Abstract
Background
Tobacco addiction is a chronic brain disorder that is characterized by a negative affective state upon smoking cessation and relapse after periods of abstinence. Previous research has shown that blockade of CRF receptors with a non-specific CRF1/CRF2 receptor antagonist prevents the deficit in brain reward function associated with nicotine withdrawal and stress-induced reinstatement of extinguished nicotine seeking in rats. The aim of these studies was to investigate the role of CRF1 and CRF2 receptors in the deficit in brain reward function associated with precipitated nicotine withdrawal and stress-induced reinstatement of nicotine seeking.
Methods
The intracranial self-stimulation (ICSS) procedure was used to assess the negative affective state of nicotine withdrawal. Elevations in brain reward thresholds are indicative of a deficit in brain reward function. Stress-induced reinstatement of nicotine seeking was investigated in animals in which responding for intravenously infused nicotine was extinguished by substituting saline for nicotine.
Results
In the ICSS experiments, the nicotinic receptor antagonist mecamylamine elevated the brain reward thresholds of the nicotine dependent rats but not those of the control rats. The CRF1 receptor antagonist R278995/CRA0450, but not the CRF2 receptor antagonist astressin-2B, prevented the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. Furthermore, R278995/CRA0450, but not astressin-2B, prevented stress-induced reinstatement of extinguished nicotine seeking. Neither R278995/CRA0450 nor astressin-2B affected operant responding for chocolate-flavored food pellets.
Conclusions
These studies indicate that CRF1 receptors, but not CRF2 receptors, play an important role in the anhedonic-state associated with acute nicotine withdrawal and stress-induced reinstatement of nicotine seeking.
Keywords: Nicotine, mecamylamine, withdrawal, anhedonia, relapse, rats
INTRODUCTION
Tobacco addiction is a chronic disorder that is characterized by loss of control over smoking, withdrawal symptoms upon smoking cessation, and relapse after periods of abstinence (1). Abrupt cessation of smoking typically mediates negative affective symptoms such as depressed mood and anxiety. It has been hypothesized that the negative affective aspects of tobacco withdrawal provide powerful motivation for the continuation of smoking (2,3). After the acute withdrawal phase, exposure to stressors increases the likelihood of relapse to smoking (4,5). Pharmacotherapies that diminish the negative affective state of tobacco withdrawal and reduce the risk for stress-induced relapse may improve long-term smoking cessation rates.
Animal models have been developed to study the negative mood state associated with drug withdrawal and stress-induced relapse. Discontinuation of cocaine, amphetamine, alcohol, fentanyl, and nicotine administration elevates brain reward thresholds in a discrete-trial intracranial self-stimulation (ICSS) procedure (6-10). Elevations in brain reward thresholds are interpreted as a deficit in brain reward function as higher current intensities are required to maintain responding for rewarding electrical stimuli (11). After the acute withdrawal phase, stressors can increase the risk for relapse (4,5). Footshocks have been shown the induce the reinstatement of extinguished cocaine, heroin, nicotine, and alcohol-seeking in rats (12-15). Extensive evidence points toward a role for the neuropeptide corticotropin-releasing factor (CRF) in stress-induced reinstatement of drug seeking. Blockade of CRF receptors with nonspecific CRF1/CRF2 receptor antagonists or small-molecule CRF1 receptor antagonists prevents stress-induced reinstatement of alcohol, cocaine, and heroin seeking (16-20).
In previous studies we demonstrated that the nonspecific CRF1/CRF2 receptor antagonist D-Phe CRF(12-41) prevents the deficit in brain reward function associated with precipitated nicotine withdrawal and stress-induced reinstatement of nicotine seeking (21,22). These studies did not indicate whether acute nicotine withdrawal or stress-induced reinstatement of nicotine seeking was mediated via the activation of CRF1 or CRF2 receptors. Substantial evidence suggests that CRF1 receptors play a role in alcohol and nicotine withdrawal-induced anxiety-like behavior and alcohol and nicotine intake in dependent animals (23-25).Conflicting findings have been reported with regard to the role of CRF2 receptors in negative emotional states and drug withdrawal (26,27). Therefore, it is not known if blockade of CRF2 receptors contributes to the anti-stress effects of non-specific CRF1/CRF2 receptor antagonists such as D-Phe CRF(12-41). During the last decade, several small-molecule CRF1 receptor antagonists have been developed that can cross the blood brain barrier and display efficacy in clinical trials for anxiety and depression (28). Therefore, it is important to know if selective CRF1 receptor antagonists can diminish the negative mood state associated with smoking cessation and prevent stress-induced relapse. In the present studies the selective CRF1 receptor antagonist R278995/CRA0450 and the selective CRF2 receptor antagonist astressin-2B were used to investigate the role of CRF1 and CRF2 receptors in the negative affective state of nicotine withdrawal and stress-induced reinstatement of drug seeking (29,30). The negative affective state of nicotine withdrawal was investigated by using a discrete-trial current-threshold ICSS procedure (31). The role of specific CRF receptors in stress-induced reinstatement of nicotine seeking was investigated by using a previously established reinstatement procedure (14,22). In order to investigate whether R278995/CRA0450 or astressin-2B induced a non-specific impairment in motor function, the effects of these CRF receptor antagonists on food responding was investigated (32).
METHODS AND MATERIALS
Subjects
Male Wistar rats (Charles River, Raleigh, NC) weighing 250-300 g were used. The ICSS rats were group-housed and maintained on a 12-hour light-dark cycle (lights off at 6 PM). The intravenous self-administration (IVSA) rats were single-housed and maintained on a 12-hour reversed light-dark cycle (lights on at 6 PM). The ICSS rats received ad libitum food and water. The IVSA rats received ad libitum food and water except during the IVSA period when they were fed 20 g of food immediately after the IVSA sessions. All subjects were treated in accordance with the National Institutes of Health guidelines regarding the principles of animal care. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) and approved by the University of Florida Institutional Animal Care and Use Committee.
