Abstract
Cholinergic activation profoundly affects vertebrate forebrain networks, but pathway, cell type, and modality specificity remain poorly understood. Here we investigated cell-specific cholinergic modulation of neurons in the zebra finch forebrain song control nucleus HVC using in vitro whole cell recordings. The HVC contains projection neurons that exclusively project to either another song motor nucleus RA (robust nucleus of the arcopallium) (HVC-RAn) or the basal ganglia Area X (HVC-Xn) and these populations are synaptically coupled by a network of GABAergic interneurons. Among HVC-RAn, we observed two physiologically distinct classes that fire either phasically or tonically to injected current. Muscarine excited phasic HVC-RAn and most HVC-Xn. Effects were observed under conditions of blockade of fast synaptic transmission and were reversed by atropine. In contrast, unlike what is commonly observed in mammalian systems, HVC interneurons were inhibited by muscarine and these effects were reversed by atropine. Thus cholinergic modulation reconfigures the HVC network in a more complex fashion than that implied by monolithic “gating.” The two projection pathways are decoupled through suppression of the inhibitory network that links them, whereas each is simultaneously predominantly excited. We speculate that fluctuating cholinergic tone in HVC could modulate the interaction of song motor commands with basal ganglia circuitry associated with song perception and modification. Furthermore, if the in vitro distinction between RA-projecting neurons that we observed is also present in vivo, then the song system motor pathway exhibits greater physiological diversity than has been commonly assumed.
INTRODUCTION
As in many behavioral model systems, spontaneous and auditory activity in the zebra finch song system is strongly regulated by behavioral state (Cardin and Schmidt 2003; Dave et al. 1998; Rauske et al. 2003; Schmidt and Konishi 1998). The mechanisms of behavioral state regulation in the song system are poorly understood, but may be important for sleep-dependent features of learning (Dave and Margoliash 2000; Shank and Margoliash 2009), reconfiguration of the song system during singing and regulation of auditory feedback (Prather et al. 2008), song perceptual mechanisms that involve the song system (e.g., Brenowitz 1991; Gentner et al. 2000), and circadian modulation of auditory responses during juvenile song learning (Nick and Konishi 2005). Cholinergic basal forebrain (BF) has been identified as one likely contributor to behavioral state-dependent changes in song system physiology, with the forebrain sensorimotor nucleus HVC implicated as a key target for cholinergic modulation (Shea and Margoliash 2003).
HVC elaborates two major output pathways, each arising from distinct classes of projection neurons. One class (HVC-RAn) projects to the robust nucleus of the arcopallium (RA), forming a premotor pathway that is obligatory for song production (Nottebohm et al. 1976). There are at least two distinct morphological types of RA-projecting HVC neurons (Fortune and Margoliash 1995; Nixdorf et al. 1989). Another class (HVC-Xn) projects to the basal ganglia Area X, forming the origin of the anterior forebrain pathway (AFP), which has been widely implicated in juvenile and adult song modification (Bottjer et al. 1984; Brainard and Doupe 2000; Kao et al. 2005; ölveczky et al. 2005; Scharff and Nottebohm 1991; Sohrabji et al. 1990; Williams and Mehta 1999;) as well as conspecific song perception (Burt et al. 2000; Prather et al. 2009; Scharff et al. 1998). HVC is necessary for singing (Nottebohm et al. 1976) and also receives song-selective auditory input from forebrain auditory regions, including the interfacial nucleus (NIf) (Coleman and Mooney 2004; Janata and Margoliash 1999), conveyed to both output pathways (Doupe and Konishi 1991; Kimpo et al. 2003; Vicario and Yohay 1993). HVC, NIf, and the Uvaeform nucleus (Uva) (which projects to HVC and NIf) are likely targets of cholinergic and/or noradrenergic modulation (Akutagawa and Konishi 2005; Cardin and Schmidt 2004; Dave et al. 1998; Shea and Margoliash 2003) and thus are well positioned to regulate song-system activity.
The state dependence of HVC auditory responses varies according to cell type. Variability with respect to circadian modulation of auditory activity has been observed among distinct putative interneuron types (HVC-In) (Rauske et al. 2003). Moreover, differences in the expression of wakeful auditory responses among RA (Dave et al. 1998) and two classes of identified HVC projection neurons (Prather et al. 2008) imply different state-dependent effects on the HVC-RAn and HVC-Xn pathways. HVC receives a projection from cholinergic cells in the BF that are part of a field considered homologous to the mammalian nucleus Basalis of Meynert (Li and Sakaguchi 1997; Reiner et al. 2004). Stimulation of the cholinergic BF suppresses auditory activity in HVC and RA, an effect that can be blocked by prior injection of cholinergic antagonists into HVC. Data from HVC injections of nicotine and muscarine further imply that each cholinergic receptor class acts on a distinct but overlapping population of neurons in HVC, suggesting pathway-specific effects (Shea and Margoliash 2003). Here we use in vitro whole cell recordings from identified neurons in HVC of adult male zebra finches to directly explore the circuit mechanisms of HVC cholinergic regulation.
METHODS
Preparation of brain slices
All experimental procedures were approved by the University of Chicago Institutional Animal Care and Use Committee. Briefly, brain slices were prepared from adult (>100 days) male zebra finches according to established methods (Farries and Perkel 2002; Livingston and Mooney 1997). Birds were deeply anesthetized with halothane and quickly decapitated and the brain was removed and transferred to chilled artificial cerebrospinal fluid (ACSF), pregassed with 95% O2-5% CO2. The two hemispheres were separated midsagittally, then each medial face was glued to the stage of a vibrating microtome (Ted Pella, Redding, CA) and sliced at a thickness of 400–500 μm. Slices were maintained 1–12 h at room temperature in a custom-built interface chamber until recording. The composition of the ACSF (in mM) was: 119 NaCl, 2.5 KCl, 1.3 MgCl2, 2.5 CaCl2, 1 NaH2PO4, 26.2 NaHCO3, and 11 glucose. The initial extraction and slicing of the tissue was performed in ACSF in which the NaCl was replaced with equiosmolar sucrose (Aghajanian and Rasmussen 1989). In a minority of experiments (n = 36/173 cells), recordings were made using a modified ACSF recipe composed of (in mM): 118 NaCl, 3 KCl, 1 MgCl2, 1.5 CaCl2, 1 NaH2PO4, 25 NaHCO3, and 30 glucose. Another minority of cells (n = 10/173) were recorded in ACSF that has been used in mammalian cortical slices to elicit network activity (Sanchez-Vives and McCormick 2000) (in mM): 126 NaCl, 3.5 KCl, 1 MgCl2, 1.2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose. No systematic differences in network or intrinsic activity were observed according to ACSF type and thus data were pooled.
Electrophysiological recordings
For recording, slices were transferred to a superfusion chamber and perfused at 10–15 ml/min with ACSF maintained at 35°C. Anecdotally, our preliminary experiments indicated improved tissue viability and stronger spontaneous synaptic activity in slices under these conditions of elevated temperature.
Intracellular recordings were achieved using “blind” whole cell recording techniques in bridge mode. Borosilicate recording pipettes were pulled on a horizontal puller (Sutter Instrument, Novato, CA) to a final resistance of 3–9 MΩ when filled with a pipette solution consisting of (in mM): 120 K-methylsulfate, 10 HEPES, 2 EGTA, 8 NaCl, 2 MgATP, and 0.3 NaGTP. This solution was adjusted to pH 7.3 with KOH. A modified pipette solution was used during some experiments (n = 36/169 cells) consisting of (in mM): 140 K-gluconate, 1 CaCl2, 10 HEPES, 10 EGTA, 2 MgCl2, and 4 Na2ATP. No differences in recordings were observed between these solutions. Data were amplified and low-pass filtered at 3 kHz with an NPI SEC-05 amplifier (ALA Scientific, Westbury, NY). Data were acquired and analyzed using the Digidata 1320/pClamp8 package (Axon Instruments, Foster City, CA). Voltage traces were corrected for an empirically measured liquid junction potential (+9 mV for standard ACSF and pipette solutions). The cocktail of drugs used to block fast synaptic transmission was: 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 10 μM R-(−)-3-(2-carboxypiperazin-4-yl)propanephosphonic acid) (CPP), and 10–20 μM (+)-bicuculline. At the concentration used, the bicuculline free base is not known to elicit the nonspecific effects that have been reported for bicuculline methiodide (Debarbieux et al. 1998; Johnson and Seutin 1997). Synaptic transmission blocker concentrations were chosen based on previous studies in mice (e.g., Tryba et al. 2008), but were also close to those used previously in zebra finch (e.g., Schmidt and Perkel 1998). Concentrations of muscarine and atropine were also similar to those used in studies of mammalian cortex in vitro (e.g., Gil et al. 1997; Hsieh et al. 2000). Aliquots of bicuculline were prepared in DMSO and all other drugs were dissolved in water. Application of the synaptic blockade cocktail occasionally resulted in a small depolarization of the neuron and always blocked spontaneous postsynaptic potentials that were often evident in recordings from cells of all types. All drugs and reagents were obtained from Sigma/RBI (St. Louis, MO), except for potassium methylsulfate (ICN Biomedicals, Aurora, OH).
