Abstract
Layer IV of the barrel cortex contains an anatomical map of the contralateral whisker pad, which serves as a useful reference in relating receptive field properties of cells to the cortical columns in which they reside. Recent studies have shown that the degree to which the surround receptive fields of layer IV cells are generated intracortically or subcortically depends on whether they lie in barrel or septal columns. To investigate whether this is true for layer V cells, we blocked intracortical activity in the barrel cortex by infusing muscimol from the cortical surface and measuring spike responses to sensory stimulation in the presence of locally iontophoresed bicuculline. Layer V cells beneath barrels had small subcortically generated single- or double-whisker center receptive fields and larger intracortically generated six to seven whisker surround receptive fields. Conversely, septally located cells received multiwhisker input from both subcortical and cortical sources. Most properties of layer Va and layer Vb cells were very similar. However, layer Vb barrel neurons showed a relative lack of phasic inhibition evoked from sensory input compared with layer Va cells. The direct thalamic input to the layer V cells was not sufficient to evoke a sensory response in the absence of input from superficial layers. These findings suggest that despite the apparent overt similarity of layer V receptive fields, barrel and septal subdivisions process different sources of information within the barrel cortex.
INTRODUCTION
Layer V of the cortex is particularly highly developed in the somatosensory system and gives rise to major projections to trigeminal, pontine, thalamic, striatal, and collicular locations (Hoffer et al. 2005; Jacquin et al. 1990; Leergaard et al. 2000; Mercier et al. 1990; Welker et al. 1988; Wright et al. 1999, 2000) as well as other cortical areas (Chakrabarti and Alloway 2006; Hoffer et al. 2005). At least two levels of spatial organization can be discerned within layer V of the barrel cortex: 1) layer Va and Vb form a sublaminar division and 2) septal and barrel columns form an orthogonal subdivision to the laminar organization. The connectivity of the layer V subdivisions differ from one another. Layer Va receives input from the posterior medial thalamic nucleus (POm), whereas layer Vb does not (Bureau et al. 2006). Layer Va tends to project to striatum, whereas layer Vb projects instead to the pontine nuclei (Mercier et al. 1990). The septal and barrel columns are also connected differently. Whereas septal columns project to motor cortex and make bilateral connections with contralateral barrel cortex, the projections to the second somatosensory area (SII) arise from both barrel and septal locations (Alloway 2008; Chakrabarti and Alloway 2006; Olavarria et al. 1984).
Although a great deal of progress has been made in mapping the afferent and efferent connections of layer V, it is not clear how the different subcortical and thalamic inputs are integrated to compose layer V receptive fields and thus to process information within the cortex. Given the morphological distinctions between the layer V subdivision in the barrel cortex, it should be possible to locate cells within the orthogonal columnar and laminar subdivisions of the cortex and test whether they have different receptive field properties. In a previous study on layer IV septal and barrel subdivisions, it was possible to separate intracortical and subcortical influences on cells by globally blocking cortical activity with muscimol (a γ-aminobutyric acid [GABA] agonist) and reactivating cells only locally with iontophoresis of bicuculline (a GABA antagonist) (Fox et al. 2003). Therefore we applied this approach to layer V and analyzed the results with respect to layer subdivisions. We found that the origin of the surround receptive field depended on whether the layer V cells lay beneath a barrel or beneath a septal location, suggesting that the distinction between septal and barrel areas, evident for layer IV of the cortex, is maintained through to layer V. In addition we found that superficial layer inputs play a major role in generating the sensory responses of layer V cells.
METHODS
Subjects
Recordings were made from adult Long–Evans rats. Twelve animals provided control data and 21 animals were used in reactivation experiments involving the inhibition of cortical activity and local reactivation by iontophoretic injection of bicuculline methochloride (BMC). An additional five animals were used in experiments to study the effect of BMC activation alone in control (untreated) cortex. Fourteen animals were used in experiments to study the early inactivation of layer V. Almost all animals were aged between 6 and 13 wk of age (n = 47) and the others between 18 and 26 wk (n = 5).
Anesthesia, surgery, electrodes, and recording
Anesthesia was induced with isoflurane (5% in O2) and maintained with urethane (administered intraperitoneally, 1.5 mg/g body weight) at an anesthetic depth equivalent to Guadel stage III-2. Anesthetic state was monitored during the experiment by spontaneous activity when present, which occurred in bursts of up–down states in the delta-frequency range of 1–4 Hz (Fox and Armstrong-James 1986), and was characterized by a weak hindlimb withdrawal reflex, the presence of a corneal reflex, and a respiration rate of about 82–92 breaths/min. We did not observe whisker movements in this state. This may correspond to a slightly deeper stage of III-2 anesthesia than reported elsewhere because whisker movements were not present and the breathing rate was below the 96–120 range, but animals were not as deep as Guadel stage III-3 because the hindlimb withdrawal reflex was present (Friedberg et al. 1999). Anesthesia was maintained by supplementary doses of 10% of the initial dose when necessary to maintain the depth of anesthesia. Body temperature was maintained at 37°C by a heating pad controlled by a rectal thermistor. Anesthetized rats were secured in a Narishige SR-6 stereotaxic frame; the skull was removed over a 2.5 × 4 mm area above the barrelfield (∼4.5–7 mm lateral to the midline and 1–5 mm posterior to bregma) and a small incision was made in the dura to permit entry of the microelectrode. We used triple-barreled glass microelectrodes with one single carbon-fiber recording electrode and two electrodes containing BMC at a pipette concentration of 10 mM, pH 5.5 (Tocris Cookson, Bristol, UK). In experiments not requiring application of BMC, we used single-barreled carbon-fiber recording electrodes. All electrodes were made on a Narishige vertical pipette puller (model PE-2). The electrode recording was amplified and filtered (600 to 6,000 Hz) before spikes were discriminated based on their amplitude between an upper and lower amplitude threshold (Neurolog, Digitimer, Welwyn Garden City, Hertfordshire, UK). Neurons were continuously monitored for spike shape by trapping an example on a digital oscilloscope and comparing subsequent discriminated spikes superimposed on the example trace. This allowed us to verify whether we had discriminated the same spike before and after muscimol treatment for the subset of experiments involving that protocol.
