Abstract
The mutations in the FMR1 gene have been described as a family of disorders called fragile X-associated disorders (FXD) including fragile X syndrome (FXS), fragile X-associated tremor/ ataxia syndrome (FXTAS), primary ovarian insufficiency and other problems associated with the premutation, such as hypothyroidism, hypertension, neuropathy, anxiety, depression, attention deficit hyperactivity disorders and autism spectrum disorders. The premutation is relatively common in the general population affecting 1 of 130–250 female individuals and 1 of 250–800 male individuals. Therefore, to provide appropriate treatment and genetic counseling for all of the carriers and affected individuals in a family, a detailed family history that reviews many of the disorders that are related to both the premutation and the full mutation should be carried out as exemplified in these cases.
To facilitate the integration of this knowledge into clinical practice, this is the first case report that demonstrates only premutation involvement across 3 generations.
Fragile X syndrome (FXS) is the most common known single gene cause of Autism Spectrum Disorders (ASD) and inherited intellectual disabilities (ID). FXS is one of a family of fragile X-associated disorders (FXD) caused by CGG-repeat expansion mutations of the fragile X mental retardation 1 (FMR1) gene at chromosome Xq27.3.1 Over 200 CGG repeats have been well described as the full mutation leading to an absence or deficiency of the fragile X mental retardation protein (FMRP). The full mutation causes the physical, behavioral, cognitive and emotional features of FXS.2,3 For a long time after the discovery of the FMR1 gene in 1991 individuals who have the fragile X premutation (55–200 CGG repeats) or gray zone alleles (45–54 CGG repeats) were thought to be clinically uninvolved by the mutation. However, advances in research in fragile X over the years have identified clinical involvement in some premutation carriers including developmental problems, such as ADHD and ASD particularly in boys,4,5 medical problems e.g. hypothyroidism, hypertension, fibromyalgia,6 seizures,7 menstrual dysfunction (primary ovarian insufficiency (POI))8 and psychological issues such as depression and anxiety9–11 and late onset neurological problems described as the fragile X-associated tremor ataxia syndrome (FXTAS).12,13 The premutation is associated with elevated FMR1-mRNA from 2 to 8 times above normal and this leads to RNA toxicity which is the molecular mechanism for clinical involvement in premutation carriers.14,15 Likewise, in the gray zone there can be more subtle increases in FMR1-mRNA levels up to almost 1.5 times normal when compared to controls.16 Clinical studies have shown that POI occurs twice as often in women who carried alleles in the gray zone compared to general population suggesting clinical attention should be warranted even in those with gray zone CGG expansions (45–54 CGG repeats).17,18 However, neurological and psychiatric problems have not been studied in individuals with gray zone alleles. To facilitate the integration of premutation involvement into clinical practice we report on three generations in a family in whom all cases of the fragile X mutation including the proband and those identified by cascade testing, were affected by premutation involvement. At least six individuals throughout 3 generations in this extended family have been confirmed with the fragile X premutation and were evaluated at our center. The terms, “carrier” and “premutation” have similar meaning and were used interchangeably in this article.
CASE DESCRIPTIONS
Clinical Presentation
The proband is a 12-year and 6-month-old boy who developed language delay and social deficits and was diagnosed with Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS) when he was three years of age and then subsequently was diagnosed with full autism at 7 years of age. Due to the diagnosis of ASD, genetic testing including fragile X DNA test was done. He was found to be a premutation carrier (63 CGG repeats) at four years of age and was confirmed to have 61 CGG repeats at 7 years of age at our center.
He had typical behaviors of fragile X and autism including excessive chewing on clothes or objects, skin picking, tactile defensiveness, hyperactivity, anxiety, perseverative behaviors, tantrums and limited eye contact.
Prenatal, Perinatal and Postnatal History
His mother experienced prolonged labor coupled with heart rate decelerations and he was born full term, vaginal delivery with an Apgar score of 7 and 9. In infancy, he did not feed well because of a weak suck.
Developmental History
His gross motor milestones were normal; he sat at 6 months, crawled at 8 months and walked at 10 months, but his speech was delayed. He did not begin talking meaningful words until after he started language intervention when he was almost 4 years.