Physiological measurement and analysis
Conventional whole cell recordings are prone to run down due to dialysis of the intracellular contents; thus we were vigilant in observing the baseline for stability. Only cells that showed a stable membrane potential (Vm) below −50 mV and that spiked readily to current injection were considered for further analysis. During many recordings (n = 138/173), we also monitored membrane resistance (Rm) with several brief (300 ms), small (20 and 80 pA) hyperpolarizing current pulses. In those cases we also excluded the cell for analysis if, over the baseline period, Rm deviated by >15%. All but four cells remained within 10%.
The basic design for all experiments was to periodically make 500-ms depolarizing current injections of a fixed amplitude and monitor Vm and evoked spike rate in an ongoing fashion. (We changed the frequency of these measurements over the course of these studies.) Once a stable baseline was established, drugs were bath applied as described and changes in Vm and spike rate were assessed. For most cells (n = 164/173), resting Vm and the response to current injection were measured every 10 s; a minority of cells (n = 9) were tested in the same manner but more frequently. In all cases, the amplitude of the depolarizing current pulse was set to roughly 20–25% above action potential threshold (mean: 250 ± 130 pA; range: 50–650 pA).
Physiological measurements for assessing cell types were taken from the first six sweeps (typically the first minute of data; see methods). The spike takeoff point was defined as the peak of the second derivative of the voltage trace. Spike afterhyperpolarization (AHP) amplitude and time-to-peak (TTP) were measured relative to this point. AHP time-to-decay (TTD) was defined as the time after the AHP peak for the voltage to decay halfway to baseline. The recovery slope of the AHP was defined as the mean instantaneous slope for 2 ms following the peak of the AHP (Spiro et al. 1999). Spike width was defined as the width halfway between the takeoff point and the apex of the spike. Sag percentage was calculated from the response to hyperpolarizing current pulse as [(ΔVm peak − ΔVm late)/(ΔVm peak)] × 100%, where Vm peak is the initial peak voltage, Vm late is the voltage at the end of the pulse, and Δ signifies the value's deviation from resting Vm. Mean firing rate during depolarizing current pulses was normalized to the magnitude of the injection as a measure of responsiveness (spikes·s−1·nA−1). The phasicness measure, ranging between 0 and 1, was 1 minus a ratio calculated by the latency to the last spike over the total duration of the pulse (for a graphical and algebraic representation see Supplemental Fig. S1).1 This measure was chosen because it is simple, yet cleanly divided our cell distribution into two disparate modes: completely accommodating phasic cells and minimally accommodating tonic cells.
Mean Vm and the rate of spiking to current injection were monitored in an ongoing fashion during drug application and washout. Mean values for each parameter for the 10 sweeps just prior to drug application were taken as the predrug value and the mean for the last 10 sweeps of drug application were taken as the postdrug value. For individual drug presentations, the significance of a given drug effect was also assessed with an unpaired t-test comparison of the 10 predrug and postdrug values and a significant effect implies P < 0.05. Population measures for drug applications were assessed with a paired t-test.
Histology
Cells recorded with pipette solution containing 0.5% Neurobiotin (Vector Laboratories, Burlingame, CA) were filled passively while recording and, when possible, at the end of each recording by applying depolarizing current pulses (1 s, 0.5 Hz, 0.3–1.0 nA) for ≤20 min. Slices were preserved using 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), cryoprotected with 30% sucrose in PBS for >12 h, and resectioned on a freezing sledge microtome at 50 μm. Some labeled cells (n = 84) were visualized using a standard avidin–peroxidase procedure (Fortune and Margoliash 1995). Other cells (n = 25) were visualized with fluorescence confocal microscopy. For the latter group, sections were washed in PBS with 0.3% Triton X-100 three times for 20 min each and were then incubated for 2–4 h with a 1:667 dilution of streptavidin-conjugated Alexafluor 594 (S-11227; Invitrogen, Carlsbad, CA) PBS with 0.3% Triton X-100. Sections were then thoroughly washed again in PBS, mounted with an aqueous medium, and screened for successful labeling using a Zeiss Axioplan2 upright scope (Carl Zeiss, Thornwood, NY) under epifluorescence. Images of all filled cells were digitally acquired. Light microscope images were taken with a Nikon D1X digital camera with a 2.5× photographic tube. Confocal stacks of fluorescent cells were taken with a Zeiss Axiovert 100 using LSM 510 software at 25× in 1-μm optical sections. Image stacks were viewed and Z-projected using ImageJ (National Institutes of Health, Bethesda, MD) or LSM Viewer (Zeiss).
RESULTS
Physiological and anatomical classes of HVC neurons
Whole cell intracellular recordings were made from neurons within the clearly visible borders of HVC. We recorded 173 cells in 157 slices of either hemisphere from 74 adult male zebra finches and these stable recordings were used to quantitatively characterize the physiological activity of each cell. The axons of filled cells were also used to classify the projection class of the cells, following the procedure of a previous in vitro study (Dutar et al. 1998). All HVC-RAn send their axons in a tight fasciculated bundle directly toward RA, which lies nearly directly ventral to HVC (Fortune and Margoliash 1995; Mooney 1992). In contrast, HVC-Xn send out axons over a more dispersed area, but generally head rostrally toward Area X through one of several rostral fiber paths (Fortune and Margoliash 1995). We recovered 28 cells that exhibited a clear axon exiting HVC ventrally through the visible RA-projecting bundle; these were categorized as RA-projecting HVC neurons. An additional 22 cells were recovered with an axon exiting HVC at a more rostral location and projecting rostrally (toward Area X); these were categorized as X-projecting HVC neurons. In contrast to projection neurons, whose major axons projected directly toward the borders of HVC, in a third group of cells (n = 7) that were recovered, the axons were elaborately branched without apparently exiting HVC; these cells were categorized as interneurons. [All HVC interneurons are believed to be GABAergic (Fortune and Margoliash 1995; Mooney 2000; Mooney and Prather 2005; Wild et al. 2005).] Cells that did not have a visible axon (n = 52) were not assigned an identity based on morphology.
Phasically firing HVC-RAn
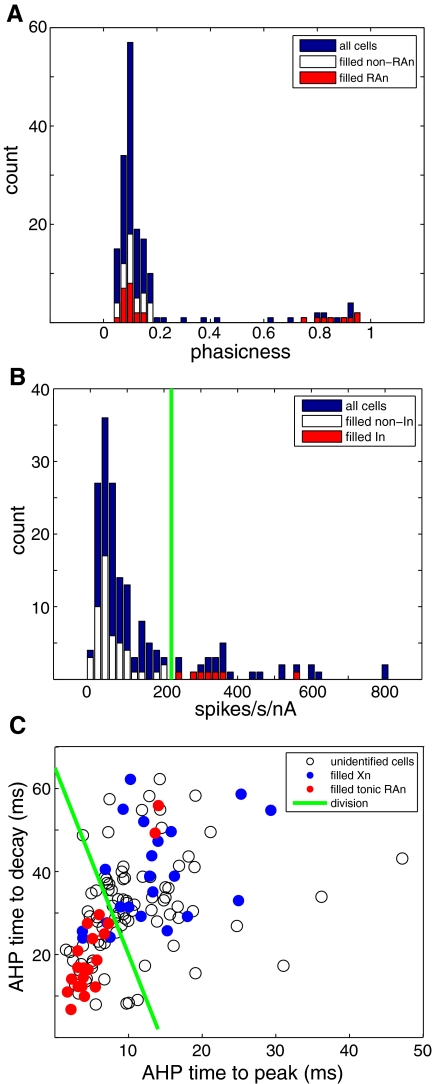
We found two distinct types of HVC-RAn with respect to phasicness (Fig. 1). Considering the 28 filled HVC-RAn, 20 had a phasicness value of ≤0.14, whereas the other 8 had a phasicness value of ≥0.74 (Fig. 2A, red bars). The latter cells are consistent with RA-projecting cells previously recorded in vitro that fired phasically (Dutar et al. 1998). We tested the significance of the unimodality of this distribution with the dip statistic (Hartigan and Hartigan 1985), demonstrating that the distribution of phasicness of HVC-RAn was statistically significantly different from a unimodal distribution (dip statistic = 0.107, P < 0.05). Although splitting the data at any particular value is somewhat arbitrary, given that the phasicness values clustered into two widely separated, distinct groups, we chose an arbitrary criterion (phasicness = 0.5) to distinguish the two groups of HVC-RAn.
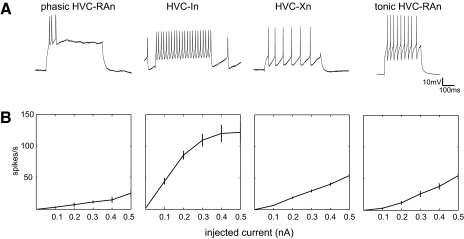
Fig. 1.
