Abstract
While methamphetamine addiction has been associated with both impulsivity and striatal dopamine D2/D3 receptor deficits, human studies have not directly linked the latter two entities. We therefore compared methamphetamine-dependent and healthy control subjects using the Barratt Impulsiveness Scale (version 11, BIS-11) and positron emission tomography with [18F]fallypride to measure striatal dopamine D2/D3 receptor availability. The methamphetamine-dependent subjects reported recent use of the drug 3.3 g per week, and a history of using methamphetamine, on average, for 12.5 years. They had higher scores than healthy control subjects on all BIS-11 impulsiveness subscales (p < 0.001). Volume-of-interest analysis found lower striatal D2/D3 receptor availability in methamphetamine-dependent than in healthy control subjects (p < 0.01) and a negative relationship between impulsiveness and striatal D2/D3 receptor availability in the caudate nucleus and nucleus accumbens that reached statistical significance in methamphetamine-dependent subjects. Combining data from both groups, voxelwise analysis indicated that impulsiveness was related to D2/D3 receptor availability in left caudate nucleus and right lateral putamen/claustrum (p < 0.05, determined by threshold-free cluster enhancement). In separate group analyses, correlations involving the head and body of the caudate and the putamen of methamphetamine-dependent subjects and the lateral putamen/claustrum of control subjects were observed at a weaker threshold (p < 0.12 corrected). The findings suggest that low striatal D2/D3 receptor availability may mediate impulsive temperament and thereby influence addiction.
Introduction
Dopamine is thought to influence impulsivity (Congdon and Canli, 2005), a category of behaviors encompassing deficits in the ability to delay immediate gratification for future larger rewards, and in response inhibition. Novelty-seeking, which includes a dimension of impulsiveness (Cloninger et al., 1993), and attention deficit/hyperactivity disorder (ADHD), which features impulsivity as a symptom (DSM-IV) (Solanto, 2002), are associated with variants of genes encoding synaptic proteins that modulate dopaminergic activity (Faraone et al., 2001, 2005; Schinka et al., 2002; Limosin et al., 2003; Congdon and Canli, 2005; Eisenberg et al., 2007; White et al., 2008). Furthermore, administration of dopaminergic agonists reduces impulsive responding by ADHD and control subjects (Tannock et al., 1989; Aron et al., 2003), and rats (Wade et al., 2000; van Gaalen et al., 2006).
Suboptimal activity (too much or too little) at D2-like (D2/D3) receptors apparently induces impulsive responses. Systemic amphetamine increases premature responding on a five-choice serial reaction time task, and administration of a D2-like receptor antagonist into subregions of the rat nucleus accumbens attenuates this effect (Pattij et al., 2007). D2/D3 receptor blockade impairs response inhibition in reversal learning tasks in various species (Ridley et al., 1981; Lee et al., 2007) and increases impulsive choice by rats on a delay-discounting task (Wade et al., 2000; van Gaalen et al., 2006). Premature responding also was associated with low D2/D3 receptor availability in the ventral striatum of rats and with high scores of “addiction-like behaviors” after chronic cocaine self-administration (Dalley et al., 2007; Belin et al., 2008). Last, the A1 allele of the DRD2/ANKK1-TaqIa gene has been associated with impulsivity (Limosin et al., 2003; Eisenberg et al., 2007; White et al., 2008; Esposito-Smythers et al., 2009).
Methamphetamine abusers exhibit losses in striatal D2/D3 receptor availability (Volkow et al., 2001); and amphetamine abusers give higher self-ratings of impulsiveness than healthy control subjects (Clark et al., 2006; Ersche et al., 2008). Stimulant abusers also underperform on tests of inhibitory control and delay of reward (Monterosso et al., 2005; Clark et al., 2006; Payer and London, 2009). Correlations of striatal D2/D3 receptor availability with glucose metabolism in the prefrontal cortex (Volkow et al., 1993, 2001, 2008), which is thought to moderate impulsivity (Brennan and Arnsten, 2008; Dalley et al., 2008), indirectly suggest a relationship. However, while novelty seeking is negatively correlated with D2/D3 receptor availability in right insular cortex (Suhara et al., 2001) and midbrain in healthy subjects (Zald et al., 2008), direct evidence linking striatal D2/D3 receptors and impulsivity in humans has been lacking.
