Abstract
The muscle protein fractional synthesis rate (FSR) is determined by monitoring the incorporation of an amino acid tracer into muscle protein during a constant-rate intravenous tracer infusion. Commonly two sequential muscle biopsies are obtained some time after starting the tracer infusion. However, other protocols, including those with an initial biopsy before starting the tracer infusion to measure the background enrichment and those with only a single biopsy after several hours of tracer infusion have been used. To assess the validity of these approaches, we compared the muscle protein FSR obtained by calculating the difference in [ring-2H5]phenylalanine and [5,5,5-2H3]leucine incorporation into muscle protein at ∼3.5 h after starting the tracer infusion and 1) at 60 min; 2) before starting the tracer infusion (background enrichment); 3) a population average muscle protein background enrichment; and 4) by measuring the tracer incorporation into muscle protein at ∼3.5 h assuming essentially no background enrichment. Irrespective of the tracer used, the muscle protein FSR calculated from the difference in the muscle protein labeling several hours after starting the tracer infusion and either the labeling at 60 min or the background enrichment were not different (e.g., 0.049 ± 0.007%/h vs. 0.049 ± 0.007%/h, respectively, with [2H5]phenylalanine; P = 0.99). However, omitting the initial biopsy and assuming no background enrichment yielded average FSR values that were ∼15% (with [2H5]phenylalanine) to 80% (with [2H3]leucine) greater (P ≤ 0.059); using a population average background enrichment reduced the difference to ∼3% (P = 0.76) and 22% (P = 0.52) with [2H5]phenylalanine and [2H3]leucine, respectively. We conclude that during basal, postabsorptive conditions, valid muscle protein FSR values can be obtained irrespective of the timing of the initial biopsy so long as the protein labeling in two sequential biopsies is measured whereas the single biopsy approach should be avoided.
Keywords: amino acid, muscle protein turnover
protein synthesis rates in human muscles are commonly determined by measuring the difference in incorporation of a stable isotope-labeled amino acid tracer (usually phenylalanine or leucine) into muscle protein between two sequential biopsies during a primed, continuous intravenous infusion of the tracer (2, 13, 15, 25, 27, 35, 37, 44, 45, 48). The muscle protein fractional synthesis rate (FSR) is then calculated by dividing the increment in label incorporation over time by the labeling of the precursor for protein synthesis [ideally the labeling of the aminoacyl- tRNA specific for the tracer amino acid infused, but a number of surrogates have been proposed and used (23, 46, 47)]. For this approach to be valid, it is necessary that label incorporation into proteins proceeds in a linear manner, which is the case if the precursor pool is steady during that time. Thus it has been recommended to administer the primed constant tracer infusion for at least 1 h before the first biopsy (47). Indeed many protocols have been designed with an initial muscle biopsy between 60 and 120 min after the start of the amino acid tracer infusion and a second one some 3 to 12 h later (7, 16, 19, 20, 26, 29, 30, 34, 41, 49). Recently, Volpi et al. (42) demonstrated that the protein labeling is linear between 120 and 360 min after starting a primed, constant phenylalanine tracer infusion, validating this approach. However, no data were provided for earlier or later time points, and the validity of studies in which the first biopsy was obtained as soon as 30 min after (2, 3) or immediately before starting the tracer infusion (6, 25, 27, 28, 32, 40) remains unclear. Theoretically these approaches might only introduce minimal error because studies in animals and human subjects have demonstrated rapid entry (within minutes) of intravenously administered labeled amino acids into the intracellular space and muscle proteins (1, 9, 38), which should ensure linearity of tracer incorporation almost instantly. However, this has never been carefully evaluated.
Moreover, several investigators have taken only a single biopsy several hours after starting the tracer infusion, apparently assuming no or only negligible background muscle protein enrichment in their calculation of the FSR (4, 14, 15, 17), or alternatively used published population average values for the background enrichment of muscle protein as the initial value in the FSR calculation (11, 12), as suggested by Rennie et al. (36). If valid FSRs can be obtained by using a single biopsy this would be advantageous to both investigators and study participant and useful in circumstances in which there might be reason to limit the number of biopsies on ethical grounds (e.g., certain patient groups) or when opportunities for an initial biopsy are limited (e.g., studies in patients intraoperatively).
