 0.001; side × group F(5,68) = 14.52, P < 0.001. One-way ANOVA contralateral side not significant in (A); (B), F(5,34) = 3.389, P = 0.014. Ipsilateral side: (A), F(5,34) = 19.81, P < 0.001; (B), F(5,34) = 14.92, P < 0.001 followed by Dunnett’s T3 post hoc test, and Bonferroni-corrected paired t-tests for within-group comparisons. Asterisk indicates different from contralateral side; hash indicates different from corresponding side of Les-Sham group. DA = dopamine.
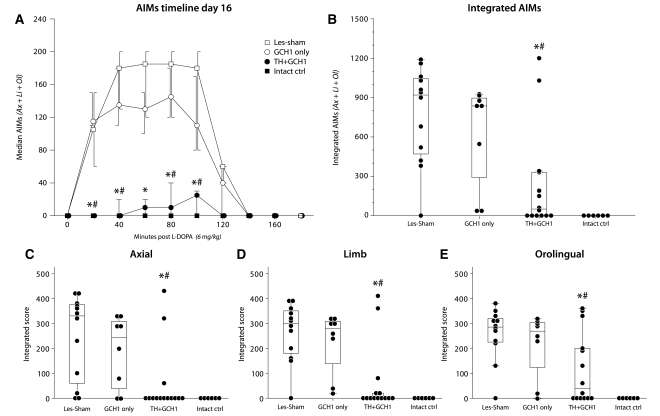
Figure 3.
Prevention of l-DOPA-induced dyskinesias. At Day 16 of daily l-DOPA treatment, 80% of the animals in the Les-Sham and GCH1-only groups displayed severe dyskinesias. In the TH + GCH1 group, the dyskinesias remained lower than in the control group during the entire peak of the l-DOPA 20–120 min post-l-DOPA injection (A). The dyskinesias were significantly lower in magnitude in this group when assessed as integrated scores (the sum of the individual scores for each assessed time point multiplied by the assessment interval in minutes, i.e. 20 min) presented as either total abnormal involuntary movements (AIM; axial + limb + orolingual) (B), or as individual axial (C), limb (D) and orolingual (E) components. Statistics: (A) Friedman test (non-parametric repeated measure); time effect P < 0.001; group effect P < 0.001. (B–E) Kruskal–Wallis (non-parametric one-way analysis) P ≤ 0.001. Kolmogorov–Smirnov post hoc analysis applied in all panels corrected for multiple comparisons (n = 6) using false discovery rate (FDR) compensation. Error bars in A represent 50% confidence interval (CI); boxes in B–E represent 50% CI, and whiskers 95% CI. Asterisk indicates different from Les-Sham group [P < FDR(0.05)], and hash indicates different from GCH1-only group [P < FDR(0.05)].
Results
Recovery of sensorimotor functions after rAAV-mediated DOPA delivery
The lesion-induced motor deficits, induced by a near complete depletion of the forebrain dopamine input, were assessed 3–5 weeks post-lesion in the stepping, cylinder and staircase tests. At 6 weeks, the animals received an injection of either rAAV-TH and rAAV-GCH1 vector mix (TH + GCH1 group) or the rAAV-GCH1 vector only, or sham surgery, and the changes in motor performance in the three tests were monitored over the subsequent 6 months. In the stepping test, the mean left (affected) forelimb performance was reduced to 0–3 steps (Fig. 2A), representing 90–100% impairment as compared with the right (intact) forelimb. In the group that received the combined TH + GCH1 vectors, the performance in the stepping test increased progressively over the first 9 weeks, and then reached a plateau at a level comparable to the performance of their intact paw (filled circles in Fig. 2A). This performance was maintained over the following 6 months. The control animals that received either the GCH1 vector alone (open circles in Fig. 2A) or followed as the lesion-only group (open boxes in Fig. 2A) showed no recovery in left forelimb stepping and remained severely impaired for the duration of the experiment (Fig. 2B and Supplementary Movie 1).
In the cylinder test, all animals showed a clear impairment in left forelimb use 3 weeks after removal of the dopamine input in the right forebrain, seen as preference of the intact (right) paw use for wall contacts during rearing (Fig. 2D). In the TH + GCH1 group, there was a significant improvement in left paw use at the testing session 12 weeks after rAAV injection. The recovery seen in this group was complete: as in the stepping test, the treated rats reached the level of performance seen in the intact animals, while the animals in the Les-Sham and GCH1-only groups showed no improvement and even a slight worsening in left paw use over time (Fig. 2E, Supplementary Movie 1).
In the staircase test prior to viral injection, all animals showed deficits in the number of sugar pellets eaten on the impaired left side, contralateral to the lesion. Out of the 40 pellets presented on each side of the stairs, they were able to successfully retrieve only 3–4 pellets, as compared to 25–30 on the intact side (represented as pellets eaten in Fig. 2F). At 12 weeks post-transduction, the animals in the TH + GCH1 group performed significantly better than they had in their baseline test prior to vector treatment, and also significantly better than the Les-Sham and GCH1-only groups at the re-test time point (Fig. 2F, G).
In order to compare the improvement obtained with gene therapy-based DOPA delivery to that obtained with peripheral l-DOPA injections, we repeated the stepping, cylinder and staircase tests after a single l-DOPA injection at the 15 week time point. ‘ON’ l-DOPA (6 mg/kg together with 15 mg/kg benserazide), about one-third of the animals in the Les-Sham and GCH1-only groups improved in the stepping test (to approximately seven left-forehand steps), whereas the remaining two-thirds did not improve significantly, yielding an average l-DOPA benefit of only 17 and 21% of normal function, respectively, in the two groups (Fig. 2C). The improvement seen in the cylinder test was similar to that in the stepping test (Fig. 2D), whereas in the staircase test, systemic l-DOPA provided no measurable improvement. The performance of the TH + GCH1 group remained unchanged in the stepping and cylinder tests after l-DOPA administration. In the staircase test, the significant difference previously seen between the TH + GCH1 and the Les-Sham groups was not seen anymore (Fig. 2F). It seems possible, however, that this effect was due to the higher variability in this test, as the animals could not be food restricted prior to this test session.