Muscimol diffusion and bicuculline application
In experiments involving the application of muscimol, an agarose gel “well” was formed over the exposed brain so that a pool of constant-concentration muscimol (200 or 500 μM; Tocris Cookson) in phosphate-buffered saline (PBS) could be applied to the barrel cortex. In these cases a larger incision was made in the dura to permit diffusion of the muscimol. The “well” was constructed by slowly applying warmed agarose over a PBS-saturated pledget of a “Lyostypt” (B. Braun, Melsungen, Germany) collagen pad. Once the agarose had set, the collagen pad was removed to leave behind a well above the surface of the brain. For a complete description of the diffusion of muscimol and iontophoresis of BMC, see Fox et al. (2003).
The progress of muscimol diffusion into the brain was monitored by stimulating the principal whisker and immediate surrounding whiskers and noting the time that the response was extinguished by direct inhibition. We plotted the wavefront of muscimol diffusion by plotting the time at which complete inhibition of sensory responses first occurred at each depth. When we estimated layer V neurons to be directly inhibited by muscimol diffusion we reactivated the cells by iontophoresis of BMC. The initial iontophoretic ejection of BMC was at +10 nA for 60 s and then reduced to about +2–5 nA for a further 60 s before whisker stimulation and recording were conducted over a period of 10 min. Iontophoretic current was continuously monitored and adjusted so that the cells were reactivated without being excited to the extent that they produced spontaneous erratic bursts of spikes: spontaneous activity of 1–2 Hz often occurred during reactivation of cells but was kept below the limit for bursts. After each 10-min recording epoch, the BMC-containing barrels were held on a negative retaining current (−12 nA) for another 10 min to ensure muscimol inhibition was reestablished before the process was repeated to measure responses from all the relevant remaining whiskers. The size of the reactivated area is about 200-μm radius at 10-min eject assuming that BMC diffuses in a manner similar to that of muscimol (see Fox et al. 2003 and methods).
Sensory responses, stimulation, and quantification
Vibrissae were stimulated with a series of fifty 200-μm vertical (upward) deflections at 1 Hz from a piezoelectric stimulator positioned at a distance of about 10 mm from the rodent's face (1° stimulus). The pulse driving the stimulator was a single 10-ms-duration monopolar pulse. The whisker stimulator deflection was calibrated using a laser position sensor (micro-Epsilon) and was found to have a rise time of <1 ms and a total duration of 10.7 ms. The principal whisker and all immediately surrounding neighbor whiskers were stimulated for each cell studied. Whiskers were stimulated sequentially one at a time with blocks of 50 stimuli. Spike2 software (Cambridge Electronic Design) was used to acquire and analyze poststimulus time histograms (PSTHs) and latency histograms. We routinely calculated the magnitude of response and the average modal latency of response for each whisker in the receptive field. The magnitude of response was calculated as the spike count during a time window 3–53 ms poststimulus minus the spontaneous activity occurring 50 ms before each stimulus (i.e., −50 to 0 ms). The average modal latency was calculated by taking the first spike in each response to a stimulus to generate a latency histogram and then ascertaining the modal or most common latency.
Histology
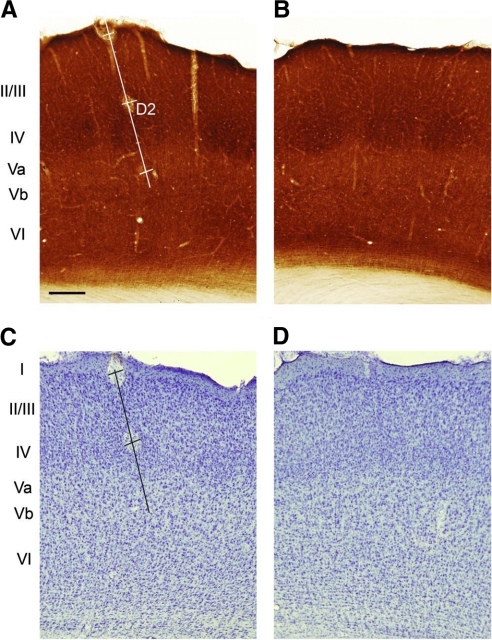
After recording from each penetration, two or three lesions were made 500 μm apart at known micromanipulator depth readings. At the end of each experiment animals were perfused with PBS followed by 4% buffered paraformaldehyde. The brain was stored overnight in 4% buffered paraformaldehyde and 20% sucrose solution and then transferred to 20% buffered sucrose solution. The corresponding cerebral cortex was coronally sectioned at 50 μm and sections were reacted for cytochrome oxidase (CO) as described previously (Fox 1994; Wong-Riley 1979). We reacted alternate sections for CO and Nissl stain (cresyl violet; see Fig. 1). The lesions were at known depths and could therefore be used to account for tissue shrinkage and to identify the recording sublayer and the septal versus barrel location. The principal whisker for the column was ascertained by identifying the whisker producing the shortest latency response for cells recorded in layer IV. Layer Va was distinguished from layer Vb in Nissl-stained sections by its lower cell density and by the greater number of large pyramidal cells in layer Vb. From alternate CO and Nissl-stained sections we noticed that the cell-sparse region of layer Va corresponded to the pale area directly below layer IV in CO-stained sections and that the border with layer Vb was characterized by larger pyramidal cells stained more darkly for CO. The Nissl and CO stains were therefore both used to judge the Va/Vb border. Layer Vb was distinguished from layer VI by the change from large pyramidal cells to denser smaller nonpyramidal cells. Recordings in layer Vb were made to a depth of about 1,250 μm and this meant that even deepest layer Vb cells we recorded were superficial to the layer Vb/VI border by a clear margin.
