Abstract
The cognitive reserve hypothesis helps to explain the incomplete relationship between brain disease and cognitive status in people with neurologic diseases, including Alzheimer's; disease and multiple sclerosis. Lifetime intellectual enrichment (estimated with education or vocabulary knowledge) lessens the negative impact of brain disease on cognition, such that people with greater enrichment are able to withstand more severe neuropathology before suffering cognitive impairment or dementia. The current research is the first to investigate directly the relationship between intellectual enrichment and an index of cerebral activity (the blood oxygen level dependent signal) in a neurologic sample. Multiple sclerosis patients completed a vocabulary-based estimate of lifetime intellectual enrichment. Disease severity was estimated with brain atrophy. Cognitive status was measured with the Symbol Digit Modalities Test. Cerebral activity (functional magnetic resonance imaging blood oxygen level dependent signal) and behavioural performance (accuracy, reaction time) were recorded during the visual N-Back working memory task (three levels of demand: 0-, 1-, 2-Back). All patients produced perfect/nearly perfect accuracy during lower demands (0- and 1-Back), and reaction time was unrelated to intellectual enrichment; however, voxelwise partial correlations controlling for brain atrophy revealed strong positive correlations between intellectual enrichment and cerebral activity within the brain's; default network (e.g. anterior and posterior cingulate corticies), indicating that patients with greater enrichment were able to maintain resting state activity during cognitive processing better. In turn, intellectual enrichment was negatively associated with prefrontal recruitment, suggesting that patients with lesser enrichment required more cerebral resources to perform the same cognitive task as patients with greater enrichment. This same pattern of enrichment-related cerebral activity was observed when cognitive demands increased (2-Back), and intellectual enrichment was negatively associated with reaction time. Principle components analysis revealed a single cognitive reserve network across tasks (greater default network, lesser prefrontal recruitment). Expression of this network almost fully mediated the positive relationship between intellectual enrichment and cognitive status (Symbol Digit Modalities Test). Also, expression of this network was positively associated with brain atrophy when controlling for cognitive status, indicating that patients with greater expression of this network can withstand more severe brain disease before exhibiting cognition similar to patients with lesser network expression. Of note, similar functional magnetic resonance imaging research with healthy adults has not found an association between intelligence and cerebral efficiency. The unique relationship between intellectual enrichment and cerebral efficiency in neurologic patients is consistent with the cognitive reserve hypothesis, which does not posit that enrichment leads to gains in neurocognitive functioning per se; rather, enrichment protects against neurocognitive decline secondarily to disease.
Keywords: cognitive reserve, functional MRI, multiple sclerosis, Alzheimer's; disease, default network
Introduction
Numerous studies have shown that intellectual enrichment (estimated with educational attainment or vocabulary knowledge) is protective against cognitive impairment in patients with neurologic disease, including Alzheimer's; disease (Stern et al., 1992; Alexander et al., 1997; Bennett et al., 2003; Stern, 2006; Roe et al., 2008), stroke (Elkins et al., 2006), and multiple sclerosis (Sumowski et al., 2009a, b). In fact, educational attainment actually reduces the negative effect of neurotic plaques (Bennett et al., 2003) and fibrillar β-amyloid (Roe et al., 2008) on cognition, and vocabulary knowledge lessens the negative effect of brain atrophy on cognitive efficiency in multiple sclerosis patients (Sumowski et al., 2009b). To explain these findings, Stern posited that intellectual enrichment is associated with cerebral efficiency, which provides a ‘cognitive reserve’ against disease-related cognitive impairment (Stern et al., 2002, 2005). In other words, when neurocognitive processing is challenged by brain disease, people with greater cerebral efficiency prior to their disease are able to withstand acquired neurocognitive challenges better, without suffering cognitive impairment. There is ample neuropsychological evidence for the cognitive reserve hypothesis across neurologic populations, but functional neuroimaging studies of cognitive reserve are limited to healthy samples (Stern, 2009). As such, there is no functional neuroimaging research we are aware of investigating the relationship between intellectual enrichment and cerebral activity in people with neurologic disease. Such research is critical to more directly investigate the cognitive reserve hypothesis more directly, namely, that intellectual enrichment protects persons with neurologic disease from disease-related cerebral inefficiency.
The current study uses functional magnetic resonance imaging (fMRI) to investigate the relationship between lifetime intellectual enrichment and patterns of cerebral activity in patients with multiple sclerosis. Several fMRI studies have already established the pattern of cerebral activity shown by multiple sclerosis patients when they are engaged in working memory tasks similar to the one used here. This provides a useful context for the current research. More specifically, to produce cognitive performance similar to healthy controls, multiple sclerosis patients require (i) greater recruitment of prefrontal cortical regions (Audoin et al., 2005; Sweet et al., 2006; Forn et al., 2007), especially the inferior frontal gyri; and (ii) greater deactivation of the anterior cingulate cortex (Sweet et al., 2006; Morgen et al., 2007) and posterior cingulate cortex (Sweet et al., 2006). The anterior and posterior cingulate cortices are the core components of the brain's; default network, which consists of brain regions more active during rest or passive thought than directed cognitive processing (Buckner et al., 2008). This network extends to the neighbouring medial frontal and precuneas regions, as well as mesial and lateral aspects of the temporal lobes. In healthy people, the default network deactivates proportionately with (i) increased cognitive task demands (Singh and Fawcett, 2008); and (ii) the increased recruitment of prefrontal cortex (Greicius et al., 2003). Taken together, the findings of the multiple sclerosis literature (patients with multiple sclerosis demonstrate greater recruitment of prefrontal cortex and greater deactivation of the brain’s default/resting state network to maintain similar cognitive performance as healthy individuals) when considered in light of the findings from the default network literature (decreased default network activity with increasing task demands and prefrontal cortex involvement) suggest an inefficient pattern of cerebral activity in multiple sclerosis.
The cognitive reserve hypothesis posits that intellectual enrichment is associated with cerebral efficiency (Stern et al., 2002, 2005). As such, multiple sclerosis patients with greater lifetime intellectual enrichment should produce a more efficient pattern of cerebral activation characterized by (i) greater preservation of activity within the brain's; default/resting network; and (ii) less recruitment of prefrontal cortical regions, even during tasks with lower cognitive demands. Owing to this greater cerebral efficiency, (iii) patients with greater intellectual enrichment should also be able to maintain behavioural performance better as cognitive task demands increase. These predicted results would help explain the preserved cognition among neurologic patients with greater intellectual enrichment in previous neurologic samples (Sumowski et al., 2009a, b).
Methods
Participants
Participants were 18 right-handed patients (15 female, 3 male) with clinically definite multiple sclerosis (McDonald et al., 2001) recruited from local multiple sclerosis clinics and the North Jersey chapter of the National Multiple Sclerosis Society. Participants were only recruited if they (i) did not have an exacerbation of their multiple sclerosis during the last month; (ii) were not currently taking glucocorticosteroid medication; (iii) were not currently under the care of a physician for any other major medical condition; and (iv) had no history of serious psychiatric illness or neurologic disease other than multiple sclerosis. English was the primary language of all participants. Mean age was 43.8 ± 7.0 years with 15.6 ± 2.3 years of education. Mean disease duration was 9.5 ± 6.3 years, and multiple sclerosis subtypes included relapsing-remitting (n = 14) and chronic progressive (n = 4) courses. The institutional review boards at the University of Medicine and Dentistry, New Jersey, and the Kessler Foundation Research Centre granted approval for the study. Informed consent was obtained from all subjects prior to participation.