Drug-induced tests were kept to a minimum in the experiment because an extensive, chronic l-DOPA administration was planned for the later part of the study. Nevertheless, in the d-amphetamine rotation test at 12 weeks post-transduction, there was more than 50% reduction in the turning response in the TH + GCH1 group (5.8 ± 1.8 turns per minute) as compared with the two control groups (12.8 ± 1.0 and 12.3 ± 1.6 for the Les-Sham and GCH1-only groups, respectively).
Prevention of l-DOPA-induced dyskinesias
Following a 16 day treatment period with 6 mg/kg l-DOPA (+15 mg/kg benserazide), 17 out of 20 animals in the Les-Sham and GCH1-only groups developed moderate to severe abnormal involuntary movements resembling peak dose dyskinesias. In the TH + GCH1 group, on the other hand, the animals developed abnormal involuntary movements at significantly lower magnitude. A total of 14 animals were studied in this group, where six had no detectable abnormal involuntary movements and another five showed only mild dyskinesias (Fig. 3A, B). The dyskinesias remained lower than in the control group during the entire observed period (Fig. 3A). The induction kinetics of dyskinesias were similar in the treatment and control groups. The magnitude of abnormal involuntary movements increased gradually until Day 12, and between Days 12 and 16 there was no significant increase. Analysis of individual abnormal involuntary movement components suggested that in the Les-Sham and GCH1-only control groups axial (Fig. 3C), limb (Fig. 3D) and orolingual (Fig. 3E) components were all prominent; the residual dyskinesias in the TH + GCH1 group were dominated by a novel stereotypic, orolingual movement that was not apparent in the lesioned controls. These stereotypic movements were similar to those seen in intact animals after treatment with high doses of dopamine receptor agonists and include excessive licking or chewing on surfaces (Canales and Graybiel, 2000; Graybiel et al., 2000). They were always possible to interrupt, and thus received low to mid points, according to the scoring system (Supplementary Movie 2). It is possible that this type of stereotypic behaviour reveals a sub-category of involuntary movements, usually masked by more severe axial and limb dyskinesias.
Involvement of the striatal serotonin innervation in therapeutic efficacy
We have recently reported that ectopic DOPA synthesis after rAAV-mediated TH + GCH1 over-expression causes a decrease in striatal serotonin levels (Björklund et al., 2009). This finding, together with the observation here that amphetamine-induced rotation was decreased in these animals, hinted at the possibility that the de novo dopamine synthesis in the TH + GCH1-treated animals could, at least in part, be mediated by the striatal serotonin terminals. In order to investigate the role of the serotonin system, we performed a unilateral lesion of the forebrain serotonin projections in a sub-group of the animals in the Les-Sham and TH+GCH1 groups 24 weeks post-rAAV injection. Changes in the animals’ performance were assessed in the stepping and cylinder tests, as in these two tests the animals in the TH+GCH1 group had already displayed complete recovery, and therefore any worsening in the therapeutic efficacy would be easily detectable. Removal of the serotonergic system by a 5,7-DHT lesion did not have any measurable effect on the performance of the animals in the stepping test, as they remained fully recovered also after the second lesion (Fig. 4A). In the cylinder test, on the other hand, we found that the functional benefits seen in the TH + GCH1 group were completely abolished 2 weeks after the second lesion (Fig. 4B). The 5,7-DHT lesion did not have any effect on the behaviour of the Les-Sham animals.
Optimized rAAV TH + GH1 gene delivery restores BH4 levels and induces robust DOPA synthesis
In order to assess the level of correction in the dopamine synthesis pathway, the animals were killed and fresh, frozen brain samples from the dorsal and ventral striatum were collected separately from each hemisphere. The ventral striatal sample contained nucleus accumbens, ventral pallidum and the olfactory tubercle. All values obtained from analysis in the ventral striatum are presented in a table as supplementary online information, while the dorsal striatal measurements are described in detail below.
Snap-frozen tissue was prepared for analysis using HPLC-EC detection. In the intact striatum, BH4 levels could be measured in a highly reproducible way. The total tissue concentration of BH4 in the dorsal striatum was 1.098 ± 0.029 pmol/mg (open bars in Fig. 5A). After near complete depletion of the forebrain dopamine projections, the BH4 levels dropped by >85%, to 0.151 ± 0.030 pmol/mg solid bar in the Les-Sham (+ /–/–) group in Fig. 5A. Most of the remaining BH4 was located in the striatal serotonin terminals, which in turn could be efficiently abolished by the 5,7-DHT lesion leaving only a little >1% of BH4 0.017 ± 0.003 pmol/mg, double lesion (+ /–/ +) group in Fig 5A. The injection of 3E10 genome copies rAAV-GCH1 alone resulted in tissue BH4 levels of about 8-fold intact levels 8.414 ± 0.979 pmol/mg, GCH1-only (+ /GCH1/–) group in Fig. 5A. In the optimized mixture of the GCH1 and TH vectors at a 1: 5 ratio, 5.8E9 genome copies of rAAV-GCH1 were injected in the TH + GCH1 group. This resulted in a striatal tissue BH4 concentration that was about 2-fold higher than the levels measured on the intact side (2.086 ± 0.233 pmol/mg, + /TH + GCH1/– group in Fig. 5A).