Physiological classes of HVC neurons recorded in vitro. A: responses of 4 HVC cells to near-threshold depolarizing current injection (tonic HVC-RAn [motor nucleus RA (robust nucleus of the arcopallium)]: +0.4 nA; all others: +0.2 nA), showing features characteristic of the 4 cell types we observed. There are notable differences in spike morphology, firing rates, and accommodation. Each cell here identified as a projection neuron was filled with Neurobiotin and visibly elaborated an axon that exited HVC in the direction of the efferent target nucleus. The interneuron was also filled; it had no extrinsically projecting axon and was morphologically consistent with known HVC-In (interneuron type). B: mean current-firing plots for each cell type shown in A.
Fig. 2.
Distinguishing physiological characteristics of anatomically identified HVC cell types. A: identification of phasic HVC-RAn. A histogram for all cells of the phasicness of the suprathreshold response to somatic current injection (see methods) reveals 2 modes: one highly phasic and one tonic. All of the filled cells with axons in the phasic mode (>0.5) projected to RA. Note that a distinct population of filled RA-projecting neurons clustered near zero in the tonic mode. B: identification of putative HVC-In. A histogram of normalized firing rate for all cells shows that all of the filled interneurons had values >225 normalized spikes·s−1·nA−1, but all projection neurons had values <225 normalized spikes·s−1·nA−1. C: a scatterplot of HVC-Xn (basal ganglia Area X) and tonic HVC-RAn on 2 measures of afterhyperpolarization (AHP) timing: time to peak (AHPttp) and time to decay (AHPttd) (see methods), showing relative separation between the 2 cell classes. Filled points indicate cells that were verified to have axons projecting to Area X (blue) or to RA (red). The green line was used to conservatively identify a region containing most HVC-Xn, while excluding HVC-RAn (with 2 notable exceptions).
Considering all 173 cells, a histogram of phasicness also exhibits two clear modes (Fig. 2A), revealing 16 cells that fired a brief phasic burst (phasicness ≥0.62) and 157 cells that fired more tonically throughout the current pulse (phasicness ≤0.42). Compared with the distribution of filled HVC-RAn, in this larger population of cells there are more cells with phasicness values intermediate between the two modes. This is expected for a larger data sample on the basis of chance alone. Thus the additional data do not violate the hypothesis that the phasic HVC-RAn represent a distinct physiological pattern of activity and support an additional hypothesis that phasic HVC-RAn are physiologically distinct from all other HVC neurons.
At least two anatomical classes of HVC-RAn have been observed previously, in canaries (Nixdorf et al. 1989) and zebra finches (Fortune and Margoliash 1995). Two classes of HVC-RAn were also observed in vitro in slices from juvenile zebra finches (Kubota and Taniguchi 1998). The differences in response properties in the prior study were not as dramatic as what we have observed, which may reflect the difference in recording from immature and mature neurons.
HVC-In
Another neuronal type we observed fired tonically at very high rates to even modest levels of depolarizing current injection (Fig. 1) and matched the previously reported properties of HVC-In (Dutar et al. 1998; Kubota and Taniguchi 1998; Mooney 2000). The characteristic steep current–firing rate relationship of these cells was captured with a measure of firing rate normalized to current injection magnitude. A histogram of normalized firing rate for all cells is shown in Fig. 2B, with anatomically confirmed projection neurons plotted in white and putative interneurons plotted in red. HVC-In had high normalized firing rates and occupied a region of the histogram that was nonoverlapping, albeit continuous with the set of confirmed projection neurons. The clustering of the 7 HVC-In at the tail of the distribution of normalized firing rates for all 57 filled cells is highly significant (Mann–Whitney U test, comparing filled HVC-In with all other filled cells, P < 0.001). Given that our normalized measures represent a ratio value, we also examined the same distribution in a log axis plot (Supplemental Fig. S2), which also showed some evidence of bimodality. In light of the complete separation between projection and interneurons on this measure and the close match with established HVC-In physiology, for further analysis we designated cells with a normalized firing rate >225 spikes·s−1·nA−1 as HVC-In (n = 29). Because the distribution of HVC-In was continuous with the distribution of other HVC cells for this measure, it is likely that our criterion misclassifies some cells near the borders of the distributions.
Our experience was that recordings from HVC-In were difficult to achieve and, in particular, it was difficult to maintain lengthy recordings. These cells were also particularly susceptible to rundown. All these properties of the recordings are consistent with what is commonly encountered while recording from interneurons.
Of the seven filled HVC-In that were recovered, four of the cells had smooth beaded dendrites; the dendrites of two others were also smooth, but thicker and not as varicose or elaborated; and one was spinous (Scott and Lois 2007). Despite the considerable morphological diversity within this group, no physiological differences were noted between these types of interneurons. Several prior studies reported that HVC-In exhibit considerable morphological and histochemical diversity (Fortune and Margoliash 1995; Scott and Lois 2007; Wild et al. 2005). Nonetheless these findings have not been correlated with any physiological differences. Rauske et al. (2003) observed differences in the behavioral state modulation of two classes of putative HVC-In recorded chronically in vivo, but the anatomical and histochemical profiles of those classes are unknown. Despite the heterogeneity of HVC-In, their properties are consistent with mammalian GABAergic cortical interneurons (Scott and Lois 2007; Wild et al. 2005) and they are believed to mediate the feedforward inhibition from HVC-RAn onto HVC-Xn (Mooney 2000; Mooney and Prather 2005). There is no evidence of excitatory HVC-In.
Tonically firing HVC-RAn and HVC-Xn
Using the above-cited criteria for phasicness and normalized firing rate, we separated the phasic HVC-RAn and HVC-In from the rest of the data set. The remaining cells included filled neurons that projected to either RA or Area X. We observed that filled HVC-Xn (n = 22) fired tonically (Fig. 1) at moderate rates during current injection, as previously reported (Dutar et al. 1998; Kubota and Taniguchi 1998; Mooney 2000). We also observed a population of filled HVC-RAn (n = 20) with similar tonic firing properties (Fig. 1).
Despite the similarity of the firing patterns of these two tonically firing cell classes, we observed some differences in their physiology. Most notably, the spike AHP kinetics typically differed such that the AHPs of the tonic HVC-RAn were sharper and faster. We visualized this attribute with a two-dimensional scatterplot of AHP time to peak (TTP) versus AHP time to decay (TTD). By this method, cells that projected in the direction of Area X or RA were separated into overlapping clusters (Fig. 2C). We conclude from these morphological and physiological data that there are both X-projecting and RA-projecting cells that fire tonically in HVC and that their anatomical class can be predicted with some probability by an examination of their AHP kinetics. Interestingly, plotting the phasic HVC-RAn on this graph (Supplemental Fig. S3) reveals that these cells cluster with the tonic HVC-RAn, raising the possibility that the two populations share some attributes.
Assessing cholinergic modulation of classes of HVC neurons
We identified four different physiological classes of HVC neurons in vitro and a panel of intrinsic property measurements for each of these classes is reported in Table 1. We assessed whether these neurons are sensitive to modulation by cholinergic drugs and whether this sensitivity varied as a function of cell class. To do this, we made whole cell recordings from HVC in vitro under current-clamp conditions. Periodically (see methods), we measured resting Vm and the spiking response to a single 500-ms depolarizing current pulse. The amplitude of the current pulse was adjusted to roughly 20–25% above threshold and fixed for the duration of the experiment.
Table 1.
Physiological properties of HVC cell types
| Vm, mV | n | Rm, MΩ | n | Spike Width, ms | n | Sag % | n | AHP Amplitude, mV | n | AHP Time to Peak, ms | n | AHP Time to Decay, ms | n | AHP Recov Slope, mV/ms | n | Spikes/s/nA | n | Phasicness | n | Firing Latency | n | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phasic HVC-RAn | −74.1 ± 5.3 | 14 | 260 ± 87 | 8 | 1.60 ± 0.28 | 14 | −1.34 ± 3.0 | 8 | 9.03 ± 3.7 | 11 | 7.72 ± 4.4 | 11 | 13.6 ± 8.8 | 11 | 0.345 ± 0.20 | 11 | 23.9 ± 14 | 14 | 0.125 ± 0.06 | 14 | 28.9 ± 14 | 14 |
| Tonic HVC-RAn | −71.9 ± 8.3 | 47 | 218 ± 88 | 32 | 0.883 ± 0.28 | 47 | 2.08 ± 3.9 | 32 | 14.2 ± 3.7 | 47 | 5.28 ± 2.7 | 47 | 20.5 ± 10 | 47 | 0.526 ± 0.55 | 47 | 75.1 ± 61 | 47 | 0.901 ± 0.03 | 47 | 73.8 ± 61 | 47 |
| HVC-Xn | −66.9 ± 8.4 | 81 | 186 ± 72 | 70 | 1.14 ± 0.34 | 81 | 3.60 ± 9.0 | 70 | 12.3 ± 3.9 | 81 | 13.2 ± 7.2 | 81 | 36.7 ± 11 | 81 | 0.125 ± 0.07 | 81 | 69.0 ± 44 | 81 | 0.859 ± 0.10 | 81 | 54.0 ± 31 | 81 |
| HVC-In | −62.2 ± 7.9 | 31 | 321 ± 140 | 28 | 0.970 ± 0.45 | 31 | 4.20 ± 4.9 | 28 | 11.9 ± 4.4 | 31 | 5.51 ± 1.8 | 31 | 10.2 ± 3.8 | 31 | 0.383 ± 0.22 | 31 | 414 ± 200 | 31 | 0.915 ± 0.06 | 31 | 23.4 ± 16 | 31 |
n, number of cells contributing to each measure.