Estimate of multiple sclerosis disease progression: brain atrophy
High-resolution 3D MRI images of the brain were acquired from all participants using a magnetization prepared rapid gradient echo sequence performed in a 3T Siemens Allegra scanner (echo time = 4.38 ms; repetition time = 2000 ms, field of veiw = 220 mm; flip angle = 8°; slice thickness = 1 mm, number of excitations = 1, matrix = 256 × 256, in-plane resolution = 0.859 mm2). Consistent with established procedures (Benedict et al., 2006a; Benedict et al., 2004), multiple sclerosis disease severity was estimated with brain atrophy measurements of third ventricle width, which is currently among the best MRI predictors of cognitive dysfunction in patients with multiple sclerosis (Bermel and Bakski, 2006). As third ventricle width is unrelated to adult intracranial volume (Gyldensted, 1977), it was not indexed on brain size in either the current or previous research (Benedict et al., 2006a, b). Third ventricle width was independently measured by two trained raters using procedures reported elsewhere (Sumowski et al., 2009b), with high intraclass correlations for both inter-rater reliability (r = 0.96) and test–retest reliability (r > 0.98). The mean third ventricle width of 5.26 ± 1.89 mm of our sample was comparable to, albeit slightly higher than, that of same-age multiple sclerosis patients in previous research (4.10 mm, Benedict et al., 2006a). Third ventricle width was used as the estimate of multiple sclerosis disease progression in subsequent analyses.
Estimate of intellectual enrichment: vocabulary knowledge
Intellectual enrichment was estimated with the vocabulary task of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), which assesses depth and breadth of acquired vocabulary knowledge. Enriching life activities such as educational attainment (Staff et al., 2004) and frequent reading (Stanvich and Cunningham, 1992, 1993) are unique and significant contributors to vocabulary knowledge in adults, even when controlling for general intellectual ability. Vocabulary knowledge is robust against decline in neurological disease (Lezak, 2004), including multiple sclerosis (Chiaravalloti and DeLuca, 2008; Sumowski et al., 2009b). As such, vocabulary tasks provide an accepted estimate of acquired knowledge/intellectual enrichment prior to the onset of neurological disease, and such tasks have been used in previous cognitive reserve research (Stern, 2006). Within the current sample, vocabulary knowledge was comparable to the normative sample of healthy adults (mean T = 51.3 ± 8.0), and ranged from slightly below normal (T = 39) to well above normal (T = 68). As expected, vocabulary knowledge was unrelated to multiple sclerosis disease progression (r = 0.31, P > 0.2; this correlation would be negative if atrophy negatively impacted vocabulary). Consistent with this non-significant correlation, recent research with a larger sample found no relationship between third ventricle width and vocabulary knowledge (r = −0.09; Sumowski et al., 2009b). Vocabulary raw scores were used to estimate lifetime intellectual enrichment in subsequent analyses.
Brief evaluation of current cognitive status
Cognitive inefficiency in the form of slowed processing speed is the most prevalent cognitive deficit among multiple sclerosis patients (Chiaravalloti and DeLuca, 2008). As such, current cognitive status was estimated using a processing speed task, namely, the Symbol Digit Modalities Test (SDMT) (Smith, 1982). Among neuropsychological tasks chosen by a consensus group for use with multiple sclerosis patients (Benedict et al., 2002), the SDMT was identified as the most sensitive task for discriminating between multiple sclerosis patients and healthy controls (Benedict et al., 2002b), and is also most strongly associated with MRI estimates of multiple sclerosis disease severity (e.g. brain atrophy; Benedict et al., 2004, 2006b). The mean SDMT performance of the current sample was below average (Z = −0.8), but ranged from severely impaired (Z = −3.4) to above average (Z = 1.8).
Blood oxygen level dependent percent signal change and behavioural performance on N-Back
The fMRI blood oxygen level dependent (BOLD) signal was acquired in the 3T MRI scanner (echo time = 30 ms; repetition time = 2000 ms; field of veiw = 22 cm; flip angle = 80°; slice thickness = 4 mm, matrix = 64 × 64, in-plane resolution = 3.438 mm2), and cerebral activity was examined during three levels of cognitive demand on the visual N-Back working memory task: 0-Back (lowest demand); 1-Back (intermediate demand); 2-Back (highest demand). The three tasks were presented in a counterbalanced block design. During the 0-Back task, participants viewed a series of single letters and responded with a button press when a target letter (e.g. ‘J’) was displayed. During the 1-Back task, participants again viewed a series of letters, but instead responded when the letter presented was the same as the letter immediately preceding it in the series (e.g. ‘F C J J’). During the 2-Back task, participants responded when the letter presented was the same as the letter presented two letters prior (e.g. ‘D J G J’). Stimuli were presented with the E-Prime presentation software, which also recorded participants’ behavioural performance (accuracy and reaction time). The high-resolution magnetization prepared rapid gradient echo image was used for localization of the fMRI activity. The fMRI data were realigned, and smoothed using the Analysis of Functional NeuroImages suite of image-analysis programs (Cox, 1996). They were then deconvolved, using a delayed boxcar function to model the haemodynamic response. The dependent variables extracted from the data were blood oxygen level dependent (BOLD) percent signal change during the 0-Back, 1-Back and 2-Back tasks relative to rest. The data were normalized to standard, Talairach space using a non-parametric method for estimating displacement fields (ART software, Ardekani et al., 2005).
Statistical analysis
Intellectual enrichment and cognitive status
We first sought to determine whether intellectual enrichment protects persons with multiple sclerosis from the negative effects of brain atrophy on cognitive status. Consistent with methods of previous research (Alexander et al., 1997), we calculated a partial correlation between brain atrophy (third ventricle width) and intellectual enrichment (Wechsler Abbreviated Scale of Intelligence vocabulary) while controlling for cognitive status (SDMT). We predict a significant positive correlation, which would indicate that multiple sclerosis patients with higher intellectual enrichment withstand more severe brain atrophy before exhibiting cognitive status similar to patients with lesser reserve.
Behavioural performance and cerebral activity
Behavioural performance during the N-Back was measured with dependent variables of accuracy (percent correct) and reaction time (milliseconds to respond during correct trials) for each N-Back trial, and these behavioural variables were analysed with separate repeated measures analyses of variance investigating the effect of N-Back trial (0-, 1-, 2-Back) on accuracy and reaction time.
To assure that the pattern of cerebral activation on the N-Back in the current sample was comparable to that which has been reported in the literature (Sweet et al., 2006), we performed a t-test for each of the N-Back trials (0-, 1-, 2-Back), in which we compared cerebral activity to the null hypothesis (activity during rest).
