Abstract
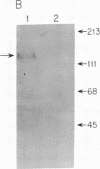
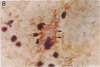
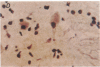
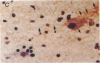
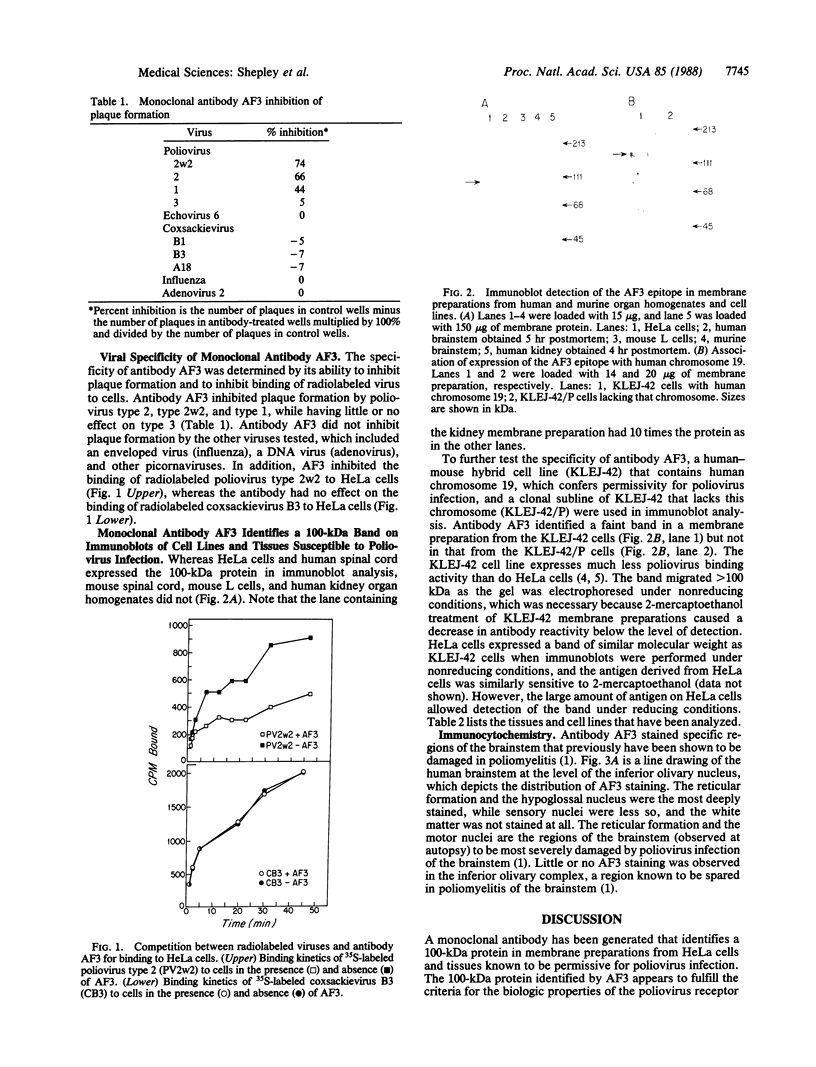
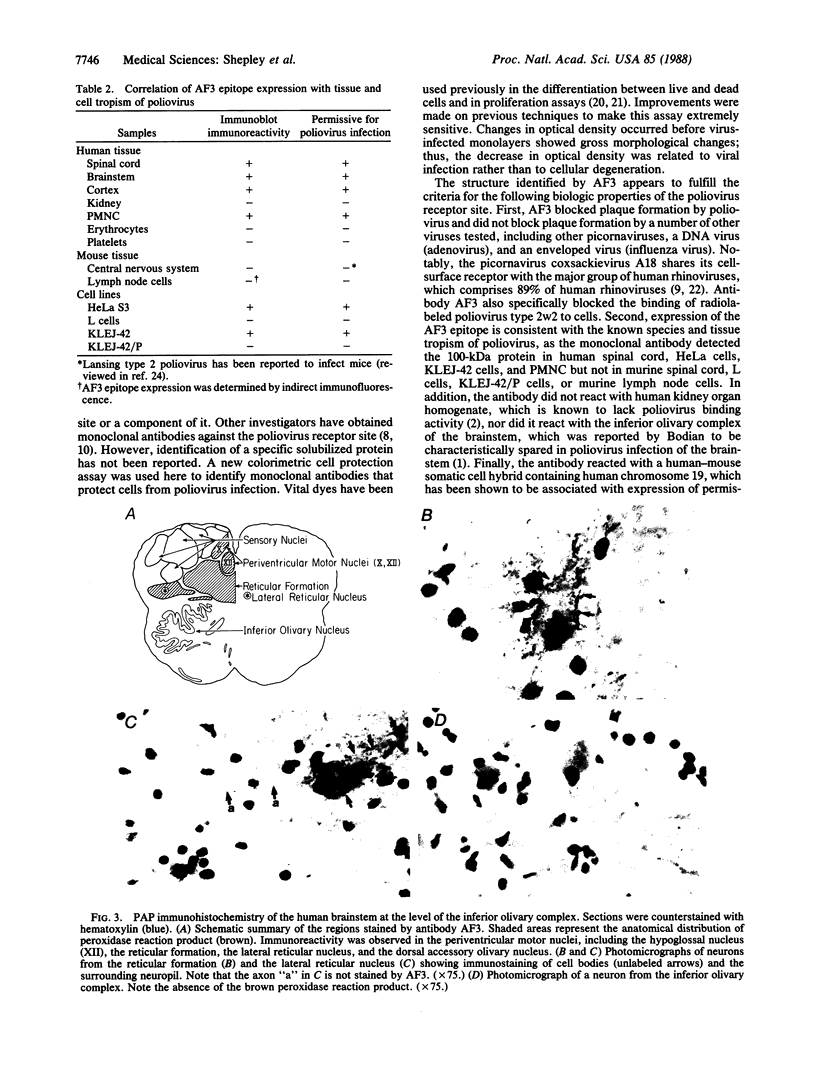
Unique receptor sites for poliovirus are considered to be the primary determinant of the virus' cell and tissue-type specificity. To study the poliovirus-cell interaction, eight monoclonal antibodies that specifically block the cytopathic effects of poliovirus were generated by using HeLa cell preparations as immunogen and a newly developed colorimetric screening assay. Plaque-inhibition assays confirmed the viral specificity of the antibodies, and when one antibody, AF3, was used as a probe in immunoblots of cell membrane preparations, it detected a 100-kDa band in only those cell lines and tissues permissive for poliovirus infection. AF3 also specifically inhibited radiolabeled poliovirus binding to cells. In terms of tissue specificity, AF3 detected the 100-kDa band in membrane preparations from human spinal cord but not in organ homogenates of human kidney or in murine tissue, including the central nervous system. Furthermore, AF3 detected the band in a human-mouse hybrid cell line containing human chromosome 19, which confers permissivity for poliovirus infection, but the antibody did not detect the band in a human chromosome 19-deficient subclone. In an immunohistochemical study of the human brainstem, AF3 stained neurons in the reticular formation and clusters of brainstem neurons, consistent with the known pattern of damage caused by poliovirus infection in the brainstem. Furthermore, AF3 reacted with human peripheral mononuclear cells, consistent with the known replication of poliovirus in Peyer's patches and tonsils. These results strongly suggest that the 100-kDa band detected by antibody AF3 is, or is closely associated with, the poliovirus receptor site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Colonno R. J. Many rhinovirus serotypes share the same cellular receptor. J Virol. 