Tissue levels of DOPA were quantified using HPLC-EC without inhibition of the AADC enzyme. Measurements under these conditions allow for more accurate analysis of the excess DOPA present in the tissue that could not be converted to dopamine rapidly (i.e. above the capacity of the AADC, or not accessible to available AADC in the host striatum). As expected, DOPA levels remained below the detection limit of the analysis, i.e. <0.015 pmol/mg, in the intact striatum. In tissue samples from 6-OHDA-lesioned animals, there was a very low but detectable amount of DOPA [0.097 ± 0.010 pmol/mg, solid bar of Les-Sham (+ /–/–) group in Fig. 5B]. Neither the GCH1 transduction nor the 5,7-DHT lesion caused a change in the tissue DOPA levels from this baseline. After rAAV-TH + GCH1 delivery, on the other hand, the levels increased two orders of magnitude. Interestingly, the vector-mediated DOPA level was not affected by serotonergic denervation and were 8.003 ± 0.956 and 8.258 ± 1.740 pmol/mg in the absence and presence of the 5,7-DHT lesion, respectively.
Optimized rAAV TH + GH1 gene delivery uses a novel site of dopamine synthesis, independent of serotonin neurons
Tissue dopamine content in the intact dorsal striatum was 68.3 ± 1.3 pmol/mg, whereas the 6-OHDA injection into the medial forebrain bundle caused a >99.7% reduction in striatal dopamine levels (0.16 ± 0.032 pmol/mg in the ipsilateral striatum; Fig. 6A). Similarly, 3,4-dihydroxyphenylacetic acid and homovanillic acid levels were decreased by >97% to 0.3 ± 0.04 and 0.09 ± 0.01 pmol/mg, respectively, compared to 13.3 ± 0.5 and 3.4 ± 0.1 pmol/mg (Fig. 6B, C). In line with previous findings that rAAV-mediated enzyme replacement does not provide a storage site for the newly synthesized dopamine in the denervated striatum, tissue dopamine levels in the TH + GCH1 group were only partially restituted to 2.1 ± 0.4 pmol/mg, i.e. a 13-fold increase from the lesion control group and 3% of the intact levels (Fig. 6A). The increase in levels of 3,4-dihydroxyphenylacetic acid and homovanillic acid, on the other hand, were much more pronounced (Fig. 6B, C). Homovanillic acid levels were no longer different from the intact control side. Interestingly, the 5,7-DHT lesion had no measurable effect on either the tissue dopamine level or the levels of the two major metabolites (Fig. 6A–C), suggesting that sufficient decarboxylase activity was still present outside this neuronal population in the dorsal striatum.
To confirm that the 5,7-DHT lesion removed the serotonergic innervation effectively, we quantified the levels of serotonin and 5-hydroxyindolacetic acid in the same striatal tissue using HPLC-EC. We found that the 6-OHDA lesion caused about 65–98% increase in serotonin levels in the Les-Sham (2.6 ± 0.3 pmol/mg) and GCH1-only (3.2 ± 0.3 pmol/mg) groups as compared with the intact striatum (1.7 ± 0.05 pmol/mg) (Fig. 7A). The 5,7-DHT lesion resulted in a robust (>97%) decrease in striatal serotonin levels (0.05 ± 0.02 pmol/mg, compared to 2.0 ± 0.01 pmol/mg in the Les-Sham group; Fig 7A). This decrease was coupled to a >87% decrease in levels of the 5-hydroxyindolacetic acid metabolite [0.16 ± 0.04 pmol/mg in the (+ /–/ +) group compared to 1.3 ± 0.2 pmol/mg in the normal control (–/–/–) group] (Fig. 7B; see also Supplementary Table 1 for additional information). As both dopaminergic and serotonergic neurons contain the AADC enzyme, we determined the changes in activity of this enzyme after the different lesioning paradigms by using an in vitro assay. The AADC activity in the intact striatum was very efficient [8.65 ± 0.74 nmol/mg·h1, (–/–/–) group; Fig. 6D] and more than two orders of magnitude higher than the corresponding TH enzyme activity as measured in homogenized tissue samples (data not shown). With the complete dopamine lesion used here, the AADC activity was reduced by 89%, to 0.96 ± 0.04 nmol/mg·h1 ( + /–/– group; Fig. 6D), and the 5,7-DHT lesion further decreased the activity, but 6% still remained after the double lesion (0.54 ± 0.04 nmol/mg·h1, + /–/ + group; Fig. 6D).
Discussion
In this study we utilized the end-stage Parkinson’s disease model in rats to address two important points for successful clinical translation of rAAV-mediated continuous DOPA delivery. First, we investigated if the functional benefits that can be obtained after the TH + GCH1 gene therapy might be compromised in animals with near complete denervation of the host forebrain dopamine system, and whether degeneration of the serotonergic system, as seen in advanced Parkinson’s disease patients, could have detrimental effects after recovery has already been established. We hypothesized that the magnitude of behavioural recovery in animals with near complete dopamine denervation could be significantly enhanced by utilization of high-titre rAAV5 vectors delivered at an optimal ratio beyond what has been possible to achieve so far using AAV2 vectors. Second, we asked whether the tolerance phenomenon described after continuous dopamine stimulation in the clinical setting could compromise the long-term benefits of this treatment approach. In conjunction with this aim, we also tested the capacity this treatment had to protect animals against the development of dyskinesias, which can be induced in the Parkinson’s disease model by chronic l-DOPA treatment.
Dopamine replacement strategies using cell- or gene-based therapies have been shown to yield robust recovery in behavioural tests in rats with intrastriatal dopamine lesions (Kirik et al., 2001). However, in transplanted animals, the same level of recovery has not been possible in models of more advanced disease (Winkler et al., 1999). In addition, while recovery of function in simple (typically drug induced) motor asymmetry tests could be robust and sometimes complete, complex motor function may remain unchanged or only partially restored when there are no spared host dopamine terminals to promote dopamine synthesis and release (Dunnett et al., 1987; Winkler et al., 1999; Kirik et al., 2002).