If baseline behavior was stable (see methods), drugs were applied and we continued to monitor changes in resting Vm and spike rate. The effects of muscarinic cholinergic receptor activation were assessed with application of the cholinergic agonists muscarine (10 μM) and carbachol (10 or 100 μM). In some experiments, to assess the selectivity of the observed effects for muscarinic receptors, we added the selective muscarinic receptor antagonist atropine (1 μM) to the bath in the continued presence of muscarine. Additionally, to assess the network dependence of drug effect, some experiments were performed in the presence of a cocktail of drugs that block fast excitatory and inhibitory synaptic transmission (see methods). In many cases, we also measured the dynamics of input resistance (Supplemental Fig. S4). Because assessing nicotinic cholinergic modulation is complicated by rapid receptor desensitization, we did not attempt to evaluate the contribution of nicotinic receptors to cholinergic modulation using our bath perfusion approach.
Cholinergic agonists excite phasic HVC-RAn
We tested 14 phasic type RA-projecting cells with cholinergic drugs in the manner described earlier and we observed a consistent, robust, and direct excitation following application of muscarine, a selective agonist for muscarinic-type cholinergic receptors. Phasic HVC-RAn showed increases in evoked spike rate and depolarization of resting Vm following muscarine application. These effects were reversed by the selective muscarinic receptor antagonist atropine and persisted in the presence of fast synaptic blockers. Figure 3A depicts a typical experiment. For this cell we continuously measured resting membrane potential and spike rate evoked by a somatic current injection pulse (+0.13 nA). These measures were stable prior to drug application and this stability continued when a cocktail of fast synaptic transmission blockers (CNQX, CPP, bicuculline; see methods) was applied and then maintained for the duration of the recording. When 10 μM muscarine was applied to the bath, the cell exhibited an abrupt increase in evoked spike rate and a sharp membrane depolarization. These effects rapidly reversed on bath application of 1 μM atropine in the continued presence of agonist. Subsequently, baseline spiking behavior reemerged and the cell repolarized to near its initial value.
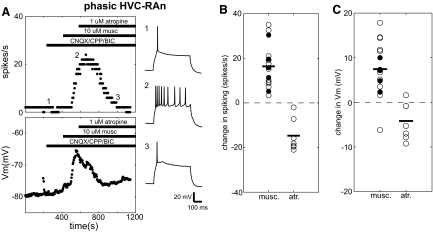
Fig. 3.
Phasic HVC-RAn are directly excited by muscarine. A: typical response of a phasic HVC-RAn to muscarine and atropine. Panels depict ongoing measurements of evoked spike rate (top left) and membrane potential (Vm, bottom left) taken regularly throughout the recording (see methods). Horizontal bars denote the times of drug application. As indicated, this experiment was performed in the presence of fast synaptic blockers. The right panels (1–3) show sample voltage sweeps in response to +0.13-nA current pulses before (1) and after (2) wash-in of 10 μM muscarine and after subsequent addition of 1 μM atropine (3). Numbers show how the traces correspond to the time points in the plots to the left. B: distribution of changes in spiking for phasic HVC-RAn in response to cholinergic agents. White and black circles denote recordings made, respectively, in the absence and presence of synaptic blockers. For muscarine experiments, changes in spike rate for each point are calculated as the difference between the predrug and postdrug values for that experiment (see methods). For atropine, the changes are also calculated as the difference between the predrug and postdrug values, relative to values measured in the presence of muscarine. Horizontal bars denote the population means. C: distribution of changes in Vm for phasic HVC-RAn in response to cholinergic agents. This panel is organized and the data were calculated as in B.
Eleven phasic HVC-RAn were treated with 10 μM muscarine alone and all were robustly excited by the drug. For the 11 cells treated with muscarine, comparing the last 10 sweeps before wash-in and the last 10 sweeps before washout (see methods), each cell showed significantly greater spiking and 10/11 also showed a significant depolarization. As a population, these cells showed significant increases in evoked spike rate (pre: 5.20 ± 4.9 spikes/s; post: 21.5 ± 12 spikes/s; paired t-test, P < 0.01) and membrane voltage (pre: −73.9 ± 13 mV; post: −65.9 ± 14 mV; paired t-test, P < 0.01) (Fig. 3, B and C). Four of the neurons fired so vigorously after muscarine wash-in that spike failure was seen (e.g., Supplemental Fig. S5). We did not discern whether this is a primary effect of muscarine or whether the mere depolarization by muscarine secondarily causes the spike failure; however, cells that showed spike failure did not differ from the others in the magnitude of depolarization. Despite the dramatic excitation elicited by muscarine, no phasic HVC-RAn ever fired spikes at rest under any condition.
The excitatory effects of muscarine on phasic HVC-RAn did not depend on synaptic network interactions. In an additional set of experiments (n = 5), 10 μM muscarine was applied in the presence of a cocktail of synaptic blockers (see methods) and all five cells showed significant increases in evoked spiking and membrane potential. Significant population changes were comparable to those seen without synaptic blockers for mean evoked spike rate (pre: 1.36 ± 1.3 spikes/s; post: 17.8 ± 9.0 spikes/s; paired t-test, P < 0.05) and membrane voltage (pre: −73.5 ± 8.2 mV; post: −67.2 ± 8.2 mV; paired t-test, P < 0.01) (Fig. 3, B and C). These data are consistent with a direct effect of muscarine on these neurons (Fig. 3, B and C).
The effects of muscarine on phasic HVC-RAn were consistently reversed by atropine, a competitive antagonist for muscarinic receptors, further supporting a specific action at muscarinic cholinergic receptors. Some cells treated with muscarine were also subsequently perfused with 1 μM atropine in the continued presence of muscarine. In each of seven cases, the effects of muscarine on spiking and Vm were significantly reversed on wash-in of atropine. Spiking behavior in all seven cells was increased by the initial muscarine application and, subsequently, atropine significantly decreased spiking in each cell. Six cells were also initially depolarized by muscarine and all were significantly hyperpolarized when treated with atropine. Population mean spiking following this treatment was significantly decreased from 23.0 ± 11 to 8.30 ± 9.6 spikes/s (n = 7; paired t-test, P < 0.01) (Fig. 3B). Population mean membrane voltage was also significantly hyperpolarized (n = 7; pre: −57.7 ± 9.7 mV; post: −61.9 ± 9.1 mV; paired t-test, P < 0.05) (Fig. 3C). These changes cannot be explained by desensitization of receptors; when presented alone, muscarine was in the bath with sustained effects for substantially longer (mean = 270 ± 74 s across all cell types) than the latency to atropine addition (mean = 170 ± 50 s across all cell types).
Although we collected substantial anatomical and intrinsic physiological data indicating that the HVC-RAn pathway includes a population of tonically firing cells in addition to these phasic cells, we had difficulty obtaining stable and consistent data from them in the presence of cholinergic drugs. Therefore we do not report their response to cholinergic stimulation here.
Cholinergic agonists excite basal ganglia–projecting HVC neurons
We also assessed cholinergic modulation in HVC neurons projecting to the basal ganglia nucleus Area X (HVC-Xn) by performing a very similar set of experiments. These cells were also repeatedly injected with brief pulses of suprathreshold depolarizing current and the evoked spike rate and resting membrane potential were measured on an ongoing basis. Like phasic HVC-RAn, HVC-Xn were excited by muscarinic cholinergic activation, albeit with somewhat lower consistency, typically showing increases in evoked spike rate and depolarization of their resting membrane potential after bath application of muscarine. These effects also persisted in the presence of blockers of fast synaptic transmission and were reversed by adding atropine to the bath in the presence of muscarine. An example of all of these effects can be seen in Fig. 4B. Resting membrane potential and spiking evoked by pulses of current injection (+0.16 nA) were continuously measured every 10 s. These measures were at baseline and after addition of fast synaptic transmission blockers. On addition of 10 μM muscarine to the bath, the cell rapidly depolarized >10 mV and nearly doubled its spike rate in response to current injection. These effects were significantly reversed when 1 μM atropine was applied in the continued presence of agonist.
