Abstract
To investigate vestibuloocular reflex (VOR) adaptation produced by changes in peripheral vestibular afference, we developed and tested a vestibular “prosthesis” that senses yaw-axis angular head velocity and uses this information to modulate the rate of electrical pulses applied to the lateral canal ampullary nerve. The ability of the brain to adapt the different components of the VOR (gain, phase, axis, and symmetry) during chronic prosthetic electrical stimulation was studied in two squirrel monkeys. After characterizing the normal yaw-axis VOR, electrodes were implanted in both lateral canals and the canals were plugged. The VOR in the canal-plugged/instrumented state was measured and then unilateral stimulation was applied by the prosthesis. The VOR was repeatedly measured over several months while the prosthetic stimulation was cycled between off, low-sensitivity, and high-sensitivity stimulation states. The VOR response initially demonstrated a low gain, abnormal rotational axis, and substantial asymmetry. During chronic stimulation the gain increased, the rotational axis improved, and the VOR became more symmetric. Gain changes were augmented by cycling the stimulation between the off and both low- and high-sensitivity states every few weeks. The VOR time constant remained low throughout the period of chronic stimulation. These results demonstrate that the brain can adaptively modify the gain, axis, and symmetry of the VOR when provided with chronic motion-modulated electrical stimulation by a canal prosthesis.
INTRODUCTION
Numerous studies have examined adaptation of the angular vestibuloocular reflex (VOR), but the vast majority of these experiments induced VOR adaptation in normal subjects by providing aberrant image motion on the retina during head rotation. The VOR gain, for example, has been modified with magnifying or minimizing lenses (e.g., Demer et al. 1989); the phase has been shifted by altering the temporal relationship between head motion and retinal slip (e.g., Kramer et al. 1995); and the axis has been changed by providing retinal image motion that is misaligned with the plane of head rotation (e.g., Quinn et al. 1996). VOR adaptation induced by changes in afferent vestibular signals, which is more relevant to clinical vestibular disorders, has received much less attention and these studies have focused exclusively on the compensation that occurs after static deficits are introduced by ablative procedures such as labyrinthectomy (e.g., Fetter and Zee 1988) or canal plugging (e.g., Broussard et al. 1999).
To investigate the brain's ability to adaptively modify the VOR when peripheral vestibular function changes, we have developed a “canal prosthesis” that senses angular head velocity and uses this information to modulate activity in vestibular afferents by direct electrical stimulation of canal ampullary nerves (Gong and Merfeld 2000, 2002). This approach offers more flexibility than ablative methodologies because it allows us to reversibly manipulate different parameters of the afferent signal, such as the baseline rate of stimulation when the head is stationary, the relationship between head velocity and the rate of electrical stimulation (e.g., the sensitivity of the prosthesis), and the temporal relationship between head motion and electrical stimulation (e.g., the time constant of the prosthetic input). This approach has a number of potential limitations, however. Since we are providing unilateral stimulation, the normal push–pull modulation provided by the contralateral vestibular afferents is absent. Furthermore, the modulation in afferent activity produced by electrical stimulation most likely affects only a subset of afferent fibers and synchronizes their firing. Finally, chronic, high-frequency electrical stimulation could have adverse effects on the peripheral or central vestibular pathways and these could potentially limit the ability of the prosthesis to generate a stable, long-term VOR response.
In prior studies we used a unilateral canal prosthesis that sensed head velocity about the yaw rotational axis and provided electrical stimulation to lateral canal afferents using biphasic current pulses (Lewis et al. 2003; Merfeld et al. 2007). The rate of stimulation had two components: 1) a baseline, tonic rate with the head stationary, that was chosen to be well above the normal resting discharge rate of the canal afferents; and 2) a motion-modulated component that increased the stimulation rate for head rotations ipsilateral to the instrumented ear and decreased the stimulation rate for contralateral head rotations (Gong and Merfeld 2000; Merfeld et al. 2007). Although these studies demonstrated that the brain can adapt to the static tone imbalance produced by the high baseline stimulation rate, the adequacy of VOR adaptation to the dynamic modulations in electrical stimulation remains uncertain. In our prior chronic squirrel monkey study (Merfeld et al. 2007), the VOR produced by motion-modulated stimulation had a low gain and an abnormally large phase lead, and the axis and symmetry characteristics were not evaluated.
Since it is highly improbable that a vestibular prosthesis, when first activated, would produce VOR responses that perfectly compensate for head motion, it is critical to determine whether the brain can engage central adaptive mechanisms during chronic prosthetic stimulation to improve the characteristics of the VOR. In this study, we have examined adaptation of the VOR gain, phase, axis, and symmetry in two squirrel monkeys with bilateral lateral canal plugs and chronic unilateral horizontal canal electrical stimulation. Since we previously observed abrupt increases in gain when the prosthesis was first activated or when the sensitivity of the prosthesis was changed (Lewis et al. 2003; Merfeld et al. 2007), we investigated the effects of multiple transitions between the off (no prosthetic stimulation), low-sensitivity, and high-sensitivity stimulation states by cycling between these states during the prolonged period of prosthetic stimulation. As detailed in the following text, prosthesis sensitivity was defined with a hyperbolic tangent function that calculates stimulation rate from angular head velocity; the slope of the curve derived from this function was twice as steep (in the central, linear range) in the high-sensitivity state than that in the low-sensitivity state.
Our results demonstrate that significant VOR adaptation occurred during chronic prosthetic stimulation, evidenced by a substantial increase in gain that was associated with an improvement in the rotational axis in the frontal plane and the symmetry of the eye-movement response. The phase lead, quantified as the dominant time constant of the VOR, did not increase over time and remained less than the time constant of the input provided by the prosthesis. Analysis of temporal bone histology and other data strongly suggests that the chronic, high-frequency electrical stimulation provided by the prosthesis did not produce progressive damage to the implanted electrode or to the peripheral or central vestibular neurons that contribute to the VOR.
METHODS
Experiments were performed in two mature male squirrel monkeys (weighing ∼1,000 g each). All experiments were approved by the institutional animal care and use committee and were in accordance with US Department of Agriculture guidelines. Both animals were healthy, with no known prior illnesses, and neither had been used in any previous experiments. The monkeys were elderly, however (10 and 13 y), and were substantially older than squirrel monkeys used in work published by other laboratories.
Instrumentation
Surgical procedures were performed under general anesthesia provided by either intravenous pentobarbital or inhaled isoflurane. A head bolt was implanted in each monkey's skull using aseptic techniques in the hospital's animal operating room. The bolt, used to immobilize the head during experiments, was oriented such that the head was pitched forward about 17° with respect to the stereotactic zero when the head was immobilized in the primate chair. This head orientation placed the lateral canals close to alignment with the earth-horizontal plane and the vertical canals nearly earth-vertical (Blanks et al. 1985). A three-turn frontal eye coil was inserted under the conjunctiva in the frontal plane in one eye using standard surgical techniques (Judge et al. 1980).
In two subsequent surgeries, both lateral canals were plugged to reduce their sensitivity to head rotation (Rabbitt et al. 1999) and a stimulating electrode was inserted into each lateral canal through a second opening in the canal more proximal to the ampulla. The electrodes were made from platinum wire (diameter: 150 μm) and about 150 μm of Teflon insulation was stripped from the tip with ophthalmic scissors while viewing the electrode under a microscope. Each electrode was slowly advanced toward the lateral canal ampullary nerve using a micromanipulator while short electrical pulse trains (biphasic current pulses at 600 Hz, duration 0.5 s) were applied to elicit eye movements. The return path for electrical stimulation was an electrode ipsilateral to the stimulating electrode inserted into the temporalis muscle. The position of the stimulating electrode in each ear was adjusted during surgery such that the maximum horizontal eye movements were elicited by the brief pulse trains, with minimal vertical eye movement or twitching of facial musculature. When the best position for each electrode was determined, it was fixed in place with dental acrylic that adhered to several implanted screws.
Electrode characterization
The electrical stimulation used for the initial electrode characterization and for all subsequent experiments consisted of charge-balanced biphasic current pulses. Each biphasic pulse consisted of cathodic, off, and anodic phases and the duration of each phase was 200 μs (Gong and Merfeld 2000, 2002). With the monkey in complete darkness, the electrodes were characterized by slowly increasing the current amplitude of short pulse trains while measuring the eye movement responses and viewing the monkey's face with an infrared camera. Three criteria were used to choose the appropriate current level for each animal: 1) the horizontal eye movements produced by the electrical stimulation should be as large as possible; 2) minimal vertical eye motion should be elicited; and 3) no facial nerve activation should be observed. Using these criteria, the current used for all subsequent experiments was fixed at 150 μA for monkey N and 140 μA for monkey S. In each animal, chronic stimulation was applied by the prosthesis to the ear electrode that elicited the greatest horizontal eye movement response during the characterization process—the left lateral canal electrode for monkey S and the right lateral canal electrode for monkey N.