Medical History Including Medications
His past medical history included an umbilical hernia, a few uncomplicated ear infections and staring spells. The latter problem began when he was five years old. An EEG demonstrated generalized poly-spike-wave discharges particularly during sleep. Valproic acid was prescribed and he subsequently improved the frequency of his spontaneous speech. His staring episodes decreased and behaviors became more attentive on valproic acid. He began speaking in sentences at five years after seizures were controlled. When he was 9 yo lamotrigine was added to valproic acid because of break through seizures which were subsequently controlled. Other medications were tried because of his autism and hyperactivity including risperidone, olanzapine and guanfacine. He developed more hyperactivity after taking risperidone and had significant sedation after taking olanzapine. Aripiprazole was tried to control tantrums, aggression, anxiety and distractibility but he developed a sleep disturbance on this so it was discontinued. Risperidone has been the most beneficial for his mood instability, irritability and tantrums. He has been on guanfacine for several years and this was noted to improve his hyperactivity. However, it was discontinued when he started lamotrigine to decrease the number of his medications. Melatonin at bedtime has helped him sleep through the night.
Physical Examination
On physical examination at 7 years of age, heart rate was 120 beats per minute. Blood pressure was 106/70 mmHg. His growth percentiles were normal with a head circumference of 52.5 cm. (50th percentile). He had very limited eye contact, difficulty with social interaction and intermittently made a high-pitched noise during the exam. He had a high-arched palate, a double-jointed thumb on the left and flat feet bilaterally. Genitalia showed normal pre-pubertal development with a testicular volume of 5 ml bilaterally. The rest of his physical examination was unremarkable.
Additional Assessments
The Leiter International Performance Scale demonstrated a moderate range of ID with a Full Scale IQ (FSIQ) of 46. The Vineland Adaptive Behavior Scales (VABS) completed with his parents yielded standard scores of 40 in Communication (age equivalent 1 year 7 months), 37 in Daily Living Skills (age equivalent 2 years 7 months), 53 in Socialization (age equivalent 1 year 10 months) and 40 on the Adaptive Behavioral Composite (age equivalent 2 years). He met the criteria for autism on the Autism Diagnostic Interview, Revised (ADI-R) and the criteria set forth in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).
Supports and Therapies
He has been in an autism program at school, which included typically developing children. He also received speech and language therapy, sensory integration therapy, occupational therapy (OT) along with behavioral intervention from the school. His current treatments include the combination of speech and language therapy along with OT and horseback riding for an hour each per week. His current medications include 0.5 mg. of risperidone, 1,450 mg. of valproic acid, 150 mg. of lamotrigine per day and 9 mg of melatonin at bedtime.
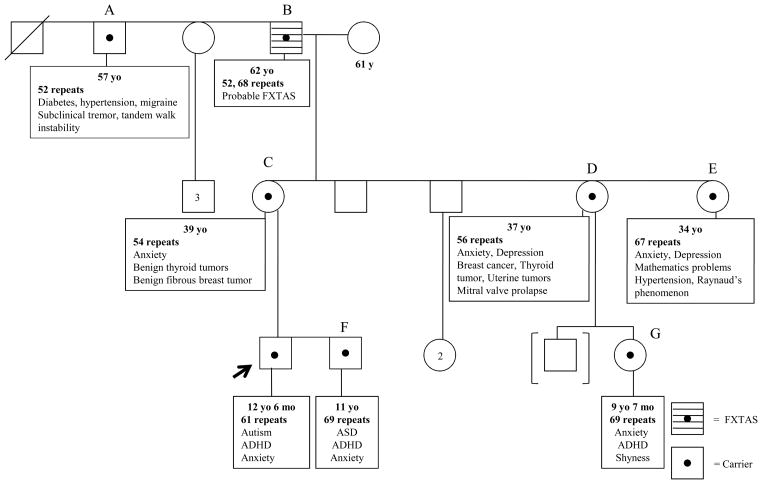
Family members who were evaluated at our center including his maternal grandfather’s brother (A), maternal grandfather (B), mother (C), both maternal aunts (D, E), biological brother (F) and cousin (G) are described in details in Table 1 and a summarized pedigree of this family is illustrated in Figure 1. Two known non-carrier members in this family including the proband’s father and maternal grandmother were also evaluated here. The biological father had hyperactivity and reading difficulties that required tutoring when he was young, but he was not diagnosed with ADHD nor treated with stimulants. The maternal grandmother had high blood pressure and depression in the past, the latter problem began after her husband was diagnosed with FXTAS. Otherwise, they both were healthy and had a normal IQ without any other current psychological diagnoses.
