Abstract
Serotonin initiates various rhythmic behaviors in vertebrates. Previously we have shown that serotonergic neurons innervate the central vocal pathway in the African clawed frog (Xenopus laevis). We also discovered that exogenous serotonin applied to isolated brains in vitro activates fictive vocalizations by activating 5-HT2C-like receptors. In this study, we examined the location of 5-HT2C-like receptors and determined whether endogenously released serotonin also initiates vocalizations by activating 5-HT2C-like receptors in male Xenopus brains. To this end, we first identified the specific location of 5-HT2C-like receptors using immunohistochemistry. We next examined which of the populations of neurons that express 5-HT2C-like receptors are functionally relevant for initiating fictive vocalizations by applying a 5-HT2C receptor agonist to brains transected at various levels. Of four populations of immunopositive neurons, we showed that 5-HT2C-like receptors located in two areas of the brain stem vocal circuit, the raphe nucleus and motor nucleus IX-X, initiate fictive vocalizations. We next showed that endogenous serotonin can also activate fictive vocalizations by increasing the extracellular concentration of endogenous serotonin using a selective serotonin reuptake inhibitor (SSRI). The SSRI-induced vocal initiation is also mediated by activation of 5-HT2C-like receptors because blockade of these receptors prevents fictive vocalization. The results suggest that in vivo release of serotonin initiates male vocalizations by activating 5-HT2C-like receptors in the brain stem vocal nuclei.
INTRODUCTION
Many behaviors such as locomotion and vocalization are episodic. Initiation of episodic behavior with appropriate timing is important for the survival of an animal. Although it has been difficult to identify the cascade of reactions from sensory input to motor output in these more complex behaviors, a “bottom-up” approach has been fruitful in identifying the mechanism of behavioral initiation. For example, rhythmic locomotor behavior in rodents was first identified to be controlled by central pattern generators in the spinal cord, which were later discovered to be activated by the mesencephalic locomotor region (MLR), the electrical stimulation of which initiates locomotion (Jordan et al. 2008). Similarly, whisking behavior in rats has been shown to require inputs from serotonergic neurons in the parapyramidal region (PPR) in the brain stem (Cramer et al. 2007).
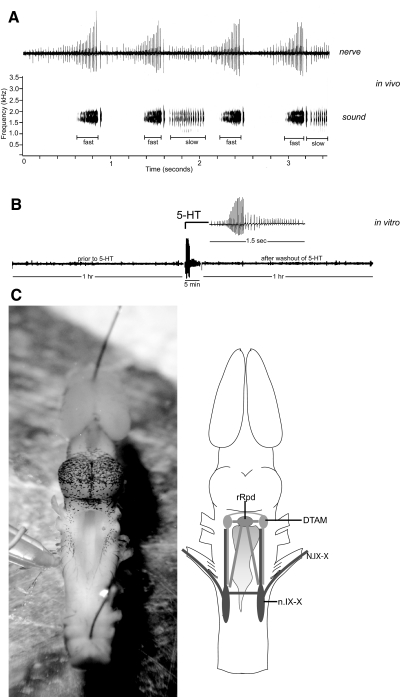
We address the issue of motor pattern initiation using the relatively simple vocal behavior of Xenopus laevis (the African clawed frog; Fig. 1A). The Xenopus vocal system is an ideal model because the behavior we observe from the isolated brain in vitro (Fig. 1B) (Rhodes et al. 2007) is representative of the behavior we observe from the animal in vivo (A), and we can readily activate fictive behavior using bath-applied serotonin.
Fig. 1.
Isolated Xenopus brain and the fictive vocal behavior observed in the presence of serotonin. A: simultaneous nerve (top) and sound (bottom, sound spectrogram) recordings of advertisement calls produced by a male Xenopus in vivo, using a recording method described in Yamaguchi and Kelley (2000). A bout of advertisement call is typically made of fast and slow trills, but it can be variable within individual; the 1st and the 3rd bout do not contain slow trills, whereas the 2nd and the 4th trills include slow trills. B: isolated Xenopus brains in vitro are typically silent (left) until serotonin (middle) is administered to the brain. Fictive vocal behavior continues until serotonin is washed out of the bath (right). An example trace obtained from a male brain. C: isolated brain photo (left), dorsal view with suction electrode placed on the left laryngeal nerve. Brain schematic (right) demonstrating reciprocal connections exist between major vocal nuclei (DTAM and n.IX-X) in Xenopus brain stem. The raphe nucleus also sends projections to both DTAM and n.IX-X, the major vocal nuclei.
The central vocal pathway of X. laevis (Fig. 1C) includes two main vocal nuclei, the laryngeal motor nucleus IX-X (n.IX-X) and the dorsal tegmental area of the medulla (premotor area: DTAM) that both reside in the brain stem. n.IX-X contains the motoneurons the axons of which comprise the laryngeal nerve (Simpson et al. 1986). n.IX-X is also reciprocally connected with DTAM (Zornik and Kelley 2007). Immunohistochemistry experiments by Rhodes et al. (2007) demonstrated that serotonergic neurons send projections to both n.IX-X and DTAM from the raphe nucleus suggesting a role for endogenous serotonin in vocal production. Recently, we have shown that 5-HT2C-like receptors in the Xenopus brain are important for the initiation of vocalizations (Yu and Yamaguchi 2009).
Based on the work described in the preceding text, we hypothesized that the critical 5-HT2C-like receptors are located in the brain stem of Xenopus in key vocal areas and that vocal behavior is initiated via activation of these receptors from endogenous serotonin sources. In this study, we first identified the specific location of the 5-HT2C-like receptors that mediate the vocal initiation using immunohistochemical and pharmacological techniques. We then determined the role of endogenous serotonin in initiating fictive vocalizations and the identity of the receptors involved. We conclude that endogenous serotonin initiates fictive vocalizations by activating 5-HT2C-like receptors in the brain stem vocal nuclei.
METHODS
Western blot
To explore the identity of the immunopositive protein in our immunohistochemical study, two male brains were used for Western blotting procedures. For whole brain extract, protein was obtained by homogenizing two male Xenopus brains in sample buffer (2% SDS/10% glycerol/0.01% bromophenol blue/10% 2-mercaptoethanol/60 mM Tris, pH 6.8) followed by sonication. After centrifuging for 15 min, the supernatant was collected and diluted 1:1 with sample buffer. Equal amounts of protein were separated on a 10% SDS-PAGE gel, transferred to a nitrocellulose membrane followed by Western blotting procedure with a 1:500 dilution of rabbit polyclonal 5-HT2C receptor antibody raised against the carboxyl-terminus of the human 5-HT2C receptor (ab32172; AbCam, Cambridge, MA) and 1:1,000 dilution of an HRP-labeled anti-rabbit antibody (Jackson Laboratories) followed by detection using a chemiluminescent HRP substrate (PicoStable, ThermoScientific). Positive control was collected from rat tissue culture cortical cells and rat brain extract, whereas negative control was collected from Xenopus stomach. All controls were prepared in the same manner as above. Actin staining was used as a loading control and to confirm the presence of protein in all lanes.
5-HT2C -like receptor immunohistochemistry
Three males (7.0 ± 0.6 cm, 41.7 ± 12.6 g; means ± SD) and three females (9.1 ± 0.3 cm, 74.4 ± 8.0 g) were perfused transcardially with 30 ml 0.1 M phosphate buffer (PB; pH 7.4) followed by 30 ml of 4% paraformaldehyde (PFA) in PB. The brain of each animal was rapidly removed and postfixed in 4% PFA for ≤2 h. Brains were embedded in O.C.T. compound (Tissue-Tek), frozen at −80°C, then sectioned in the horizontal plane at 20 μm on a cryostat. Tissue sections were mounted directly onto slides and were then processed for immunohistochemistry.
