Abstract
Evidence suggests that the nervous system controls motor tasks using a low-dimensional modular organization of muscle activation. However, it is not clear if such an organization applies to coordination of human walking, nor how nervous system injury may alter the organization of motor modules and their biomechanical outputs. We first tested the hypothesis that muscle activation patterns during walking are produced through the variable activation of a small set of motor modules. In 20 healthy control subjects, EMG signals from eight leg muscles were measured across a range of walking speeds. Four motor modules identified through nonnegative matrix factorization were sufficient to account for variability of muscle activation from step to step and across speeds. Next, consistent with the clinical notion of abnormal limb flexion-extension synergies post-stroke, we tested the hypothesis that subjects with post-stroke hemiparesis would have altered motor modules, leading to impaired walking performance. In post-stroke subjects (n = 55), a less complex coordination pattern was shown. Fewer modules were needed to account for muscle activation during walking at preferred speed compared with controls. Fewer modules resulted from merging of the modules observed in healthy controls, suggesting reduced independence of neural control signals. The number of modules was correlated to preferred walking speed, speed modulation, step length asymmetry, and propulsive asymmetry. Our results suggest a common modular organization of muscle coordination underlying walking in both healthy and post-stroke subjects. Identification of motor modules may lead to new insight into impaired locomotor coordination and the underlying neural systems.
INTRODUCTION
Human movements exhibit considerable variability from trial to trial (Bernstein 1967) and are highly complex in terms of both neural activation and biomechanical output (Gottlieb 1998; Winter and Yack 1987). However, much evidence now indicates that a relatively low-dimensional organizational structure may underlie the rich complexity of neuromechanical output. Computer simulations have shown that modulating the timing and amplitude of a few sets of distinct muscle groupings is sufficient to produce pedaling at different cadences (Raasch and Zajac 1999) and pedaling backward (Neptune et al. 2000; Ting et al. 1999). Furthermore, these muscle groupings are shown to correspond to particular biomechanical functions critical to the propulsion and phase transitions during pedaling. Experimental studies using decomposition techniques have shown that muscle activation patterns during natural behaviors may in fact be organized in a similar modular fashion during locomotor and postural tasks in animal and humans (Bizzi et al. 2008; Ivanenko et al. 2004, 2005; Merkle et al. 1998; Olree and Vaughan 1995; Ting and Macpherson 2005; Ting and McKay 2007; Tresch et al. 1999). Moreover, a few experimental studies in postural control have shown direct correlations between the activity of motor modules (also referred to as muscle synergies) with kinetic and kinematic outputs (Krishnamoorthy et al. 2003; Ting and Macpherson 2005; Torres-Oviedo et al. 2006). Accordingly, these motor modules may reflect a neural strategy of coordinating muscles in a low-dimensional set of patterns that facilitate control of functional motor behaviors.
Although modular organization of muscle coordination during human walking has been proposed (Merkle et al. 1998; Olree and Vaughan 1995), the robustness of such an organization and the relationship to task-level goals is not well established. Prior work has shown consistent timing of motor patterns across various walking tasks but suggests that the coactivation of muscle varies considerably (Ivanenko et al. 2004). However, studies of different postural tasks suggest that stable modules do exist (Ting and Macpherson 2005; Torres-Oviedo et al. 2006), which is consistent with our simulations of pedaling and simulations of central pattern generator control of locomotor patterns (McCrea and Rybak 2008). There are clear repeatable characteristics of locomotor muscle activity from step to step that suggest stable modular organization is a feasible control solution. However, it is not known whether such an organization can account for the inherent variability that is also characteristic of repeated stepping or walking at different speeds.
If muscle modules are indeed mechanisms by which task-level biomechanical goals are implemented, we would hypothesize that impairments to the neural control and organization of such modules would directly result in impaired biomechanical outputs. Simulations have shown that independent activation of primary extensors and bifunctional leg muscles is necessary to allow for smooth flexion–extension transitions during pedaling. Indeed, in post-stroke subjects, abnormal coactivation of bifunctional thigh muscles with extensors and difficulty with flexion–extension transition phases is observed post-stroke along with reduced external mechanical work production. Although these results suggest a link between abnormal muscle coactivation and impaired biomechanical output, the degree to which such observations would apply to impaired walking (e.g., in persons with post-stroke hemiparesis) is unknown. Moreover, if motor modules do produce biomechanical functions, it would be critical to know whether patients have access to a subset of modules available to healthy subjects or whether the modules themselves are also impaired (Ting and McKay 2007).
The causal relationships between neuromotor impairments and locomotor performance post-stroke have yet to be shown. post-stroke motor impairment has traditionally been characterized in the clinic as being caused by “abnormal synergies,” in reference to stereotypical patterns of limb flexion and extension, but the relationship of such observations to the muscle synergies/modules identified during walking through quantitative analysis of EMG is unknown. Additionally, motor impairments in persons post-stroke are typically evaluated during isolated voluntary movements (Fugl-Meyer et al. 1975), which may have limited relevance to motor coordination during walking (Bowden et al. 2009). Although post-stroke locomotor impairments are assumed to be caused by abnormal patterns of muscle coordination, prior studies typically focused on kinematic or spatiotemporal patterns (De Quervain et al. 1996; Mulroy et al. 2003) or on muscle activation magnitude and timing patterns in individual muscles (Den Otter et al. 2006; Knutsson and Richards 1979).
We hypothesized that walking is produced through the variable activation of a small set of motor modules in healthy subjects. Therefore we predicted that the step-by-step variability in muscle activation patterns and changes in muscle activity across different walking speeds could all be accounted for by the same set of motor modules identified through decomposition techniques. We further hypothesized that the changes in motor modules post-stroke underlies the specific motor deficits observed and the reduced complexity in the muscle activation signals. Accordingly, we predicted that differences in the modular organization between post-stroke and healthy persons would be associated with various measures of walking performance.
METHODS
Participants
Participants in this study included 55 adults with post-stroke hemiparesis and 20 healthy adults (see Table 1 for demographic information). Inclusion criteria for persons post-stroke included hemiparesis secondary to a single unilateral stroke and absence of lower extremity joint pain, contractures, major sensory deficits, cardiovascular or respiratory symptoms contraindicative of walking, and any other significant non–stroke-related impairment affecting walking. All participants were capable of walking independently for 10 m on a level surface, showing that dynamic postural stability was largely intact and that our study was primarily addressing locomotor coordination. Study procedures were approved by the Institutional Review Boards of the Department of Veterans Affairs and the University of Florida. Participants provided informed consent in accordance with the Declaration of Helsinki.
Table 1.
Participant demographics
| Mean | SD | Range | ||
|---|---|---|---|---|
| Hemiparetic group (n = 55) | ||||
| Age (yr) | 59.5 | 11.7 | 36–82 | |
| Time since stroke (mo) | 57.8 | 64.8 | 7–411 | |
| Lower extremity Fugl-Meyer score (out of 34) | 22.0 | 8.3 | 8–34 | |
| Lower extremity Fugl-Meyer synergy score (out of 22) | 14.9 | 5.5 | 6–22 | |
| Self-selected overground walking speed (m/s) | 0.58 | 0.26 | 0.14–1.16 | |
| Sex (male/female) | 35/20 | |||
| Side affected (left/right) | 34/21 | |||
| Control group (n = 20) | ||||
| Age | 65.5 | 9.8 | 51–83 | |
| Self-selected overground walking speed (m/s) | 1.25 | 0.15 | 0.99–1.62 | |
| Sex (male/female) | 4/16 |
Procedures
Walking trials of 30-s duration were performed on an ADAL split-belt motor driven treadmill (Techmachine, Andrezieux Boutheon, France), and all subjects were secured by a safety harness to eliminate the risk of falling. No body weight unloading was provided, and participants did not hold onto a bar or use any other supporting devices. Participants with post-stroke hemiparesis walked at self-selected comfortable speed (SS, 3 separate trials) and at fastest comfortable speed (FC, 2 separate trials). Healthy participants walked at SS (3 trials), FC (2 trials), and six additional speeds (1 trial each) ranging from 0.3 to 1.8 m/s, which allowed us to test the robustness of our methodology across different walking speed conditions. In addition, overground walking at self-selected speed was assessed using a GAITRite walkway (CIR Systems, Havertown, PA). Bipolar Ag-AgCl surface electrodes were used to record EMG from the tibialis anterior (TA), soleus (SO), medial gastrocnemius (MG), vastus medialis (VM), rectus femoris (RF), medial hamstrings (MH), lateral hamstrings (LH), and gluteus medius (GM) of each leg using a telemetered EMG acquisition system (Konigsberg Instruments, Pasedana, CA). Each skin site was shaved and cleaned with alcohol before electrode placement (Perroto 1994). All data were measured at 2,000 Hz using Vicon Workstation v4.5 software and saved to disk for off-line analysis. Data were analyzed using Matlab 7.0 (The Mathworks, Natick, MA) and JMP statistical software (v. 7.0, SAS Institute, Cary, NC).