Table 1.
A summary of clinical data of other family members
| Characteristic | Grandfather’s brother (A) | Grandfather (B) | Mother (C) | Aunt (D) | Aunt (E) | Brother (F) | Cousin (G) |
|---|---|---|---|---|---|---|---|
| Age (years) | 57 | 62 | 39 | 37 | 34 | 11 | 9 years 7 months |
| Number of CGG repeats | 52 CGG repeats | 52, 68 CGG repeats (mosaic premutation) | 54 CGG repeats | 56 CGG repeats | 67 CGG repeats | 69 CGG repeats | 69 CGG repeats |
| mRNA level | 1.83 ± 0.081 | 2.62 ± 0.34 | 1.9 ± 0.63 | 1.88 ± 0.30 | 1.95 ± 0.04 | 1.51± 0.43 | 1.91 ± 0.43 |
| Activation Ratio (AR2) | - | - | 0.54 | - | 0.55 | - | - |
| Clinical presentation/ involvement |
|
|
|
|
|
|
|
| Physical examination |
|
|
Unremarkable | Not examined |
|
|
|
| Psychological testing/ other tests |
|
|
|
Not tested | Not tested |
|
|
| Management | enalapril 0.5 mg, lipitor 20 mg/ day and insulin | metoprolol 50 mg, dutasteride 0.5 mg and fenofibrate 145 mg/ day | sertraline 75 mg/ day | escitalopram20 mg/ day | sertraline 50 mg/ day | sertraline 37.5 mg/ day, individualized educational program | recommend start sertraline 12.5 mg and methylphenidate extended release 18 mg/ day, work with the therapist |
Mean ± SD. of the mRNA level and every sample was normalized to a normal control which is 1.26 ± 0.23
AR: fraction of normal FMR1 allele as the active allele,
WAIS-III: The Wechsler Adult Intelligence Scale-Third Edition, VIQ: Verbal IQ, PIQ: Performance IQ, FSIQ: Full Scale IQ
SCL-90-R: Symptom Checklist-90-Revised demonstrated a clinically significant level (>63) of depression (DEP) and irritability (HOS) and almost a clinically significant level of obsessive compulsive problems (O-C), psychological difficulties (PSY) and Global Severity Index (GSI).
WPPSI: Wechsler Preschool and Primary Scale of Intelligence,
WASI: Wechsler Abbreviated Scale of Intelligence
VABS: The Vineland Adaptive Behavior Scales, C: Communication, DLS: Daily Living Skills, S: Socialization, M: Motor and ABC: Adaptive Behavioral Composite
Figure 1.
A summarized pedigree of a family with fragile X mutation demonstrating a broad spectrum of clinical involvement throughout generations
DISCUSSION
This family demonstrated various types of the premutation involvement without any individuals with the full mutation. Premutation involvement is a relatively new concept for clinicians and it deserves further review so that it can be incorporated into screening and treatment endeavors of physicians. Data in the literature which compares those with the premutation to controls without the premutation will be reviewed to further validate whether these problems are associated with the premutation.