Fig. 1.
Histological determination of layers and septal/barrel subdivisions. A: coronal section through barrel cortex showing an electrode penetration track (white line) passing through the D2 barrel. The track is marked by 3 lesions: at the surface and at 500 and 1,000 μm. Layer IV is clear from the location of the 3 barrels (darker stain) in this cytochrome oxidase–stained section and the septal locations (lighter stain in layer IV) can be distinguished in between. B: cytochrome oxidase section from the opposite hemisphere to A showing the location of 3 barrels (dark stain) and intervening septae (lighter stain within layer IV). C: coronal section next in series to that shown in A, stained with cresyl violet, showing the layer Va and layer Vb subdivisions and the electrode penetration (black line). Note that the lesion at 1,000 μm is not visible on this particular section. D: the next section in series to B stained for cresyl violet. Scale bar in A = 250 μm.
Nomenclature for whiskers, principal barrel assignment, and septal/barrel column assignment
The term “central receptive fields” (CRFs) refers here to responses produced by stimulating the principal whisker (PW) for the cell. The PW is defined by the anatomically related barrel. The boundary of the column was defined as the line perpendicular to a tangent at the surface that bisected the CO-delineated barrels in layer IV (see Fig. 2). The cell was assigned the closest barrel column as the principal barrel. In most cases the PW determination was straightforward. However, in cases where the principal barrel was indistinct, for example for a septal cell on the edge of a column, the shortest latency response was used to define the PW (as described in results). The septal barrel boundaries were anatomically defined in a similar way, by projecting the edge of the barrel from layer IV down to layer V. The term “surround receptive fields” (SRFs) refers to responses produced by stimulating whiskers immediately adjacent (either by row or column, or both row and column) to the PW. Most recordings were made from D-row barrels and therefore most neurons had eight immediate SRF whiskers.
Fig. 2.
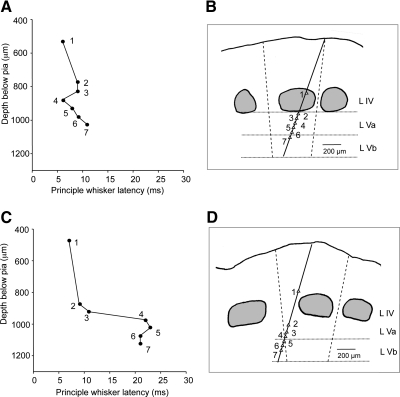
Latency of response to principal whisker (PW) stimulation within and across barrel columns. A: the latencies to D1 whisker stimulation are plotted against the recording depth for the penetration shown in B. In this penetration the electrode track stayed within the D1 barrel column. Responses to PW stimulation were ≤11 ms for all cells encountered within the penetration. C: latencies are plotted for stimulation of the C2 whisker for the electrode track shown in D. In this penetration the electrode track moved from the C2 barrel column at the top of layer IV and into the septal region within layer V. The response to the PW (established from the layer IV short latency responses to be C2), increased from 11 to >20 ms as the electrode track moved into the neighboring column at a point approximately halfway between the 2 barrel columns. At this point the C2 whisker is no longer the PW but a surround whisker. The vertical dashed lines represent an estimate of the boundary between columns and are placed halfway between the edges of the adjacent barrels and angled to the vertical axis of the column (see methods).
Data analysis and statistics
To analyze the SRFs of layer V cells under different experimental criteria, responses were ranked in order of magnitude, from S1 giving the greatest response to S8, the weakest. Responses were quantified as average response levels across cells that had been subject to the same treatment and/or anatomical location. To examine the kurtosis of SRF changes under various conditions, average SRFs were normalized in relation to their own individual CRF responses before taking averages for each group of cells. To examine the magnitude of the SRF changes under various conditions, we averaged responses of the PW and successive SRF whiskers (S1, S2, … , S8) across treatment groups. Treatment groups included local BMC application alone, BMC application during global muscimol diffusion, and the control condition in the absence of drugs.
We divided the data into cases where we could be confident that muscimol had diffused to the site of the recording and those where inhibition of responses occurred too fast for direct diffusion based on a linear fit of the square root of inactivation time versus depth in the cortex (see Fig. 6). The concentration (C) as a function of depth (x) and time (t) is given by
where C0 is the surface concentration (held constant in this case), D is the diffusion constant for muscimol modified for volume fraction and tortuosity (taken as 8.7 × 10−6 cm2/s; see Fox et al. 2003), and erfc is the complementary error function. In general, the time taken for layer II/III and layer IV cells to become inactivated were used to generate a linear fit of depth versus the square root of time. The linear fit was then extrapolated to predict the extinction of layer V responses. Occasionally, layer V cells responded normally for >1 or 2 h after the muscimol was applied to the surface before losing their response. In these cases, we checked to see whether the inactivation time and depth fell close to the linear fit of the data gathered for layer II/III and layer IV cells in the same penetration; if they did, we included them to improve the linear prediction and, if they did not, we excluded them from the linear fit. In total, 20 of 34 plots included cells only >750 μm, which was the approximate location of the layer IV/V border. The average depth of the deepest cell used for each plot was 736 ± 146 μm (range, 400 to 1,040 μm).
