Abstract
Recent epidemiologic studies report that regular exercise may be associated with substantial reductions in cancer-specific and all-cause mortality following a breast cancer diagnosis. The mechanisms underlying this relationship have not been identified. We investigated the effects of long-term voluntary wheel running on growth and progression using an animal model of human breast cancer. We also examined effects on the central features of tumor physiology, including markers of tumor blood perfusion/vascularization, hypoxia, angiogenesis, and metabolism. Athymic female mice fed a high-fat diet were orthotopically (direct into the mammary fat pad) implanted with human breast cancer cells (MDA-MB-231 at 1 × 106) into the right dorsal mammary fat pad and randomly assigned (1:1) to voluntary wheel running (n = 25) or a nonintervention (sedentary) control group (n = 25). Tumor volume was measured every three days using digital calipers. All experimental animals were killed when tumor volume reached ≥1,500 mm3. Kaplan-Meier (KM) analysis indicated that tumor growth (survival) was comparable between the experimental groups (exercise 44 days vs. control 48 days; KM proportional hazard ratio = 1.41, 95% confidence interval, 0.77–2.58, P = 0.14). However, tumors from exercising animals had significantly improved blood perfusion/vascularization relative to the sedentary control group (P < 0.05). Histological analyses indicated that intratumoral hypoxia levels (as assessed by hypoxia-inducible factor 1) were significantly higher in the exercise group relative to sedentary control (P < 0.05). Aerobic exercise can significantly increase intratumoral vascularization, leading to “normalization” of the tissue microenvironment in human breast tumors. Such findings may have important implications for inhibiting tumor metastasis and improving the efficacy of conventional cancer therapies.
Keywords: survival, orthotopic, physical activity, carcinogenesis
investigation into the role of physical activity and exercise across the entire cancer continuum (i.e., prevention to survivorship) has been the subject of considerable research and clinical interest over the past several decades. Initial studies focused on the association between physical activity and the primary risk of common forms of cancer. There is now a wealth of evidence suggesting that regular moderate-intensity physical activity is associated with reduced risk of several forms of cancer, including breast, colon, and endometrial (7, 9, 12). Based on this evidence, several large-scale randomized trials were launched and demonstrated that structured exercise training can significantly change biomarkers of cancer risk among individuals at high risk of breast or colon cancer (4, 13).
More recently, several research groups have started to investigate the role of exercise following a cancer diagnosis either during or following the cessation of systemic (e.g., chemotherapy) and locoregional (e.g., radiation) therapy. Systematic reviews conclude that exercise is a safe and feasible supportive intervention associated with significant improvements in patient-reported outcomes (e.g., quality of life, fatigue) as well as physiological outcomes (exercise tolerance, muscle strength) in cancer populations (3, 21). Moreover, several landmark epidemiologic studies have provided the first evidence that regular moderate-intensity exercise is associated with a 30–50% reduction in the risk of cancer-specific mortality and all-cause mortality following a diagnosis of early breast or colorectal cancer (14, 16, 28). As such, elucidation of the molecular mechanisms underlying this association is of paramount importance to optimize the safety and efficacy of exercise in cancer control.
To this end, several preclinical studies have investigated the effects of exercise, alone or in combination with caloric restriction, on the mechanisms that influence the induction, initiation, and growth of experimentally induced cancer (10, 18–20, 35, 39). Such experimental data, however, are only applicable to the early stages of tumorigenesis (i.e., primary risk of cancer) and are likely not appropriate for deciphering the effects of exercise in a postdiagnosis setting. Indeed, advanced stages of tumorigenesis (clinically detectable disease) is controlled by a unique set of gene regulation pathways involving acquired capabilities such as sustained angiogenesis, tissue invasion, and metastatic dissemination to distant sites in the body (11). It follows logically that the mechanistic properties of exercise on tumor biology and progression are likely different in the pre- vs. postdiagnosis setting.