Physical Involvement
The proband, his brother and cousin have at least one of the subtle physical features associated with FXS, such as flat feet, a high arched palate, pectus excavatum and a double jointed thumb, which can often be seen with the premutation because the level of FMRP can sometimes be deficient in the premutation range.2,5 Although these physical features are also common in the general population, hyperextensibility of finger joints, ear prominence and enlarged testicles can also be presented in individuals with the premutation and these features are correlated with FMRP levels in both the premutation and the full mutation.2,5,19–21
Developmental and Behavioral Involvement
Although the presence of autism in those with the full mutation has been thoroughly studied this is not true of those with the premutation. Two to six percent of children with autism of unknown etiology will have the fragile X full mutation22–24 but the rate of the premutation in autism is not known. Approximately 30% of children with the full mutation and FXS will have autism25,26 and an additional 30% will have PDD-NOS.27 Developmental and behavioral problems including developmental delay, speech and language impairments, ASD, ADHD, and social deficits, are relatively common in young individuals with the premutation particularly in boys as reported in the literature.4,5,28–30 Farzin et al. (2006) found that ASD and ADHD was significantly more common in boys with the premutation who presented as probands compared to their brothers without the premutation.4 In a large survey study of over 1,200 fragile X families it was found that 45% of male individuals over 6 years of age with the premutation had been diagnosed or treated for attention problems, 36% for anxiety, 32% for developmental delay, 30% for hyperactivity and 19% for autism and aggression.7
Cognitive Involvement
Although cognitive deficits and even dementia are common in individuals with FXTAS,9,31 other cognitive deficits including executive function, working memory, selective attention and social cognition have been demonstrated across the lifespan in premutation carriers without FXTAS compared to controls.21,31–37 One exception to these reports is a study by Hunter et al. (2008) who found no cognitive deficits in a cohort of individuals with the premutation who were younger than age 50 compared to controls.38 Most individuals do well with the premutation during early life and are able to overcome ADHD or social deficits to go on to higher education.39
Interesting work has been done in relating the cognitive impairments in premutation carriers without FXTAS to neuroimaging findings suggesting a strong relationship among gene, brain and cognitive profiles. For instance, decreased amygdala activation while viewing fearful human faces, or decreased left hippocampal activation in addition to increased right parietal activation during a recall task when compared to controls have been illustrated in men with the premutation but without FXTAS.40,41 These functional changes were associated with both elevated FMR1 RNA and psychiatric symptoms in these men. These findings suggest that the connectivity in the brain linked to the amygdala and the hippocampus in those with the premutation is mildly dysfunctional from a neurodevelopmental perspective and may contribute to clinically signficicant impairment especially in those with higher CGG repeat alleles. Studies of premutation neuron cell cultures have shown that branching of the dendrites is decreased and the synaptic size is increased in neurons with the premutation compared to controls.42 These connectivity changes likely make the individual with the premutation more vulnerable to additional genetic or environmental problems that further interfere with development. We also know that the premutation neuron will die more easily with toxicity or stress in the environment so these cells are more vulnerable because of the RNA toxicity.43,44
Those with the premutation usually have a normal IQ, but this proband’s IQ was in a moderate range of ID. This is a more of significant deficit than what is typically seen in premutation carriers and in contrast to his brother’s higher level of function, so additional additive effects must be considered here such as the proband’s history of recurrent seizures in addition to autism.4,5,45 ID has been documented in male individuals with the premutation in previous literature5,15,46,47 and the prevalence of ID was approximately 12% as reported in one large study.46 The effect of each factor including severe social impairment, seizures comorbidity, perhaps a second gene effect, and other environmental factors can be additive to the premutation to predispose this proband to have more intellectually impaired when compared to his biological brother even though they both have similar range of CGG repeats. Taken together, CGG repeats alone do not predict or determine the entire outcome of those with fragile X mutations. To improve the long-term outcome, early multidisciplinary management including early intervention, speech and language therapy, occupational therapy, physical therapy, special education, tailored behavioral and medical interventions should be recommended particularly in those who have behavioral and neurodevelopmental problems.48,49
Neurological Involvement
Seizures have been reported to be increased in those with autism and FXS compared to FXS without autism.50 Seizures are more likely to occur in males with the premutation compared to controls when matched on children’s age and family income (11.3% vs. 1.2%) in a recent large survey study.7 This proband had seizures and a clinical response to valproic acid with improvement in seizures in addition to language abilities and social skills. Such a response is often seen in those with a full mutation and seizures48,49 and also in the autism population without fragile X syndrome.51 Anticonvulsants should be part of the treatment program in those with the fragile X mutation (premutation or full mutation) and clinical seizures.48,49
FXTAS, a progressive late onset neurological disorder has been documented for years in a subgroup of older individuals with the premutation affecting some carriers over 50 years of age.12 The proband’s maternal grandfather was diagnosed with probable FXTAS because of his tremors and other symptoms52 and his FMR1 testing showed premutation mosaicism with allele sizes of 52 and 68 CGG repeats. FXTAS features include intention tremor and/ or gait ataxia, peripheral neuropathy, parkinsonism, psychiatric symptoms including anxiety, irritability, or disinhibition, autonomic dysregulation including hypertension, orthostatic hypotension and incontinence, executive function and memory deficits.13,31,37,52 In cases of FXTAS, 60% of males and 13% of females have an MRI with symmetric white matter lesions involving the middle cerebellar peduncles (MCP sign).53 Presence of intranuclear inclusions in both neurons and astrocytes throughout the brain are also reported from post-mortem neuropathological studies of those with FXTAS.54 The maternal grandfather’s brother also had a gray zone allele with 52 repeats and he had subtle tremor and balance problems but they were not of the severity of what is typically seen in FXTAS (See Table 1). We do not yet know whether neurological problems are more common in those with a gray zone allele.