Fig. 6.
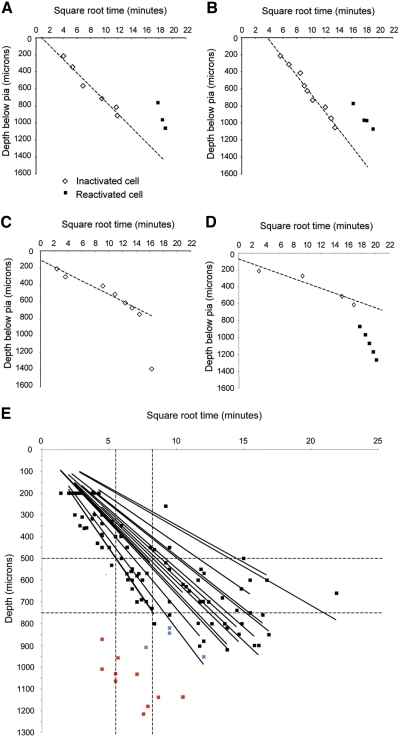
Examples of the estimated time course of muscimol diffusion and the time cells were reactivated by iontophoresis of BMC. A: muscimol diffusion is monitored by measuring spontaneous activity and responses to whisker stimulation. The white diamonds indicate the time muscimol inactivates the cell located at that particular depth. The line shows the best fit through the points (note the time axis is square root time). The times at which 3 cells were reactivated with BMC are shown by the black squares. At the time the reactivated cells were recorded the muscimol had diffused to a depth of ≥1,400 μm (estimated by extrapolation of the earlier recordings). B: a similar case to that shown in A where diffusion is monitored to a depth of 1,100 μm and the cells are locally reactivated during inactivation to an extrapolated depth of about 1,300 μm. C: in some cases the rate of inactivation deviated greatly from that expected from simple diffusion (dashed line). The cell located at 1,400 μm was inactivated far sooner than predicted from direct diffusion of muscimol to the soma. D: in this example 5 cells were reactivated in layer V (black squares) at a time when muscimol was extremely unlikely to have diffused directly to the location of the soma (dashed line) and layer V was not inactivated directly by muscimol. Nevertheless, the reactivated cells (black squares) showed no responses before BMC iontophoresis. E: diffusion rate of muscimol and the related loss of cortical activity. Muscimol was held at a constant concentration (200 or 500 μM) at the surface of the brain and diffused into the brain at a rate monitored by the loss of evoked and spontaneous activity. Cell responses to whisker stimulation were recorded until totally abolished in layer II/III and IV (black squares). The linear fit of the inactivation times for the individual recording tracks are plotted and give an indication of the spread of diffusion rates across experiments. The red squares indicate recordings made in cases where the electrode was positioned in layer V before applying muscimol to the surface. Note that the cells are inactivated earlier than the time taken for muscimol to diffuse to layer V. The leftmost vertical line indicates the fastest time taken to inactivate to the bottom of layer III (at ∼500 microns; horizontal dashed line). The rightmost vertical line indicates the fastest time taken to inactivate to the bottom of layer IV (at ∼750 microns; horizontal dashed line). Some cells were inactivated at a time when muscimol might have diffused directly to the location of recording (blue squares).
To test for any effect of the treatments used in this study and the location of the cells relative to sublayer or barrel versus septal subdivision we ran two- or three-way ANOVAs (as described in results). We used post hoc t-tests to check the origins of effects and interactions using JMP-IN software (Cary, NC). Where the distributions were not normal we used chi-squared (χ2) nonparametric statistics to judge the significance of differences. Cumulative distribution functions were analyzed using the Kolmogorov–Smirnov (K-S) two-sample test.
RESULTS
Receptive field properties of layer V neurons
We quantified the receptive fields of layer V neurons by measuring PSTHs for the principal whisker and the eight immediate neighboring whiskers (see methods). We identified the anatomical location of the recordings and subdivided the subsequent receptive field analysis into four subcategories based on whether neurons were located in layer Va or layer Vb and whether they lay beneath either a barrel or a septal location (see Fig. 1).
CENTER RECEPTIVE FIELDS.
The principal whisker was defined as the whisker corresponding to the name of the barrel column in which the cell was located i.e., the E1 whisker for a cell in the E1 barrel column. The anatomically defined principal whisker was ascertained from recordings as the electrode penetration passed through the barrel in layer IV, where the principal whisker is most easily identified as producing the shortest latency response and the largest response.
For some cells in layer V, the whisker evoking the largest response was not always the same as the anatomically defined principal whisker from layer IV recordings earlier in the penetration. In layer Va barrel locations, the principal whisker evoked the greatest response in 74% of the cases and for layer Vb barrel locations 68% of the cases. The correspondence between principal whisker and largest whisker response was lower for septal locations at 60 and 45% for Va and Vb sublaminae, respectively (see Table 1 for sample sizes).
Table 1.