To directly assess the link between striatal D2/D3 receptors and impulsivity, we administered the Barratt Impulsiveness Scale (BIS-11) and performed positron emission tomography (PET) to assess striatal D2/D3 receptor availability in healthy control subjects and in participants who met criteria for methamphetamine dependence. We hypothesized a negative association between striatal D2/D3 receptor availability and impulsiveness extending across groups, consistent with a role of these receptors in impulsive temperament.
Materials and Methods
Subjects.
Fifty-one methamphetamine-dependent [age = 33.6 ± 8.8 (SD) years, 30 men] and 66 healthy control subjects [age = 31.3 ± 8.3 (SD) years, 34 men] completed the BIS-11. PET scanning was initiated later in the study; participants in each group (22 methamphetamine subjects, 30 healthy control subjects) completed PET scanning. Following a thorough explanation of the study, each subject gave informed written consent to participate. The study was approved by the University of California, Los Angeles (UCLA) Office for Protection of Research Subjects. The Structured Clinical Interview for DSM-IV was used to identify and exclude healthy control subjects with axis I psychiatric diagnoses other than nicotine dependence, and methamphetamine subjects with any axis I diagnoses unrelated to drug abuse or dependence. The methamphetamine subjects all had positive urine tests for methamphetamine upon entry to the study. They resided at the UCLA General Clinical Research Center for the course of the study; healthy control subjects participated on a nonresidential basis.
Impulsiveness measure.
The BIS-11 (Patton et al., 1995), which includes three subscales (cognitive, motor, nonplanning), was administered; and each item was rated from 1 (absent) to 4 (most extreme). Self-reports of impulsivity were obtained during the first 2 weeks (0–12 d) after entry to the study. At this time, methamphetamine subjects were abstinent from methamphetamine for an average of 2.5 d (range of 0–15). There was no significant relationship between duration of abstinence and BIS scores (all p values >0.236).
PET scanning.
Dopamine D2/D3 receptor availability was assayed using [18F]fallypride, a radioligand with high affinity for D2/D3 receptors (Mukherjee et al., 1995). The methamphetamine subjects were abstinent from the drug for 4–10 d when scanned. Images were acquired using a Siemens ECAT EXACT HR+ scanner [in-plane resolution full-width at half-maximum (FWHM) = 4.6 mm, axial FWHM = 3.5 mm, axial field of view = 15.52 cm] in three-dimensional (3D) mode. Subjects were placed in the supine position, with the brain centered in the transaxial field of view. A 7 min transmission scan was acquired using a rotating 68Ge/68Ga rod source for attenuation correction. PET dynamic data acquisition was initiated with a bolus injection of [18F]fallypride (∼5 mCi in 30 s). To minimize discomfort, the subject was given a 20 min break after the first 80 min of data acquisition, and then returned to the scanner bed. After a second 7 min transmission scan, emission data were collected for another 80 min. The total dynamic scanning sequence consisted of six 30 s frames, seven 1 min frames, five 2 min frames, four 5 min frames, and 12 10 min frames. Data were reconstructed using ECAT v7.3 OSEM (Ordered Subset Expectation Maximization; 3 iterations, 16 subsets) after corrections for decay, attenuation, and scatter.
Structural magnetic resonance imaging.
Participants who had PET scans also had structural scans on a 1.5-T magnetic resonance imaging (MRI) scanner (SIEMENS Sonata). A high-resolution sagittal T1-weighted 3D volumetric scan was acquired using a whole brain MPRAGE sequence (repetition time/echo time = 25/11 ms, number of excitations = 1, slice thickness = 1.2 mm contiguous, in-plane resolution = 1 × 1 mm2, runtime = 10 min).
MRI processing and definition of volumes of interest.
All images were aligned to a standardized stereotactic space defined relative to the anterior and posterior commissures, such that the axial plane became the default plane; and the origin of the image was set to the anterior commissure. The caudate nucleus, putamen and nucleus accumbens were defined as volumes of interest (VOIs) using an automated procedure (FSL FIRST; FMRIB Integrated Registration and Segmentation Tool, Oxford University, Oxford UK) that provides a 3D binary mask for these regions in native space (http://www.fmrib.ox.ac.uk/fsl/first/index.html). Two investigators (AVB and BL) manually refined the automatically generated VOIs, following methods described in the Segmentation Manual by the Center for Morphometric Analysis at Harvard Medical School and MA General Hospital (http://www.cma.mgh.harvard.edu/manuals/segmentation/) (intraclass correlation coefficients: 0.900, 0.985, 0.886, for caudate nucleus, putamen, and nucleus accumbens, respectively). All VOIs were drawn from the most anterior slice in which the structure appeared. The nucleus accumbens was sampled until one slice before the anterior commissure clearly appeared. The caudate nucleus VOI included the head and body of caudate, and the putamen VOI included the entire putamen.
