Abstract
Background
Most of the millions of oral contraceptive (OC) users are under age 30 years and in the critical period for bone mass accrual.
Study Design
This cross-sectional study of 606 women aged 14–30 years examined both OC duration and estrogen dose and their association with bone mineral density (BMD) at the hip, spine, and whole-body (DEXA).
Results
Of 389 OC users and 217 nonusers enrolled, 50% were adolescents (14–18 years). Of OC users, 38% used “low-dose” OCs [<30 mcg ethinyl estradiol (EE)]. In adolescents, mean BMD differed by neither OC duration nor EE dose. However, 19–30 year-old women’s mean BMD was lower with longer OC use for spine and whole-body (p=0.004, p=0.02, respectively) and lowest for >12 months of low-dose OCs for the hip, spine and whole-body (p=0.02, 0.003 and 0.002, respectively).
Conclusions
Prolonged use of today’s OCs, particularly <30 mcg EE, may adversely impact young adult women’s bone density while ingesting these agents.
Keywords: bone mineral density, oral contraceptives, hormones, peak bone density, adolescents
1. Introduction
Nearly 12 million U.S. women use oral contraceptives (OCs) [1]. OC use is highest in women under age 30 years, a critical time for bone mass accrual. OCs’ effects on bone remain unclear, with increasing evidence suggesting current OC formulations may act differently in women who have not yet achieved peak bone mass as compared to skeletally mature women [2– 5]. Studies in adolescent and young adult women report mixed results, with some bone density studies reporting an OC benefit or no difference from nonusers [6–11], but others noting that OCs may inhibit bone mass accrual [3, 4, 12–17].
We report results of a population-based cross-sectional evaluation of both OC duration and estrogen dose on bone mineral density (BMD) in 606 women ages 14 and 30 years. To our knowledge, this is the first study to examine these factors and their possible different roles in adolescent and young adult women within a single study population.
2. Materials and methods
Study participants were enrollees of Group Health Cooperative, a mixed model managed-care system in the Pacific Northwest. The Group Health Human Subjects Committee approved all study procedures. We obtained written consent from participants (or from parents, with assent from minors).
We used a population-based sampling strategy to select potential participants from the health system’s computerized databases. We aimed to enroll 600 participants, including 200 OC users and 100 nonusers equally distributed in two age strata: adolescents (aged 14–18 years) and young adult women (19–30 years). We used the health system’s pharmacy data files to identify current OC users and classify them as “initiators” if ≤3 months of use; or “prevalent users” if >3 months of use. We preferentially enrolled women using “low-dose” OCs [<30 mcg ethinyl estradiol (EE)] in order to evaluate these agents adequately as well as the predominately-used 30– 35 mcg EE formulations [18]. The non-OC using women (the comparison group) were sampled concurrently from the health plan enrollment files, frequency matching on age and primary clinic base. Nonusers were required to have no hormonal contraception use in the previous 2 years and less than 8 years in their lifetime.
Potential participants were recruited by mailed invitation with telephone follow-up to ascertain willingness to participate, eligibility, and to obtain consent. We excluded women who were pregnant, lactating, intending to become pregnant, using other methods of hormonal contraception, or who had conditions or were using medications known to affect bone density. We sent invitation letters to 1,549 women who had current OC prescriptions and 1,199 potential comparison women. We enrolled 606 participants: 389 current OC users and 217 nonusers; 301 participants (50%) were 14–18 years old and 305 were 19–30 years. Of the OC use group, 164 (42%) were initiators and 225 (58%) prevalent users. Also, 148 women (38%) were using <30 mcg EE OCs and 241 (62%) were using 30–35 mcg EE OCs.
Participants completed questionnaires to collect information on health history, reproductive and menstrual history, smoking, alcohol use, physical activity, caffeine intake, and demographics; and a validated food frequency questionnaire of dietary consumption [19]. We obtained detailed information about lifetime hormonal contraceptive use via survey and an in-person interview using a life events calendar to assist in pinpointing start and stop dates [20–22]. We measured height and weight. We used dual energy x-ray absorptiometry (Hologic Delphi, Waltham, MA) to assess bone density at the hip, lumbar spine and whole body, following manufacturer’s protocols. The densitometer provides the areal bone mineral density (BMD, g/cm2) and the bone mineral content (BMC, g). At the spine, we accounted for navel jewelry using previously reported methods [23]. Participants received $30 for the visit.