Relationship between intellectual enrichment, behavioural performance and cerebral activity
For our primary analyses investigating the effect of intellectual enrichment on behavioural performance and cerebral activity, we performed partial correlations between reaction time and intellectual enrichment (Wechsler Abbreviated Scale of Intelligence vocabulary raw score) for each N-Back trial (0-, 1-, 2-Back), while controlling for brain atrophy (third ventricle width). Regarding cerebral activation, voxelwise partial correlations were performed between intellectual enrichment and percent signal change during each N-Back trial (0-, 1-, 2-Back), while controlling for brain atrophy. For the fMRI analyses, the threshold for significance was set at an alpha of 0.01 and we controlled for multiple comparisons by setting the required cluster size at 10 voxels in native space (3.438 × 3.438 × 4 mm), or 500 voxels after the data were resampled to 1 mm isotropic voxels. This cluster threshold correction technique controls false positives while also sparing statistical power (Forman et al., 1995).
Neural basis of cognitive reserve
After identifying areas of cerebral activation which are positively and negatively associated with intellectual enrichment, we investigated whether such patterns represent the neural basis of cognitive reserve. Percent signal change was quantified and averaged within the identified brain regions for each subject, and values were entered into a principle component analysis to create a cognitive reserve network variable. If this network represents the neural basis of cognitive reserve, then (i) expression of this network (i.e. increased activity in areas positively correlated, decreased activity in areas negatively correlated) should mediate the relationship between intellectual enrichment and current cognitive status; and (ii) patients with greater expression of this network should be able to withstand more severe brain disease before showing cognitive impairment. We used multiple regressions to determine the amount of variance in current cognitive status (SDMT) that is explained by (i) intellectual enrichment alone; and then (ii) intellectual enrichment controlling for expression of the identified network. We expect the identified network to explain most (if not all) of the relationship between intellectual enrichment and SDMT performance, thereby representing the neural basis for the relationship between enrichment and cognitive status. Finally, we investigated whether expression of the identified network protects patients with multiple sclerosis' multiple sclerosis cognitive status from the effects of disease. More specifically, we predict a positive association between expression of the identified network and brain atrophy when controlling for SDMT performance. In other words, patients with greater expression of this network should be able to withstand more severe multiple sclerosis disease before their cognitive status declines to the level of patients with lesser expression of this network. This would support inferences about this network as the neural basis of cognitive reserve, at least for multiple sclerosis patients.
Results
Intellectual enrichment and cognitive status
As stated in the Methods section, there is no relationship between intellectual enrichment and brain atrophy in the current study (P > 0.2) or in previous research (Sumowski et al., 2009b). Despite this, a significant positive partial correlation between intellectual enrichment and brain atrophy emerged when controlling for cognitive status (r = 0.66, P < 0.005). In other words, it takes more severe brain atrophy to bring multiple sclerosis patients with higher intellectual enrichment down to the same cognitive status of patients with lesser enrichment. This is consistent with the hypothesis that people with higher intellectual enrichment are able to withstand more severe brain disease before demonstrating cognitive dysfunction.
Behavioural performance and cerebral activity
There was main effect of N-Back trial on performance accuracy (F = 31.5, P < 0.001), as all patients produced perfect/nearly perfect accuracy during the 0-Back (0.99 ± 0.03) and 1-Back (0.99 ± 0.03) tasks, but accuracy declined during the more challenging 2-Back task (0.85 ± 0.11; P < 0.001). There was a similar main effect of N-Back trial on reaction time (F = 19.3, P < 0.001), such that reaction times on the 0-Back (640.9 ± 140.1) and 1-Back (680.3 ± 168.3) tasks were significantly faster than during the 2-Back (806.1 ± 175.7; P < 0.001).
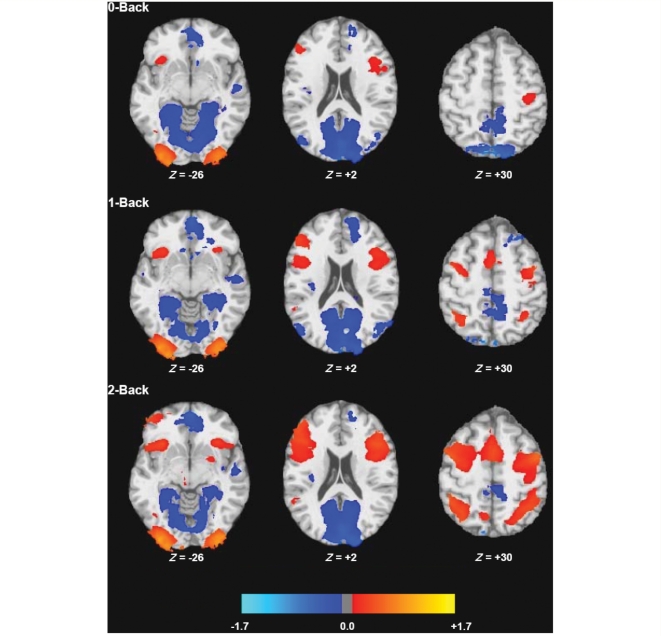
Patterns of cerebral activation were consistent with N-Back results in previous research (Sweet et al., 2006; Forn et al., 2007). More specifically, patients with multiple sclerosis deactivated regions of the brain's; default network across N-Back trials, including the anterior cingulate cortex/medial frontal, posterior cingulate cortex/precuneas, and mesial/lateral temporal areas (Fig. 1 and Table 1). Increased activation was most prominent in prefrontal regions, and appeared to increase with increased task demands. In other words, the behavioural and fMRI characteristics of our sample are consistent with previous samples of multiple sclerosis patients.
Figure 1.
Statistical parametric maps for the BOLD percent signal changes for each of the N-Back tasks relative to rest (P < 0.01, cluster size ≥500 ml). Colour shading represents percent signal change, with negative and positive values indicating deactivation and activation, respectively. Specific brain areas are presented in Table 1.
Table 1.