Receptive field (RF) properties for layer V cells recorded in control animals
| Control | Cells (n) | PW Magnitude, spikes/50 stimuli | PW Latency, time/ms | RF Size, Responding Whiskers | Spontaneous Activity, Hz |
|---|---|---|---|---|---|
| Va barrel | 47 | 36.91 ± 3.36 | 10.33 ± 0.300 | 4.98 ± 0.42 | 1.41 ± 0.18 |
| Va septum | 30 | 35.67 ± 4.53 | 9.73 ± 0.368 | 4.53 ± 0.48 | 1.49 ± 0.30 |
| Vb barrel | 25 | 43.64 ± 4.16 | 9.52 ± 0.320 | 5.48 ± 0.43 | 1.62 ± 0.40 |
| Vb septum | 33 | 35.61 ± 3.52 | 9.68 ± 0.430 | 4.97 ± 0.41 | 1.68 ± 0.28 |
Values are means ± SE.
The whisker that evoked the shortest latency response was a slightly better predictor of the anatomically defined principal whisker than the whisker that produced the largest response. For those cases where the anatomical location was clear, we found that the shortest latency response was produced by the principal whisker in 78% of cases for layer Va barrel cells, 67% for Va septal cells, 76% for Vb barrel cells, and 58% for Vb septal cells. Figure 2 shows two example penetrations where the electrode was directed at an angle across the cortical column. When the penetration remains within the column the latency is relatively constant (Fig. 2, A and B). However, if the penetration moves out of the anatomically defined barrel, the latency of the principal whisker for that barrel increases substantially (Fig. 2, C and D). In general, the latency of the principal whisker response was consistent within layer Vb barrel locations (mean ± SE = 9.52 ± 0.32 ms), suggesting that latency is a good predictor of principal whisker for the deepest layer V locations recorded. Therefore in cases where the anatomical location was ambiguous, the shortest latency response was used to define the principal whisker. This definition was particularly useful for cells in septal locations where identification of the principal whisker from the histological assessment of the principal barrel column was more difficult.
The principal whisker response magnitude was remarkably similar across different subregions of layer V (average response = 37.6 ± 1.93 spikes/50 stimuli, n = 132) (Fig. 3 and Table 1). In contrast, principal whisker responses in layer V differed from those in other cortical layers. The principal whisker response magnitude was significantly lower in layer V than that in either layer IV [54.8 + 3.3 spikes/50 stimuli, n = 58, t(188) = 1.973; P < 0.001] or layer II/III [53.8 ± 7.4 spikes/50 stimuli, n = 33, t(163) = 2.0; P < 0.003] recorded in the same animals. The principal whisker responses in layer V were 32% smaller than those in layer IV CRF and 30% smaller than those in layer II/III. The center receptive field is therefore less responsive in layer V than that in other cortical layers.
Fig. 3.
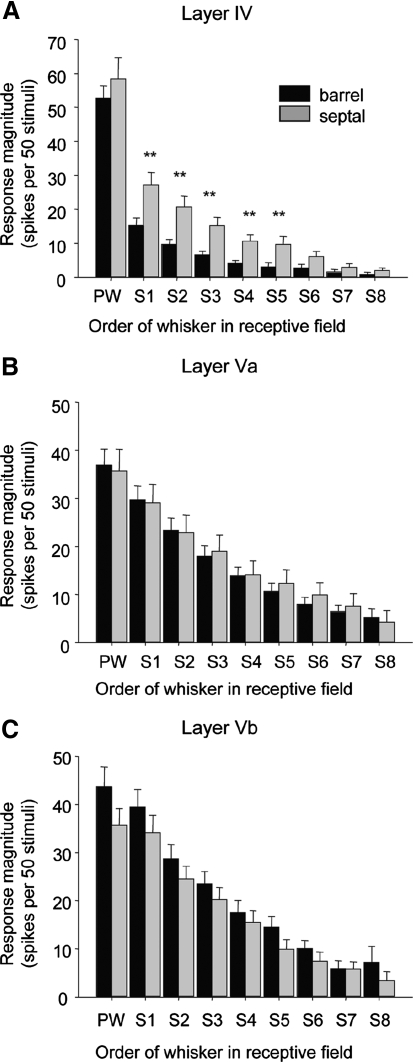
Control receptive field properties of layer V neurons in the rat barrel cortex. A: receptive field (RF) properties of neurons in layer IV barrel (n = 36, black bars) and septal regions (n = 22, gray bars). B: RF properties of neurons in layer Va barrel (n = 47) and septal regions (n = 30). C: RF properties of neurons in layer Vb barrel (n = 25) and septal regions (n = 33). The response magnitude is plotted for the PW and the surrounding whiskers (S1, S2, etc.) are ranked in order of magnitude for each cell before averaging. Student's t-tests revealed no significances between responses of septal and barrel subdivisions of layer V cells, but significant differences between the 2 subdivisions for layer IV cells (**P < 0.01). In barrels, an average of 2.2 whiskers composed the RFs (n = 36, SE = 0.3), whereas in septae an average of 4.1 whiskers were involved (n = 22, SE = 0.4). Consequently, whereas layer IV barrel RFs differed from those in all subdivisions of layer V [Va barrel, t(81) = 1.990, P < 0.001; Vb barrel t(59) = 2.001; P < 0.001; Va septum, t(64) = 1.998; P < 0.001, and Vb septum, t(67) = 1.997; P < 0.001], layer IV septal RFs were similar in size to those in layer V (t-test, α = 0.05).