To date, few studies have investigated the effects of exercise in a postdiagnosis animal model of carcinogenesis involving the initiation of exercise following establishment of primary tumor growth or metastatic disease. Most, but not all, report that exercise is associated with inhibition of primary tumor growth and a decrease in metastatic dissemination (25, 26, 32, 36, 40). However, the majority of studies investigated either the effects of short-term (<14 days) exercise, adopting forced exercise paradigms (e.g., treadmill running), on primary growth and/or dissemination of tumor cells artificially implanted outside the organ of origin (i.e., subcutaneous or tail-vein injection of tumor cells), which may not be clinically relevant. Here, we extend prior work by investigating the effects of moderate to long-term (6 wk) voluntary wheel running on growth and progression using an orthotopic model of human breast cancer. Specifically, human breast cancer cells were implanted directly into the mammary fat pad of recipient animals fed a high-fat diet, and exercise was only initiated after tumor establishment. In addition, we also examined effects on the central features of tumor physiology, including markers of tumor blood perfusion/vascularization, hypoxia, angiogenesis, and metabolism. We hypothesized that exercise would inhibit breast cancer growth and progression.
EXPERIMENTAL PROCEDURES
Animals.
Fifty athymic homozygous female mice (3–4 wk of age; mean body weight, 19 ± 1.3 g) were obtained from the Duke Cancer Center Isolation Facility (Durham, NC). Aythmic mice have compromised immune function; immune deficit mice are required to study tumorigenicity of human cancer cells in a murine model. All mice were housed individually and fed a Western diet (40% fat, 44% carbohydrate, 16% protein; TestDiet, Indianopolis, IN) to reflect the typical dietary intake of women with breast cancer for the study duration (∼6 wk). The diet was freshly prepared weekly to prevent the fat from becoming rancid. Food levels were monitored daily and provided ad libitum as required. Animal food consumption was not monitored. Animal care was approved and in accordance with the Institutional Animal Care and Use Guidelines at Duke University Medical Center.
Cell culture.
The human mammary adenocarcinoma cell line MDA-MB-231 was purchased from American Type Culture Collection (Rockville, MD). Cells were grown in 5% CO2 at 37°C and harvested by trypsinization at 70–80% confluence in log phase growth on the day of tumor injection.
Study procedures.
On day 0, all experimental animals were injected orthotopically into the right dorsal mammary fat pad with MDA-MB-231 (1 × 106) in suspension. Two days following tumor implantation, all animals were randomly assigned (1:1) to exercise (n = 25) or a nonintervention (sedentary) control group (n = 25).
Exercise protocol.
The exercise modality in this experiment was voluntary wheel running as opposed to forced exercise paradigms such as treadmill running. Voluntary wheel running, as opposed to forced exercise paradigms, in our opinion, may be more reflective of normal as well as individual exercise behavior of mice; is less stressful; and a higher and more variable dose of exercise can be investigated. Murine voluntary wheel running is characterized by intermittent exercise performed for relatively short time periods at high speed, against a low load, throughout the entire dark cycle. Previous work has reported that voluntary wheel running is associated with significant improvements in exercise tolerance (i.e., time to exhaustion and peak oxygen consumption) as well as histological improvements in skeletal muscle enzyme activity (e.g., citrate synthase) (8).
Animals randomized to exercise were given voluntary access to a wheel measuring 11.5 cm in diameter, with wheel revolutions monitored continuously by magnetic sensor using the VitalView data acquisition program (Respironics, Murrysville, PA). Mice randomized to the control group were housed individually in cages without wheels.
Assessment of tumor volume.
Tumor volume was measured every 3 days using digital calipers. Tumor volume was measured in two orthogonal dimensions. The greatest dimension of the tumor was recorded as tumor length, with the other dimension (at a 90° angle) recorded as width. Tumor volume was calculated as π/6 × width × length2, which is a standard formula for calculating tumor volume in mouse models of breast cancer.
Necropsy.