Medical and Endocrinological Involvement
There is an increased risk for medical and endocrinological problems in individuals with the premutation compared to age matched controls.6,55 The proband’s mother and one maternal aunt had thyroid problems and another maternal aunt had hypertension and both conditions have a higher prevalence in female carriers with neurological symptoms compared to controls.6 These findings have been replicated in a subsequent study56 although an earlier study did not report these medical problems, but many of these problems were not specifically questioned.57 POI has been well validated by many studies as increased in premutation carriers compared to controls.8,55,58 POI affects approximately 20% of female carriers but was not seen in the 3 females we evaluated here.
Psychological Involvement
The biological brother and cousin of the proband developed an overanxious disorder and obsessive compulsive rituals. Likewise, mother and both maternal aunts of the proband experienced anxiety and depression, which are more common in the premutation carriers, varying from 18–43% in numerous studies of carriers compared to controls.9–11,59,60 Furthermore, psychological difficulties in those with the premutation were significantly associated with elevated FMR1-mRNA level suggesting FMR1 dysfunction is an important pathogenic mechanism of these problems, over and above the well-known stresses associated with caring for children with intellectual impairment.11,59,60
Mechanism of Clinical Involvement in the Premutation
As we described in the introduction, the mechanism of the premutation involvement is different from those with the full mutation in that an RNA toxic gain-of-function effect is seen due to enhanced levels of FMR1 mRNA (2–8 times higher than normal individuals). The elevated mRNA levels leads to dysregulation of several proteins and subsequent neuronal and astrocyte toxicity and eventually cell death.15,43 The mRNA toxicity can involve many areas in the CNS including the limbic system, pituitary, the autonomic ganglia of the peripheral nervous system in addition to the testicles, thyroid and adrenal gland.61,62 Therefore, the elevated mRNA in the maternal grandfather’s case is likely leading to the symptoms of FXTAS including head and hands tremors, hypertension, hypoglycemic episodes, sleep apnea and anxiety although he does not have the MCP sign.
Signs of premutation involvement including psychopathology, endocrine dysfunction, autonomic dysfunction, and neuropathy typically occur even before the FXTAS problems of tremor and ataxia begin. The fragile X or FMR1 DNA test should be considered in individuals at high risk of having fragile X mutations particularly in children with ASD, developmental and intellectual disabilities, women with early menopause, or older adults with intention tremor or ataxia.63 This family tree is unusual because there is only premutation and gray zone involvement. With more widespread screening for fragile X mutations we predict many more similar pedigrees with significant premutation involvement will be seen because the premutation is far more prevalent (1/250–800) than the full mutation (1/2500–4000).64
In conclusion, there is often a wide range of clinical involvement in extended family members in all generations when a proband is identified with a fragile X mutation. Therefore, to provide appropriate treatment and genetic counseling for all of the affected individuals in a family, a detailed family history that reviews many of the disorders that are related to both the premutation and the full mutation should be carried out in addition to referral to a genetic counselor and multidisciplinary professionals as necessary.48,65 Early treatment of premutation and full mutation disorders are likely to be beneficial for their long-term outcome.13,48
Acknowledgments
This work was supported by grants from NICHD HD036071, HD02274, NIA AG032115, NIMH 77554, NCRR DE019683 and the Health and Human Services Administration of Developmental Disabilities grant 90DD0596.