Drugs
Nicotine, the nicotinic acetylcholine receptor antagonist mecamylamine (33,34), and pentobarbital were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in sterile saline. R278995/CRA0450 (1-[8-(2,4-dichlorophenyl)-2-methylquinolin-4-yl]-1,2,3,6-tetrahydropyridine-4-carboxamide benzenesulfonate) was synthesized by Taisho Pharmaceutical Co. (Saitama, Japan). Astressin-2B (cyclo(31-34)[DPhe11,His12,Nle17,CαMeLeu13,39, Glu31,Lys34]Ac-Sau(8-40)) was synthesized at The Salk Institute for Biological Studies (La Jolla, CA). R278995/CRA0450 and astressin-2B were dissolved in distilled water and administered within one hour after being dissolved. The astressin-2B solution was kept on ice until being used. R278995/CRA0450 is a selective CRF1 receptor antagonist (IC50 of 53.2 nM for CRF1 receptor, Ki of >10,000 nM for CRF2 receptor) and astressin-2B is a selective CRF2 receptor antagonist (IC50 > 500 nM for CRF1 receptor, IC50 of 1.3 nM for CRF2 receptor) (29,30).
Surgical procedures
For experiments 1 and 2, the rats were prepared with an 11 mm electrode in the medial forebrain bundle and an 11 mm cannula above the lateral ventricle (21). The rats were anesthetized with an isoflurane/oxygen vapor mixture and placed in a stereotaxic frame with the incisor bar set 3.3 mm below the interaural line (flat skull). The cannulae were implanted above the lateral ventricle using the following flat skull coordinates: anterior posterior (AP) -0.9 mm, medial lateral (ML) ±1.4 mm, dorsal ventral (DV) -3.0 mm from skull (35). The electrodes were implanted in the medial forebrain bundle by using the following coordinates: AP -0.5 mm, ML ±1.7 mm, DV -8.3 mm from dura (incisor bar 5 mm above interaural line). For experiments 3 and 4, the rats were prepared with a cannula above the lateral ventricle and a chronic catheter in the right jugular vein as described previously (22).
Intracranial self-stimulation procedure
Rats were trained on a modified discrete-trial ICSS procedure (36), as described previously (31). The operant conditioning chambers were housed in sound-attenuating chambers (Med Associates, Georgia, VT). The operant conditioning chambers had a 5 cm wide metal response wheel centered on a sidewall and a photobeam detector recorded every 90 degrees of rotation. Brain stimulation was delivered by constant current stimulators (Model 1200C, Stimtek, Acton, MA). The rats were trained on a discrete-trial current-threshold procedure. Each test session provided a brain reward threshold and a response latency. The brain reward threshold was defined as the midpoint between stimulation intensities that supported responding and current intensities that failed to support responding. The response latency was defined as the time interval between the beginning of the non-contingent stimulus and a positive response.
Nicotine self-administration, extinction, and stress-induced reinstatement
Drug self-administration sessions were conducted as described previously (22). Briefly, rats were trained to respond for food pellets under a fixed-ratio 5, time-out 20 second (FR5 TO20-s) schedule of reinforcement. After completion of food training the rats were allowed to self-administer nicotine at the 0.03 mg/kg/infusion (free base) dose. Responding on the active lever resulted in the delivery of a nicotine infusion (0.1 ml infused over a 5.6-second time-period). Responding for nicotine was extinguished by replacing nicotine with saline. Nicotine seeking was reinstated by the administration of footshocks (8 shocks in a 10-minute time-period, 0.8 mA, 1 second shocks, mean off period 37 seconds) immediately prior to the self-administration session.
Intracranial drug administration
For intracerebroventricular injections, stainless steel injectors projecting 2.5 mm beyond the guide cannulae were used. All injections were made by gravity induced by raising the 10 μl Hamilton syringe. Five μl of solution was administered over a 30-60 second period, and the injector was left in place for another 30 seconds to allow diffusion from the injector tip.
Statistical analyses
ICSS parameters were analyzed by two-way repeated-measures analyses of variance (ANOVA) with the dose of the CRF receptor antagonist as the within-subjects factor and pump content (saline or nicotine) as the between-subjects factor. The self-administration of nicotine over time was analyzed by one-way repeated-measures ANOVA with time as the within-subjects factor. The effect of extinction training on lever pressing was analyzed by one-way repeated-measures ANOVA with time as the within subjects factor. The effect of footshocks on lever pressing was analyzed using a paired sample t-test. The effect of the CRF receptor antagonists on lever pressing after the administration of footshocks was analyzed by one-way repeated-measures ANOVA with the dose of the antagonist as the within subjects factor. The effect of the CRF receptor antagonists on food responding was analyzed by one-way repeated-measures ANOVA with the dose of the CRF antagonist as the within subjects factor. Statistically significant results in the ANOVA's were followed by the Newman-Keuls post-hoc test.
Experimental design
Experiment 1: Effect of R278995/CRA0450 on precipitated nicotine withdrawal
The rats were trained on the ICSS procedure and when stable baseline brain reward thresholds were achieved (defined as less than 10% variation within a 5 day period), the rats were prepared with 28-day osmotic minipumps containing either saline (n = 12) or nicotine (n = 14, 9 mg/kg/day of nicotine salt, Alzet model 2ML4). ICSS parameters were assessed daily between 9:00 AM and 12:00 noon. Mecamylamine (3 mg/kg, sc) injections started at least 6 days after the implantation of the minipumps to allow the development of nicotine dependence. The CRF1 receptor antagonist R278995/CRA0450 (1 – 20 μg, icv) was administered 15 minutes prior treatment with mecamylamine. The rats were placed in the ICSS test chambers 5 minutes after mecamylamine administration. To allow the reestablishment of nicotine dependence the minimum time interval between the mecamylamine injections was at least 72 hours.
Experiment 2. Effect of astressin-2B on precipitated nicotine withdrawal
This experiment was the same as experiment 1, with the exception that the CRF2 receptor antagonist astressin-2B (1 – 20 μg, icv) was administered to rats treated with nicotine (n=8) or saline (n=8).