Regions that exhibited activation or deactivation on the N-Back task relative to rest
| Task | Sign | Voxels | X | Y | Z | Hemisphere | Anatomical location |
|---|---|---|---|---|---|---|---|
| 0-Back | Activation | 14538 | +32 | −76 | −31 | Right | Occipital pole |
| 14530 | −33 | −75 | −36 | Left | Occipital pole | ||
| 4577 | −45 | +28 | +7 | Left | IFG, precentral gyrus | ||
| 1512 | +47 | +51 | +3 | Right | MFG | ||
| 1405 | +35 | +39 | −21 | Right | IFG, insula | ||
| 1060 | −36 | −5 | +37 | Left | Precentral gyrus | ||
| Deactivation | 174283 | −2 | −51 | +6 | Right | PCC, precuneas, MTG | |
| Left | PCC, precuneas, MTG, PHG | ||||||
| 15154 | −6 | +68 | −19 | Right | ACC, medial frontal | ||
| Left | ACC, medial frontal | ||||||
| 4924 | −20 | +49 | +17 | Left | Medial frontal | ||
| 3890 | +23 | +13 | −46 | Right | PHG | ||
| 2882 | +38 | 0 | −7 | Right | Insula | ||
| 1879 | +23 | +43 | +19 | Right | Cingulate | ||
| 662 | +65 | +1 | −16 | Right | STG | ||
| 648 | −27 | +17 | −47 | Left | PHG | ||
| 544 | +5 | −9 | +44 | Right | Medial frontal | ||
| 1-Back | Activation | 24471 | +44 | +35 | +5 | Right | IFG, MFG, insula, precentral gyrus |
| 16083 | −44 | +25 | +13 | Left | IFG, insula, precentral gyrus | ||
| 15642 | +32 | −76 | −31 | Right | Occipital pole | ||
| 13799 | −33 | −75 | −35 | Left | Occipital pole | ||
| 10577 | +38 | −33 | +25 | Right | IPL, SPL | ||
| 1663 | −45 | −20 | +24 | Left | IPL | ||
| 1582 | +43 | +69 | −20 | Right | IFG | ||
| 1398 | −41 | −47 | −64 | Left | Cerebellar tonsil | ||
| 787 | +47 | −44 | −43 | Right | Declive | ||
| 511 | +55 | −29 | −5 | Right | STG | ||
| Deactivation | 135451 | −1 | −46 | +1 | Right | PCC, precuneas, PHG, insula | |
| Left | PCC, precuneas, PHG | ||||||
| 33709 | −8 | +63 | −12 | Right | ACC, medial frontal | ||
| Left | ACC, medial frontal | ||||||
| 7939 | −55 | +15 | −40 | Left | MTG, ITG | ||
| 5440 | +3 | +33 | +27 | Right | Cingulate, medial frontal | ||
| Left | Cingulate, medial frontal | ||||||
| 3454 | −32 | −38 | +27 | Left | IPL, SPL | ||
| 3234 | +49 | −56 | −3 | Right | MTG | ||
| 2152 | +58 | +11 | −38 | Right | MTG | ||
| 1951 | +23 | +45 | +18 | Right | Cingulate | ||
| 1669 | +3 | −4 | +32 | Right | Cingulate | ||
| 1010 | −39 | +3 | −9 | Left | Insula | ||
| 1010 | +13 | +73 | +3 | Right | Medial frontal | ||
| 921 | +38 | −8 | +52 | Right | Precentral gyrus | ||
| 694 | −57 | −11 | −10 | Left | STG | ||
| 664 | +63 | +8 | +15 | Right | Precentral gyrus | ||
| 2-Back | Activation | 76669 | +32 | +36 | +13 | Right | IFG, insula, precentral gyrus |
| 41656 | −43 | +26 | +15 | Left | IFG, MTG, insula, precentral gyrus | ||
| 32075 | −36 | −67 | −42 | Left | Occipital pole | ||
| 24662 | +35 | −72 | −34 | Right | Occipital pole | ||
| 20626 | +37 | −35 | +24 | Right | IPL, SPL | ||
| 17311 | −36 | −34 | +28 | Left | IPL, SPL | ||
| 2802 | −19 | +17 | −16 | Left | Lentiform nucleus, putamen | ||
| 1943 | +14 | +14 | −11 | Right | Thalamus | ||
| 745 | +51 | −13 | −36 | Right | MTG | ||
| 683 | +68 | −19 | −33 | Right | |||
| 657 | +9 | −45 | +34 | Right | Precuneas | ||
| Deactivation | 118810 | −2 | −53 | −2 | Right | PCC, precuneas, cuneas | |
| Left | PCC, precuneas, cuneas | ||||||
| 18944 | −5 | +65 | −18 | Right | ACC, medial frontal | ||
| Left | ACC, medial frontal | ||||||
| 4212 | −54 | +12 | −36 | Left | MTG | ||
| 2795 | +48 | +4 | −11 | Right | Insula | ||
| 879 | +52 | −58 | −10 | Right | MTG |
IFG = inferior frontal gyrus; MFG = middle frontal gyrus; PCC = posterior cingulate cortex, ACC = anterior cingulate cortex, ITG = inferior temporal gyrus; MTG = middle temporal gyrus; STG = superior temporal gyrus; PHG = parahippocampal gyrus; IPL = inferior parietal lobule; SPL = superior parietal lobule. Cluster sizes are reported as the number of active voxels in millilitres. Coordinates, reported in Talairach space, emerged at a threshold of P < 0.01, cluster size ≥ 500 ml.
Relationship between intellectual enrichment, behavioural performance and cerebral activity
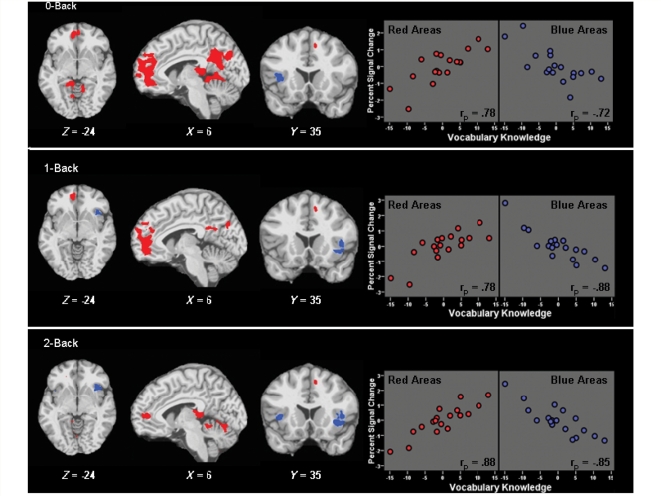
One of our primary questions was whether neurocognitive functioning differs due to differences in intellectual enrichment. All patients produced perfect/near-perfect accuracy during the lower cognitive demands of the 0-Back and 1-Back tasks, and reaction time was unrelated to intellectual enrichment during both tasks (0-Back: rp = −0.03, P > 0.5; 1-Back: rp = −0.36, P > 0.1). In other words, multiple sclerosis patients produced comparable behavioural performance regardless of intellectual enrichment. In contrast, fMRI results during the lower cognitive demands of the 0-Back and 1-Back tasks revealed strong positive partial correlations between intellectual enrichment and activation within the brain's; default network (anterior cingulate cortex/medial frontal, posterior cingulate cortex/precuneas, lateral temporal), and strong negative associations with prefrontal cortex (inferior frontal gyri; Fig. 2 and Table 2). To characterize the strength of these associations, we used Analysis of Functional NeuroImages to create masks of these areas of significant positive (red) and negative (blue) association, calculated the percent signal change for all participants within each masked area, and performed partial correlations between vocabulary knowledge and the total percent signal change within masked areas while controlling for brain atrophy. As depicted in the scatterplots within Fig. 2, intellectual enrichment accounted for 52–77% of the variance within these masked areas for the 0-Back and 1-Back tasks. Taken together, behavioural performance was similar for all multiple sclerosis patients during the lower cognitive demands of the 0-Back and 1-Back tasks, but multiple sclerosis patients with greater intellectual enrichment met these demands with less deactivation of the default network and less recruitment of prefrontal cortex.
Figure 2.
