Abstract
We hypothesized that episodic hypoxia (EH) leads to alterations in chemoreflex characteristics that might promote the development of central apnea in sleeping humans. We used nasal noninvasive positive pressure mechanical ventilation to induce hypocapnic central apnea in 11 healthy participants during stable nonrapid eye movement sleep before and after an exposure to EH, which consisted of fifteen 1-min episodes of isocapnic hypoxia (mean O2 saturation/episode: 87.0 ± 0.5%). The apneic threshold (AT) was defined as the absolute measured end-tidal Pco2 (PetCO2) demarcating the central apnea. The difference between the AT and baseline PetCO2 measured immediately before the onset of mechanical ventilation was defined as the CO2 reserve. The change in minute ventilation (V̇I) for a change in PetCO2 (ΔV̇I/ ΔPetCO2) was defined as the hypocapnic ventilatory response. We studied the eupneic PetCO2, AT PetCO2, CO2 reserve, and hypocapnic ventilatory response before and after the exposure to EH. We also measured the hypoxic ventilatory response, defined as the change in V̇I for a corresponding change in arterial O2 saturation (ΔV̇I/ΔSaO2) during the EH trials. V̇I increased from 6.2 ± 0.4 l/min during the pre-EH control to 7.9 ± 0.5 l/min during EH and remained elevated at 6.7 ± 0.4 l/min the during post-EH recovery period (P < 0.05), indicative of long-term facilitation. The AT was unchanged after EH, but the CO2 reserve declined significantly from −3.1 ± 0.5 mmHg pre-EH to −2.3 ± 0.4 mmHg post-EH (P < 0.001). In the post-EH recovery period, ΔV̇I/ΔPetCO2 was higher compared with the baseline (3.3 ± 0.6 vs. 1.8 ± 0.3 l·min−1·mmHg−1, P < 0.001), indicative of an increased hypocapnic ventilatory response. However, there was no significant change in the hypoxic ventilatory response (ΔV̇I/ΔSaO2) during the EH period itself. In conclusion, despite the presence of ventilatory long-term facilitation, the increase in the hypocapnic ventilatory response after the exposure to EH induced a significant decrease in the CO2 reserve. This form of respiratory plasticity may destabilize breathing and promote central apneas.
Keywords: long-term facilitation, chemoresponse, nonrapid eye movement sleep
acute exposure to episodic hypoxia (EH) elicits a sustained increase in ventilatory motor output that lasts for up to 90 min after the termination of hypoxia, which is referred to as long-term facilitation (LTF) (12, 27). The occurrence and manifestations of LTF are influenced by the prevailing end-tidal Pco2 (PetCO2) level, experimental paradigm, animal species, and baseline upper airway mechanics (1, 4–6, 10–12, 15, 17–21, 27, 34, 36, 37, 41, 48, 57). Manifestations of LTF in sleeping humans include increased minute ventilation (V̇I; i.e., ventilatory LTF) (4), decreased inspiratory upper airway resistance (1, 4, 5, 7, 48), and increased genioglossus electromyographic (EMG) activity (i.e., upper airway LTF) (15). Ventilatory LTF during sleep may have a role in stabilizing breathing by reducing plant gain, whereby a greater change in ventilation is required to elicit a reduction in Pco2. Thus, the development of hypocapnia-induced apnea during sleep is less likely (16, 38). Likewise, upper airway muscle LTF might promote breathing stability during sleep by enhancing upper airway patency (1, 45).
However, as recently hypothesized (32), an exposure to acute EH may lead to alterations in chemoreflex characteristics that could potentially counteract the stabilizing impact that ventilatory and upper airway muscle LTF have on breathing during sleep. More specifically, a number of studies (20, 31, 38, 40) in both animals and humans during wakefulness have indicated that chemoreflex sensitivity is enhanced after an exposure to EH. Enhancement of chemoreflex sensitivity could lead to the development of hypocapnia and, ultimately, central apnea (38). In addition to the induction of apnea, hypocapnia could render upper airway muscle LTF ineffective (32), which in due course could lead to the development of an obstructive event (8, 46). Likewise, it is possible that an exposure to EH could decrease the CO2 reserve, which also increases the incidence of an apnea.
Despite these possibilities, no studies to date have ascertained the effect of EH on the susceptibility to develop hypocapnic central apnea in healthy humans during sleep. We hypothesized that an exposure to EH would increase the hypocapnic ventilatory response and decreases the CO2 reserve despite the presence of ventilatory LTF.
METHODS
Participants
The Human Investigation Committees of Wayne State University School of Medicine and Detroit Veterans Affairs Medical Center approved the experimental protocols. Informed written consent was obtained from 11 healthy participants free of daytime sleepiness, sleep-disordered breathing (i.e., apnea-hypopnea index of <5/h), or other medical disorders.
Breathing Circuit
Each participant was connected to the breathing circuit via a nasal mask. An appropriately sized, airtight silicone nasal mask (Respironics, Murrysville, PA) was glued to the participant's face to prevent mask leaks. The mask was connected to a plateau exhalation valve (Respironics) via a heated pneumotachometer. The valve, which provides a continuous leak path in the breathing circuit and serves as an exhaust vent, was connected to the inspiratory line. Participants were restricted to nasal breathing by placing tape over the mouth. During the mechanical ventilation (MV) protocol (see below), hyperventilation was achieved using a pressure support ventilator (Quantum PSV, Healthdyne Technologies, Marietta, GA) (44, 63). During the EH Protocol (see below), two cylinders containing 100% N2 or 100% O2 were connected to the inspiratory line. To maintain isocapnia, supplemental CO2 (fraction of inspired CO2: 0.07, balanced with N2) (41) was added to the inspiratory line from an external source to maintain PetCO2 at or near control levels.