Contributor Information
WEERASAK CHONCHAIYA, M.I.N.D. Institute, University of California-Davis Medical Center, Sacramento, CaliforniaDivision of Growth and Development, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
AGUSTINI UTARI, M.I.N.D. Institute, University of California-Davis Medical Center, Sacramento, CaliforniaDivision of Human Genetics, Center for Biomedical Research, Faculty of Medicine, Diponegoro University, Semarang, Indonesia
GABRIELA MARQUES PEREIRA, Department of Pediatrics, São Marcos Hospital, Braga, Portugal
FLORA TASSONE, M.I.N.D. Institute, University of California-Davis Medical Center, Sacramento, CaliforniaDepartment of Biochemistry and Molecular Medicine, University of California, Davis, School of Medicine, Davis, California
DAVID HESSL, M.I.N.D. Institute, University of California-Davis Medical Center, Sacramento, CaliforniaDepartment of Psychiatry and Behavioral Sciences, University of California-Davis Medical Center, Sacramento, California
RANDI J. HAGERMAN, M.I.N.D. Institute, University of California-Davis Medical Center, Sacramento, CaliforniaDepartment of Pediatrics, University of California-Davis Medical Center, Sacramento, California
References
- 1.Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 2.Tassone F, Hagerman RJ, Iklé DN, et al. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 1999;84:250–261. [PubMed] [Google Scholar]
- 3.Hagerman RJ. Physical and behavioral phenotype. In: Hagerman RJ, Hagerman PJ, editors. Fragile X syndrome: Diagnosis, treatment and research. Baltimore: The Johns Hopkins University Press; 2002. pp. 3–109. [Google Scholar]
- 4.Farzin F, Perry H, Hessl D, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27:S137–144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- 5.Aziz M, Stathopulu E, Callias M, et al. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet. 2003;121B:119–127. doi: 10.1002/ajmg.b.20030. [DOI] [PubMed] [Google Scholar]
- 6.Coffey SM, Cook K, Tartaglia N, et al. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey DB, Jr, Raspa M, Olmsted M, et al. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008;146A:2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- 8.Wittenberger MD, Hagerman RJ, Sherman SL, et al. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–465. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Bourgeois JA, Coffey SM, Rivera SM, et al. A review of fragile X premutation disorders: expanding the psychiatric perspective. J Clin Psychiatry. 2009;70:852–862. doi: 10.4088/JCP.08m04476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts JE, Bailey DB, Jr, Mankowski J, et al. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:130–139. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- 11.Hessl D, Tassone F, Loesch DZ, et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. 2005;139:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- 12.Berry-Kravis E, Abrams L, Coffey SM, et al. Fragile X-associated tremor/ataxia syndrome: Clinical features, genetics, and testing guidelines. Mov Disord. 2007;22:2018–2030. doi: 10.1002/mds.21493. [DOI] [PubMed] [Google Scholar]
- 13.Hagerman RJ, Hall DA, Coffey S, et al. Treatment of fragile X-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Clin Interv Aging. 2008;3:251–262. doi: 10.2147/cia.s1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tassone F, Hagerman RJ, Taylor AK, et al. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagerman PJ, Hagerman RJ. The fragile-X premutation: a maturing perspective. Am J Hum Genet. 2004;74:805–816. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loesch DZ, Bui QM, Huggins RM, et al. Transcript levels of the intermediate size or grey zone fragile X mental retardation 1 alleles are raised, and correlate with the number of CGG repeats. J Med Genet. 2007;44:200–204. doi: 10.1136/jmg.2006.043950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. 2005;117:376–382. doi: 10.1007/s00439-005-1326-8. [DOI] [PubMed] [Google Scholar]
- 18.Streuli I, Fraisse T, Ibecheole V, et al. Intermediate and premutation FMR1 alleles in women with occult primary ovarian insufficiency. Fertil Steril. 2009;92:464–470. doi: 10.1016/j.fertnstert.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Loesch DZ, Huggins RM, Bui QM, et al. Relationship of deficits of FMR1 gene specific protein with physical phenotype of fragile X males and females in pedigrees: A new perspective. Am J Med Genet. 2003;118A:127–134. doi: 10.1002/ajmg.a.10099. [DOI] [PubMed] [Google Scholar]