Experiment 3. Effect of R278995/CRA0450 on stress-induced reinstatement of nicotine seeking
Drug naïve rats (n = 12) were trained to respond for food pellets and then allowed to self-administer nicotine (FR5 TO20-s, 0.03 mg/kg of nicotine per infusion; free base) for 14 consecutive days. Nicotine-seeking behavior was extinguished by substituting saline for nicotine. Extinction training was considered completed when the average number of infusion was less than 2. Reinstatement sessions started one day after extinction training was completed. R278995/CRA0450 (5, 20 μg, icv) was administered according to a Latin-square design 15 minutes before the footshock sessions. There were at least 2 off-days between test days and on these days the rats were left undisturbed. The design of this study was based on previous studies that showed that repeated footshock sessions or repeated exposure to cues associated with nicotine delivery reliably reinstates extinguished nicotine-seeking (14,22,37). At the end of the experiment the rats were euthanized with an overdose of pentobarbital (150 mg/kg, intraperitoneal) and cannulae placement were verified by administering 5 μl of an 0.5% aqueous methyl blue solution at the injection site.
Experiment 4. Effect of astressin-2B on stress-induced reinstatement of nicotine seeking
The design of this experiment was the same as that of experiment 3, with the exception that astressin-2B (0, 5, 20 μg, icv) was administered to the animals (n = 16) 15 minutes prior the footshock session.
Experiment 5: Effects of R278995/CRA0450 and astressin-2B on responding for food pellets
The rats from experiment 3 were used to investigate the effect of R278995/CRA0450 on food responding (n=11 [1 rat died before the onset of experiment 5]) and the rats from experiment 4 were used to investigate the effect of astressin-2B on food responding (n = 16). First, the rats were allowed to respond for chocolate-flavored food pellets under an FR5 TO20-s schedule of reinforcement. After approximately one week, the test sessions with R278995/CRA0450 or astressin-2B were inititiated. R278995/CRA0450 (0, 5, 20 μg, icv) or astressin-2B (0, 5, 20 μg, icv) was administered according to a Latin-square design 15 minutes before the rats were placed in the operant conditioning chambers. There were at least 2 off-days between the drug days. The animals were allowed to respond for chocolate-flavored food pellets on off-days. The rats were allowed to respond for food 7 days per week and all the sessions were 20 minutes. At the end of the experiment the rats were euthanized using an overdose of pentobarbital and cannulae placement were verified.
RESULTS
Experiment 1. Effect of R278995/CRA0450 on precipitated nicotine withdrawal
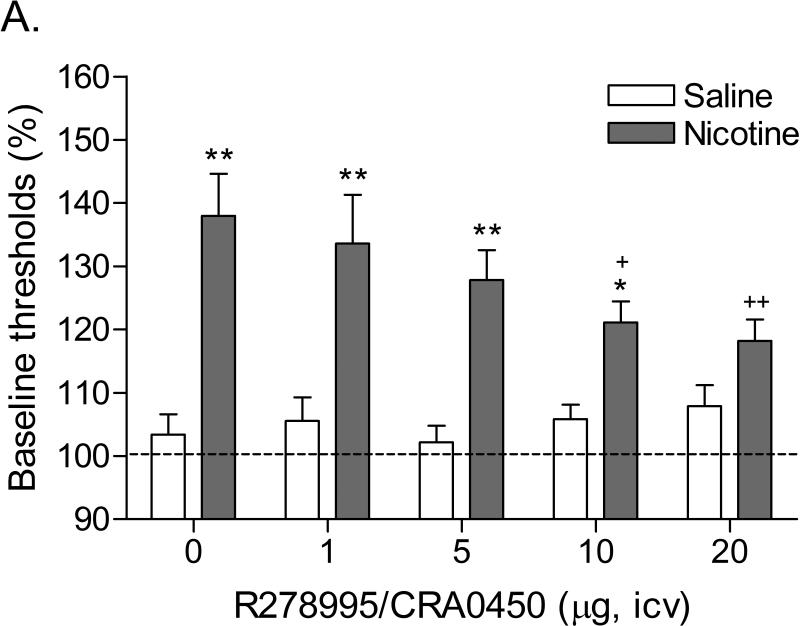
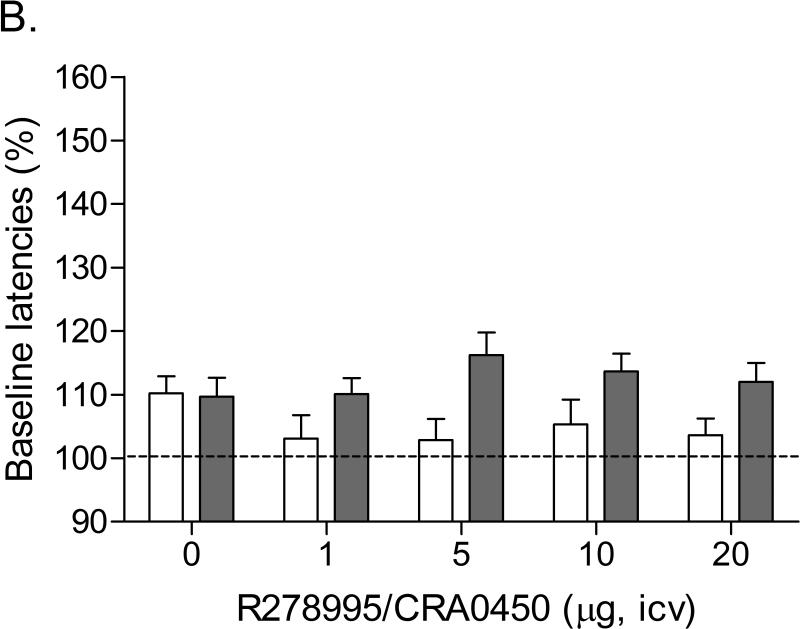
Mean (±S.E.M.) absolute brain reward thresholds before minipump-implantation for saline and nicotine-treated rats were 101.01 ± 6.53 and 101.61 ± 8.72 μA, respectively. Mean (±S.E.M.) absolute response latencies for saline and nicotine-treated rats were 3.18 ± 0.10 and 3.30 ± 0.11 seconds, respectively. Figure 1 indicates that mecamylamine elevated the brain reward thresholds of the nicotine-treated rats and did not affect the brain reward thresholds of the saline-treated rats (Figure 1A; Treatment: F1,24=28.491, P<0.0001). Pretreatment with R278995/CRA0450 prevented the mecamylamine-induced elevations in brain reward thresholds in the nicotine-treated rats and did not affect the brain reward thresholds of the saline-treated rats (Dose × Treatment interaction: F4,96=3.162, P<0.017). Newman-Keuls post-hoc comparisons indicated that 20 μg of R278995/CRA0450 attenuated the elevations in brain reward thresholds associated with nicotine withdrawal. Mecamylamine increased the response latencies of the nicotine-treated rats compared to those of the saline-treated rats (Figure 1B; Treatment: F1,24=16.381, P<0.0005). R278995/CRA0450 did not affect the response latencies of the nicotine or saline-treated rats (Figure 1B).