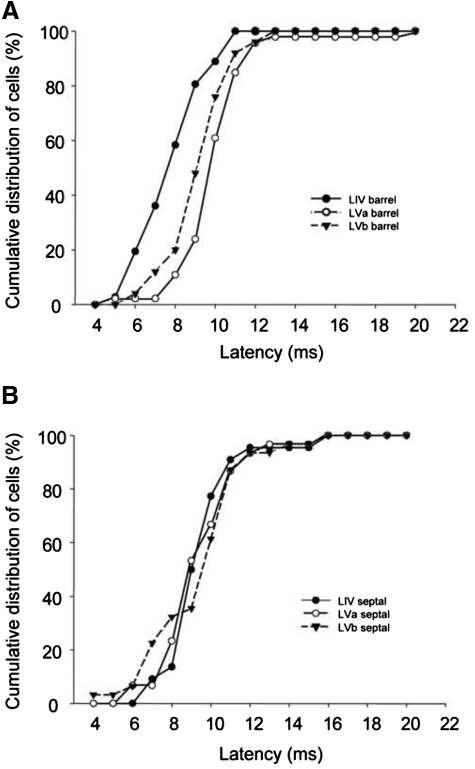
The timing of responses in different layers can give some clue to the sequence of activation within the cortex. As a population, layer IV barrel cells respond earlier than those in layer V barrel-column locations (Fig. 4A). In the septal subdivisions, layer IV and layer V cells respond at approximately the same time as one another (Fig. 4B). A small number of layer Vb cells in septal locations responded with very short latencies characteristic of layer IV cells (<8 ms; see Fig. 4B), which might indicate a direct thalamic input to this location.
Fig. 4.
Cumulative distribution functions for PW response latencies for cells in septal and barrel subdivisions of layers IV and V. A: response latency distributions are shown for cells located in barrel columns (black circle solid line: layer IV cells; white circle solid lines: layer Va cells; inverted triangle dashed line: layer Vb cells). Note that layer IV cells are activated first followed by layer Vb and layer Va. B: response latency distributions for septally located cells in layers IV, Va, and Vb. Note that septal column cells do not show a clear sequence of activation like the barrel-located cells. A subset of layer Vb cells are activated earlier (hump in curve between 6 and 9 ms).
Finally, we looked to see whether the latency of principal whisker responses varied across subdivisions of layer V (Fig. 4). We found that average latencies were remarkably similar across subdivisions of layer V and varied from 10.3 ms for layer Va barrel locations to 9.5 ms for layer Vb barrel locations (see Table 1). There were no significant differences between the CRF latencies in any subregion of layer V (α = 0.05). Nonparametric analysis of the population principal whisker latencies led to a similar conclusion. Layer Va and layer Vb latencies were not different in general (Dmax = 0.14, P = 0.56; K-S two-sample test) and neither were barrel and septal locations in Va (Dmax = 0.29, P = 0.09) or barrel and septal locations in layer Vb (Dmax = 0.147, P = 0.926). These results contrast with those in layer IV, where we found a highly significant difference between the latencies of response in the barrel versus septal locations (Dmax = 0.45, P < 0.01).
SURROUND RECEPTIVE FIELD PROPERTIES.
Layer V neurons had significantly larger receptive fields than those recorded in layer IV from the same animals (Fig. 3). A whisker was defined as part of the receptive field if it evoked a response of ≥10 spikes per 50 stimuli. Previous studies have used a threshold of 5 spikes per 50 stimuli for inclusion in the receptive field (Armstrong-James and Fox 1987), but even with our more restrictive criterion, the receptive fields were generally quite large in layer V. Receptive field sizes differ between barrel and septal locations in layer IV (Fox et al. 2003) and thus we checked to see whether the same applied in layer V. We found that average receptive field sizes were remarkably similar across different subdivisions of layer V, with values ranging from 4.5 whiskers for layer Va septum to 5.5 for layer Vb barrel cells (Table 1). There were no significant differences between septal or barrel regions in any of these cases (t-test, α = 0.05).
In contrast, in layer IV, septal and barrel region receptive fields were different (see Fig. 3).
In addition to similar receptive field sizes across layer V boundaries, the magnitude of response of the individual whiskers within the surround receptive fields were also relatively uniform. As an initial analysis we used a two-way ANOVA for the surround whisker responses across sublaminae (Va vs. Vb) and septal/barrel subdivisions (total degrees of freedom [df] = 130). There were no significant differences for S2–S8 (α = 0.05), although there was an effect of sublamina position on S1 [F(1,1) = 4.6, P < 0.05]. Post hoc t-test revealed this to be because the S1 whisker response (strongest surround whisker; see methods) was larger in layer Vb (39.4 spikes/50 stimuli) than that in layer Va [29.7 spikes/50 stimuli; t(69) = 1.995; P = 0.045], combining septal and barrel cases. These data show that receptive fields are relatively uniform in size across septal/barrel boundaries in layer V and (with the exception of S1) uniform across sublaminae in layer V.
Inactivation of layer V neurons with muscimol superfusion
To estimate layer V receptive field properties in the absence of intracortical synaptic transmission we continuously superfused the cortex with the GABAA agonist muscimol (200 or 500 μM), dissolved in isotonic PBS (Fig. 5A). The objective of this procedure was to prevent intracortical transmission of information between neighboring barrels and within the barrel column, so that we could reactivate cells with local application of bicuculline and observe its response to thalamic input. We monitored diffusion of muscimol by recording evoked response from an electrode as we gradually advanced it into the cortex. When whisker responses and spontaneous activity became inhibited at a particular depth, we advanced the electrode until whisker responses reappeared at deeper locations. In this way we were able to plot the progress and time course of diffusion through the depth of the cortex (Fig. 6).
Fig. 5.