All sections were washed in PB prior to incubation in 3% H2O2-PB. Sections were incubated in blocking buffer composed of 0.1% Triton-X/PB and 5% normal goat serum (Sigma, St. Louis, MO) for 30 min. Primary antibody was prepared just before incubation.
The antibody used in these studies was designed from a synthetic peptide derived from within residues 400 to the C-terminus of the human 5-HT2C receptor. A BLAST search showed that this peptide shared 68% homology with the terminal amino acid sequence of the 5-HT2C receptor of X. laevis. The BLAST search also revealed no significant similarities between the immunizing peptide and the X. tropicalis 5-HT2B receptor sequence or the X. tropicalis 5-HT2A receptor sequence nor were there any other X. laevis peptides that showed sequence similarities with the immunizing peptide. Although the entire sequence of X. laevis 5-HT2C receptors is not yet fully known, we refer to these receptors throughout this study as 5-HT2C-like.
For visualization of 5-HT2C-like immunoreactivity, rabbit anti-human-5-HT2C polyclonal antibody (Abcam, Cambridge, MA) was prepared at a concentration of 1:5,000 in 0.1% Triton-X/PB. Tissue was incubated for three nights in primary antibody solution at 4°C, washed in 0.3% Triton-X/PB and then incubated in goat anti-rabbit biotinylated secondary antibody (1:500) for 1 h. Sections were washed and then incubated in Vectastain ABC reagent (prepared according to manufacturer's instructions) for 1 h. Sections were then washed and incubated in 3′-3′ diaminobenzidine (DAB/Ni) reagent, prepared according to manufacturer's directions (Vector Labs). All sections were allowed to incubate in DAB/Ni for ∼7 min. Slides were then rinsed and allowed to dry completely before coverslipping with Permount (Fisher Scientific, Pittsburgh, PA). Adjacent sections from the same brains were used as control tissue. This tissue was treated the same as antibody-treated tissue except that the tissue was incubated with primary antibody that had been preadsorbed with the immunizing peptide (ab21171; AbCam).
Photomicroscopy
Image acquisition was performed by using an Olympus microscope BX140. High-magnification images were acquired using Magnafire. Composite photomicrographs (Fig. 3) of immunohistochemistry were created using Adobe Illustrator (Adobe).
Fig. 3.
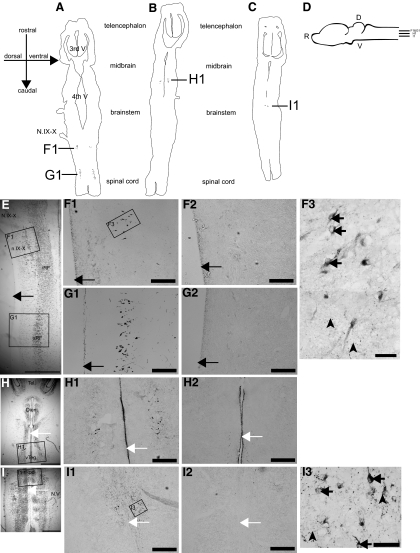
Immunohistochemistry for 5-HT2C-like receptors in Xenopus brain. 5-HT2C-like receptors are located in 4 distinct regions of the Xenopus brain. A–C: line drawings of horizontal sections of a Xenopus brain showing the locations of the receptor populations (left to right is dorsal to ventral; top to bottom is rostral to caudal). D: schematic shows the relative plane of section for schematics drawn in A–C. E, H, and I: Nissl-stained tissue sections representing the location of each 5-HT2C receptor population. Boxed area corresponds with photomicrographs shown in F, and 2, G, 1 and 2, H, 1 and 2, and I, 1 and 2. N.IX-X, nerve IX-X; iRF, inferior reticular formation; Tel., telencephalon; Dien., diencephalon;.N.V, nerve V. Scale for Nissl stained images = 800 μm. F1: antero-medial portion of n.IX-X (n.IX-Xam). F3: higher magnification of area enclosed in F1. Black arrows point to immunopositive somata, black arrowheads point to immunopositive puncta. G1: rostral reticular formation of spinal cord (sRF). H1: ventral tegmental area (VTeg) of the midbrain. I1: rostral raphe nucleus (rRpd). I3: higher magnification of area enclosed in I1. Black arrows point to immunopositive somata, black arrowheads point to immunopositive puncta. Scale for low- and high-magnification images = 50 μm. F2–I2: adjacent control tissue sections of F1–I1 (respectively) preadsorbed with immunizing peptide lacked 5-HT2C-like receptor staining. White arrows,e tissue midline; black arrows, lateral edge of tissue. Scale 50 μm.
Whole-brain preparation
Sexually mature Xenopus males (n = 24; 35.8 ± 3.7 g; 6.95 ± 0.3 cm) and females (n = 2; 72.4 ± 10.0 g; 8.8 ± 0.6 cm) were purchased from Nasco (Fort Atkinson, WI). The animals were kept in glass aquaria on a 12:12 light:dark cycle at room temperature. All experimental procedures were approved by the Boston University Institutional Animal Care and Use Committee and performed in compliance with guidelines published by the National Institute of Health. Frogs were anesthetized with MS-222, 0.15 mg/g body wt, injected subcutaneously (Sigma), and brains were rapidly removed in oxygenated (99% O2-1% CO2) ice-cold saline composed of (in mM) 96 NaCl, 20 NaHCO3, 2 CaCl2, 2 KCl, 0.5 MgCl2, 10 HEPES, and 11 glucose with pH 7.8. Brains were then transferred to a recording chamber where they were continually superfused with fresh oxygenated saline (150 ml/h) and allowed to return to room temperature (∼22°C) during the following hour.
In vitro nerve recordings
Methods of recording the population activity of motor nucleus IX-X were described previously (Rhodes et al. 2007). Briefly, a suction electrode was placed on the most caudal rootlet of nerve IX-X to record compound action potentials (CAPs); this nerve rootlet contains the axons of the laryngeal and glottal motoneurons (Simpson et al. 1986). The recorded signal was amplified 1,000 times (A-M Systems differential amplifier 1700), high-pass filtered (1 Hz), digitized at 10 kHz (Digidata 1322A, Molecular Devices, Sunnyvale, CA), and recorded on a PC using AxoScope software (Axon Instruments). All recordings were made at room temperature (∼22°C).
Drugs
Stock solutions of Serotonin hydrochloride (5-hydroxytryptamine; Sigma Aldrich), 6-chloro-2-(1-piperazinyl) pyrazine hydrochloride (MK-212; Tocris Bioscience, Ellsville, MO), (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI hydrochloride; Sigma), (αS)-6-chloro-5-fluoro-α-methyl-1H-indole-1-ethanamine fumarate (Ro 60–0175; Tocris), and citalopram hydrobromide were dissolved in deionized water and 8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulphonamido) phenyl-5-oxopentyl)-1,3,8-trizaspiro[4.5] decane-2,4-dione hydrochloride (RS 102221 hydrochloride; Tocris) was dissolved in DMSO. Application of DMSO (20 μl) alone to isolated whole brains had no effect on the ability of serotonin to initiate fictive vocalizations (data not shown). Serotonin used in this study was made fresh as a stock solution on the day of use and was kept on ice until its final dilution in oxygenated saline at room temperature. MK-212, DOI, Ro 60–0175, citalopram and RS102221 were made as stock solutions and kept at 4°C until final dilution in oxygenated saline.