Back-voltages were measured in both monkeys at regular intervals during the experimental protocol. Since the current supplied to the stimulating electrode was controlled, the back-voltage reflects the impedance of the electrode and the surrounding tissues and thus provides information about the integrity of the electrode and its ability to provide current to the surrounding tissues during the long period of chronic stimulation. To avoid potential adverse effects on the electrode caused by chronic electrical stimulation, such as loss of platinum or formation of bubbles around the electrode's tip, we calculated the safe charge limit using standard methods (e.g., Robblee and Rose 1990). The charge delivered by the electrode during chronic stimulation was less than one half of the calculated safe charge limit.
Vestibular stimulation
As previously described (Gong and Merfeld 2002; Lewis et al. 2003; Merfeld et al. 2007), the prosthesis was chronically mounted on the monkey's head inside a hollow headcap and contained a piezoelectrical angular rate sensor that was oriented with its sensitive axis aligned with that of the lateral canals. The rate sensor measured angular head velocity in the yaw axis and this signal was digitized and then filtered with a digital high-pass filter with a time constant of 5 s, to approximate the dynamics of the afferent cue normally provided by the lateral canal. The filtered head velocity signal was then used to modulate the rate of the biphasic current pulses applied to the lateral canal ampullary nerve via the implanted electrode.
Using the approach we previously applied to squirrel monkeys (Lewis et al. 2003; Merfeld et al. 2007), we chose to use a nonzero tonic rate of stimulation when the head was stationary. Consistent with our prior observations, we hypothesized that the monkeys would adapt to the high level of tonic stimulation and that, by increasing the rate of stimulation for head turns toward the electrode and decreasing it for head turns in the opposite direction, we could provide a bidirectional afferent cue to the brain with a unilateral prosthesis. This approach recapitulates in a general sense the normal modulation of canal afferents during head rotations (Fernandez and Goldberg 1971), but requires that we stimulate the nerve at a rate substantially above its normal resting discharge rate to allow an adequate reduction in stimulation below the tonic rate during contralateral head turns. The tonic level of stimulation was chosen to be 250 pulses/s (pps) in monkey S and 200 pps in monkey N, rates that are about midway between the average baseline firing rate of canal afferents (∼100 spikes/s) and the maximal firing rate of these afferents (∼400–450 spikes/s) (Goldberg and Fernandez 1971). These tonic rates of stimulation therefore allowed us to provide a relatively symmetric range of modulation upward and downward about the baseline pulse rate.
As previously described (Gong and Merfeld 2000; Merfeld et al. 2007), the motion-modulated component of the electrical stimulation was derived from a hyperbolic tangent function, which provided a nearly linear modulation over the central range of head velocities and saturated gradually as larger velocities were approached in either direction. For monkey S, the frequency of electrical stimulation (f) was defined as f = 250 ± 200 tanh (ω/500/3) for the low-sensitivity state and as 250 ± 200 tanh (ω/250/3) for the high-sensitivity state, where ω is the high-pass filtered angular head velocity. The frequency of stimulation for monkey S ranged from a minimum of 50 pps to a maximum of 450 pps and the prosthesis sensitivity in the central linear range was 1.2 pps·deg−1·s−1 (low sensitivity) and 2.4 pps·deg−1·s−1 (high sensitivity). For monkey N, the frequency of stimulation was defined as f = 200 ± 150 tanh (ω/500/3) for the low-sensitivity state and as 200 ± 150 tanh (ω/250/3) for the high-sensitivity state. For this monkey, the stimulation rate ranged from a minimum of 50 pps to a maximum of 350 pps and the prosthesis sensitivity in the central linear range of was 0.9 pps·deg−1·s−1 (low sensitivity) and 1.8 pps·deg−1·s−1 (high sensitivity).
Motion paradigms
The monkeys were passively rotated about an earth-vertical yaw axis in complete darkness. Since the modulation in electrical stimulation provided by the prosthesis was twice as sensitive to head velocity changes in the high compared with the low-sensitivity state, we always halved the velocity of head rotation in the former state to ensure that approximately the same pattern of electrical stimulation was provided by the prosthesis during testing in both the low- and high-sensitivity states. Peak velocity of rotation was 80°/s when the prosthesis was in the low-sensitivity state and was reduced to 40°/s in the high-sensitivity state. Sinusoidal head rotations were provided at frequencies of 0.01, 0.02, 0.05, 0.1, 0.5, 0.2, and 1.0 Hz. Each test session also included one velocity step in each direction. The steps consisted of accelerations from zero to 80°/s (low-sensitivity state) or 40°/s (high-sensitivity state), each occurring over 1.0 s. The velocity was held constant for 60 s and then symmetrically decelerated to zero over 1.0 s.
Eye movement recordings and analysis
Horizontal and vertical eye movements were recorded from one eye using a Robinson-style coil system (CNC Engineering). We used the right-hand-rule sign convention, with positive directions defined as leftward and downward. Eye movements were calibrated by mounting a coil identical to that implanted in the monkeys on a gimbal. Voltages were set to zero with the eye centered and the amplifiers were adjusted so that 20° deviations in the horizontal or vertical planes produced deflections of 5 V. A linear calibration was performed based on this voltage–degree relationship. Eye movements were filtered at 50 Hz with an eight-pole Bessel filter and sampled at a rate of 200 Hz for off-line analysis. Eye position data were then digitally differentiated and quick phases were removed from the velocity data with an interactive algorithm. Spontaneous nystagmus—defined as the nystagmus recorded with the monkey upright, stationary, and in the dark—was characterized by calculating the mean slow-phase velocity during the 30 s that immediately preceded the first motion profile of each test session. For the sinusoidal motion paradigms, the slow-phase eye-velocity traces were fit to a sinusoid plus a constant using a least-mean-squared error algorithm to calculate the VOR gain and phase at each frequency of head rotation. To characterize the overall sinusoidal response for each test session, the gain and phase results for each frequency over the 0.01- to 1.0-Hz range were fit (using an iterative nonlinear algorithm, fmins in Matlab) to a transfer function having the form Thp(s) = Gs/(ts + 1), where G is the transfer function gain, t is the transfer function time constant, and s is the Laplace variable. For the velocity steps, the gain was defined as the (peak eye velocity)/(peak head velocity) and was calculated separately for head rotations that were ipsilateral or contralateral to the stimulated ear.
Single-pulse paradigm
Eye movements elicited by single cathodic pulses were recorded in monkey N before the prosthesis was first activated and again each time the prosthetic stimulation was stopped. The pulses used the same amplitude (150 μA) as that of the chronic stimulation in this animal and a pulse width of 1,000 μs. These eye movements were recorded at 18,000 Hz and were digitally differentiated and then low-pass filtered with a digital fourth-order low-pass Bessel filter having a cutoff frequency of 160 Hz to calculate eye velocity.
Experimental protocol
After the monkeys were instrumented with the head bolt and eye coils, their VOR was measured several times using the two types of motion stimuli described earlier. Then both lateral canals were plugged and stimulating electrodes were implanted into each plugged canal. After recovery from the surgeries (between 2 and 4 wk), the VOR was remeasured. The electrodes were then characterized and the single-pulse responses were recorded in monkey N. For each monkey, the prosthesis was initially activated in the low-sensitivity configuration, with the monkey upright and stationary in the dark, the spontaneous nystagmus produced by the tonic stimulation was recorded for 30 min and then the first set of prosthesis-mediated VOR responses was recorded. After the initial data were obtained, the prosthesis remained activated and the monkeys returned to their normal lighted cages, where they were free to move without restriction. The VOR was recorded several times during the first day of stimulation and, subsequently, was recorded one to three times per week. For monkey S, the initial low-sensitivity prosthetic state was maintained for 70 days; for monkey N it was maintained for 20 days. At the end of the low-sensitivity period of stimulation, the prosthesis was switched to the high-sensitivity state while the monkeys were stationary in the dark. The peak velocity of all motion paradigms was halved in the high-sensitivity state, as described earlier. The VOR was characterized three times during the first day that the sensitivity of the prosthetic stimulation was increased and, subsequently, one to three times per week. The first period of high-sensitivity stimulation lasted 49 days in monkey S and 14 days in monkey N. Then the prosthesis was turned off while the monkey was stationary in the dark, the VOR was measured, and in monkey N the single-pulse protocol was carried out. The prosthesis remained off for 1 wk, after which the stimulation pattern described earlier was repeated. For the subsequent stimulation periods, the low-sensitivity stimulation was provided for 14 to 20 days, the high-sensitivity stimulation was provided for 14 to 28 days, and the prosthesis was inactivated for 1 wk. These stimulation patterns were repeated a total of four times in monkey S and three times in monkey N.
Temporal bone histology
After the monkeys were killed, both temporal bones were removed from each animal and were prepared for light microscopy. They were fixed with 10% formalin, decalcified using ethylenediaminetetraacetic acid, and sectioned in the axial plane. Monkey N was stained with hematoxylin and eosin to optimize the appearance of the neural tissue and monkey S was stained with toluidine blue to optimize visualization of the electrode.