2. 1. Statistical methods
We compared characteristics of adolescent (14 to 18 year-old) and young adult (19 to 30 year-old) women currently using OCs to nonusers of the same age. Within each age group, we examined unadjusted mean BMD values by OC duration and dose. Current duration of OC use, defined as how many months a participant had continuously used the formulation as of her clinic visit, was categorized as 0 (comparison group), >0–3, >3–12, >12–24, and >24 months. OC dose was categorized as 0 (comparison women), <30 mcg, and 30–35 mcg EE OCs. We then examined dose-duration groups, comparing nonusers to: short OC duration (≤12 months) and 30–35 mcg EE; short duration and lower (<30 mcg) EE; longer duration (>12 months) and 30–35 mcg EE; and longer duration and lower EE pill content. We adjusted for potential confounders, which were selected a priori or associated with either BMD or OC use, and tested for a group linear trend using least squares linear regression. Because, in the youngest participants, the bone size as well as bone density might be altered with OC use, we also evaluated bone mineral content (BMC, g). Self-reported study data and health plan computerized pharmacy data on current OC dose at the time of sampling agreed 100%.
All analyses were conducted in SAS Version 9 (Cary, NC) and accounted for the sampling design. All statistical tests were two-sided.
3. Results
Overall, relative to nonusers, women currently using OCs were more likely to be white, married, smoke, wear navel jewelry, older and have lower BMI (Table 1). Among adolescents, OC users were more likely than nonusers to have a relative with a fracture history. Young adult OC users had more irregular menstrual periods, physical activity, dietary calcium intake, and alcohol consumption compared to nonusers. Mean BMD and BMC for OC users vs. nonusers was slightly higher among adolescents and slightly lower or the same among young adult women at all anatomic sites.
Table 1.
Participant characteristics by age group and OC exposure status
| Adolescents | Young women | |||
|---|---|---|---|---|
| Age 14–18 years (n=301) | Age 19–30 years (n=305) | |||
| OC status | OC user (n=194) |
OC nonuser b (n=107) |
OC user (n=195) |
OC nonuser b (n=110) |
| N (weighted %) a | ||||
| Ethnicity | ||||
| Asian | 4 (1.1) | 8 (7.5) | 13 (7.4) | 14 (12.7) |
| Black/African American | 7 (3.4) | 8 (7.5) | 4 (0.2) | 5 (4.5) |
| White | 157 (85.4) | 68 (64.2) | 153 (81.6) | 72 (65.5) |
| Other | 26 (10.1) | 22 (20.8) | 24 (10.8) | 19 (17.3) |
| Education ≤HS or GED | 179 (90.8) | 182 (93.4) | 8 (3.6) | 4 (3.6) |
| Never married | 188 (95.9) | 106 (99.1) | 133 (68.1) | 88 (80.0) |
| Current smoker | 9 (5.9) | 5 (4.7) | 23 (13.1) | 6 (5.5) |
| Relative with fracture | 31 (22.3) | 13 (16.9) | 43 (30.0) | 22 (30.1) |
| Ever pregnant | 1 (1.1) | 2 (1.9) | 26 (14.1) | 17 (15.6) |
| Regular periods | 160 (80.6) | 89 (83.2) | 173 (87.7) | 102 (92.7) |
| Navel jewelry | 23 (15.3) | 6 (5.6) | 18 (12.4) | 7 (6.4) |
| Current OC 30–35 mcg EE | 121 (86.6) | NA | 120 (89.3) | NA |
| Weighted mean a(SE) | ||||
| Age (yrs) | 16.8 (0.1) | 16.4 (0.2) | 24.6 (0.3) | 24.1 (0.3) |
| Age at menarche (yrs) | 12.1 (0.1) | 12.4 (0.1) | 12.7 (0.1) | 12.5 (0.1) |
| Body mass index (kg/m2) | 23.3 (0.4) | 23.9 (0.4) | 24.3 (0.5) | 25.2 (0.5) |
| Height (in) | 65 (0.3) | 65 (0.2) | 65 (0.2) | 65 (0.2) |
| Wgt-bearing phys activity | 1189 (57) | 1178 (61) | 839 (56) | 820 (48) |
| Calcium (mg/day) | 1167 (49) | 1181 (58) | 1045 (49) | 1001 (48) |
| Alcohol intake (g/d) | 1.5 (0.5) | 0.7 (0.2) | 9.1 (1.2) | 5.7 (0.8) |
| Protein, total (g/d) | 74 (3) | 72 (3) | 70 (2) | 74 (3) |
| Current OC use (mos.) | 9 (1) | NA | 18 (2) | NA |
| Total hip BMD (g/cm2) | 0.995 (0.010) | 0.994 (0.010) | 0.980 (0.012) | 0.994 (0.010) |
| Spine BMD (g/cm2) | 1.011 (0.010) | 1.001 (0.008) | 1.026 (0.011) | 1.044 (0.011) |
| Whole-body BMD (g/cm2) | 1.090 (0.007) | 1.083 (0.007) | 1.112 (0.008) | 1.112 (0.008) |
| Whole-body BMC (g) | 2146.7 (30.4) | 2120.8 (26.7) | 2179.8 (30.3) | 2192.4 (27.1) |
Ns are actual number of participants; percentages and means are weighted to account for the study sampling frame.