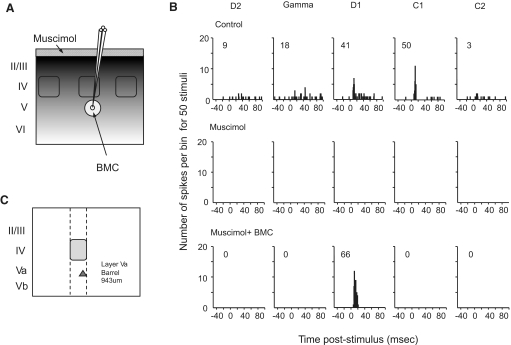
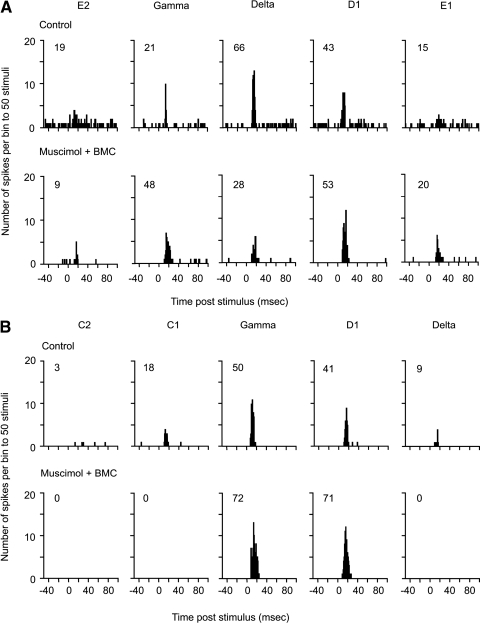
Method used to inactivate the cortex globally and reactivate cells locally. A: schematic diagram indicating the method of reactivating small areas of layer V with iontophoretic release of bicuculline methochloride (BMC, indicated by arrow) into barrel cortex inhibited by muscimol (applied at the cortical surface as indicated by the small arrow). B: poststimulus time histograms (PSTHs) show the principal whisker (D1) and the 4 strongest surround whisker responses from an individual cell throughout control conditions (top row). Complete inactivation by surface application of muscimol (middle row) and reactivation by iontophoretic injection of BMC under continued muscimol inactivation is shown (bottom row). The principal whisker evoked the shortest latency response in the control condition (12 ms) and the principal whisker latency decreased to 9 ms in the BMC + muscimol condition. Inset numbers show the number of spikes produced in reply to 50 stimuli minus spontaneous activity. Bin width 1 ms. C: the location of recorded cell within the cortical column (D1 barrel column cell layer Va).
Once all whisker-evoked (and spontaneous) activity was abolished in layers II/III, IV, and V, we iontophoresed the GABAA antagonist BMC at low currents to reactivate a neuron close to the electrode tip (see methods; Fig. 5). In the example shown of a layer Va barrel cell (Fig. 5), a single whisker receptive field returned after reactivation. As described in the following text, the reactivated receptive field could vary in size dependent on several factors.
It took several hours for muscimol to diffuse down to layer V (a minimum distance of ∼750 μm) at sufficient concentration to directly inactivate the cells. Layer III neurons were usually inactivated within 10–40 min of surface application, whereas layer IV cells often took between 50 and 200 min before they were inhibited. These results are to be expected from the natural rate of diffusion of muscimol in the cortex, as shown in Fig. 6 (note that the black data points in Fig. 6E represent the location of cells used to track diffusion, not cells where we studied reactivated receptive field properties). From a consideration of diffusion alone, layer V requires somewhere between 200 and 400 min for complete block to a depth of 1,500 μm and this is approximately the period we normally allowed before reactivating the cells. The exact time muscimol took to diffuse to layer V depended on the size of the dural opening, the muscimol concentration, and thereby on the rate of diffusion into the brain, which varied between experiments. For the cases included in the analysis of the reactivation experiments, the times before reactivation ranged from 153 to 457 min (average time before reactivating the first cell: 256 ± 16 min). Figure 6 shows examples of the linear relationship between depth and the square root of the time before the cell became unresponsive, as predicted by simple diffusion. In most cases, we reactivated the cells after muscimol had inactivated cells in layer V (Fig. 6, A and B).
However, a complicating factor arose in the estimation of muscimol diffusion into layer V because these cells often became inhibited much earlier than predicted by direct diffusion of muscimol to the depth of the cell body (Fig. 6C). In early experiments, this inadvertently led to reactivating the cells before muscimol could have diffused to the location of layer V based on its rate of diffusion through layers II/III and IV in the same animal (Fig. 6D). To check whether layer V cells were inactivated earlier than predicted by diffusion, we placed an electrode in layer V before applying muscimol to the surface and measured the time at which responses to center and surround receptive field components were lost. As shown in Fig. 6E, complete loss of receptive fields occurred rapidly for most of the cells tested in this subset of experiments (10/14; red points). We found four cases were sufficiently close to the diffusion curve that the cells may have been directly inhibited by muscimol (blue points), but in the majority of cases both central and surround receptive field activity was lost in layer V earlier than might be expected from diffusion to the site of the electrode recording. In each experiment where we found rapid inactivation (10 cases), we retracted the electrode into layer IV after losing the responses in layer V and found that layer IV sensory responses were still intact. Therefore to analyze the rest of the studies we categorized layer V cells into cases where muscimol had diffused directly to layer V before reactivating them with BMC (e.g., Fig. 6, A and B) and cases where we calculated that insufficient time had elapsed for direct inhibition to have caused the loss of response (see Fig. 6D and red points in Fig. 6E for examples of the latter).