Association between intellectual enrichment and cerebral activity during the N-Back. Statistical maps depict brain regions with significant positive (red) or negative (blue) partial correlations between vocabulary knowledge and BOLD percent signal change, controlling for brain atrophy (P < 0.01; voxel cluster ≥ 500 ml). Scatterplots depict partial correlations between vocabulary knowledge and percent signal change within masked areas of positive (red) and negative (blue) association (P < 0.001). Within scatterplots, percent signal change values (Y-axis) are presented as sample-based z-scores, and vocabulary knowledge (X-axis) is centred at 0. Specific brain regions are listed in Table 2.
Table 2.
Regions positively or negatively associated with vocabulary knowledge across N-Back trials, controlling for disease severity
| Task | Sign | Voxels | X | Y | Z | Hemisphere | Anatomical location |
|---|---|---|---|---|---|---|---|
| 0-Back | Positive | 19643 | −1 | −40 | +1 | Right | PCC, precuneas, cingulate |
| Left | PCC, precuneas, cingulate | ||||||
| 11904 | +6 | +71 | −8 | Right | ACC, medial frontal | ||
| Left | ACC, medial frontal | ||||||
| 4926 | +59 | +11 | −51 | Right | ITG, MTG | ||
| 2989 | −9 | +51 | +23 | Left | Medial frontal, cingulate | ||
| 1608 | +7 | −13 | −11 | Right | PCC | ||
| 760 | +21 | +48 | +21 | Right | Medial frontal | ||
| Negative | 931 | +40 | +35 | −11 | Right | IFG, insula | |
| 544 | +33 | +44 | +4 | Right | MFG | ||
| 1-Back | Positive | 9020 | +7 | +69 | −8 | Right | ACC, medial frontal |
| Left | ACC | ||||||
| 1792 | +63 | +5 | −42 | Right | ITG, MTG | ||
| 1722 | +56 | +18 | −60 | Right | ITG, MTG | ||
| 1585 | −12 | +50 | +21 | Left | Medial frontal, cingulate | ||
| 836 | −11 | −28 | +12 | Left | PCC, cingulate | ||
| 767 | +8 | −32 | +8 | Right | PCC | ||
| 675 | +2 | −59 | +14 | Right | Precuneas, cuneas | ||
| Left | Precuneas, cuneas | ||||||
| Negative | 1030 | −39 | +39 | +19 | Left | IFG, insula | |
| 2-Back | Positive | 2839 | +60 | +13 | −50 | Right | ITG, MTG |
| 2039 | +7 | +65 | −14 | Right | ACC | ||
| Left | ACC | ||||||
| 1349 | +11 | −15 | +10 | Right | Retrosplenial cortex | ||
| 1108 | +11 | −31 | −29 | Right | Culmen | ||
| 862 | +14 | −37 | −71 | Right | Cerebellar tonsil | ||
| 801 | −8 | +45 | +23 | Left | Medial frontal, cingulate | ||
| 630 | +5 | −49 | −26 | Right | Lingual gyrus | ||
| Negative | 1748 | −39 | +37 | −19 | Left | IFG, insula | |
| 711 | −18 | −48 | +41 | Left | SPL | ||
| 503 | +40 | +33 | −16 | Right | Insula |
IFG = inferior frontal gyrus; MFG = middle frontal gyrus; PCC = posterior cingulate cortex; ACC = anterior cingulate cortex; ITG = inferior temporal gyrus; MTG = middle temporal gyrus; SPL = superior parietal lobule. Cluster sizes are reported as the number of active voxels in millilitres. Coordinates, reported in Talairach space, emerged at a threshold of P < 0.01, cluster size ≥ 500 ml.
When cognitive demands increased during the 2-Back, intellectual enrichment was again positively associated with default network activity and negatively associated with prefrontal recruitment (Fig. 2, Table 2). As depicted in the scatterplots within Fig. 2, intellectual enrichment accounted for 72–77% of the variance within areas of significant positive and negative association, respectively. Regarding behavioural performance, there was an inverse association between intellectual enrichment and reaction time (2-Back: rp = −0.49, P < 0.05), such that patients with greater intellectual enrichment processed the task more quickly. There was also a trend toward greater intellectual enrichment in patients who retained at least 75% accuracy (rp = 0.45, P = 0.07). Taken together, patients with multiple sclerosis with greater intellectual enrichment continued to show less deactivation of the default network and less recruitment of prefrontal cortex during the higher cognitive demands of the 2-Back task; however, unlike lower task demands, patients with multiple sclerosis with greater intellectual enrichment were also able to maintain cognitive performance better.
Neural basis of cognitive reserve
The network of brain areas identified in previous analyses may represent the neural basis of cognitive reserve. Percent signal change in each of the six masks (i.e. positive and negative masks for each N-Back trial, Fig. 2) were entered into a principle component analysis, which yielded one component accounting for 80.3% of the variance in all masked areas. Positive masks had positive loadings (0.85–0.94) and negative masks had negative loadings (−0.82 to −0.93) with the derived neural network component score. If expression of this network (i.e. higher default network, lower prefrontal recruitment) represents the neural basis of cognitive reserve, then expression of this network should mediate the relationship between intellectual enrichment and current cognitive status. Although intellectual enrichment accounted for about one-third of the variance in SDMT performance controlling for brain atrophy (R2 = 0.32, P < 0.005), this association essentially disappeared when controlling for expression of the identified neural network (R2 = 0.01, P > 0.1). Stated differently, the identified neural network accounted for about 97% of the relationship between intellectual enrichment and cognitive status, suggesting little to no unique contribution of intellectual enrichment to cognitive status over-and-above the identified network. Finally, in a stepwise regression entering brain atrophy, intellectual enrichment, and the identified neural network, only brain atrophy (R2 = 0.27, P < 0.01) and expression of the neural network (R2 = 0.35, P < 0.01) accounted for significant unique variance.
These findings suggest that the identified neural network mediates the relationship between intellectual enrichment and cognitive status, and that the network may therefore represent the neural basis of cognitive reserve. As one final test of this hypothesis, we repeated the partial correlation between intellectual enrichment and brain atrophy controlling for SDMT (see first paragraph of ‘Results’ section); however, we replaced intellectual enrichment with the identified neural network. As expected, there was no association between expression of the neural network and brain atrophy alone (r = −0.04, P > 0.5); however, a significant positive partial correlation emerged when controlling for SDMT (rp = 0.42, P < 0.05, one-tailed). In other words, it takes more severe brain disease to bring multiple sclerosis patients with greater expression of this network down to the cognitive status of patients with lesser expression of this network. Therefore, greater maintenance of the default network and lower need for prefrontal recruitment may well represent the neural basis of cognitive reserve in people with multiple sclerosis.