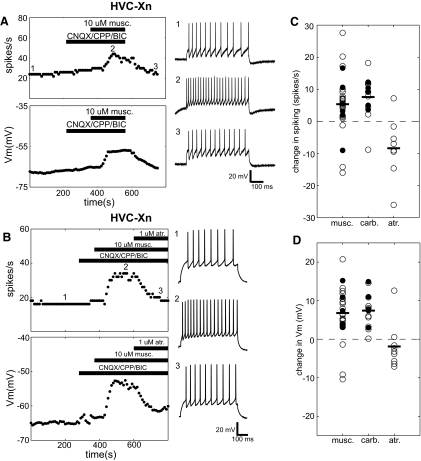
Fig. 4.
HVC-Xn are directly excited by muscarine. A: typical response of an anatomically identified HVC-Xn to muscarine. Panels depict ongoing measurements of evoked spike rate (top left) and Vm (bottom left) measurements taken every 10 s throughout the recording (see methods). Horizontal bars denote the times of drug application. As indicated, this experiment was performed in the presence of fast synaptic blockers. The right panels (1–3) show sample voltage sweeps in response to +0.25-nA current pulses before (1) and after (2) wash-in of 10 μM muscarine and after subsequent washout of all drugs (3). Numbers show how the traces correspond to the time points in the plots to the left. B: typical response of an HVC-Xn to muscarine and atropine. Panels are organized as in A. The right panels (1–3) show sample voltage sweeps in response to +0.16-nA current pulses before (1) and after (2) wash-in of 10 μM muscarine in the presence of fast synaptic blockers and after subsequent addition of 1 μM atropine (3). C: distribution of changes in spiking for HVC-Xn in response to cholinergic agents. White and black circles denote recordings made, respectively, in the absence and presence of synaptic blockers. For muscarine and carbachol experiments, changes in spike rate for each point are calculated as the difference between the predrug and postdrug value for that experiment (see methods). For atropine, the changes are also calculated as the difference between the predrug and postdrug values, that is relative to values measured in the presence of muscarine. Horizontal bars denote the population means. D: distribution of changes in Vm for HVC-Xn in response to cholinergic agents. This panel is organized as in C.
Given the fact that our analysis of projection class and physiological properties did not allow a completely unambiguous separation of HVC-Xn and tonic-firing RA-projecting HVC neurons, here we first restricted our data set to anatomically identified HVC-Xn. In all, 10 identified HVC-Xn were tested with 10 μM bath-applied muscarine. Of these cells, most (9/10) were significantly depolarized by the drug and 6 additionally showed a significant increase in spiking. One exceptional cell showed a dramatic effect that was consistently of the opposite sign, exhibiting significant decreases in both spiking and membrane potential. When this cell is included with the other data, filled HVC-Xn still showed a significant depolarization (pre: −64.7 ± 7.8 to −57.4 ± 12 mV; paired t-test, P < 0.01), although the change in spike rate was not significant (pre: 14.2 ± 6.2 spikes/s; post: 18.3 ± 11 spikes/s; paired t-test, P = 0.17) (Fig. 4, C and D). When the outlier was excluded, the remaining cells (n = 9) showed significant changes in both measures (paired t-test, P < 0.05). An example of typical muscarine effects on an anatomically identified HVC-Xn is shown in Fig. 4A. At baseline and after adding fast synaptic blockers to the bath, this cell showed steady tonic spiking in response to somatic current injection. Bath application of 10 μM muscarine evoked a significant increase in this tonic firing rate and also significantly depolarized the cell. Both measures steadily returned to their baseline values following washout of all drugs.
The results obtained from the larger corpus of physiologically identified HVC-Xn cells were consistent with results obtained from morphologically identified HVC-Xn, including the inhibition seen in a small percentage of cells. In this data set, we included all cells whose physiological properties fall within the region of Fig. 2C above the green line (almost exclusively occupied by identified HVC-Xn), with the exception of the 2 that exhibited an axon projecting to RA. Considering all experiments with muscarine in putative HVC-Xn (n = 22), mean spiking was significantly increased from 12.2 ± 4.6 to 17.8 ± 5.2 spikes/s (paired t-test, P < 0.05) and mean membrane voltage depolarized from −63.7 ± 8.0 to 57.1 ± 12 mV (paired t-test, P < 0.001) (Fig. 4, C and D). Of 22 putative HVC-Xn, most were excited by muscarine application: 19 showed a significant depolarization and 17/19 of these also showed a significant increase in spiking. There were also 3 cells (of 22) that showed pronounced and significant inhibition in response to the application of muscarine. These cells showed a mean 10.5 ± 8.0 spikes/s decrease in spiking and a mean hyperpolarization of 6.99 ± 4.9 mV.
Both the predominant excitatory and occasional inhibitory effects on HVC-Xn were very likely direct because they persisted in the presence of blockade of fast synaptic transmission. Six cells were presented with 10 μM muscarine in the presence of synaptic blockers and five of them were significantly excited by this treatment, with all five of them showing significant depolarization and four of them additionally showing increased evoked spiking. The sixth cell was initially inhibited by muscarine alone and, again, hyperpolarized and reduced its spiking in muscarine under conditions of synaptic blockade. Thus the full range of cholinergic effects in HVC-Xn were observed in fast synaptic blockers. Over all cells (n = 6) there was a significant membrane depolarization from −62.7 ± 9.1 to −55.3 ± 9.8 mV (paired t-test, P < 0.05); again, however, as a result of the bimodal distribution of effects, mean spiking was not significantly changed (pre: 12.0 ± 6.3 to 16.6 ± 11 spikes/s; paired t-test, P = 0.23) (Fig. 4, C and D). Changes in both measures were significant for the five excited cells (paired t-test, P < 0.05).
Further confirming the cholinergic modulation of HVC-Xn, very similar predominantly excitatory effects were observed in 13 putative HVC-Xn that were tested with carbachol, an unselective cholinergic receptor agonist. Nine cells were treated with 10 μM (n = 3) or 100 μM (n = 6) carbachol (similar effects were seen with both concentrations and these were combined for analysis). In addition, 5 neurons were treated with 100 μM carbachol in the presence of synaptic blockers. Among all carbachol experiments, 13 of 14 (including all 5 with synaptic blockers) showed a significant depolarization and a significant increase in spiking in response to current injection, suggesting direct, predominantly excitatory effects of carbachol on the recorded neuron. The exceptional neuron (tested with carbachol alone) showed a strong and significant decrease in spikes to current injection that resembled the occasional inhibitory effects of muscarine. Overall, the population mean spike rate was significantly increased from 12.0 ± 4.0 to 19.7 ± 8.1 spikes/s (paired t-test, P < 0.001) and the population mean membrane voltage was elevated from −64.5 ± 8.6 to −57.1 ± 9.6 mV by carbachol application (paired t-test, P < 0.001) (Fig. 4, C and D).
The effects of muscarine on HVC-Xn were reversed by atropine, consistent with a specific action at muscarinic cholinergic receptors. Nine cells tested with muscarine were additionally subsequently perfused with 1 μM atropine in the continued presence of muscarine, leading to a reversal of the initial effects. The robust and significant depolarization and increase in spikes evoked by current injection that appeared shortly after addition of muscarine to the bath abruptly and nearly completely reversed when 1 μM atropine was added in the continued presence of muscarine. On addition of atropine, all cells showed a significant change in membrane potential that was opposite in sign to the primary effects of muscarine. This was also true with respect to firing rate changes for eight of nine cells. Considering the cells that were initially excited by muscarine (n = 8/9), population mean spike rate was significantly reduced by atropine (n = 8; pre: 18.4 ± 8.8 spikes/s; post: 8.15 ± 7.2 spikes/s; paired t-test, P < 0.001) and the population mean membrane voltage was also significantly lower following atropine application (n = 8; pre: −56.5 ± 8.7 mV; post: −60.2 ± 9.3 mV; paired t-test, P < 0.01) (Fig. 4, C and D). The ninth cell tested with atropine was initially inhibited by muscarine and this effect was also reversed by atropine; the cell exhibited significantly more spiking and a significantly more depolarized membrane potential following atropine addition. A typical example of the atropine reversal of muscarine effects is depicted in the latter time points of Fig. 4B.
Cholinergic agonists inhibit HVC interneurons
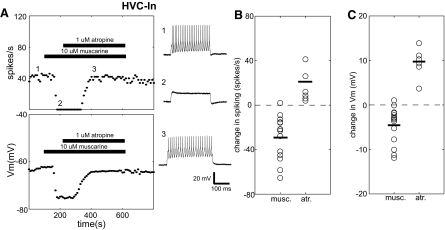
The strong cholinergic modulation in HVC projection neurons raises the question of whether this modulation extends to the HVC network of GABAergic interneurons (HVC-In), which serve as a synaptic intermediary between the RA-projecting and X-projecting pathways (Mooney 2000; Mooney and Prather 2005). To assess this, HVC-In were tested in the same manner by delivering repeated brief pulses of suprathreshold depolarizing current and continuously monitoring the evoked spike rate and resting membrane potential. In contrast to the muscarine effects on projection neurons seen in these experiments, we observed strong inhibition of interneurons following application of muscarine, which was reversed by atropine. One example showing both effects is depicted in Fig. 5A. Prior to drug application, this HVC-In cell showed tonic high-frequency firing in response to current injection. Shortly after bath application of 10 μM muscarine, there was a large, sudden hyperpolarization, accompanied by complete cessation of spiking. Subsequently, after addition of 1 μM atropine there was a complete recovery of baseline physiology, with the membrane potential and spike rate ultimately stably returning to their initial values.