All experimental animals were killed when tumor volume reached 1,500 mm3 as required by institutional guidelines. Before death, all animals were given Hoechst 33342 (20 mg/ml, 100 μl iv) and anesthetized with pentobarbital (75 mg/kg). Tumors were excised, weighed, and snap-frozen in liquid nitrogen and stored at −80°C. Histological analysis was only performed on tumors obtained from the 10 animals recording the highest mean exercise running distance and 10 random control animals. We choose to only conduct histological analysis on the top 10 “runners” because these animals received the highest exercise “dose” and thus provided the optimal examination of the effects of exercise on breast cancer tumorigenesis in the present context.
Immunohistochemistry.
Hematoxylin and eosin (H and E) staining was used for identification of tissue architecture, including viable and necrotic tumor tissue. Blood vessels were identified with CD31 staining using a rat-anti-mouse primary antibody (BD no. 550274) with Cy2-conjugated donkey-anti-rat secondary antibody (Jackson Immunoresearch no. 712–225-153). Next, this slide was then also imaged for perfused blood vessels shown with Hoechst given before death. Hypoxia was identified using an immunohistochemistry for the endogenous protein marker, carbonic anhydrase isoform IX (CAIX), as previously described (5).
Western blotting.
Homogenized tumors from both groups were analyzed using commercially available ELISAs for content of VEGF (R&D Systems, kit DVE00) and hypoxia-inducible factor (HIF)-1, (Panomics, Fremont, CA), whereas Western blotting was used to assess AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α (Santa Cruz Biotechnology, Santa Cruz, CA) as previously described (38).
Assessment of tumor energy status (metabolism).
The energy status of the tumor was measured using bioluminescence for ATP as described previously (31). In brief, bioluminescence enzymatic reaction was conducted with a temperature-controlled, black box-encased fluorescence microscope using a cooled 16-bit CCD camera with photon-counting capability. Tissue sections were brought into contact with reaction solution for ATP, and light emission from the enzymatic activity of the luciferase reporter was detected and quantified to ATP concentration based on previous calibration (30).
Image analysis.
Necrotic fraction, defined as the necrotic area (pixels) divided by the total tumor area, was assessed using H and E images. Actively perfused vessels were assessed via the presence of Hoechst staining. Perfused (Hoechst positive) blood vessels were counted manually, and the percent perfused area was calculated using individual region of interest for each vessel divided by the viable and/or total tumor area. Previous work by our laboratory (33) found that viable tumor tissue, as opposed to perinecrotic tumor tissue, is more sensitive to chemotherapy and radiotherapy. Thus whether the effects of exercise on markers of tumor physiology were different for viable vs. total tumor tissue was similarly of great interest. The area of positive threshold CD31 expression divided by the viable and/or total tumor area yielded percent vessel area of the tissue. CAIX and HIF-1 expression were assessed in an identical manner and reported as percentage of viable and/or total tumor area. All images were processed using identical methods by personnel blinded to group assignment.
Statistical analysis.
Tumor growth and survival curves were analyzed using the Kaplan-Meier (KM). All immunohistochemistry/protein results were initially analyzed with Shapiro-Wilk for normality (values with P < 0.05 were significant for nonnormal distribution). Those that were nonnormally distributed were compared between experimental groups using nonparametric Mann-Whitney tests. Normally distributed results were compared with Student t-test (33). Two-tailed tests were used for the analysis with a P < 0.05 considered significant.
RESULTS
Voluntary wheel running exercise behavior and body weight.
Median running distance ranged from ∼4 to ∼6 km/day for the entire duration of the study and did not change during the experiment (P > 0.05). Body weight increased over the course of the experiment in both groups (P < 0.05) but with no differences between groups.
Effect on tumor growth and survival.
The median tumor volume of 1,500 mm3 was reached 44 ± 3 days after randomization in the exercise group. At this point, the median tumor volume in the sedentary (nonexercise) group was 1,186 mm3 (Fig. 1). KM analysis indicated no significant survival differences between the experimental groups [exercise 44 days vs. control 48 days; KM proportional hazard ratio = 1.41, 95% confidence interval (CI), 0.77–2.58, P = 0.14; Fig. 1]. KM analysis indicated no statistical difference in tumor growth (survival) between animals selected for histological analysis (46 vs. 48 days; KM proportional hazard ratio = 1.62, 95% CI 0.59–4.39, P = 0.62).