Data analysis
NONNEGATIVE MATRIX FACTORIZATION.
Muscle activation signals (EMGs) were high-pass filtered (40 Hz) with a zero lag fourth-order Butterworth filter, demeaned, rectified, and smoothed with a zero lag fourth-order low-pass (4 Hz) Butterworth filter. To facilitate comparisons between subjects and among different walking speeds, the EMG from each muscle was normalized to its peak value from self-selected walking and resampled at each 1% of the gait cycle. For each subject, leg, and walking speed, the EMGs were combined into an m × t matrix (EMGo), where m indicates the number of muscles and t is the time base (t = no. of strides × 101).
For each subject, an NNMF algorithm (Lee and Seung 1999; Ting and Macpherson 2005) was applied to the m × t matrix corresponding to all gait cycles from SS trials 1 and 2 (trial 3 was reserved for later analysis). A priori, the number of modules, n, is specified, and the NNMF algorithm finds the properties of the modules by populating two matrices: an m × n matrix, which specifies the relative weighting of a muscle in each module, with each muscle weight invariant across all gait cycles, and an n × t matrix, which specifies the activation timing of each module over each of the gait cycles.
When these two matrices are multiplied, an m × t matrix is produced that attempts to reconstruct the EMGs over all of the consecutive gait cycles. Notice that NNMF allows muscles to belong to more than one module and that a muscle's reconstructed EMG is the summed contributions from all the modules (Fig. 1). The m × t matrix of the reconstructed EMGs (EMGr) is compared with the original EMG matrix (EMGo) and the agreement quantified by calculating the sum of the squared errors: (EMGo – EMGr)2. Within this framework, the NNMF algorithm performed an iterative optimization until it converged on the muscle weights and the activation timings of the modules that minimized the error.
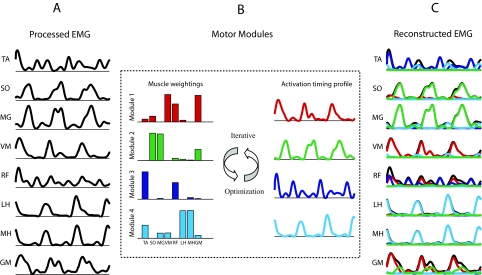
Fig. 1.
Reconstruction of EMGs by nonnegative matrix factorization (NNMF). Only 3 consecutive cycles of reconstruction are shown. A: normalized muscle activation signals from eight unilateral leg muscles over a series of cycles were analyzed. B: muscle activity was processed by an NNMF algorithm, which applied an iterative optimization procedure to best reconstruct the activation signals using a small set of motor modules. For each module, the adjusted parameters include muscle weightings and an activation timing profile across the gait cycle. The contribution of any given module to a muscle's activation over the gait cycle is the product of the muscle weighting for that module times the module's activation timing profile. C: for each muscle, the summed contributions from the modules constitute the reconstructed EMG signal.
Determining the number of modules needed for EMG reconstruction
We made no a priori assumptions regarding the number of modules that would be required to adequately reconstruct the EMG signals. Therefore separate NNMF analyses were performed with the output constrained to one, two, three, four, and five modules. To determine the minimum number of modules needed to adequately reconstruct EMGo in each leg of each subject, we calculated the variability accounted for (VAF) as the ratio of the sum of the squared error values to the sum of the squared EMGo values [VAF = 1 − (EMGo–EMGr)2/EMGo2]. VAF was calculated for each muscle across all gait cycles. To ensure that EMG was adequately reconstructed within each region of the gait cycle, VAF was also calculated as the cumulative of all muscles within each of six regions for all gait cycles. The regions were defined as 1) first double support, 2) first half of ipsilateral single leg stance, 3) second half of ipsilateral single leg stance, 4) second double support, 5) first half of ipsilateral swing, and 6) second half of ipsilateral swing. The analysis for each subject began by assuming that only one module was needed for EMG reconstruction. If VAF was ≥90% for each of the eight muscles and six regions, it was concluded that additional modules were not needed. Otherwise, the number of modules assumed was increased until all muscles and regions achieved 90% VAF or until adding an additional module did not increase VAF by >5% for the muscle(s) and/or region(s) with the lowest VAF. This approach is conservative and ensures a strong agreement between the original and reconstructed EMG signals.
Robustness of module definitions across walking speeds
To determine the robustness of the SS module definitions across different SS trials and at other walking speeds, we performed an EMG reconstruction on walking data not originally used to define the modules (i.e., SS trial 3 and all fixed speed trials). This was accomplished by holding the muscle weightings of the modules (which were defined using SS trials 1 and 2) constant across all conditions while allowing the activation timing in each cycle to vary. This analysis was performed for each individual subject and leg, and the VAF of the reconstructed EMG was used to quantify the success of the original identified muscle weightings and the newly computed activation timings to reconstruct the EMGs.
Locomotor performance measures
To assess whether the number of modules accounting for the paretic leg EMGs was related to locomotor performance, we examined the self-selected overground walking speed (Perry et al. 1995), change in speed between self-selected and fastest comfortable walking (Jonkers et al. 2009), step length asymmetry (Balasubramanian et al. 2007), and forward propulsive asymmetry (Bowden et al. 2006). To quantify step length asymmetry, we first calculated the ratio of the paretic step length to the overall stride length. We then subtracted this paretic step ratio from 0.5 (which would indicate perfectly symmetrical step lengths) to determine the deviation from symmetry. A similar method was used for calculating forward propulsive asymmetry, where propulsion is defined as the integral of the anteriorly directed component of the horizontal ground reaction force (Bowden et al. 2006). The amount of paretic propulsion relative to the sum of paretic and nonparetic propulsion (Bowden et al. 2006) was computed and subtracted from 0.5 (where 0.5 indicates symmetrical propulsion) to determine the deviation from symmetry.
Activation characteristics contributing to reduced complexity
To further examine the characteristics of muscle activation responsible for reduced locomotor output complexity, we separated our post-stroke participants into groups based on the number of modules required for EMG reconstruction (2, 3, or 4 modules; see results). After establishing these post-stroke groups, we performed the NNMF analysis assuming four modules for all of the participants to assess how the muscle weightings differed among groups and how activation profiles differed among modules within the same group. The muscle weightings for each module were correlated across groups to quantify the similarity (i.e., module 1 across groups, module 2 across groups, etc.). High correlations indicate similar module composition. Within each group, correlations of activation timing profiles between modules were computed to assess whether modules are independently active. High correlations indicate a lack of independent module activation.
EMG magnitude
To assess the potential effect of the EMG magnitude on the number of modules identified, raw EMG in millivolts from each muscle was averaged by leg (i.e., paretic or nonparetic) over all the gait cycles for each participant. The values from all muscles were averaged together to obtain a single composite measure for each leg of each participant.
Statistics
Group differences in the number of modules accounting for muscle activation during self-selected walking were assessed using a Pearson's χ2 test. The association between the number of modules and walking performance was assessed using a Spearman's correlation. A two-factor ANOVA and Tukey's post hoc analysis were used to compare EMG magnitude across control, paretic, and nonparetic legs with different levels of locomotor output complexity.