Fig. 5.
HVC interneurons are inhibited by muscarine. A: typical response of an HVC-In to muscarine and atropine. Panels depict ongoing measurements of evoked spike rate (top left) and Vm (bottom left) measurements taken every 10 s throughout the recording (see methods). Horizontal bars denote the times of drug application. The right panels (1–3) show sample voltage sweeps in response to +0.15-nA current pulses before (1) and after (2) wash-in of 10 μM muscarine and after subsequent addition of 1 μM atropine (3). Numbers show how the traces correspond to the time points in the plots to the left. B: distribution of changes in spiking for HVC-In in response to cholinergic agents. For muscarine experiments, changes in spike rate for each point are calculated as the difference between the predrug and postdrug values for that experiment (see methods). For atropine, the changes are also calculated as the difference between the predrug and postdrug values, relative to values measured in the presence of muscarine. Changes indicated for atropine are relative to values measured after muscarine application. Horizontal bars denotes the population means. C: distribution of changes in Vm for HVC-In in response to cholinergic agents. This panel is organized as in B.
Fourteen HVC-In were tested with 10 μM muscarine and most were dramatically inhibited. Of the 14 cells, 13 showed a significant decrease in spike rate to current injection and 12 were significantly hyperpolarized after muscarine wash-in. Considering pre- and postdrug population mean values for all muscarine applications (n = 14), spiking to current injection was significantly reduced (pre: 46.0 ± 27 spikes/s; post: 17.2 ± 18 spikes/s; paired t-test, P < 0.001) and membrane voltage was significantly hyperpolarized (pre: −63.8 ± 10 mV; post: −68.3 ± 11 mV; paired t-test, P < 0.01) (Fig. 5, B and C). We were not able to test the effect of muscarine on HVC-In under conditions of fast synaptic blockade.
The inhibition of HVC-In by muscarine was also consistently reversed by atropine. Six neurons that were inhibited by muscarine initially were subsequently treated with atropine in the continued presence of muscarine. Each of these cells was initially inhibited by muscarine; all of them were significantly depolarized when atropine was added and five of six showed a significant increase in spikes to current injection. Population mean membrane voltage was significantly increased from −70.0 ± 14 to −60.5 ± 14 mV (n = 6; paired t-test, P < 0.01) after atropine wash-in (Fig. 5C). Population mean spiking was also significantly increased (n = 6; pre: 20.4 ± 9.0 spikes; post: 36.6 ± 12 spikes; paired t-test, P < 0.05) (Fig. 5B).
DISCUSSION
We have observed strong effects of cholinergic agents on three classes of neurons tested in the sensorimotor song control nucleus HVC of adult male zebra finches. Experiments performed in conjunction with a cholinergic antagonist or with blockade of fast synaptic transmission suggest that these effects may be direct and demonstrate that they are specifically mediated by muscarinic cholinergic receptors. Our data identify specific cellular substrates for the dramatic effects of cholinergic modulation by basal forebrain (BF) that we observed in vivo (Shea and Margoliash 2003) and motivate speculation on its consequences from a circuit-level perspective.
The effects of cholinergic agonists vary according to HVC cell type, involving robust excitation of one projection neuron class (phasic HVC-RAn) and most neurons of a second projection class (HVC-Xn), with concomitant inhibition of interneurons. Interestingly, a minority of HVC-Xn showed a dramatic inhibition by muscarine. It is difficult to evaluate the biological significance of this minority pattern. To some extent, this variability may be explained by miscategorization of some HVC-RAn as HVC-Xn, but it cannot be completely explained by cell-type categorization error since it was observed in one neuron that unambiguously projected to Area X. Some relatively subtle anatomical differences have been reported among variants of HVC-Xn (Fortune and Margoliash 1995; Nixdorf et al. 1989). These pathway-specific effects raise the possibility that a fluctuating level of acetylcholine (ACh) reconfigures HVC circuitry for different behaviors. One specific effect of this reconfiguration may be suppression of the inhibitory interneuron network, which is likely to affect selectivity for autogenous song (Rosen and Mooney 2003) and uncouple song motor commands from the basal ganglia–projecting pathway, which is implicated in song perception and modification (e.g., Brainard and Doupe 2000; Burt et al. 2000; Kao et al. 2005; Mooney and Prather 2005; ölveczky et al. 2005; Scharff et al. 1998). The strong and divergent (excitatory vs. inhibitory) cell-type specificity of cholinergic effects could point to a diversity of muscarinic receptor subtypes in HVC, potentially explaining its modest and variable muscarinic receptor binding (Ball et al. 1990; Ryan and Arnold 1981).
Cholinergic input to HVC is thought to arise from cholinergic basal forebrain, a conclusion supported by anatomical studies (Li and Sakaguchi 1997), and by observations that injections of cholinergic antagonists into HVC block the effects of BF stimulation on HVC neurons (Shea and Margoliash 2003). Recent anatomical data (Akutagawa and Konishi 2005) have been taken to suggest that BF modulation of HVC occurs multisynaptically through the thalamic nucleus Uvaeformis (Uva) (Coleman and Mooney 2006). This cannot explain the present results of direct cholinergic modulation of HVC neurons. Akutagawa and Konishi (2005) reported no choline acetyltransferase–positive neurons in Uva, nor did we observe them in our own material (T. Levin, S. D. Shea, and D. Margoliash, unpublished data). We propose that the cholinergic input to Uva from the thalamic reticular formation reported by Akutagawa and Konishi (2005) is best interpreted to indicate that cholinergic mechanisms act at several levels of the song system.
HVC cell types
We have identified four physiologically distinct classes of HVC neurons. Three of these classes appear to correspond to projection neuron and interneuron types previously described with in vitro intracellular recordings of adult zebra finch HVC (Dutar et al. 1998; Mooney and Prather 2005). The present results include all three types observed in those studies, with the addition of a distinct class of tonic HVC-RAn. Kubota and Taniguchi (1998) reported four types of HVC cells in juvenile zebra finches, including two types of HVC-RAn, but some differences in the physiological phenotypes they report (neither type of HVC-RAn bursts phasically) suggest that cellular properties in the song system mature as birds move into adulthood and song crystallizes. It is noteworthy that the maturation of physiological properties in HVC during development occur in parallel with complex ontogenetic patterns of neuromodulatory expression (e.g., Harding et al. 1998; Sakaguchi and Saito 1991).
Although an anatomical division of the HVC-RAn pathway has been noted in several studies, including in canaries (Nixdorf et al. 1989) and zebra finches (Fortune and Margoliash 1995), the status of functional diversity in this pathway is not resolved. To date, most physiological and theoretical studies of the HVC-RA circuit have recognized only a unitary class of HVC-RAn (e.g., Dutar et al. 1998; Fiete et al. 2004; Hahnloser et al. 2002; Mooney 2000; Mooney and Prather 2005; Troyer and Doupe 2000a,b). Mooney (2000) reported properties of HVC-RAn in vivo in an anesthetized zebra finch preparation. Some cells illustrated have morphologies that we recognize in our tonic HVC-RAn (small with thin dendrites), but in vivo current injection produced variable, temporally patterned activity that did not resemble the physiology of either type we observed in vitro. This could suggest that the physiological categories we observed in vitro change in the context of in vivo network activity in the anesthetized animal or that there are additional cell types that have yet to be recorded in that preparation. Using a different approach, in two studies of extracellular recordings of identified HVC-RAn during singing (Hahnloser et al. 2002), most cells had highly phasic patterns of activity similar to responses of phasic HVC-RAn to somatic current injection, although roughly 10% of the cells had notably more tonic responses suggestive of the tonic HVC-RAn we report here. In light of our observation that muscarine excites phasic HVC-RAn and elicits a more tonic firing pattern, one intriguing possibility is that to some extent the variable physiological patterns exhibited by HVC-RAn may be under the influence of cholinergic or other neuromodulatory control. This is possibly consistent with the similar AHP kinetics in each class (Supplemental Fig. S2). It is also possible that these cells represent different stages of development, given that HVC-RAn undergo massive neurogenesis and apoptosis throughout adulthood (Alvarez-Buylla et al. 1990; Kirn et al. 1991). In any case, these unanswered questions and discrepancies across different data sets should motivate further studies of how RA-projecting cell types differ with regard to their in vivo physiology.