The purpose of the present study was to 1) monitor during the early stages of a primed constant infusion of labeled leucine the plasma α-ketoisocaproate (α-KIC) labeling and the KIC-to-leucine enrichment ratio as an index of the equilibration of plasma and intracellular amino acid labeling, and 2) to evaluate how the timing, or omission, of the initial muscle biopsy affects the calculated FSR. Specifically, we measured in healthy men during basal, postabsorptive conditions 1) plasma leucine and α-KIC labeling before and at frequent time points immediately after the start of a primed, continuous [5,5,5-2H3]leucine infusion, and 2) the muscle protein FSR during primed, continuous intravenous infusions of [ring-2H5]phenylalanine or [5,5,5-2H3]leucine with muscle biopsies obtained either immediately before and several hours after starting the tracer infusion or 60 min and several hours after starting the tracer infusion.
METHODS
Subjects.
No subject was enrolled specifically for the purpose of this study; instead, we used data collected from healthy men for other purposes (unpublished data). Data from five men [age 34 ± 5 yr; body mass index (BMI) 31 ± 2 kg/m2; means ± SE], who participated in lipoprotein metabolism studies that included a primed constant leucine tracer infusion and frequent blood sampling, were analyzed to determine the time course and extent of plasma α-KIC labeling (as a measure of the labeled plasma amino acid entry into the intracellular pool). Data from 22 men (age 70 ± 1 yr; BMI 28 ± 1 kg/m2), who received a phenylalanine or leucine tracer infusion in combination with muscle biopsies as part of currently ongoing studies, were analyzed to determine the effect of biopsy timing on the measured muscle protein FSR. All subjects were considered to be in good health after completing a comprehensive medical evaluation. All studies were approved by the Human Research Protection Office and the Center for Applied Research Services Scientific Advisory Committee at Washington University School of Medicine. Written informed consent was obtained from each subject before participation in the study.
Experimental protocol.
All subjects were instructed to adhere to their regular diet and to refrain from vigorous exercise for 3 days before they were admitted to the clinical research unit on the evening before the metabolic study. At 2000 they consumed a standard meal providing 12 kcal/kg body wt; 55% of the total meal energy was provided as carbohydrates, 30% as fat, and 15% as protein. Subjects then rested in bed and fasted (except for water) until completion of the tracer infusion study the next day.
In the five men who were studied to determine the relationship between plasma leucine and α-KIC labeling early after the start of a leucine tracer infusion, a cannula was inserted into an antecubital vein at ∼0600 for infusion of the stable isotope labeled amino acid; another one was inserted into a vein of the contralateral forearm for blood sampling. At ∼0700, a primed constant infusion of [5,5,5-2H3]leucine (priming dose 7.2 μmol/kg body wt, infusion rate 0.12 μmol·kg body wt −1·min−1) was started and maintained until completion of the study. Blood samples (4 ml each) were obtained before and at 5, 15, 30, 60, 90, 120, 150, and 180 min after the start of the tracer infusion.
In the 22 men studied to determine the effect of biopsy timing on the measured muscle protein FSR, a cannula was inserted into an antecubital vein for infusion of the stable isotope-labeled amino acids at ∼0600. At ∼0700, a primed constant infusion of either l-[ring-2H5]phenylalanine (priming dose 2.03 ± 0.03 μmol/kg body wt, infusion rate 0.055 ± 0.002 μmol·kg body wt−1·min−1) or [5,5,5-2H3]leucine (priming dose 4.23 ± 0.21 μmol/kg body wt, infusion rate 0.091 ± 0.004 μmol·kg body wt−1·min−1) was started and maintained until completion of the study. Two muscle biopsies from the quadriceps femoris (one from each leg) were taken (during local anesthesia with 2% lidocaine solution) immediately before and 210 min after the start of the tracer infusion (n = 5 with phenylalanine and n = 6 with leucine) or at 60 min and 240 min after the start of the tracer infusion (n = 6 with phenylalanine and n = 5 with leucine) to determine the leucine and phenylalanine labeling of muscle protein and muscle tissue fluid (as incorporation of labeled amino acids into proteins; see Calculations).
Sample collection, processing and analyses.
Blood samples (∼3 ml) were collected in prechilled tubes containing EDTA; plasma was separated by centrifugation within 30 min of collection and then stored at −80°C until final analyses were performed. Muscle tissue samples were rinsed in ice-cold saline immediately after collection, cleared of visible fat and connective tissue, frozen in liquid nitrogen, and stored at −80°C until final analysis.