Nonusers had no OC use in the prior 2 years. They were allowed some use prior to that time: In adolescents, 94% of nonusers had no prior use at all; only one had >3 months of use before the 2 most recent years (11.9 mos.); in young adult women, 64% of the 110 nonusers had no prior use at all; 9 had >36 months of use before the 2 most recent years (range 37–86 months).
Mean use of the current OC was 9 months (range 0.3–37) for adolescents and 18 months (range 0.3–135) for young adult women. Average lifetime OC use was 12 months (range 0.3–62) and 55 months (0.3–159), for adolescent and young adult women, respectively.
Among 14- to 18-year-olds, adjusted mean BMD values for duration of use and dose for current OC users vs. nonusers did not differ significantly at any anatomic site (Table 2). This was also true for adjusted mean BMC (data not shown). However, in the 19- to 30-year-old group, adjusted mean BMDs at the spine and whole-body were significantly lower with increasing OC duration (p-value for linear trend=0.02 and 0.004, respectively). Mean BMD was 5.9% and 2.3% lower at these sites, respectively, for women with >24 months of OC use, relative to nonusers. Trends were similar for the hip (p-value, trend=0.09). Our evaluation of BMC yielded similar results, but trends for duration in young adult women were non-significant (p-value, trend=0.45, 0.16, 0.13 for hip, spine and whole-body respectively). Mean BMD at all anatomic sites was lower, relative to nonusers, for women using 30–35 mcg EE OCs and lowest for women using <30 mcg EE OCs, but adjusted results were not significant (p≥0.10, all sites). Mean BMC also showed no significant differences by OC EE dose. Results did not differ when we restricted the nonuser group to women who had never used OCs.
Table 2.
Adjusted mean BMD by age group, duration, and dose of current OC use
| Hip BMD a | Spine BMD a | Whole-body BMD a | ||
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| Adolescents, 14–18 years old | ||||
| N b | ||||
| OC duration c | ||||
| Nonuser | 107 | 0.984 (0.960 –1.008) | 1.003 (0.984–1.022) | 1.085 (1.069–1.100) |
| > 0–3 months | 117 | 0.994 (0.977 –1.012) | 1.016 (0.997–1.035) | 1.089 (1.076–1.101) |
| >3–12 months | 46 | 1.012 (0.974–1.051) | 1.016 (0.974–1.057) | 1.101 (1.075–1.128) |
| >12–24 months | 23 | 0.979 (0.941–1.018) | 0.999 (0.960–1.039) | 1.084 (1.055–1.113) |
| >24 months | 8 | 0.971 (0.900–1.043) | 1.010 (0.948–1.073) | 1.094 (1.042–1.145) |
| P for linear trend | 0.77 | 0.86 | 0.75 | |