Fig. 1.
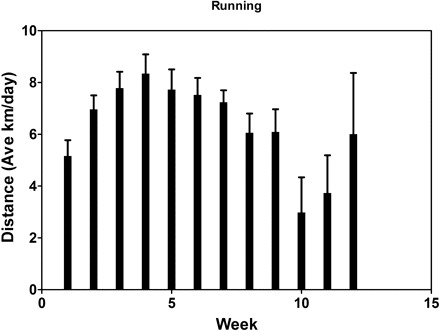
Kaplan-Meier (KM) survival analysis. Median tumor volume of 1,500 mm3 was reached 44 ± 3 days after randomization in the exercise group. At this point, the median tumor volume in the sedentary (nonexercise) group was 1,186 mm3 (44 vs. 48 days; KM proportional hazard ratio = 1.41, 95% confidence interval, 0.77–2.58, P = 0.14).
Effect on intratumoral blood perfusion/vascularization and necrosis.
Mean number of perfused vessels was significantly higher in the exercise group compared with sedentary control counterparts when based on the viable tumor (P = 0.023) and normalized to total tumor volume (P = 0.03; Fig. 2A). The percentage of perfused area of total tumor was 7.4% in the exercise group compared with 3.0% in the sedentary control group (P = 0.034; Fig. 2B). A similar trend was observed in the perfused area of viable tumor but was nonsignificant (Fig. 2B). There were no differences for proteins levels of CD-31 (22.9 ± 4.0 for the exercise group vs. 18.9 ± 3.0 for the sedentary control group, P = 0.66) or tumor necrosis (58 ± 5% for the exercise group vs. 57 ± 7% for the sedentary control group, P = 0.89) between groups.
Fig. 2.
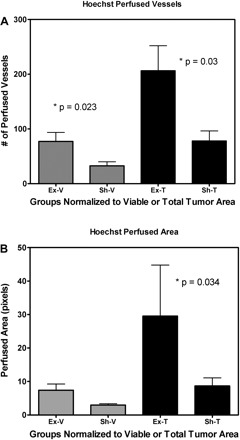
Mean vessel perfusion of athymic female mice randomized to voluntary wheel running or sedentary control (n = 9–10/per group). A: mean number of perfused vessels of viable tumor (P = 0.023) and normalized to total tumor volume (P = 0.03). B: mean perfused area of viable tumor (P > 0.05) and normalized to total tumor volume (P = 0.034). Abbreviations: Ex-V, exercise group-viable tissue; Sh-V, sham group-viable tissue; Ex-T, exercise group-total tissue; Sh-T, sham group-total tissue.
Effect on intratumoral angiogenesis (VEGF) and hypoxia (HIF-1, CAIX).
Protein levels of HIF-1 were significantly higher in the exercise group compared with sedentary control counterparts when normalized to viable tumor volume (11.4 ± 4.0% vs. 5.4% ± 2.0%, respectively, P = 0.02; Fig. 3) but not when based on total tumor (4.2 ± 1.3% vs. 2.1 ± 1.3%, respectively, P = 0,16; Fig. 3). The percent stained area for CAIX was not significantly different between experimental groups. Similarly, protein levels of VEGF, as measured by ELISA, were not significantly different between groups (47.0 ± 6.0 pg/ml for the exercise group vs. 48.6 ± 8.8 pg/ml for the sedentary control group, P = 0.86). There was, however, a significant correlation between protein levels of VEGF and the total number of perfused blood vessels in the exercise group (r = 0.64, P = <0.01) but not the sedentary control group (r = 0.17, P = 0.27).
Fig. 3.