PET image processing.
The dynamic frames were spatially aligned to a reference image (middle frame) using SPM5 (Wellcome Department of Cognitive Neurology, The Wellcome Trust Centre for Neuroimaging, London, UK) to correct for head motion and differences in positioning (before vs after the break). All realigned PET images were then reoriented to a standardized stereotactic space defined relative to the anterior and posterior commissures, such that the axial plane became the default plane, and the origin of the image was set to the anterior commissure. A reference VOI was drawn on the cerebellar hemispheres (including left and right cerebellum), which have very low D2/D3 receptor availability (Mukherjee et al., 2002). The VOIs were moved from the MRI space to the PET space using a linear transformation that coregistered the MRI images to the PET images. The volumes of the coregistered VOIs were the same as those drawn on the MRI images, except for slight changes due to interpolation (differences <1%). Time-activity curves (TACs) were extracted from coregistered VOIs in the realigned PET frames. The Logan reference-tissue model (Logan et al., 1996) implemented in PMOD 3.0 (PMOD Technologies) was used to calculate binding potentials (from the TACs) and generate parametric images of binding potentials.
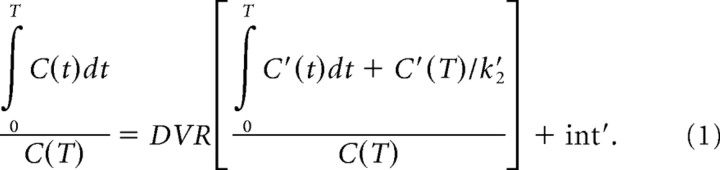 |
Briefly, the distribution volume ratio (DVR) between a VOI (or voxel) and the reference region can be directly calculated with the graphical method using linear regression (Eq. 1), where C(T) is the VOI (or voxel) tracer activity, C′(T) is the average tracer activity in the reference region, both at time T, and the regression slope is DVR. The quantity DVR−1 is defined as binding potential (BPND), an index for in vivo receptor availability (Mintun et al., 1984; Logan et al., 1996; Innis et al., 2007). This model requires the average rate constant (k′2) for transfer from the reference tissue to arterial plasma as an input parameter. Since the k′2 determined from the simplified reference-tissue model is a suitable estimate for the average k′2 (Wu and Carson, 2002), we used the average k′2 obtained from this method applied to the caudate nucleus and putamen, as suggested by Ichise et al. (2008).
Spatial normalization of BPND images.
The parametric BPND images were warped into a common stereotactic space [Montreal Neurological Institute (MNI) space] using MRI-guided spatial normalization (SPM5). The T1-weighted MRI of each subject was first coregistered to the mean PET image of the realigned PET frames, from which the BPND image was generated. The coregistered MRI was then spatially normalized to MNI305 space using nonlinear registration, and the same transformation matrix was applied to the BPND image to normalize it. No additional smoothing was applied to the spatially normalized BPND images.
Statistical analyses.
We tested the group difference in age by an independent-samples t test, and group differences in sex and smoking status by Fisher's exact test. Since the methamphetamine subjects were predominantly tobacco smokers, smoking was a potential confound. We examined the healthy control group, which contains comparable numbers of smokers and nonsmokers, for the effect of smoking on the BIS subscales, and on BPND for the three striatal VOIs.
We examined group differences in the three BIS subscales with multivariate analysis of covariance (MANCOVA), with group as the factor and smoking status and age as covariates as appropriate. We applied Holm–Bonferroni corrections to post hoc independent-samples analysis of covariance (ANCOVA). BIS total score was similarly analyzed by ANCOVA. Using MANCOVA/ANCOVA we analyzed the BIS subscales and BIS total score for the effect of cumulative exposure (self-reported years of abuse) and of recent exposure to methamphetamine (self-reported days of methamphetamine abuse in the 30 d before the study), controlling for age in the former since cumulative exposure is expected to be strongly correlated with age.
We examined group differences in BPND for the three striatal VOIs with MANCOVA, with group as the factor and smoking status and age as covariates as appropriate, followed by Holm–Bonferroni-corrected post hoc independent-samples ANCOVA. Using MANCOVA of BPND in the three striatal volumes, we tested for effects of cumulative and recent exposure to methamphetamine, controlling for age in the former assessment.