The integrity of serotonergic innervation could have an impact on both the therapeutic efficacy and side effect profile of l-DOPA treatment. First, serotonin neurons contain the AADC enzyme and vesicular monoamine transporter, which can convert exogenous l-DOPA to dopamine, and store and release it in an activity dependent manner (Ng et al., 1970; Hollister et al., 1979; Arai et al., 1994, 1995, 1996). Non-dopaminergic degeneration, especially loss of the serotonin innervation of the striatum, has been observed in Parkinson’s disease patients (Bernheimer et al., 1961; Chase, 1972; Scatton et al., 1983; Kish et al., 2008). Serotonin neurons have also been shown to play an important role in the induction of l-DOPA-induced dyskinesias in animal models (Carta et al., 2007; Carlsson et al., 2009). Furthermore, our results show that the functional efficacy of gene-based DOPA delivery is maintained in part when serotonin innervation is removed. Therefore, the localization and magnitude of AADC enzyme activity that generates the functional pool of dopamine in TH + GCH1 transduced animals and the functional results obtained after removal of the striatal serotonin innervation are two important findings in this study.
The 5,7-DHT lesion used here efficiently reduced the striatal serotonin content by >97%. Interestingly, the decrease in AADC enzyme activity was less pronounced. The 6-OHDA lesion reduced AADC activity in the dorsal striatum to ∼11% of that measured in the intact striatum. The addition of a second 5,7-DHT lesion further decreased this to ∼6% of normal, suggesting that there is at least one additional compartment with significant AADC activity in this part of the brain. In fact, the presence of this third compartment has been studied extensively and is suggested to be a combination of a population of striatal neurons that have been shown to contain the AADC enzyme (Melamed et al., 1980; Mura et al., 1995), and a non-neuronal fraction (e.g. present in the vascular endothelium; Bertler et al., 1966). Striatal astrocytes may also contribute to this residual AADC activity (Juorio et al., 1987, 1993). Non–AADC-mediated decarboxylase activity seems unlikely since AADC has been shown to be the only contributor to DOPA decarboxylation in the rodent striatum (Kang et al., 1992). It should be noted that while reduction of serotonin innervation is nearly complete in our 5,7-DHT-treated animals, it has been shown to be highly variable in post-mortem analysis of advanced Parkinson’s disease brains (Kish et al., 2008). Therefore, in patients where the serotonin system is spared, striatal serotonin innervation should be able to sustain full therapeutic effect of TH + GCH1 delivery, even in advanced patients. However, as the functional benefits of TH + GCH1 gene therapy were maintained in part also in animals with complete combined dopamine and serotonin denervation, our data suggest that significant motor functional restoration should be possible even in advanced cases where the serotonin system may be affected.
In this study we have seen that complete recovery in a number of spontaneous sensorimotor tests can be achieved by rAAV-mediated striatal DOPA delivery without restoring the storage and release mechanisms for the newly synthesized dopamine. In fact, the level of recovery seen in the stepping and staircase tests is superior to that observed after peripheral l-DOPA delivery or transplantation of foetal midbrain dopamine neurons to the striatum of the same model (Winkler et al., 2002). The remarkable level of behavioural recovery seen in this study clearly demonstrates that anatomical reconstitution of the nigrostriatal pathway is not a necessary requirement for robust functional improvement in a range of motor tests, nor does it seem that restoration of synaptic contacts from dopamine neurons, such as obtained by cell transplantation, is crucial.
The reconstitution of total stored dopamine seen after this mode of DOPA delivery is minor, relative to the intact striatum. However, as the lack of storage and re-uptake mechanisms dramatically increases the dopamine turnover rate, dopamine levels measured from tissue homogenates do not accurately reflect the available dopamine at the synaptic site and thus the therapeutic efficacy. This is supported by a high level of reconstitution of the metabolites (Björklund et al., 2009) and positron emission tomography studies showing that this mode of DOPA delivery fully restores synaptic dopamine neurotransmission (Leriche et al., 2009). The lack of storage and release mechanisms also explains the incomplete normalization observed in the amphetamine-induced rotation test. d-amphetamine acts through a reversal of the dopamine transporter and partial induction of vesicular release (Schwarz et al., 1980; Kuhr et al., 1985). Thus, transplants containing even few dopamine neurons have been very efficient at normalizing this parameter. In the case of TH + GCH1 gene transfer, neither the transporters nor the storage sites are reconstituted, therefore recovery observed in the amphetamine rotation test remains somewhat elusive; but could be attributed to the release of dopamine from the serotonin terminals as a false neurotransmitter.
The level of DOPA reconstitution seen after optimized TH + GCH1 delivery exceeded 8 pmol/mg in the striatal tissue, which was measured without the use of a centrally acting AADC inhibitor (such as NSD-1015), and thus reflects saturation of the available AADC enzyme in the denervated striatum. We have previously estimated l-DOPA accumulation in the striatum to be 1.3 pmol/mg tissue when measured 60 min after peripheral l-DOPA injection without central AADC inhibition in rats with a partial lesion (Kirik et al., 2002). A direct comparison between the two measurements is difficult because vector-mediated DOPA delivery is measured at an equilibrium state whereas the peripheral administration is transient. Nevertheless, the levels obtained after the optimized rAAV-mediated delivery are in the therapeutic range.