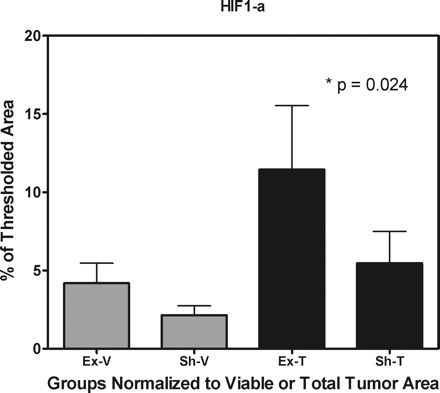
Protein levels of hypoxia-inducible factor (HIF)-1α of athymic female mice randomized to voluntary wheel running or sedentary control (n = 9–10/per group) when normalized to total tumor volume (P = 0.024) but not when based on viable tumor (P > 0.05).
Effect on tumor energy status (metabolism).
There were no significant differences in protein level of ATP concentration in viable tumor (0.14 ± 0.25 mmol/g for the exercise group vs. 0.18 ± 0.16 mmol/g for the sedentary control group, P = 0.21) or when normalized to total tumor volume (0.42 ± 0.53 mmol/g for the exercise group vs. 0.34 ± 0.28 mmol/g for the sedentary control group, P = 0.97). Similarly, intratumoral protein levels of PGC-1α and AMPK, when normalized both to β-actin and viable tumor, were not significantly different between experimental groups (P = 0.66 and P = 0.78, respectively).
DISCUSSION
Recent epidemiological studies have provided the first evidence that regular exercise may be strongly inversely associated with mortality following a breast cancer diagnosis (14–16). However, the molecular mechanisms underlying this association have not been elucidated. As an initial step, we investigated the effects of voluntary wheel running on tumor growth (survival), as well as key features of tumor physiology, using a model of human breast cancer.
In contrast to existing clinical epidemiological data, we found breast tumors grew at comparable rates in exercising and sedentary animals. These results are also in contrast with several preclinical investigations reporting that exercise significantly inhibits tumor growth and metastatic dissemination in human and murine models of cancer (25, 26, 40). For example, Zielinski et al. (40) found that high-intensity forced daily treadmill running performed for 14 days significantly delayed lymphoma tumor growth relative to sedentary controls. Nevertheless, not all preclinical studies have reported an inverse association between exercise and tumor growth. Woods et al. (36) reported no significant differences in breast cancer tumor growth among mice randomized to 14 days of moderate-intensity or high-intensity treadmill running, relative to sedentary control. The reasons for the contrasting findings are not clear but are likely explained by differences in the tumor model (breast vs. other cancer types), exercise paradigm (forced vs. voluntary exercise) and stimulus (short-term vs. long-term training), site of tumor implantation (orthotopic vs. subcutaneous), and dietary background (high-fat vs. low-fat diet). In terms of the latter, it is not clear whether similar findings would be observed among animals receiving a normal chow diet (i.e., low-fat diet) since we did not control for this variable in the present study. Clearly, further research elucidating the effects and underlying molecular mechanisms of exercise in postdiagnosis models of cancer is required.
An intriguing finding in our study was that exercise significantly increased intratumoral perfusion/vascularization and hypoxia relative to sedentary control counterparts. It is well-established that the tumor microenvironment plays a pivotal role in solid tumor progression and metastatic dissemination (37). Investigating the interaction between exercise and the tissue environment that supports tumor cells may therefore provide insight into the exercise-tumorigenesis relationship. To our knowledge, only one other study has examined the effects of exercise on the tumor microenvironment. As described previously, Zielinski et al. (40) evaluated intratumoral levels of von Willebrand factor VIII, a marker of tumor vascularization (blood-vessel density) in mice implanted with lymphoma. In contrast to our findings, exercise significantly reduced intratumoral vascularization. The authors speculated that exercise-induced reductions in VEGF may have mediated this effect, which, in turn, led to a reduction in tumor angiogenesis (development of new blood vessels) and ultimately tumor growth inhibition. Such an explanation seems reasonable; however, an exercise-induced reduction in intratumoral vascularization could augment rather than inhibit tumor growth over the long-term.