Discussion
The cognitive reserve hypothesis states that people with greater intellectual enrichment are able to withstand more severe brain disease before suffering cognitive impairment (Stern, 2009). This finding has been demonstrated for patients with multiple sclerosis in the current study, as well as in previous research (Sumowski et al., 2009b). To explain this phenomenon, Stern posited that intellectual enrichment is associated with greater cerebral efficiency, which helps patients to cope better with the neurocognitive challenges associated with brain disease (Stern et al., 2002, 2005). The current study used fMRI to investigate the relationship between intellectual enrichment and cerebral activity more directly in patients with neurological disease. We show that intellectual enrichment is positively associated with default/resting state activity and negatively associated with prefrontal recruitment during cognitive processing in patients with multiple sclerosis. Stated differently, multiple sclerosis patients with greater intellectual enrichment require less deactivation of the brain's; default/resting state and less recruitment of prefrontal cortices to perform the same tasks as patients with lesser enrichment. We propose that the identified pattern of enrichment-related cerebral activity (maintenance of default network, lower prefrontal recruitment) may represent the neural basis of cognitive reserve (at least for multiple sclerosis patients), especially because (i) expression of this network appears to mediate the relationship between intellectual enrichment and current cognitive status; and (ii) persons with greater expression of this network are able to withstand more severe brain disease before exhibiting cognitive status similar to patients with lesser enrichment. Furthermore, the pattern of cerebral activity associated with intellectual enrichment is opposite to the pattern associated with multiple sclerosis disease in previous research (lower default network, greater prefrontal recruitment; e.g. Sweet et al., 2006), thereby suggesting that multiple sclerosis patients with higher intellectual enrichment show reduced expression of multiple sclerosis-related patterns of cerebral activity.
Previous cognitive reserve research has shown that intellectual enrichment protects against observable cognitive dysfunction in neurologic samples (Stern, 2009). By directly measuring an index of cerebral activity (the BOLD signal), we showed that intellectual enrichment is strongly related to differences in cerebral activity even before changes in cognitive functioning are evident (0-Back and 1-Back). Consistent with the cognitive reserve hypothesis, we posit that greater cerebral efficiency evident during simple cognitive tasks represents a reserve against cognitive decline on more challenging tasks (e.g. 2-Back). In other words, due to greater cerebral efficiency in the face of disease, multiple sclerosis patients with greater enrichment are less likely to exhaust cerebral resources when task demands increase (e.g. 2-Back), thereby permitting greater maintenance of observable cognitive performance. We posit, therefore, that cerebral efficiency is the vehicle by which intellectual enrichment protects against disease-related cognitive dysfunction.
Prefrontal recruitment and default network activity: markers of cerebral efficiency
A key premise of this research is that increased prefrontal recruitment and decreased default network activity are markers of cerebral inefficiency. As reviewed, multiple sclerosis patients require greater prefrontal recruitment to maintain cognitive performance relative to healthy controls (Audoin et al., 2005; Sweet et al., 2006; Forn et al., 2007). This pattern of increased prefrontal recruitment is also typical of neurological diseases in general, including traumatic brain injury (McAllister et al., 1999; Christodoulou et al., 2001; Maruishi et al., 2007; Turner et al., 2008), HIV (Chang et al., 2001; Ernst et al., 2002) and Alzheimer's; disease (Bookheimer et al., 2000; Grady et al., 2003). In fact, patients with left temporal lobe epilepsy exhibit greater right prefrontal recruitment relative to controls, but convert to lesser recruitment more typical of healthy controls after neurosurgical intervention (Maccotta et al., 2007). Among healthy individuals, prefrontal recruitment is positively associated with age (Rypma et al., 2005) and negatively associated with performance on neuropsychological measures of cognitive efficiency (Rypma et al., 1999, 2006). Taken together, people with reduced cognitive efficiency, whether due to neurological disease, ageing or developmental variation, appear to require greater prefrontal recruitment to perform cognitive tasks (Hillary et al., 2006). Within this context, we argue that (i) greater prefrontal recruitment is a marker of cerebral inefficiency; and (ii) based on the strong negative association of intellectual enrichment and prefrontal recruitment in the current study, greater enrichment is associated with greater cerebral efficiency in patients with neurological disease. In other words, multiple sclerosis patients with greater intellectual enrichment require fewer cerebral resources to perform the same cognitive task as compared to patients with lesser enrichment.
The default network consists of brain regions more active during rest or passive thought than during directed cognitive processing (Buckner et al., 2008). The default network is deactivated proportionately to cognitive processing demands in healthy subjects (Singh and Fawcett, 2008), and is inversely associated with prefrontal recruitment (Greicius et al., 2003). Patients with multiple sclerosis require greater deactivation of the default network (and increased prefrontal recruitment) to perform the same cognitive tasks as healthy controls (Sweet et al., 2006; Morgen et al., 2007). We posit that reduced default network activation is a marker of cerebral inefficiency, as people who require more cerebral resources for cognitive processing will also show greater deactivation of their default/resting state. This premise is consistent with a rapidly growing literature on default network activity in healthy individuals and patients with neurological disease in general. For instance, default network activity is negatively associated with advancing age (Lustig et al., 2003; Damoiseaux et al., 2008), and positively associated with performance on neuropsychological measures of memory in healthy young adults (Wig et al., 2008) and executive attention/processing speed in older healthy adults (Damoiseaux et al., 2008). Furthermore, default network activity is reduced in adults with attention deficit/hyperactivity disorder (Castellanos et al., 2008), mild cognitive impairment (Rombouts et al., 2005; Celone et al., 2006; Sorg et al., 2007; Bai et al., 2008), and mild Alzheimer's; disease (Lustig et al., 2003; Greicius et al., 2004; Rombouts et al., 2005; Celone et al., 2006; Wang et al., 2007). Adults at genetic risk for Alzheimer's disease estimated with apolipoprotein-E4 also show reduced default network activity, even before the emergence of observable cognitive dysfunction (Reiman et al., 2005). The default network is becoming an important construct in the fields of neuroscience and neurology, as reduced default network activity is associated with ageing, neurological disease and poorer neuropsychological performance. Our results indicate a strong positive relationship between intellectual enrichment and default network activity in patients with multiple sclerosis. In other words, intellectual enrichment appears to protect neurological patients from a pattern of disease-related cerebral activity.
Reiman et al. (2005) suggested that reduced default network activity may be a useful biomarker for predicting Alzheimer's; disease, because such a biomarker may ‘help overcome the confounding effects of individual differences in cognitive reserve capacity, which may compensate for Alzheimer's; disease neuropathology and mask its clinical expression’. On the contrary, our results indicate that default network activity is strongly associated with intellectual enrichment (i.e. cognitive reserve). In fact, based on the current and previous research, default network activity may be a biomarker of cerebral efficiency, which is essentially an fMRI marker of cognitive reserve itself. Default network activation may predict cognitive impairment in neurological patients (e.g. mild cognitive impairment, Alzheimer's; disease) in part because such activation represents the degree of remaining cognitive reserve. When this cognitive reserve capacity falls below a certain threshold, persons with neurological disease begin to clinically express their neuropathology via cognitive impairment or dementia.
Is intellectual enrichment related to cerebral efficiency in healthy individuals?