Measurements
Electroencephalograms (EEGs), electrooculograms (EOGs), and chin EMGs were recorded using the international 10-20 system of electrode placement (EEG: C3-A2 and C4-A1; EOG: O-A2). Inspiratory airflow was measured by a heated pneumotachometer (model 3700A, Hans Rudolph, Kansas City, MO) attached to a pressure transducer (Validyne, Northridge, CA). The tidal volume (VT) was obtained from the electronic integration of the flow signal (model FV156 Integrator, Validyne). To confirm the central etiology of apnea and to ascertain upper airway mechanics, supraglottic pressure was measured using a pressure transducer-tipped catheter (model TC-500XG, Millar Instruments, Houston, TX) with the tip positioned in the hypopharynx. The hypopharyngeal position was obtained by advancing the catheter tip for 2 cm after it disappeared behind the tongue. PetCO2 readings were obtained continuously by an infrared analyzer (model CD-3A, AEI Technologies, Pittsburgh, PA) from tubing placed in the nares via a port in the nasal mask. Arterial O2 saturation (SaO2) was measured by a pulse oximeter (Biox 3700, Ohmeda). Signals were displayed on a polygraph recorder (Grass model 15, Astro-Med, West Warwick, RI) and recorded using Powerlab data-acquisition software (AD Instruments, Colorado Springs, CO) for detailed analysis.
Experimental Protocols
Overview.
The study was conducted during normal nocturnal sleep. Study participants were instructed to limit total sleep time to a maximum of 5 h on the night before the study. A screening polysomnography was performed on night 1 to confirm the absence of sleep apnea. The experimental procedure was performed on night 2 (EH) and night 3 (sham hypoxia). Participants assumed the supine position for the entire experimental protocol, which was conducted during stable nonrapid eye movement (NREM) sleep; hence, all trials were conducted while participants were in stable stage 2 or stage 3 sleep. Eight participants agreed to return for the sham protocols on night 3. The experimental protocol was conducted in three phases during NREM sleep, which included a determination of the apneic threshold (AT) before and after the exposure to EH or a comparable duration of room air representing sham hypoxia.
MV protocol: prehypoxia.
A representative polygraph segment obtained from a participant in stage N2 sleep before, during, and after the MV trial is shown in Fig. 1. We used nasal noninvasive positive pressure mechanical ventilation to produce hyperventilation to determine the AT before and after the exposure to EH. MV was applied for 3 min as previously described (44, 45, 63) in the spontaneous-timed mode. In this mode, a backup respiratory rate is preset; timed breaths are delivered if the participant‘s respiratory rate falls below the set rate. The ventilator respiratory rate was set at 6–8 breaths/min, which was below the participant’s eupneic rate, to prevent neuromechanical inhibition of the ventilatory motor output. During MV, the inspiratory positive airway pressure was increased gradually in 1- to 2-cmH2O increments starting from 2 cmH2O at the beginning of each MV trial while keeping the expiratory positive airway pressure fixed at 2 cmH2O throughout the MV. MV was terminated after 3 min during expiration by returning the inspiratory positive airway pressure to the baseline expiratory positive airway pressure of 2 cmH2O. The ensuing hypocapnia resulted in either a hypopnea or central apnea. If an apnea was not induced, additional hyperventilation trials were completed until an apnea was evident. Central apnea was defined as an expiratory time ≥ 5 s. After the MV protocol, each participant was switched to spontaneous room air breathing for 15 min. Subsequently, the participants were exposed to EH.
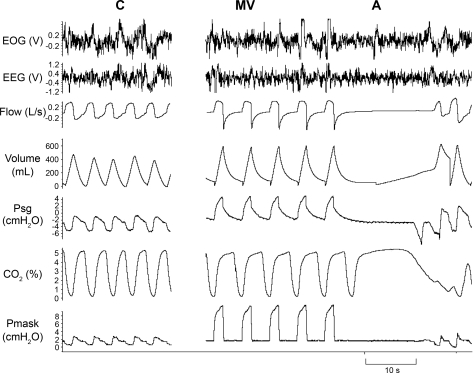
Fig. 1.
Representative polygraph segment from a subject during stable nonrapid eye movement (NREM) sleep at different time points: room air control condition (C) and mechanical ventilation (MV) leading to central apnea (A) after the cessation of MV. Note the 10-s central apnea [absence of respiratory effort on the supraglottic pressure (PSG) tracing]. EOG, electrooculogram; EEG, electroencephalogram; Pmask: mask pressure.
EH protocol.
After the completion of the MV protocol, the participants breathed room air for a 5-min control period followed by 15 episodes of 1-min isocapnic hypoxia separated by room air breathing for 1–2 min (41). Hypoxia was induced rapidly by adding 100% N2 for four to six breaths to the breathing circuit to produce a hypoxic mixture. Supplemental 7% CO2 was added to the circuit to maintain isocapnia (Fig. 2). The administration of 100% N2 was terminated when the oxyhemoglobin saturation decreased to 89%; thereafter, the O2 saturation declined spontaneously an additional 3–6% in the milieu of the hypoxic gas mixture in the circuit. Hypoxia was terminated abruptly at the end of 1 min with two breaths of 100% O2. Once the O2 saturation had returned to baseline levels, a short recovery period in room air followed before the next hypoxic episode (41). The number of acceptable EH episodes with a stable sleep stage was 11.8 ± 0.7 hypoxic episodes/subject, with a duration of 63.6 ± 4.3 s and separated by room air breathing during a recovery period of 110.1 ± 4.2 s.
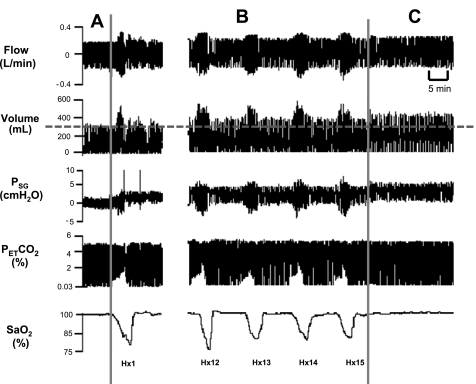
Fig. 2.