We studied the relationship between BIS total score and BPND for the three striatal VOIs, controlling for age. Multiple VOI analyses were performed by canonical correlation for the methamphetamine and healthy control groups separately, followed by post hoc assessments of correlations for each VOI. To obtain sensitivity in substructures within VOIs, BPND images (thresholded to exclude voxels with negligible D2/D3 binding, i.e., BPND < 0.2) were subjected to linear regression to test associations with BIS total scores. Control for familywise error was implemented using nonparametric randomization tests with the FSL RANDOMISE v2.1 tool (Permutation-based nonparametric inference, Oxford University, Oxford UK), with variance smoothing of 10 mm (FWHM Gaussian) (http://www.fmrib.ox.ac.uk/fsl/randomize). This method repeatedly reorders the rows of the design matrix and computes the whole-brain statistical map for each sample, recording the maximum statistic across the brain for each sample to obtain an empirical null distribution (Nichols and Holmes, 2002). The methamphetamine and healthy control subjects were treated as “exchangeability blocks” within a single model; the design matrix was resampled within each group. Threshold-free cluster enhancement (TFCE) (Smith and Nichols, 2009) was used to detect significant clusters of activation; this method provides the ability to perform cluster-based inference without the need to specify an arbitrary cluster-forming threshold, as is necessary when using Gaussian random field theory. For each analysis, 5000 randomization runs were performed. Statistical maps were thresholded at one-tailed p < 0.05 corrected for the entire search volume, though some results are reported at more lenient thresholds as noted in the results section.
Results
For subjects completing the BIS-11, the groups did not differ in sex (Fisher's Exact test: p = 0.46) or age (t(115) = 1.498, p = 0.14) (Table 1). The PET subgroups also did not differ in sex (Fisher's Exact test: p = 0.78) or age (t(50) = 0.265, p = 0.79) (Table 1). A significantly larger proportion of the methamphetamine subjects were daily tobacco smokers (Fisher's exact test: p < 0.001). On average, the methamphetamine subjects reported methamphetamine abuse for 12.5 years (SD = 7.4), and used 3.3 g of methamphetamine (SD = 4.5) per week during the month before study. For the methamphetamine subjects, age and cumulative exposure to methamphetamine were strongly correlated (r = 0.63, p < 0.001).
Table 1.
Characteristics of research subjects
| Group | Entire sample(data on BIS-11) |
Subsample(data on BIS-11 and BP) |
||
|---|---|---|---|---|
| HC (n = 66) | MA (n = 51) | HC (n = 30) | MA (n = 22) | |
| Sex (M/F) | 34/32 | 30/21 | 16/14 | 13/9 |
| Age (year) | 31.3 ± 8.3 | 33.6 ± 8.8 | 34.9 ± 8.9 | 35.6 ± 8.4 |
| Number of smokers/group | 35/66 | 46/51* | 11/30 | 19/22* |
| Duration of MA abuse(year) | N/A | 12.5 ± 7.42 | N/A | 14.6 ± 8.2 |
| Days of MA abuse inlast 30 d | N/A | 21.6 ± 8.18 | N/A | 20.4 ± 8.3 |
| Intensity of MA abuse(g/week) | N/A | 3.3 ± 4.48 | N/A | 3.0 ± 3.2 |
Data are given as mean ± SD, except for sex and smoking status. *Significant group difference, p < 0.05. HC, Healthy controls; MA, methamphetamine; M, male; F, female.
Effects of smoking status and age
The effect of age was not significant for BIS subscales (multivariate regression: χ2(3) = 7.2, p = 0.07) but highly significant for BPND (multivariate regression: χ2(3) = 13.3, p = 0.004). For subsequent tests of BPND we controlled for age. For the healthy control group, the effect of smoking was significant for neither BIS subscales (MANOVA: Wilks' lambda = 0.93, χ2(3) = 4.2, p = 0.24) nor BPND (MANOVA: Wilks' lambda = 0.97, χ2(3) = 0.87, p = 0.83).
BIS subscales and BIS total score
The BIS subscales showed a significant effect of group (MANOVA: Wilks' lambda = 0.73, χ2(3) = 35.2, p < 0.001). Post hoc, independent-samples ANCOVA showed the methamphetamine subjects scoring significantly above the healthy control subjects on all three subscales (all F(1,115) ≥ 13.1, all p values < 0.001). For BIS total score, the methamphetamine subjects scored above the healthy control subjects [69.3 (SD = 10.1) vs 57.4 (SD = 10.3), respectively] (t(115) = 6.2, p < 0.001). For the methamphetamine group, the cumulative effect of methamphetamine, controlling for age, was nearly significant (multivariate regression: χ2(3) = 7.7, p = 0.053), but the effect of recent methamphetamine exposure was not significant (multivariate regression: χ2(3) = 2.2, p = 0.53).