Figure 1.
Effect of the CRF1 receptor antagonist R278995/CRA0450 (saline, n = 12; nicotine, n = 14) on the elevations in brain reward thresholds associated with mecamylamine-precipitated nicotine withdrawal (A). Effect of R278995/CRA0450 on the response latencies of rats chronically treated with saline (n = 12) or nicotine (n = 14) and acutely treated with mecamylamine (B). Brain reward thresholds and response latencies are expressed as a percentage of the pre-test day values. Asterisks (* P<0.05, ** P<0.01) indicate elevations in brain reward thresholds compared to those of the corresponding saline-treated control group. Plus signs (+ P<0.05, ++ P<0.01) indicate lower brain reward thresholds compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and vehicle (0 μg of R278995/CRA0450). Data are expressed as means ± SEM.
Experiment 2. Effect of astressin-2B on precipitated nicotine withdrawal
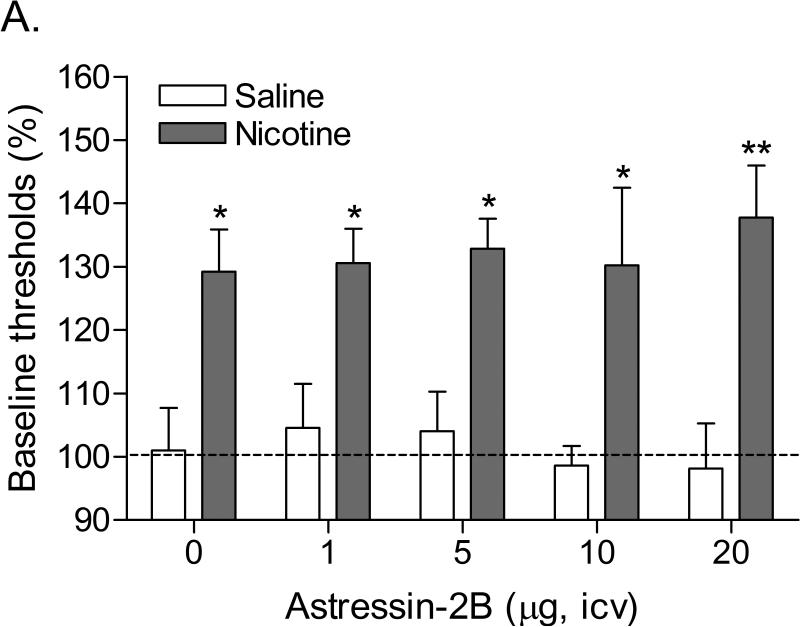
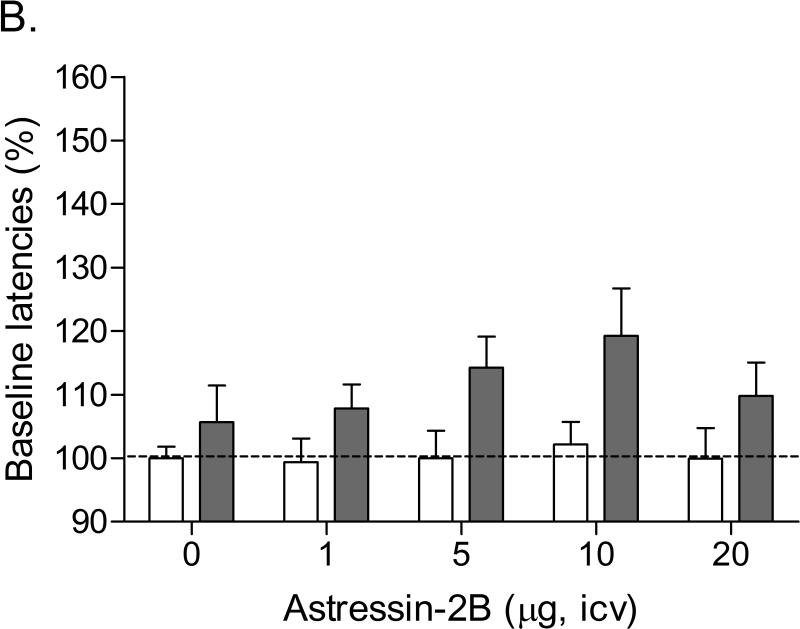
Mean (±S.E.M.) absolute brain reward thresholds before pump-implantation for the saline and nicotine-treated rats were 123.07 ± 3.24 and 122.80 ± 3.13 μA, respectively. Mean (±S.E.M.) absolute response latencies for saline and nicotine-treated rats were 3.24 ± 0.10 and 3.13 ± 0.10 seconds, respectively. Mecamylamine elevated the brain reward thresholds of the nicotine-treated rats and did not affect the brain reward thresholds of the saline-treated rats (Figure 2A; Treatment: F1,14=29.931, P<0.0001). Pretreatment with astressin-2B did not affect the brain reward thresholds of the nicotine-treated rats or the saline-treated rats (Figure 2A). Mecamylamine increased the response latencies of the nicotine-treated rats compared to those of the saline-treated rats (Figure 2B; Treatment: F1,14=15.824, P<0.001). Astressin-2B did not affect the response latencies of the nicotine-treated rats or the saline-treated rats (Figure 2B).
Figure 2.
Effect of the CRF2 receptor antagonist astressin-2B (saline, n = 8; nicotine, n = 8) on the elevations in brain reward thresholds associated with mecamylamine-precipitated nicotine withdrawal (A). Effect of astressin-2B on the response latencies of rats chronically treated with saline (n = 8) or nicotine (n = 8) and acutely treated with mecamylamine (B). Brain reward thresholds and response latencies are expressed as a percentage of the pre-test day values. Asterisks (* P<0.05, ** P<0.01) indicate elevations in brain reward thresholds compared to those of the corresponding saline-treated control group. Data are expressed as means ± SEM.
