Abstract
It is known that after spinalization animals lose their ability to maintain lateral stability when standing or walking. A likely reason for this is a reduction of the postural limb reflexes (PLRs) driven by stretch and load receptors of the limbs. The aim of this study was to clarify whether spinal networks contribute to the generation of PLRs. For this purpose, first, PLRs were recorded in decerebrated rabbits before and after spinalization at T12. Second, the effects of epidural electrical stimulation (EES) at L7 on the limb reflexes were studied after spinalization. To evoke PLRs, the vertebrate column of the rabbit was fixed, whereas the hindlimbs were positioned on the platform. Periodic lateral tilts of the platform caused antiphase flexion–extension limbs movements, similar to those observed in intact animals keeping balance on the tilting platform. Before spinalization, these movements evoked PLRs: augmentation of extensor EMGs and increase of contact force during limb flexion, suggesting their stabilizing postural effects. Spinalization resulted in almost complete disappearance of PLRs. After EES, however, the PLRs reappeared and persisted for up to several minutes, although their values were reduced. The post-EES effects could be magnified by intrathecal application of quipazine (5-HT agonist) at L4–L6. Results of this study suggest that the spinal cord contains the neuronal networks underlying PLRs; they can contribute to the maintenance of lateral stability in intact subjects. In acute spinal animals, these networks can be activated by EES, suggesting that they are normally activated by a tonic supraspinal drive.
INTRODUCTION
In quadrupeds the most common posture of the body, with its dorsal side oriented upward (basic posture), is maintained by a postural system. This closed-loop control system is driven by sensory feedback signals and compensates for deviations from the desired body orientation by producing corrective motor responses (for review, see Deliagina et al. 2006a; Horak and Macpherson 1996; Massion 1994, 1998). Both spinal mechanisms and supraspinal motor centers participate in the maintenance of body posture (Beloozerova et al. 2003b, 2005; Horak and Macpherson 1996; Jacobs and Horak 2007). Recent studies have shown that decerebrated animals can produce appropriate postural responses to the lateral tilt and horizontal translation of the supporting surface (Honeycutt et al. 2009; Musienko et al. 2008), suggesting that basic postural mechanisms reside in the brain stem, cerebellum, and spinal cord. Although it is clear that postural responses can be modified and overwritten by supraspinal structures to ensure whole body stability, a specific role of the spinal and supraspinal levels of the postural system is not quite clear (for discussion, see Lyalka et al. 2005, 2008, 2009; Macpherson et al. 1997, 2007; Stapley et al. 2006).
Elucidation of relationships between the spinal and supraspinal postural mechanisms is important for understanding the consequences of spinal cord injury (SCI). From lesion studies on quadrupeds it is known that spinalization at the lower thoracic level causes severe impairment of postural control, so that these animals lose their ability to maintain the lateral stability of the hindquarters when standing or walking, an ability that does not recuperate over time (see e.g., Edgerton et al. 2001; Grillner 1973; Lyalka et al. 2009b; Pratt et al. 1994; Rossignol et al. 1999). There are several ways to explain this finding. On one extreme is the hypothesis implying that the spinal reflex mechanism is competent and potent enough to maintain the basic body posture, although it requires a supraspinal excitatory drive to be activated.
On the other extreme is the hypothesis implying that the sole function of spinal networks is the transformation of the specific phasic supraspinal commands into the motor pattern of postural corrections. This principle underlies the postural control in the lamprey (a lower vertebrate) (Deliagina et al. 2008). A possible way to test these hypotheses is to substitute the missing supraspinal excitatory drive by the artificial drive—i.e., by stimulation of the spinal cord below the lesion. This approach was used in the present study.
It is known that epidural electrical stimulation (EES) applied to the dorsal surface of the lumbar spinal cord can induce stepping movements of the hindlimbs in acute and chronic spinal animals with severely impaired locomotor functions (Gerasimenko et al. 2003; Ichijama et al. 2005; Lavrov et al. 2008). In spinal patients, EES can evoke stepping-like movements (Dimitrijevic et al. 1998; Minassian et al. 2007). These studies (reviewed by Gerasimenko et al. 2008) support the concept that a substantial part of the locomotor mechanisms of the hindlimbs (known as the central pattern generators [CPGs]; see Grillner and Zangger 1979; Orlovsky et al. 1999) resides in the spinal cord. When properly activated, the CPGs are able to generate the basic pattern of stepping. It was suggested (Gerasimenko et al. 2008) that EES provides an excitatory drive to the spinal locomotor networks that substitutes a normal (supraspinal) drive and activates these networks. It seems likely that EES affects not only the CPGs but also their peripheral feedback (Gerasimenko et al. 2006; Lavrov et al. 2006; Musienko et al. 2007).
Can EES also activate the spinal components of the postural system? We addressed this issue in the rabbit. For rabbits and cats, functional organization of the postural system responsible for lateral stability, as well as the effects of different SCIs on this system, were characterized in our previous studies (Beloozerova et al. 2003a; Deliagina et al. 2000, 2006b; Lyalka et al. 2005, 2009a,b). It was shown that the system consists of three relatively independent subsystems, which stabilize orientation of the head, of the anterior part of the trunk, and of its posterior part (Beloozerova et al. 2003a; Deliagina et al. 2000, 2006b). The trunk stabilizing subsystems are driven by somatosensory inputs from the corresponding limbs and reflex mechanisms of individual limbs play an important role in the generation of postural corrections (Beloozerova et al. 2003a; Deliagina et al. 2006b). It was also demonstrated that extensive SCIs lead to dramatic impairment of this system (Lyalka et al. 2005, 2009a,b). In this study we describe postural limb reflexes in the decerebrated rabbit, disappearance of these reflexes after spinalization, and their partial restoration caused by EES.
It is well known that all spinal reflexes are subjected to considerable changes during the postlesion period, starting from their dramatic reduction during the initial phase of the spinal shock, which is followed by partial restoration and reorganization of the reflexes accompanied by the development of spasticity (Ditunno et al. 2004; Harkema 2008; Ko et al. 1999; Valero-Cabre et al. 2004). One can therefore expect that the effects of EES also depend on time. Herein we describe the effects of EES observed in acute experiments, whereas the effects observed in chronic conditions (≤2 mo postlesion) will be described separately. We report here that, at the early postlesion stages, EES resulted in facilitation of limb reflexes.
Another possible way for activation of spinal networks below transection is application of different pharmacological agents to this area. This approach was successfully used for activation of the hindlimb locomotor CPGs (Rossignol et al. 1998, 2001). It was also shown that the two approaches (electrical and pharmacological stimulation) could be combined to enable well-coordinated stepping (Courtine et al. 2009; Gerasimenko et al. 2007). We have also found that application of serotonergic drugs facilitates postural limb reflexes in chronic rabbits with extensive SCIs (Lyalka et al. 2008, 2009b). In the present study we compared, in acute spinal rabbits, the effect on limb reflexes produced by EES alone, quipazine (a 5-hydroxytryptamine [5-HT1,2,3; serotonin] agonist) application alone, and by EES combined with quipazine application.
A brief account of a portion of this study was previously published in abstract form (Musienko et al. 2007b).
METHODS
Experiments were carried out on 17 adult New Zealand rabbits (weighing 2.5–3.5 kg). All experiments were conducted with the approval of the local ethical committee (Norra Djurförsöksetiska Nämden) in Stockholm.
Surgical procedures
The animal was injected with propofol (average dose, 10 mg/kg, administered intravenously) for induction of anesthesia, which was continued on isoflurane (1.5–2.5%) delivered in O2. The trachea was cannulated. For all subsequent procedures, the animal was positioned in a metal frame and its head and vertebral column were rigidly fixed (Fig. 1A). The first laminectomy was performed at the lower lumbar level and a ball electrode (d = 0.5 mm) was later positioned on the dorsal aspect of the spinal cord for epidural stimulation. The second laminectomy was performed at the lower thoracic and upper lumbar level, for subsequent transection of the spinal cord at T12, which was performed under local anesthesia (xylocaine). Later, through this opening, an intrathecal cannula (silicone tube 200 mm in length, OD = 0.7 mm, filled with Ringer solution) was inserted under the dura and protracted caudally to reach the lower lumbar segments. Later, the cannula was used for drug injection.