Application of pharmacological agents
While recordings were made from the laryngeal nerve of the isolated brain, 5-HT was applied by replacing half the saline in the recording chamber (20 ml) with 60 μM 5-HT dissolved in oxygenated saline to achieve a final concentration of 30 μM. 5-HT application took 5–10 s, after which 5-HT remained in the recording chamber for 5 min; during this time superfusion of saline was suspended. 5-HT2C receptor agonists (MK-212, Ro 60–0175, and DOI) were bath-applied to isolated whole brain preparations in the same manner as 5-HT and were allowed to incubate on the preparation for ≤15 min during which superfusion was also suspended. To wash all drugs out of the bath after the treatment period, saline superfusion was reinstated at a high rate (10–20 ml/min) for 5–10 min, which is sufficient to completely exchange the solution in the recording chamber several times. All brains were continually superfused with oxygenated saline (100–150 ml/h) for 1 h between repeated applications of 5-HT or 5-HT2C receptor agonist. Citalopram (30 μM) was also applied to the bath in the same way as 5-HT with the exception that it remained in the recording chamber for 30 min (during this time, superfusion was suspended). In experiments where the 5-HT2C receptor antagonist was used to block citalopram-induced fictive vocalizations, citalopram was first bath-applied to the isolated brain (30 min), which was then immediately followed by administration of oxygenated saline containing RS102221 (50 μM) and citalopram (30 μM) for 15 min. This time period has previously been demonstrated to be effective for 5-HT2C receptor antagonist blockade of 5-HT-induced fictive vocalizations (Yu and Yamaguchi 2009). Immediately after antagonist application, the perfusion (containing 5-HT2C receptor antagonist and citalopram) was reinstated at the rate of ∼100 ml/h for the following hour while the laryngeal nerve activity was recorded. Control experiments were performed in the same way except vehicle (DMSO) replaced the antagonist (RS102221). All final concentrations of drugs used in this study are well within the concentration ranges used for other in vitro intact preparations (Calvino et al. 2005; Katz and Frost 1995).
In vitro transection experiments
To determine the location of 5-HT2C-like receptors that mediate initiation of fictive vocalizations, transection experiments were carried out using the isolated whole brain preparation. Iridectomy scissors were used to perform each transection under a Leica stereoscope (Model Mz75). All brains were superfused by oxygenated saline for ≥30 min after the transection prior to application of agonists. If the 5-HT2C receptor agonist activated fictive vocalizations in the transected brain, then we concluded that the receptors expressed in the remaining tissue are sufficient to activate vocalizations. After the experiment, the transected brains were fixed in 4% paraformaldehyde, sectioned at 50 μm using a Vibratome (Series 1000), and stained with Nissl to confirm the accuracy of transections. An Olympus microscope (Model BX41) was used to observe Nissl stained tissue to confirm the accuracy of transections.
Analyses of fictive calls for transected brains
In this study, we focused on the initiation of vocal behavior. In males, advertisement calls are the most common and best studied vocalization, whereas in females, release calls (sometimes called ticking) are the most common vocalizations. In vivo advertisement calls are produced exclusively by males, whereas ticking is produced occasionally by males but mostly by females (Tobias et al. 2004a). Similarly on bath application of serotonin, fictive advertisement calls are elicited only from male brains, whereas fictive ticking can be evoked occasionally from male (∼30% of male brains showed 5-HT-induced fictive ticking) but mostly from female brains (Rhodes et al. 2007).
In these pharmacological studies, we assessed whether or not fictive vocal behavior was activated in the presence of pharmacological agents. Typically, isolated Xenopus brains are silent in vitro, but when 5-HT or a 5-HT2C receptor agonist is administered, fictive vocalization is induced (Rhodes et al. 2007; Yu and Yamaguchi 2009) (Fig. 2A). Fictive advertisement calls in males are characterized by a series of CAPs repeated at a slow rate (∼30 Hz; slow trill), followed by a train of CAPs repeated at faster rates (∼60 Hz; fast trill) with progressively larger amplitude (Rhodes et al. 2007; Yamaguchi and Kelley 2000) (Fig. 1A). In contrast, fictive ticking is characterized by CAPs repeated at a very slow, often monotonous rate (<10 Hz) without any systematic AM (Rhodes et al. 2007; Yamaguchi and Kelley 2000).
Fig. 2.
The size of Xenopus 5-HT2C-like receptor is similar to 5-HT2C receptor of human and rat. A: BLAST alignment of Xenopus laevis, human, and rat 5-HT2C receptor. Sequence is the C-terminus of the 5-HT2C receptor protein. C-termini of X. tropicalis 5-HT2A and 5-HT2B sequences are also provided for comparison. Boxed regions indicate the same amino acids. B: Western blot of 5-HT2C receptor. Whole-brain extracts of Xenopus (middle) and rat (right) demonstrate a band of the appropriate size (∼52 kDa) but not in Xenopus stomach tissue (left). C: actin served as loading control for the presence of protein in all lanes.
Because there is inter- and intraindividual variation in the temporal structure of fictive vocalizations, we first assessed the normal range of variation in 5-HT-induced fictive vocalizations and compared them to those induced by other pharmacological agents (for details, see Yu and Yamaguchi 2009). To this end, 5-HT-induced fictive advertisement calls and ticking recorded from two male brains and two female brains were analyzed by sampling bouts of advertisement calls and ticking (range: 3–10 bouts) from each male and female brain, respectively. Instantaneous CAP rates (reciprocal of inter-CAP interval) were measured using Clampfit 10.0 (Axon Instruments), and frequency histograms were plotted for each animal with bin size of 1 Hz (as in Rhodes et al. 2007). The frequency histograms of males were fit well with two Gaussian curves (R2 > 0.9) with two means μ1 and μ2. These μ's were used as estimates of mean slow and mean fast trill rates in males, respectively. The frequency histogram of females can be fit well with single Gaussian curves (R2 > 0.9), and μ's used as estimates for mean ticking rate in females. In addition, maximum sustained CAP rate (calculated using a sliding window—i.e., by averaging 10 consecutive instantaneous CAP rates and taking the maximum) was used to characterize fictive vocalizations. μ's and maximum sustained CAP rates were used for statistical analyses to determine whether fictive vocalizations initiated in the presence of pharmacological agents differ from those evoked by serotonin.
Statistical analysis
All statistical analyses were done using StatView software (SAS Institute, Cary, NC). For experiments in which the agonist initiated vocal behavior, we examined if there was any quantitative difference in the fictive vocalizations evoked by the agonist and by 5-HT (5 males and 5 females) using a Mann-Whitney U test.
To determine if citalopram induces fictive advertisement calls in male brains, we compared the number of bouts (including bouts that only include fast trill as well as complete bouts that contain both fast and slow trills) produced in 1 h after treatment with citalopram by experimental brains to those produced in 1 h after saline application by control brains using a Mann-Whitney U test. To determine if antagonist blocks citalopram-evoked fictive vocalizations, a Mann-Whitney U test was used to compare the number of bouts produced by the control brains in the presence of citalopram alone to those produced by the experimental brains in the presence of citalopram and the antagonist.