RESULTS
Modular organization of locomotor muscle activity in healthy individuals
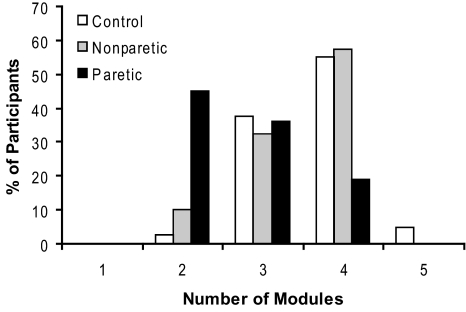
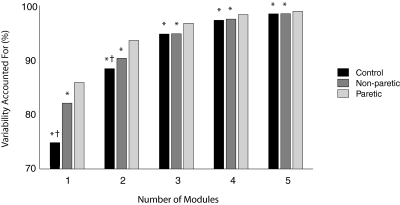
In healthy participants, four modules were typically required to reconstruct unilateral lower extremity muscle activation during walking at self-selected speed (3.6 ± 0.6 modules for the right leg, 3.7 ± 0.7 for the left leg). Of the 40 healthy legs measured, 2.5% required two, 37.5% required three, 55% required four, and 5% required five modules (Fig. 2). The variability accounted for by any given number of modules was always lower in the control and nonparetic legs compared with the paretic legs, indicating less complexity in the patterned activity of the paretic leg during walking (Fig. 3).
Fig. 2.
Number of modules at self-selected walking speed. Four modules were needed to account for cycle-by-cycle variability of muscle activity recorded from 8 unilateral leg muscles during self-selected walking in the majority of healthy control and nonparetic legs. Significantly fewer modules were required for the paretic legs.
Fig. 3.
Total variability accounted for based on the number of modules extracted by NNMF. For each number of modules, the control and nonparetic legs had lower variability accounted for (VAF) than the paretic leg (*P < .001), and with 1 or 2 modules, the control legs had lower VAF than the nonparetic legs (†P < .001). Lower VAF for a particular number of modules indicates that the analysis is less able to account for the overall variability because of greater complexity in the muscle activation patterns during walking.
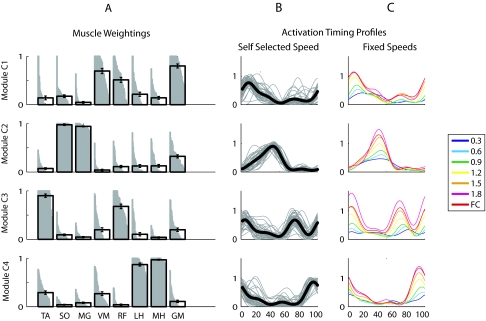
To characterize typical module composition and timing in the healthy legs, we extracted four independent modules from all of the subjects from the two SS trials, regardless of the actual number of modules required to account for our minimum criterion of 90% of the variability of each muscle and each region of the gait cycle. Each module was phased to a particular region of the gait cycle, and the characteristics of each module were quite similar across the healthy participants (Fig. 4, A and B). Our results showed the following properties of each module.
Fig. 4.
Module muscle weightings and activation timing profiles for each of the modules C1–C4 in healthy individuals (n = 40 legs) at self-selected walking speed and individual activation timing profiles with walking speed. A: for each module C1–C4, muscle weightings indicate the strength of representation for each muscle. Gray bars show the representation of that muscle within the module (all left and right healthy legs are shown). If a muscle was fully represented within a module across all subjects, the gray region would form a perfect rectangle. The black bar for each muscle weighting indicates group mean and SE. B: timing profiles indicate how activation of a module varies over the gait cycle. Thin gray lines show the profiles for each individual leg, with each line representing the average of the leg over all the gait cycles. Thick black lines show the group mean. C: group mean activation timing profiles of modules C1–C4 at various fixed speeds ranging from 0.3 to 1.8 m/s and at the fastest comfortable speed (FC). The muscle weightings shown in A were constrained to be identical for the EMG reconstruction performed at each of the walking speeds.
Module C1 consisted mainly of extensor activity from the GM (hip extensor and abductor), VM (knee extensor), and to a lesser extent, RF (knee extensor and hip flexor). This module was active primarily in early stance and likely provides body support during weight acceptance (Neptune et al. 2009).
Module C2 consisted mainly of calf muscles SO (ankle plantarflexor) and MG (ankle plantarflexor and knee flexor) and was active during late stance. It likely contributes to body support, forward propulsion, and swing initiation (Neptune et al. 2009).
Module C3 primarily consisted of activity in the TA (ankle dorsiflexor) and RF during early stance, which likely provides dorsiflexion during and immediately after heel strike and early swing, where it likely contributes to ground clearance of the foot (Neptune et al. 2009).
Module C4 consisted mainly of medial and lateral hamstring (MH and LH; knee flexor and hip extensor) activation during late swing and early stance and may decelerate the leg at the end of swing and propel the body during early stance (Neptune et al. 2009).
For each subject, the same set of module weightings was able to reproduce muscle activity for a range of walking speeds between 0.3 and 1.8 m/s (85–98% of variability was accounted for in all muscles and regions of the gait cycle). As walking speed increased, the activation timing profile of each module increased in amplitude and showed more defined peaks (Fig. 4C).
Modular organization of locomotor muscle activity in persons post-stroke
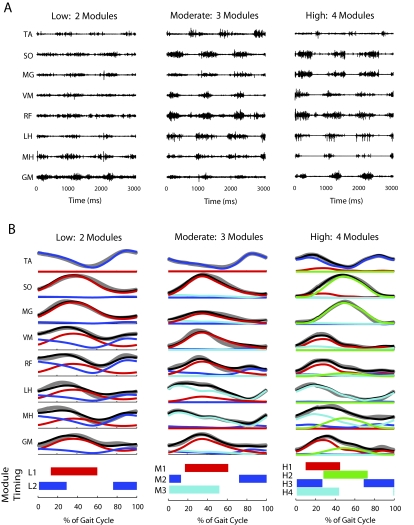
Fewer modules were needed to account for walking muscle activity in the paretic leg of persons post-stroke (2.7 ± 0.8) relative to nonparetic (3.5 ± 0.7) and healthy control legs (3.6 and 3.7 for right and left legs, respectively; P < 0.001) The majority of nonparetic legs (58%) required four modules, which is comparable to the healthy controls. However, most paretic legs required just two (45%) or three (36%) modules (Fig. 2). Henceforth, we refer to the two-, three-, and four-module paretic subgroups as the low, moderate, and high complexity paretic subgroups, respectively, because the presence of more independent modules reflects greater locomotor output complexity. Participants requiring fewer modules had more muscle coactivation (Fig. 5, cf. unprocessed, processed, and reconstructed EMGs).
Fig. 5.
Comparison of muscle activity and module timing in paretic legs with low, moderate, and high locomotor output complexity. A: unprocessed muscle activity from 3 s of walking in individual subjects with low (2 modules), moderate (3 modules), and high (4 modules) locomotor output complexity. B: processed muscle activity (gray lines), reconstructed muscle activity (black lines), and the contribution from each module to the reconstructed activity (colored lines) for each muscle averaged over 10 consecutive gait cycles. In both A and B, note that the activation patterns appear broader and less differentiated in the low complexity subject. The horizontal bars at the bottom of the figure indicate where in the gait cycle each module is highly active (defined as >50% of the mean activity).
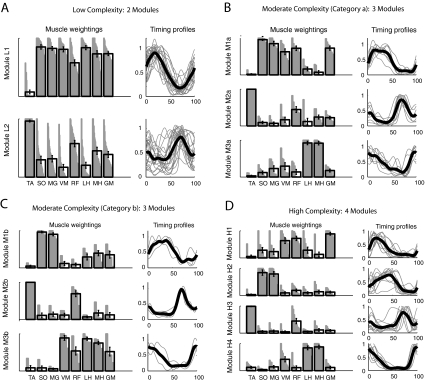
In the low complexity group, two modules with independent timing were found. One included strong representation of all of the muscles measured except for ankle dorsiflexor TA (module L1; Fig. 6A) and was active primarily in the stance phase. The other was dominated by TA and RF activity (module L2) and active primarily in the swing phase, similar to module C3 in healthy controls. However, the representation of other muscles within L2 was generally higher than in C3 in healthy control legs (cf. Fig. 4A, module C3, to Fig. 6A, module L2).