1984 Aug;51(2):340–345. doi: 10.1128/jvi.51.2.340-345.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. H., Jr, Johnson D., Ogonowski M., Weiner H. L. Type 1 human poliovirus binds to human synaptosomes. Ann Neurol. 1987 Jan;21(1):64–70. doi: 10.1002/ana.410210112. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Callahan P. L., Long W. J. Isolation of a monoclonal antibody that blocks attachment of the major group of human rhinoviruses. J Virol. 1986 Jan;57(1):7–12. doi: 10.1128/jvi.57.1.7-12.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell R. L., Field A. K., Schleif W. A., Long W. L., Colonno R. J., Mapoles J. E., Emini E. A. Monoclonal antibody that inhibits infection of HeLa and rhabdomyosarcoma cells by selected enteroviruses through receptor blockade. J Virol. 1986 Feb;57(2):438–445. doi: 10.1128/jvi.57.2.438-445.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. Receptor affinities as major determinants of enterovirus tissue tropisms in humans. Virology. 1961 Nov;15:312–326. doi: 10.1016/0042-6822(61)90363-4. [DOI] [PubMed] [Google Scholar]
- Heeg K., Reimann J., Kabelitz D., Hardt C., Wagner H. A rapid colorimetric assay for the determination of IL-2-producing helper T cell frequencies. J Immunol Methods. 1985 Mar 18;77(2):237–246. doi: 10.1016/0022-1759(85)90036-5. [DOI] [PubMed] [Google Scholar]
- La Monica N., Meriam C., Racaniello V. R. Mapping of sequences required for mouse neurovirulence of poliovirus type 2 Lansing. J Virol. 1986 Feb;57(2):515–525. doi: 10.1128/jvi.57.2.515-525.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Mapoles J. E., Krah D. L., Crowell R. L. Purification of a HeLa cell receptor protein for group B coxsackieviruses. J Virol. 1985 Sep;55(3):560–566. doi: 10.1128/jvi.55.3.560-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLAREN L. C., HOLLAND J. J., SYVERTON J. T. The mammalian cell-virus relationship. I. Attachment of poliovirus to cultivated cells of primate and non-primate origin. J Exp Med. 1959 May 1;109(5):475–485. doi: 10.1084/jem.109.