Representative compressed polygraph segment from a subject showing the hypoxia protocol, including the control room air period (A), the beginning (Hx1) and end of the hypoxia episodes (Hx12–Hx15) (B), and the recovery period (C). Note that isocapnia was maintained during the hypoxia episodes. Also note that the tidal volume (VT) was elevated during each hypoxia episode and remained elevated during recovery. The dotted line represents the control VT. PetCO2, end-tidal Pco2; SaO2, arterial O2 saturation.
MV protocol: posthypoxia.
After the 15th hypoxic episode, breathing was monitored for 10 min of recovery (i.e., recovery period) in room air. Thereafter, the participant was returned to an expiratory positive airway pressure of 2 cmH2O, and the MV protocol was repeated using a similar protocol as described above in MV protocol: prehypoxia. This portion of the protocol was completed within 60 min of the last hypoxic exposure while stable NREM sleep was maintained, since other forms of respiratory plasticity have reportedly persisted for this length of time after EH (6, 21, 57). The MV trials that produced apnea were repeated once to ensure repeatability of the determination of the AT.
Sham protocol.
To ensure that the changes during the recovery period were not due to time-dependent phenomena independent of EH, eight of the subjects underwent a sham protocol on a different night with identical measurements but without the hypoxia intervention (night 3). The remaining three subjects were unavailable for the sham protocol. The sham protocol involved switching of gases except that the composition was room air instead of the hypoxia mixture. Overall, the flow of room air was similar to the flow of gases during the hypoxia runs.
Data Analysis
MV protocols: pre- and post-EH.
Sleep staging (43) and scoring of arousals (56) were completed using standard criteria, analyzing trials with stable NREM sleep, as confirmed by a blinded observer. During the control period, five breaths recorded immediately before the onset of the MV were averaged. Likewise, during the MV period, the last five mechanically ventilated breaths before the return to baseline expiratory positive airway pressure were averaged (Fig. 1). The data analysis methodology has been previously described (44, 45, 63). The AT was defined as the PetCO2 that demarcated the central apnea closest to the eupneic PetCO2. The CO2 reserve was defined as the change in PetCO2 (ΔPetCO2) between eupneic PetCO2 (control) and AT. The “hypocapnic ventilatory response” was defined as the change in V̇I between control and a hypopnea or an apnea divided by ΔPETCO2 (ΔV̇I/ΔPetCO2 relationship), i.e., this is the slope of the ventilatory response (see Fig. 6). The hypocapnic ventilatory response was calculated for each hypopnea trial and the apnea trial with the smallest CO2 reserve and then averaged for each individual; the values for each individual subject were used for the analysis.
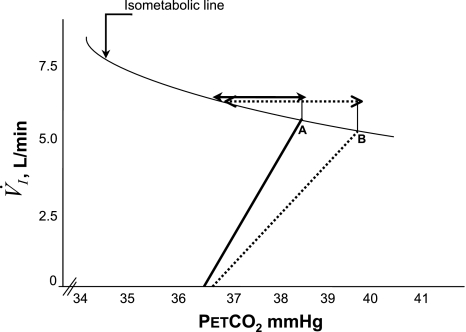
Fig. 6.
Diagramatic representation of the relationship between V̇I and PetCO2 along the isometabolic curve. A steeper slope post-EH (solid line) compared with the pre-EH exposure (dotted line) indicates a higher hyocapnic ventilatory chemoresponse post-EH. An increase in the slope decreased the magnitude of the CO2 reserve below eupnea post-EH (solid arrow) versus the CO2 reserve pre-EH (dotted arrow), thereby increasing the susceptibility to the development of apnea, despite hyperventilation and the reduced eupneic PetCO2, indicative of reduced plant gain (point A vs. point B) (see text for further explanation).
EH protocol.
Data recorded during the exposure to EH were used for analysis only if they were measured during stable NREM sleep. Inspired VT, inspiration time, total breath time, breathing frequency, V̇I, PetCO2, and SaO2 were calculated breath by breath. For each variable, an average value was computed during a control period, during each hypoxic episode, and during the recovery periods that followed each hypoxic episode. Average values during the control period were determined by averaging the data obtained from 20 breaths recorded immediately before the onset of the first hypoxic episode. This analysis has also been described previously (41). During each hypoxic episode, the variables outlined above were averaged using 11.5 ± 1.0 breaths recorded; the number of breaths analyzed depended on the duration of hypoxia during a given hypoxic episode. Similarly, average values for the variables were obtained using the last five breaths recorded during each normoxic period that preceded each hypoxic episode. Finally, an average value for each variable was obtained from 10 consecutive breaths after 10 min of recovery from the last hypoxic episode.
Additionally, we measured the hypoxic ventilatory response (HVR). This was defined as the change in V̇I for a change in SaO2 during a given hypoxic episode (55). The ventilation during a given hypoxic episode was compared with the room air control period immediately preceding the hypoxic period (ΔV̇I/ΔSaO2) for each hypoxic episode. HVR was determined during the initial five breaths of the hypoxia episode [acute HVR (AHVR)], representing the immediate effect of hypoxia, and during the nadir O2 desaturation period at the end of the trial (HVRtot), representing the net hypoxic effect.
Sham protocol.
During the sham protocol, the AT, CO2 reserve, V̇I, and hypocapnic ventilatory response were obtained before and after participants breathed room air for 30 min.
Statistical Analysis
Results are presented as means ± SE unless otherwise specified. A commercially available computer statistical package was used to analyze the data (SigmaStat 3.11.0, SPSS). The level of statistical significance was set at P ≤ 0.05.
MV protocols.
For normally distributed data, paired t-tests were performed to compare the eupneic V̇I, eupneic PetCO2, AT, and CO2 reserve recorded during the MV protocols completed before and after EH. For non-normally distributed data [hypocapnic ventilatory response (ΔV̇I/ΔPetCO2)], the Wilcoxon signed-rank test was used.
EH protocol.