Effects of cholinergic agents
Cholinergic drugs evoked differential effects according to cell type. Most HVC-Xn and all the phasic class of HVC-RAn were robustly excited by cholinergic stimulation; HVC-In were prominently inhibited as were a minority of HVC-Xn. The persistence of cholinergic effects on projection neurons in the presence of CNQX, CPP, and bicuculline suggests the cholinergic effects were mediated by postsynaptic receptors on these neurons.
Neuromodulatory processes have previously been implicated in the behavioral state dependence of auditory responses, observed at several song system nuclei including HVC (Cardin and Schmidt 2004; Dave et al. 1998; Shea and Margoliash 2003). The predominant effect of arousal on HVC auditory activity appears to be suppression, suggesting that auditory input to HVC is “gated” by arousal (Cardin and Schmidt 2003; Rauske et al. 2003; Schmidt and Konishi 1998). However, suppression was assessed with recordings that were likely to have overrepresented HVC-In, based on their high firing rates; thus the effects on projection neurons were not necessarily ascertained. Indeed, although we observed HVC-In suppression by muscarine, the cell-type–dependent mixture of excitatory and inhibitory effects we observe is inconsistent with a unitary “gate.” Thus if cholinergic inputs participate in state-dependent changes in HVC, they are likely to elicit more complex regulation via network reconfiguration.
Our effects in an isolated and reduced network are difficult to extrapolate to a fully functional in vivo HVC network. Nevertheless, the results motivate speculation about how suppression of HVC interneurons will alter auditory processing intrinsic to HVC. In anesthetized birds, excitatory auditory input to all cell types is thought to arise via extrinsic input, possibly via axons from NIf or the caudal mesopallium (CM) (Bauer et al. 2008; Mooney 2000). Although the extrinsic input, from NIf in particular, strongly drives HVC (Coleman and Mooney 2004; Janata and Margoliash 1999), activity is then strongly sculpted by the highly pervasive network of HVC-In (Rosen and Mooney 2003). The critical role of inhibition in auditory responses of HVC-Xn is well documented, but there is also evidence of feedback contacts between HVC-In and HVC-RAn (Mooney and Prather 2005). These intrinsic network interactions may explain why muscarine injections into HVC in vivo suppress auditory activity in RA (Shea and Margoliash 2003). It is also worth noting that the cholinergic response of one major class of HVC-RAn is unaccounted for here. Finally, the rules that govern the relationship of temporally patterned firing in HVC and RA are still unclear (Hahnloser et al. 2002; Leonardo and Fee 2005), which hampers simple predictions about the consequences of enhanced (or reduced) HVC-RAn excitability.
Functional significance of cholinergic input
State-dependent activity has by now been well established in the song system, although connecting this to neuromodulatory regulation of the song system remains speculative. In adult sleeping zebra finches, auditory responses to song playback have been observed in HVC and RA; these responses are not present in awake zebra finches except for some HVC interneurons (Dave and Margoliash 2000; Dave et al. 1998; Kozhevnikov and Fee 2007; Rauske et al. 2003). Spontaneous activity of RA neurons during sleep in adults mimics the response to auditory playback (and singing) of those same neurons (Dave and Margoliash 2000) and, in juveniles, carries information about the tutor song model (Shank and Margoliash 2009).
HVC-RAn that have been recorded during singing show phasic motor activity patterns that are precisely locked to specific song features (Hahnloser et al. 2002). On the other hand, HVC-Xn show phasic patterns of activity that resemble a motor estimate of auditory feedback (Prather et al. 2008) that may be used by the basal ganglia and downstream regions to modify song (Kao et al. 2005; ölveczky et al. 2005). The phasic activity of HVC-Xn is heavily sculpted by feedforward inhibition from HVC-RAn (Rosen and Mooney 2003); thus suppression of the inhibitory network during singing may modulate corollary discharge and uncouple it from motor commands and modulate thresholds for plasticity. A separate perceptual function has also been specifically proposed for HVC-Xn. Prather et al. (2009) reported that categorical neuronal responses to song in HVC-Xn of awake swamp sparrows were correlated with categorical perceptual behavior in field studies. This is consistent with lesion studies that also implicate HVC and the AFP in song perception (Brenowitz 1991; Burt et al. 2000; Gentner et al. 2000; Scharff et al. 1998). Depending on when it is exerted, cholinergic control of the X-projecting pathway could therefore modulate this distinct HVC function. Measurement and manipulation of cholinergic activity in a temporally and spatially precise fashion during singing or song perception are technically challenging but can serve to test such hypotheses.
Comparison with cholinergic modulation of mammalian cortical circuits
Our pattern of results across cell types is somewhat surprising given the observed effects of ACh and its agonists on mammalian cortical circuitry. Whereas we observed dramatic and consistent suppression of HVC-In by muscarine, several types of cortical interneurons are excited by cholinergic stimulation in vitro (Fanselow et al. 2008; Kawaguchi 1997; Xiang et al. 1998). Due to the combination of this network synaptic effect with the direct effects of ACh, modulation of principal excitatory cortical cells is complex. Briefly, the increased output of the GABAergic network is reflected as a short-term hyperpolarization and reduction in excitability in principal cells (Lucas-Meunier 2009; Xiao et al. 2009), occurring alongside a direct hyperpolarization (Gulledge and Stuart 2005). However, sustained exposure to muscarine also directly causes a slow depolarization (Haj-Dahmane and Andrade 1996; McCormick and Prince 1986). Further complexity is observed in a differential modulation of spontaneous and evoked synaptic inhibition onto the principal neurons (Xiao et al. 2009). There are great diversities of cortical and HVC interneurons. Indeed, some cortical interneurons show inhibition by muscarine (Xiang et al. 1998; Xiao et al. 2009), so it remains unclear whether the full range of HVC-In modulation has yet been appreciated or whether the differences in our results reflect a fundamentally different role for ACh in the circuit that might be meaningfully related to behavior.
GRANTS
This work was supported by National Institutes of Health Grants DC-05098 to S. D. Shea and MH-59831 to D. Margoliash.
Supplementary Material
ACKNOWLEDGMENTS
We thank R. Mooney for critical initial training of S. D. Shea and S. M. Sherman (along with R. Mooney) for valuable comments on the manuscript.
Footnotes
1The online version of this article contains supplemental data.