Experiment 3. Effect of R278995/CRA0450 on stress-induced reinstatement of nicotine seeking
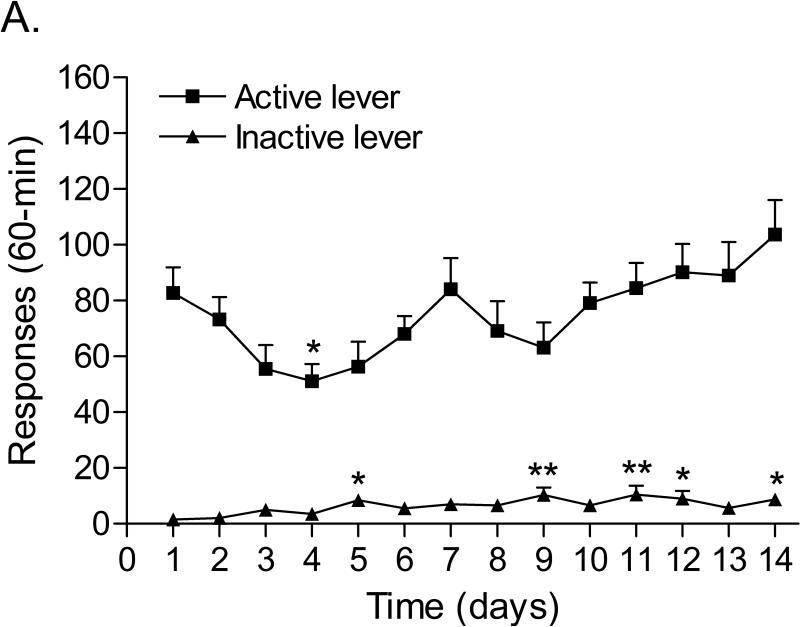
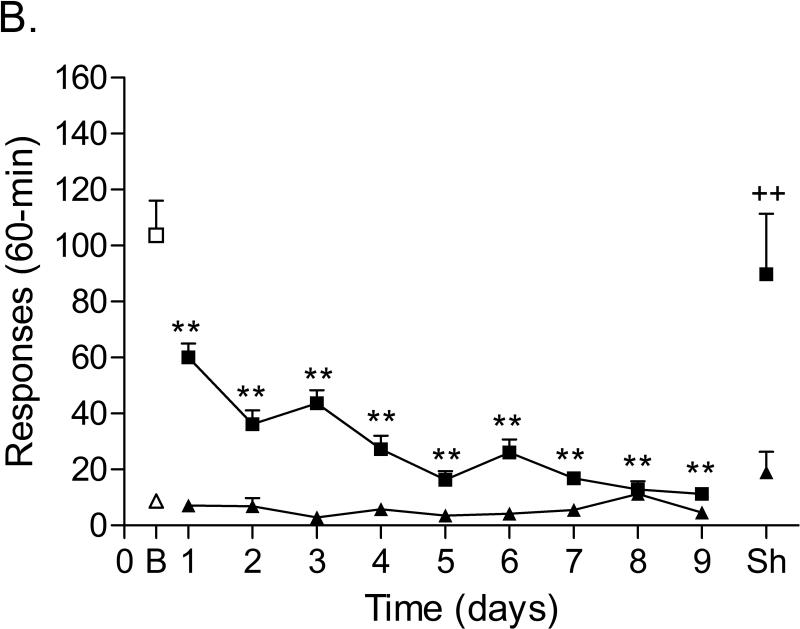
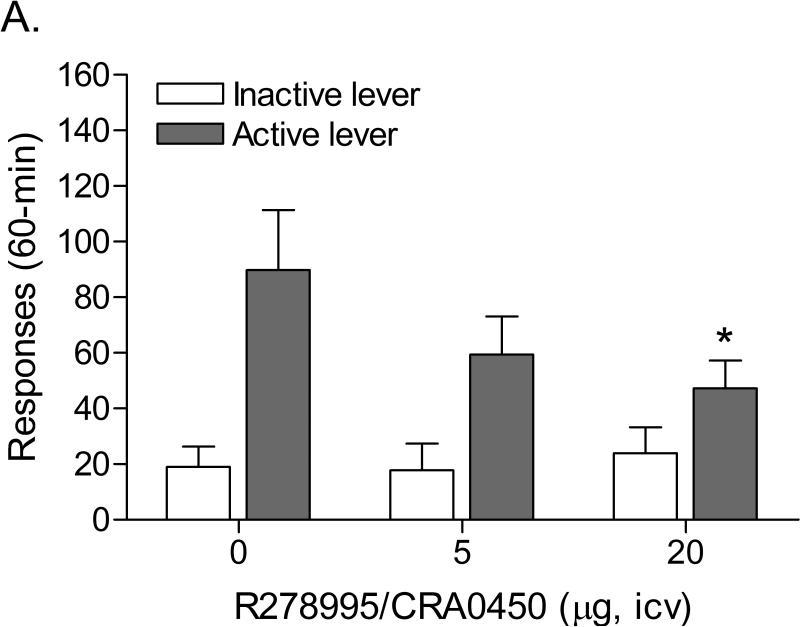
The mean (±S.E.M.) number of infusions of 0.03 mg/kg of nicotine, responses on the active lever, and responses on the inactive lever during the last day of nicotine self-administration were 20.50 ± 2.48, 103.67 ± 12.37, 8.75 ± 1.86, respectively (Figure 3A). After the onset of nicotine self-administration, responding on the active lever initially decreased and then increased (Time: F13,143=5.222, p<0.0001). Responding on the inactive lever gradually increased (Time: F13,143=3.347, p<0.0002). Substituting saline for nicotine caused a rapid decline in the number of responses on the active lever (Figure 3B; F8,88=28.184, P<0.0001) and did not affect the number of responses on the inactive lever. The extinction criterion (less than 2 infusions) was met 9 days after the onset of extinction training. The administration of footshocks increased the number of responses on the active lever (Figure 3B; last day of extinction vs. footshocks vehicle condition; [t(11)=3.59, P<0.004]) and did not affect the number of responses on the inactive lever. R278995/CRA0450 decreased stress-induced responding on the active lever (Figure 4A; F2,22=3.908, P<0.035) and did not affect responding on the inactive lever.
Figure 3.