It is well established that solid tumors have “abnormal” blood vessels that impair blood perfusion, the delivery of oxygen, and removal of by-products of glycolysis. The resultant hypoxic microenvironment promotes an aggressive tumor phenotype characterized by invasion and metastasis (17). As such, strategies that can “normalize” the tumor microenvironment (i.e., increase tumor perfusion) may reverse this phenotype and inhibit metastasis. In support of this notion, Mazzone et al. (27) reported that normalization of the tumor endothelial layer [via heterogeneous deficiency of oxygen-sensing prolyl hydroxylase (PHD)] improved oxygenation and reduced glycolysis, leading to inhibition of distant metastases in animal models of lung cancer and melanoma. Against this background, an exciting speculation is that exercise may induce similar tumor “normalizing” effects and inhibit metastasis. Unfortunately, we did not evaluate the extent or incidence of metastases in this study.
It is also noteworthy that the abnormal vasculature and resulting hypoxia also pose a formidable barrier to the delivery and efficacy of systemic (e.g., chemotherapy) and locoregional (i.e., radiotherapy) anticancer therapies (29). As such, the application of exercise to improve the therapeutic index of these treatments (i.e., a therapy sensitizer) may also be plausible. In an initial study, we found that tumor growth (survival) was comparable between mice bearing human breast cancer xenografts receiving exercise in combination with doxorubicin (a common chemotherapeutic agent) or doxorubicin alone (22). Although these results do not support our hypothesis, this study had several cautionary limitations (e.g., use of forced exercise, subcutaneous tumor implantation, no tumor histological analysis) and is by no means conclusive. Investigation of the effects of exercise on primary tumor growth, metastasis, and response to anticancer therapies is the subject of ongoing investigation in our laboratory.
The molecular mechanisms underlying the effects of exercise on tumorigenesis remain to be elucidated. Chronic aerobic training causes favorable adaptations in the organ components that govern cardiorespiratory fitness (i.e., cardiac-pulmonary-vasculature-skeletal muscle axis). These adaptations are mediated by changes in numerous gene-expression pathways across multiple different systems, including metabolism, immune surveillance, and inflammation, etc. Of relevance to this experiment, exercise also improves the negative consequences associated with a high-fat diet (i.e., insulin resistance, weight gain, etc.). Together, these adaptations, in turn, induce favorable systemic (whole body) changes in the host (2). Given that the organ location of most malignancies, other than lung cancer and possibly sarcomas, are not directly involved in the exercise response, it appears reasonable to suggest that these systemic (secondary) effects are primarily responsible for our observed effects of exercise-induced increases in intratumoral vascularization. For example, exercise is a major modulator of whole body (systemic) metabolism. The skeletal muscle accounts for ∼80% of glucose disposal under insulin-stimulated conditions while glucose uptake can increase 20- to 100-fold during exercise (34). As a consequence, exercise causes significant changes in metabolic hormone concentrations (e.g., insulin, insulin-like growth factors), which, in turn, would be expected to preferentially influence growth of tumor cells that that are highly reliant on glycolysis (23). A whole body change in substrate availability may either inhibit tumor growth or paradoxically indirectly stimulate a metabolic and/or angiogenic switch in tumor cells because of exercise-induced tumor “metabolic energy crises,” leading to increased expression of VEGF and increased vascularization.
In concert with changes in metabolism, exercise is also a potent modulator of systemic angiogenesis. Muscle cells are exquisitely sensitive to changes in whole body and intracellular O2 availability. During strenuous exercise, O2 delivery may be unable to meet mitochondrial O2 demand. The mismatch activates several highly preserved adaptive gene pathways, including a potent angiogenic response centrally mediated via VEGF which acts locally to augment the pathways for O2 delivery (1). Of relevance, VEGF is also released systemically from skeletal muscle, as well as from the vascular endothelium via shear stress, where it exerts further effects, including the mobilization of bone marrow-derived cells (24). These responses significantly contribute to the cardiovascular benefits of exercise but could, in theory, also contribute to pathological tumor angiogenesis (6) and our observed improvements in intratumoral perfusion/vascularization. The significant correlation between intratumoral VEGF levels and blood perfusion in the exercise group but not the sedentary control group partially supports this notion. Unfortunately, we did not assess changes in systemic metabolic hormones or angiogenic factors in this study. Such measurements should be included in future studies. Clearly, the molecular mechanisms underlying the exercise-tumor-genesis relationship are complex, and proposed explanations remain speculative.