| OC dose (EE) | ||||
| Nonuser | 107 | 0.983 (0.958–1.007) | 1.002 (0.983–1.021) | 1.084 (1.069–1.100) |
| 30–35 mcg | 121 | 0.994 (0.973–1.016) | 1.012 (0.991–1.033) | 1.092 (1.077–1.107) |
| <30 mcg | 73 | 1.000 (0.974–1.026) | 1.011 (0.990–1.033) | 1.094 (1.076–1.111) |
| P for global test | 0.52 | 0.69 | 0.65 | |
| Young Adults, 19–30 years old | ||||
| OC durationc | ||||
| Nonuser | 110 | 0.983 (0.959–1.006) | 1.040 (1.016–1.065) | 1.107 (1.089–1.125) |
| >0–3 months | 100 | 0.990 (0.950–1.031) | 1.037 (1.004–1.071) | 1.132 (1.109–1.156) |
| >3–12 months | 29 | 1.008 (0.966–1.050) | 1.067 (1.030–1.104) | 1.136 (1.107–1.166) |
| >12–24 months | 34 | 0.965 (0.926–1.005) | 1.037 (0.996–1.078) | 1.109 (1.074–1.143) |
| >24 months | 32 | 0.945 (0.902–0.988) | 0.979 (0.940–1.017) | 1.082 (1.056–1.108) |
| P for linear trend | 0.09 | 0.02 | 0.004 | |
| OC dose (EE) | ||||
| Nonuser | 110 | 0.982 (0.959–1.006) | 1.040 (1.016–1.064) | 1.107 (1.089–1.125) |
| 30–35 mcg | 120 | 0.978 (0.956–1.001) | 1.030 (1.007–1.052) | 1.116 (1.100–1.131) |
| <30 mcg | 75 | 0.952 (0.924–0.980) | 1.004 (0.979–1.029) | 1.095 (1.073–1.117) |
| P for global test | 0.21 | 0.10 | 0.32 | |
Adjusted for age, race (White vs. Non-white), BMI, calcium intake, alcohol intake, weight-bearing physical activity, age at menarche, ever smoke (yes/no), and regular periods (yes/no). Mean results are based on OC variable as categorical term in the model.
N reflects the number of enrolled study participants by baseline current OC duration and dose. The actual N is slightly lower in some models due to missing data for covariates and/or outcomes.
OC duration is months of continuous use of the OC being used at the study visit.
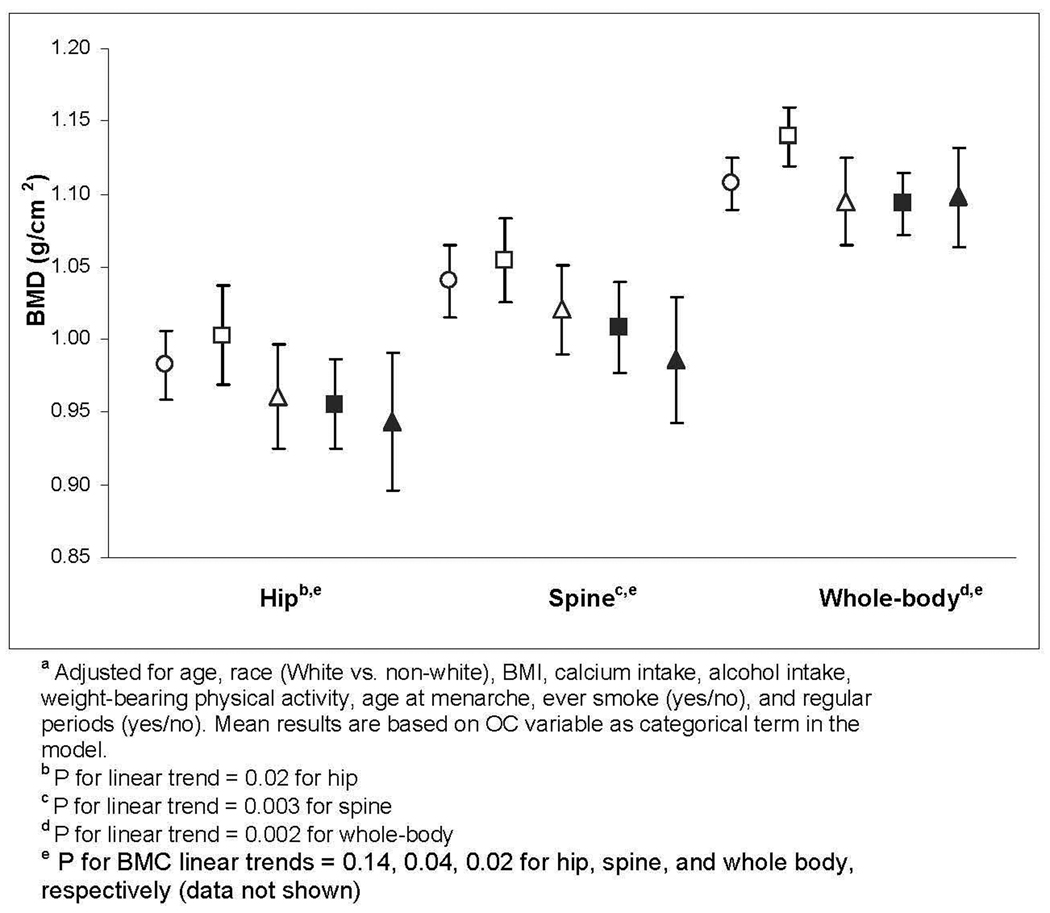
Among adolescents, we saw no trend across the dose-duration categories for any anatomic site (data not shown), but few women had used OCs for >12 months. Among young adult women, mean BMD trended lower with lower dose and longer duration (Figure 1). Trends were significant at all anatomic sites (p-value, trend = 0.02, 0.003, and 0.002 for hip, spine and whole-body, respectively) (Figure 1). Mean spine BMD in the women with >12 months of low-dose OC use was 5.2% lower than in non-OC comparison women. Our evaluation of mean BMC showed similar, although less significant, trends (p-value, trend=0.14, 0.04, and 0.02 for hip, spine, and whole-body BMC, respectively).
Figure 1.
Mean bone density for 19–30 year-old participants, by current OC dose and duration category.a