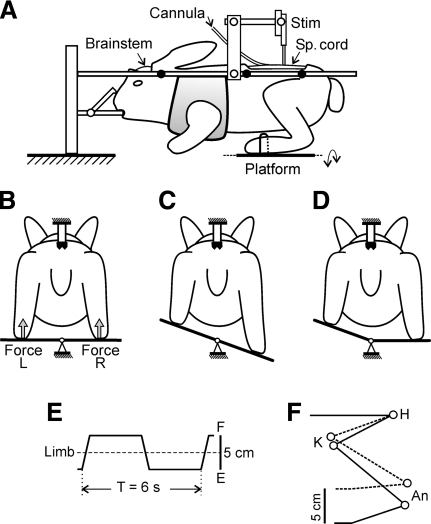
Fig. 1.
Experimental design. A: the decerebrated animal was positioned in a rigid frame and its head and vertebral column were firmly fixed. To affect the spinal reflex mechanisms, the spinal cord was stimulated by means of the electrode (Stim) and the drug (quipazine) was injected by means of the intrathecal cannula. The hindlimbs were positioned on the platform, which could be periodically tilted in the frontal plane. B and C: tilt of the platform caused flexion of one limb and extension of the other limb. The contact force under each limb was measured by the force sensor (Force). D: the left and the right parts of the platform could be tilted independently. E: trajectory of the foot movement. F: limb configuration at the 2 extreme platform positions (H, K, and An: hip, knee, and ankle joints, respectively).
Up to eight bipolar electromyographic (EMG) electrodes were inserted into the hindlimb muscles. We systematically recorded (bilaterally) the EMGs from gastrocnemius lateralis (Gast, ankle extensor) and vastus lateralis (Vast, knee extensor). In a portion of the experiments, we also recorded the EMGs from gracilis (Grac, acting as hip extensor), semitendinosus (St, knee flexor and hip extensor), and tibialis anterior (TA, ankle flexor).
The animal was then decerebrated at the precollicular–postmammillary level (Musienko et al. 2008). After decerebration, the anesthesia was discontinued. During the experiment, the rectal temperature and mean blood pressure of the animal were continuously monitored and were kept at 37–38°C and >90 mmHg, respectively. Recordings were started not <1 h after cessation of anesthesia.
Experimental design
The experimental design is shown in Fig. 1. The head and vertebral column of the decerebrated rabbit were rigidly fixed in several points and the forequarters were suspended in a hammock. The hindlimbs were hemiflexed and positioned on the horizontal platform. The limb configuration was similar to that observed in normal rabbits during standing, with the hip, knee, and ankle angles about 25, 60, and 50°, respectively (Beloozerova et al. 2003a). The distal part of each foot was gently fastened to the platform, so that the interfeet distance (11 cm) was similar to that observed in normal rabbits of this size when standing.
The platform as a whole, or its right or left part separately, could be tilted periodically by rotation about the medial axis (Fig. 1, B–D), which led to close-to-vertical displacements of the distal point of the corresponding limb. Since the position of the proximal point was fixed, tilts of the platform caused passive flexion/extension (F/E) movements at the limb joints, resulting in the corresponding changes of the functional length of the limb. In the tests with tilting only one part of the platform, we could activate separately the sensory input from each of the limbs. With tilting of the platform as a whole, the two limbs were affected in antiphase (Fig. 1C), like that in the normal rabbit maintaining balance on the tilting platform (Beloozerova et al. 2003a). In this test, the reflex mechanisms of both limbs were activated and their possible interaction could be assessed by comparing with the reflexes caused by single-limb movements.
A time trajectory of tilting the platform and thus a trajectory of foot displacements was trapezoidal (Fig. 1E), which revealed both dynamic and static components of postural limb reflexes. A distance between the two extreme positions (up and down) was 5 cm; these positions were symmetrical in relation to the horizontal platform position. Each position was maintained for about 2.5 s and the transition between them lasted for about 0.5 s. As demonstrated by video recording, the imposed foot displacements (5 cm peak-to-peak) caused F/E movements at the hip, knee, and ankle joints, with a magnitude of about 10° (Fig. 1F). These F/E limb movements were repeated periodically (T = 6 s).
The tilt angles of the left and right parts of the platform were monitored by mechanical sensors, scaled, and recorded as changes in the vertical position of the foot (Fig. 1E). The contact forces under the limbs were measured by means of force sensors (Force in Fig. 1B).
Electrical and pharmacological stimulation
Epidural electrical stimulation (EES) of the spinal cord was performed by means of the ball electrode (d = 0.5 mm) positioned on the dorsal surface of the cord in L5–L7 segments, close to the midline (for the choice of stimulation parameters, see results).
Quipazine (concentration: 5 mM; volume of bolus: 100 μl) was injected into the subarachnoid space of the spinal cord through the inlet of cannula, as described by Lyalka et al. (2008). A subsequent bolus injection of saline (100 μl) was made to flush the drug outside the cannula; the dead space of the cannula was 80 μl. Position of the cannula was verified postmortem. In all cases, the tip was positioned in the L5–L7 segments.
Experimental protocol, recordings, and data analysis
Each decerebrated animal was subjected to three main tests. First, the limb reflexes were recorded before spinalization. This was done for antiphase F/E movements of the two limbs and (in part of the experiments) also for F/E movements of each of the limbs alone. Second, the limb reflexes were recorded after spinalization. Third, in the spinal animals, the effect of EES on the limb reflexes was examined. In addition, in some of the spinal animals, the effects on the limb reflexes produced by application of quipazine alone or in combination with EES were characterized. To estimate the passive force, its value was measured during F/E limb movements in a few minutes after sacrificing the animal.
In each trial, we recorded reflex responses (EMGs and ground reaction forces) in many (from 10 to 100) sequential F/E movement cycles. The signals from EMG electrodes and from position and force sensors were amplified, digitized with a sampling frequency of 5 kHz (EMGs) and 1 kHz (sensors), and recorded on a computer disk using the data acquisition and analysis system (Power-1401/Spike2; Cambridge Electronic Design, Cambridge, UK). The EMG signals were rectified and smoothed. The smoothed output at time t was the average value of the input from time t − τ to time t + τ; the value of τ was 50 ms.
All quantitative data in this study are presented as means ± SE. Student's paired t-test was used to characterize the statistical significance when comparing different means; the significance level was set at P = 0.05. For each test, we indicate the number of animals used (N) and the number of individual limbs examined in repeated tests (n).
RESULTS
Postural limb reflexes in decerebrated rabbit
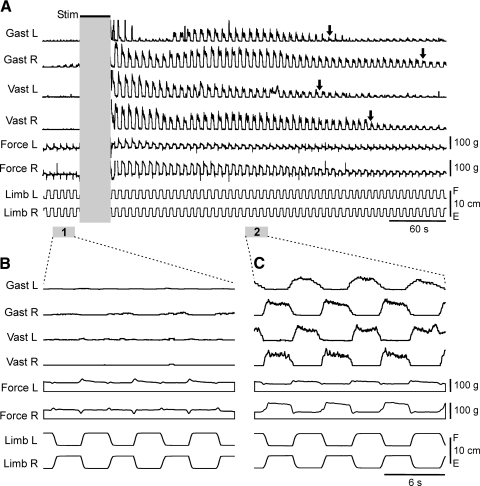
In all tested decerebrated rabbits (n = 17), reflex responses to antiphase flexion/extension movements of the two limbs (postural limb reflexes) were well pronounced, as illustrated in Fig. 2A. Flexion of each of the limbs was accompanied by activation of its extensor muscles, Gast and Vast, as well as by an increase in the force developed by the limb. The responses usually contained both dynamic and static components (see also Fig. 5, A and B). By contrast, extension of the limb caused not only an almost complete inactivation of its extensors, but also a considerable decrease in the force value. These responses persisted with repeated, periodic application of F/E stimuli. The averaged values of dynamic and static components of the force responses were 680 ± 195 and 545 ± 110 g, respectively (Decereb in Fig. 3, A and B). These values were much larger than the corresponding values measured after sacrificing the animal (109 ± 10 and 93 ± 8 g, Passiv in Fig. 3, A and B), suggesting that the contribution of the passive force to the force responses in decerebrated animals could be neglected.