To compare vocal activity induced by SSRI and serotonin, we applied a 5-min sliding window to SSRI-evoked vocal traces and determined the maximum number of bouts produced within any given window. This maximum value was compared with the total number of calls evoked in response to serotonin within 5 min. Such adjustment was necessary because SSRI, unlike serotonin, evokes fictive vocalizations slowly, and SSRI-treated brains were observed for 1 h, while exogenous serotonin is only administered for 5 min. A Mann-Whitney U test was used to compare bout counts for unpaired comparisons.
These experiments comply with the Principles of Animal Care, Publication 86–23, revised 1985, of the National Institutes of Health and also with the current laws of the United States.
RESULTS
Western blot of Xenopus 5-HT2C -like receptors
To evaluate the ability of the antibody to detect 5-HT2C-like receptors in Xenopus, we compared the sequence homology of 5-HT2C receptors among X. laevis, human, and rat. A BLAST alignment with the reported sequences for Xenopus, human, and rat revealed similarities in the region coding for the C-terminus in all three species (Fig. 2A). The antibody used in the present experiments recognizes a 22 amino acid sequence from amino acids 400 to the C-terminus of the human 5-HT2C receptor, and showed 68% similarity to that of X. laevis C-terminus. An alignment of the 5-HT2A and 5-HT2B terminal sequences of X. tropicalis (a closely related species to X. laevis) indicates very little sequence homology with any of the 5-HT2C receptor sequences (Fig. 2A).
To confirm the identity of immunopositive protein in Xenopus brain, we performed a western blot analysis on Xenopus and rat tissue extracts. Immunoblotting with Xenopus and rat brain tissue extracts revealed a band at ∼52 kDa (Fig. 2B), the size expected for Xenopus 5-HT2C-like receptors, whereas no immunopositive band was detected in the tissue extracts of Xenopus stomach. Actin served as a loading control and confirmed the presence of protein in all lanes (Fig. 2C). The CNS-specific detection of the protein is consistent with the idea that the antibody specifically labeled 5-HT2C receptors as they are known to be expressed exclusively in the CNS (Hoyer et al. 2002) and not in peripheral tissue. Furthermore the results of the negative control indicate the specificity of the antibody. Stomach tissue is known to contain high levels of a closely related receptor, the 5-HT2B receptor (Hoyer et al. 2002). However, the receptor was not detected with the 5-HT2C receptor antibody we used. The size of the 5-HT2C receptor is conserved across the phylogeny and falls within the range from 52.0 to 54.1 kDa in vertebrates (Table 1). Thus our Western blot results demonstrate that the antibody shows specific affinity for a CNS-specific protein of a size that is comparable to 5-HT2C-like receptor in Xenopus brains.
Table 1.
Comparison of amino acid sequence length and protein size of 5-HTx receptors across species
| Amino Acids | Protein Size, kDa | |
|---|---|---|
| H. sapiens | 458 | 52.0 |
| G. gailus | 472 | 53.7 |
| R. norvegicus | 460 | 52.3 |
| D. rerio | 476 | 54.1 |
| T. guttata | 472 | 53.7 |
The number of amino acids comparising the 5-HT2C receptor and the subsequent protein size is conserved across vertebrate phylogenies.
5-HT2C-like receptors are located in the midbrain, hindbrain, and the spinal cord
We first examined the distribution of 5-HT2C-like receptors using immunohistochemical methods. The results showed four distinct populations of cells that were positively stained for 5-HT2C-like receptor in both male and female Xenopus (n = 3 each, male example shown). Two populations were found in the brain stem (antero-medial nucleus IX-X, n.IX-Xam; Fig. 3F1, and rostral raphe nucleus, rRpd; I1). The remaining two populations were found in the midbrain (ventral tegmentum, Fig. 3H1) and in the spinal reticular formation, (sRF; G1). Importantly, two of these brain stem nuclei, rRpd and n.IX-Xam, are excellent candidates for the site of action of serotonin. rRpd sends projections to n.IX-X and DTAM (Brahic and Kelley 2003), suggesting its involvement in activation of vocal neurons, while n.IX-Xam contains interneurons that project to and receive input from DTAM (Zornik and Kelley 2008), indicating its importance in coordinating the vocal behavior. We found no obvious sex differences in the distribution and numbers of positively stained neurons (not shown). Furthermore, the 5-HT2C-like receptor staining was absent from the telencephalon in all brains examined (not shown). The locations of the four populations of 5-HT2C receptor staining were compared with Nissl stained tissue sections (Fig. 3, E, H, and I).
The staining of 5-HT2C-like receptors was present in somata but absent from the nucleus of the neurons (Fig. 3, F3 and I3), and detailed observation revealed numerous puncta throughout each region consistent with receptor staining on projection terminals (I1). The specificity of the staining was confirmed by comparing each immunopositive stained tissue section with its adjacent negatively controlled immuno-negative tissue section for each brain; all control tissue had an absence of label (Fig. 3, F2–I2).
5-HT2C -like receptors expressed in the brain stem are functionally important for fictive vocalizations in both sexes
We next investigated what region of the brain contains the 5-HT2C-like receptors that are functionally involved in initiating vocalizations. To this end, we eliminated populations of immunopositive neurons by transecting the brain at various positions, and examined the effect of the 5-HT2C receptor agonist in activating fictive vocalizations from the remaining tissue in both sexes. For reasons not well understood, the 5-HT2C receptor agonist Ro 60–0175 never initiates fictive ticking in healthy female brains yet always initiates fictive advertisement calls in healthy male brains (Yu and Yamaguchi 2009). Thus we used Ro 60–0175 or MK-212 as agonists in male brains and MK-212 or DOI as agonists in female brains. DOI is a 5-HT2A/C agonist but acts as a 5-HT2C receptor agonist in this preparation for we previously demonstrated (Yu and Yamaguchi 2009) that the effect of DOI in eliciting fictive ticking from a female brain is mediated by activation of 5-HT2C, but not 5-HT2A receptors.
Using brains that originally responded to the 5-HT2C receptor agonist (Ro 60–0175 or MK-212, males; MK-212 or DOI, females; 50 μM, ≤10 min, see preceding text) with fictive vocalizations, we first removed the telencephalon and diencephalon from male (n = 2) and female (n = 4) brains, respectively (Fig. 4, top cartoon), to determine if there are any 5-HT2C receptors in these regions that mediate vocal activation that were not stained in our immunohistochemical study. In response to Ro 60–0175 or MK-212 (males) or MK-212 or DOI (females), fictive advertisement call or ticking was elicited from all transected male and female brains, respectively (Fig. 4A, 1, male; 2, female). Thus 5-HT2C-like receptors expressed in the region caudal to the diencephalon can initiate vocalizations in males and females.
Fig. 4.
5-HT2C-like receptors in the brain stem initiate fictive vocalizations in the sexes. A, 1 and 2: progressive transection reveals that agonists to 5-HT2C receptors can initiate fictive vocal behavior even in the absence of telencephalon and diencephalon. B, 1 and 2: further transections to remove the midbrain and rostral spinal cord reveal that 5-HT2C-like receptors contained in the brain stem can still initiate fictive vocal behavior in isolated male and female brains.