For normally distributed data, comparisons between time points for control, hypoxia, and recovery were made using one-way ANOVA with repeated measures followed by a post hoc analysis for all pair-wise comparisons using the Holm-Sidak method. When the normality test failed (for inspiration time, expiratory time, SaO2, and upper airway resistance), then ANOVA on ranks was performed followed by pairwise multiple-comparison tests again using the Holm-Sidak method. AHVR values from the first three hypoxic episodes were compared with the final three hypoxic episodes using one-way repeated-measures ANOVA. Similarly, HVRtot values of the first three hypoxic trials versus the last three hypoxic trials were compared.
Sham protocol.
For the eight subjects who underwent both the experimental and sham protocols, paired t-tests were performed to compare the eupneic PetCO2, AT, and eupneic V̇I between the pre- and post-sham MV protocols. To evaluate for the effect of timing in the two groups, the CO2 reserve and hypocapnic ventilatory response were analyzed using repeated-measures ANOVA with two factors: 1) timing (pre vs. post) × 2) protocol (experimental vs. sham) followed by a post hoc analysis for all pairwise comparisons using the Holm-Sidak method. Specifically, for the hypocapnic ventilatory response, the two-way repeated-measures ANOVA was done after log transformation, as the values were not normally distributed.
RESULTS
Participant characteristics and the apnea/hypopnea index from their baseline polysomnography experiments are shown in Table 1. All participants were healthy and without sleep-disordered breathing. Results from the EH protocol are shown initially followed by the results obtained from the MV protocols completed before and after the EH and, finally, from the sham protocol.
Table 1.
Subject characteristics
| Variable | |
|---|---|
| Age, yr | 26.1 ± 4.2 |
| Sex | 4 men/7 women |
| Body mass index, kg/m2 | 22.1 ± 2.0 |
| Apnea-hypopnea index, number/h | 0.7 ± 1.4 |
Values are means ± SD; n = 11 subjects.
EH
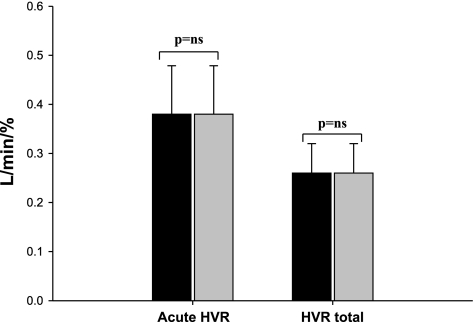
Ventilatory and timing data are shown in Table 2. The oxyhemoglobin saturation decreased to 87.0 ± 0.5% during hypoxia, resulting in increased VT and V̇I compared with the room air control; the remaining measured parameters were unchanged. In addition, a significant increase in VT and V̇I compared with the baseline persisted into the recovery period after the last hypoxic episode; this was associated with a reduction in PetCO2 (Table 2). However, the AHVR was not altered by repeated hypoxic exposure [AHVR: first 3 hypoxia episodes vs. last 3 hypoxia episodes, 0.38 ± 0.1 vs. 0.38 ± 0.09 l·min−1·%−1, P = not significant (NS); and HVRtot: first 3 hypoxia episodes vs. last 3 hypoxia episodes, 0.26 ± 0.06 vs. 0.24 ± 0.04 l·min−1·%−1, P = NS; Fig. 3].
Table 2.
Results of episodic hypoxia presented as grouped data for timing and ventilation during the three periods: room air control, hypoxia, and recovery periods
| Variables | Control | Hypoxia | Recovery |
|---|---|---|---|
| Inspiratory time, s | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.1 |
| Expiratory time, s | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.1 |
| Frequency of breathing, breaths/min | 15.4 ± 0.9 | 16.0 ± 0.9* | 15.3 ± 0.9 |
| End-tidal Pco2, mmHg | 40.1 ± 0.9 | 39.5 ± 0.9 | 38.9 ± 0.9* |
| Arterial O2 saturation, % | 97.7 ± 0.1§ | 87.0 ± 0.5 | 97.9 ± 0.2† |
| Tidal volume, liters | 0.397 ± 0.02 | 0.497 ± 0.03* | 0.446 ± 0.28*† |
| Minute ventilation, l/min | 6.0 ± 0.4 | 7.9 ± 0.5* | 6.7 ± 0.4*† |
| Upper airway resistance, l·s−1·cmH2O−1 | 9.6 ± 3.1 | 6.2 ± 1.3 | 6.5 ± 1.7 |
Values are means ± SE; n = 11 subjects.
P < 0.05 vs. control;
P < 0.05 vs. hypoxia.
Fig. 3.
Acute and net hypoxic ventilatory response (HVR) expressed as the change in minute ventilation (V̇I) for a corresponding change in SaO2 (see text). No significant changes were noted between the first 3 hypoxia episodes (solid bar) and the last 3 hypoxia episodes (shaded bar). NS, not significant.
Pre- and Post-EH AT and CO2 Reserve
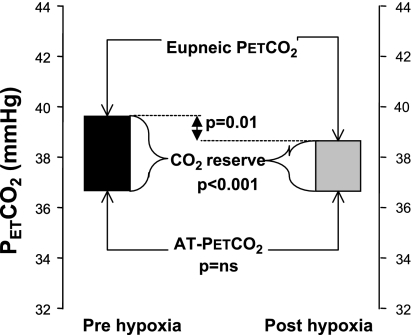
The eupneic V̇I after the exposure to EH was significantly greater compared with measures obtained before EH (6.3 ± 0.3 vs. 6.1 ± 0.3 l/min, P = 0.01). Likewise, the eupneic PetCO2 during the MV protocol after the exposure to EH was significantly less than the eupneic PetCO2 during the MV protocol before EH (38.3 ± 0.9 vs. 39.7 ± 0.9 mmHg, P = 0.01; Fig. 4). The hypocapnic ventilatory response measured during the MV protocol after the exposure to EH was greater than pre-EH values (3.3 ± 0.6 vs. 1.8 ± 0.3 l·min−1·mmHg−1, P < 0.001). This difference remained significant (2.9 ± 0.4 vs. 1.8 ± 0.3 l·min−1·mmHg−1, P < 0.01) even after one outlier subject (who showed the greatest increase) was excluded from the analysis. Subsequently, the CO2 reserve decreased significantly after the exposure to EH relative to pre-EH values (−2.3 ± 0.4 vs. −3.1 ± 0.5 mmHg, P < 0.001; Fig. 4). Notably, the PetCO2 that demarcated the AT was not altered after EH compared with before EH (36.4 ± 0.7 vs. 36.6 ± 0.7 mmHg, P = NS; Fig. 4). In summary, EH resulted in decreased eupneic PetCO2, an increased hypocapnic ventilatory response, and narrowing of the CO2 reserve.