RESULTS
Normal and canal-plugged states
NORMAL MONKEYS.
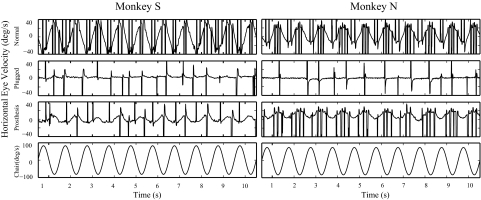
Prior to ear surgery, spontaneous nystagmus was recorded in the dark in monkey N (mean slow-phase velocity [SPV] = 1.8°/s) but not monkey S (mean SPV = 0°/s). Both monkeys had relatively low VOR gains during yaw-axis rotation. For sinusoidal rotations (Fig. 1, Normal), monkey S had a VOR gain constant of 0.41 and a time constant of 46 s; monkey N had a VOR gain constant of 0.26 and a time constant of 23.4 s. For velocity steps (Fig. 2, Normal), VOR gains were 0.44 for monkey S and 0.28 for monkey N. VOR responses were symmetric in both animals during both sinusoidal and step stimuli, but the spontaneous nystagmus in monkey N (a normal finding in nonhuman primates) biased his VOR responses in the positive direction (Figs. 1 and 2). The size of the bias varied since the amplitude of the spontaneous nystagmus depended on the length of time spent in the dark prior to the specific trial. The angle of the VOR rotational axis in the frontal plane, calculated from the step data, was 86° in monkey S and 88° in monkey N (where horizontal eye movements are defined to have an angle of 90° and vertical eye movements an angle of 0°).
Fig. 1.
Horizontal eye velocity plotted against time during yaw-axis sinusoidal rotation at 0.5 Hz. Traces show the eye movement responses in the normal monkeys, following bilateral plugging of the lateral canals and after the prosthesis was first activated in the low-sensitivity state. Peak velocity of head rotation was 80°/s. Vestibuloocular reflex (VOR) responses were symmetric for monkey S in the normal state, but monkey N had a spontaneous nystagmus in the dark in the normal state, which measured 4.0°/s during this test session, and this biased the VOR in the positive direction. The VOR response in monkey N was symmetric, however, about this biased zero. After the prosthesis was activated, monkey N had a leftward (positive) VOR bias that reflected the small, residual spontaneous nystagmus produced by the tonic electrical stimulation of the right ear that remained after 30 min of tonic stimulation in the dark.
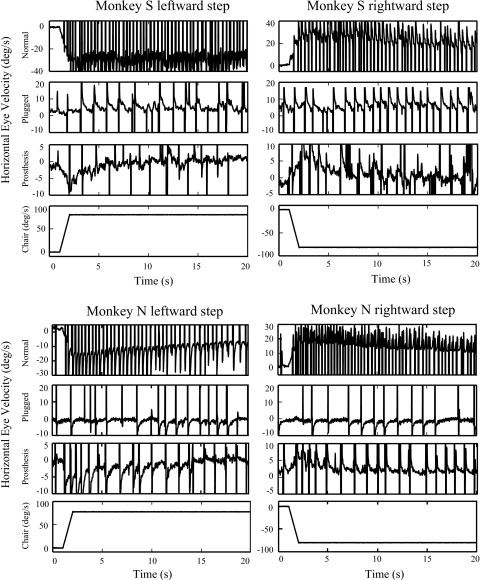
Fig. 2.
Horizontal eye velocity vs. time during leftward (positive) and rightward (negative) yaw-axis velocity steps (0–80°/s) in the normal, plugged, and prosthesis-on states. The amplitude of the step response was symmetric in the normal state in both monkeys, although monkey N again demonstrated a positive bias due to spontaneous nystagmus in the dark. After the prosthesis was activated, both animals show evidence of a small VOR bias due to the residual spontaneous nystagmus that was present after 30 min of tonic stimulation in the dark. The small bias (∼2°/s) is negative (rightward) in monkey S due to left ear stimulation and is positive (leftward) in monkey N due to right ear stimulation.
CANAL-PLUGGED, INSTRUMENTED MONKEYS.
After bilateral plugging of the lateral canals and insertion of a stimulating electrode in each plugged canal, a small change occurred in the spontaneous nystagmus (monkey S: mean SPV = 3.75°/s; monkey N: mean SPV = −0.08°/s), and the horizontal VOR response was markedly attenuated (Figs. 1 and 2, Plugged). For monkey S, the VOR gain in the plugged state was 0.016 (sinusoids) and 0.04 (velocity steps); for monkey N the gains were 0.001 (sinusoids) and 0.025 (steps). The VOR time constant could not be calculated due to the very low gains. Small off-axis (vertical) eye-movement responses were also recorded in both animals during yaw-axis rotation. In monkey S the vertical VOR gain during yaw-axis sinusoidal rotation increased from 0.015 (normal) to 0.043 (bilateral canal plugged), whereas the vertical gain increased slightly from 0.009 (normal) to 0.012 (bilateral canal plugged) in monkey N. These results demonstrate that the canal-plugging procedure rendered the lateral canals minimally sensitive to yaw-axis head rotation over the frequency range we used, but evoked a very small off-axis VOR response as previously described (Angelaki et al. 1996; Merfeld et al. 2007).
First period of prosthetic electrical stimulation
SPONTANEOUS NYSTAGMUS.
When the prosthesis was first activated with the monkey stationary and in the dark, a brisk spontaneous nystagmus was recorded with slow phases directed away from the stimulated ear. The initial slow-phase velocities were 27°/s for monkey S and 32°/s for monkey N. The spontaneous nystagmus gradually attenuated while the animals were maintained stationary in the dark and decreased rapidly after they were returned to their lighted cages. The mean SPV dropped to <2°/s after 6 h of stimulation in monkey S and after 1 day of stimulation in monkey N. The spontaneous nystagmus remained relatively small in both animals during the subsequent prolonged periods of chronic prosthetic stimulation.
VOR GAIN, SINUSOIDAL ROTATION.
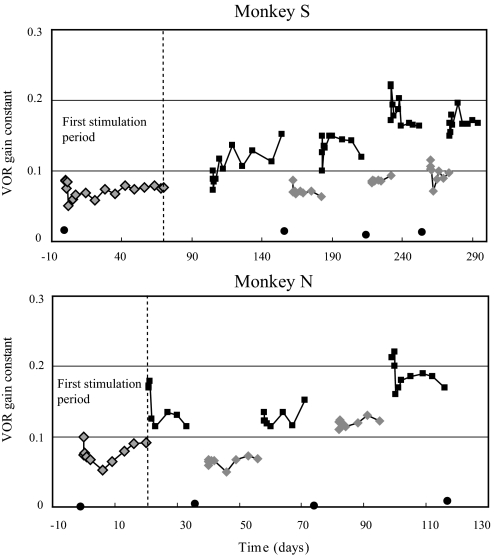
When the prosthesis was activated in the dark, a horizontal VOR response was recorded during sinusoidal yaw-axis rotation that was not present in the canal-plugged state (Fig. 1, Prosthesis). The frequency dependence of the gain was qualitatively similar to that of normal squirrel monkey responses, reflecting the high-pass characteristics of the input provided by the prosthesis (Merfeld et al. 2007), although the gains were substantially smaller than those recorded in the normal state. The initial VOR gain constants calculated from the transfer function fits of the sinusoidal data were 0.09 (monkey S) and 0.1 (monkey N). Subsequent testing performed during the first period of chronic prosthetic stimulation showed that the VOR gains rapidly declined in both monkeys and then slowly increased and plateaued at values that were slightly smaller than those present when the prosthesis was first activated (Fig. 3, first stimulation period).
Fig. 3.
VOR gain constants during the entire period of prosthetic stimulation, calculated from the transfer function fit to the VOR gains measured during sinusoidal rotation over a frequency range of 0.01 to 1.0 Hz. The first stimulation period is to the left of the vertical dashed line and these data were previously published for monkey S (Merfeld et al. 2007; Fig. 7). Circles are values in the canal-plugged state with the prosthesis off, light diamonds are the low-sensitivity state of prosthetic stimulation, and dark squares are the high-sensitivity state of prosthetic stimulation. For monkey S, the gap between the end of the first period of low-sensitivity stimulation and the start of high-sensitivity stimulation indicates a period where the power supply of the prosthesis failed.
VOR GAIN, VELOCITY STEPS.
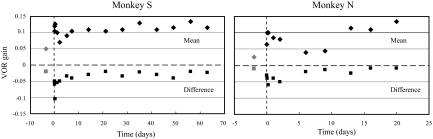
After the prosthesis was activated, VOR responses to velocity steps in both directions were clearly present (Fig. 2, Prosthesis), but the gains were lower than those recorded in the normal state (0.12 for monkey S and 0.07 for monkey N). During the first period of chronic stimulation, the mean step response (average of the gains produced by rotations ipsilateral and contralateral to the stimulated ear; Fig. 4) followed a pattern that was qualitatively similar to that of the sinusoidal responses (Fig. 3), although the gains produced by the velocity steps were characteristically larger than those produced by sinusoidal rotations. The symmetry of the VOR response was also assessed with velocity steps and, immediately after prosthesis activation, a substantial VOR asymmetry was observed in both monkeys. Larger VOR gains were recorded for head turns away from the stimulated ear compared with head turns toward the stimulated ear, evidenced by negative values for the [ipsilateral gain − contralateral gain] difference (Fig. 4). In both animals, this asymmetry became much smaller during the first period of electrical stimulation (Fig. 4); the difference between the ipsilateral and contralateral gains attenuated 71% by day 14 in monkey S and 78% by day 9 in monkey N.