To determine whether 5-HT2C-like receptor containing neurons in the brain stem are sufficient for vocal initiation, we removed the most rostral (ventral tegmentum in the midbrain) and most caudal (reticular formation of the spinal cord) population of 5-HT2C-positive neurons by isolating brain stems of additional male and female brains (Fig. 4, bottom cartoon) and applied the 5-HT2C receptor agonist to the remaining brain stem. Of all the brains that later responded to serotonin (2 male brains: 1 Ro 60–0175 and 1 MK-212, and 2 female brains: 1 MK-212 and 1 DOI), each 5-HT2C receptor agonist elicited fictive advertisement calls in male brains (1 of which also produced fictive ticking), and fictive ticking in female brains (Fig. 4B, 1, male; 2, female). In addition, a third isolated brain stem of a male (n = 1) responded to a broad-spectrum 5-HT2 receptor agonist (α-Me-5-HT; 30 μM; 5 min) with fictive advertisement calls that were subsequently blocked by the 5-HT2C receptor antagonist (RS 102221 50 μM; 15 min; not shown), indicating that α-Me-5-HT initiates fictive vocalizations mainly through activation of 5-HT2C-like receptors in the isolated brain stem. The fictive vocalizations that we did elicit from isolated brain stems in response to the 5-HT2C agonists (Ro 60–0175, MK-212, and DOI) did not differ significantly from those induced by 5-HT in intact control brains (male brains: μ1: U = −1.12, P = 0.25, μ2: U = −0.78, P = 0.44; maximum sustained CAP rate: U = −1.55, P = 0.12, number of bouts: U = −0.78, P = 0.44; female brains: μ: U = −0.78, P = 0.44; maximum sustained CAP rate: U = −1.55, P = 0.12.). For reasons that are not clear, some isolated brain stems failed to respond to serotonin or 5-HT2C receptor agonists (n = 9 males, 8 females) even though post hoc Nissl staining did not show any systematic difference in the plane of transection between successful and unsuccessful tissues. One possibility is that there are some 5-HT receptors at the edge of the isolated brain stem that were excluded from the failed tissues but included in the successful tissues. Alternatively, the transections caused mechanical damage to the DTAM in failed tissues so that activation of the receptors by 5-HT did not elicit fictive vocalizations. We consider the latter to be more likely because the plane of transection was consistent in all the tissues, and the plane of section is very close to DTAM, a nucleus that is critical for vocal production. Therefore we conclude that 5-HT2C receptor agonists initiate fictive vocalizations by binding to 5-HT2C-like receptors expressed in the brain stem in both sexes.
Bath application of citalopram initiates fictive vocal behavior in isolated male brains
Next we wished to determine if endogenous release of 5-HT can also initiate fictive vocalizations. To address this question, we applied a selective serotonin reuptake inhibitor (SSRI), citalopram, to male brains. SSRIs such as citalopram act to block the serotonin transporter required for serotonin reuptake and recycling. Thus serotonin is allowed to accumulate in the extracellular space where it can exert its effect. Citalopram was used in this study because it is highly specific for the serotonin transporter (Bezchlibnyk-Butler et al. 2000; Laakso et al. 1996; Owen et al. 2001) and has been used successfully in other intact (Calvino et al. 2005) and slice (Galzin et al. 1985) preparations to increase extracellular accumulation of 5-HT and synaptic responses evoked by serotonergic neurons. Citalopram is also known to evoke synaptic potentials with a faster onset and for a longer duration of time compared with other SSRIs such as fluoxetine (Calvino et al. 2005). We first recorded untreated isolated brains to demonstrate a lack of vocal behavior prior to treatment with citalopram (Fig. 5A). We then incubated each preparation with citalopram for 30 min to allow the drug to penetrate the brain and to allow for sufficient accumulation of endogenous serotonin. During the following 60 min, nerve activity was recorded (Fig. 5A). Control brains were treated the same as experimental brains and incubated for the same amount of time (1 h) with saline, while nerve recordings were made (Fig. 5A).
Fig. 5.
Application of citalopram initiates male fictive advertisement call. A: timeline of experiment. N.IX-X recording indicates the duration of time during which a number of fictive calls were recorded from control and experimental brains. B: example traces from a citalopram-treated brain (left) and a saline-treated brain (right). Scale for both traces is 2 s. C: enlarged view of the 1st bout from the citalopram-treated brain shown in B, left. Scale 1 s. Both fast and slow trills are present in citalopram-induced fictive advertisement call. D: a box plot showing the number of advertisement call bouts generated in the presence (n = 7) and in the absence (saline treatment, n = 5) of citalopram during 1 h posttreatment. Brains treated with citalopram produced a significantly larger number of call bouts compared with brains treated with saline alone.
Under normal circumstances, isolated Xenopus brains are typically quiescent until serotonin or 5-HT2C agonists are applied (see Fig. 1B). When citalopram (30 μM) was applied to male brains (n = 7) for 30 min, six of the seven brains (86%) initiated bouts of advertisement call without any further treatment (Fig. 5, B, left, and C). In contrast, all control male brains treated with saline for 30 min (n = 5) failed to produce any advertisement call during or after the treatment (Fig. 5B, right). All control and experimental brains later responded to exogenous serotonin with fictive advertisement calls. When the total number of call bouts produced by experimental and control brains was compared, they were significantly different (Fig. 5D; Mann-Whitney U test; P ≪ 0.01). Furthermore, the temporal structure of fictive vocalizations elicited by citalopram did not differ significantly from those induced by 5-HT in intact control brains (Mann-Whitney U test; μ1: U = 17.00, P = 0.35; μ2: U = 14.00, P = 0.75; maximum sustained CAP rate; U = 25.50, P = 0.95). The only difference that we can detect between citalopram and serotonin in their effectiveness in evoking fictive vocalizations was the overall amount of vocal activity. When the maximum number of bouts produced in a 5-min window (see methods) was compared with the total number of bouts evoked by serotonin in the same period of time, Citalopram-treated brains produced a significantly smaller number of call bouts compared with naïve control brains given 5-HT (Mann-Whitney U test; U = 38.00, P = 0.04). Taken together, we conclude that treatment with citalopram does initiate fictive advertisement calls of intact temporal morphology, although the overall vocal activity induced is less than exogenously delivered 5-HT. The results indicate that endogenous serotonin is released at appropriate locations within the brains of Xenopus to initiate fictive vocalizations.