Fig. 6.
Module muscle weightings and activation timing profiles in the paretic leg of persons post-stroke at self-selected walking speed. Refer to Fig. 4 for meaning of gray and black bars and lines. A: the low complexity subgroup had a stance module (L1) that resembled a combination of control modules C1, C2, and C4 and a swing module (L2) that resembled control module C3. B: 1 of the moderate complexity subgroups (category a) had a stance module (M1a) that resembled a combination of control modules C1 and C2 and a swing module (M2a) that resembled control module C3. The 3rd module (M3a) resembled control module C4. C: the 2nd moderate complexity subgroup (category b) had a stance module (M1b) that resembled control module C2 and a swing module (M2b) that resembled control module C3. The 3rd module (M3b) resembled a combination of control modules C1 and C4. D: the high complexity subgroup had 4 modules (H1–H4) that resembled control modules C1–C4, respectively.
Within the moderate complexity subgroup, we observed two general categories of modular organization that were shared by a number of participants. Eight of 19 individuals (category a) had a module active throughout stance with strong representation of the ankle plantarflexors, as well as the more proximal extensor muscles (Fig. 6B, module M1a). This differed from the two separate modules (C1 and C2) observed in healthy controls with activation restricted to late and early stance, respectively. The remaining modules (Fig. 6B, modules M2a and M3a) were similar to healthy control modules C3 and C4, respectively. In four individuals (category b), a different modular organization was observed. The proximal extensors appeared in the same module as the hamstring muscles (Fig. 6C, module M3b), resembling a combination of healthy control modules C1 and C4. The remaining modules (Fig. 6C, modules M1b and M2b) resembled modules C2 and C3 in healthy controls. Seven individuals in the moderate complexity subgroup had more variable patterns that did not fall into an obvious classification, although it is notable that all maintained a distinct swing module dominated by TA activity.
Modules from the high complexity subgroup (Fig. 6D, modules H1–H4) appeared similar to those observed in the healthy controls in both the muscle weightings and activation timing profiles (cf. Figs. 4 and 6D).
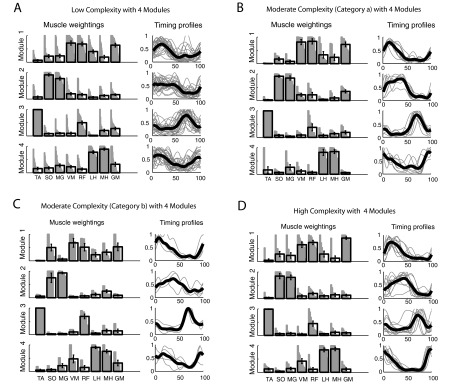
Activation characteristics contributing to reduced complexity
Although four modules were not needed to account for the variability of activation in most paretic legs (as described earlier), we proceeded to run the NNMF analysis using four modules on all study participants to examine why merging of modules was observed in the paretic legs. The muscle weightings and activation timing profiles of each paretic subgroup were found to be very strongly associated with those of the healthy control group (Table 2, A and B, and cf. Fig. 7 to Fig. 4). When assessing the module characteristics within each subgroup, we found that the muscle weightings of each module were completely independent (Table 2C; Fig. 7) but the activation timing profiles became progressively more similar (stronger correlations between module timing) in the high, moderate, and low complexity paretic subgroups, respectively (Table 2D; Fig. 7). These findings indicate that, although modules with similar muscle weightings are present in both healthy and paretic legs, the ability to differentially activate the modules was compromised in many of the paretic legs and led to merging of module activation timing.
Table 2.
Comparison of four-module results in healthy and paretic legs
| A Correlation of Muscle Weightings Across Groups | B Correlation of Module Timing Across Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Module 1 | Module 1 | ||||||||
| Group | Healthy | Low | Moderate “a” | Moderate “b” | Group | Healthy | Low | Moderate “a” | Moderate “b” |
| Low | 0.90 | Low | 0.87 | ||||||
| Moderate “a” | 0.92 | 0.98 | Moderate “a” | 0.93 | 0.98 | ||||
| Moderate “b” | 0.78 | 0.78 | 0.82 | Moderate “b” | 0.95 | 0.90 | 0.95 | ||
| High | 0.93 | 0.93 | 0.90 | 0.76 | High | 0.79 | 0.96 | 0.95 | 0.84 |
| Module 2 | Module 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Healthy | Low | Moderate “a” | Moderate “b” | Group | Healthy | Low | Moderate “a” | Moderate “b” |
| Low | 0.97 | Low | 0.72 | ||||||
| Moderate “a” | 0.97 | 0.98 | Moderate “a” | 0.88 | 0.92 | ||||
| Moderate “b” | 0.95 | 0.94 | 0.91 | Moderate “b” | 0.93 | 0.88 | 0.96 | ||
| High | 0.98 | 0.99 | 0.97 | 0.97 | High | 0.95 | 0.82 | 0.91 | 0.97 |
| Module 3 | Module 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Healthy | Low | Moderate “a” | Moderate “b” | Group | Healthy | Low | Moderate “a” | Moderate “b” |
| Low | 0.94 | Low | 0.49 | ||||||
| Moderate “a” | 0.92 | 0.98 | Moderate “a” | 0.54 | 0.99 | ||||
| Moderate “b” | 0.98 | 0.97 | 0.95 | Moderate “b” | 0.65 | 0.96 | 0.97 | ||
| High | 0.94 | 0.98 | 0.99 | 0.97 | High | 0.64 | 0.95 | 0.98 | 0.96 |
| Module 4 | Module 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Healthy | Low | Moderate “a” | Moderate “b” | Group | Healthy | Low | Moderate “a” | Moderate “b” |
| Low | 0.94 | Low | 0.62 | ||||||
| Moderate “a” | 0.96 | 0.97 | Moderate “a” | 0.92 | 0.82 | ||||
| Moderate “b” | 0.89 | 0.91 | 0.88 | Moderate “b” | 0.65 | 0.92 | 0.85 | ||
| High | 0.97 | 0.93 | 0.94 | 0.96 | High | 0.87 | 0.86 | 0.98 | 0.85 |
| C Correlation of Muscle Weightings Within Each Group | D Correlation of Module Timing Within Each Group | ||||||
|---|---|---|---|---|---|---|---|
| Healthy | Healthy | ||||||
| Module | 1 | 2 | 3 | Module | 1 | 2 | 3 |
| 2 | −0.40 | 2 | −0.06 | ||||
| 3 | 0.07 | −0.45 | 3 | 0.45 | −0.63 | ||
| 4 | −0.32 | −0.48 | −0.30 | 4 | 0.31 | −0.54 | 0.14 |
| Low Complexity Paretic | Low Complexity Paretic | ||||||
|---|---|---|---|---|---|---|---|
| Module | 1 | 2 | 3 | Module | 1 | 2 | 3 |
| 2 | −0.30 | 2 | 0.73 | ||||
| 3 | −0.26 | −0.40 | 3 | −0.68 | −0.82 | ||
| 4 | −0.22 | −0.35 | −0.40 | 4 | 0.82 | 0.48 | −0.67 |
| Moderate Complexity “Category a” Paretic | Moderate Complexity “Category a” Paretic | ||||||
|---|---|---|---|---|---|---|---|
| Module | 1 | 2 | 3 | Module | 1 | 2 | 3 |
| 2 | −0.25 | 2 | 0.66 | ||||
| 3 | −0.25 | −0.45 | 3 | −0.65 | −0.78 | ||
| 4 | −0.32 | −0.42 | −0.26 | 4 | 0.50 | −0.11 | −0.39 |
| Moderate Complexity “Category b” Paretic | Moderate Complexity “Category b” Paretic | ||||||
|---|---|---|---|---|---|---|---|
| Module | 1 | 2 | 3 | Module | 1 | 2 | 3 |
| 2 | −0.28 | 2 | 0.43 | ||||
| 3 | −0.33 | −0.46 | 3 | −0.53 | −0.77 | ||
| 4 | 0.04 | −0.30 | −0.48 | 4 | 0.78 | 0.26 | −0.64 |
| High Complexity Paretic | High Complexity Paretic | ||||||
|---|---|---|---|---|---|---|---|
| Module | 1 | 2 | 3 | Module | 1 | 2 | 3 |
| 2 | −0.22 | 2 | 0.58 | ||||
| 3 | −0.30 | −0.38 | 3 | −0.61 | −0.71 | ||
| 4 | −0.31 | −0.43 | −0.33 | 4 | 0.32 | −0.29 | −0.31 |
Bold italicized text indicates significant positive correlation (P < 0.001).