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano L., Green H. Picornavirus receptors and picornavirus multiplication in human-mouse hybrid cell lines. Virology. 1973 Aug;54(2):515–524. doi: 10.1016/0042-6822(73)90161-x. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C., Johnson B., Lionetti K. A., Nobis P., Wimmer E., Racaniello V. R. Transformation of a human poliovirus receptor gene into mouse cells. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7845–7849. doi: 10.1073/pnas.83.20.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P. D., Pipkin P. A., Hockley D., Schild G. C., Almond J. W. Monoclonal antibodies which block cellular receptors of poliovirus. Virus Res. 1984;1(3):203–212. doi: 10.1016/0168-1702(84)90039-x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nobis P., Zibirre R., Meyer G., Kühne J., Warnecke G., Koch G. Production of a monoclonal antibody against an epitope on HeLa cells that is the functional poliovirus binding site. J Gen Virol. 1985 Dec;66(Pt 12):2563–2569. doi: 10.1099/0022-1317-66-12-2563. [DOI] [PubMed] [Google Scholar]
- Sherry B., Rueckert R. Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J Virol. 1985 Jan;53(1):137–143. doi: 10.1128/jvi.53.1.137-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini J. E., Colonno R. J. Isolation of a receptor protein involved in attachment of human rhinoviruses. J Virol. 1986 May;58(2):290–295. doi: 10.1128/jvi.58.2.290-295.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems F. T., Melnick J. L., Rawls W. E. Replication of poliovirus in phytohemagglutinin-stimulated human lymphocytes. J Virol. 1969 May;3(5):451–457. doi: 10.1128/jvi.3.5.451-457.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T., Papahadjopoulos D., Taber R. Biological properties of poliovirus encapsulated in lipid vesicles: antibody resistance and infectivity in virus-resistant cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3471–3475. doi: 10.1073/pnas.74.8.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]