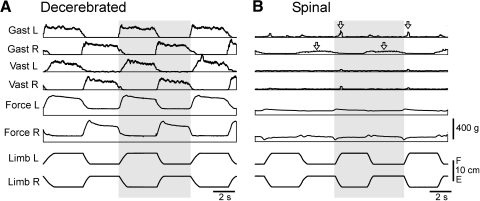
Fig. 2.
Effect of spinalization on postural limb reflexes in the decerebrated rabbit. A: reflex electromyographic (EMG) and force responses to flexion/extension (F/E) antiphase movements of the hindlimbs before spinalization. B: reflex responses (in the same rabbit as in A) after spinalization. For each muscle, the scales in A and B are the same. Arrows indicate remaining small responses in gastrocnemius lateralis, left and right (Gast L and Gast R, respectively).
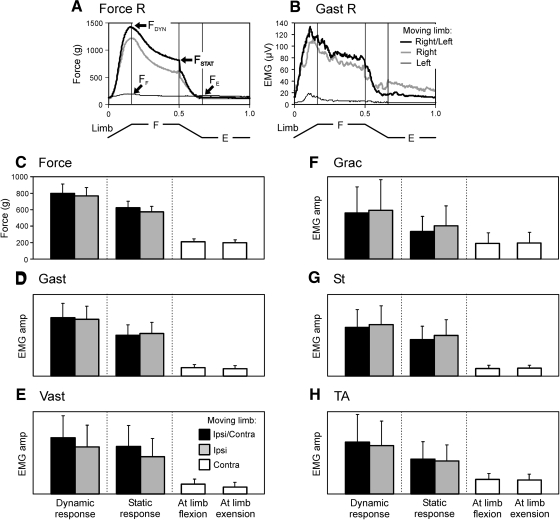
Fig. 5.
Contribution of sensory inputs from the ipsi- and contralateral limbs to generation of postural limb reflexes. A and B: an example of the Force R (A) and Gast R (B) responses, elicited by antiphase F/E movements of both limbs (Right/Left), of the right limb (Right), and of the left limb (Left). The beginning of flexion of the right limb was taken as the cycle onset in the tests Right/Left and Right and the beginning of extension of the left limb in the test Left. C– H: force responses (C) and EMG responses in Gast (D), in Vast (E), in Grac (F), in St (G), and in TA (H), observed in the tests Ipsi/Contra, Ipsi, and Contra (mean ± SE). In C and D: N = 6, n = 14; in E: N = 6, n = 8; in F: N = 3, n = 5; in G: N = 5, n = 7; in H: N = 4, n = 8. EMG amplitude was measured in arbitrary units the same for a given muscle.
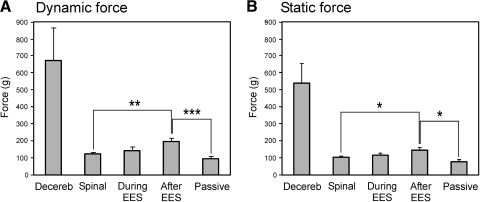
Fig. 3.
Dynamic (A) and static (B) force responses under different conditions. The force magnitude (mean ± SE) was measured before spinalization (Decereb, N = 9, n = 36), after spinalization (Spinal, N = 7, n = 30), during epidural stimulation (During EES, N = 7, n = 30), after stimulation (After EES, N = 7, n = 30), and after sacrificing the animal (Passive, N = 5, n = 5). Indication of significance level: *P < 0.05; **P < 0.01; ***P < 0.001.
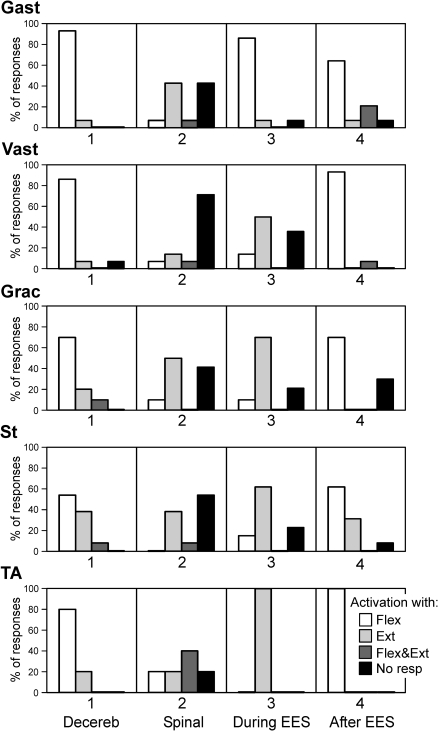
For all five types of studied muscles (Gast, Vast, Grac, St, and TA), we qualitatively characterized the pattern of EMG responses, i.e., the phase of EMG in the cycle of F/E movements of the ipsilateral limb. Four categories were used: 1) activation of the muscle with flexion of the ipsilateral limb, 2) activation with limb extension, 3) activation with both flexion and extension, and 4) no response to F/E stimuli. In Fig. 4, the first column (Decereb) shows the proportion of different categories of responses in each particular type of muscle, which were recorded in seven decerebrated rabbits before spinalization. In the overwhelming majority of cases, Gast and Vast were activated with limb flexion (93 and 86% of cases, respectively). Grac was most often activated with flexion (70%), but in 20% of cases it was activated with extension. Responses in St were less uniform: this muscle was activated with limb flexion in 54% of cases and with its extension in 38% of cases. TA was most often activated with limb flexion (80%) and less often with its extension (20%).
Fig. 4.
Pattern of EMG responses in different muscles to F/E antiphase movements of both limbs. Four categories of responses were distinguished: activation of the muscle with flexion of the ipsilateral limb (Flex), activation with limb extension (Ext), activation with both flexion and extension (Flex&Ext), and no response to F/E stimuli (No resp). On the y-axis is shown a proportion of responses of different categories. The analysis was done for different conditions designated as in Fig. 3. Number of animals and tests: Gast (N = 7, n = 14); Vast (N = 7, n = 14); Grac (N = 5, n = 10); St (N = 7, n = 13); TA (N = 3, n = 5). Gast, gastrocnemius lateralis; Vast, vastus lateralis; Grac, gracilis; St, semitendinosus; TA, tibialis anterior.
To reveal a contribution of sensory inputs from each of the limbs to the generation of these reflex responses, we compared the results of three tests: with antiphase F/E movements of both limbs (the Ipsi/Contra test), with movements of only the limb under study (the Ipsi test), and with movements of the opposite limb (the Contra test). The examples of force and Gastr EMG responses in these tests in one of the rabbits are shown in Fig. 5, A and B . When F/E stimuli were applied only to the limb under study, the responses slightly decreased compared with bilateral application of F/E stimuli. By contrast, the F/E movements of the opposite limb caused very weak responses in the limb under study.