In summary, aerobic exercise can significantly increase intratumoral vascularization, leading to “normalization” of the tissue microenvironment in human breast xenografts implanted in animals fed a high-fat diet. Although the underlying molecular mechanisms are not known, such findings may have important implications for inhibiting tumor metastasis and improving the efficacy of conventional cancer therapies. Mechanistically based preclinical research is now required to gain further understanding of the effects and underlying molecular mechanisms of exercise on tumor-genesis as well as the interaction with anticancer therapy to guide hypothesis-driven clinical trials.
GRANTS
This study was supported, in part, with funds from the Tug McGraw Research Center (L. W. Jones) and National Institutes of Health Grant CA 40355 (M. W. Dewhirst)
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451: 1008–1012, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Booth FW, Lees SJ. Fundamental questions about genes, inactivity, and chronic diseases. Physiol Genomics 28: 146–157, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Brown JK, Byers T, Doyle C, Coumeya KS, Demark-Wahnefried W, Kushi LH, McTieman A, Rock CL, Aziz N, Bloch AS, Eldridge B, Hamilton K, Katzin C, Koonce A, Main J, Mobley C, Morra ME, Pierce MS, Sawyer KA. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin 53: 268–291, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Campbell KL, McTiernan A, Li SS, Sorensen BE, Yasui Y, Lampe JW, King IB, Ulrich CM, Rudolph RE, Irwin ML, Surawicz C, Ayub K, Potter JD, Lampe PD. Effect of a 12-month exercise intervention on the apoptotic regulating proteins Bax and Bcl-2 in colon crypts: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev 16: 1767–1774, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Cao Y, Sonveaux P, Liu S, Zhao Y, Mi J, Clary BM, Li CY, Kontos CD, Dewhirst MW. Systemic overexpression of angiopoietin-2 promotes tumor microvessel regression and inhibits angiogenesis and tumor growth. Cancer Res 67: 3835–3844, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Carmeliet P. Angiogenesis in health and disease. Nat Med 9: 653–660, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Cummings SR, Tice JA, Bauer S, Browner WS, Cuzick J, Ziv E, Vogel V, Shepherd J, Vachon C, Smith-Bindman R, Kerlikowske K. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst 101: 384–398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davidson SR, Burnett M, Hoffman-Goetz L. Training effects in mice after long-term voluntary exercise. Med Sci Sports Exerc 38: 250–255, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr 132: 3456S–3464S, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Gillette CA, Zhu Z, Westerlind KC, Melby CL, Wolfe P, Thompson HJ. Energy availability and mammary carcinogenesis: effects of calorie restriction and exercise. Carcinogenesis 18: 1183–1188, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 100: 57–70, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Harriss DJ, Atkinson G, Batterham A, George K, Cable NT, Reilly T, Haboubi N, Renehan AG. Lifestyle factors and colorectal cancer risk (2): a systematic review and meta-analysis of associations with leisure-time physical activity. Colorectal Dis 11: 689–701, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Hawkins VN, Foster-Schubert K, Chubak J, Sorensen B, Ulrich CM, Stancyzk FZ, Plymate S, Stanford J, White E, Potter JD, McTiernan A. Effect of exercise on serum sex hormones in men: a 12-month randomized clinical trial. Med Sci Sports Exerc 40: 223–233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, Baron JA, Egan KM, Willett WC. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev 17: 379–386, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA 293: 2479–2486, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, Baumgartner RN, Baumgartner KB, Bernstein L. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol 26: 3958–3964, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307: 58–62, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res 68: 5492–5499, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang W, Zhu Z, Thompson HJ. Effect of energy restriction on cell cycle machinery in 1-methyl-1-nitrosourea-induced mammary carcinomas in rats. Cancer Res 63: 1228–1234, 2003 [PubMed] [Google Scholar]