5-HT2C receptor antagonists block citalopram-induced vocalizations in isolated male brains
Are citalopram-induced fictive vocalizations also mediated by the activation of 5-HT2C–like receptors? To address this question, we applied 5-HT2C receptor antagonist to male brains (n = 5) treated with citalopram. We first recorded untreated isolated brains to establish a lack of vocal behavior as in the preceding text (Fig. 6A). Then these silent brains were administered citalopram (30 μM) for 30 min as before. After the 30 min treatment of citalopram, the brains were immediately administered a 5-HT2C receptor antagonist RS102221 (50 μM, citalopram concentration remained the same) for 15 min, a treatment previously shown to block 5-HT-initiated fictive vocal behavior in all brains (Yu and Yamaguchi 2009). Then all brains were recorded for one hour during which the brain was continually superfused with oxygenated saline that contained 5-HT2C receptor antagonist (50 μM) at a rate ∼100 ml/h (Fig. 6A). In control experiments, five male brains initially treated with citalopram (30 μM, 30 min) were administered vehicle alone for 15 min (DMSO, 0.02%, while the concentration of citalopram remained the same) then vehicle was perfused into the recording chamber for 1 h immediately following this treatment (Fig. 6A). In all experimental brains, citalopram failed to initiate fictive advertisement calls in the presence of 5-HT2C receptor antagonist (Fig. 6B, right), whereas in four of five control brains, citalopram did initiate fictive advertisement calls throughout the 1 h of vehicle treatment (Fig. 6, B, left, and C). When the 5-HT2C receptor antagonist was washed out for 10 min from the recording chamber of the experimental brains, fictive advertisement calls were recorded from these brains during the following hour, presumably due to the long-lasting effect of the citalopram that was no longer opposed by the effect of the antagonist (not shown). Currently, it is not clear how long the effect of citalopram lasts in this in vitro preparation but in this study, we observed citalopram-evoked advertisement calls for ≤two h after treatment in the brains used for the preceding experiments (not shown). Later application of serotonin induced fictive advertisement calls from all control and experimental brains, indicating that the failure to induce fictive vocalizations from experimental brains is not due to ill health of the tissue. When the number of call bouts produced in the presence of 5-HT2C receptor antagonist and in the presence of vehicle alone (control) were compared, the control brains produced significantly larger number of calls than experimental brains (Fig. 6D; Mann-Whitney U test, U = −2.353, P = 0.02). Interestingly, the number of bouts produced by citalopram seems to be enhanced in some male brains in the presence of DMSO (compare Figs. 5D and 6D), but the effect was highly variable. Accordingly, when the maximum number of bouts induced by citalopram in the presence of DMSO was compared with those induced by citalopram alone, or by 5-HT alone, they were not significantly different (Mann-Whitney U test; U = 14.00, P = 0.75). It is possible that DMSO may facilitate the ability of citalopram to penetrate the tissue but the effect is contingent on individual differences in endogenous serotonin release. Regardless of the possible effects of DMSO in enhancing citalopram-induced vocal activity, the results showed that the application of 5-HT2C receptor antagonist blocked citalopram-induced vocalizations. Thus we conclude that vocalizations induced by the endogenously released serotonin are also mediated by the activation of 5-HT2C–like receptors.
Fig. 6.
5-HT2C receptor antagonist blocks citalopram-induced fictive vocalizations. A: timeline of the experiment. N.IX-X recording indicates the duration of the time during which a number of fictive call bouts were counted and compared in control and experimental brains. B: example traces from a citalopram-treated brain with DMSO control (left) and a citalopram-treated brain with 5-HT2C receptor antagonist (right). Scale for both traces is 2 s. C: enlarged view of the 5th bout from the citalopram plus DMSO-treated brain shown in B, left. Scale is 0.5 s. D: a box plot showing the number of advertisement call bouts generated in the presence of 5-HT2C receptor antagonist (n = 5), and in the presence of vehicle (DMSO) alone (n = 5). Brains treated with vehicle alone generated significantly more bouts of advertisement calls compared with brains treated with 5-HT2C receptor antagonist.
DISCUSSION
Identifying 5-HT2C -like receptors in the Xenopus brain stem
The results of the immunohistochemistry experiments demonstrated that there are four distinct immuno-positive neuronal populations in Xenopus brains. The 5-HT2C receptor protein of X. laevis is only partially sequenced. However, we were able to detect specific proteins in distinct regions of the Xenopus CNS using a 5-HT2C receptor antibody developed against the human 5-HT2C receptor sequence. Our Western blot results indicate that the antibody recognizes ∼52-kDa proteins only found within the Xenopus CNS (Hoyer et al. 2002) and does not recognize the 5-HT2B receptor of X. laevis (De Lucchini et al. 2005) that shares little sequence similarity to the 5-HT2C receptor. The size of 5-HT2C receptors are phylogenetically conserved; human, rat, chicken, zebrafinch, and zebrafish all express the 5-HT2C receptor with a protein size that falls within a narrow range of 52 to 54 kDa. Thus the size of the Xenopus protein recognized by the antibody on the Western blot fits the predicted size of the 5-HT2C receptor protein. Short of cloning the 5-HT2C receptor of X. laevis and developing the antibody against it, we used commercially available tools developed for the human 5-HT2C receptor in this study and discovered that proteins of a size similar to 5-HT2C receptors are expressed exclusively in the CNS of X. laevis, and a region of the brain that contain immunopositive populations of neurons responds functionally to the 5-HT2C receptor agonists. Putting the immunohistochemistry and Western blot results together, it is likely that what we stained in the tissue are 5-HT2C-like receptors.
Locating the critical 5-HT2C-like receptors in the Xenopus brain
Our immunohistochemistry results suggest that 5-HT2C-like receptors are located in four distinct regions in Xenopus brain, two of which [antero-medial n.IX-X (n.IX-Xam) and rRpd] overlap with the central vocal pathways. Anatomically, the dorsal raphe nucleus is known to send serotonergic projections to both DTAM and n.IX-X (Brahic and Kelley 2003; Rhodes et al. 2007). In addition, it is assumed that some local circuit neurons within the dorsal raphe nucleus also release serotonin to postsynaptic neurons within the nucleus including projection neurons. Therefore 5-HT2C-like receptor-mediated activation of raphe neurons may result in further release of serotonin into key parts of the vocal pathways, namely DTAM and n.IX-X. This is possible in light of other experiments that showed serotonergic neurons in the dorsal raphe nucleus can form dendro-dendritic synapses with other serotonergic neurons (Chazal and Ralston 1987), and it has been observed that serotonergic neurons can form synapses with other serotonergic neurons in the raphe nucleus (Kapadia et al. 1985). Such synapses, if present, can provide a mechanism for controlling release of and amplification of serotonin in Xenopus. Thus activation of 5-HT2C-like receptors in the raphe nucleus may function to amplify the serotonergic signals via a positive feedback mechanism.
Currently we do not know what role serotonergic innervation of DTAM plays in vocal production. We expect to find other serotonin receptors in DTAM because dense serotonergic projections from the dorsal raphe nucleus to DTAM (Rhodes et al. 2007) are likely to be translated into physiological action by some type of serotonergic receptors. Although we predict the presence of other types of 5-HT receptors in DTAM, our data indicate that activation of these non-5-HT2C receptors is not sufficient to initiate vocalizations because pharmacological blockade of 5-HT2C-like receptors blocks all 5-HT-induced fictive vocalizations (Yu and Yamaguchi 2009).
Neurons located in antero-medial n.IX-X (n.IX-Xam) are known to send projections to both DTAM (for reciprocal connections) as well as to contralateral n.IX-X (for commissural connection, Zornik and Kelley 2007). Thus the possible impact of n.IX-Xam activation is far-reaching. Activation of 5-HT2C-like receptors in n.IX-Xam may initiate network communication between DTAM and n.IX-X, which in turn initiates vocalizations. This hypothesis is in line with that previously proposed by Schmidt (1992), who proposed a two-part CPG that involves both n.IX-X and DTAM for vocal production in leopard frogs (Rana pipiens).
It is not clear whether the somatic staining we observed represents functional receptors expressed in membranes or those that are yet to be inserted into membranes at dendritic terminals. Also it is unclear whether the punctate staining we observe represents receptors expressed by the local neurons (i.e., those the cell bodies and dendritic arborization of which remain within a nucleus) or by dendrites invading from neurons the cell bodies of which lie outside of the nucleus. Although further anatomical research is necessary, we believe that the most parsimonious explanation for our staining pattern is that the somatic staining represents cytosolic receptors that are yet to be transported, and punctuate staining represents the receptors expressed by the local neurons as has been seen in 5-HT2A receptors (Huang and Pickel 2003).