Fig. 4.
VOR gains produced by velocity steps in the canal-plugged state (gray icons) and during the first cycle of low-sensitivity prosthetic stimulation (black icons). Diamonds are the average of the gains produced by rotations ipsilateral and contralateral to the stimulated ear ([gainipsi + gaincontra]/2); squares are the difference between the gains produced by ipsilateral and contralateral rotations (gainipsi − gaincontra) and reflect the asymmetry of the VOR response to velocity steps.
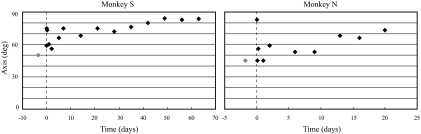
VOR AXIS.
When the prosthesis was first activated, there was a substantial increase in the horizontal VOR gain and a small increase in the vertical gain, shifting the eye's rotational axis in the frontal plane close to the compensatory orientation of 90° (Fig. 5). The rapid reduction in the horizontal VOR gain that followed shifted the rotational axis away from 90°, but during the subsequent chronic period of electrical stimulation, the horizontal gain slowly increased whereas the vertical gain decreased slightly, shifting the rotational axis in the frontal plane closer to the compensatory orientation (Fig. 5). For example, monkey S had a horizontal gain of 0.075 and a vertical gain of 0.05 to velocity steps on day 2 of stimulation, resulting in an axis of 56°. At the conclusion of the stimulation period the horizontal gain had increased to 0.17, whereas the vertical gain decreased to 0.02, producing an axis of 83.5°. Similarly, on day 1 of electrical stimulation monkey N had a horizontal VOR gain of 0.05 and a vertical gain of 0.05 to velocity steps, yielding a rotational axis of 45°. At the end of the stimulation period, the horizontal response had increased to 0.13, whereas the vertical gain was minimally smaller (0.04), resulting in a rotational axis of 73°.
Fig. 5.
Rotational axis of the eye in the frontal plane calculated from the velocity step data, in the canal-plugged state (gray diamonds) and during the first period of low-sensitivity prosthetic stimulation (black diamonds). The axis is defined as the tan−1 (peak horizontal eye velocity/peak vertical eye velocity). A purely horizontal eye movement response, which is perfectly compensatory for the yaw-axis head rotation, has an axis of 90° and a purely vertical response has an axis of 0°.
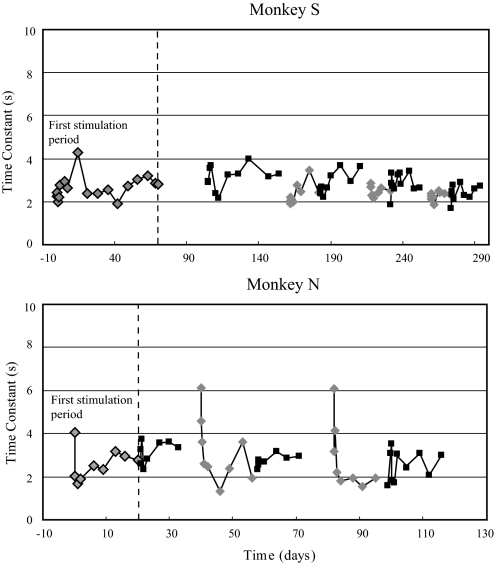
VOR PHASE.
When the prosthesis was first activated, the phase leads for the sinusoidal motion paradigms reflected the high-pass characteristics of the prosthetic input, but were larger than expected, particularly at the lower frequencies. The time constant of the VOR response when the prosthesis was first activated, calculated from the transfer function fit of the sinusoidal data, was smaller than the time constant of the prosthesis in both monkeys (2.3 s in monkey S and 4.0 s in monkey N). Similar short time constants were evidenced by the rapid attenuation of the VOR response produced by velocity steps (Fig. 2). During the first period of low-sensitivity prosthetic stimulation, the time constant for monkey S varied irregularly between a maximum of 4.3 s and a minimum of 1.9 s (Fig. 6) and there was no evidence of a systematic increase in value during the 70-day period of stimulation. In monkey N, the initial time constant was close to the 5-s time constant of the high-pass filter applied to the head velocity signal by the prosthesis, but it rapidly dropped to a value of 2.0 s during the first 2 h of prosthetic stimulation and then remained between 1.7 and 3.2 s throughout the subsequent 20 days of the first stimulation period (Fig. 6).
Fig. 6.
VOR time constants during the entire period of prosthetic stimulation, calculated from the transfer function fit to the VOR phase measured during sinusoidal rotation over a frequency range of 0.01 to 1.0 Hz. The first stimulation period is to the left of the vertical dashed line. Light diamonds are the low-sensitivity state of prosthetic stimulation and dark squares are the high-sensitivity state of prosthetic stimulation. Time constants could not be accurately calculated in the canal-plugged state with the prosthesis off because of the very low VOR gains.
Cycling prosthetic stimulation between the off, low-sensitivity, and high- sensitivity states
VOR GAIN.
When the prosthesis was first changed from the low to the high-sensitivity state at the end of the first stimulation period, the horizontal VOR gain increased substantially (Fig. 3), as would be predicted from the increased slope of the curve relating stimulation rate to filtered head velocity. Following the transition to high-sensitivity state, the VOR gain dropped rapidly in both animals and then either gradually increased (e.g., first high-sensitivity period for monkey S) or remained relatively stable (e.g., first high-sensitivity period for monkey N). During the subsequent periods of stimulation in the low-sensitivity and high-sensitivity prosthetic states, significant increases in the VOR gain were observed (Fig. 3). The VOR gain typically increased, then dropped rapidly after the start of each stimulation period, and then either increased slowly or remained relatively stable. Generally the gains at the start and end of each stimulation period were larger than those recorded at the start and end of the prior stimulation period with the same prosthetic sensitivity.
In monkey S, the VOR gains gradually increased in both prosthetic stimulation states. In the low-sensitivity state, the mean VOR gain increased 43%, from 0.07 ± 0.01 during the first stimulation period to 0.1 ± 0.01 during the fourth period (Mann–Whitney rank-sum test: P < 0.001). In the high-sensitivity state, the VOR gain increased 55%, from 0.11 during the first stimulation period to 0.17 during the fourth period (t-test: P < 0.001). A two-way ANOVA confirmed that the VOR gain was significantly affected by both the sensitivity of the prosthesis (P < 0.001) and the stimulation period (P < 0.001). In monkey N, the VOR gains remained approximately the same during the first two low- and high-sensitivity stimulation periods, but then increased substantially during the third, final stimulation period. In the low-sensitivity state, the mean VOR gain increased 50%, from 0.08 ± 0.01 during the first stimulation period to 0.12 ± 0.01 during the third period (t-test: P < 0.001). In the high-sensitivity state, the VOR gain increased 36%, from 0.14 ± 0.03 during the first period to 0.19 ± 0.02 during the third period (t-test: P < 0.001). A two-way ANOVA confirmed that, like monkey S, both the sensitivity of the prosthesis (P < 0.001) and the stimulation period (P < 0.001) significantly influenced the VOR gain.
Although the VOR gains increased in both monkeys during cycling of prosthetic stimulation, when the prosthesis was inactivated after each period of high-sensitivity stimulation and the monkeys were tested in the canal-plugged state, the gains did not increase over time but rather remained extremely small (Fig. 3). In both monkey S and monkey N, the regression slope relating the VOR gain in the plugged state to time (day in the paradigm when the data were acquired) was not significantly different from zero.
VOR PHASE.
Unlike the gain, there was no evidence that the VOR time constant increased over time when the stimulation was repeatedly cycled between the off, low-sensitivity, and high-sensitivity states. In monkey S the time constant varied in an essentially random fashion between 2 and 4 s throughout the entire stimulation period (Fig. 6). In monkey N, the time constant was maximal immediately after each transition from the off to the low-sensitivity state (Fig. 6). In each of the three stimulation cycles, the initial time constant was close to the time constant of the input signal provided by the prosthesis (5 s), but rapidly dropped to the 2- to 3-s range within several hours of stimulation and remained low throughout the remainder of the stimulation cycle.
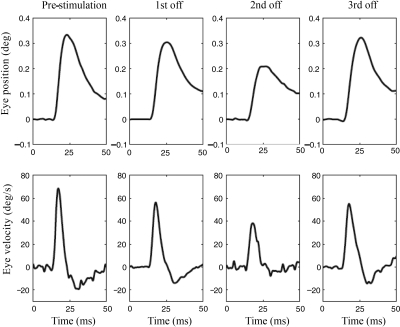
EYE MOVEMENT RESPONSES TO SINGLE PULSES.