REFERENCES
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse 3: 331–338, 1989 [DOI] [PubMed] [Google Scholar]
- Akutagawa E, Konishi M. Connections of thalamic modulatory centers to the vocal control system of the zebra finch. Proc Natl Acad Sci USA 102: 14086–14091, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science 249: 1444–1446, 1990 [DOI] [PubMed] [Google Scholar]
- Ball GF, Nock B, Wingfield JC, McEwen BS, Balthazart J. Muscarinic cholinergic receptors in the songbird and quail brain: a quantitative autoradiographic study. J Comp Neurol 298: 431–442, 1990 [DOI] [PubMed] [Google Scholar]
- Bauer EE, Coleman MJ, Roberts TF, Roy A, Prather JF, Mooney R. A synaptic basis for auditory-vocal integration in the songbird. J Neurosci 28: 1509–1522, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton S, Cardin JA, DeVoogd TJ. Lucifer Yellow filling of Area X projecting neurons in the high vocal center of female canaries. Brain Res 799: 138–147, 1998 [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224: 901–903, 1984 [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature 404: 762–766, 2000 [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Altered perception of species-specific song by female birds after lesions of a forebrain nucleus. Science 251: 303–305, 1991 [DOI] [PubMed] [Google Scholar]
- Burt JM, Lent KL, Beecher MD, Brenowitz EA. Lesions of the anterior forebrain song control pathway in female canaries affect song perception in an operant task. J Neurobiol 42: 1–13, 2000 [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Song system auditory responses are stable and highly tuned during sedation, rapidly modulated and unselective during wakefulness, and suppressed by arousal. J Neurophysiol 90: 2884–2899, 2003 [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. J Neurosci 24: 7745–7753, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Mooney R. Synaptic transformations underlying highly selective auditory representations of learned birdsong. J Neurosci 24: 7251–7265, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Mooney R. Thalamic gating of auditory responses in telencephalic song control nuclei. J Neurosci 27: 10024–10036, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science 290: 812–816, 2000 [DOI] [PubMed] [Google Scholar]
- Dave AS, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science 282: 2250–2254, 1998 [DOI] [PubMed] [Google Scholar]
- Debarbieux F, Brunton J, Charpak S. Effect of bicuculline on thalamic activity: a direct blockade of IAHP in reticularis neurons. J Neurophysiol 79: 2911–2918, 1998 [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Konishi M. Song-selective auditory circuits in the vocal control system of the zebra finch. Proc Natl Acad Sci USA 88: 11339–11343, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P, Petrozzino JJ, Vu HM, Schmidt MF, Perkel DJ. Slow synaptic inhibition mediated by metabotropic glutamate receptor activation of GIRK channels. J Neurophysiol 84: 2284–2290, 2000 [DOI] [PubMed] [Google Scholar]
- Dutar P, Vu HM, Perkel DJ. Multiple cell types distinguished by physiological, pharmacological, and anatomic properties in nucleus HVc of the adult zebra finch. J Neurophysiol 80: 1828–1838, 1998 [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol 100: 2640–2652, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farries MA, Perkel DJ. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J Neurosci 22: 3776–3787, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete IR, Hahnloser RH, Fee MS, Seung HS. Temporal sparseness of the premotor drive is important for rapid learning in a neural network model of birdsong. J Neurophysiol 92: 2274–2282, 2004 [DOI] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D. Parallel pathways and convergence onto HVc and adjacent neostriatum of adult zebra finches (Taeniopygia guttata). J Comp Neurol 360: 413–441, 1995 [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Bentley GE, Ball GF. Individual vocal recognition and the effect of partial lesions to HVc on discrimination, learning, and categorization of conspecific song in adult songbirds. J Neurobiol 42: 117–133, 2000 [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron 19: 679–686, 1997 [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Cholinergic inhibition of neocortical pyramidal neurons. J Neurosci 25: 10308–10320, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419: 65–70, 2002 [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Andrade R. Muscarinic activation of a voltage-dependent cation nonselective current in rat association cortex. J Neurosci 16: 3848–3861, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CF, Barclay SR, Waterman SA. Changes in catecholamine levels and turnover rates in hypothalamic, vocal control, and auditory nuclei in male zebra finches during development. J Neurobiol 34: 329–346, 1998 [PubMed] [Google Scholar]
- Hartigan JA, Hartigan PM. The dip test of unimodality. Annals Stat 13: 70–84, 1985 [Google Scholar]
- Hsieh CY, Cruikshank SJ, Metherate R. Differential modulation of auditory thalamocortical and intracortical synaptic transmission by cholinergic agonist. Brain Res 880: 51–64, 2000 [DOI] [PubMed] [Google Scholar]
- Janata P, Margoliash D. Gradual emergence of song selectivity in sensorimotor structures of the male zebra finch song system. J Neurosci 19: 5108–5118, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V. Bicuculline methiodide potentiates NMDA-dependent burst firing in rat dopamine neurons by blocking apamin-sensitive Ca2+-activated K+ currents. Neurosci Lett 231: 13–16, 1997 [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature 433: 638–643, 2005 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol 78: 1743–1747, 1997 [DOI] [PubMed] [Google Scholar]
- Kimpo RR, Theunissen FE, Doupe AJ. Propagation of correlated activity through multiple stages of a neural circuit. J Neurosci 23: 5750–5761, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, Alvarez-Buylla A, Nottebohm F. Production and survival of projection neurons in a forebrain vocal center of adult male canaries. J Neurosci 11: 1756–1762, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhevnikov AA, Fee MS. Singing-related activity of identified HVC neurons in the zebra finch. J Neurophysiol 97: 4271–4283, 2007 [DOI] [PubMed] [Google Scholar]
- Kubota M, Taniguchi I. Electrophysiological characteristics of classes of neuron in the HVc of the zebra finch. J Neurophysiol 80: 914–923, 1998 [DOI] [PubMed] [Google Scholar]
- Leonardo A, Fee MS. Ensemble coding of vocal control in birdsong. J Neurosci 25: 652–661, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Sakaguchi H. Cholinergic innervation of the song control nuclei by the ventral paleostriatum in the zebra finch: a double-labeling study with retrograde fluorescent tracers and choline acetyltransferase immunohistochemistry. Brain Res 763: 239–246, 1997 [DOI] [PubMed] [Google Scholar]
- Livingston FS, Mooney R. Development of intrinsic and synaptic properties in a forebrain nucleus essential to avian song learning. J Neurosci 17: 8997–9009, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Meunier E, Monier C, Amar M, Baux G, Fregnac Y, Fossier P. Involvement of nicotinic and muscarinic receptors in the endogenous cholinergic modulation of the balance between excitation and inhibition in the young rat visual cortex. Cereb Cortex 19: 2411–2427, 2009 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Mechanisms of action of acetylcholine in the guinea-pig cerebral cortex in vitro. J Physiol 375: 169–194, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. Different subthreshold mechanisms underlie song selectivity in identified HVc neurons of the zebra finch. J Neurosci 20: 5420–5436, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Prather JF. The HVC microcircuit: the synaptic basis for interactions between song motor and vocal plasticity pathways. J Neurosci 25: 1952–1964, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Neural song preference during vocal learning in the zebra finch depends on age and state. J Neurobiol 62: 231–242, 2005 [DOI] [PubMed] [Google Scholar]
- Nixdorf BE, Davis SS, DeVoogd TJ. Morphology of Golgi-impregnated neurons in hyperstriatum ventralis, pars caudalis in adult male and female canaries. J Comp Neurol 284: 337–349, 1989 [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol 165: 457–486, 1976 [DOI] [PubMed] [Google Scholar]
- ölveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol 3: 902–909, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Nowicki S, Anderson RC, Peters S, Mooney R. Neural correlates of categorical perception in learned vocal communication. Nat Neurosci 12: 221–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature 451: 305–310, 2008 [DOI] [PubMed] [Google Scholar]
- Rauske PL, Shea SD, Margoliash D. State and neuronal class-dependent reconfiguration in the avian song system. J Neurophysiol 89: 1688–1701, 2003 [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED, of the Avian Brain Nomenclature Forum Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol 473: 377–414, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MJ, Mooney R. Inhibitory and excitatory mechanisms underlying auditory responses to learned vocalizations in the songbird nucleus HVC. Neuron 39: 177–194, 2003 [DOI] [PubMed] [Google Scholar]
- Rosen MJ, Mooney R. Synaptic interactions underlying song-selectivity in the avian nucleus HVC revealed by dual intracellular recordings. J Neurophysiol 95: 1158–1175, 2006 [DOI] [PubMed] [Google Scholar]
- Ryan SM, Arnold AP. Evidence for cholinergic participation in the control of birdsong: acetylcholinesterase distribution and muscarinic receptor autoradiography in the zebra finch brain. J Comp Neurol 202: 211–219, 1981 [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Saito N. Developmental change of cholinergic activity in the forebrain of the zebra finch during song learning. Brain Res Dev Brain Res 62: 223–228, 1991 [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3: 1027–1034, 2000 [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci 11: 2896–2913, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F, Cynx J. Conspecific and heterospecific song discrimination in male zebra finches with lesions in the anterior forebrain pathway. J Neurobiol 36: 81–90, 1998 [PubMed] [Google Scholar]
- Schmidt MF, Konishi M. Gating of auditory responses in the vocal control system of awake songbirds. Nat Neurosci 1: 513–518, 1998 [DOI] [PubMed] [Google Scholar]
- Schmidt MF, Perkel DJ. Slow synaptic inhibition in nucleus HVc of the adult zebra finch. J Neurosci 18: 895–904, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BB, Lois C. Developmental origin and identity of song system neurons born during vocal learning in songbirds. J Comp Neurol 502: 202–214, 2007 [DOI] [PubMed] [Google Scholar]
- Shank SS, Margoliash D. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature 458: 73–77, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SD, Margoliash D. Basal forebrain cholinergic modulation of auditory activity in the zebra finch song system. Neuron 40: 1213–1226, 2003 [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol 53: 51–63, 1990 [DOI] [PubMed] [Google Scholar]
- Troyer TW, Doupe AJ. An associational model of birdsong sensorimotor learning. I. Efference copy and the learning of song syllables. J Neurophysiol 84: 1204–1223, 2000a [DOI] [PubMed] [Google Scholar]
- Troyer TW, Doupe AJ. An associational model of birdsong sensorimotor learning. II. Temporal hierarchies and the learning of song sequence. J Neurophysiol 84: 1224–1239, 2000b [DOI] [PubMed] [Google Scholar]
- Tryba AK, Peña F, Lieske SP, Viemari JC, Thoby-Brisson M, Ramirez JM. Differential modulation of neural network and pacemaker activity underlying eupnea and sigh-breathing activities. J Neurophysiol 99: 2114–2125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario DS, Yohay KH. Song-selective auditory input to a forebrain vocal control nucleus in the zebra finch. J Neurobiol 24: 488–505, 1993 [DOI] [PubMed] [Google Scholar]
- Wild JM, Williams MN, Howie GJ, Mooney R. Calcium-binding proteins define interneurons in HVC of the zebra finch (Taeniopygia guttata). J Comp Neurol 483: 76–90, 2005 [DOI] [PubMed] [Google Scholar]
- Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol 39: 14–28, 1999 [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science 281: 985–988, 1998 [DOI] [PubMed] [Google Scholar]
- Xiao Z, Deng PY, Yang C, Lei S. Modulation of GABAergic transmission by muscarinic receptors in the entorhinal cortex of juvenile rats. J Neurophysiol 102: 659–669, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.