- 20.Kenneson A, Zhang F, Hagedorn CH, et al. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10:1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- 21.Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Ment Retard Dev Disabil Res Rev. 2004;10:31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- 22.Wassink TH, Piven J, Patil SR. Chromosomal abnormalities in a clinic sample of individuals with autistic disorder. Psychiatr Genet. 2001;11:57–63. doi: 10.1097/00041444-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Reddy KS. Cytogenetic abnormalities and fragile-X syndrome in Autism Spectrum Disorder. BMC Med Genet. 2005;6:3. doi: 10.1186/1471-2350-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagerman RJ, Rivera SM, Hagerman PJ. The fragile X family of disorders: A model for autism and targeted treatments. Current Pediatric Reviews. 2008;4:40–52. [Google Scholar]
- 25.Kaufmann WE, Cortell R, Kau AS, et al. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet. 2004;129A:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- 26.Rogers SJ, Wehner EA, Hagerman RJ. The behavioral phenotype in fragile X: Symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Harris SW, Hessl D, Goodlin-Jones B, et al. Autism profiles of males with fragile X syndrome. Am J Mental Retardation. 2008;113:427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clifford S, Dissanayake C, Bui QM, et al. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. J Autism Dev Disord. 2007;37:738–747. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- 29.Loesch DZ, Bui QM, Dissanayake C, et al. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neurosci Biobehav Rev. 2007;31:315–326. doi: 10.1016/j.neubiorev.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagerman RJ, Goodlin-Jones BL, Spence S, et al. The fragile X premutation and autistic spectrum disorders. Am J Hum Genet. 2002;(Suppl 71):A679, 287. [Google Scholar]
- 31.Grigsby J, Brega AG, Engle K, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- 32.Loesch DZ, Bui MQ, Grigsby J, et al. Effect of the fragile X status categories and the FMRP levels on executive functioning in fragile X males and females. Neuropsychology. 2003;17:646–657. doi: 10.1037/0894-4105.17.4.646. [DOI] [PubMed] [Google Scholar]
- 33.Cornish KM, Kogan C, Turk J, et al. The emerging fragile X premutation phenotype: Evidence from the domain of social cognition. Brain Cogn. 2005;57:53–60. doi: 10.1016/j.bandc.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Moore CJ, Daly EM, Schmitz N, et al. A neuropsychological investigation of male premutation carriers of fragile X syndrome. Neuropsychologia. 2004;42:1934–1947. doi: 10.1016/j.neuropsychologia.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Cornish KM, Li L, Kogan CS, et al. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex. 2008;44:628–636. doi: 10.1016/j.cortex.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loesch D, Huggins R, Bui QM, et al. Effect of the fragile X status categories and FMRP deficits on cognitive profiles estimated by robust pedigree analysis. Am J Med Gen. 2003;122A:12–23. doi: 10.1002/ajmg.a.20214. [DOI] [PubMed] [Google Scholar]
- 37.Brega AG, Goodrich G, Bennett RE, et al. The primary cognitive deficit among males with fragile X-associated tremor/ataxia syndrome (FXTAS) is a dysexecutive syndrome. J Clin Exp Neuropsychol. 2008:1–17. doi: 10.1080/13803390701819044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter JE, Allen EG, Abramowitz A, et al. No evidence for a difference in neuropsychological profile among carriers and noncarriers of the FMR1 premutation in adults under the age of 50. Am J Hum Genet. 2008;83:692–702. doi: 10.1016/j.ajhg.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grigsby J, Brega AG, Jacquemont S, et al. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS) J Neurol Sci. 2006;248:227–233. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Hessl D, Rivera S, Koldewyn K, et al. Amygdala dysfunction in men with the fragile X premutation. Brain. 2007;130:404–416. doi: 10.1093/brain/awl338. [DOI] [PubMed] [Google Scholar]
- 41.Koldewyn K, Hessl D, Adams J, et al. Reduced hippocampal activation during recall is associated with elevated FMR1 mRNA and psychiatric symptoms in men with the fragile X premutation. Brain Imaging and Behavior. 2008;2:105–116. doi: 10.1007/s11682-008-9020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Tassone F, Berman RF, et al. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. doi: 10.1093/hmg/ddp479. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arocena DG, Iwahashi CK, Won N, et al. Induction of inclusion formation and disruption of lamin A/C structure by premutation CGG-repeat RNA in human cultured neural cells. Hum Mol Genet. 2005;14:3661–3671. doi: 10.1093/hmg/ddi394. [DOI] [PubMed] [Google Scholar]