Fig. 4.
Schematic representation of the observed eupneic CO2, apneic threshold (AT) PetCO2, and CO2 reserve (eupneic PetCO2 minus AT PetCO2) during the pre-episodic hypoxia (pre-EH; solid bars) and post-EH (grey bars) periods (n = 11). The top horizontal line represents the eupneic CO2, the bottom horizontal line represents the AT, and boxes between these two lines represent the CO2 reserve [pre-EH (solid box) and post-EH (shaded box)]. The CO2 reserve was significantly smaller post-EH as a result of the significantly lower eupneic PetCO2, without a change in the AT.
Experimental Versus Sham Protocol
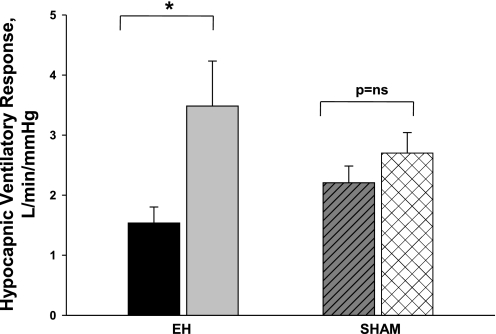
The eight participants who underwent both the experimental and sham protocols were similar to the remaining three nonparticipants in terms of their individual characteristics. There were no significant changes in eupneic control PetCO2 (39.2 ± 0.9 vs. 38.7 ± 0.7 mmHg, P = NS), AT (36.1 ± 1.0 vs. 36.5 ± 1.0 mmHg, P = NS), and eupneic V̇I (5.8 ± 0.2 vs. 5.6 ± 0.2 l/min, P = NS) during MV completed after sham intervention compared with measures obtained before sham intervention. Comparison of the hypocapnic ventilatory response in these eight subjects revealed a significant interaction between the time of intervention (pre vs. post) and the type of intervention (EH vs. sham, P = 0.05), indicating that the change over time in the hypocapnic ventilatory response was not the same for the two levels of the protocol. Likewise, a comparison of the CO2 reserve revealed a significant interaction between the time of intervention (pre vs. post) and the type of intervention (EH vs. sham, P < 0.01). The hypocapnic ventilatory response was significantly higher (P < 0.01; Fig. 5) posthypoxia within the experimental protocol but not within the sham protocol. Additionally, the CO2 reserve was significantly decreased post- versus preintervention within the experimental protocol (−2.1 ± 0.4 vs. −2.9 ± 0.5 mmHg, P < 0.05) but not within the sham protocol (−2.7 ± 0.6 vs. −2.7 ± 0.5 mmHg, P = NS). Thus, the results clearly indicate that the observed reciprocal changes in the hypocapnic ventilatory response and CO2 reserve after EH were due to the EH intervention per se and were not time-dependent phenomena (unchanged results during sham).
Fig. 5.
Comparison of the hypocapnic ventilatory responses under the two conditions: 1) the EH protocol [pre-EH (solid bar) vs. post-EH (shaded bar)] and 2) the sham protocol [before (hatched bar) vs. after (cross-hatched bar) the sham intervention]. The major finding was that the hypocapnic ventilatory response was significantly increased after isocapnic EH but not after the sham protocol (see text for explanation).
DISCUSSION
Summary of Findings
Our study revealed several significant findings after EH during NREM sleep. First, EH was followed by a period of sustained increase in V̇I and a reduction in the eupneic PetCO2, indicative of ventilatory LTF. Second, EH was associated with an increased hypocapnic ventilatory response and narrowing of the CO2 reserve; this was not observed after the sham exposure. Third, the AT did not change after EH. Finally, the increase in the hypocapnic ventilatory response after the exposure to EH was observed even though progressive enhancement of the HVR was not evident during the exposure to EH. To our knowledge, this is the first report of the effect of LTF on the AT in sleeping humans.
Methodological Considerations
Several methodological factors could potentially have influenced our findings. First, sleep state instability may influence the AT and hypocapnic ventilatory response; however, we analyzed trials with a stable sleep state only. In addition, 8 of 11 participants during sleep clearly demonstrated no evidence of a time-dependent increase in ventilatory motor output after sham EH. The lack of change in ventilation after sham EH is consistent with our previous findings (15, 41). Second, our experimental paradigm resembles but does not mimic the myriad of physiological perturbations associated with sleep apnea, including arousals, increased sympathetic nervous system activity, changes in intrathoracic pressures, and increased blood pressure. Additionally, our study is focused on acute EH and does not address the effects of chronic intermittent hypoxia (25). Third, we used moderate sleep curtailment on the night preceding the sleep study to facilitate natural, unaided sleep. We excluded the use of hypnotics, given the potential effects on ventilatory control. Our previous experience demonstrated no differences in the findings between subjects who obtained normal versus curtailed nocturnal sleep. The available literature supports our observations (49). In addition, eight subjects underwent similar sleep curtailment for both the EH and sham nights, so any potential alteration in chemoresponsiveness would be present in both nights and, thus, would not alter our conclusions. Finally, mechanical ventilation may be associated with neuromuscular inhibition. However, such experiments (24) used larger VT than our study, inducing neuromechanical inhibition, even in the absence of hypocapnia, but only when VT was increased to two times the eupneic VT (1.2 liters). Therefore, we doubt that neuromechanical inhibition was responsible for our findings. Indeed, if present, neuromechanical inhibition would have been applicable to both the experimental and sham protocols of the study and would not explain the differential response seen post-EH.