Mean ± 95% Confidence Interval
○ Nonuser (N=110)
□ 30–35 mcg, ≤12mo (N=79)
Δ <30 mcg EE, ≤12mo (N=50)
■ 30–35mcg, >12mo (N=41)
▲ <30 mcg, >12mo (N=25)
4. Discussion
This cross-sectional analysis of a population-based sample of 606 adolescent and young adult women associated current longer-term use of OCs containing ≤35 mcg EE with lower BMD in young adult women but not adolescents. The longer-use/lower-dose group had the strongest associations. We also noted stronger associations for the spine than for the hip, consistent with peak bone mass attainment occurring later at the spine [6, 24, 25]. In the 301 adolescents 14 to 18 years old—the group most actively gaining bone—neither current OC duration nor dose was associated with lower BMD.
Despite the highly prevalent and long-term use of OCs in the U.S. and worldwide, the available evidence of their effects on bone health in adolescent and young adult women—the groups with highest use—continues to be inconclusive [2]. To date, study methodology and populations have varied, complicating interpretation of results. Generally, the relatively few studies focused on younger adolescents have been prospective [9, 11, 16, 17]. Two with relatively short follow-up (12–24 months) reported no significant between-group differences, as in our study. In our study, only 8 of our adolescent participants reported >24 months’ use. However, two studies with longer-term follow-up of 4 and 5 years noted significantly smaller increases in spine and femoral neck BMC [16] and distal radius BMD [17] in adolescent OC users vs. nonusers.
More investigations have been conducted in older adolescents and young adult women who are still gaining bone, with more disparate findings, some of which may be due to differences in OC dose and duration. Two of the longest prospective studies found adverse impacts from low-dose (20 mcg EE) OCs. A 5-year prospective study of 200 women aged 19–22 years, found no gains in spine BMD in users of a 20-mcg EE OC and a gain of 7.8% in comparison women [4]. A recent 36-month prospective study [15] of women ages 16–34 years reported BMD losses at both the spine and femoral neck (−0.5% and −1.3%, respectively) among users of a 20-mcg EE OC compared to BMD gains (1.9% and 0.6%, respectively) in nonusers. In contrast, in our prior prospective cohort analyses of women aged 18–39 years, BMD did not differ significantly in the 89 OC users (mostly using 30–35 mcg EE) vs. 156 nonusers after 36 months [8].