VOI Analysis of D2/D3 receptor availability (BPND)
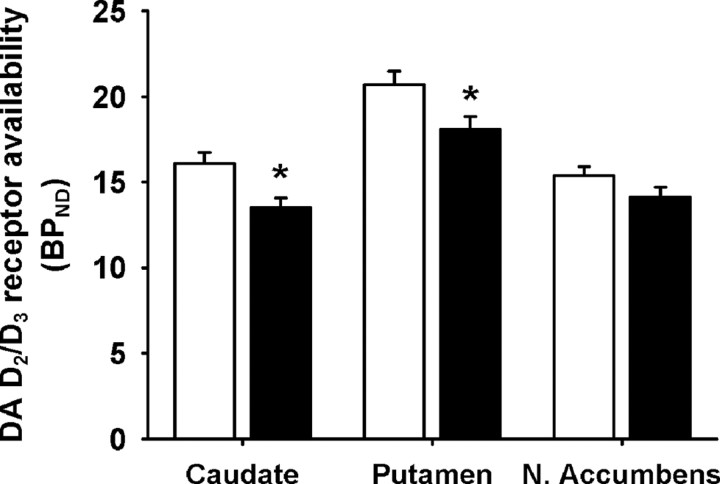
Striatal BPND in the methamphetamine subjects was lower than in the healthy control subjects, by 16.1% in the caudate nucleus, 12.6% in the putamen, and 8.4% in the nucleus accumbens (Fig. 1). MANCOVA of BPND, for the three VOIs, controlling for age, gave p = 0.008 (Wilks' lambda = 0.79, χ2(3) = 9.9). Post hoc, independent ANCOVA yielded p = 0.0015 (F(1,49) = 11.1), 0.013 (F(1,49) = 6.6), 0.10 (F(1,49) = 2.8), for the caudate nucleus, putamen, and nucleus accumbens respectively; the first two reaching statistical significance when Holm–Bonferroni corrected. Controlling for age, BPND for the methamphetamine group was significantly negatively affected by cumulative exposure to methamphetamine (multivariate regression: χ2(3) = 3.3, p = 0.048). Post hoc univariate regression yielded p = 0.011, 0.005, 0.032 (χ2(1) = 2.99, 7.9, 4.6) for the caudate nucleus, putamen and nucleus accumbens, respectively, all three reaching corrected significance. There was no significant relationship between striatal BPND and frequency of recent use (multivariate regression: χ2(3) = 0.33, p = 0.95).
Figure 1.
Dopamine D2/D3 receptor availability (BPND) in healthy control (white bars) and methamphetamine-dependent (black bars) subjects. Data are presented as means ± SE. Values from left and right hemispheres were averaged (weighted by number of voxels) as they were highly correlated (all p values <0.001; all correlation coefficients >0.932). *Significant group difference after correcting for age by ANCOVA, p < 0.05 (after Holm–Bonferroni correction for 3 comparisons). Smoking status was not used as a covariate because smoking status had no main effects.
Relation between BIS and BPND (VOI analysis)
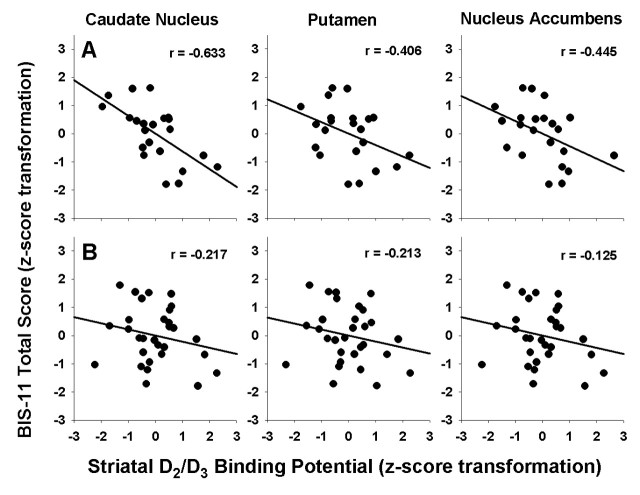
The relation between BIS total score and BPND in each group separately was assessed by canonical correlation, controlling for age. For the methamphetamine group, we obtained significant negative correlation (r = −0.71, p = 0.0044), lower receptor availability being associated with higher impulsiveness (Fig. 2). The post hoc Pearson correlations were as follows: caudate nucleus (r = −0.63, p = 0.002), putamen (r = −0.41, p = 0.068), nucleus accumbens (r = −0.45, p = 0.043). Of the individual regions, only the caudate nucleus showed significance after Holm–Bonferroni correction. For the healthy control group we obtained p = 0.64 (r = −0.25). Age and BIS total score were sufficient to explain the variance in BPND; after controlling for age and BIS total score, we found no group effect (MANCOVA: Wilks' lambda = 0.93, χ2(3) = 3.77, p = 0.29).