Similar results were obtained in all experiments, in which the contribution of somatosensory information from individual limbs to the generation of postural limb reflexes was assessed (Fig. 5, C–H). To quantitatively characterize the force and EMG responses in both the Ipsi/Contra and Ipsi tests, we measured both a dynamic component of the response (its peak value in the beginning of movement cycle) and a static component (the response value just before its rapid decrease in the middle of movement cycle). These components of force response (FDYN and FSTAT) are indicated in Fig. 5A. To characterize the low-level activity in the Contra test, we used two values: the maximal activity in the flexor and in the extensor parts of the movement cycle (FF and FE in Fig. 5A). The response values were averaged over five to six sequential cycles of a given test and then over all experiments (N = 6, n = 14). Both the averaged force responses and the EMG responses in different muscles are shown in Fig. 5, C–H. One can see that there was no significant difference between the responses in both the Ipsi/Contra and the Ipsi tests. By contrast, both EMG and force responses in the Contra test were much smaller than those in the Ipsi and Ipsi/Contra tests; moreover, there was no significant difference between the activity levels in the flexor and extensor parts of the cycle. These data indicate that postural reflexes in a given limb are generated mainly in response to somatosensory input from the same limb.
Effect of spinalization on postural limb reflexes
Transection of the spinal cord at T12 caused a dramatic effect on postural limb reflexes. This is illustrated in Fig. 2B for the rabbit, whose reflexes before spinalization are shown in Fig. 2A. Spinalization resulted in complete disappearance of the reflex EMG responses in both left and right Vast, whereas very small responses persisted in Gast (a dynamic one in the left limb and a static one in the right limb, indicated by arrows). Reflex force responses also disappeared almost completely in both limbs.
Similar results—i.e., a considerable reduction in the reflex force responses and a pronounced reduction or complete disappearance of EMG responses—were observed after spinalization in all rabbits (n = 17). Figure 3, A and B shows the average values of dynamic and static components of the force response observed after spinalization (Spinal in Fig. 3): 122 ± 9 and 105 ± 8 g, respectively, and that practically did not differ from the passive force (109 ± 10 and 93 ± 8 g, respectively) (Passive in Fig. 3).
Spinalization caused not only a dramatic reduction or complete disappearance of EMG responses to antiphase F/E movements of the limbs, but also a change of the patterns in the remaining responses. To characterize these patterns, we used the same four categories as those before spinalization (see earlier text). In Fig. 4, the second column (Spinal) shows a proportion of different types of EMG responses in each particular type of muscle, which were recorded in the spinal rabbits. The response in Gast was typically either absent (43% of cases) or reversed, compared with that before spinalization, i.e., Gast in spinal rabbits was activated with limb extension (43% of cases). Vast in spinal rabbits usually did not respond (71% of cases). The response in Grac was either absent (43% of cases) or reversed (50%). St was either activated with limb extension (38% of cases) or did not respond (54%). Responses in TA were very diverse: this muscle was activated with limb flexion (20%), extension (20%), with both flexion and extension (40%), or did not respond (20%).
Effects of EES on limb reflexes
POSTSTIMULATION EFFECTS.
The main result of this study was that EES in spinal rabbits was followed by a long-lasting enhancement of reflex EMG and force responses to F/E limb movements, with the pattern very close to that observed before spinalization. An example of these effects is shown in Fig. 6. Before stimulation, limb movements caused very small or no EMG and force responses (Fig. 6, A and B). Then stimulation of the dorsal surface of the spinal cord at L7 was performed (train duration, 40 s; pulse duration, 2 ms; frequency, 3 pps; current, 450 μA). After cessation of EES, flexion of each of the limbs caused activation of Gast and Vast in this limb, as well as an increase in force developed by the limb (Fig. 6, A and C). These EMG and force responses persisted for a long period of time after cessation of stimulation. For each individual muscle, we defined this period as the time required for a fivefold reduction of EMG bursts (relative to the burst value immediately after cessation of stimulation). In the example shown in Fig. 6A, the duration of post-EES facilitation ranged from 220 s (in the left Vast) to 330 s (in the right Gast).
Fig. 6.
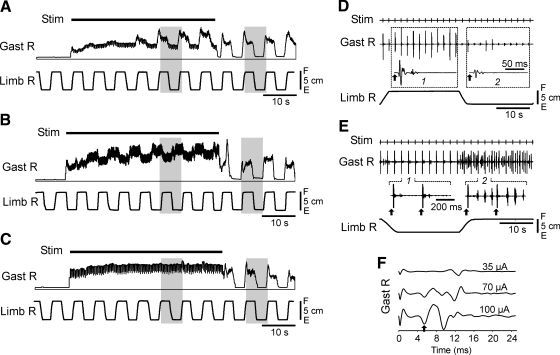
Facilitation of limb reflexes after epidural electrical stimulation (EES). A: stimulation (Stim) of the dorsal surface of the spinal cord at L7 resulted in facilitation of reflex EMG and force responses to F/E antiphase movements of both limbs. Parts of the recording marked by 1 and 2 in A are presented in B and C with better time resolution. For each muscle, the scales in B and C are the same. The part of recording during EES is not shown because it contains distortions caused by large synchronized responses to stimulating pulses. Arrows in A indicate the duration of post-EES enhancement of responses in different muscles.
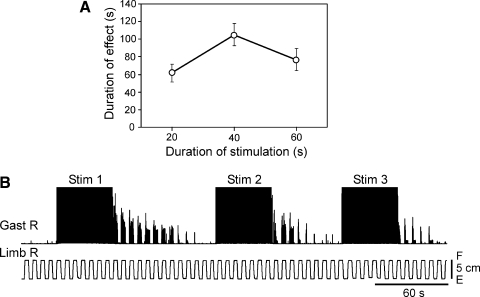
The poststimulation effects of EES—i.e., the long-lasting facilitation of force and EMG responses—were observed in all spinal rabbits, provided the stimulation was applied to L7, and the parameters of stimulation were similar to those indicated earlier. Typically, the facilitation lasted for 50–100 s (see Figs. 8A, 9C, and 10C). Both dynamic and static components of the force response increased after stimulation by roughly 60 and 40%, respectively (After EES in Fig. 3), compared with those before stimulation (Spinal in Fig. 3), but they were many times smaller than the responses before spinalization (Decereb in Fig. 3).
Fig. 8.
Effect of the duration of stimulation train and repeated stimulation on the post-EES reflex facilitation. A: duration of reflex facilitation (mean ± SE, n = 9) as a function of the length of stimulation train. B: example of the reduction of post-EES facilitation with repeated stimulation.
Fig. 9.
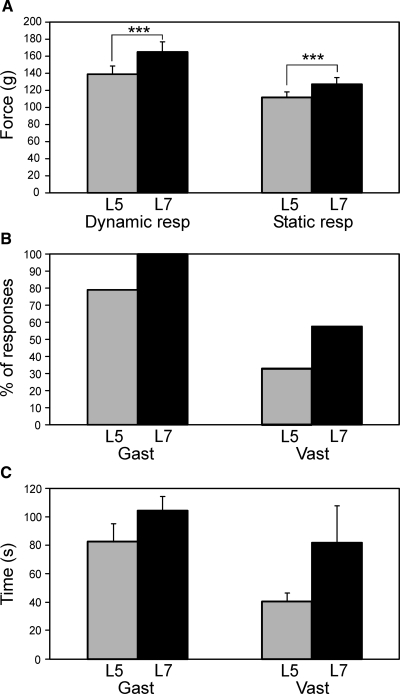
Comparison of post-EES effects produced by L5 and L7 stimulation. A: dynamic and static components of the force response to antiphase F/E limb movements observed after EES, in the tests with L5 and with L7 stimulation (mean ± SE, N = 4, n = 12). B: number of cases (%) with post-EES activation of Vast and Gast, in the tests with L5 and with L7 stimulation (for Gast: N = 4, n = 14; for Vast: N = 4, n = 12). C: duration of the period of post-EES facilitation of EMG responses in the tests with L5 stimulation and with L7 stimulation (mean ± SE). For Gast: L7 simulation, N = 4, n = 14; L5 stimulation, N = 4, n = 11; for Vast: L7 stimulation, N = 3, n = 7; L5 stimulation, N = 2, n = 4.
Fig. 10.