Varying results likely also reflect the complexity of the interactions between OCs and endogenous hormones. Notably, estrogenic effects from lower dose OCs (~20 mcg EE) resemble those from native ovarian production of 17β-estradiol [26] more than do those of OCs containing higher (>30 mcg) EE doses [27, 28]. Either dose suppresses the mid-cycle estrogen peak that occurs with normal ovarian function. Estrogens have multiple actions on bone. Within cancellous bone, estrogen decreases bone turnover, which increases bone density. Estrogens also cause closure of the growth plates [29], which determines the length of the bones, and have complex effects on periosteal tissue [30]. OCs may affect all these aspects of bone development.
OCs’ non-estrogenic actions also may affect the skeleton. OCs cause 2- to 6-fold increases in sex hormone binding globulin (SHBG), decreasing free androgen levels. Hepatic production of insulin-like growth factor (IGF) also appears to be inhibited by OCs in young women [26] but not in women older than 35 years [31]. These non-estrogenic actions of OCs would be predicted to have negative, potentially age-related, effects on bone density. Both androgens and IGF are important for normal growth plate development [32] and periosteal expansion [30].
Thus, through their effects on gonadal hormones, OCs may exert different—and not necessarily concurrent—effects on the skeleton depending on initial bone turnover rate, growth plate status, IGF and androgen activity, and OC dose and length of use.
Evaluating the immediate clinical importance of the approximately 5% difference in spinal bone density we observed between OC users and nonusers in young adults is difficult. Young women have few fractures, and data from clinical trials are insufficient to determine fracture risk related to steroidal contraceptive use [33]. Two large prospective epidemiological studies of fracture found higher risk ratios for incident fractures in mostly premenopausal women using OCs than in nonusers [34, 35]. However, a large case-control study using automated data [36] reported no increased fracture risk for younger OC users. Current OC formulations are just beginning to have been in use long enough to study their impact on fractures in postmenopausal women. A 5% lower bone density after menopause is associated with approximately 50% more osteoporotic fractures [37]. Possible impacts of OC use on bone also must be considered in the context of risks accompanying an unintended pregnancy and use of other hormonal contraceptive options such as depot medroxyprogesterone acetate.
Strengths of the current study include its size, a sample structured to evaluate both adolescent and young adult age groups and two OC pill strengths, multivariable adjustment for numerous potentially related covariates, and selection of OC-exposed and comparison group participants from the same defined population. To our knowledge, no prior study of young women has examined the combination of OC duration and estrogen dose together with the benefit of a comparison group.
This study’s main limitation is that these cross-sectional associations between OC dose and duration and lower bone density may not be causal. Also, our data on duration of current OC use relied on self-report. However, comparing self-report and available automated pharmacy data showed complete agreement regarding current dose, and we feel recall of the current OC duration is also likely to be high, particularly with use of the Life Events calendar [20–22].
Osteoporosis is increasingly common and OC use is an exceptionally prevalent exposure. Thus, continued investigation of the optimal duration, dose, and routes of administration of hormonal contraceptives as well as bone changes following discontinuation is critical.
Acknowledgments
We acknowledge the invaluable contributions of: Patty Yarbro, project manager; Holly Roberts and Linda Wehnes, bone densitometrists; Jane Grafton, programmer; Kelli O’Hara, Shirley Meyer, and Mary Lyons, study recruiters; and our participants.