- 44.Handa V, Goldwater D, Stiles D, et al. Long CGG-repeat tracts are toxic to human cells: implications for carriers of Fragile X premutation alleles. FEBS Lett. 2005;579:2702–2708. doi: 10.1016/j.febslet.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Goodlin-Jones B, Tassone F, Gane LW, et al. Autistic spectrum disorder and the fragile X premutation. J Dev Behav Pediatr. 2004;25:392–398. doi: 10.1097/00004703-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Rousseau F, Heitz D, Tarleton J, et al. A multicenter study on genotype-phenotype correlations in the fragile X syndrome, using direct diagnosis with probe StB12.3: the first 2,253 cases. Am J Hum Genet. 1994;55:225–237. [PMC free article] [PubMed] [Google Scholar]
- 47.Tassone F, Hagerman RJ, Taylor AK, et al. Clinical involvement and protein expression in individuals with the FMR1 premutation. Am J Med Genet. 2000;91:144–152. doi: 10.1002/(sici)1096-8628(20000313)91:2<144::aid-ajmg14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 48.Hagerman RJ, Berry-Kravis E, Kaufmann WE, et al. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome--present and future. Ment Retard Dev Disabil Res Rev. 2004;10:42–48. doi: 10.1002/mrdd.20007. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Nonell C, Ratera ER, Harris S, et al. Secondary medical diagnosis in fragile X syndrome with and without autism spectrum disorder. Am J Med Genet A. 2008;146A:1911–1916. doi: 10.1002/ajmg.a.32290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Martino A, Tuchman RF. Antiepileptic drugs: affective use in autism spectrum disorders. Pediatr Neurol. 2001;25:199–207. doi: 10.1016/s0887-8994(01)00276-4. [DOI] [PubMed] [Google Scholar]
- 52.Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adams JS, Adams PE, Nguyen D, et al. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS) Neurology. 2007;69:851–859. doi: 10.1212/01.wnl.0000269781.10417.7b. [DOI] [PubMed] [Google Scholar]
- 54.Greco CM, Berman RF, Martin RM, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan AK, Marcus M, Epstein MP, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, et al. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. [Accessed August 17, 2009];Eur J Hum Genet. 2009 April; doi: 10.1038/ejhg.2009.51. [serial online] Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19367323. [DOI] [PMC free article] [PubMed]
- 57.Hundscheid RD, Smits AP, Thomas CM, et al. Female carriers of fragile X premutations have no increased risk for additional diseases other than premature ovarian failure. Am J Med Genet A. 2003;117A:6–9. doi: 10.1002/ajmg.a.10862. [DOI] [PubMed] [Google Scholar]
- 58.Cronister A, Schreiner R, Wittenberger M, et al. Heterozygous fragile X female: historical, physical, cognitive, and cytogenetic features. Am J Med Genet. 1991;38:269–274. doi: 10.1002/ajmg.1320380221. [DOI] [PubMed] [Google Scholar]
- 59.Abbeduto L, Seltzer MM, Shattuck P, et al. Psychological well-being and coping in mothers of youths with autism, Down syndrome, or fragile X syndrome. Am J Ment Retard. 2004;109:237–254. doi: 10.1352/0895-8017(2004)109<237:PWACIM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez-Revenga L, Madrigal I, Alegret M, et al. Evidence of depressive symptoms in fragile-X syndrome premutated females. Psychiatr Genet. 2008;18:153–155. doi: 10.1097/YPG.0b013e3282f97e0b. [DOI] [PubMed] [Google Scholar]
- 61.Gokden M, Al-Hinti JT, Harik SI. Peripheral nervous system pathology in fragile X tremor/ataxia syndrome (FXTAS) Neuropathology. 2009;29:280–284. doi: 10.1111/j.1440-1789.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 62.Greco CM, Soontarapornchai K, Wirojanan J, et al. Testicular and pituitary inclusion formation in fragile X associated tremor/ataxia syndrome. The Journal of urology. 2007;177:1434–1437. doi: 10.1016/j.juro.2006.11.097. [DOI] [PubMed] [Google Scholar]
- 63.Hagerman RJ, Hagerman PJ. Testing for fragile X gene mutations throughout the life span. JAMA. 2008;300:2419–2421. doi: 10.1001/jama.2008.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandez-Carvajal I, Walichiewicz P, Xiaosen X, et al. Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. J Mol Diagn. 2009;11:324–329. doi: 10.2353/jmoldx.2009.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McConkie-Rosell A, Abrams L, Finucane B, et al. Recommendations from multi-disciplinary focus groups on cascade testing and genetic counseling for fragile X-associated disorders. J Genet Couns. 2007;16:593–606. doi: 10.1007/s10897-007-9099-y. [DOI] [PubMed] [Google Scholar]