To determine plasma leucine and α-KIC tracer-to-tracee ratios (TTR) plasma proteins were precipitated, and the supernatant, containing free amino acids and their keto analogs, was collected to prepare the t-butyldimethylsilyl (t-BDMS) and O-t-butyldimethylsilyl quinoxalinol derivatives, respectively. Leucine and α-KIC TTR were determined by gas-chromatography/mass-spectrometry (GC-MS; MSD 5973 System, Hewlett-Packard) (22, 31). To determine the phenylalanine or leucine TTR in muscle proteins and muscle tissue fluid, muscle samples (∼20 mg) were homogenized in 1 ml trichloroacetic acid solution (3% wt/vol), proteins were precipitated by centrifugation, and the supernatant, containing free amino acids, was collected. The pellet containing muscle proteins was washed and then hydrolyzed in 6 N HCl at 110°C for 24 h. Amino acids in the protein hydrolysate and supernatant samples were purified on cation-exchange columns (Dowex 50W-X8–200, Bio-Rad Laboratories, Richmond, CA), and their t-BDMS derivatives were prepared to determine their TTR by GC-MS (MSD 5973 System, Hewlett-Packard) (31).
Calculations.
The muscle protein FSR was calculated based on the incorporation rate of [2H5]phenylalanine or [2H3]leucine into muscle proteins by using a standard precursor-product model as follows: FSR = (ΔEp/EIC)× (1/t) × 100, where ΔEp is the change in protein-bound phenylalanine or leucine labeling over time, t is the time, and EIC is the enrichment of the precursor pool for protein synthesis (i.e., phenylalanine or leucine labeling in muscle tissue fluid). The FSR was calculated as follows: 1) ΔEp in the above equation is the change in the measured TTR of protein-bound phenylalanine or leucine between 60 min and 240 min; 2) ΔEp in the above equation is the change in the measured TTR of protein-bound phenylalanine or leucine between 0 min and 210 min; 3) ΔEp in the above equation is the measured TTR of protein-bound phenylalanine or leucine at 210 or 240 min (assuming the background enrichment of amino acids in muscle protein before the start of the tracer infusion is negligible, i.e., zero); and 4) ΔEp in the above equation is the difference in the measured TTR of protein-bound phenylalanine or leucine at 210 min or 240 min, respectively and the “population” background TTR {i.e., average background TTR of protein-bound phenylalanine or leucine obtained from 40 studies conducted in healthy subjects in our laboratory between 2005 and 2008 in which a muscle biopsy was obtained in “tracer virgins” before the start of either a [ring-2H5]phenylalanine (n = 15) or [5,5,5-2H3]leucine (n = 25) infusion}. For consistency we used the phenylalanine or leucine labeling in the final muscle tissue fluid sample as the precursor pool enrichment for each of these approaches. Using the average precursor pool enrichment at 60 min and 240 min for the calculation of the FSR in the first of these four approaches did not affect the conclusions from our study.
Statistical analysis.
All data sets were tested for normality. ANOVA and Tukey's post hoc analysis were used to examine changes over time in the plasma leucine and α-KIC TTR. FSR values were compared by using ANOVA and Student t-test for post hoc analysis. Coefficients of variation (in percent) for the FSR values obtained by the different methods were calculated by dividing the SD by the mean and multiplying this value by 100. A P value ≤ 0.05 was considered statistically significant. All data are presented as means ± SE.
RESULTS
Plasma α-KIC labeling during intravenous infusion of labeled leucine.
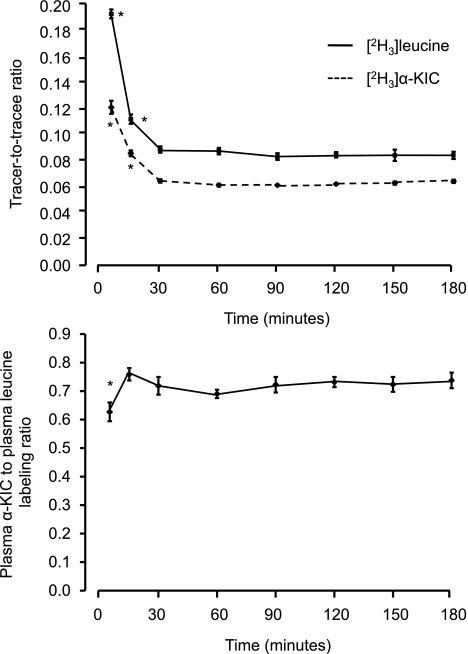
Significant conversion of [2H3]leucine to α-[2H3]KIC was observed within 5 min after starting the [2H3]leucine infusion, and the α-KIC labeling in relationship to the plasma leucine labeling was constant between 15 and 180 min (Fig. 1). After an initial peak at 5 min, the plasma α-KIC labeling returned to near steady-state values by 15 min (35 ± 4% above the mean value between 30 and 180 min; P < 0.001) with no change in the TTR between 30 min and 180 min (Fig. 1).