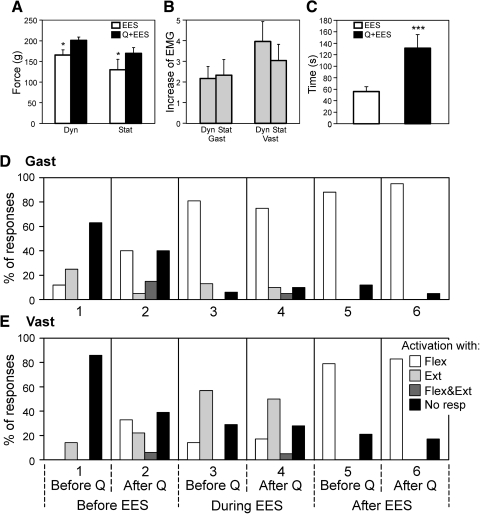
Effects of quipazine injection. A and B: effects of quipazine on the force and EMG responses to F/E limb movements observed after EES. A: dynamic (Dyn) and static (Stat) components of force response after stimulation alone (EES) and after stimulation combined with quipazine application (Q+EES). B: ratio of EMG values for Q+EES and EES conditions. C: duration of the period of post-EES reflex facilitation (mean ± SE). In A, N = 4, n = 8; in B, N = 8, n = 18 and 16; in C, N = 8, n = 17 and 15. D and E: pattern of EMG responses to F/E limb movements in Gast and Vast. Four categories of responses were distinguished: activation of the muscle with flexion of the ipsilateral limb (Flex), activation with limb extension (Ext), activation with both flexion and extension (Flex&Ext), and no response to F/E stimuli (No resp). On the y-axis is shown a proportion of different categories of responses. Analysis was done for 6 conditions indicated below the graphs. Number of animals and tests: Gast (N = 8, n = 18); Vast (N = 8, n = 15).
The patterns of EMG responses in different muscle types, observed after cessation of stimulation, were very similar to those found in decerebrated rabbits before spinalization. In Fig. 4, the fourth column (After EES) shows the proportion of different categories of EMG responses in each particular muscle type, which were observed in spinal rabbits during the period of poststimulation facilitation. In all studied muscles, the most frequent category of response was activation of the muscle with limb flexion, which was also typical for the rabbits before spinalization (Decereb in Fig. 4).
When F/E stimuli were applied to only one limb, in all cases (n = 5) the facilitated responses in this limb persisted practically unchanged compared with bilateral application of F/E stimuli, suggesting that in spinal rabbit, the postural limb reflexes are generated in response to somatosensory information from the own limb, as shown for decerebrated rabbits (see earlier text).
EFFECTS DURING STIMULATION.
During stimulation, there was a gradual increase of the tonic extensor activity that reached a plateau in 10–20 s after onset of EES (Fig. 7, A–C). The antiphase F/E limb movements caused the EMG responses in most muscles, but the responses during EES were usually smaller than those after EES (Fig. 7, A–C). Similarly, EES caused no significant change in the value of force responses compared with those before EES (Fig. 3).
Fig. 7.
Responses in the right Gast to F/E antiphase movements of both limbs during EES (only Limb R trace is shown). A–C: different patterns of Gast responses: activation with limb flexion (A); activation with limb extension (B); no response (C). D–F: fine structure of EMG activity caused by F/E movements. D: movement-related modulation of synchronized responses to EES pulses (arrows). Traces 1 and 2 show averaged responses in the corresponding parts of the tilt cycle. E: a part of EMG activity caused by EES was not synchronized by stimuli and was modulated by F/E movements. Parts 1 and 2 of the Gast EMG are also shown with higher time resolution (EES pulses are indicated by arrows). F: Gast responses to EES pulses recorded at different values of stimulating current (Limb R was flexed).
The effects observed during EES were usually complicated due to the fact that both EMG and force responses were often synchronized by the stimulating pulses (3 Hz) and the magnitude of these synchronized responses could be modulated by F/E limb movements. This is illustrated in Fig. 7D. The Gast EMG contained the rhythmic pulses that were delayed by a few milliseconds relative to the stimulating pulses; their magnitude was large when the limb was flexed and small when the limb was extended. Figure 7E shows the case when the Gast EMG contained both synchronized responses and a nonsynchronized activity. The latter one was modulated by F/E movements and was present when the limb was flexed. The synchronized response had a complex shape and consisted of a few components (Fig. 7F). The latencies of these components ranged from 4 to 10 ms and their relative values depended on the strength of stimulation.
To classify EMG responses in individual muscles during EES, we used the same four categories as those in the other conditions (see earlier text). In Fig. 4, the third column (During EES) shows the EMG pattern—i.e., the proportion of different categories of responses in each particular muscle type, which were observed in all rabbits during EES. One can see that the majority of muscles (four of five) were activated predominantly by limb extension. This was in strong contrast to the pattern of postural limb reflexes (activation of the majority of muscles with limb flexion), which was observed not only in decerebrated rabbits before spinalization (Decereb), but also in spinal rabbits after cessation of stimulation (After EES) (Fig. 4).
Role of different characteristics of EES
PARAMETERS OF STIMULATION.
Parameters of stimulation were not very critical for the main effect of EES (poststimulation facilitation of limb reflexes) to occur. We found that the optimal frequencies were within the range of 3–5 pps. The lower frequencies were less effective (a stronger current was needed), whereas the higher frequencies (10 and 50 pps were tested) could occasionally evoke a rhythmic, locomotor-like bursting in EMGs. In a few initial experiments, we used the current pulses of 0.2 ms in duration. Later we found that 2-ms pulses were more effective and used this pulse duration in all subsequent experiments. To set the optimal current strength in each experiment, we initially determined the threshold current (that elicited motor responses in the hindlimbs) and then used the current three- to fivefold stronger, which usually was within the range of 100–500 μA.
The poststimulation effects of EES (the duration of poststimulation facilitation of reflex responses and the degree of facilitation of force and EMG responses) depended on the duration of EES. In three rabbits, we qualitatively characterized how this factor affected the duration of facilitation. It was found that an optimal length of the stimulation train was about 40 s and, with shorter or longer stimulation, the duration of post-EES reflex facilitation decreased (Fig. 8A). The duration of post-EES facilitation also decreased with a repetitive application of the stimulation train, if the period of repetition was less than a few minutes, as illustrated in Fig. 8B.
ELECTRODE POSITION.
In the transverse plane, the stimulating electrode was always positioned close to the midline to avoid a left/right asymmetry in the effect of stimulation. To estimate the role of the rostrocaudal position of the electrode, in four rabbits we compared the effects of stimulation of two different segments, L5 and L7. We applied identical stimuli to each of these two points and recorded bilaterally the EMG (from Gast and Vast) and force responses. As shown in Fig. 9 A, post-EES facilitation of the dynamic and static force responses (mean ± SE, n = 12) was larger with L7 stimulation than that with L5 stimulation. As shown in Fig. 9B, facilitation of EMG responses occurred more often after L7 stimulation than that after L5 stimulation (for Gast, 100 vs. 79%; for Vast, 58 vs. 33%, respectively). Finally, as shown in Fig. 9C, the period of post-EES facilitation (mean ± SE) was longer with L7 stimulation than that with L5 stimulation in both muscles. Because of its higher efficacy, the L7 stimulation was used in most experiments of the present study.
Effect of quipazine on limb reflexes
The effect of quipazine was studied in eight spinal rabbits. Figure 10, A–C shows the main characteristics of the post-EES responses to F/E stimuli in this group of animals (mean ± SE) under two conditions: when EES was performed alone and when it was performed in 1–2 h after quipazine application. Quipazine caused a moderate increase in the dynamic and static components of the force responses (Fig. 10A) and a considerable (two- to fourfold) increase in the EMG responses (Fig. 10B). Quipazine also caused a more than twofold increase in the duration of post-EES facilitation of reflexes (Fig. 10C).