Eye movements elicited by single cathodic pulses were measured in monkey N before the prosthesis was first activated and then three times subsequently when prosthesis was turned off after each period of high-sensitivity stimulation (see Fig. 3). The eye movements had a mean latency of 5.4 ms, suggesting that they reflected activation of the three neuron/two synapse VOR pathway in the brain stem (Broussard et al. 1992). Although the VOR gains measured at the end of each period of high-sensitivity stimulation clearly increased in monkey N during subsequent stimulation periods (Fig. 3), the peak amplitude and velocity of the eye movements evoked by single pulses did not show a similar increase (Fig. 7). These results thus demonstrate a lack of correlation between the VOR gain produced by prosthetic stimulation and the amplitude of the eye-movement responses elicited by single cathodic pulses.
Fig. 7.
Horizontal eye position and velocity responses in monkey N elicited by single cathodic electrical pulses with durations of 1,000 μs and amplitudes of 150 μA. Traces are the average of 20–25 individual responses and were obtained with the prosthesis off, prior to electrical stimulation (first column) and after each of the 3 periods of high-sensitivity stimulation were completed (see Fig. 3; monkey N, circles = prosthesis-off).
BACK-VOLTAGE MEASUREMENTS.
In both monkeys, the back-voltages were observed by the experimenters each time the animals were tested, and were recorded at regular intervals. For monkey S, the back-voltages were recorded nine times during the period of chronic stimulation, with the last measurement made on day 237 of stimulation. The positive and negative back-voltages did not vary substantially during this prolonged period of stimulation. Specifically, the means and SDs for the positive and negative back-voltages were 2.3 ± 0.13 and −2.7 ± 0.28, respectively. No positive or negative measurement was >20% from the respective means and linear regression of voltage versus duration of stimulation yielded slopes that were not significantly different from zero for either the positive or negative values. For monkey N, back-voltages were recorded five times during chronic stimulation and the findings were similar to those of monkey S. Specifically, no individual value differed from the positive or negative mean by >20% (positive: 1.8 ± 0.1; negative: −2.2 ± 0.1) and linear regression of voltage versus duration of stimulation yielded slopes that did not differ from zero.
Temporal bone histology
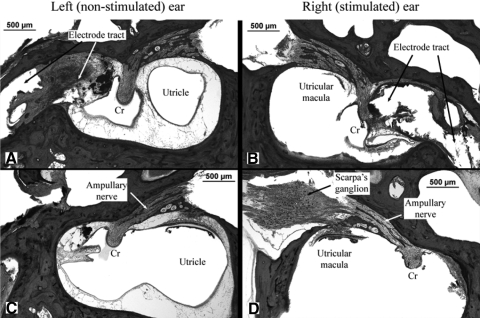
The temporal bones of both monkeys were examined using light microscopy. The two ears were compared in each animal: one ear had an implanted electrode that had been used for long-term, high-frequency electrical stimulation, whereas the other ear had an implanted electrode that had not been used for chronic stimulation. In both animals, as noted previously, the ear that was chosen for chronic stimulation was the one that generated larger-amplitude horizontal eye movements during the electrode characterization procedures. Pertinent histologic findings for monkey N are illustrated in Fig. 8. The electrode tracts in both ears are clearly visible and are surrounded by a capsule of fibrous tissue that also contained foreign body giant cells (Fig. 8, A and B). There is no acute or chronic inflammation around the electrodes. The electrode tract in the stimulated right ear (Fig. 8B) is closer to the crista of the lateral canal, thus causing more disruption of the sensory epithelium than that observed in the nonstimulated left ear (Fig. 8A). The ampullary nerves (of the lateral canals) are well visualized and normal nerve fibers are evident in both ears, although the density of nerve fibers appears to be reduced on the stimulated side (Fig. 8D) compared with that on the nonstimulated side (Fig. 8C). Scarpa's ganglion was preserved in the right ear only (the stimulated ear). Ganglion cells are observed and appeared qualitatively normal in number, but comparison with the nonstimulated ear (left ear) was not possible.
Fig. 8.
Temporal bone histology in monkey N showing anatomic details of the left (nonstimulated) and right (stimulated) ears. The top panels show the position of the electrode tract with respect to the crista (Cr). The bottom panels show the lateral canal ampullary nerves in both ears and Scarpa's ganglion on the right.
Similar histologic findings were observed in monkey S. The electrode tract was closer to the cupula in the stimulated ear and normal nerve fibers were present in both ampullary nerves. Their density could not be compared, however, because the toluidine blue stain used in this animal was not optimal for visualizing nerve fibers. Scarpa's ganglion was not preserved on either side in monkey S.
DISCUSSION
The principal finding of this study is that the brain is capable of adapting the VOR when the peripheral input is provided by motion-modulated electrical stimulation of canal afferents, evidenced by gradual improvements in the VOR gain, rotational axis, and symmetry during chronic stimulation. We first consider vestibular function in the monkeys prior to ear surgery, after bilateral lateral canal plugging and during chronic electrical stimulation, and then discuss possible neural mechanisms that could underlie the VOR changes observed in this study.
Vestibular function in the normal and canal-plugged states
NORMAL MONKEYS.
Prior to ear surgery, the VOR gain in both animals was relatively low (particularly in monkey N), but time constant and symmetry measurements were normal. The low gains could potentially reflect preexisting peripheral or central vestibular damage or could be a manifestation of the monkeys' relatively advanced age. The characteristics of the VOR were not consistent with peripheral vestibular damage, either unilateral or bilateral. Unilateral peripheral damage is invariably associated with a shortening of the VOR time constant (typically <12 s) because velocity storage is reduced (Fetter and Zee 1988). In addition, after the acute period the gain reduction associated with unilateral loss is limited to very low frequencies (e.g., 0.01 Hz), whereas at higher frequencies (e.g., velocity steps like those we used), the gain is asymmetric (smaller when the head is rotated toward the impaired ear). None of these changes was observed in either monkey. Bilateral peripheral damage lowers the gain across the frequency range we tested but the time constant invariably becomes very short (e.g., <6 s; Dimitri et al. 2001/2002), which was not observed in either animal. For these reasons, we reject the hypothesis that the low gains resulted from a pathologic unilateral or bilateral loss of peripheral vestibular function.
Central vestibular damage could reduce the VOR gain if the vestibular nerve root entry zones or the vestibular nuclei were damaged bilaterally. Although this occurs in some rare conditions (e.g., severe vitamin B1 deficiency), the gain reduction is never isolated and is always associated with a marked reduction in the VOR time constant (Furman and Becker 1989). In addition, other evidence of brain stem damage would be expected, such as abnormal saccades and quick phases, postural instability, and other cranial nerve and long-tract abnormalities. Since the VOR time constants were normal in both monkeys, they appeared to have normal balance prior to ear surgery, and their VOR quick phases and random saccades appeared normal, it is improbable that they suffered from central vestibular damage that affected the VOR gain in isolation.
The monkeys used in this study were part of a cohort of four elderly squirrel monkeys (age range: 10–14 y) and all of these animals had low VOR gains when tested prior to ear surgery (see Lewis et al. 2003/2004; Merfeld et al. 2007). None of these monkeys had been used for any prior studies and all were healthy according to detailed veterinary records. Since it has been well documented that the VOR gain in humans declines with advancing age (e.g., Dimitri et al. 1996; Paige 1992), it is most probable that the monkeys used in our current study were normal but that their VOR gains were low because of their advanced age. Although these animals were not explicitly chosen as a model system to test prosthetic adaptation in an elderly population, their results may better reflect the adaptive capabilities of older humans, who have the greatest incidence of vestibular dysfunction (Agrawal et al. 2009).
BILATERAL CANAL PLUGGING.
Canal plugging has two well-documented effects on the VOR. First, the gain is attenuated in a frequency-dependent manner, such that responses are small at lower frequencies but are essentially normal at higher frequencies (Rabbitt et al. 1999). In our monkeys, minimal VOR responses were recorded in the canal-plugged state, indicating that the combination of plugging and electrode insertion rendered the lateral canals minimally responsive to head rotation over the range of frequencies we used. The second effect of canal plugging involves the velocity storage integrator in the brain stem. Although velocity storage appears to be intact after canal plugging, evidenced by normal optokinetic after-nystagmus, VOR responses measured after unilateral plugging in squirrel monkeys (Paige 1983) and bilateral plugging in rhesus monkeys (Angelaki et al. 1996) suggest that vestibular inputs do not engage the velocity storage integrator. Even though the VOR gains measured after bilateral canal plugging in our monkeys were too low to make accurate calculations of the VOR phase, this observation may be relevant when we consider the dynamics of the VOR produced by prosthetic stimulation in the canal-plugged preparation.