Fig. 7.
Module muscle weightings and activation timing profiles identified when NNMF was performed using 4 modules in all paretic legs. Refer to Fig. 4 for meaning of gray and black bars and lines. Associations within each group for muscle weightings and activation timing profiles are quantified in Table 2, C and D, respectively. A: the low complexity subgroup had modules with independent composition (muscle weightings) but similar timing of modules 1, 2, and 4. B: the category a moderate complexity subgroup had modules with independent composition but similar timing of modules 1 and 2. C: the category b moderate complexity subgroup had modules with independent composition but similar timing of modules 1 and 4. D: the high complexity subgroup had modules with independent composition and activation timing profiles that were less correlated than in the moderate and low complexity subgroups.
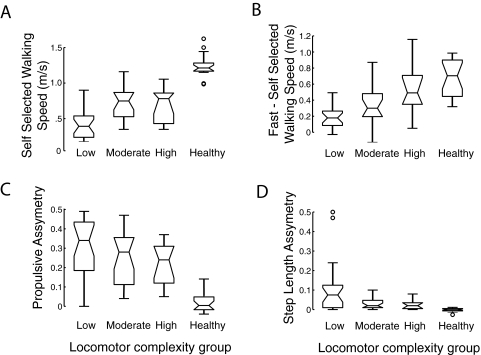
Locomotor output complexity and task performance
The level of locomotor output complexity in the paretic leg of persons post-stroke (i.e., the number of independent modules) predicted locomotor performance, including self-selected walking speed (Fig. 8A; ρ = 0.50, P = 0.0002), speed modulation between self-selected and fast walking (Fig. 8B; ρ = 0.47, P = 0.0008), propulsive asymmetry (Fig. 8C; ρ = −0.28, P = 0.04), and step length asymmetry (Fig. 8D; ρ = −0.32, P = 0.02). Even within the moderate complexity subgroup where the number of independent modules is three, differences in modular organization highlight functional consequences. When the distal and proximal extensors were not independently modulated (i.e., persons in category a), the ratio of propulsion generated by the paretic leg relative to the nonparetic leg was low (0.27 ± 0.16). In contrast, when the distal extensors were modulated independently (i.e., persons in category b), the ratio tended to be higher (0.52 ± 0.32), although this did not achieve statistical significance (P = 0.09).
Fig. 8.
Locomotor output complexity and walking performance. Locomotor output complexity in the paretic leg of persons post-stroke is associated with measures of walking performance, including (A) overground self-selected walking speed, (B) speed difference between self-selected and fast walking, (C) propulsive asymmetry, and (D) step length asymmetry. In C and D, 0 represents perfect symmetry, whereas higher values indicate asymmetry. Healthy control data are shown as a reference but were not included in the statistical analysis. The 3 horizontal bars representing each variable indicate, from bottom to top, the lower quartile, median, and upper quartile. Error bars indicate ±1.5 · interquartile range. Circles represent outlying data points.
EMG magnitude
A significant effect of group was found for the measure of composite muscle activation magnitude (P = 0.0004), but post hoc analysis showed only that the moderate complexity nonparetic subgroup had greater activation amplitude than the low and moderate complexity paretic subgroups. No significant difference was found between the low and high complexity subgroups for either the paretic or nonparetic legs or between the four-module controls and low complexity paretic and nonparetic subgroups (all P > 0.05; Table 3).
Table 3.
Mean EMG of all muscles by subgroup
| Control | |
|---|---|
| 3 Modules (15) | 203.1 ± 67.9 mV |
| 4 Modules (20) | 160.05 ± 81.8 mV |
| Nonparetic | |
| 2 Modules (2) | 269.3 ± 194.8 mV |
| 3 Modules (14) | 206.5 ± 57.1 mV |
| 4 Modules (23) | 142.8 ± 46.3 mV |
| Paretic | |
| 2 Modules (21) | 127.1 ± 50.9 mV |
| 3 Modules (13) | 125.3 ± 75.3 mV |
| 4 Modules (6) | 178.4 ± 67.9 mV |
Values are means ± SD for mean EMG. Numbers in parentheses indicate all muscles.
DISCUSSION
Our results support a common, low-dimensional modular organization of muscle coordination underlying walking in both healthy and post-stroke subjects with hemiparesis. Four independently timed modules generally accounted for cycle-by-cycle variability in muscle activation across a range of walking speeds in healthy persons, whereas in persons post-stroke, the number of independently timed modules was often less because of the coincident timing of some modules. Fewer modules correspond to an overall reduction in complexity of locomotor control, and this reduction in locomotor output complexity was associated with poorer walking performance. These results suggest that the modular organization of muscle activation serves as a quantitative indicator of complex changes in multiple muscle coordination and also underlies walking ability. Furthermore, this technique takes advantage of the inherent trial-to-trial variability that typically poses a problem for reliable clinical assessment, showing common low-dimensional structure underlying the variability. Taken together with both basic science and simulation studies, such an approach may prove promising for identifying and monitoring locomotor impairments in a range of patient populations.
Modular control of locomotion in healthy and neurologically impaired populations
Our data suggest that the fundamental modular organization of muscle coexcitation in healthy and post-stroke nervous systems is qualitatively similar but that the difference lies in the extent to which modules can be activated independently. Indeed, when the data for all post-stroke participants were analyzed using four modules (even when our VAF criteria could be met with fewer modules), we found that the module muscle weightings were remarkably similar between the healthy and post-stroke groups. A similar result has also been reported in a recent study that used NNMF to examine control of reaching in persons post-stroke (Cheung et al. 2009). The activation timing profiles of the four modules were relatively independent in the control and high complexity paretic groups, but less independent in the moderate and low complexity paretic subgroups. This lack of modular independence led to the identification of merged modules (and therefore reduced locomotor output complexity) in most of the post-stroke participants when the number of modules was determined according to our VAF criteria. Although reduced dimension modular organization of muscle activation in healthy persons may facilitate execution of the primary biomechanical subtasks of walking, impaired ability to independently control the modules post-stroke reduces locomotor output complexity to the degree that it may excessively constrain motor output and disrupt proper biomechanics.
Our results indicate that the level of locomotor output complexity in persons post-stroke is predictive of walking performance, because persons with fewer independently timed modules walk more slowly and have more asymmetrical step lengths and propulsion generation. Indeed, we have recently shown that locomotor output complexity is a superior predictor of walking performance than the Fugl-Meyer assessment (Bowden et al. 2009), which is generally considered the gold standard for assessing post-stroke motor impairment. Furthermore, within the moderate complexity paretic subgroup, we observed two distinct types of modular organization that yielded predictable biomechanical results. Poorer generation of propulsion resulted if the early and late stance modules were combined, because this indicates interference between the weight acceptance and propulsion subtasks. In contrast, better propulsion resulted when the late stance module remained independent. It is possible that such differences in modular organization may occur based on the neural pathways/systems affected by stroke. Conversely, specific behavioral deficits may provide insight to the neural pathways or systems affected by injury.