Interestingly, the serotonin lesion had no effect on the recovery of the animals in the stepping test. This may suggest that the motor function required to carry out this task is modulated via a tonic stimulation of the dopamine receptors through volume transmission rather than activity-dependent vesicular release at synaptic sites (Ahlenius et al., 1973; Ahlenius, 1974). However, other behaviours appear to be dependent on the release of dopamine (from exogenous DOPA) from the spared striatal serotonin fibres. The reduction in amphetamine-induced turning response in the TH + GCH1-treated animals was coupled to a stress-induced, spontaneous rotation in the opposite direction to that seen in lesion control animals under baseline conditions (clockwise compared to counter-clockwise; data not shown). This stress-evoked rotation disappeared with the lesion to the forebrain serotonin system. Taken together, these data suggested that at least some of the dopamine ectopically synthesized in the TH + GCH1-transduced animals was stored in a form (or at a site) that could be modulated by stress and/or released by d-amphetamine (Kalén et al., 1989; Kirby et al., 1997). Further, the functional recovery initially seen in the cylinder test was lost after lesioning the serotonin system. Thus, it appears that the integrity of the striatal serotonin innervation may determine the magnitude of recovery after this gene therapy. It is interesting to observe the association between the behavioural outcome dependent on the synthesis and release of dopamine from serotonergic terminals and the therapeutic efficacy that can be obtained by single peripheral l-DOPA injections. Pharmacotherapy is very efficient in improving paw use in the cylinder test at the expense of inducing rotational bias. In the forelimb use assessed by the stepping test (not directly dependent on striatal serotonin innervation), on the other hand, the effect of peripheral l-DOPA is minor compared to the full recovery seen after gene therapy. Thus, one could argue that pulsatile l-DOPA provides symptomatic relief via one of the two mechanisms operational after continuous DOPA stimulation, and that this mechanism is serotonin dependent.
Continuous l-DOPA infusion or dopamine stimulation by receptor agonists may induce tolerance, leading to a reduced therapeutic efficacy over time (Mouradian et al., 1990; Nutt et al., 2000), although not all clinical studies reported this phenomenon (Parkinson Study Group, 2003). In cases where tolerance was observed, it could be avoided by dose level switches between wake and sleep hours (Stocchi et al., 2002, 2005). However, dose adjustments in the gene therapy product after initial delivery would not be possible with the available vector constructs. Therefore, if tolerance to DOPA would develop after gene therapy, it would have important implications for its use in patients. In order to address this issue, we monitored the therapeutic efficacy over a 6 month follow-up period after rAAV delivery and found that the animals showed no loss in therapeutic efficacy after TH + GCH1 gene transfer, as the recovery in the cylinder and stepping tests were sustained 23 to 26 weeks post-rAAV injection.
Interestingly, while rAAV-mediated transgene expression is expected to reach near maximum during the first 4 weeks (Mandel et al., 1997; Reimsnider et al., 2007), the performance of the animals in the stepping test continued to improve gradually until 9 to 12 weeks after transduction. This observation seems to suggest that TH + GCH1 gene therapy resulted in slowly developing post-synaptic changes with impact on the therapeutic outcome beyond simply providing a continuous DOPA source. This phenomenon appears to be accompanied by normalization of the lesion-induced dopamine receptor supersensitivity, as the animals are resistant to the development of dyskinesia when subjected to daily peripheral l-DOPA administration. This effect is particularly important as it may allow widening the therapeutic window for peripherally administered l-DOPA, which would possibly yield to further improvements in motor function.
Clinical outlook
To date, all clinical trials using rAAV-mediated gene transfer to the CNS have been carried out using serotype 2 capsids. By utilizing AAV serotype 5 capsids and delivering the two genes GCH1 and TH at an optimized ratio of 1: 5, we show here that therapeutic efficacy could match and surpass previous results obtained using rAAV2 vectors (Kirik et al., 2002; Carlsson et al., 2005), and that this is obtained with a significantly reduced injection volume and reduced number of injection tracts. In a previous study, utilizing the same batches of rAAV5 vectors, we have shown that two 2 µl injections are sufficient to cover >50% of the whole striatal volume and transduce close to 700 000 cells (Björklund et al., 2009). This should be compared with a transduction of 320 000 cells when a larger volume (15 µl in five sites) of rAAV2 was injected (Kirik et al., 2002). In addition, we have recently shown that the serotype 5 vectors are very effective when injected into the striatum of non-human primates, both in the tissue volume covered by each injection and number of cells transduced (Dodiya et al., 2009). These results suggest that TH + GCH1 gene therapy using rAAV5 vectors can readily be applied for studies in non-human primates and also scaled up for use in the larger human brain. Studies in non-human primates are now in progress in order to provide data needed to estimate the minimum therapeutic dose for application in Parkinson’s disease patients.
The present results point to several factors of importance for the selection of patients suitable for AAV-mediated DOPA therapy. Although any patient with Parkinson’s disease showing a robust response to peripheral l-DOPA would be expected to respond positively to TH + GCH1 gene therapy, patients with advanced disease are likely to benefit the most. In these cases, the dopamine denervation is more extensive, and the therapeutic window of standard l-DOPA therapy becomes very narrow, and is complicated by the emergence of severe dyskinesias. The ability to provide symptomatic relief in advanced Parkinson’s disease patients by striatal DOPA delivery is in contrast to alternative gene therapy approaches aimed to modify disease progression (i.e. via delivery of neurotrophic factors). Such therapies depend on the presence of significant residual dopaminergic nigrostriatal projection neurons and should therefore ideally target patients with Parkinson’s disease at early disease stages. Therefore, restorative and neuroprotective gene therapy approaches are complementary and should be pursued for the development of radically new therapies for patients with Parkinson’s disease in the clinic.