Responding on the active and inactive lever during nicotine self-administration (A, n = 12), extinction training, and relapse (B, n = 12). In figure 3A, asterisks (* P<0.05, ** P<0.01) indicate a decrease in responding on the active lever or an increase in responding on the inactive lever compared to the first day of nicotine self-administration. In figure 3B, asterisks (** P<0.01) indicate a decrease in responding on the active lever compared to baseline responding (last day of nicotine self-administration). Plus signs (++ P<0.01) indicate a footshock-induced increase in responding on the active lever compared to day 9 of extinction training. Abbreviations: B, baseline; Sh, footshocks. Data are expressed as means ± SEM.
Figure 4.
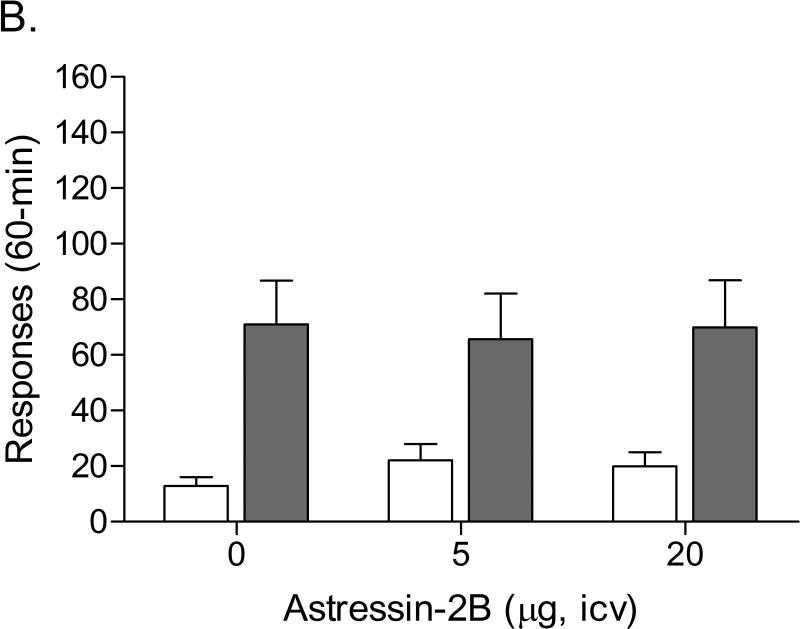
Effects of the CRF1 receptor antagonist R278995/CRA0450 (A, n = 12) and the CRF2 receptor antagonist astressin-2B (B, n = 16) on footshock-induced reinstatement of nicotine-seeking behavior. Asterisks (* P<0.05) indicate a decrease in responding on the active lever compared to rats that were pretreated with vehicle. Data are expressed as means ± SEM.
Experiment 4. Effect of astressin-2B on stress-induced reinstatement of nicotine seeking
The mean (±S.E.M.) number of infusions of 0.03 mg/kg of nicotine, responses on the active lever, and responses on the inactive lever during the last day of nicotine self-administration were 14.94 ± 1.72, 75.06 ± 8.49, 12.56 ± 2.80, respectively. The role of the CRF2 receptor in stress-induced reinstatement of nicotine seeking was investigated in two separate groups of 8 rats. Substituting saline for nicotine caused a rapid decline in the number of responses on the active lever in the first group (F13,91=5.152, P<0.0001) and the second group (F6,42=3.553, P<0.006). The extinction criterion (less than 2 infusions) was met after 14 days in the first group and after 7 days in the second group. During the extinction period the number of responses on the inactive lever decreased in the first group (F13,91=3.836, P<0.0001) and the second group (F6,42=3.868, P<0.004). For further data analyses the first and second group were combined. The administration of footshocks increased the number of responses on the active lever (last day of extinction vs. footshocks vehicle condition)[t(15)=7.70, P<0.0001] and did not affect the number of responses on the inactive lever. Astressin-2B did not affect footshock-induced responding on the active lever or the inactive lever (Figure 4B). This suggests that blockade of the CRF2 receptor does not affect stress-induced reinstatement of nicotine seeking.
Experiment 5: Effects of R278995/CRA0450 and astressin-2B on responding for food pellets
Table 1 shows the effects of R278995/CRA0450 and astressin-2B on responding for food pellets under an FR5 TO20-s schedule of reinforcement. CRA0450/R278995 or astressin-2B did not affect responding on the active or the inactive lever.
Table 1.
Effects of R278995/CRA0450 and astressin-2B on food responding.
| Dose (μg, icv) | Active lever |
Inactive lever |
|---|---|---|
| R278995/CRA0450 | ||
| 0 | 249.3 ± 13.9 | 2.7 ± 1.7 |
| 5 | 260.5 ± 17.7 | 0.5 ± 0.2 |
| 20 | 256.0 ± 30.1 | 1.9 ± 0.9 |
| Astressin-2B | ||
| 0 | 244.2 ± 7.3 | 0.8 ± 0.4 |
| 5 | 241.6 ± 10.8 | 1.4 ± 0.8 |
| 20 | 246.1 ± 4.5 | 1.7 ± 0.8 |
Data are expressed as means ± SEM.
DISCUSSION
The CRF1 receptor antagonist R278995/CRA0450 prevented the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. In contrast, the CRF2 receptor antagonist astressin-2B did not affect the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. Furthermore, the CRF1 receptor antagonist R278995/CRA0450, but not the CRF2 receptor antagonist astressin-2B, prevented stress-induced reinstatement of extinguished nicotine seeking. Neither R278995/CRA0450 nor astressin-2B affected operant responding for food pellets. This indicates that neither compound induced motor impairments or sedative effects. The present findings extend and corroborate previous findings by demonstrating that the nicotine withdrawal induced deficit in brain reward function and stress-induced reinstatement of nicotine seeking are at least partly mediated by the activation of central CRF1 receptors.