If intellectual enrichment is equally related to cerebral efficiency in healthy persons as in neurological patients, then patients with higher intellectual enrichment are simply less likely to cross the threshold of cognitive impairment because their cerebral functioning was more efficient at the outset. In other words, brain disease may cause the same negatively sloped effect on cognition in all patients, but patients with higher enrichment may simply have further to fall before impairment is evident. Contrary to this idea, previous research has not supported a link between intellectual enrichment and cerebral efficiency in healthy persons. Despite early electrophysiological data linking intelligence and cerebral efficiency in adults, subsequent fMRI research has not supported the notion that intelligent healthy persons put forth less effort to perform cognitive tasks (Neubauer and Fink, 2009). For instance, a recent fMRI study examining the relationship between intelligence and cerebral activation (BOLD) during the N-Back task in a large sample of older healthy adults (n = 37) found no relationship between intelligence and brain activation (Waiter et al., 2009). Also, even when scientists used a much more challenging version of the N-Back (3-Back) in a large sample of healthy adults (n = 48), there was still essentially no relationship between intelligence and cerebral activation (Gray et al., 2003). The sole exception was a positive correlation between intelligence and prefrontal recruitment when the 3-Back task was made even more difficult by employing lures designed to increase task interference (at which point accuracy was also correlated with intelligence). Despite presumably high statistical power and methods very similar to our own, these studies did not show an association between intellectual enrichment and cerebral efficiency in healthy individuals. [Note that some fMRI studies have shown a negative association between behaviourally-measured cognitive efficiency and prefrontal recruitment in healthy people, e.g. Rypma and D'E;sposito (1999) and Rypma et al. (2006), but such studies did not examine associations with intelligence.]
The notion that intellectual enrichment is more associated with cerebral efficiency in neurological patients than healthy controls is entirely consistent with the cognitive reserve hypothesis. According to the reserve hypothesis, intellectual enrichment does not lead to gains in cognition per se; rather, higher enrichment protects patients from cognitive decline due to disease. As such, if there is no brain disease to protect against, then the association between intellectual enrichment and efficiency should be weak or absent. Consistent with this notion, we demonstrated in a previous study that intellectual enrichment was only weakly associated with cognitive efficiency in healthy adults, but strongly related to efficiency in patients with multiple sclerosis (Sumowski et al., 2009a). Furthermore, in a separate study, the association between intellectual enrichment and cognitive efficiency was much weaker among multiple sclerosis patients with less severe brain disease (atrophy) relative to patients with more severe disease (Sumowski et al., 2009b). Again, this is because intellectual enrichment protects against the negative effects of disease on cognition, rather than improving cognition per se. Within this context, it makes sense that intellectual enrichment is more related to cerebral efficiency in neurological patients (i.e. multiple sclerosis) than in healthy people. It also supports the notion that higher enrichment moderates/lessens the negative impact of brain disease on cognition (Bennett et al., 2003; Roe et al., 2008; Sumowski et al., 2009b).
How is intellectual enrichment related to cerebral efficiency?
What is it about intellectual enrichment that leads to greater cerebral efficiency? One hypothesis is that cognitively stimulating lifestyles result in greater elaboration of synaptic networks within the brain. As one popular example, posterior hippocampal volumes of London taxi drivers are positively correlated with the amount of time spent as a taxi driver, suggesting that development of the brain region responsible for spatial memory is affected by life experience (Maguire et al., 2000). An alternative hypothesis is that vocabulary knowledge may simply be a proxy of a more genetically-based intellectual capacity, and that greater cerebral efficiency from a young age may help people complete more school and learn more information. Although this nature–nurture theme may seem like a chicken-and-egg problem at first, longitudinal research has shown that educational attainment contributes to cognition in the sixth decade of life over-and-above measured intelligence at age 11 years (Staff et al., 2004). Moreover, engagement in cognitive leisure activities such as reading, writing and board games uniquely protects against cognitive decline later in life (Scarmeas et al., 2003; Verghese et al., 2003; Wilson et al., 2007). It appears, therefore, that life experiences make unique contributions to cognitive reserve over-and-above early genetic advantages.
How can patient with neurological disease increase their cognitive reserve to help protect against cognitive impairment? This question has important implications for those with acquired brain injuries and newly diagnosed neurodegenerative diseases. To date, cognitive reserve has generally been conceptualized as the cumulative effect of lifetime intellectual enrichment, including educational and occupational attainment, literacy and cognitive leisure activities (Stern, 2009). At first glance, this conceptualization of cognitive reserve seems to preclude any intensive intervention aimed at increasing a patient's; cognitive reserve in the short term. On the other hand, cognitive reserve has also been described as a repertoire of cognitive processing strategies resulting in greater cerebral efficiency (Stern, 2002). The brain is a limited capacity system and if subcomponents of a task are performed more efficiently (e.g. math fact retrieval), then more resources are available for other aspects of the task to be performed (e.g. working memory), thereby increasing the likelihood that the whole task could be accomplished successfully (e.g. mentally adding prices in a supermarket). In other words, cognitive rehabilitation aimed at improving cognitive efficiency of subcomponents of a task may preserve cerebral resources for other aspects of the task, thereby increasing cognitive reserve, albeit in a domain-specific fashion.
Conclusion
The current research utilized fMRI to demonstrate that intellectual enrichment is associated with cerebral efficiency in neurological patients, thereby supporting the cognitive reserve hypothesis. Intellectual enrichment was positively associated with fMRI correlates of default network activity and negatively associated with prefrontal recruitment in multiple sclerosis patients during simple cognitive tasks, suggesting that intellectual enrichment is related to patterns of cerebral activity even in the absence of observable cognitive deficits. Enrichment-related differences in observable cognitive performance did emerge during more challenging task demands. As reviewed, the brain's; default network has become an important construct in neuroscience and neurology, especially because activity within the default network is reduced among patient with neurological disease. Our results show that default network activity is strongly related to intellectual enrichment/cognitive reserve, at least in patients with multiple sclerosis. Moreover, the identified pattern of cerebral activity associated with intellectual enrichment (less prefrontal recruitment, greater default network maintenance) almost completely mediates the relationship between intellectual enrichment and cognitive efficiency, thereby supporting our hypothesis that it represents the neural basis of cognitive reserve. Perhaps most importantly, multiple sclerosis patients with greater expression of the identified network appear able to withstand multiple sclerosis disease better before showing cognitive impairment. Future research is needed to investigate the relationship between intellectual enrichment and cerebral activity in other neurological diseases (e.g. Alzheimer's; disease), and assess the unique contribution of other proxies of cognitive reserve, including participation in cognitive leisure activities. Finally, future research should investigate whether cognitive interventions (i.e. cognitive strategy training) can improve cerebral efficiency (i.e. cognitive reserve) in patients newly diagnosed with neurological diseases, thereby delaying the onset of cognitive impairment and improving quality of life.
Funding
National Multiple Sclerosis Society (RG3330A1/3 to N.C., MB0003 to J.D.); the National Institutes of Health (HD060765-01 to J.F.S.). Funding for open access charge: Kessler Foundation Research Center.
Acknowledgements
The authors thank Christine Zakrzewski for her assistance with brain atrophy measurements and data processing.