Grant support: This study was funded by grant 1R01-HD31165-11 (Scholes) from the National Institute for Child Health and Human Development (NICHD), National Institutes of Health (NIH). Dr. Beasley is a postdoctoral fellow supported by T-32 AG027677 from the National Institute on Aging (NIA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005;23:1–160. [PubMed] [Google Scholar]
- 2.Martins SL, Curtis KM, Glasier AF. Combined hormonal contraception and bone health: a systematic review. Contraception. 2006;73:445–469. doi: 10.1016/j.contraception.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Hartard M, Kleinmond C, Wiseman M, Weissenbacher ER, Felsenberg D, Erben RG. Detrimental effect of oral contraceptives on parameters of bone mass and geometry in a cohort of 248 young women. Bone. 2007;40:444–450. doi: 10.1016/j.bone.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Polatti F, Perotti F, Filippa N, Gallina D, Nappi RE. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception. 1995;51:221–224. doi: 10.1016/0010-7824(95)00036-a. [DOI] [PubMed] [Google Scholar]
- 5.Register TC, Jayo MJ, Jerome CP. Oral contraceptive treatment inhibits the normal acquisition of bone mineral in skeletally immature young adult female monkeys. Osteoporos Int. 1997;7:348–353. doi: 10.1007/BF01623776. [DOI] [PubMed] [Google Scholar]
- 6.Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women. JAMA. 1992;268:2403–2408. [PubMed] [Google Scholar]
- 7.Nappi C, Di Spiezio Sardo A, Greco E, Tommaselli GA, Giordano E, Guida M. Effects of an oral contraceptive containing drospirenone on bone turnover and bone mineral density. Obstet Gynecol. 2005;105:53–60. doi: 10.1097/01.AOG.0000148344.26475.fc. [DOI] [PubMed] [Google Scholar]
- 8.Reed SD, Scholes D, LaCroix AZ, Ichikawa L, Barlow WE, Ott SM. Longitudinal changes in bone density in relation to oral contraceptive use. Contraception. 2003;68:177–182. doi: 10.1016/s0010-7824(03)00147-1. [DOI] [PubMed] [Google Scholar]
- 9.Cromer BA, Bonny AE, Stager M, et al. Bone mineral density in adolescent females using injectable or oral contraceptives: a 24-month prospective study. Fertil Steril. 2008;90:2060–2067. doi: 10.1016/j.fertnstert.2007.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petitti DB, Piaggio G, Mehta S, Cravioto MC, Meirik O The WHO Study of Hormonal Contraception and Bone Health. Steroid hormone contraception and bone mineral density: a cross-sectional study in an international population. Obstet Gynecol. 2000;95:736–744. doi: 10.1016/s0029-7844(00)00782-1. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd T, Taylor DS, Lin HM, Matthews AE, Eggli DF, Legro RS. Oral contraceptive use by teenage women does not affect peak bone mass: a longitudinal study. Fertil Steril. 2000;74:734–738. doi: 10.1016/s0015-0282(00)00719-6. [DOI] [PubMed] [Google Scholar]
- 12.Prior JC, Kirkland SA, Joseph L, et al. Oral contraceptive use and bone mineral density in premenopausal women: cross-sectional, population-based data from the Canadian Multicentre Osteoporosis Study. Can Med Assoc J. 2001;165:1023–1029. [PMC free article] [PubMed] [Google Scholar]
- 13.Cromer BA, Stager M, Bonny A, et al. Depot medroxyprogesterone acetate, oral contraceptives and bone mineral density in a cohort of adolescent girls. J Adolesc Health. 2004;35:434–441. doi: 10.1016/j.jadohealth.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Almstedt Shoepe H, Snow CM. Oral contraceptive use in young women is associated with lower bone mineral density than that of controls. Osteoporos Int. 2005;16:1538–1544. doi: 10.1007/s00198-005-1868-6. [DOI] [PubMed] [Google Scholar]
- 15.Berenson AB, Rahman M, Breitkopf CR, Bi LX. Effects of depot medroxyprogesterone acetate and 20-microgram oral contraceptives on bone mineral density. Obstet Gynecol. 2008;112:788–799. doi: 10.1097/AOG.0b013e3181875b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pikkarainen E, Lehtonen-Veromaa M, Mottonen T, Kautiainen H, Viikari J. Estrogen-progestin contraceptive use during adolescence prevents bone mass acquisition: a 4-year follow-up study. Contraception. 2008;78:226–231. doi: 10.1016/j.contraception.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Beksinska ME, Kleinschmidt I, Smit JA, Farley TM. Bone mineral density in a cohort of adolescents during use of norethisterone enanthate, depot-medroxyprogesterone acetate or combined oral contraceptives and after discontinuation of norethisterone enanthate. Contraception. 