To classify the EMG responses in individual muscles (Gast and Vast) in the experiments with quipazine application, we used the same four categories as those in the other experiments (see earlier text). Figure 10, D and E shows the EMG pattern—i.e., the proportion of different categories of responses in Gast and Vast observed in all eight rabbits under different conditions. Before EES and before quipazine (column 1), the muscles were most often not activated by F/E stimuli. Before EES and after quipazine (column 2), about 60% of muscles responded to F/E stimuli and activation with limb flexion prevailed. During EES and before quipazine (column 3), activation with limb flexion prevailed in Gast and, with extension, prevailed in Vast. This pattern remained almost unchanged after quipazine application (column 4). Finally, quipazine practically did not affect the EMG pattern after EES: both muscles were activated with limb flexion (compare columns 5 and 6), although the responses under quipazine were facilitated considerably (Fig. 10, A–C).
DISCUSSION
Facilitation of spinal limb reflexes by electrical stimulation of the spinal cord
A complete rupture of the spinal cord (spinalization) in mammals causes a spinal shock. A characteristic feature of the initial period of spinal shock is a dramatic reduction or complete disappearance of the muscle tone and numerous limb reflexes. Later on, some reflex activity in the spinal cord below the lesion reappears (Ditunno et al. 2004; Ko et al. 1999).
In the present study, we addressed the question of whether, during the initial period of the spinal shock, the spinal reflex mechanisms can be facilitated by electrical stimulation of the spinal cord below the lesion. We used the technique of epidural electrical stimulation (EES) of the dorsal surface of the spinal cord in the lumbar region. Earlier, this technique was successfully used for activation of locomotor networks in spinal animals (Gerasimenko et al. 2008). A specific aim of this study was to activate the reflexes relevant for postural control. To evoke these reflexes, the hindlimbs of the decerebrated rabbit with a fixed vertebrate column were positioned on the platform. Periodic lateral tilts of the platform caused antiphase flexion–extension (F/E) movements of the limbs, with the amplitude and limb configuration similar to those in the previously studied task, in which the intact rabbit maintained balance on the periodically tilting platform (Beloozerova et al. 2003a).
In decerebrated rabbits before spinalization, these F/E movements elicited vigorous reflex responses, including activation of the ankle, knee, and hip extensors (Gast, Vast, Grac) in the flexing limb, which resulted in a significant increase of the force produced by this limb (Figs. 2–4). Augmentation of extensor EMGs and increase of contact force during limb flexion suggest stabilizing postural effects—thus these reflex responses can be termed “postural limb reflexes.” Coactivation of extensors and some bifunctional muscles, in particular St (Lyalka, Deliagina, and Orlovsky, unpublished data), is a characteristic feature of the postural reactions to lateral tilts in intact rabbits (Beloozerova et al. 2003a; Deliagina et al. 2000). In decerebrated rabbits, limb flexion was also associated with activation of the ankle flexor (TA). Activation of TA together with extensors was not observed in intact rabbits, suggesting that the muscular synergy of postural limb reflexes formed in decerebrated rabbits differs slightly from the normal postural synergy.
After spinalization, the force responses to limb flexion were practically absent (they did not differ from the passive force; Fig. 3). The EMG responses either disappeared or their magnitude considerably decreased. Moreover, many of the residual responses in extensor muscles exhibited a reversed pattern—these muscles were activated not with limb flexion (as in the decerebrated rabbit) but with limb extension (Fig. 4), suggesting their destabilizing postural effects. Thus spinalization leads to the disappearance of postural limb reflexes. Similar results—a dramatic decrease of the magnitude and probability of EMG responses to tilts, as well as the appearance of incorrectly phased responses—were observed in awake rabbits with severe SCIs, when tested on the tilting platform (Lyalka et al. 2008, 2009a,b).
The main result of this study was that, in spinal rabbits, EES caused facilitation of limb reflexes, both during the period of stimulation and during a long period (up to a few minutes) afterward. However, the patterns of reflexes facilitated during these two periods were essentially different.
During the post-EES facilitation period, all muscles (Gast, Vast, Grac, St, and TA) were activated with limb flexion. This pattern was similar to that exhibited by decerebrated rabbits before spinalization (Fig. 4), suggesting that postural limb reflexes were facilitated. The EMG and force responses contained both dynamic and static components (Fig. 5). The presence of a static component suggests that EES resulted in a reappearance of the extensor tone. However, the magnitude of these facilitated limb reflexes in spinal rabbits was much smaller than that in decerebrated rabbits (Fig. 3).
During the period of EES, most muscles (Vast, Grac, St, and TA) exhibited a reversed response pattern—i.e., they were activated with limb extension and only one muscle (Gast) was activated with limb flexion (Fig. 4). In general, EMG responses during EES were weaker and more complex (due to synchronization by EES pulses) than those observed during the post-EES facilitation period. No increase of force responses was observed during EES (Fig. 3). Since the activation of extensors with their shortening may have destabilizing postural effects, the reflex responses facilitated during EES cannot be considered as postural limb reflexes.
These results have shown that EES can facilitate two different groups of limb reflexes, with prevailing muscular responses either to limb extension (nonpostural reflexes, facilitated during EES) or to limb flexion (postural limb reflexes, facilitated after cessation of EES). Thus the spinal cord contains the networks underlying postural limb reflexes. One can suggest that, in intact rabbits, a specific supraspinal tonic drive is used for selection and activation of these networks. The obtained data imply that EES partly substitutes this supraspinal drive (abolished by spinalization), which results in activation of the reflex mechanisms of the postural control system residing in the spinal cord. A low efficacy of postural limb reflexes in spinal animals can be caused for two reasons: 1) insufficient activation of spinal postural networks by EES and 2) the absence of supaspinal corrective commands that supplement spinal postural reflexes.
Sensory origin of reflex responses
Different groups of limb afferents can be responsible for postural reactions in the normal rabbit keeping balance on the tilting platform (Duysens et al. 2000; Horak and Macpherson 1996), including stretch receptors (muscle spindles) and load receptors (Golgi tendon organs, pressure receptors in the foot sole). The postural limb reflexes caused by the whole-limb antiphase F/E movements in decerebrated rabbits (activation of extensors with limb flexion and loading; Fig. 2A) were basically similar not only to the responses in normal rabbits, but also to those caused by multijoint movements of a single limb in decerebrated cats (Hyngstrom et al. 2008). These authors argued that the most likely origin for these responses was the input from stretch receptors of muscle spindles, transmitted by group Ia and group II afferents.
It seems likely that the postural limb reflexes, which we observed in spinal animals during the post-EES facilitation period (activation of extensors with flexion and loading the limb; Fig. 6C), were caused primarily by the input from stretch receptors of extensor muscles, whereas the input from load receptors played a secondary role. First, the magnitude of force produced by the limb was relatively small compared with that in the standing intact animals (Beloozerova et al. 2003a). This force seems to be insufficient for elicitation of a significant response in Golgi tendon organs (Matthews 1972). Second, in resting animals, the main effect of 1b fibers from these receptors is inhibition of extensor motoneurons (Matthews 1972). To evoke their excitation, a reversal of 1b reflexes is necessary. Such reflex reversal was observed during elicitation of locomotion caused not only by stimulation of the mesencephalic locomotor region in decerebrated cats, but also by drug application in spinal cats (Gossard et al. 1994).
It remains unclear, however, whether the post-EES effects, in addition to facilitation of limb reflexes, also included the 1b reflex reversal. The answer to this question is important for understanding the possible contribution of limb reflexes to postural control. For example, in the task of keeping balance on the tilting platform, the feedback based on stretch receptors would promote the lateral stability by increasing the rigidity of the limb when it is flexing under the effect of load. By contrast, the feedback based on load receptors could cause extension of the loaded limb, thus promoting the maintenance of normal body posture on an inclined plane (for discussion, see Lyalka et al. 2008, 2009; Musienko et al. 2008).
During the EES period, inverse responses—i.e., activation of extensor muscles with their passive shortening—were observed. The afferent source of these responses remained unclear as well. It seems unlikely that the activation of muscles was caused by their own spindle and tendon afferents. It seems more likely that activation was caused by the afferents of antagonistic muscles.