Effects of chronic high-frequency electrical stimulation
Little is known about the effects of long-term, high-frequency electrical stimulation of vestibular afferents on the tissues surrounding the electrode, the primary vestibular afferents and central vestibular neurons, and on synaptic transmission in the vestibular pathways. Our histologic data suggest that the crista may have been damaged during electrode insertion (O'Leary et al. 1991), particularly in the ear used for chronic stimulation (where the electrode tip was in direct contact with the crista). Although the relatively low VOR gains produced by the prosthesis may reflect damage to the sensory epithelium, temporal bone histology demonstrates that a large number of normal primary afferent neurons remained in both ears.
Despite the long-term presence of a foreign body (the electrode) in the ampulla, there was no evidence of acute or chronic inflammation in the labyrinth and only minimal new bone formation (Li et al. 2007) was observed. The stable back-voltage measurements and single-pulse responses indicate that neither the conductivity of the tissues nor the efficacy of afferent nerve stimulation changed significantly during the course of the experiment.
It is possible that chronic high-frequency electrical stimulation reduced the excitability of primary vestibular afferents (Tykocinski et al. 1995) or caused their degeneration (Shepard and Clark 1987). In monkey N, structural damage in the ampulla was more pronounced in the ear that generated larger eye movements during electrical stimulation (and was therefore used for chronic stimulation) because the electrode was in direct contact with the crista in that ear. As noted earlier, the density of nerve fibers in the ampullarly nerve was less in this ear than that in the nonstimulated ear (which had the more distally located electrode tip). We cannot conclude from this one monkey whether trauma from electrode insertion or whether chronic electrical stimulation caused the reduction in nerve fiber density in the stimulated ear. Since the eye movements produced by single pulses did not change during chronic stimulation in monkey N, however, it is probable that the nerve fiber density in the ampullary nerve (or at least that subset of neurons that were activated electrically) did not change substantially during the period of chronic stimulation.
A final concern is that the efficacy of synaptic transmission in the vestibular pathways may have been degraded by the constant release of synaptic vesicles caused by the high level of tonic electrical stimulation. Such synaptic effects could certainly contribute to, or be responsible for, the initial rapid drop in gain that occurred when stimulation began and could affect the time constant as well (particularly in monkey N; see following text). It should be emphasized, however, that despite the initial drop, the VOR gain recovered and gradually increased over time with repeated cycles of stimulation. In particular, large but short-lived increases in gain (and time constant in monkey N) occurred almost every time stimulation was initiated after the 1-wk-off period, implying that any impairment in synaptic transmission during chronic stimulation was reversible.
In sum, our data suggest that the ability of the electrodes to deliver current and to stimulate the direct (three-neuron) VOR pathway was not degraded during chronic electrical stimulation. Conversely, the increase in VOR gain and the improvement in VOR rotational axis and symmetry provide compelling evidence that central adaptive mechanisms were engaged by prosthetic stimulation and served to improve the compensatory nature of the VOR response.
Adaptation of vestibular tone and gain
During the first period of prosthetic stimulation there was a rapid attenuation of spontaneous nystagmus and a concomitant rapid decline in VOR gain followed by a gradual increase in the VOR gain. Although we did not record activity of peripheral or central vestibular neurons during prosthetic stimulation, we can formulate a hypothesis about the neural mechanisms that may underlie these behavioral observations. In this regard, it is helpful to compare the neural consequences of a destructive lesion such as a labyrinthectomy, which have been extensively studied, with the possible neural changes that could occur during prosthetic stimulation.
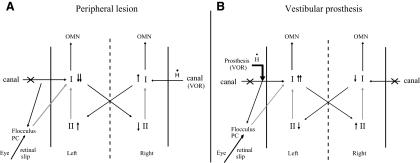
With a destructive lesion, the tone imbalance is caused by a loss of spontaneous activity in the type I neurons in the ipsilateral vestibular nucleus and the gain reduction reflects loss of input from the destroyed labyrinth, with the residual VOR driven by signals from the intact labyrinth that modulate activity in the type I neurons contralateral to the lesion (Fig. 9A) (Fetter and Zee 1988). The reduction in ipsilateral type I activity probably reflects both the loss of excitatory input from the lesioned labyrinth and the inhibition from the commissural pathways that connect the vestibular nuclei on both sides (Ris and Godaux 1998). Recovery of spontaneous activity in the ipsilateral type I neurons, which occurs over a period of several days (Ris et al. 1995), contributes to the rebalancing of vestibular tone (eliminating the spontaneous nystagmus) and to the recovery of the VOR, by partially restoring the normal push–pull modulation in activity in the central vestibular neurons on both sides that are driven by the remaining labyrinth (Dieringer 1995; Vidal et al. 1998). There is evidence that these compensatory changes are produced, at least in part, by modifications in the inhibitory commissural pathways (Berquist et al. 2008). In contrast, activation of the vestibular prosthesis in monkeys with bilateral canal plugs stimulates the ipsilateral type I neurons at rates that are higher than normal, and the VOR response is driven by the prosthesis via these same ipsilateral type I neurons (Fig. 9B). The increased activity in the ipsilateral type I neurons may be augmented by the positive feedback produced by the commissural connections.
Fig. 9.
Schematic diagram showing changes in activity in the vestibular nuclei after a unilateral labyrinthectomy (A) and the changes in nuclear activity we hypothesized may occur after prosthesis activation in an animal with bilateral lateral canal plugs (B). Labyrinthectomy eliminates both the tonic and motion-modulated activity in the afferent vestibular nerve; canal plugging does not affect the tonic level of activity but appreciably attenuates the motion-modulated component. Both types of lesions are indicated by an “ ” through the canal ampullary nerve. I, type I vestibular neurons; II, type II vestibular neurons; OMN, ocular motor neurons; PC, Purkinje cells. Black arrows represent excitatory connections and gray arrows inhibitory connections.
” through the canal ampullary nerve. I, type I vestibular neurons; II, type II vestibular neurons; OMN, ocular motor neurons; PC, Purkinje cells. Black arrows represent excitatory connections and gray arrows inhibitory connections.
The changes in VOR gain we documented could potentially be explained by two neural processes with differing dynamics. First, when the lateral canal afferents are tonically stimulated at a high rate, it is probable that either the primary afferents or the central vestibular neurons that receive their input become less responsive to the electrical stimulation fairly rapidly (Courjon et al. 1987; Goldberg et al. 1984). This decline in the response to electrical stimulation could result from reduced efficacy of synaptic transmission in the VOR pathway, as discussed earlier, or could parallel the habituation that occurs when vestibular cues are maintained tonically (e.g., Jeannerod et al. 1976). Independent of the underlying mechanism, this reduced response to electrical stimulation would help to rebalance the central vestibular tone because it would lower the rate of activity in the ipsilateral type I neurons and thus should reduce the spontaneous nystagmus produced by the tonic stimulation (Fig. 9B). Another consequence of this reduced sensitivity to electrical stimulation, however, would be an early decrease in the eye-movement (VOR) response produced by motion-modulated prosthetic stimulation, which we observed in both monkeys soon after the prosthesis was activated.
To increase the VOR gain during head turns while eliminating spontaneous nystagmus when the head is stationary, the brain must increase its sensitivity to the dynamic modulations in electrical stimulation associated with head rotations while reducing its sensitivity to the high rate of tonic stimulation. One way these changes could be achieved is by increasing the sensitivity of the central vestibular pathways to the prosthetic input while high-pass filtering the prosthetic signal in a manner that attenuates the static (DC) bias introduced by the high level of tonic stimulation. Somewhat analogous forms of frequency-specific adaptation have been described in the VOR in normal animals (Lisberger et al. 1983). As discussed in the following text, we suggest that this central processing is an adaptive response driven by the brain's attempt to concurrently minimize the retinal slip error signal when the head is stationary (suppressing spontaneous nystagmus by restoring tone balance) and moving (improving the compensatory nature of the VOR response).
In summary, the changes in spontaneous nystagmus and the VOR that occurred after the initiation of prosthetic stimulation are consistent with a superposition of two processes: 1) reduced sensitivity of the afferents or their central connections to electrical stimulation (which attenuates the spontaneous nystagmus but causes the initial reduction in the VOR gain) and 2) a slower form of associative learning that minimizes retinal slip when the head is stationary (further reducing the spontaneous nystagmus) and rotating (increasing the VOR gain).
Effects of cycling the stimulation state
In a prior study we provided long-term prosthetic stimulation of the lateral canal in monkeys with bilateral lateral canal plugs and found that the VOR gain did not increase significantly over time (Merfeld et al. 2007). When the prosthetic stimulation was first turned on or when its sensitivity was modified, however, substantial but transient elevations in the VOR gain were observed (Lewis et al. 2003; Merfeld et al. 2007). For this reason, in the current study we chose to repeatedly cycle the prosthetic stimulation in the monkeys between the off, low-sensitivity, and high-sensitivity states. We found that after each off-to-on transition, the VOR gain at the onset of stimulation was generally greater than that recorded after the previous off-to-on transition. The VOR gain typically decreased rapidly after each on transition but then gradually increased and plateaued at a new level that was higher than that recorded in the previous on state. Since we did not independently examine the effects of each type of transition (off to low-sensitivity, low-sensitivity to high-sensitivity), we do not know whether one or both of these transitions are necessary for the gain adaptation observed in the two monkeys.