EH, Ventilatory LTF, and the CO2 Reserve
The susceptibility to hypocapnia-induced central apnea is a function of multiple variables including background ventilatory drive, chemoreflex sensitivity, and sleep state (16, 35, 47). Figure 6 shows the resting levels of ventilation and PetCO2 along with the CO2 reserve and slope of the hypocapnic ventilatory chemoresponse before and after EH in our study. The combination of a higher V̇I and lower eupneic PetCO2 after EH would shift the resting Pco2 upward on the isometabolic curve and lead to an increased CO2 reserve. Thus, if all other factors remain constant, increased respiratory motor output produces a reduction in the eupneic PetCO2 and a reduced plant gain (16). Decreased plant gain stabilizes respiration as a greater change in ventilation is required to induce a given change in PetCO2 (decreased ΔPetCO2/ΔV̇I). Accordingly, a decrease in PetCO2 after EH could mitigate the propensity to central apnea and lead to an increased CO2 reserve if the hypocapnic ventilatory response remained unchanged (16, 23). This is the physiological response noted when hyperventilation is elicited by stimulation of the central chemoreceptors with metabolic acidosis or stimulation of the peripheral chemoreceptor with almitrine (38).
Hypocapnic Ventilatory Response
Our study revealed that the ventilatory response to hypocapnia increased after EH (steeper slope), resulting in a closer proximity of the hypocapnic AT to the eupneic PetCO2 (narrowed CO2 reserve, as shown by the solid arrow in Fig. 6) and, therefore, a greater propensity to develop hypocapnic central apnea, despite an increase in the resting ventilation (point A on the graph in Fig. 6). This finding is similar to the decreased CO2 reserve noted after an exposure to hypoxia in a canine model (38) and in patients with congestive heart failure (50, 59). Interestingly, in the canine model (38), continuous hypoxia, not EH, sensitized the ventilatory responsiveness to CO2 below eupnea and narrowed the CO2 reserve. Thus, an increased hypocapnic ventilatory response promotes the development of central apnea because the decrease in ventilation for a given reduction in PetCO2 is enhanced (13, 14, 23). Despite a decrease in plant gain, an increased hypocapnic ventilatory response was noted after EH, thereby increasing the tendency for central apnea.
Mechanisms of Decreased CO2 Reserve After EH
The increase in the hypocapnic ventilatory response after EH might represent an increase in central or peripheral chemoreflex sensitivity. Collectively, the results from previous studies in humans during wakefulness have indicated that peripheral chemoreflex sensitivity is enhanced after exposure to both EH (20, 31, 40) and sustained hypoxia (2, 22, 28, 29, 54). Conversely, studies have also indicated that central chemoreflex sensitivity remains unaltered after relatively short exposures to EH (31) or sustained hypoxia (28, 29).
Lack of peripheral chemoresponsiveness.
Enhancement of the peripheral chemoreflex sensitivity would potentially be reflected by a progressive increase in the HVR from the beginning to the end of the EH protocol (31, 32). However, we noted that no change in the HVR was evident throughout the EH protocol. This finding is in contrast to the increased peripheral chemoreflex sensitivity after EH in healthy subjects during wakefulness (20, 32, 58). Interestingly, this increase was observed 1 h after EH and only when PetCO2 levels were at least 3 mmHg above the ventilatory recruitment threshold (20, 58) and not under hypocapnic conditions. Subsequent findings revealed that the expression of an enhanced HVR during and after exposure to EH under conditions of wakefulness is clearly dependent on the level of CO2 sustained throughout the hypoxic exposure (20, 31, 41, 58). Thus, manifestation of the enhancement is absent under hypo- and isocapnic conditions (20, 31) and only becomes clearly evident when CO2 levels are sustained slightly above baseline values. This has also been noted in animal studies (51–53). Although these findings are limited to the wakefulness condition, it is possible that the absence of an enhanced HVR in the present study was in part related to the absence of hypercapnia throughout the protocol.
Potential contribution of cerebrovascular CO2 reactivity.
Cerebrovascular reactivity and the ventilatory response to arterial Pco2 are tightly linked, so that the regulation of cerebral blood flow has an important role in stabilizing breathing during fluctuating levels of chemical stimuli, including hypoxia and hypercapnia (3). One study (42) demonstrated that the cerebral blood flow response to step changes in CO2 in humans was much faster (6-s delay) than that documented in previous reports. Studies have indicated that the cerebrovascular responsiveness to CO2, primarily via its effects at the level of the central chemoreceptors, is an important determinant of eupneic ventilation and the hypercapnic ventilatory response in otherwise healthy humans during wakefulness (60), sleep (61), and at high altitude (3, 62). Hypoxia per se is a cerebral vasodilator that induces a rise in cerebral blood flow in proportion to the severity of isocapnic hypoxia (62). Conversely, the hypoxia-induced activation of peripheral chemoreceptor activity leads to a hyperventilation-induced lowering of arterial Pco2 and subsequent cerebral vasoconstriction. Specifically, reductions in the normal cerebral vascular response to hypocapnia may increase the susceptibility to apneas and breathing instability during sleep (61). Thus, it is possible that alterations in cerebrovascular reactivity to CO2, specifically a reduced cerebrovascular reactivity rather than an increase in peripheral chemoreflex sensitivity, might be responsible for the observed changes in ventilation in our study, since no significant change in the HVR was observed. In our study, there was an increased responsiveness to CO2, probably via increased chemoreflex sensitivity due to the hypoxia. A change in the hypocapnic ventilatory response may potentially occur without a change in peripheral chemoresponsiveness if cerebral vascular reactivity is decreased, resulting in an increased gradient between cerebral and arterial CO2. Increased central chemoreflex sensitivity is another possible mechanism for the increased hypocapnic ventilatory response. However, evidence for increased central chemoreflex sensitivity after EH is lacking (28, 29, 31).
Other potential mechanisms.