In quadrupeds, lateral stability in the hindquarters is based not only on the activity of reflex mechanisms of individual limbs but also on their interaction due to mutual influences mediated by crossed reflexes (Deliagina et al. 2006b). In the present study, however, we have not revealed any significant interlimb influences either in decerebrated rabbits or in spinal rabbits subjected to EES. One can conclude that the postural limb reflexes were caused by the inputs from afferents of the ipsilateral limb. This finding suggests that EES does not facilitate the crossed reflexes contributing to the maintenance of lateral stability in intact rabbits.
Combination of EES with quipazine application
It is known that serotonergic drugs promote activation of some spinal mechanisms related to postural control. As shown by Miller et al. (1996), the extensor excitability in the acute spinal cat can be increased by 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI, a 5-HT2 agonist). Our recent studies (Lyalka et al. 2008) have shown that both serotonin and quipazine (a broad but predominantly 5-HT2 agonist), when applied to the lumbar spinal cord in the chronic rabbits with a partial SCI, facilitated postural limb reflexes.
In the present study we addressed the question of whether quipazine is also efficient in acute spinal rabbits and whether quipazine can augment the effects of EES. It was found that quipazine, when applied alone, could facilitate limb reflexes, including both postural limb reflexes (activation of extensors with limb flexion and loading) and nonpostural (reversed) responses (Fig. 10, D and E). Thus activation of limb reflexes with quipazine was less selective than their post-EES activation.
When application of quipazine was combined with EES, the main effect of EES (i.e., the post-EES facilitation of the postural limb reflexes) was considerably magnified (Fig. 10, A–C), despite quipazine itself facilitated both normal and reversed responses (compare columns 1 and 2 in Fig. 10, D and E).
Comparison of postural and locomotor effects of EES
Previously it was shown that EES in the chronic spinal cats and rats promoted activation of spinal locomotor mechanisms, which resulted in coordinated stepping movements of the hindlimbs on the moving treadmill. When tested in the acute spinal cats, EES appeared less efficient: although EES could induce stepping movements as early as in a few hours after spinalization, these movements were very weak and poorly coordinated and there was no weight bearing during stepping (for review, see Gerasimenko et al. 2008). In the chronic spinal rats, the locomotor effects of EES could be enforced by serotonergic drug administration (Courtine et al. 2009; Gerasimenko et al. 2007b).
In contrast to those results, the main effect of EES observed in the present study was post-EES facilitation of limb reflexes relevant for postural control, whereas the spinal locomotor mechanisms were not activated. A number of factors could be responsible for this difference between the effects of EES, including for example the difference in species used, the difference in characteristics of EES (strength, frequency, site of stimulation), and the difference in somatosensory stimulation of limbs (by moving the treadmill or by tilting the platform). The most important factor, in our opinion, was the time passed after spinalization. In the present study, enhancement of limb reflexes was observed in acute experiments (the postlesion time did not exceed 10 h). This period corresponds to the initial phase of the spinal shock, when the spinal networks are not yet subjected to plastic changes (Ditunno et al. 2004; Valero-Cabre et al. 2004). During this period, the locomotor effects of EES were weak (Musienko et al. 2007a). By contrast, stronger locomotor effects of EES were observed in chronic experiments, with the postlesion time from 2 wk to 2 mo (Gerasimenko et al. 2008). It is known that, at the later postlesion stages, significant plastic changes occur in the spinal cord, resulting in development of spasticity, which includes not only impairment of the system of spinal reflexes, but also augmentation of oscillatory properties of spinal neurons and thus instability of their networks (Harkema 2008; Ko et al. 1999; Lyalka et al. 2008). These dramatic changes in the functional state of the spinal networks toward the rhythmogenesis could be responsible for the changes in the effect of EES—from postural to locomotor ones. To test this hypothesis, a study of the effects of EES in the rabbit at later postlesion stages is now in progress. We expect that postural effects of EES will decrease over time along with augmentation of its locomotor effects.
Structures responsible for the effects of EES
It is not clear which neuronal groups are responsible for the effects of EES. The likely neuronal structures activated by EES have been considered in the studies devoted to the locomotor effects of EES in the rat (Gerasimenko et al. 2006, 2008; Lavrov et al. 2006). It was found that EMG responses were partly synchronized by the EES pulses and contained three components (early, middle, and late), with their latency about 3, 5, and 9 ms, respectively. It was suggested that the early component reflected a direct excitation of motor axons or motoneurons, whereas the middle and late components reflected a mono- and polysynaptic excitation of motoneurons.
In the present study it was found that the synchronized EMG responses during EES also consisted of a few components, with their latencies ranging from 4 to 10 ms (Fig. 7F). By analogy with the studies on the rat, the earliest component could be attributed to a direct excitation of motoneurons (or their axons), whereas the later components could be ascribed to their mono- and polysynaptic excitation. The nonsynchronized component of EMG response (Part 2 in Fig. 7E) was most likely a result of the activity in polysynaptic pathways. This issue, however, requires further study.
As shown in the present study, the effects of EES considerably outlasted the duration of stimulation. Moreover, the effects after cessation of EES were usually much more uniform and prominent than those during EES. Our interpretation of these findings is that there are two different spinal circuits activated by EES. The first circuit accumulates the effects of EES and causes a long-lasting activation of postural limb reflexes. The second circuit is active only during EES. It does not participate in the activation of postural limb reflexes; rather it perturbs normal functioning of the first circuit. One can suggest that the second circuit participates in the locomotor effects of EES (Gerasimenko et al. 2008).
In the present study we compared the effectiveness of stimulation of L5 and L7 segments and found that L7 stimulation was more effective (Fig. 9). This result suggests that the neuronal circuits causing facilitation of postural limb reflexes are distributed unevenly in the lumbar region of the spinal cord. A specific rostrocaudal distribution of motoneuron pools in rabbits (Portal et al. 1991) can also contribute to the intersegmental differences. A similar conclusion was made for the neuronal circuits responsible for the locomotor effects of EES (Gerasimenko et al. 2007a; Ichiyama et al. 2005).
To summarize, the present study has shown that the postural circuits are present in the spinal cord of the rabbit, but they are not active in the spinal animals because of the lack of supraspinal excitatory drive. The EES can partly substitute this excitatory drive and, to a certain extent, activate the spinal postural circuits, suggesting that these circuits in intact animals are most likely activated by tonic supraspinal drive. When activated, these circuits generate the limb reflexes relevant for postural functions (postural limb reflexes), which in intact animals contribute to the generation of postural corrections. The effects of EES could be increased by application of quipazine, but this increase is not sufficient for restoration of postural functions in spinal subjects. A search for other factors promoting activation of spinal postural reflexes seems meaningful.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-049884 and Swedish Research Council (Karolinska Institutet) Grants 11554 to T. G. Deliagina and 21076 to P. Zelenin.