2009;79:345–349. doi: 10.1016/j.contraception.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien SH, Kaizar EE, Gold MA, Kelleher KJ. Trends in prescribing patterns of hormonal contraceptives for adolescents. Contraception. 2008;77:264–269. doi: 10.1016/j.contraception.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 20.Marcus AC. Memory aids in longitudinal health surveys: results from a field experiment. Am J Public Health. 1982;72:567–573. doi: 10.2105/ajph.72.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coulter A, Vessey M, McPherson K, Crossley B. The ability of women to recall their oral contraceptive histories. Contraception. 1986;33:127–137. doi: 10.1016/0010-7824(86)90079-x. [DOI] [PubMed] [Google Scholar]
- 22.Cook LS, White JL, Stuart GC, Magliocco AM. The reliability of telephone interviews compared with in-person interviews using memory aids. Ann Epidemiol. 2003;13:495–501. doi: 10.1016/s1047-2797(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 23.Ott SM, Ichikawa LE, LaCroix AZ, Scholes D. Navel jewelry artifacts and intravertebral variation in spine bone densitometry in adolescents and young women. J Clin Densitom. 2009;12:84–88. doi: 10.1016/j.jocd.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons TJ, Prentice A, Smith EA, Cole TJ, Compston JE. Bone mineral mass consolidation in young British adults. J Bone Miner Res. 1996;11:264–274. doi: 10.1002/jbmr.5650110216. [DOI] [PubMed] [Google Scholar]
- 25.Henry YM, Fatayerji D, Eastell R. Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: relative contributions of bone size and volumetric bone mineral density. Osteoporos Int. 2004;15:263–273. doi: 10.1007/s00198-003-1542-9. [DOI] [PubMed] [Google Scholar]
- 26.Mah PM, Webster J, Jonsson P, Feldt-Rasmussen U, Koltowska-Haggstrom M, Ross RJ. Estrogen replacement in women of fertile years with hypopituitarism. J Clin Endocrinol Metab. 2005;90:5964–5969. doi: 10.1210/jc.2005-1207. [DOI] [PubMed] [Google Scholar]
- 27.Mishell DRJ, Thorneycroft IH, Nakamura RM, Nagata Y, Stone SC. Serum estradiol in women ingesting combination oral contraceptive steroids. Am J Obstet Gynecol. 1972;114:923–928. doi: 10.1016/0002-9378(72)90098-1. [DOI] [PubMed] [Google Scholar]
- 28.Spona J, Elstein M, Feichtinger W, et al. Shorter pill-free interval in combined oral contraceptives decreases follicular development. Contraception. 1996;54:71–77. doi: 10.1016/0010-7824(96)00137-0. [DOI] [PubMed] [Google Scholar]
- 29.Perry RJ, Farquharson C, Ahmed SF. The role of sex steroids in controlling pubertal growth. Clin Endocrinol (Oxf) 2008;68:4–15. doi: 10.1111/j.1365-2265.2007.02960.x. [DOI] [PubMed] [Google Scholar]
- 30.Vanderschueren D, Venken K, Ophoff J, Bouillon R, Boonen S. Clinical Review: Sex steroids and the periosteum--reconsidering the roles of androgens and estrogens in periosteal expansion. J Clin Endocrinol Metab. 2006;91:378–382. doi: 10.1210/jc.2005-1766. [DOI] [PubMed] [Google Scholar]
- 31.Walsh JS, Eastell R, Peel NF. Effects of Depot medroxyprogesterone acetate on bone density and bone metabolism before and after peak bone mass: a case-control study. J Clin Endocrinol Metab. 2008;93:1317–1323. doi: 10.1210/jc.2007-2201. [DOI] [PubMed] [Google Scholar]
- 32.van der Eerden BC, Karperien M, Wit JM. Systemic and local regulation of the growth plate. Endocr Rev. 2003;24:782–801. doi: 10.1210/er.2002-0033. [DOI] [PubMed] [Google Scholar]
- 33.Lopez LM, Grimes DA, Schulz KF, Curtis KM. Steroidal contraceptives: effect on bone fractures in women. Cochrane Database Syst Rev. 2009:CD006033. doi: 10.1002/14651858.CD006033.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Cooper C, Hannaford P, Croft P, Kay CR. Oral contraceptive pill use and fractures in women: a prospective study. Bone. 1993;14:41–45. doi: 10.1016/8756-3282(93)90254-8. [DOI] [PubMed] [Google Scholar]
- 35.Vessey M, Mant J, Painter R Findings in a large cohort study. Oral contraception and other factors in relation to hospital referral for fracture. Contraception. 1998;57:231–235. doi: 10.1016/s0010-7824(98)00026-2. [DOI] [PubMed] [Google Scholar]
- 36.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in very young women using combined oral contraceptives. Contraception. 2008;78:358–364. doi: 10.1016/j.contraception.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]