Fig. 1.
Plasma leucine and α-ketoisocaproic acid (KIC) tracer-to-tracee ratio (TTR; top) and the ratio between the α-KIC and leucine labeling in plasma (bottom) during a primed, constant infusion of [5,5,5-2H3]leucine. Values are means ± SE. *Value significantly different from all other corresponding values, P < 0.05.
Muscle free amino acid and protein labeling and muscle protein FSR.
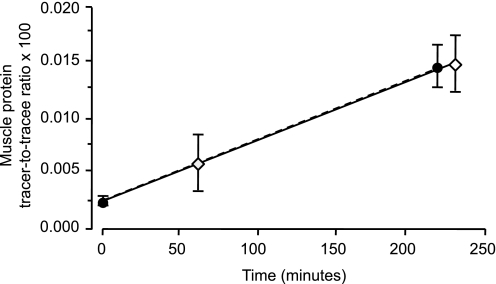
The muscle free phenylalanine labeling was not different at 60 min and 240 min (TTR 0.0532 ± 0.0060 and 0.0642 ± 0.0078, respectively; P = 0.12). When plotting the average TTR of protein-bound phenylalanine over time (Fig. 2), the lines connecting the TTR between 0 min and 210 min and 60 min and 240 min, respectively, were superimposable; the intercept (0.000022 ± 0.000012) was significantly different from zero (P = 0.05) but similar to the average background TTR (0.000018) from a previously studied cohort (P = 0.75).
Fig. 2.
Muscle protein phenylalanine labeling [tracer-to-tracee ratio (TTR)] measured immediately before and 210 min after the start of a primed constant [ring-2H5]phenylalanine infusion or at 60 min and 240 min after the start of the tracer infusion. Values are means ± SE. There were no differences in either the slopes (P = 0.99) or the intercepts (P = 0.98) of the two lines. The intercept (0.000022 ± 0.000012) was significantly different from zero (P = 0.05) but similar to the average background TTR (0.000018) from a previously studied cohort (P = 0.75).
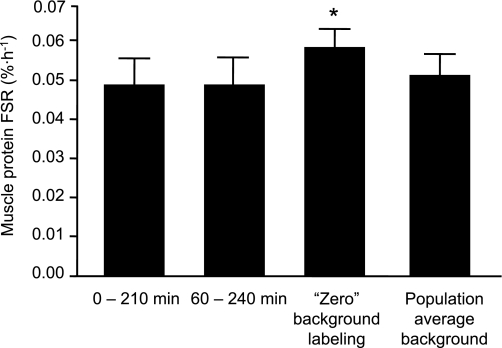
The muscle protein FSRs obtained by using the [2H5]phenylalanine tracer are presented in Fig. 3. The muscle protein FSR calculated from the labeled phenylalanine incorporation into protein between 0 min (measured TTR) and 210 min was not different (P = 0.99) from the FSR calculated from the label incorporation into protein between 60 min and 240 min. Power analysis revealed that the sample size required to detect a difference of the observed magnitude with sufficient power (≥0.80) to reject the null hypothesis and a sufficiently small probability of Type-I error (α < 0.05) is ≥1 million. Omitting the initial biopsy and assuming no phenylalanine labeling in muscle protein before the start of the tracer infusion gave FSR values that were on average ∼15% (0.009 ± 0.005%/h) greater (P = 0.059) than the FSR values obtained by measuring the labeling of muscle protein in two sequential biopsies whereas using the average background TTR from a previously studied cohort resulted in FSR values that were on average not different (∼3% difference, P = 0.76) from those obtained from measuring the muscle protein labeling in both an initial and final biopsy. The coefficients of variation for each of these methods were similar: 33% (biopsies immediately before and at 210 min after the start of the tracer infusion), 34% (biopsies at 60 min and 240 min after the start of the tracer infusion), 27% (omitting the first biopsy and assuming zero background labeling), and 32% (omitting the first biopsy and using a population average background enrichment). The average values of the variables used to calculate the FSR (i.e., the muscle free phenylalanine labeling in the final biopsy, the change in muscle protein bound phenylalanine labeling over time, and the duration between two biopsies or the time lapse from beginning the tracer infusion and the final biopsy for the single biopsy approach) are presented in Table 1.