Extensive evidence suggests that a hyperactivity of brain CRF systems plays a role in negative emotional states (38). Substantial evidence also points toward a role for brain stress systems in drug addictions. Alcohol, nicotine, and cannabinoid withdrawal increases extracellular CRF levels in the central nucleus of the amygdala (24,39,40). The withdrawal-induced increase in CRF levels has been suggested to mediate increased anxiety-like behavior and drug intake in dependent animals (41,42). The non-specific CRF1/CRF2 receptor antagonist α-helical CRF(9-41) has been shown to decrease alcohol withdrawal-induced anxiety-like behavior in rats (43). In addition, specific CRF1 receptor antagonists decrease alcohol intake in alcohol dependent animals (25). In a previous study we reported that the nonspecific CRF1/CRF2 receptor antagonist D-Phe CRF(12-41) prevents the elevations in brain reward thresholds associated with nicotine withdrawal (21). The results presented here indicate that CRF mediates the nicotine withdrawal-induced deficit in brain reward function at least partly by activating central CRF1 receptors. These findings suggest that antagonism of CRF1 receptors could be an efficacious treatment for the anhedonic-state associated with smoking cessation. Although a great number of studies have investigated the role of CRF2 receptors in anxiety-like behavior (42,44-46), very few studies have reported on the role of CRF2 receptors in drug withdrawal. Evidence indicates that the activation of CRF2 receptors diminishes alcohol withdrawal-induced anxiety-like behavior (26). In contrast, somatic morphine withdrawal signs are attenuated in CRF2 receptor deficient mice, thus suggesting that CRF2 receptor activation contributes to withdrawal symptomatology (47). In a previous study we reported that blockade of CRF1/CRF2 receptors prevents the negative affective state associated with nicotine withdrawal and in the present study we demonstrated that a CRF1 receptor antagonist, but not a CRF2 receptor antagonist, prevents the negative affective state associated with nicotine withdrawal (21). Therefore, these studies suggest that CRF2 receptors do not play a role in the deficit in brain reward function associated with nicotine withdrawal. The icv administration of CRF elevates brain reward thresholds and induces conditioned place aversion and these effects can be prevented by pretreatment with nonspecific CRF1/CRF2 receptor antagonists (48,49). Although there is strong evidence for a role of CRF1 receptors in negative affective states (41), a recent study reported that blockade of CRF2 receptors, but not CRF1 receptors, prevents icv CRF-induced conditioned place aversion (50). This would suggest that drug withdrawal induced negative affective states are mediated via the activation of CRF1 receptors and exogenous CRF may mediate negative affective states via the activation of CRF2 receptors. A possible explanation for this discrepancy could be that exogenous and endogenous CRF may stimulate different CRF receptor populations. Exogenous CRF may induce activation of CRF receptors that are located in close proximity to the lateral ventricles (e.g., CRF2 receptors in lateral septum). In contrast, drug withdrawal may lead to the activation of CRF1 receptors in very specific brain sites such as the central nucleus of the amygdala (24,39,40).
Stressors play an important role in relapse to drug abuse in humans. Footshocks have been shown to induce the reinstatement of extinguished drug seeking behavior in rats (14,20,51). Footshocks activate brain CRF systems as indicated by an increased release of CRF in the ventral tegmental area and an increased expression of c-Fos protein in CRF-immunoreactive neurons (52,53). In a previous study, we reported that the CRF1/CRF2 receptor antagonist D-Phe CRF(12-41) prevents stress-induced reinstatement of nicotine seeking (22). This follow-up study suggests that footshocks mediate stress-induced reinstatement of nicotine seeking via the activation of CRF1 and not CRF2 receptors. The outcome of this study suggests that small-molecule CRF1 receptors antagonists may decrease the risk for stress-induced relapse to smoking. The outcome of this study is in line with previous studies that investigated the role of CRF1 receptors in stress-induced reinstatement of drug seeking. Blockade of CRF1 receptors has been shown to attenuate stress-induced reinstatement of heroin, cocaine, and alcohol seeking (18,19). Furthermore, blockade of CRF1 receptors attenuates stress-induced reinstatement of morphine and cocaine-induced conditioned place preference (54,55). The icv administration of a CRF2 receptor antagonist does not attenuate stress-induced reinstatement of cocaine-induced conditioned place preference (55). A recent study by Wise and colleagues suggests that blockade of CRF2 receptors, but not CRF1 receptors, in the ventral tegmental area (VTA) prevents footshock-induced reinstatement of cocaine seeking (56). The latter study suggests that the administration of specific CRF receptor antagonists into brain sites may have a different effect on stress-induced reinstatement of drug seeking then the icv administration of CRF receptor antagonists. Follow up studies are needed to investigate if CRF2 receptors in the VTA also play a critical role in stress-induced reinstatement of nicotine seeking. Drugs and cues associated with the self-administration of drugs of abuse can induce the reinstatement of drug seeking (57). Evidence suggests that CRF1 receptor activation may play a role in drug and cue-induced reinstatement of drug seeking. The non-specific CRF1/CRF2 receptor antagonist D-Phe CRF(12-41) attenuates drug-induced reinstatement of cocaine seeking (58). Furthermore, the specific CRF1 receptor antagonist CP-154,526 blocks cue and drug-induced reinstatement of cocaine and methamphetamine seeking (59,60). Additional studies are warranted to investigate if CRF1 receptors also play a role in cue and drug-induced reinstatement of nicotine seeking.
The present studies demonstrated that R278995/CRA0450 prevents stress-induced reinstatement of extinguished nicotine seeking. It is unlikely that R278995/CRA0450 prevented stress-induced nicotine seeking by inhibiting motor output. The dose of R278995/CRA0450 (20 μg, icv) that prevented stress-induced reinstatement of nicotine seeking did not affect the response latencies in the ICSS procedure and did not decrease operant responding for food pellets. In the present studies we did not investigate the effect of R278995/CRA0450 on baseline operant responding in rats in which intravenous nicotine self-administration was extinguished. However, based on the aforementioned findings it is extremely unlikely that R278995/CRA0450 would have affected baseline responding. Furthermore, a previous study reported that doses of the CRF1 receptor antagonist CP-154,526 that attenuated stress-induced reinstatement of extinguished heroin and cocaine seeking did not affect baseline responding in animals in which drug seeking was extinguished (19).
Taken together, these findings indicate that blockade of CRF1 receptors prevents the deficit in brain reward function associated with nicotine withdrawal and stress-induced reinstatement of extinguished nicotine seeking. These studies also suggest that activation of CRF2 receptors does not play a role in acute nicotine withdrawal and stress-induced reinstatement of nicotine seeking. These studies have significant clinical implications for the treatment of tobacco addiction. The present findings suggest that small-molecule CRF1 receptor antagonists may diminish the anhedonic-state associated with smoking cessation, prevent stress-induced reinstatement of tobacco smoking, and thereby could improve smoking cessation rates.