Our findings are consistent with the seminal work of Knuttson and Richards (1979), who established distinct classifications that qualitatively described the locomotor activation patterns of most of their post-stroke research participants. Each of their classifications were characterized by abnormal amplitude and/or timing of activation (e.g., coactivation of several limb muscles), although their classifications were based on observation of EMG data rather than a quantitative algorithm. Subsequently, other investigators have developed quantitative methods for detecting abnormal activation, but these approaches generally analyze one muscle at a time or compare pairs of muscles (Den Otter et al. 2006; Fung and Barbeau 1989). Decomposition analyses, such as the one use here, offer a more comprehensive methodology for dissociating the changes in coactivation and timing of a large number of muscles, providing insight to the underlying organization of the locomotor activation pattern.
The modules identified in our healthy control group are qualitatively very similar to the modular organization of muscle activation presented by earlier studies of human walking (Cappellini et al. 2006; Davis and Vaughan 1993; Ivanenko et al. 2004; Merkle et al. 1998; Wootten et al. 1990). This is despite the fact that these studies have recorded from a larger set of muscles and have used normalization and decomposition procedures that differ somewhat from ours. Ivanenko and colleagues have extensively studied control of human walking using decomposition procedures (Cappellini et al. 2006; Ivanenko et al. 2004, 2006), and our results in healthy controls compares favorably with their work. Like us, they showed an early stance component with high activity of the quadriceps group and gluteus medius, a late stance component with high activity in the triceps surae, an early swing component with high activity of ankle and hip flexors, and a late swing component with high activity in the hamstrings. The most notable discrepancy in the data is that they have identified five modules, whereas we identified four. This can be accounted for by the fact that we recorded from fewer muscles, because the additional component from their study contains high weightings for the erector spinae and iliopsoas muscles. Consistent with this finding, we recently performed a study in which the healthy modules were used to control a forward dynamics simulation of walking and showed that an additional (fifth) module comprised of iliopsoas activity contributed to walking during pre- and early swing (Neptune et al. 2009).
In persons with incomplete spinal cord injury, Ivanenko et al. (2003, 2004) found the same five muscle timing patterns that were observed in healthy persons. This is in contrast to our own results in which a number of individuals with incomplete spinal cord injury exhibited reduced locomotor output complexity much like our post-stroke subjects (Fox et al. 2009). Although the discrepancy in their results to ours may simply reflect differences between the participants in our respective studies, they are likely caused by differences in the ways that similar analysis techniques were used to test different hypotheses. We hypothesized that muscle groupings would remain constant and thus allowed the timing patterns to vary to identify common muscle modules. Ivanenko et al. hypothesized the timing patterns to remain the same, allowing muscle groupings to vary while identifying common timing patterns across conditions (equivalent to applying the decomposition to the transpose of the data matrix). Whereas our methodology shows that muscle groupings remain fixed despite cycle-by-cycle differences in timing, the use of averaged data (Ivanenko et al. 2004) may mask any observed variations in timing. Finally, our criterion for choosing the number of modules was to use the minimum number that accounted for ≥90% of the variability for each muscle and each region of the gait cycle. In contrast, their criterion was to accept any component that explained more than a particular amount of variability (generally 5% or more of the total variability), without the explicit goal of accounting for most of the variability in the data set (Ivanenko et al. 2003).
Modular control and biomechanical output
In the way that we and others have applied and interpreted the NNMF and similar decomposition techniques, fixed groupings of muscles are coactive to elicit a particular biomechanical function (Ting and Macpherson 2005; Tresch et al. 1999). Although we do not view muscle weightings as a fixed parameter over long time scales, we expect that weightings will remain relatively similar from stride to stride during steady-state walking or across different speeds when the biomechanical requirements remain similar. During postural responses, the number of modules used by a subject and the muscle weightings have been shown to constrain trial by trial variability in muscle activation patterns and to be consistent across days (Torres-Oviedo and Ting 2007). Similarly, we showed that the same muscle weightings can be used to account for the variability in the locomotor activation pattern across strides and across speeds in healthy individuals. However, if the biomechanical requirements change to a larger extent, we expect that the muscle weightings will also change. For example, it has been shown that if a person's weight is increased (i.e., by wearing a weighted belt) while a body weight support system offloads an equal amount of weight, the task mechanics require an increase of horizontally directed ground reaction force (i.e., propulsion) but unchanged vertically directed ground reaction force (i.e., body support) (McGowan et al. 2009). The contribution of the plantarflexor muscle group to these mechanical changes was shown to be dominated by enhanced activation of soleus, with considerably smaller changes in gastrocnemius activation. These changes in activation are consistent with their known biomechanical functions (Neptune et al. 2001) in that soleus contributes more to horizontal propulsion than does gastrocnemius. Thus variation occurred in the muscle activation space to best produce the biomechanical function (which likely is an effective way to reduce the variability in the performance of the biomechanical function in each task). The concept that variability in the activation space can be used to reduce variability in the output space is consistent with the use of the term “synergy” by Latash (Danna-Dos-Santos et al. 2009; Latash et al. 2007). During walking, modulation of modular organization on a step by step basis is facilitated by ongoing feedback (dynamic state of the system and environment) and feed-forward (task objectives) neural signals to yield a set of modules that performs well the biomechanical functions to produce successful walking.
Several simulation studies showed the feasibility of using low-dimensional modular muscle control in producing robust biomechanical outputs for movement. Using a computer simulation of healthy walking where muscles are excited using module control, we have previously shown that the timing and muscle composition of the experimentally identified healthy modules result in appropriate biomechanical output to meet the task requirements of steady-state walking (Neptune et al. 2009). The biomechanical role that we proposed for each module, which was confirmed by the simulation, are weight acceptance and/or body support for module C1, body support, propulsion and swing initiation for module C2, flexion/ground clearance for module C3, and leg deceleration for module C4 (Neptune et al. 2004, 2009; Zajac et al. 2003). This finding suggests that the modules represent a functional transformation between sensorimotor signals and biomechanical output. Similarly, in physiological tests in frogs, a small set of independently controlled motor modules rather than time-varying muscle synergies is competent to reproduce a wide range of limb trajectories (Kargo and Giszter 2008). Moreover, motor modules may be organized to capture the natural dynamics of the limb (Berniker et al. 2009). In simulations of force generation during postural control in cats, muscle synergy constraints for nominal postural configurations were shown to constrain force output direction when the postural configuration changed (McKay and Ting 2008). Together with experimentally measured correlation between motor module activity and biomechanical outputs (Ivanenko et al. 2007; Ting and Macpherson 2005; Torres-Oviedo et al. 2006), these studies suggest that complex biomechanical interactions during natural movements can be controlled by a small set of task-appropriate biomechanical components that both exploit and constrain the dynamics of the limb (Ting and McKay 2007). This premise concurs with the findings of Krouchev et al. (2006), who used cluster analysis and NNMF to examine modularity in the hindlimb of cats during walking. Although their cluster analysis identified seven synergies, some of these synergies were active during the same region of the gait cycle and were concluded to contribute to the same biomechanical function. Consistent with our findings in human walking, they identified four separate biomechanical functions with associated coactive muscle groupings. Furthermore, when they applied the NNMF algorithm using seven factors to their data, they found strong similarities with the synergies identified by their cluster analysis.
Neural basis for impaired modular control post-stroke
Although studies in animal locomotion suggest that the modular organization of muscle coordination is encoded in the spinal cord (Dietz 2003; Kargo and Giszter 2008; McCrea and Rybak 2008; Tresch et al. 1999), the impaired modular organization that we observed in the paretic leg of our post-stroke participants is likely related to changes in processes at multiple levels of the neural axis. Some of the most critical issues post-stroke that may disrupt the typical modular output are altered supraspinal drive (and its secondary consequences) and impaired sensory-motor control. Thus descending supraspinal input, spinal pattern generation, and processing of afferent information may all be impaired.