Fig. 3.
Muscle protein fractional synthesis rate (FSR) during basal, postabsorptive conditions assessed by collecting muscle biopsies 1) immediately before and 210 min after the start of a primed constant [ring-2H5]phenylalanine infusion or 2) at 60 min and 240 min after the start of the tracer infusion, 3) omitting the first biopsy and assuming zero background labeling, and 4) omitting the first biopsy and using a population average background enrichment. Values are means ± SE. *Value different (P = 0.059) from values obtained by measuring the labeling of muscle protein in two sequential biopsies.
Table 1.
Muscle free phenylalanine/leucine labeling in the final biopsy, the change in muscle protein bound phenylalanine/leucine labeling over time, and the duration between two biopsies or the time lapse from beginning the tracer infusion and the final biopsy for the single-biopsy approach
| Two Biopsies |
Single Biopsy |
|||
|---|---|---|---|---|
| 0 min and 210 min | 60 min and 240 min | “Zero” background | Population average background | |
| [ring-2H5]phenylalanine | ||||
| Muscle free TTR | 0.0639 ± 0.0037 | 0.0642 ± 0.0078 | 0.0640 ± 0.0043 | 0.0640 ± 0.0043 |
| Time for FSR calculation, min | 224 ± 4 | 171 ± 3 | 229 ± 3 | 229 ± 3 |
| ΔMuscle protein TTR | 0.000119 ± 0.000022 | 0.000094 ± 0.000022 | 0.000147 ± 0.000021 | 0.000129 ± 0.000021 |
| [5,5,5-2H3]leucine | ||||
| Muscle free TTR | 0.0327 ± 0.0020 | 0.0530 ± 0.0078 | 0.0419 ± 0.0047 | 0.0419 ± 0.0047 |
| Time for FSR calculation, min | 218 ± 3 | 177 ± 4 | 227 ± 4 | 227 ± 4 |
| ΔMuscle protein TTR | 0.000046 ± 0.000005 | 0.000056 ± 0.000008 | 0.000107 ± 0.000023 | 0.000051 ± 0.000023 |
Values are means ± SE. TTR, tracer-to-tracee ratio; FSR, fractional synthesis rate.
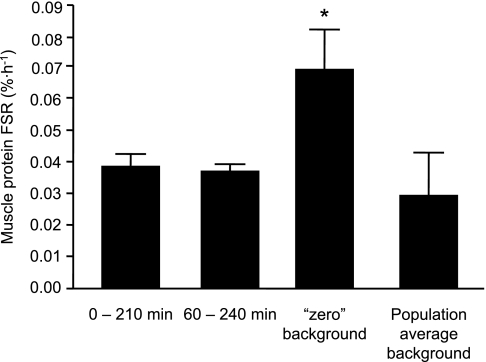
With the leucine tracer, the results were essentially the same, except that the results were more variable with the single-biopsy approaches, most likely because of the greater natural abundance of [2H3]leucine compared with [2H5]phenylalanine in muscle proteins. The muscle free leucine labeling was not significantly different at 60 min and 240 min (TTR 0.0417 ± 0.0042 and 0.0530 ± 0.0078, respectively; P = 0.057). The muscle protein FSRs obtained by using the [2H3]leucine tracer are presented in Fig. 4. The FSR values obtained with two biopsies taken either before and 210 min after the start of the tracer infusion or at 60 and 240 min after the start of the tracer infusion were not different (P = 0.66). Power analysis revealed that the sample size required to detect a difference of the observed magnitude with sufficient power (≥0.80) to reject the null hypothesis and a sufficiently small probability of Type-I error (α < 0.05) is ≥ 160 (assuming the smaller SD) and ≥ 269 (assuming the larger SD). Omitting the initial biopsy and assuming no background leucine enrichment in muscle protein before the start of the tracer infusion gave FSR values that were on average ∼80% (0.0031 ± 0.0012%/h, P = 0.03) greater than the FSR values obtained by measuring the labeling of muscle protein in two sequential biopsies. This difference was significantly greater than the corresponding value obtained when using the phenylalanine tracer (P = 0.05). The FSR values obtained by using the average background [2H3]leucine enrichment in muscle protein from a previously studied cohort (TTR = 0.000056) were on average ∼22% different (P = 0.52) from those obtained by measuring the muscle protein labeling in two sequential biopsies. The coefficients of variations for each of these methods, in ascending order, were 16% with biopsies at 60 min and 240 min after the start of the tracer infusion, 25% with biopsies immediately before and at 210 min after the start of the tracer infusion, 59% when omitting the first biopsy and assuming zero background labeling, and 145% when omitting the first biopsy and using a population average background enrichment. The average values of the variables used to calculate the FSR (i.e., the muscle free leucine labeling in the final biopsy, the change in muscle protein bound leucine labeling over time, and the duration between two biopsies or the time lapse from beginning the tracer infusion and the final biopsy for the single biopsy approach) are presented in Table 1.