Funding
The research leading to these results has received funding from the Swedish Research Council (K2007-61X-14552-05-3, K2008-75SX-20860-01-3, K2009-61P-20945-03-1), the National Institutes of Health (R01 NS055143), the Söderberg foundation (MN45/06) and the European Community’s Seventh Framework Program FP7/2007-2013 under the grant agreement no HEALTH-F5-2008-222925 (NEUGENE program).
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
The authors thank Anneli Josefsson for technical support.
Glossary
Abbreviations
- 5,7-DHT
5,7-dihydroxytyramine
- 6-OHDA
6-hydroxydopamine
- AADC
aromatic l-amino acid decarboxylase
- BH4
5,6,7,8-tetrahydro-l-biopterin
- GCH1
GTP cyclohydrolase-1
- HPLC-EC
high performance liquid chromatography with electrochemical detection
- l-DOPA
l-3,4-dihydroxyphenylalanine
- rAAV
recombinant adeno-associated virus
- TH
tyrosine hydroxylase.
References
- Ahlenius S. Reversal by l-DOPA of the suppression of locomotor activity induced by inhibition of tyrosine-hydroxylase and DA-beta-hydroxylase in mice. Brain Res. 1974;69:57–65. doi: 10.1016/0006-8993(74)90370-9. [DOI] [PubMed] [Google Scholar]
- Ahlenius S, Andén NE, Engel J. Restoration of locomotor activity in mice by low l-DOPA doses after suppression by alpha-methyltyrosine but not by reserpine. Brain Res. 1973;62:189–99. doi: 10.1016/0006-8993(73)90627-6. [DOI] [PubMed] [Google Scholar]
- Arai R, Karasawa N, Geffard M, Nagatsu I. l-DOPA is converted to dopamine in serotonergic fibers of the striatum of the rat: a double-labeling immunofluorescence study. Neurosci Lett. 1995;195:195–8. doi: 10.1016/0304-3940(95)11817-g. [DOI] [PubMed] [Google Scholar]
- Arai R, Karasawa N, Geffard M, Nagatsu T, Nagatsu I. Immunohistochemical evidence that central serotonin neurons produce dopamine from exogenous l-DOPA in the rat, with reference to the involvement of aromatic L-amino acid decarboxylase. Brain Res. 1994;667:295–9. doi: 10.1016/0006-8993(94)91511-3. [DOI] [PubMed] [Google Scholar]
- Arai R, Karasawa N, Nagatsu I. Aromatic L-amino acid decarboxylase is present in serotonergic fibers of the striatum of the rat. A double-labeling immunofluorescence study. Brain Res. 1996;706:177–9. doi: 10.1016/0006-8993(95)01281-8. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O. Distribution of 5-hydroxytryptamine (serotonin) in the human brain and its behavior in patients with Parkinson’s syndrome. Klin Wochenschr. 1961;39:1056–9. doi: 10.1007/BF01487648. [DOI] [PubMed] [Google Scholar]
- Bencsics C, Wachtel SR, Milstien S, Hatakeyama K, Becker JB, Kang UJ. Double transduction with GTP cyclohydrolase I and tyrosine hydroxylase is necessary for spontaneous synthesis of L-DOPA by primary fibroblasts. J Neurosci. 1996;16:4449–56. doi: 10.1523/JNEUROSCI.16-14-04449.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertler A, Falck B, Owman C, Rosengrenn E. The localization of monoaminergic blood-brain barrier mechanisms. Pharmacol Rev. 1966;18:369–85. [PubMed] [Google Scholar]
- Björklund T, Hall H, Breysse N, Soneson C, Carlsson T, Mandel RJ, et al. Optimization of continuous in vivo DOPA production and studies of ectopic DA synthesis using rAAV5 vectors in parkinsonian rats. J Neurochem. 2009;111:355–67. doi: 10.1111/j.1471-4159.2009.06340.x. [DOI] [PubMed] [Google Scholar]
- Björklund T, Kirik D. Scientific rationale for the development of gene therapy strategies for Parkinson’s disease. Biochim Biophys Acta. 2009;1792:703–13. doi: 10.1016/j.bbadis.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Breysse N, Carlsson T, Winkler C, Björklund A, Kirik D. The functional impact of the intrastriatal dopamine neuron grafts in parkinsonian rats is reduced with advancing disease. J Neurosci. 2007;27:5849–56. doi: 10.1523/JNEUROSCI.0626-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377–83. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- Carlsson T, Carta M, Muñoz A, Mattsson B, Winkler C, Kirik D, et al. Impact of grafted serotonin and dopamine neurons on development of l-DOPA-induced dyskinesias in parkinsonian rats is determined by the extent of dopamine neuron degeneration. Brain. 2009;132(Pt 2):319–35. doi: 10.1093/brain/awn305. [DOI] [PubMed] [Google Scholar]
- Carlsson T, Carta M, Winkler C, Björklund A, Kirik D. Serotonin neuron transplants exacerbate l-DOPA-induced dyskinesias in a rat model of Parkinson’s disease. J Neurosci. 2007;27:8011–22. doi: 10.1523/JNEUROSCI.2079-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson T, Winkler C, Burger C, Muzyczka N, Mandel RJ, Cenci A, et al. Reversal of dyskinesias in an animal model of Parkinson’s disease by continuous l-DOPA delivery using rAAV vectors. Brain. 2005;128(Pt 3):559–69. doi: 10.1093/brain/awh374. [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of l-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130(Pt 7):1819–33. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. l-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–706. [PubMed] [Google Scholar]
- Chase TN. Serotonergic mechanisms in Parkinson’s disease. Arch Neurol. 1972;27:354–6. doi: 10.1001/archneur.1972.00490160082011. [DOI] [PubMed] [Google Scholar]