We propose that the two mechanisms described earlier (rapid loss of sensitivity to electrical stimulation; slower frequency-dependent increased central sensitivity to electrical stimulation) can potentially explain these results if we hypothesize that retention of these two changes differed during the periods when the prosthetic stimulation was off. Specifically, we suggest that during the 1-wk-off periods, the general sensitivity to electrical stimulation improved but the increased frequency-dependent sensitivity to dynamic stimulation persisted. The mechanism underlying the proposed recovery of sensitivity to electrical stimulation during the prosthesis-off periods is unclear, but it is probable that the period without electrical stimulation allowed synaptic transmission in the VOR pathway, which may have been attenuated during chronic electrical stimulation, to significantly improve its efficacy. In this scenario, when the prosthesis was reactivated after 1 wk in the subsequent cycles of stimulation, the initial VOR gain should be greater each time, since a relatively normal sensitivity to electrical stimulation was applied to a progressively more sensitive central vestibular circuit.
Another possible explanation for the gradual increase in gain that occurred when the stimulation state was cycled could relate to the three signals used by the brain to estimate the dynamics of head motion during voluntary head turns: vestibular cues from the canals, proprioceptive inputs from the neck, and a copy of the efferent command sent to the neck muscles (efference copy) (Sadeghi et al. 2007). Normally these three cues should be congruent, allowing the animal to generate an accurate estimate of head motion. After bilateral canal plugging, the canal afferent cue is markedly attenuated, but presumably over time the animal learns to associate the diminished canal cue with the proprioceptive and efferent signals to calibrate a reasonably accurate estimate of head dynamics. When the prosthesis is turned on in the low-sensitivity state or changes from the low to high-sensitivity state, a large, abrupt discrepancy is introduced between the velocity dependence of the new prosthetic canal cue and the unchanged proprioceptive and efferent signals. Each of these transitions thus requires the brain to recalibrate its internal estimate of head motion so that the new vestibular cue is congruent with the proprioceptive and efferent cues. It is reasonable to consider that the adaptive processes that recalibrate estimated head motion may be associated with the central vestibular adaptation that increases the VOR gain. Repeating these transitions many times may therefore augment the VOR adaptive process and ultimately result in a larger VOR gain than that obtained after one off-to-on transition followed by chronic prosthetic stimulation (Merfeld et al. 2007).
Mechanisms that may underlie changes in VOR gain
It is probable that the gradual increase in VOR gain reflects, at least in part, the learning that occurs when retinal slip signals are temporally associated with the vestibular cues that encode head motion (e,g., Kawato and Gomi 1992). This form of learning has been extensively studied in normal subjects using optical devices that produce image motion on the retina during head turns (e.g., Miles and Eighmy 1980). Since the bilateral canal plugs dramatically reduced the horizontal VOR response and the gain produced by the prosthesis when it was initially activated was much less than unity, substantial horizontal retinal slip occurred during horizontal head movements. Smaller vertical retinal slip was also present during horizontal head rotations, since these head rotations produced relatively small vertical eye movements. Our results suggest that an associative form of learning based on a reduction in retinal slip contributed to the changes in VOR, since the horizontal eye movements produced by yaw-axis head rotation gradually increased in magnitude (reducing horizontal retinal slip), whereas the off-axis vertical eye movements decreased (reducing vertical retinal slip). These changes shifted the eyes' rotational axis in the frontal plane toward alignment with the head's rotational axis.
A second potential mechanism for VOR adaptation is that chronic, high-frequency electrical stimulation of canal afferents produced a generalized increase in the sensitivity of the VOR pathways to electrical stimulation. In this scenario, the electrical stimulation alone—not the temporal coupling of prosthetic stimulation with retinal slip cues during head motion—is responsible for changes in synaptic efficacy in the VOR pathways (Capocchi et al. 1992). This mechanism cannot be entirely excluded but it is unlikely to be solely responsible for the observed changes in VOR gain, since it would be predicted to nonspecifically increase both the horizontal and vertical eye-movement responses that were produced at the onset of prosthetic stimulation. As noted earlier, the vertical eye movements remained the same or decreased their amplitude during prosthetic stimulation, whereas the horizontal eye movements simultaneously increased their size, findings that are inconsistent with a generalized increase in sensitivity to electrical stimulation.
A third potential mechanism relates to the canal-plugged preparation we used. It has been demonstrated in rhesus monkeys and other species that a frequency-dependent recovery of the VOR occurs gradually after unilateral or bilateral canal plugging (Angelaki et al. 1996; Broussard et al. 1999). It is thus possible that the adaptation we observed occurred in response to the canal-plugged preparation, not the chronic electrical stimulation, and resulted from either a peripheral or central change in sensitivity in the VOR pathways (Hess et al. 2000). Our results are generally inconsistent with this mechanism of adaptation. First, over the frequency range we tested, there was no significant increase in VOR gain over time when the prosthesis was turned off. This implies that the gain change observed with the prosthesis on did not simply reflect recovery from the canal plug. Second, when we used the prosthesis for many months but did not cycle between the off, low-sensitivity, and high-sensitivity states, we did not observe any meaningful increases in VOR gain (Merfeld et al. 2007). The pattern of electrical stimulation thus appears to be responsible for the changes in VOR gain over time, not simply adaptation engendered by the canal-plugging procedure.
Whereas the VOR gain increased substantially over time in both monkeys, the peak velocity of the eye movements evoked with single cathodic pulses in monkey N did not follow a similar pattern. The initial eye movement produced by the single pulse had a latency of about 5 ms and it has been argued that this early component of the response, reflected in the peak eye velocity, results from activation of the disynaptic VOR pathway in the brain stem (Broussard and Hong 2003; Broussard et al. 1992). Our results therefore imply that the adaptive changes in VOR gain primarily resulted from changes in polysynaptic VOR pathways, which could include cerebellar and/or commissural projections (Broussard et al. 1992). Similar results were previously described in animals that underwent optically induced modification of the VOR gain because the early eye-movement response produced by single electrical pulses depended weakly on the VOR gain in monkeys (Broussard et al. 1992) and was independent of the gain in cats (Broussard and Hong 2003).
Temporal characteristics of the VOR response
The time constant of the VOR produced by the prosthesis was shorter than the time constant used by the prosthesis to generate the electrical stimulation of the canal afferents. In monkey N, the time constant showed a characteristic pattern, in that it was largest at the onset of each stimulation period but then rapidly declined (Fig. 6). This pattern was not as prominent or consistent in monkey S, although several stimulation cycles did show a small but rapid decline in the time constant at the onset of stimulation (see, for example, the onset of the third and fourth low-sensitivity cycles in Fig. 6).
The mechanism underlying these results is uncertain, but is almost certainly multifactorial. Since the time constant of the VOR was not increased beyond that of the peripheral input when prosthetic stimulation was first activated, it appears that the velocity storage integrator was not engaged by the prosthetic input. It has been well documented that vestibular cues in animals with canal plugs do not access the velocity storage integrator (Angelaki et al. 1996; Paige 1983), so it is likely that either the canal-plugged preparation or the nonphysiologic characteristics of the electrical stimulation provided by the prosthesis were responsible for the absence of velocity storage at the onset of stimulation. The subsequent rapid decline of the time constant observed most stereotypically in monkey N could reflect reduced efficacy of synaptic transmission in the central vestibular pathways resulting from the high level of tonic stimulation. This effect was clearly reversible, however, since the time constant returned to a similar value at the start of each stimulation cycle in monkey N.
Conclusions
Our results demonstrate that the VOR produced by a unilateral canal prosthesis shows adaptive capabilities during chronic stimulation, evidenced by an increase in the gain and improvement in the rotational axis and symmetry of the eye-movement response. These adaptive changes indicate that the brain can use the artificial information provided by the prosthesis to improve behavioral performance.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grants DC-6909 and DC-8362 to R. F. Lewis and DC-8167 to D. M. Merfeld.
ACKNOWLEDGMENTS
We thank S. Merchant, B. Silveira, M. Lankow, L. Zupan, D. Channer, and R. Terry.