Previously we have shown that the central pattern generator (CPG) for vocalization is contained in the brain stem of Xenopus (Rhodes et al. 2007). We have also shown that the activation of 5-HT2C-like receptors is sufficient for initiating fictive vocal behavior in male and female Xenopus (Yu and Yamaguchi 2009). In this study, our transection results demonstrate that the primary action of 5-HT2C receptor agonists for the initiation of vocal behavior takes place in the brain stem in both sexes. The telencephalon and diencephalon, as well as the midbrain and rostral spinal cord do not seem to part of the vocal CPG in Xenopus because 5-HT2C receptor agonists can still activate fictive vocal behavior after removal of these regions. It remains unclear at this point if rRpd or n.IX-Xam or both play a role in the initiation of vocal behavior. Because these nuclei lie in close proximity to key parts of the CPG (DTAM and n.IX-X), we were unable to transect these locations to ascertain the role of rRpd and n.IX-Xam in initiation of vocal behavior in this study. Future work will utilize small lesions rather than transections to emphasize the role of rRpd and n.IX-Xam; our study lays the groundwork for these important future experiments.
Endogenous serotonin is sufficient to initiate fictive vocalizations in male brains
Previous results showed that endogenous serotonin is available in key vocal nuclei (n.IX-X and DTAM) (Rhodes et al. 2007), and the receptors that initiate fictive vocalizations are also located in the central vocal pathways. Based on these results, we hypothesized that release of endogenous stores of serotonin can initiate fictive advertisement call in male brains by activating 5-HT2C-like receptors in raphe nucleus, n.IX-X, or both.
To facilitate endogenous release of serotonin, we used a SSRI, citalopram. Citalopram is one of the most selective blockers of the serotonin transporter (Owen et al. 2001) and has been used successfully to increase extracellular serotonin concentrations in other physiological preparations (Calvino et al. 2005). In the present study, we showed that citalopram significantly increases the number of fictive call bouts generated by male brains.
For reasons that are unclear to us, a small proportion of citalopram-treated brains failed to initiate fictive advertisement calls in our experiments (14% of all experiments). Although citalopram likely exerts its effect by causing accumulation of serotonin in the extracellular space, the amount of accumulation likely depends on the firing frequency of the raphe neurons, which are known to fire spontaneously at rates that vary across the sleep-wake cycle (Urbani et al. 2006). Individual differences in the spontaneous firing rates of raphe neurons may account for the individual variation in responsiveness to citalopram, which may be more pronounced in brains also administered DMSO. Citalopram produces bouts of advertisement calls with temporal morphology similar to those induced by exogenous 5-HT. The only difference between citalopram and exogenous 5-HT that we detected was that the overall vocal activity induced by citalopram was less than those evoked by exogenous 5-HT. The reduced effectiveness of citalopram may be partly due to the poor penetration of citalopram to the target site (i.e., synaptic terminals of serotonergic neurons) or to the decreased concentration of serotonin available at the receptors. If we combine electrical stimulation delivered to rostral raphe nucleus with citalopram application, we may obtain vocal activity levels comparable to those induced by exogenous 5-HT. Regardless of overall vocal activity level, we conclude that endogenously released serotonin evokes fictive advertisement calls.
Citalopram-induced fictive vocalizations were blocked by application of a 5-HT2C receptor antagonist, indicating that fictive vocalizations activated by endogenously released serotonin are also mediated by the activation of 5-HT2C-like receptors in Xenopus. We suggest that endogenous serotonin released from the neurons of dorsal raphe nucleus within the nucleus itself and into n.IX-X initiate fictive vocalizations by activating 5-HT2C-like receptors in these two locations. Although the cellular response of the serotonergic target neurons is not clear, it is possible that vocal neurons are excited in response to the activation of 5-HT2C-like receptors by reducing leak currents as shown in rat respiratory system (Ptak et al. 2009). Based on these results, we conclude that the receptor mechanism by which endogenous 5-HT and exogenous 5-HT exert their effect is similar.
How does endogenous serotonin initiate vocal behavior in Xenopus?
Based on our findings in the present study and previous work we suggest that initiation of vocal behavior in vivo involves serotonergic transmission mediated by 5-HT2C-like receptors in key vocal nuclei of the Xenopus brain stem. Binding of serotonin to 5-HT2C-like receptors in n.IX-X may activate the neurons that project to DTAM (a critical part of the Xenopus vocal CPG) (Rhodes et al. 2007) either directly or indirectly or decrease the threshold of the n.IX-X neurons that receive inputs from DTAM neurons. Binding of serotonin to neurons in the raphe may serve to amplify the serotonergic transmission so that more serotonin is released to target neurons in both n.IX-X and DTAM (Rhodes et al. 2007).
Locating the initiation site of a motor behavior
In recent years, much research has been focused on how a particular motor behavior is activated. Some research takes a top-down approach, trying to understand motor pattern selection starting at the level of the basal ganglia. For example, in rats, the mesencephalic locomotor region (MLR) has been implicated in the initiation of walking. Stimulation of this region upstream of the walking CPG activates the walking motor program in spinal cord preparations (Bem et al. 1993; Jell et al. 1985). Likewise, electrical stimulation of the diencephalic locomotor region (DLR) in lamprey initiates fictive swimming (Menard and Grillner 2008). Closely related to our work, it has been shown that rhythmic whisking behavior in rats is governed by how strongly the serotonergic neurons of the parapyramidal region (PPR) are stimulated (Liu and Jordan 2005). It was demonstrated that the facial nucleus receives innervation from the serotonergic neurons of the PPR and is activated by the release of endogenous serotonin onto 5-HT2 receptors (Hattox et al. 2003).
The approach that we describe in this study is a bottom-up approach. By identifying the types of receptors that mediate the action of serotonin for initiating behavior, and the types of neurons that receive serotonergic input, we can begin to understand the mechanisms by which the central pattern generator for the motor program can be turned on. For example, 5-HT2C receptors are often localized to GABAergic neurons (Bubar and Cunningham 2007; Liu et al. 2007; Serrats et al. 2005). Inhibitory interneurons are commonly part of CPGs often providing reciprocal inhibition for a simple oscillator (Marder and Bucher 2001). This possibility is particularly interesting given that many episodic rhythmic behaviors such as swallowing (Wang and Bieger 1991) are under constant tonic inhibition and the removal of inhibition caused by the state-dependent change in nuclei upstream of or within the CPG results in initiation of behavior (Staras et al. 2003). Thus 5-HT2C receptors expressed by GABAergic neurons may initiate the vocalizations by inhibiting the neurons that provide tonic inhibition to the CPG system.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant RO1 NS-048834, a Clare Luce Booth Professorship, and startup funds from Boston University Biology Department to A Yamaguchi.
ACKNOWLEDGMENTS
We thank S. Tsunoda, H. Man, and the members of their laboratories for help with Western blotting procedures. We also thank M. Baum, J.-W. Lin, M. Wachowiak, E. Zornik, and A. Katzen for providing comments on the earlier version of this manuscript. We also thank S. Zhang and M. Portalatin for assistance with tissue sectioning.