- Chen L, Ding Y, Cagniard B, Van Laar AD, Mortimer A, Chi W, et al. Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. J Neurosci. 2008;28:425–33. doi: 10.1523/JNEUROSCI.3602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodiya H, Bjorklund T, Stansell Iii J, Mandel R, Kirik D, Kordower J. Differential transduction following basal ganglia administration of distinct pseudotyped AAV capsid serotypes in nonhuman primates. Mol Ther. 2009 doi: 10.1038/mt.2009.216. Sep 22. [Epub ahead of print] doi:10.1038/mt.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett SB, Whishaw IQ, Rogers DC, Jones GH. Dopamine-rich grafts ameliorate whole body motor asymmetry and sensory neglect but not independent limb use in rats with 6-hydroxydopamine lesions. Brain Res. 1987;415:63–78. doi: 10.1016/0006-8993(87)90269-1. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23(10 Suppl):S71–7. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Grimm D, Kern A, Rittner K, Kleinschmidt JA. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 1998;9:2745–60. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- Hauswirth WW, Lewin AS, Zolotukhin S, Muzyczka N. Production and purification of recombinant adeno-associated virus. Meth Enzymol. 2000;316:743–61. doi: 10.1016/s0076-6879(00)16760-6. [DOI] [PubMed] [Google Scholar]
- Hollister AS, Breese GR, Mueller RA. Role of monoamine neural systems in L-dihydroxyphenylalanine-stimulated activity. J Pharmacol Exp Ther. 1979;208:37–43. [PubMed] [Google Scholar]
- Hornykiewicz O. Chemical neuroanatomy of the basal ganglia—normal and in Parkinson's disease. J Chem Neuroanat. 2001;22:3–12. doi: 10.1016/s0891-0618(01)00100-4. [DOI] [PubMed] [Google Scholar]
- Howells DW, Hyland K. Direct analysis of tetrahydrobiopterin in cerebrospinal fluid by high-performance liquid chromatography with redox electrochemistry: prevention of autoxidation during storage and analysis. Clin Chim Acta. 1987;167:23–30. doi: 10.1016/0009-8981(87)90081-7. [DOI] [PubMed] [Google Scholar]
- Juorio AV, Li XM, Walz W, Paterson IA. Decarboxylation of L-dopa by cultured mouse astrocytes. Brain Res. 1993;626:306–9. doi: 10.1016/0006-8993(93)90592-b. [DOI] [PubMed] [Google Scholar]
- Juorio AV, Walz W, Sloley BD. Absence of decarboxylation of some aromatic-L-amino acids by cultured astrocytes. Brain Res. 1987;426:183–6. doi: 10.1016/0006-8993(87)90440-9. [DOI] [PubMed] [Google Scholar]
- Kageyama T, Nakamura M, Matsuo A, Yamasaki Y, Takakura Y, Hashida M, et al. The 4F2hc/LAT1 complex transports l-DOPA across the blood-brain barrier. Brain Res. 2000;879:115–21. doi: 10.1016/s0006-8993(00)02758-x. [DOI] [PubMed] [Google Scholar]
- Kang UJ, Park DH, Wessel T, Baker H, Joh TH. Dopa-decarboxylation in the striata of rats with unilateral substantia nigra lesions. Neurosci Lett. 1992;147:53–7. doi: 10.1016/0304-3940(92)90773-z. [DOI] [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Burger C, Winkler C, Muzyczka N, Mandel RJ, et al. Reversal of motor impairments in parkinsonian rats by continuous intrastriatal delivery of L-dopa using rAAV-mediated gene transfer. Proc Natl Acad Sci USA. 2002;99:4708–13. doi: 10.1073/pnas.062047599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Rosenblad C, Björklund A. Delayed infusion of GDNF promotes recovery of motor function in the partial lesion model of Parkinson’s disease. Eur J Neurosci. 2001;13:1589–99. doi: 10.1046/j.0953-816x.2001.01534.x. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Björklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–77. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Kirik D, Winkler C, Björklund A. Growth and functional efficacy of intrastriatal nigral transplants depend on the extent of nigrostriatal degeneration. J Neurosci. 2001;21:2889–96. doi: 10.1523/JNEUROSCI.21-08-02889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Tong J, Hornykiewicz O, Rajput A, Chang LJ, Guttman M, et al. Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain. 2008;131(Pt 1):120–31. doi: 10.1093/brain/awm239. [DOI] [PubMed] [Google Scholar]
- Kuhr WG, Ewing AG, Near JA, Wightman RM. Amphetamine attenuates the stimulated release of dopamine in vivo. J Pharmacol Exp Ther. 1985;232:388–94. [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443–62. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Leriche L, Björklund T, Breysse N, Besret L, Grégoire MC, Carlsson T, et al. Positron emission tomography imaging demonstrates correlation between behavioral recovery and correction of dopamine neurotransmission after gene therapy. J Neurosci. 2009;29:1544–53. doi: 10.1523/JNEUROSCI.4491-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd K, Hornykiewicz O. Parkinson’s disease: activity of L-dopa decarboxylase in discrete brain regions. Science. 1970;170:1212–3. doi: 10.1126/science.170.3963.1212. [DOI] [PubMed] [Google Scholar]
- Mandel RJ, Spratt SK, Snyder RO, Leff SE. Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson’s disease in rats. Proc Natl Acad Sci U S A. 1997;94:14083–8. doi: 10.1073/pnas.94.25.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed E, Hefti F, Wurtman RJ. Nonaminergic striatal neurons convert exogenous L-dopa to dopamine in parkinsonism. Ann Neurol. 1980;8:558–63. doi: 10.1002/ana.410080603. [DOI] [PubMed] [Google Scholar]
- Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991;36:219–28. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- Mouradian MM, Heuser IJ, Baronti F, Chase TN. Modification of central dopaminergic mechanisms by continuous levodopa therapy for advanced Parkinson’s disease. Ann Neurol. 1990;27:18–23. doi: 10.1002/ana.410270105. [DOI] [PubMed] [Google Scholar]