Acknowledgements
This work was funded by National Institute on Drug Abuse grants (DA023575 and DA020504) to Adrie Bruijnzeel. The authors would like to thank Dr. Shigeyuki Chaki (Taisho Pharmaceutical Co., Saitama, Japan) and Dr. Thomas Steckler (Johnson & Johnson Pharmaceuticals Research & Development, Beerse, Belgium) for generously providing R278995/CRA0450 and Dr. Jean Rivier (The Clayton Foundation Laboratories for Peptide Biology, The Salk Institute for Biological Studies, San Diego, CA) for providing astressin-2B.
Footnotes
Financial Disclosures
The author(s) declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Press; Washington, DC: 2000. text revision ed. [Google Scholar]
- 2.Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- 4.Swan GE, Denk CE, Parker SD, Carmelli D, Furze CT, Rosenman RH. Risk factors for late relapse in male and female ex-smokers. Addict Behav. 1988;13:253–266. doi: 10.1016/0306-4603(88)90052-4. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S, Lichtenstein E. Perceived stress, quitting smoking, and smoking relapse. Health Psychol. 1990;9:466–478. doi: 10.1037//0278-6133.9.4.466. [DOI] [PubMed] [Google Scholar]
- 6.Bruijnzeel AW, Lewis B, Bajpai LK, Morey TE, Dennis DM, Gold M. Severe deficit in brain reward function associated with fentanyl withdrawal in rats. Biol Psychiatry. 2006;59:477–480. doi: 10.1016/j.biopsych.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 8.Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- 9.Wise RA, Munn E. Withdrawal from chronic amphetamine elevates baseline intracranial self-stimulation thresholds. Psychopharmacology (Berl) 1995;117:130–136. doi: 10.1007/BF02245178. [DOI] [PubMed] [Google Scholar]
- 10.Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barr AM, Markou A. Psychostimulant withdrawal as an inducing condition in animal models of depression. Neurosci Biobehav Rev. 2005;29:675–706. doi: 10.1016/j.neubiorev.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- 13.Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- 14.Buczek Y, Le AD, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- 15.Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- 16.Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- 17.Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl- imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- 22.Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12-41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;58:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, et al. CRF CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-Releasing Factor 1 Antagonists Selectively Reduce Ethanol Self-Administration in Ethanol-Dependent Rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP. Antagonism of CRF(2) receptors produces anxiolytic behavior in animal models of anxiety. Brain Res. 2001;902:135–142. doi: 10.1016/s0006-8993(01)02405-2. [DOI] [PubMed] [Google Scholar]
- 28.Holsboer F, Ising M. Central CRH system in depression and anxiety--evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 29.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, et al. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- 30.Chaki S, Nakazato A, Kennis L, Nakamura M, Mackie C, Sugiura M, et al. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur J Pharmacol. 2004;485:145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 31.Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- 32.Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther. 1994;270:209–218. [PubMed] [Google Scholar]
- 33.Martin BR, Onaivi ES, Martin TJ. What is the nature of mecamylamine's antagonism of the central effects of nicotine? Biochem Pharmacol. 1989;38:3391–3397. doi: 10.1016/0006-2952(89)90106-8. [DOI] [PubMed] [Google Scholar]
- 34.Varanda WA, Aracava Y, Sherby SM, VanMeter WG, Eldefrawi ME, Albuquerque EX. The acetylcholine receptor of the neuromuscular junction recognizes mecamylamine as a noncompetitive antagonist. Mol Pharmacol. 1985;28:128–137. [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. Academic Press; San Diego: 1998. [Google Scholar]
- 36.Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- 37.Paterson NE, Froestl W, Markou A. Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine-seeking in rats. Neuropsychopharmacology. 2005;30:119–128. doi: 10.1038/sj.npp.1300524. [DOI] [PubMed] [Google Scholar]
- 38.Stahl SM, Wise DD. The potential role of a corticotropin-releasing factor receptor-1 antagonist in psychiatric disorders. CNS Spectr. 2008;13:467–483. doi: 10.1017/s1092852900016709. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- 40.Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev. 2005;49:505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- 44.Henry B, Vale W, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003;980:206–212. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- 47.Papaleo F, Ghozland S, Ingallinesi M, Roberts AJ, Koob GF, Contarino A. Disruption of the CRF(2) Receptor Pathway Decreases the Somatic Expression of Opiate Withdrawal. Neuropsychopharmacology. 2008;33:2878–2887. doi: 10.1038/npp.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macey DJ, Koob GF, Markou A. CRF and urocortin decreased brain stimulation reward in the rat: reversal by a CRF receptor antagonist. Brain Res. 2000;866:82–91. doi: 10.1016/s0006-8993(00)02229-0. [DOI] [PubMed] [Google Scholar]
- 49.Cador M, Ahmed SH, Koob GF, Le MM, Stinus L. Corticotropin-releasing factor induces a place aversion independent of its neuroendocrine role. Brain Res. 1992;597:304–309. doi: 10.1016/0006-8993(92)91487-y. [DOI] [PubMed] [Google Scholar]
- 50.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- 52.Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi IY, Lee S, Rivier C. Novel role of adrenergic neurons in the brain stem in mediating the hypothalamic-pituitary axis hyperactivity caused by prenatal alcohol exposure. Neuroscience. 2008;155:888–901. doi: 10.1016/j.neuroscience.2008.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu L, Ceng X, Huang M. Corticotropin-releasing factor receptor type I mediates stress-induced relapse to opiate dependence in rats. Neuroreport. 2000;11:2373–2378. doi: 10.1097/00001756-200008030-00008. [DOI] [PubMed] [Google Scholar]
- 55.Lu L, Liu D, Ceng X. Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats. Eur J Pharmacol. 2001;415:203–208. doi: 10.1016/s0014-2999(01)00840-8. [DOI] [PubMed] [Google Scholar]
- 56.Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- 57.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 58.Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moffett MC, Goeders NE. CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue-and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacology (Berl) 2007;190:171–180. doi: 10.1007/s00213-006-0625-7. [DOI] [PubMed] [Google Scholar]
- 60.Goeders NE, Clampitt DM. Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2002;161:222–232. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]