- 20. Jiang W, Zhu Z, Thompson HJ. Modulation of the activities of AMP-activated protein kinase, protein kinase B, and mammalian target of rapamycin by limiting energy availability with 2-deoxyglucose. Mol Carcinog 47: 616–628, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Jones LW, Demark-Wahnefried W. Diet, exercise, and complementary therapies after primary treatment for cancer. Lancet Oncol 7: 1017–1026, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Jones LW, Eves ND, Courneya KS, Chiu BK, Baracos VE, Hanson J, Johnson L, Mackey JR. Effects of exercise training on antitumor efficacy of doxorubicin in MDA-MB-231 breast cancer xenografts. Clin Cancer Res 11: 6695–6698, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell 13: 472–482, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 109: 220–226, 2004 [DOI] [PubMed] [Google Scholar]
- 25. MacNeil B, Hoffman-Goetz L. Effect of exercise on natural cytotoxicity and pulmonary tumor metastases in mice. Med Sci Sports Exerc 25: 922–928, 1993 [PubMed] [Google Scholar]
- 26. MacNeil B, Hoffman-Goetz L. Exercise training and tumour metastasis in mice: influence of time of exercise onset. Anticancer Res 13: 2085–2088, 1993 [PubMed] [Google Scholar]
- 27. Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, De Smet F, Vinckier S, Aragones J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, Carmeliet P. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136: 839–851, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, Schilsky RL, Fuchs CS. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 24: 3535–3541, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer 6: 583–592, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, Dewhirst MW. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell 8: 99–110, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Mueller-Klieser W, Walenta S. Geographical mapping of metabolites in biological tissue with quantitative bioluminescence and single photon imaging. Histochem J 25: 407–420, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Murphy EA, Davis JM, Brown AS, Carmichael MD, Mayer EP, Ghaffar A. Effects of moderate exercise and oat beta-glucan on lung tumor metastases and macrophage antitumor cytotoxicity. J Appl Physiol 97: 955–959, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Ponce AM, Viglianti BL, Yu D, Yarmolenko PS, Michelich CR, Woo J, Bally MB, Dewhirst MW. Magnetic resonance imaging of temperature-sensitive liposome release: drug dose painting and antitumor effects. J Natl Cancer Inst 99: 53–63, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Ryder JW, Chibalin AV, Zierath JR. Intracellular mechanisms underlying increases in glucose uptake in response to insulin or exercise in skeletal muscle. Acta Physiol Scand 171: 249–257, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Thompson HJ, McGinley JN, Spoelstra NS, Jiang W, Zhu Z, Wolfe P. Effect of dietary energy restriction on vascular density during mammary carcinogenesis. Cancer Res 64: 5643–5650, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Woods JA, Davis JM, Kohut ML, Ghaffar A, Mayer EP, Pate RR. Effects of exercise on the immune response to cancer. Med Sci Sports Exerc 26: 1109–1115, 1994 [PubMed] [Google Scholar]
- 37. Yang FC, Ingram DA, Chen S, Zhu Y, Yuan J, Li X, Yang X, Knowles S, Horn W, Li Y, Zhang S, Yang Y, Vakili ST, Yu M, Burns D, Robertson K, Hutchins G, Parada LF, Clapp DW. Nf1-dependent tumors require a microenvironment containing Nf1+/−- and c-kit-dependent bone marrow. Cell 135: 437–448, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413: 131–138, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Zhu Z, Jiang W, Sells JL, Neil ES, McGinley JN, Thompson HJ. Effect of nonmotorized wheel running on mammary carcinogenesis: circulating biomarkers, cellular processes, and molecular mechanisms in rats. Cancer Epidemiol Biomarkers Prev 17: 1920–1929, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zielinski MR, Muenchow M, Wallig MA, Horn PL, Woods JA. Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. J Appl Physiol 96: 2249–2256, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