Fig. 4.
Muscle protein FSR during basal, postabsorptive conditions assessed by collecting muscle biopsies 1) immediately before and 210 min after the start of a primed constant or [5,5,5-2H3]leucine infusion or 2) at 60 min and 240 min after the start of the tracer infusion, 3) omitting the first biopsy and assuming zero background labeling, and 4) omitting the first biopsy and using a population average background enrichment. Values are means ± SEM. *Value significantly different (P < 0.05) from values obtained by measuring the labeling of muscle protein in two sequential biopsies.
DISCUSSION
In the present study we compared the calculated basal skeletal muscle protein FSR obtained by using different experimental protocols for measuring the rate of label incorporation into muscle protein during a primed constant intravenous infusion of a tracer amino acid. Specifically we examined whether an initial muscle biopsy is necessary and whether the timing of the initial biopsy affects the calculated muscle protein FSR. We found that similar muscle protein synthesis rates are obtained irrespective of whether the initial muscle biopsy is taken immediately before or 60 min after starting a primed, constant amino acid tracer infusion, suggesting that the timing of the initial biopsy does not affect the final FSR value. However, omitting the initial biopsy and either assuming no background enrichment or using a population average background enrichment in lieu of the actual measured background enrichment yields average FSR values that are greater than those based on two biopsies, with the magnitude of error dependent on the amino acid tracer used.
The fact that independent of the tracer used the FSR measured between 0 and 210 min after starting the tracer infusion was not different from the FSR measured between 60 and 240 min after starting the tracer infusion indicates that either the major underlying assumption for the FSR approach (i.e., linear tracer incorporation) was met or it was violated only to a minimal and undetectable extent, which did not affect the results. This implies that the tracer labeled the aminoacyl tRNA pool very rapidly and was incorporated into muscle protein almost instantaneously after starting the infusion. We indeed observed rapid equilibration between the plasma leucine and α-KIC labeling and near steady-state labeling of the KIC pool within 15–30 min after the leucine tracer administration in the present study. This is consistent with earlier work that demonstrated that 70% of a phenylalanine tracer given intravenously in rats appeared in muscle within 3 min and tracer incorporation into muscle protein occurred as soon as 2 min after administration (1, 38) and that the half-life of methionine (which is similar in its metabolism and transport to the branched-chain amino acids) uptake in human muscle is 6 min (9). The FSR values obtained by using two biopsies are in good agreement with those previously reported by us and other investigators. For example, we have previously reported that in young and middle-aged healthy adults in the postabsorptive state at rest the mixed muscle protein FSR measured by using a phenylalanine tracer is ∼0.045%/h (39); most others who use a phenylalanine tracer and two biopsies report average values in the range of 0.04%/h to 0.06%/h (e.g., 2, 8, 21, 24,33, 45) although some reports include somewhat smaller or greater values (e.g., 2, 5, 10, 18). This strengthens our confidence that the data obtained are robust and that it is possible to accurately measure the muscle protein FSR within a few hours of the start of the tracer infusion without the need for an “equilibration period” before the initial biopsy.
The degree to which the average FSR deviates from the “true” FSR when relying on a single biopsy measurement depends on the choice of tracer, the derivative for MS analysis, and the labeling value used as a surrogate for the background enrichment of muscle protein. Relatively small errors result when using a tracer and derivative adduct with a low natural abundance of the analyzed isotope and assuming essentially no background enrichment (i.e., when using a [2H5]phenylalanine tracer the average FSR values fell within ∼15% of the actual FSR) whereas the average error can be in excess of 50% when using a tracer and derivative with a high natural abundance (i.e., when using a [2H3]leucine tracer the average FSR values were ∼80% different from the actual FSR). In contrast, the use of a population average background enrichment when omitting the initial biopsy reduces, but does not entirely abolish, this error.