Figure 2.
Regression lines illustrate the correlations for the methamphetamine (row A) and healthy control (row B) groups. The values for BIS total impulsiveness scores and BP were corrected for age and then normalized (i.e., converted to z-scores) within each group. As shown above, negative correlations in the methamphetamine group were of similar magnitude for the three striatal regions, reaching statistical significance even with Holm–Bonferroni correction in the caudate nucleus (p values = 0.002, 0.068, and 0.043 for caudate nucleus, putamen, and nucleus accumbens, respectively). The difference in correlations between methamphetamine and healthy control subjects was not significant for any region (p values = 0.086, 0.485, and 0.249 for caudate nucleus, putamen, and nucleus accumbens, respectively).
Relation between BIS and BPND (voxelwise analysis)
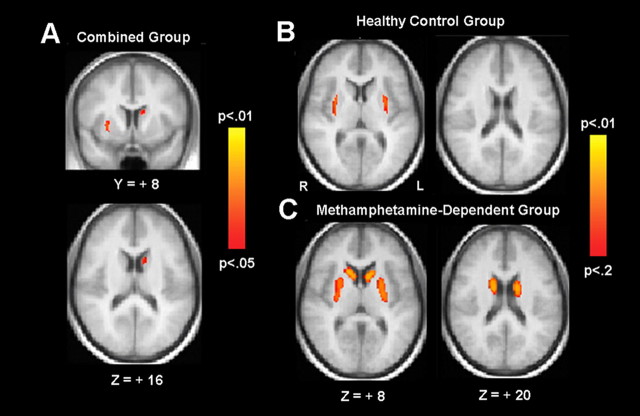
We found a significant correlation (p < 0.05, TFCE-corrected) between BPND and BIS total scores in the left caudate nucleus and right lateral putamen/claustrum when data from both groups were combined (Fig. 3A). Modeling the groups separately, there were significant effects in both groups at a slightly weaker corrected threshold of p < 0.12 (Fig. 3, B and C, illustrates results at p < 0.20). For healthy control subjects, the region of significant correlation was limited to the lateral putamen/claustrum, though a region in the caudate was apparent at a weaker corrected threshold of p < 0.25. For methamphetamine subjects, the region of significant correlation included the head and body of caudate as well as putamen, bilaterally. A direct comparison of the BIS/BPND relation across groups showed no significant voxels, even at p < 0.005 uncorrected, suggesting that any differences between groups are minimal despite the apparent differences in the activation maps.
Figure 3.
Results from voxelwise regression of BPND on BIS total scores, shown for the combined (n = 52) (A), the healthy control (n = 30) (B), and the methamphetamine-dependent (n = 22) (C) groups. TFCE probability maps are overlaid on the averaged normalized anatomy. Statistical maps for the combined group are shown with results thresholded at TFCE-corrected p < 0.05. Data for the individual group are shown at a more liberal threshold (p < 0.2, corrected) for illustration only.
Discussion
In this study, methamphetamine subjects viewed themselves as more impulsive than did healthy control subjects; and they exhibited lower striatal D2/D3 receptor availability. VOI analysis indicated a significant negative relationship between striatal D2/D3 receptor availability and impulsiveness in methamphetamine-dependent subjects, while voxelwise analysis revealed striatal regions where D2/D3 receptor availability was significantly related to impulsiveness across both subject groups.
PET with [18F]fallypride replicated findings of deficits in D2/D3 receptor availability in the caudate nucleus and putamen, observed with [11C]raclopride (Volkow et al., 2001), and extended analysis to the nucleus accumbens, where there was no group difference. The nucleus accumbens is smaller and less well defined than the caudate nucleus and putamen, leading to greater variability in determining its D2/D3 BPND, therefore providing less power to detect a group difference in that structure.