REFERENCES
- Bem T, Orsal D, Cabalquen JM. Fictive locomotion in the thalamic rat. Exp Brain Res 97: 301–304, 1993 [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk-Butler K, Aleksic I, Kennedy SH. Citalopram—a review of pharmacological and clinical effects. J Psychiatry Neurosci 25: 241–254, 2000 [PMC free article] [PubMed] [Google Scholar]
- Boothman L, Raley J, Denk F, Hirani E, Sharp T. In vivo evidence that 5-HT(2C) receptors inhibit 5-HT neuronal activity via a GABAergic mechanism. Br J Pharmacol 149: 861–869, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience 146: 286–297, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic CJ, Kelley DB. Vocal circuitry in Xenopus laevis: telencephalon to laryngeal motor neurons. J Comp Neurol 464: 115–130, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvino MA, Iscla IR, Szczupak L. Selective serotonin reuptake inhibitors induce spontaneous interneuronal activity in the leech nervous system. J Neurophysiol 93: 2644–2655, 2005 [DOI] [PubMed] [Google Scholar]
- Chazal G, Ralston HJ. Serotonin-containing structures in the nucleus raphe dorsalis of the cat: an ultrastructural analysis of dendrites, presynaptic dendrites, and axon terminals. J Comp Neurol 259: 317–329, 1987 [DOI] [PubMed] [Google Scholar]
- Chen J, Condron BG. Drosophila serotonergic varicosities are not distributed in a regular manner. J Comp Neurol 515: 441–453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer NP, Li Y, Keller A. The whisking rhythm generator: a novel mammalian network for the generation of movement. J Neurophysiol 97: 2148–2158, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Feldman JL. Respiratory rhythm: an emergent network property? Neuron 34: 821–830, 2002 [DOI] [PubMed] [Google Scholar]
- De Lucchini S, Ori M, Cremisi F, Nardini M, Nardi I. 5-HT2B-mediated serotonin signaling is required for eye morphogenesis in Xenopus. Mol Cell Neurosci 29: 299–312, 2005 [DOI] [PubMed] [Google Scholar]
- Fetcho JR. Spinal network of the Mauthner cell. Brain Behav Evol 37: 298–316, 1991 [DOI] [PubMed] [Google Scholar]
- Galzin AM, Moret C, Verzier B, Langer SZ. Interaction between tricyclic and nontricyclic 5-hydroxytryptamine uptake inhibitors and the presynaptic 5-hydroxytryptamine inhibitory autoreceptors in the rat hypothalamus. J Pharmacol Exp Ther 235: 200–211, 1985 [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom M. Mechanisms for selection of basic motor programs—roles for the striatum and pallidum. Trends Neurosci 28: 364–370, 2005 [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallén P, Saitoh K, Kozlov A, Robertson B. Neural bases of goal-directed locomotion in vertebrates–an overview. Brain Res Rev 57: 2–12, 2008 [DOI] [PubMed] [Google Scholar]
- Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull 78: 69–74, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattox A, Li Y, Keller A. Serotonin regulates rhythmic whisking. Neuron 39: 343–352, 2003 [DOI] [PubMed] [Google Scholar]
- Herberholz J, Sen MM, Edwards DH. Escape behavior and escape circuit activation in juvenile crayfish during predator-prey interactions. J Exp Biol 207: 4543–4550, 2004 [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71: 533–554, 2002 [DOI] [PubMed] [Google Scholar]
- Huang J, Pickel VM. Ultrastructural localization of serotonin 2A and N-methyl-d aspartate receptors in somata and dendrites of single neurons within rat dorsal motor nucleus of the vagus. J Comp Neurol 455: 270–280, 2003 [DOI] [PubMed] [Google Scholar]
- Jell RM, Elliott C, Jordan LM. Initiation of locomotion from the mesencephalic locomotor region: effects of selective brain stem lesions. Brain Res 328: 121–128, 1985 [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57: 183–191, 2008 [DOI] [PubMed] [Google Scholar]
- Kapadia SE, de Lanerolle NC, LaMotte CC. Immunocytochemical and electron microscopic study of serotonin neuronal organization in the dorsal raphe nucleus of the monkey. Neuroscience 15: 729–746, 1985 [DOI] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Intrinsic neuromodulation in the Tritonia swim CPG: serotonin mediates both neuromodulation and neurotransmission by the dorsal swim interneurons. J Neurophysiol 74: 2281–2294, 1995 [DOI] [PubMed] [Google Scholar]
- Laakso A, Palvimaki E-P, Kuoppamaki M, Syvalahti E, Hietala J. Chronic citalopram and fluoxetine treatments upregulate 5-HT2C receptors in the rat choroids plexus. Neuropsychopharmacology 15: 143–151, 1996 [DOI] [PubMed] [Google Scholar]
- Li WC, Sautois B, Roberts A, Soffe SR. Reconfiguration of a vertebrate motor network: specific neuron recruitment and context-dependent synaptic plasticity. J Neurosci 27: 12267–12276, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J Neurophysiol 94(2): 1392–404, 2005 [DOI] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience 146: 1677–1688, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol 11: R986–R996, 2001 [DOI] [PubMed] [Google Scholar]
- Menard A, Grillner S. Diencephalic locomotor region in the lamprey—afferents and efferent control. J Neurophys 100: 1343–1353, 2008 [DOI] [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychol 50: 345–350, 2001 [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes HJ, Yu HJ, Yamaguchi A. Xenopus vocalizations are controlled by a sexually differentiated hindbrain central pattern generator. J Neurosci 27: 1485–1497, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Soffe SR, Clarke JD, Dale N. Initiation and control of swimming in amphibian embryos. Symp Soc Exp Biol 37: 261–284, 1983 [PubMed] [Google Scholar]
- Schmidt RS. Neural correlates of frog calling: production by two semi-independent generators. Behav Brain Res 50: 17–30, 1992 [DOI] [PubMed] [Google Scholar]
- Serrats J, Mengod G, Cortes R. Expression of serotonin 5-HT2C receptors in GABAergic cells of the anterior raphe nuclei. J Chem Neuroanat 29: 83–91, 2005 [DOI] [PubMed] [Google Scholar]
- Simpson HB, Tobias ML, Kelley DB. Origin and identification of fibers in the cranial nerve IX-X complex of Xenopus laevis: Lucifer Yellow backfills in vitro. J Comp Neurol 244: 430–444, 1986 [DOI] [PubMed] [Google Scholar]
- Staras K, Kemenes I, Benjamin PR, Kemenes G. Loss of self-inhibition is a cellular mechanism for episodic rhythmic behavior. Curr Biol 13: 116–124, 2003 [DOI] [PubMed] [Google Scholar]
- Tobias ML, Barnard C, O'Hagan R, Horng SH, Rand M, Kelley DB. Vocal communication between male Xenopus laevis. Anim Behav 67: 353–365, 2004a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias ML, Viswanathan SS, Kelley DB. Rapping, a female receptive call initiates male-female duets in the South African clawed frog. Proc Natl Acad Sci USA 95: 1870–1875, 2004b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaini N, Creamer K, Debonnel G. Electrophysiological diversity of the dorsal raphe cells across the sleep-wake cycle of the rat. J Physiol 573: 679–695, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Bieger D.Role of solitarial GABAergic mechanisms in control of swallowing. Am J Physiol Regulatory Integrative Comp Physiol 261: R639–646, 1991 [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Kelley DB. Generating sexually differentiated vocal patterns: laryngeal nerve and EMG recordings from vocalizing male and female African clawed frogs (Xenopus laevis). J Neurosci 20: 1559–1567, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HJ, Yamaguchi A. 5-HT2C-like receptors initiate sex-typical vocalizations in Xenopus laevis. J Neurophysiol 102: 752–765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornik E, Kelley DB. Breathing and calling: neuronal networks in the Xenopus laevis hindbrain. J Comp Neurol 501: 303–315, 2007 [DOI] [PubMed] [Google Scholar]
- Zornik E, Kelley DB. Regulation of respiratory and vocal motor polls in the isolated brain of Xenopus laevis. J Neurosci 28: 612–621, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]