These findings have important implications not only for the design of studies in which the basal muscle protein FSR at rest is to be determined but also studies in which the muscle protein FSR will be determined under conditions other than fasting at rest (e.g., postexercise or during feeding) or studies with a sequential study design (e.g., a basal period followed by feeding, etc.). First, it is unlikely that the conclusions from our study would have been different had we made our measurements during conditions other than the postabsorptive state, at rest (e.g., after exercise or during intravenous nutrient infusion) unless there were reason to believe that the time it takes to reach isotopic equilibrium between the plasma and intracellular pool were greatly extended (which could adversely affect the result) by these circumstances. Second, our findings suggest (although we do not have direct proof for this) that sequential study designs, particularly those in which care is taken to minimize changes in the precursor pool enrichment (e.g., 3, 39, 43), are most likely valid because we have demonstrated that small initial temporary imbalances in the precursor pool enrichment do not affect the measured muscle protein FSR.
In summary, the plasma and intracellular amino acid pools equilibrate quickly during a primed, continuous intravenous amino acid tracer infusion, and valid basal muscle protein FSR values can be obtained irrespective of the timing of the initial biopsy when the protein labeling in two sequential biopsies is measured. Under some circumstances valid average muscle protein FSR values can be obtained by measuring the labeling of protein in a single biopsy after some time of continuous tracer infusion, although this approach could potentially result in highly erroneous results and should therefore be avoided.
GRANTS
This study was supported by National Institutes of Health Grants AR-49869, HD-057796, AG-025501, AG-025721, DK-56341 (Clinical Nutrition Research Unit), and RR-00954 (Biomedical Mass Spectrometry Resource) and by National Center for Research Resources (NCRR) Grant UL1-RR-024992. G. I. Smith was supported by an Ellison Medical Foundation/American Federation for Aging Research Postdoctoral Fellowship. D. N. Reeds was supported by an American Society of Nutrition Physician Nutrition Support Specialist Award.
DISCLOSURES
None of the authors had conflicts of interest.
ACKNOWLEDGMENTS
We thank the staff of the Center for Applied Research Services for technical assistance and the study subjects for their participation.
REFERENCES
- 1. Banos G, Daniel PM, Moorhouse SR, Pratt OE. The movement of amino acids between blood and skeletal muscle in the rat. J Physiol 235: 459–475, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol 552: 315–324, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol 532: 575–579, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boirie Y, Short KR, Ahlman B, Charlton M, Nair KS. Tissue-specific regulation of mitochondrial and cytoplasmic protein synthesis rates by insulin. Diabetes 50: 2652–2658, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Carroll CC, Fluckey JD, Williams RH, Sullivan DH, Trappe TA. Human soleus and vastus lateralis muscle protein metabolism with an amino acid infusion. Am J Physiol Endocrinol Metab 288: E479–E485, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19: 422–424, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Dillon EL, Sheffield-Moore M, Paddon-Jones D, Gilkison C, Sanford AP, Casperson SL, Jiang J, Chinkes DL, Urban RJ. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab 94: 1630–1637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587: 1535–1546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fischman AJ, Yu YM, Livni E, Babich JW, Young VR, Alpert NM, Tompkins RG. Muscle protein synthesis by positron-emission tomography with l-[methyl-11C]methionine in adult humans. Proc Natl Acad Sci USA 95: 12793–12798, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab 292: E77–E83, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) 72: 503–509, 1987 [DOI] [PubMed] [Google Scholar]
- 12. Gibson JN, Morrison WL, Scrimgeour CM, Smith K, Stoward PJ, Rennie MJ. Effects of therapeutic percutaneous electrical stimulation of atrophic human quadriceps on muscle composition, protein synthesis and contractile properties. Eur J Clin Invest 19: 206–212, 1989 [DOI] [PubMed] [Google Scholar]