REFERENCES
- Beloozerova IN, Sirota MG, Orlovsky GN, Deliagina TG. Activity of pyramidal tract neurons in the cat during postural corrections. J Neurophysiol 93: 1831–1844, 2005 [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Swadlow H, Orlovsky GN, Popova LB, Deliagina TG. Activity of different classes of neurons in the motor cortex during postural corrections. J Neurosci 23: 7844–7853, 2003b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloozerova IN, Zelenin PV, Popova LB, Orlovsky GN, Grillner S, Deliagina TG. Postural control in the rabbit maintaining balance on the tilting platform. J Neurophysiol 90: 3783–3793, 2003a [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12: 1333–1342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Popova LB, Sirota MG, Swadlow H, Grant G, Orlovsky GN. Role of different sensory inputs for maintenance of body posture in sitting rat and rabbit. Motor Control 4: 439–452, 2000 [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN. Spinal and supraspinal postural networks. Brain Res Rev 57: 212–221, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Zelenin PV, Beloozerova IN. Neural bases of postural control. Physiology 21: 216–225, 2006a [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Sirota MG, Zelenin PV, Orlovsky GN, Beloozerova IN. Interlimb postural coordination in the standing cat. J Physiol 573: 211–224, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic M, Gerasimenko Y, Pinter M. Evidence for a spinal central pattern generator in humans. Ann NY Acad Sci 860: 360–376, 1998 [DOI] [PubMed] [Google Scholar]
- Ditunno JF, Little JW, Tessler A, Burns AS. Spinal shock revisited: a four-phase model. Spinal Cord 42: 383–395, 2004 [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80: 83–133, 2000 [DOI] [PubMed] [Google Scholar]
- Edgerton VR, de Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J Physiol 533: 15–22, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko YP, Avelev VD, Nikitin OA, Lavrov IA. Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neurosci Behav Physiol 33: 247–254, 2003 [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, Roy RR, Edgerton VR. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol 98: 2525–2536, 2007 [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Lavrov IA, Courtine G, Ichiyama RM, Dy CJ, Zhong H, Roy RR, Edgerton VR. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Methods 157: 253–263, 2006 [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Moshonkina TR, Musienko PE. Capacity of lumbar-sacral spinal cord to produce locomotor pattern in response to electrical epidural stimulation and step training in decerebrated and spinal animals. Soc Neurosci Abstr 754, 2007 [Google Scholar]
- Gerasimenko YP, Roy RR, Edgerton VR. Epidural stimulation: comparison of the spinal circuits that generate and control locomotion in rats, cats and humans. Exp Neurol 209: 417–425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard JP, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group 1b pathway from hindlimb extensor muscles in the cat. Exp Brain Res 98: 213–228, 1994 [DOI] [PubMed] [Google Scholar]
- Grillner S. Locomotion in the spinal cat. In: Control of Posture and Locomotion, edited by Stein RB, Pearson KG, Smith RS, Redford JB. New York: Plenum Press, 1973, p. 515–535 [Google Scholar]
- Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res 34: 241–261, 1979 [DOI] [PubMed] [Google Scholar]
- Harkema SJ. Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res Rev 57: 255–264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Gottschall JS, Nichols TR. Electromyographic responses from the hindlimb muscles of the decerebrate cat to horizontal support surface perturbations. J Neurophysiol 101: 2751–2761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak F, Macpherson J. Postural orientation and equilibrium. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Neural Control of Movement Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, p. 255–292 [Google Scholar]
- Hyngstrom A, Johnson M, Schuster J, Heckman CJ. Movement-related receptive fields of spinal motoneurons with active dendrites. J Physiol 586: 1581–1593, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett 383: 339–344, 2005 [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm 114: 1339–1348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H-Y, Ditunno JF, Graziani V, Little JW. The pattern of reflex recovery during spinal shock. Spinal Cord 37: 402–409, 1999 [DOI] [PubMed] [Google Scholar]
- Lavrov I, Courtine G, Dy CJ, van den Brand R, Fong AJ, Gerasimenko Y, Zhong H, Roy RR, Edgerton VR. Facilitation of stepping with epidural stimulation in spinal rats: role of sensory input. J Neurosci 28: 7774–7780, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrov I, Gerasimenko Y, Ichiyama RM, Courtine G, Zhong H, Roy RR, Edgerton VR. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J Neurophysiol 96: 1699–1710, 2006 [DOI] [PubMed] [Google Scholar]
- Lyalka VF, Karayannidou A, Zelenin PV, Orlovsky GN, Deliagina TG. Facilitation of postural limb reflexes in spinal rabbits. Soc Neurosci Abstr 35: 766.13, 2009b [Google Scholar]
- Lyalka VF, Musienko PE, Orlovsky GN, Grillner S, Deliagina TG. Effect of intrathecal administration of serotoninergic and noradrenergic drugs on postural performance in rabbits with spinal cord lesions. J Neurophysiol 100: 723–732, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyalka VF, Orlovsky GN, Deliagina TG. Impairment of postural control in rabbits with extensive spinal lesions. J Neurophysiol 101: 1932–1940, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyalka VF, Zelenin PV, Karayannidou A, Orlovsky GN, Grillner S, Deliagina TG. Impairment and recovery of postural control in rabbits with spinal cord lesions. J Neurophysiol 94: 3677–3690, 2005 [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Everaert DG, Stapley PJ, Ting LH. Bilateral vestibular loss in cats leads to active destabilization of balance during pitch and roll rotations of support surface. J Neurophysiol 97: 4357–4367, 2007 [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Fung J, Jacobs R. Postural orientation, equilibrium, and the spinal cord. In: Advances in Neurology: Neuronal Regeneration, Reorganization, and Repair, edited by Seil FJ. Philadelphia, PA: Lippincott–Raven, 1997, vol. 72, p. 227–232 [PubMed] [Google Scholar]
- Massion J. Postural control system. Curr Opin Neurobiol 4: 877–888, 1994 [DOI] [PubMed] [Google Scholar]
- Massion J. Postural control systems in developmental perspective. Neurosci Biobehav Rev 2: 465–472, 1998 [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and Their Central Actions.London: Arnold, 1972 [Google Scholar]
- Miller JF, Paul KD, Lee RH, Rymer WZ, Heckman CJ. Restoration of extensor excitability in the acute spinal cat by the 5HT2 agonist DOI. J Neurophysiol 75: 620–628, 1996 [DOI] [PubMed] [Google Scholar]
- Minassian K, Persy I, Rattay F, Pinter MM, Kern H, Dimitrijevic MR. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum Mov Sci 26: 275–295, 2007 [DOI] [PubMed] [Google Scholar]
- Musienko PE, Bogacheva IN, Gerasimenko YP. Significance of peripheral feedback in the generation of stepping movements during epidural stimulation of the spinal cord. Neurosci Behav Physiol 37: 181–190, 2007a [DOI] [PubMed] [Google Scholar]
- Musienko PE, Zelenin PV, Lylalka VF, Orlovsky GN, Deliagina TG. Postural performance in decerebrated rabbit. Behav Brain Res 190: 124–134, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko PE, Zelenin PV, Orlovsky GN, Deliagina TG. Enhancement of limb reflexes in the spinal rabbit by stimulation of the spinal cord. Soc Neurosci Abstr 33: 75.17, 2007b [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion. From Mollusc to Man.Oxford, UK: Oxford Univ. Press, 1999 [Google Scholar]
- Portal JJ, Corio M, Viala D. Localization of the lumbar pools of motoneurons which provide hindlimb muscles in the rabbit. Neurosci Lett 124: 105–107, 1991 [DOI] [PubMed] [Google Scholar]
- Pratt CA, Fung J, Macpherson JM. Stance control in the chronic spinal cat. J Neurophysiol 71: 1981–1985, 1994 [DOI] [PubMed] [Google Scholar]
- Rossignol S, Chau C, Brustein E, Giroux N, Bouyer L, Barbeau H, Reader TA. Pharmacological activation and modulation of the central pattern generator for locomotion in the cat. Ann NY Acad Sci 860: 346–359, 1998 [DOI] [PubMed] [Google Scholar]
- Rossignol S, Drew T, Brustein E, Jiang W. Locomotor performance and adaptation after partial or complete spinal cord lesions in the cat. In: Peripheral and Spinal Mechanisms in the Neural Control of Movement, edited by Binder MD.Amsterdam: Elsevier, 1999, p. 349–365 [DOI] [PubMed] [Google Scholar]
- Rossignol S, Giroux N, Chau C, Marcoux J, Brustein E, Reader TA. Pharmacological aids to locomotor training after spinal injury in the cat. J Physiol 533: 65–74, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapley PJ, Ting LH, Kuifu C, Everaert DG, Macpherson JM. Bilateral vestibular loss leads to active destabilization of balance during voluntary head turns in the standing cat. J Neurophysiol 95: 3783–3797, 2006 [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Fores J, Navarro X. Reorganization of reflex responses mediated by different afferent sensory fibers after spinal cord transection. J Neurophysiol 91: 2838–2848, 2004 [DOI] [PubMed] [Google Scholar]