Sensory LTF may be due to enhanced production of ROS after EH. There is no available evidence that acute EH, per se, elicits sensory LTF of the carotid body; however, acute EH does produce phrenic LTF (18, 25). ROS formation is necessary for the induction and/or maintenance of phrenic LTF (26). Acute EH may increase the production of ROS, enhancing excitatory neurotransmission and facilitation of ventilatory plasticity (an indicator of phrenic LTF). Whether ROS played a role in the increased hypocapnic ventilatory response after acute EH in our protocol is unconfirmed at this time. Additionally, enhanced ventilatory chemoreflex sensitivity could be a result of sensory LTF that manifests as a long-lasting activation of sensory discharge from the carotid bodies, activated by acute EH after preconditioning with chronic intermittent hypoxia (25, 26). However, our model did not address chronic intermittent hypoxia. Finally, studies have identified regions of the caudal hypothalamus and rostral ventrolateral medulla, in addition to the pons, that are directly excited by hypoxia (39) and that, when activated, increase sympathetic and respiratory activity; enhanced chemoreflex sensitivity may occur at the level of the integration of the afferent output at these central nervous system sites via oxygen-sensing neurons and the subsequent translation of chemoreceptor afferent information to appropriate ventilatory changes (11). All of the above are potential explanatory mechanisms; however, our protocol and findings do not permit us to definitively identify a specific mechanism to explain the increased chemoreflex sensitivity after EH.
Physiological Significance
The aforementioned findings have significant clinical implications regarding the pathophysiology of central apnea. Increasing the hypocapnic ventilatory response and narrowing the CO2 reserve in the aftermath of EH renders the ventilatory control system more susceptible to recurrent central apnea and may perpetuate instability, even after the initial perturbation is removed. Likewise, this susceptibility to apnea may be evident in the presence of ventilatory LTF, indicating that LTF may be a marker for ventilatory instability after EH. Therefore, enhanced susceptibility to central apnea after EH may contribute to increased apnea severity across the night in patients with sleep apnea. We believe that the increased hypocapnic ventilatory response and the concomitant decrease in the CO2 reserve observed in the present study were consequences of the exposure to EH, since these did not change significantly after the sham intervention. There is evidence that LTF may be mediated via serotonergic receptors (6, 25, 26), N-methyl-d-aspartate receptors (33), or ROS (26), at least in animal models, and these may provide potential targets for pharmacological manipulations. Our study allowed us to clarify the role of LTF on breathing stability in humans during sleep. Elucidation of the components and mechanisms of LTF in humans during sleep potentially provides an incremental understanding toward the development of pharmacological therapies for sleep apnea.
In summary, our findings confirm our hypothesis: that EH in sleeping humans promotes breathing instability and the development of central apnea by narrowing the CO2 reserve. While ventilatory LTF, per se, may mitigate central apnea by decreasing plant gain, the increased ventilatory chemoreflex sensitivity offsets the protective effect.
GRANTS
This work was supported by the Department of Veterans Affairs and the National Heart, Lung, and Blood Institute.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGEMENTS
The authors thank Dr. Sukanya Pranathiageswaran for the technical assistance provided.
REFERENCES
- 1. Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol 91: 2751–2757, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Ainslie PN, Kolb JC, Ide K, Poulin MJ. Effects of five nights of normobaric hypoxia on the ventilatory responses to acute hypoxia and hypercapnia. Respir Physiol Neurobiol 138: 193–204, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex. Control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep 21: 709–716, 1998 [PubMed] [Google Scholar]
- 5. Babcock M, Shkoukani M, Aboubakr SE, Badr MS. Determinants of long-term facilitation in humans during NREM sleep. J Appl Physiol 94: 53–59, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Badr MS, Skatrud Dempsey JB. J. Determinants of poststimulus potentiation in humans during NREM sleep. J Appl Physiol 73: 1958–1971, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 78: 1806–1815, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529: 215–219, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Behan M, Zabka AG, Mitchell GS. Age and gender effects on serotonin-dependent plasticity in respiratory motor control. Respir Physiol Neurobiol 131: 65–77, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Bavis RW, Mitchell GS. Intermittent hypoxia induces phrenic long-term facilitation in carotid-denervated rats. J Appl Physiol 94: 399–409, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Cao KY, BerthonJones M, Zwillich CW, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dog. J Appl Physiol 73: 2083–2088, 1992 [DOI] [PubMed] [Google Scholar]
- 13. Cherniack NS, Edelman NH, Lahiri S. Hypoxia and hypercapnia as respiratory stimulants and depressants. Respir Physiol 11: 113–126, 1970 [DOI] [PubMed] [Google Scholar]
- 14. Cherniack NS, Longobardo GS. Cheyne-Stokes breathing. An instability in physiologic control. N Engl J Med 288: 952–957, 1973 [DOI] [PubMed] [Google Scholar]
- 15. Chowdhuri S, Pierchala L, Aboubakr SE, Shkoukani M, Badr MS. Long-term facilitation of genioglossus activity is present in normal humans during NREM sleep. Respir Physiol Neurobiol 160: 65–75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol 90: 13–24, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol 477: 469–479, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fuller D, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol 121: 135–146, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Fuller D. Episodic hypoxia induces long-term facilitation of neural drive to tongue protrudor and retractor muscles. J Appl Physiol 98: 1761–1767, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol 291: R1111–R1119, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebrate rats. Am J Physiol Regul Integr Comp Physiol 265: R811–R819, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Howard LS, Robbins PA. Alterations in respiratory control during 8 h of isocapnic and poikilocapnic hypoxia in humans. J Appl Physiol 78: 1098–1107, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Khoo MCK, Gottschalk A, Pack AI. Sleep-induced breathing and apnoea: a theoretical study. J Appl Physiol 70: 2014–2024, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Leevers AM, Simon PM, Dempsey JA. Apnea after normocapnic mechanical ventilation during NREM sleep. J Appl Physiol 77: 2079–2085, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21: 5381–5388, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MacFarlane PM, Wilkerson JE, Lovett-Barr MR, Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol 164: 263–271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maltais F, Dinh L, Cormier Y, Series F. Changes in upper airway resistance during progressive normocapnic hypoxia in normal men. J Appl Physiol 70: 548–553, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Mahamed S, Cunningham DA, Duffin J. Changes in respiratory control after three hours of isocapnic hypoxia in humans. J Physiol 547: 271–281, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mahamed S, Duffin J. Repeated hypoxic exposures change respiratory chemoreflex control in humans. J Physiol 534: 595–603, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mateika JH, Fregosi RH. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol 82: 419–425, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Mateika JH, Mendello C, Obeid D, Badr MS. Peripheral chemoreflex responsiveness is increased at elevated levels of carbon dioxide after episodic hypoxia in awake humans. J Appl Physiol 96: 1197–1205, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp Physiol 94: 279–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McGuire M, Zhang Y, White DP, Ling L. Phrenic long-term facilitation requires NMDA receptors in the phrenic motonucleus in rats. J Physiol 567: 599–611, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McKay LC, Janczewski WA, Feldman JL. Episodic hypoxia evokes long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol 557: 13–18, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meza S, Mendez M, Ostrowski M, Younes M. Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol 85: 1929–1940, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Millhorn DE, Eldridge FL, Waldro TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol 41: 87–103, 1980 [DOI] [PubMed] [Google Scholar]
- 37. Morris KF, Gozal D. Persistent respiratory changes following intermittent hypoxic stimulation in cats and human beings. Respir Physiol Neurobiol 140: 1–8, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med 165: 1251–1260, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Neubauer JA, Sunderram J. Oxygen-sensing neurons in the central nervous system. J Appl Physiol 96: 367–374, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J Appl Physiol 97: 2020–2025, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respir Physiol Neurobiol 160: 259–266, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Poulin MJ, Liang PJ, Robbins PA. Fast and slow components of cerebral blood flow response to step decreases in end-tidal Pco2 in humans. J Appl Physiol 85: 388–397, 1998. [DOI] [PubMed] [Google Scholar]
- 43. Rechtschaffen A, Kales A. Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: National Institute of Neurological Disease and Blindness, 1968 [Google Scholar]
- 44.Rowley JA, Zhou XS, Diamond MP, Badr MS. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep 29: 95–103, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Rowley JA, Deebajah I, Parikh S, Najar A, Saha R, Badr MS. The influence of episodic hypoxia on upper airway collapsibility in subjects with obstructive sleep apnea. J Appl Physiol 103: 911–916, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Sankri-Tarbichi AG, Rowley JA, Badr MS. Expiratory pharyngeal narrowing during central hypocapnic hypopnea. Am J Respir Crit Care Med 179: 313–319, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Skatrud JB, Dempsey JA. Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. J Appl Physiol 55: 813–822, 1983 [DOI] [PubMed] [Google Scholar]
- 48. Shkoukani M, Babcock MA, Badr MS. Effect of episodic hypoxia on upper airway mechanics in humans during NREM sleep. J Appl Physiol 92: 2565–2570, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Spengler CM, Shea SA. Sleep deprivation per se does not decrease the hypercapnic ventilatory response in humans. Am J Respir Crit Care Med 161: 1124–1128, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Solin P, Roebuck T, Johns DP, Haydn Walters E, Naughton MT. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med 162: 2194–2200, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Smith CA, Bisgard GE, Nielsen AM, Daristotle L, Kressin NA, Forster HV, Dempsey JA. Carotid bodies are required for ventilatory acclimatization to chronic hypoxia. J Appl Physiol 60: 1003–1010, 1986 [DOI] [PubMed] [Google Scholar]
- 52. Smith CA, Harms CA, Henderson KS, Dempsey JA. Ventilatory effects of specific carotid body hypoxia and hypocapnia in the awake dog. J Appl Physiol 82: 791–798, 1997 [DOI] [PubMed] [Google Scholar]
- 53. Smith CA, Chenuel BJ, Henderson KS, Dempsey JA. The apneic threshold during non-REM sleep in dogs: sensitivity of carotid body vs. central chemoreceptors. J Appl Physiol 103: 578–586, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Tansley JG, Fatemian M, Howard LS, Poulin MJ, Robbins PA. Changes in respiratory control during and after 48 h of isocapnic and poikilocapnic hypoxia in humans. J Appl Physiol 85: 2125–2134, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Tarbichi AG, Rowley JA, Shkoukani MA, Mahadevan K, Badr MS. Lack of gender difference in ventilatory chemoresponsiveness and post-hypoxic ventilatory decline. Respir Physiol Neurobiol 137: 41–50, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Task Force of the American Sleep Disorders Association EEG arousals: scoring and examples. A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 15: 174–184, 1992 [PubMed] [Google Scholar]
- 57. Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goat. J Physiol 499: 543–550, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wadhwa H, Gradinaru C, Gates GJ, Badr MS, Mateika JH. Impact of intermittent hypoxia on long-term facilitation of minute ventilation and heart rate variability in men and women: do sex differences exist? J Appl Physiol 104: 1625–1633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med 165: 1245–1250, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Xie A, Skatrud JB, Morgan B, Chenuel B, Khayat R, Reichmuth K, Lin J, Dempsey JA. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J Physiol 577: 319–329, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xie A, Skatrud JB, Barczi SR, Reichmuth K, Morgan BJ, Mont S, Dempsey JA. Influence of cerebral blood flow on breathing stability. J Appl Physiol 106: 850–856, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang SP, Bergo GW, Krasney E, Krasney JA. Cerebral pressure-flow and metabolic responses to sustained hypoxia: effect of CO2. J Appl Physiol 76: 303–313, 1994 [DOI] [PubMed] [Google Scholar]
- 63. Zhou XS, Rowley JA, Demirovic F, Diamond MP, Badr MS. Effect of testosterone on the apneic threshold in women during NREM sleep. J Appl Physiol 94: 101–107, 2003. [DOI] [PubMed] [Google Scholar]