- 13. Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol 586: 6049–6061, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guillet C, Delcourt I, Rance M, Giraudet C, Walrand S, Bedu M, Duche P, Boirie Y. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J Clin Endocrinol Metab 94: 3044–3050, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J 18: 1586–1587, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab 278: E620–E626, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Hellstern G, Kaempf-Rotzoll D, Linderkamp O, Langhans KD, Rating D. Parenteral amino acids increase albumin and skeletal muscle protein fractional synthetic rates in premature newborn minipigs. J Pediatr Gastroenterol Nutr 35: 270–274, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Henderson GC, Dhatariya K, Ford GC, Klaus KA, Basu R, Rizza RA, Jensen MD, Khosla S, O'Brien P, Nair KS. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J 23: 631–641, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katsanos CS, Aarsland A, Cree MG, Wolfe RR. Muscle protein synthesis and balance responsiveness to essential amino acids ingestion in the presence of elevated plasma free fatty acid concentrations. J Clin Endocrinol Metab 94: 2984–2990, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 291: E381–E387, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Kim PL, Staron RS, Phillips SM. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol 568: 283–290, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langenbeck U, Luthe H, Schaper G. Keto acids in tissues and biological fluids: O-t-butyldimethylsilyl quinoxalinols as derivatives for sensitive gas chromatographic/mass spectrometric determination. Biomed Mass Spectrom 12: 507–509, 1985 [DOI] [PubMed] [Google Scholar]
- 23. Ljungqvist OH, Persson M, Ford GC, Nair KS. Functional heterogeneity of leucine pools in human skeletal muscle. Am J Physiol Endocrinol Metab 273: E564–E570, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol 107: 1655–1662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mittendorfer B, Andersen JL, Plomgaard P, Saltin B, Babraj JA, Smith K, Rennie MJ. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol 563: 203–211, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89: 161–168, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol 587: 897–904, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nair KS, Halliday D, Griggs RC. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol Endocrinol Metab 254: E208–E213, 1988 [DOI] [PubMed] [Google Scholar]
- 29. Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol 41: 215–219, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol 91: 1041–1047, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism 46: 943–948, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Petersen AM, Magkos F, Atherton P, Selby A, Smith K, Rennie MJ, Pedersen BK, Mittendorfer B. Smoking impairs muscle protein synthesis and increases the expression of myostatin and MAFbx in muscle. Am J Physiol Endocrinol Metab 293: E843–E848, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J 20: 768–769, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rennie MJ, Edwards RH, Millward DJ, Wolman SL, Halliday D, Matthews DE. Effects of Duchenne muscular dystrophy on muscle protein synthesis. Nature 296: 165–167, 1982 [DOI] [PubMed] [Google Scholar]
- 36. Rennie MJ, Smith K, Watt PW. Measurement of human tissue protein synthesis: an optimal approach. Am J Physiol Endocrinol Metab 266: E298–E307, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab 286: E92–E101, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Simon O, Munchmeyer R, Bergner H, Zebrowska T. Distribution of radioactivity in the body and rate of incorporation of radioactivity into the tissue proteins of monogastric animals following intravenous injection of tracer amino acids [in German]. Arch Tierernahr 26: 599–609, 1976 [DOI] [PubMed] [Google Scholar]
- 39. Smith GI, Atherton P, Reeds DN, Mohammed BS, Jaffery H, Rankin D, Rennie MJ, Mittendorfer B. No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia-hyperaminoacidemia in middle-aged adults. J Appl Physiol 107: 1308–1315, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith GI, Atherton P, Villareal DT, Frimel TN, Rankin D, Rennie MJ, Mittendorfer B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS ONE 3: e1875, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr 86: 451–456, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Volpi E, Chinkes DL, Rasmussen BB. Sequential muscle biopsies during a 6-h tracer infusion do not affect human mixed muscle protein synthesis and muscle phenylalanine kinetics. Am J Physiol Endocrinol Metab 295: E959–E963, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 101: 2000–2007, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 85: 4481–4490, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286: 1206–1212, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Watt PW, Lindsay Y, Scrimgeour CM, Chien PA, Gibson JN, Taylor DJ, Rennie MJ. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: Use in studies of human tissue protein synthesis. Proc Natl Acad Sci USA 88: 5892–5896, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wolfe RR, Chinkes D. Isotope Tracers in Metabolic Research: Principles and Practices of Kinetic Analysis. Hoboken, NJ: Wiley, 2005 [Google Scholar]
- 48.Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol Endocrinol Metab 265: E210–E214, 1993 [DOI] [PubMed] [Google Scholar]
- 49. Zachwieja JJ, Witt TL, Yarasheski KE. Intravenous glutamine does not stimulate mixed muscle protein synthesis in healthy young men and women. Metabolism 49: 1555–1560, 2000. [DOI] [PubMed] [Google Scholar]