Glossary
Abbreviations
- BOLD
blood oxygen level dependent
- fMRI
functional magnetic resonance imaging
- SDMT
Symbol Digit Modalities Test
References
- Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, et al. Association of premorbid intellectual function with cerebral metabolism in Alzheimer's; disease: implications for the cognitive reserve hypothesis. Am J Psychiat. 1997;154:165–172. doi: 10.1176/ajp.154.2.165. [DOI] [PubMed] [Google Scholar]
- Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtaszek M, Nierenberg J. Quantitative comparison of inter-subject volumetric MRI registration methods. J Neurosci Meth. 2005;142:67–76. doi: 10.1016/j.jneumeth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Audoin B, Van Au Duong M, Ranjeva J, Iborrola D, Malikova I, Confort-Gouny A, et al. Magnetic resonance study of the influence of tissue damage and cortical reorganization on PASAT performance at the earliest stage of multiple sclerosis. Hum Brain Mapp. 2005;24:216–228. doi: 10.1002/hbm.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Zhang Z, Yu H, Shi Y, Yuan Y, Zhu W, et al. Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: a combined structural and resting-state functional MRI study. Neurosci Lett. 2008;438:111–115. doi: 10.1016/j.neulet.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Bruce JM, Dwyer MG, Abdelrahman N, Hussein S, Weinstock-Guttman B, et al. Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol. 2006a;63:1301–1306. doi: 10.1001/archneur.63.9.1301. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Cookfair D, Gavett R, Gunther M, Munschauer F, Garg N, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis. J Int Neuropsych Soc. 2006b;12:549–558. doi: 10.1017/s1355617706060723. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Fischer JS, Archibald CJ, Arnett PA, Beatty WW, Bobholz J, et al. Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol. 2002;16:381–397. doi: 10.1076/clin.16.3.381.13859. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Weinstock-Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R. Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol. 2004;61:226–230. doi: 10.1001/archneur.61.2.226. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- Bermel R, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol. 2006;5:158–170. doi: 10.1016/S1474-4422(06)70349-0. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, et al. Patterns of brain activation in people at risk for Alzheimer's; disease. New Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiat. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- Christodoulou C, DeLuca J, Ricker JH, Madigan NK, Bly BM, Lange G, et al. Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. J Neurol Neurosurg PS. 2001;71:161–168. doi: 10.1136/jnnp.71.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Elkins JS, Longstreth WT, Monolio TA, Newman AB, Bhadelia RA, Johnson SC. Education and the cognitive decline associated with MRI-defined brain infarct. Neurology. 2006;67:435–440. doi: 10.1212/01.wnl.0000228246.89109.98. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant change in functional magnetic resonance imaging (fMRI): use of a cluster size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Forn C, Barros-Loscertales A, Escudero J, Benlloch V, Campos S, Parcet MA, et al. Compensatory activations in patients with multiple sclerosis during preserved performance on the auditory N-back task. Hum Brain Mapp. 2007;28:424–430. doi: 10.1002/hbm.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's; disease. J Neurosci. 2003;23:986–993. doi: 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's; disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyldensted C. Measurements of the normal ventricular system and hemispheric sulci of 100 adults with computed tomography. Neuroradiology. 1977;14:183–192. doi: 10.1007/BF00496982. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Genova HM, Chiaravalloti ND, Rypma B, DeLuca J. Prefrontal modulation of working memory performance in brain injury and disease. Hum Brain Mapp. 2006;27:837–847. doi: 10.1002/hbm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 4th. New York: Oxford University Press; 2004. [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'B;rien KC, McAvoy M, Raichle ME, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL, Gilliam FG, Ojemann JG. Changing frontal contributions to memory before and after medial temporal lobectomy. Cereb Cortex. 2007;17:443–456. doi: 10.1093/cercor/bhj161. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruishi M, Miyatani M, Nakao T, Muranaka H. Compensatory cortical activation during performance of an attention task by patients with diffuse axonal injury: a functional magnetic resonance imaging study. J Neurol Neurosur PS. 2007;78:168–173. doi: 10.1136/jnnp.2006.097345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, et al. Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology. 1999;53:1304–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- Morgen K, Sammer G, Courtney SM, Wolters T, Melchior H, Blecker CR, et al. Distinct mechanisms of altered brain activation in patients with multiple sclerosis. Neuroimage. 2007;37:937–946. doi: 10.1016/j.neuroimage.2007.05.045. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Fink A. Intelligence and neural efficiency. Neurosci Bibehav R. 2009;33:1004–1023. doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci USA. 2005;102:8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Mintun MA, D'A;ngelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11 labeled Pittsburgh compound B uptake. Arch Neurol. 2008;65:1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's; disease: an fMRI study. Hum Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Genova H, Rebbechi D, D’Esposito M. Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI. Cortex. 2005;41:582–594. doi: 10.1016/s0010-9452(08)70198-9. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, et al. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–979. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'E;sposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Natl Acad Sci USA. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, et al. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol. 2003;60:359–365. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KD, Fawcett IP. Transient and linearly-graded deactivation of the human default-mode network by a visual detection task. Neuroimage. 2008;41:100–112. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- Smith A. Los Angeles: Western Psychological Services; 1982. Symbol digit modalities test manual. [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's; disease. Proc Natl Acad Sci USA. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Deary IJ, Whalley LJ. What provides cerebral reserve? Brain. 2004;127:1191–1199. doi: 10.1093/brain/awh144. [DOI] [PubMed] [Google Scholar]
- Stanovich KE, Cunningham AE. Studying the consequences of literacy within a literate society: the cognitive correlates of print exposure. Mem Cognit. 1992;20:51–68. doi: 10.3758/bf03208254. [DOI] [PubMed] [Google Scholar]
- Stanovich KE, Cunningham AE. Where does knowledge come from? Specific associations between print exposure and information acquisition. J Educ Psychol. 1993;85:211–229. [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsych Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alz Dis Assoc Dis. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's; disease. Ann Neurol. 1992;32:371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, et al. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex. 2005;15:394–402. doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumowski JF, Chiaravalloti N, DeLuca J. Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. J Clin Exp Neuropsyc. 2009a;31:913–926. doi: 10.1080/13803390902740643. [DOI] [PubMed] [Google Scholar]
- Sumowski JF, Chiaravalloti N, Wylie GR, DeLuca J. Cognitive reserve moderates the negative effect of brain atrophy on cognitive efficiency in multiple sclerosis. J Int Neuropsych Soc. 2009b;15:606–612. doi: 10.1017/S1355617709090912. [DOI] [PubMed] [Google Scholar]
- Sweet LH, Rao SM, Primeau M, Durgerian S, Cohen RA. Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Hum Brain Mapp. 2006;27:28–36. doi: 10.1002/hbm.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GR, Levine B. Augmented neural activity during executive control processing following diffuse axonal injury. Neurology. 2008;71:812–818. doi: 10.1212/01.wnl.0000325640.18235.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, et al. Leisure activities and the risk of dementia in the elderly. New Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- Waiter GD, Deary IJ, Staff RT, Murray AD, Fox HC, Starr JM, et al. Exploring possible neural mechanisms of intelligence differences using processing speed and working memory tasks: an fMRI study. Intelligence. 2009;37:199–106. [Google Scholar]
- Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, et al. Altered functional connectivity in early Alzheimer's; disease: a resting-state fMRI study. Hum Brain Mapp. 2007;28:967–978. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Wolford GL, Petersen SE, Kelley WM. Medial temporal lobe BOLD activity at rest predicts individual differences in memory ability in healthy young adults. Proc Natl Acad Sci USA. 2008;105:18555–18560. doi: 10.1073/pnas.0804546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69:1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]