Increased reliance on supraspinal pathways other than corticospinal may contribute to the merged modules that we observed in persons post-stroke. Corticospinal drive (Canedo 1997) influences the overall level of muscle activity during normal walking (Petersen et al. 2001) and, at times, individual muscle activity (Pijnappels et al. 1998). Reduced corticospinal drive post-stroke could contribute to a reduced ability to activate paretic modules (Nielsen et al. 2008). Corticospinal pathways may also fractionate individual movement from more complex multijoint patterns (Lemon 2008). Compromised corticospinal control may lead to a reliance on other descending brain stem pathways that produce a more diffuse output. Thus loss of descending drive from corticospinal pathways to the interneurons and motoneuron pools responsible for generating the extensor phase modules may cause the propulsion and/or leg deceleration modules to no longer be independent from the weight acceptance module. Increased reliance on other descending brain stem pathways, such as reticulospinal and bulbospinal tracts (Dewald and Beer 2001; Lum et al. 2003), has been suggested to cause post-stroke abnormal coupling of torque generation (Cruz and Dhaher 2008).
Impaired sensory-motor integration likely induced changes in reflexes and afferent processing and may have also affected the organization of motor modules. Indeed, research of the hindlimb wiping reflex in frogs has shown that sensory feedback alters phasing of premotor primitives (Kargo and Giszter 2008) and that deafferentation leads to the loss of kinematic phases of limb movements and abnormal synchronization of muscle activation (Kargo and Giszter 2000). Although another study of frogs performing voluntary movements suggests that the contribution of sensory afferents to motor module organization is limited (Cheung et al. 2005), recent studies in human stroke patients showed that abnormalities in muscle cocontraction are observed in both reflex responses and voluntary arm movements (Trumbower et al. 2008). Similarly, dysfunction of excitatory Ia afferent projections and recurrent inhibition from the quadriceps to the soleus motoneurons (Dyer et al. 2009) could lead to coexcitation of plantarflexors and knee extensors and contribute to the merging of the support and propulsion modules that we observed. Similarly, impaired function of heteronymous (Gordon et al. 2009; Nichols et al. 2002), reciprocal (Finley et al. 2008) and afferent (Lewek et al. 2006) reflex pathways may lead to atypical coactivation, and these changes will be evident within the modular organization. The transition between the stance and swing phases of gait, which has been shown to be facilitated by stretch of the limb flexor muscles during late stance (Hiebert et al. 1996), may be adversely affected by diminished proprioception post-stroke. For example, the inability to advance the center of mass over the stance leg may reduce or eliminate the proprioceptive signal for flexion.
Methodological considerations
Although methodological factors may influence quantitative aspects of our analysis, it is unlikely that such differences would qualitatively alter our results. It is possible the slower walking speed in persons post-stroke contributed to the identification of fewer modules. However, we believe that this is not the case, because each subject in essence served as their own control with respect to this question, as their nonparetic leg required a greater number of modules than their paretic leg even at slow walking speeds. Thus walking speed was probably not a major determinant for fewer modules being identified in the paretic leg. It is possible that we may have identified additional modules had we collected from a larger set of muscles (Ivanenko et al. 2004) or from a more diverse set of motor tasks (Ivanenko et al. 2005). Although the identification of additional modules would have the benefit of improving the resolution for quantifying locomotor output complexity, it would not be expected to influence the finding that locomotor output complexity is reduced post-stroke and is related to locomotor performance. Another methodological consideration is the effect of EMG signal amplitude on the number of modules identified. Despite a possible reduction in the signal-to-noise ratio of EMG in persons post-stroke, we found that muscle activation magnitude was comparable across groups. Indeed, the overall activation magnitude (nonnormalized, rectified, and averaged across muscles) did not differ between the low and high complexity subgroups for either the paretic or nonparetic legs, or between the four-module control group and the low complexity paretic and nonparetic subgroups. Furthermore, the stringent criteria used for choosing the number of modules required the algorithm to account for subtleties in the activation signals that were independent of the effects of signal amplitude. Therefore activation magnitude does not seem to have a substantial influence on the number of modules needed to account for signal variability. Finally, it should be noted that the primary goal of NNMF is to identify general commonalities underlying a complex data set, and that small intersubject differences in these common elements are not necessarily crucial to the interpretation. Accordingly, it can be challenging to apply a rigorous statistical analysis to some aspects of the data set (e.g., module composition), particularly between participants who exhibit a different number of modules.
GRANTS
This work was supported by National Institutes of Health Grants HD-46820 to S. A. Kautz and NS-053822 to L. H. Ting and the Department of Veterans Affairs Rehabilitation Research and Development Service (Merit Review B3983-R to S. A. Kautz, Career Development Award B4888M to D. J. Clark, and Brain Rehabilitation Research Center of Excellence Grant F2182C).
REFERENCES
- Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil 88: 43–49, 2007 [DOI] [PubMed] [Google Scholar]
- Berniker M, Jarc A, Bizzi E, Tresch MC. Simplified and effective motor control based on muscle synergies to exploit musculoskeletal dynamics. Proc Natl Acad Sci USA 106: 7601–7606, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein NA. The Coordination and Regulation of Movements Oxford, UK: Pergamon Press, 1967 [Google Scholar]
- Bizzi E, Cheung VC, d'Avella A, Saltiel P, Tresch M. Combining modules for movement. Brain Res Rev 57: 125–133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke 37: 872–876, 2006 [DOI] [PubMed] [Google Scholar]
- Bowden MG, Clark DJ, Neptune RR, Kautz SA. Evaluation of abnormal synergy patterns post-stroke: relationship of clinical examination to hemiparetic locomotion. Neurorehabil Neural Repair 2009. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canedo A. Primary motor cortex influences on the descending and ascending systems. Prog Neurobiol 51: 287–335, 1997 [DOI] [PubMed] [Google Scholar]
- Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. J Neurophysiol 95: 3426–3437, 2006 [DOI] [PubMed] [Google Scholar]
- Cheung VC, d'Avella A, Tresch MC, Bizzi E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci 25: 6419–6434, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VC, Piron L, Agostini M, Silvoni S, Turolla A, Bizzi E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc Natl Acad Sci USA 106: 19563–19568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz TH, Dhaher YY. Evidence of abnormal lower-limb torque coupling after stroke: an isometric study. Stroke 39: 139–147, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna-Dos-Santos A, Shapkova EY, Shapkova AL, Degani AM, Latash ML. Postural control during upper body locomotor-like movements: similar synergies based on dissimilar muscle modes. Exp Brain Res 193: 565–579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BL, Vaughan CL. Phasic behavior of EMG signals during gait: use of multivariate statistics. J EMG Kinesiol 3: 51–60, 1993 [DOI] [PubMed] [Google Scholar]
- Den Otter AR, Geurts AC, Mulder T, Duysens J. Gait recovery is not associated with changes in the temporal patterning of muscle activity during treadmill walking in patients with post-stroke hemiparesis. Clin Neurophysiol 117: 4–15, 2006 [DOI] [PubMed] [Google Scholar]
- De Quervain IA, Simon SR, Leurgans S, Pease WS, McAllister D. Gait pattern in the early recovery period after stroke. J Bone Joint Surg Am 78: 1506–1514, 1996 [DOI] [PubMed] [Google Scholar]
- Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve 24: 273–283, 2001 [DOI] [PubMed] [Google Scholar]
- Dietz V. Spinal cord pattern generators for locomotion. Clin Neurophysiol 114: 1379–1389, 2003 [DOI] [PubMed] [Google Scholar]