The deficit in striatal D2/D3 receptor availability in the methamphetamine group was related to cumulative methamphetamine exposure but not frequency of recent methamphetamine abuse. This observation contrasts with some findings that some indices of methamphetamine exposure were not related to various outcome measures, including cerebral blood flow (Chang et al., 2002), striatal dopamine transporter availability (McCann et al., 2008), or microglial activation in the brain (Sekine et al., 2008), and with a few reports linking cognitive deficits to frequency of recent methamphetamine use (Simon et al., 2000; Monterosso et al., 2005). Finding of a cumulative effect, however, is consistent with prior findings of deficits in frontal white matter structure (Ernst et al., 2000) and metabolic activity (Kim et al., 2009), cerebral N-acetylaspartate (Nordahl et al., 2005), striatal size (Chang et al., 2005), and dopamine and serotonin transporter availability (Sekine et al., 2006).
We also found a negative relationship between striatal D2/D3 receptor availability and impulsiveness. VOI analysis revealed such a relationship involving the caudate nucleus and nucleus accumbens in the methamphetamine group only. However, correlation coefficients also were negative for the putamen of the methamphetamine group and for striatal structures of the healthy control group, and our lack of a significant finding may reflect inadequate power. Along this line, effects at discrete sites can be diluted when sampling an entire striatal volume in VOI analysis; and voxelwise analysis revealed a negative association between D2/D3 receptor availability and impulsivity in the putamen of the methamphetamine subjects (p < 0.05, TFCE corrected) and in striatal regions in the control group albeit at a statistical threshold of p < 0.12 (TFCE corrected).
The negative correlation between impulsivity and striatal D2/D3 receptor availability in humans, reported here, is in line with a relationship observed between impulsivity and D2/D3 receptor availability of the ventral striatum in rats (Dalley et al., 2007), although the present study reports changes in additional regions of the striatum. The discrepancy in subregions of the striatum linked to impulsive behavior in the two species likely reflects profound differences between the subjective self-report of impulsivity taken here and in operant measures of impulsivity in experimental animals as well as possible lack of homology in striatal substructures across species.
As the negative relationship between striatal D2/D3 receptor availability and impulsivity extends to healthy control subjects, it appears to be fundamental (albeit exaggerated in subjects who self-administer methamphetamine). In addition, the association between duration of methamphetamine abuse and striatal D2/D3 receptor availability suggests that methamphetamine exposure has a negative impact on the D2/D3 receptor system, thereby promoting impulsivity. This view is consistent with findings that stimulant administration promotes impulsive behavior in animals (Jentsch and Taylor, 1999). Nonetheless, we cannot exclude the possibility that lower D2/D3 receptor availability (with attendant greater impulsivity) contributes to the self-administration of methamphetamine.
The mechanism by which striatal D2/D3 receptors can influence impulsivity is not completely clear, but accumulating evidence points to a “frontostriatal system” in influencing various dimensions of impulsivity (Aron et al., 2007; Dalley et al., 2008). Prefrontal cortical inhibition of subcortical and posterior cortical structures is thought to play a key role in modulating behavior, and lesions of the prefrontal cortex have been linked with deficits in motor response inhibition (Aron et al., 2004). In addition, striatal lesions in rats increase impulsivity, as measured on the delayed discounting task (Cardinal et al., 2001; Pothuizen et al., 2005) and the Stop Signal Task (Eagle and Robbins, 2003). Similarly, patients with basal ganglia lesions also have impaired performance on the Stop Signal Task (Rieger et al., 2003). Neuroimaging studies have provided evidence for striatocortical link in a network that may be related to impulsivity. In this regard, striatal D2/D3 receptor availability was positively related to glucose metabolism in cortical regions involved in inhibitory control (e.g., orbitofrontal cortex, cingulate gyrus, and dorsolateral prefrontal cortex). This positive correlation has been observed in individuals who engage in stimulant abuse (Volkow et al., 1993, 2001) and in obese subjects (Volkow et al., 2008).
Our results provide direct empirical evidence that striatal D2/D3 receptor availability is associated with impulsiveness in humans, suggesting an important link between a striatal dopaminergic system and cortical influence on a wide variety of disorders that involve uninhibited maladaptive behavior.
Footnotes
This work was supported by National Institutes of Health Grants P20DA022539, R01 DA020726, and RL1DA024853 (to E.D.L.) and M01 RR00865 (University of California, Los Angeles General Clinical Research Center), by an endowment from the Thomas P. and Katherine K. Pike Chair in Addiction Studies, and by a generous gift from the Marjorie M. Greene Trust.