- Muñoz A, Carlsson T, Tronci E, Kirik D, Björklund A, Carta M. Serotonin neuron-dependent and -independent reduction of dyskinesia by 5-HT1A and 5-HT1B receptor agonists in the rat Parkinson model. Exp Neurol. 2009;219:298–307. doi: 10.1016/j.expneurol.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Mura A, Jackson D, Manley MS, Young SJ, Groves PM. Aromatic L-amino acid decarboxylase immunoreactive cells in the rat striatum: a possible site for the conversion of exogenous l-DOPA to dopamine. Brain Res. 1995;704:51–60. doi: 10.1016/0006-8993(95)01104-8. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Yamamoto T, Kato T. A new and highly sensitive voltammetric assay for aromatic L-amino acid decarboxylase activity by high-performance liquid chromatography. Anal Biochem. 1979;100:160–5. doi: 10.1016/0003-2697(79)90126-x. [DOI] [PubMed] [Google Scholar]
- Ng KY, Chase TN, Colburn RW, Kopin IJ. L-Dopa-induced release of cerebral monoamines. Science. 1970;170:76–7. doi: 10.1126/science.170.3953.76. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Obeso JA, Stocchi F. Continuous dopamine-receptor stimulation in advanced Parkinson’s disease. Trends in Neurosciences. 2000;23(Suppl 10):S109–15. doi: 10.1016/s1471-1931(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lancet neurology. 2006;5:677–87. doi: 10.1016/S1474-4422(06)70521-X. [DOI] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15(Pt 2):3863–75. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford Biomedica. 2009. Oxford Biomedica Announces Update On Phase I/II Study Of ProSavin® In Parkinson’s Disease. Available from: http://www.oxfordbiomedica.co.uk/page.asp?pageid=59&newsid=236 (12 October 2009) [Google Scholar]
- Parkinson study group. A controlled trial of rotigotine monotherapy in early Parkinson’s disease. Arch Neurol. 2003;60:1721–8. doi: 10.1001/archneur.60.12.1721. [DOI] [PubMed] [Google Scholar]
- Piccini P, Pavese N, Hagell P, Reimer J, Björklund A, Oertel WH, et al. Factors affecting the clinical outcome after neural transplantation in Parkinson’s disease. Brain. 2005;128(Pt 12):2977–86. doi: 10.1093/brain/awh649. [DOI] [PubMed] [Google Scholar]
- Reimsnider S, Manfredsson FP, Muzyczka N, Mandel RJ. Time course of transgene expression after intrastriatal pseudotyped rAAV2/1, rAAV2/2, rAAV2/5, and rAAV2/8 transduction in the rat. Mol Ther. 2007;15:1504–11. doi: 10.1038/sj.mt.6300227. [DOI] [PubMed] [Google Scholar]
- Romero-Ramos M, Venero JL, Cano J, Machado A. Low selenium diet induces tyrosine hydroxylase enzyme in nigrostriatal system of the rat. Brain Res Mol Brain Res. 2000;84:7–16. doi: 10.1016/s0169-328x(00)00171-6. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275:321–8. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Schallert T, De Ryck M, Whishaw IQ, Ramirez VD, Teitelbaum P. Excessive bracing reactions and their control by atropine and l-DOPA in an animal analog of parkinsonism. Exp Neurol. 1979;64:33–43. doi: 10.1016/0014-4886(79)90003-7. [DOI] [PubMed] [Google Scholar]
- Schallert T, Tillerson JL. Intervention strategies for degeneration of dopamine neurons in parkinsonism: Optimizing behavioral assessment of outcome. In: Emerich DF, Dean RL III, Sanberg PR, editors. Central Nervous System Diseases: Innovative models of CNS diseases from molecule to therapy. Totowa, NJ: Humana Press; 2000. pp. 131–51. [Google Scholar]
- Schwarz RD, Uretsky NJ, Bianchine JR. The relationship between the stimulation of dopamine synthesis and release produced by amphetamine and high potassium in striatal slices. J Neurochem. 1980;35:1120–7. doi: 10.1111/j.1471-4159.1980.tb07867.x. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Ruggieri S, Vacca L, Olanow CW. Prospective randomized trial of lisuride infusion versus oral levodopa in patients with Parkinson’s disease. Brain. 2002;125(Pt 9):2058–66. doi: 10.1093/brain/awf214. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Vacca L, Ruggieri S, Olanow CW. Intermittent vs continuous levodopa administration in patients with advanced Parkinson disease: a clinical and pharmacokinetic study. Arch Neurol. 2005;62:905–10. doi: 10.1001/archneur.62.6.905. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970;24:485–93. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, Bencsics C, Kang UJ. Role of aromatic L-amino acid decarboxylase for dopamine replacement by genetically modified fibroblasts in a rat model of Parkinson’s disease. J Neurochem. 1998;69:2055–63. doi: 10.1046/j.1471-4159.1997.69052055.x. [DOI] [PubMed] [Google Scholar]
- Watson C, Paxinos G. Academic Press San Diego. 1986. The rat brain in stereotaxic coordinates. [Google Scholar]
- Winkler C, Bentlage C, Nikkhah G, Samii M, Bjorklund A. Intranigral transplants of GABA-rich striatal tissue induce behavioral recovery in the rat Parkinson model and promote the effects obtained by intrastriatal dopaminergic transplants. Exp Neurol. 1999;155:165–86. doi: 10.1006/exnr.1998.6916. [DOI] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Björklund A, Cenci MA. l-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson’s disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2002;10:165–86. doi: 10.1006/nbdi.2002.0499. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin, Potter M, Chesnut K, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–85. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ, et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–67. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.