- Dyer JO, Maupas E, de Andrade Melo S, Bourbonnais D, Fleury J, Forget R. Transmission in heteronymous spinal pathways is modified post-stroke and related to motor incoordination. PLoS One 4: e4123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JM, Perreault EJ, Dhaher YY. Stretch reflex coupling between the hip and knee: implications for impaired gait following stroke. Exp Brain Res 188: 529–540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EJ, Kautz SA, Clark DJ, Suter S, Day K, Gill L, Behrman A. Reduced modular control accounts for muscle activation during walking in persons with incomplete spinal cord injury. XIX Conference of the International Society for Posture and Gait Research, Bologna, Italy, June 21–25, 2009 [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. I. A method for evaluation of physical performance. Scand J Rehabil Med 7: 13–31, 1975 [PubMed] [Google Scholar]
- Fung J, Barbeau H. A dynamic EMG profile index to quantify muscular activation disorder in spastic paretic gait. Electroencephalogr Clin Neurophysiol 73: 233–244, 1989 [DOI] [PubMed] [Google Scholar]
- Gordon KE, Wu M, Kahn JH, Dhaher YY, Schmit BD. Ankle load modulates hip kinetics and EMG during human locomotion. J Neurophysiol 101: 2062–2076, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb GL. Muscle activation patterns during two types of voluntary single-joint movement. J Neurophysiol 80: 1860–1867, 1998 [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol 75: 1126–1137, 1996 [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Cappellini G, Dominici N, Poppele RE, Lacquaniti F. Coordination of locomotion with voluntary movements in humans. J Neurosci 25: 7238–7253, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Cappellini G, Dominici N, Poppele RE, Lacquaniti F. Modular control of limb movements during human locomotion. J Neurosci 27: 11149–11161, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Grasso R, Zago M, Molinari M, Scivoletto G, Castellano V, Macellari V, Lacquaniti F. Temporal components of the motor patterns expressed by the human spinal cord reflect foot kinematics. J Neurophysiol 90: 3555–3565, 2003 [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol 556: 267–282, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F. Motor control programs and walking. Neuroscientist 12: 339–348, 2006 [DOI] [PubMed] [Google Scholar]
- Jonkers I, Delp S, Patten C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture 29: 129–137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ, Giszter SF. Afferent roles in hindlimb wipe-reflex trajectories: free-limb kinematics and motor patterns. J Neurophysiol 83: 1480–1501, 2000 [DOI] [PubMed] [Google Scholar]
- Kargo WJ, Giszter SF. Individual premotor drive pulses, not time-varying synergies, are the units of adjustment for limb trajectories constructed in spinal cord. J Neurosci 28: 2409–2425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsson E, Richards C. Different types of disturbed motor control in gait of hemiparetic patients. Brain 102: 405–430, 1979 [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V, Latash ML, Scholz JP, Zatsiorsky VM. Muscle synergies during shifts of the center of pressure by standing persons. Exp Brain Res 152: 281–292, 2003 [DOI] [PubMed] [Google Scholar]
- Krouchev N, Kalaska JF, Drew T. Sequential activation of muscle synergies during locomotion in the intact cat as revealed by cluster analysis and direct decomposition. J Neurophysiol 96: 1991–2010, 2006 [DOI] [PubMed] [Google Scholar]
- Latash ML, Scholz JP, Schoner G. Toward a new theory of motor synergies. Motor Control 11: 276–308, 2007 [DOI] [PubMed] [Google Scholar]
- Lee DD, Seung HS. Learning the parts of objects by non-negative matrix factorization. Nature 401: 788–791, 1999 [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008 [DOI] [PubMed] [Google Scholar]
- Lewek MD, Schmit BD, Hornby TG, Dhaher YY. Hip joint position modulates volitional knee extensor muscle activity after stroke. Muscle Nerve 34: 767–774, 2006 [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Shor PC. Evidence for strength imbalances as a significant contributor to abnormal synergies in hemiparetic subjects. Muscle Nerve 27: 211–221, 2003 [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57: 134–146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan CP, Kram R, Neptune RR. Modulation of leg muscle function in response to altered demand for body support and forward propulsion during walking. J Biomech 42: 850–856, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JL, Ting LH. Functional muscle synergies constrain force production during postural tasks. J Biomech 41: 299–306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle LA, Layne CS, Bloomberg JJ, Zhang JJ. Using factor analysis to identify neuromuscular synergies during treadmill walking. J Neurosci Methods 82: 207–214, 1998 [DOI] [PubMed] [Google Scholar]
- Mulroy S, Gronley J, Weiss W, Newsam C, Perry J. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait Posture 18: 114–125, 2003 [DOI] [PubMed] [Google Scholar]
- Neptune RR, Clark DJ, Kautz SA. Modular control of human walking: a simulation study. J Biomech 42: 1282–1287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Muscle contributions to specific biomechanical functions do not change in forward versus backward pedaling. J Biomech 33: 155–164, 2000 [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech 34: 1387–1398, 2001 [DOI] [PubMed] [Google Scholar]
- Neptune RR, Zajac FE, Kautz SA. Muscle force redistributes segmental power for body progression during walking. Gait Posture 19: 194–205, 2004 [DOI] [PubMed] [Google Scholar]
- Nichols TR, Wilmink RJ, Burkholder TJ. Multidimensional and temporal regulation of limb mechanics by spinal circuits. In: Progress in Motor Control, edited by Latash ML. Champaign, IL: Human Kinetics, 2002, p. 179–194 [Google Scholar]
- Nielsen JB, Brittain JS, Halliday DM, Marchand-Pauvert V, Mazevet D, Conway BA. Reduction of common motoneuronal drive on the affected side during walking in hemiplegic stroke patients. Clin Neurophysiol 119: 2813–2818, 2008 [DOI] [PubMed] [Google Scholar]
- Olree KS, Vaughan CL. Fundamental patterns of bilateral muscle activity in human locomotion. Biol Cybern 73: 409–414, 1995 [DOI] [PubMed] [Google Scholar]
- Perroto AO. Anatomical Guide for the Electromyographer Springfield, IL: Charles C Thomas, 1994 [Google Scholar]
- Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke 26: 982–989, 1995 [DOI] [PubMed] [Google Scholar]
- Petersen NT, Butler JE, Marchand-Pauvert V, Fisher R, Ledebt A, Pyndt HS, Hansen NL, Nielsen JB. Suppression of EMG activity by transcranial magnetic stimulation in human subjects during walking. J Physiol 537: 651–656, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnappels M, Van Wezel BM, Colombo G, Dietz V, Duysens J. Cortical facilitation of cutaneous reflexes in leg muscles during human gait. Brain Res 787: 149–153, 1998 [DOI] [PubMed] [Google Scholar]
- Raasch CC, Zajac FE. Locomotor strategy for pedaling: muscle groups and biomechanical functions. J Neurophysiol 82: 515–525, 1999 [DOI] [PubMed] [Google Scholar]
- Ting LH, Kautz SA, Brown DA, Zajac FE. Phase reversal of biomechanical functions and muscle activity in backward pedaling. J Neurophysiol 81: 544–551, 1999 [DOI] [PubMed] [Google Scholar]
- Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophysiol 93: 609–613, 2005 [DOI] [PubMed] [Google Scholar]
- Ting LH, McKay JL. Neuromechanics of muscle synergies for posture and movement. Curr Opin Neurobiol 17: 622–628, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol 96: 1530–1546, 2006 [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. J Neurophysiol 98: 2144–2156, 2007 [DOI] [PubMed] [Google Scholar]
- Tresch MC, Saltiel P, Bizzi E. The construction of movement by the spinal cord. Nat Neurosci 2: 162–167, 1999 [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Ravichandran VJ, Krutky MA, Perreault EJ. Altered multijoint reflex coordination is indicative of motor impairment level following stroke. Conf Proc IEEE Eng Med Biol SocVancouver, BC, 2008, p. 3558–3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DA, Yack HJ. EMG profiles during normal human walking: stride-to-stride and inter-subject variability. Electroencephalogr Clin Neurophysiol 67: 402–411, 1987 [DOI] [PubMed] [Google Scholar]
- Wootten ME, Kadaba MP, Cochran GV. Dynamic electromyography. I. Numerical representation using principal component analysis. J Orthop Res 8: 247–258, 1990 [DOI] [PubMed] [Google Scholar]
- Zajac FE, Neptune RR, Kautz SA. Biomechanics and muscle coordination of human walking. II. Lessons from dynamical simulations and clinical implications. Gait Posture 17: 1–17, 2003 [DOI] [PubMed] [Google Scholar]