REFERENCES
- Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutritional Examination Survey, 2001–2004. Arch Intern Med 169: 938–944, 2009 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Hess BJM, Arai Y, Suzuki J-I. Adaptation of primate vestibuloocular reflex to altered peripheral vestibular inputs. I. Frequency-specific recovery of horizontal VOR after inactivation of the lateral semicircular canals. J Neurophysiol 76: 2941–2953, 1996 [DOI] [PubMed] [Google Scholar]
- Bergquist F, Ludwid M, Dutia MB. Role of commissural inhibitory system in vestibular compensation in the rat. J Physiol 586: 4441–4452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanks RH, Curthoys IS, Bennett ML, Markham CH. Planar relationship of the semicircular canals in rhesus and squirrel monkeys. Brain Res 340: 315–324, 1985 [DOI] [PubMed] [Google Scholar]
- Broussard DM, Bhatia JK, Jones GEG. The dynamics of the vestibulo-ocular reflex after peripheral vestibular damage. I. Frequency-dependent asymmetry. Exp Brain Res 125: 353–364, 1999 [DOI] [PubMed] [Google Scholar]
- Broussard DM, Bronte-Stewart HM, Lisberger SG. Expression of motor learning in the response of the primate vestibuloocular reflex pathway to electrical stimulation. J Neurophysiol 67: 1493–1508, 1992 [DOI] [PubMed] [Google Scholar]
- Broussard DM, Hong JA. The response of vestibulo-ocular reflex pathways to electrical stimulation after canal plugging. Exp Brain Res 149: 237–248, 2003 [DOI] [PubMed] [Google Scholar]
- Capocchi G, Della Torre G, Grassi S, Pettorossi VE, Zampolini M. NMDA receptor-mediated long term modulation of electrically evoked field potentials in the rat medial vestibular nuclei. Exp Brain Res 90: 546–550, 1992 [DOI] [PubMed] [Google Scholar]
- Courjon JH, Precht W, Sirkin DW. Vestibular nerve and nuclei unit responses and eye movement responses to repetitive galvanic stimulation of the labyrinth in the rat. Exp Brain Res 66: 41–48, 1987 [DOI] [PubMed] [Google Scholar]
- Demer J, Porter F, Goldberg J, Jenkins H, Schmidt K. Adaptation to telescopic spectacles: vestibulo-ocular reflex plasticity. Invest Ophthalmol Vis Sci 30: 159–170, 1989 [PubMed] [Google Scholar]
- Dieringer N. “Vestibular compensation”: Neural plasticity and its relations to functional recovery after labyrinthine lesions in frogs and other vertebrates. Prog Neurobiol 46: 97–129, 1995 [PubMed] [Google Scholar]
- Dimitri PS, Wall C, 3rd, Oas JG. Classification of human rotation test results using parametric modeling and multivariate statistics. Acta Otolaryngol 116: 497–506, 1996 [DOI] [PubMed] [Google Scholar]
- Dimitri PS, Wall C, 3rd, Rauch SD. Multivariate vestibular testing: thresholds for bilateral Ménière's disease and aminoglycoside ototoxicity. J Vestib Res 11: 391–404, 2001/2002 [PubMed] [Google Scholar]
- Fernandez C, Goldberg J. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol 34: 661–675, 1971 [DOI] [PubMed] [Google Scholar]
- Fetter M, Zee DS. Recovery from unilateral labyrinthectomy in rhesus monkey. J Neurophysiol 59: 370–393, 1988 [DOI] [PubMed] [Google Scholar]
- Furman JM, Becker JT. Vestibular responses in Wernicke's encephalopathy. Ann Neurol 26: 669–674, 1989 [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol 34: 635–660, 1971 [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of squirrel monkey. J Neurophysiol 51: 1236–1256, 1984 [DOI] [PubMed] [Google Scholar]
- Gong W, Merfeld DM. Prototype neural semicircular canal prosthesis using patterned electrical stimulation. Ann Biomed Eng 28: 572–581, 2000 [DOI] [PubMed] [Google Scholar]
- Gong W, Merfeld DM. System design and performance of a unilateral horizontal semicircular canal prosthesis. IEEE Trans Biomed Eng 49: 175–181, 2002 [DOI] [PubMed] [Google Scholar]
- Hess BJM, Lysakowski A, Minor LB, Angelaki DE. Central versus peripheral origin of vestibulo-ocular reflex recovery following semicircular canal plugging in rhesus monkeys. J Neurophysiol 84: 3078–3082, 2000 [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Magnin M, Schmid R, Stefanelli M. Vestibular habituation to angular velocity step in the cat. Biol Cybern 22: 39–48, 1976 [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980 [DOI] [PubMed] [Google Scholar]
- Kawaato M, Gomi H. The cerebellum and VOR/OKR learning models. Trends Neurosci 15: 445–453, 1992 [DOI] [PubMed] [Google Scholar]
- Kramer PD, Shelhamer M, Zee DS. Short-term adaptation of the phase of the vestibulo-ocular reflex (VOR) in normal human subjects. Exp Brain Res 106: 318–326, 1995 [DOI] [PubMed] [Google Scholar]
- Lewis RF, Gong W, Ramsey M, Minor L, Boyle R, Merfeld DM. Vestibular adaptation studied with a prosthetic semicircular canal. J Vestib Res 12: 87–94, 2003/2004 [PubMed] [Google Scholar]
- Lewis RF, Haburcakova C, Gong W, Merfeld DM. Effects of semicircular canal activation on perceived head orientation. ARO Abstr 1107, 2006 [Google Scholar]
- Li PM, Somdas MA, Eddington DK, Nadol JR., Jr Analysis of intracochlear new bond and fibrous tissue formation in human subjects with cochlear implants. Ann Otol Rhinol Laryngol 116: 731–738, 2007 [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Miles FA, Optican LM. Frequency-selective adaptation: evidence for channels in the vestibulo-ocular reflex? J Neurosci 3: 1234–1244, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merfeld DM, Gong W, Morrissey J, Saginaw M, Haburcakova C, Lewis RF. Acclimation to chronic constant-rate peripheral stimulation provided by a vestibular prosthesis. IEEE Trans Biomed Eng 53: 2362–2372, 2006 [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Haburcakova C, Gong W, Lewis RF. Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEE Trans Biomed Eng 54: 1005–1015, 2007 [DOI] [PubMed] [Google Scholar]
- Miles FA, Eighmy BB. Long-term adaptive changes in primate vestibuloocular reflex. I. Behavioral observations. J Neurophysiol 43: 1406–1425, 1980 [DOI] [PubMed] [Google Scholar]
- Minor LB, Goldberg JM. Vestibular-nerve inputs to the vestibule-ocular reflex: a functional-ablation study in the squirrel monkey. J Neurosci 11: 1636–1648, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary MJ, Fayad J, House WF, Linthicum FH., Jr Electrode insertion trauma in cochlear implantation. Ann Otol Rhinol Laryngol 100: 695–699, 1991 [DOI] [PubMed] [Google Scholar]
- Paige GD. Vestibuloocular reflex and its interactions with visual following mechanisms in the squirrel monkey. II. Response characteristics and plasticity following unilateral inactivation of horizontal canal. J Neurophysiol 49: 152–168, 1983 [DOI] [PubMed] [Google Scholar]
- Paige GD. Senescence of human visual-vestibular interactions. 1. Vestibulo-ocular reflex and adaptive plasticity with aging. J Vestib Res 2: 131–151, 1992 [PubMed] [Google Scholar]
- Quinn KJ, Helminski JO, Didier AJ, Baker JF, Peterson BW. Changes in sensitivity of vestibular nucleus neurons induced by cross-axis adaptation of the vestibulo-ocular reflex in the cat. Brain Res 718: 176–180, 1996 [DOI] [PubMed] [Google Scholar]
- Rabbitt RD, Boyle R, Highstein SM. Influence of surgical plugging on horizontal semicircular canal mechanics and afferent response dynamics. J Neurophysiol 82: 1033–1053, 1999 [DOI] [PubMed] [Google Scholar]
- Ris L, de Waele C, Serafin M, Vidal P-P, Godaux E. Neuronal activity in the ipsilateral vestibular nucleus following unilateral labyrinthectomy in the alert guinea-pig. J Neurophysiol 74: 2087–2099, 1995 [DOI] [PubMed] [Google Scholar]
- Ris L, Godaux E. Neuronal activity in the vestibular nuclei after contralateral or bilateral labyrinthectomy in the alert guinea pig. J Neurophysiol 80: 2352–2367, 1998 [DOI] [PubMed] [Google Scholar]
- Robblee LS, Rose TL. Electrochemical guidelines for selection of protocols and electrode materials for neural stimulation. In: Neural Prostheses: Fundamental Studies, edited by Agnew WF, McCreery DB. Englewood Cliffs, NJ: Prentice Hall, 1990, p. 25–66 [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol 97: 1503–1514, 2007 [DOI] [PubMed] [Google Scholar]
- Shepard RK, Clark GM. Effect of high electrical stimulus intensities on the auditory nerve suing brain stem response audiometry. Ann Otol Rhinol Laryngol 96, Suppl. 128: 50–52, 1987 [Google Scholar]
- Tykocinski M, Shepard RK, Clark G. Reduction in excitability of the auditory nerve following electrical stimulation at high stimulus rates. Hear Res 88: 124–142, 1995 [DOI] [PubMed] [Google Scholar]
- Vidal P-P, De Waele C, Vibert N, Muhlethaler M. Vestibular compensation revisted. Otolaryngol Hean Neck Surg 119: 34–42, 1998 [DOI] [PubMed] [Google Scholar]