References
- Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci. 2008;1129:236–245. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller EN. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Res. 2002;114:65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biol Psychiatry. 2006;60:515–522. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Congdon E, Canli T. The endophenotype of impulsivity: reaching consilience through behavioral, genetic, and neuroimaging approaches. Behav Cogn Neurosci Rev. 2005;4:262–281. doi: 10.1177/1534582305285980. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Eisenberg DT, Mackillop J, Modi M, Beauchemin J, Dang D, Lisman SA, Lum JK, Wilson DS. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behav Brain Funct. 2007;3:2. doi: 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology. 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito-Smythers C, Spirito A, Rizzo C, McGeary JE, Knopik VS. Associations of the DRD2 TaqIA polymorphism with impulsivity and substance use: preliminary results from a clinical sample of adolescents. Pharmacol Biochem Behav. 2009;93:306–312. doi: 10.1016/j.pbb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Doyle AE, Mick E, Biederman J. Meta-analysis of the association between the 7-repeat allele of the dopamine D(4) receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1052–1057. doi: 10.1176/appi.ajp.158.7.1052. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Ichise M, Cohen RM, Carson RE. Noninvasive estimation of normalized distribution volume: application to the muscarinic-2 ligand [(18)F]FP-TZTP. J Cereb Blood Flow Metab. 2008;28:420–430. doi: 10.1038/sj.jcbfm.9600530. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kim YT, Lee SW, Kwon DH, Seo JH, Ahn BC, Lee J. Dose-dependent frontal hypometabolism on FDG-PET in methamphetamine abusers. J Psychiatr Res. 2009;43:1166–1170. doi: 10.1016/j.jpsychires.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D(2)/D(3) receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Limosin F, Loze JY, Dubertret C, Gouya L, Adès J, Rouillon F, Gorwood P. Impulsiveness as the intermediate link between the dopamine receptor D2 gene and alcohol dependence. Psychiatr Genet. 2003;13:127–129. doi: 10.1097/01.ypg.0000066963.66429.00. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–227. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Yang ZY, Das MK, Brown T. Fluorinated benzamide neuroleptics–III. Development of (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-[18F]fluoropropyl)-2, 3-dimethoxybenzamide as an improved dopamine D-2 receptor tracer. Nucl Med Biol. 1995;22:283–296. doi: 10.1016/0969-8051(94)00117-3. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, Mantil J. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, Kile S, Buonocore MH. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2005;62:444–452. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM. Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology. 2007;191:587–598. doi: 10.1007/s00213-006-0533-x. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Payer D, London ED. Methamphetamine and the brain. In: Roll JJ, Rawson RA, Ling W, Shoptaw S, editors. Methamphetamine addiction: from basic science to treatment. New York: Guilford; 2009. pp. 61–91. [Google Scholar]
- Pothuizen HH, Jongen-Rêlo AL, Feldon J, Yee BK. Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. Eur J Neurosci. 2005;22:2605–2616. doi: 10.1111/j.1460-9568.2005.04388.x. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Haystead TA, Baker HF. An analysis of visual object reversal learning in the marmoset after amphetamine and haloperidol. Pharmacol Biochem Behav. 1981;14:345–351. doi: 10.1016/0091-3057(81)90401-9. [DOI] [PubMed] [Google Scholar]
- Rieger M, Gauggel S, Burmeister K. Inhibition of ongoing responses following frontal, nonfrontal, and basal ganglia lesions. Neuropsychology. 2003;17:272–282. doi: 10.1037/0894-4105.17.2.272. [DOI] [PubMed] [Google Scholar]
- Schinka JA, Letsch EA, Crawford FC. DRD4 and novelty seeking: results of meta-analyses. Am J Med Genet. 2002;114:643–648. doi: 10.1002/ajmg.10649. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav Brain Res. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- Suhara T, Yasuno F, Sudo Y, Yamamoto M, Inoue M, Okubo Y, Suzuki K. Dopamine D2 receptors in the insular cortex and the personality trait of novelty seeking. Neuroimage. 2001;13:891–895. doi: 10.1006/nimg.2001.0761. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol. 1989;17:473–491. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- White MJ, Morris CP, Lawford BR, Young RM. Behavioral phenotypes of impulsivity related to the ANKK1 gene are independent of an acute stressor. Behav Brain Funct. 2008;4:54. doi: 10.1186/1744-9081-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22:1440–1452. doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, Kessler RM. Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J Neurosci. 2008;28:14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]