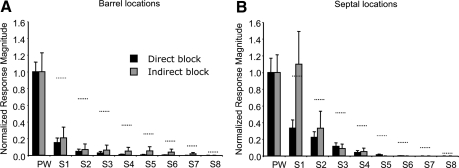
We compared the receptive fields of directly and indirectly inhibited layer V cells and found that those located within the barrel column had similar size receptive fields when reactivated with BMC, irrespective of whether the muscimol had diffused sufficiently long enough to inactivate the cell directly in layer V or had diffused only as far as the superficial layers (Fig. 7A). In general, the response to the principal whisker returned with BMC application, but the responses to the surround whiskers were absent or attenuated compared with control (see following text). This suggests a large component of layer V barrel column excitation by surround whiskers is dependent on input from superficial cortical layers. However, responses were very different for septally located layer V cells, depending on whether muscimol had directly diffused to inactivate layer V (Fig. 7B). Surround receptive field responses were smaller if muscimol had been given sufficient time to diffuse directly to the site of the recording [for S1, t(32) = 2.2, P < 0.05]. This suggests that layer V septal cells depend less on superficial layer input for their surround receptive fields than barrel-column cells.
Fig. 7.
The effect of direct or indirect block of layer V cells on the reactivated receptive field. A: the average normalized responses of cells located in layer Va and Vb in barrel columns. Black bars show responses to the principal whisker (PW) and surround whiskers (S1–S8) during BMC application at a time when muscimol had diffused at least to the site of the somatic recording and usually beyond. Gray bars show the responses of cells that were indirectly inactivated by muscimol at the time of BMC reactivation, i.e., they were inactivated without muscimol having diffused to the site of the somatic recording. B: layer V cells located in septal columns show a larger surround receptive field response when indirectly inactivated by muscimol (gray bars) compared with when directly inactivated (black bars). The horizontal dashed lines indicate the normalized control responses of layer V cells in untreated animals. Note that normalization occurs after averaging the responses of different cells.
In conclusion, many layer V cells lost their sensory response before layer V activity was directly inhibited at the somatic level, but at a time corresponding with the loss of activity in layers II/III and IV. If a cell was chosen for recording in layer V before application of muscimol, the probability of the cell losing its response to principal and surround whisker stimulation before direct diffusion of muscimol was high (78%). This suggests that a significant component of the receptive field of layer V cells relies on intracortical relay from layers II/III and IV. However, if cells were searched for in layer V after inhibition of layer IV, some were found to respond to whisker stimulation and to lose their responses much later at a time that could have been related to diffusion (15 cells in 34 penetrations). All these cells were located above 1,040 μm (average depth: 867 ± 72 μm) and were located in layer Va. This suggests that many cells in layer Va do not rely on superficial layers for sensory responses. Unfortunately, an estimate of their frequency in this case is biased by the fact that many cells may already have been silenced in layer V by the time a search was made. Therefore to simplify the analysis in the following sections, we just consider the responses of the 69 reactivated cells where muscimol was predicted to have directly inhibited the neurons in layer V based on a linear extrapolation of diffusion through superficial layers.
Reactivated receptive field properties of layer V neurons
CENTER RECEPTIVE FIELDS.
Figures 5 and 9 show examples of the three most common types of reactivated receptive field, where the receptive field of a cell located at the same recording site is measured before and after muscimol treatment. The most common occurrence was the emergence of a single whisker receptive field in the absence of global intracortical activity (Fig. 5B, 68.1% of cases). However, occasionally either one other whisker returned in the reactivated receptive field (Fig. 8B, 13.0%) or several (Fig. 8A, 18.8%). The latter case was more common in septal locations (36% of septal cases) but could also occur within the barrel column (9.1% of barrel cases) (see Fig. 8B).
Fig. 9.
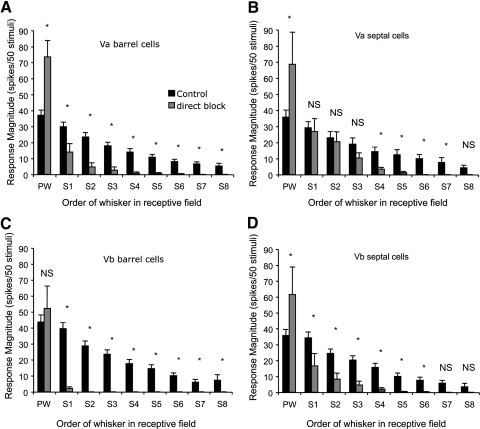
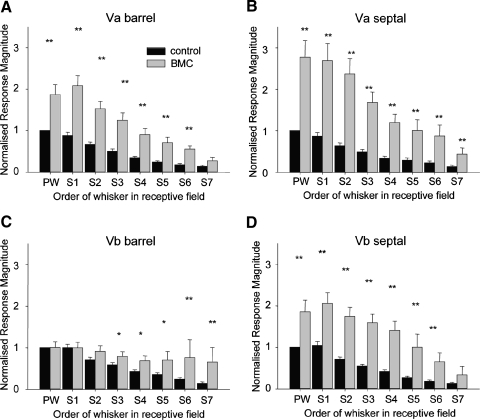
Effect of global cortical inactivation on receptive fields in different subdivisions of layer V. The average response is plotted for stimulation of principal whisker (PW) and surround whiskers (S1–S8) of control cells recorded in normal animals (black bars) and cells that were locally reactivated by BMC in cortex that had been directly inactivated with muscimol to a depth of at least the recorded cell (gray bars). A: layer Va barrel cells respond more strongly to the PW when treated with BMC, but all surround whisker responses are decreased significantly by cortical inactivation (α = 0.05, t-test; see results). B: layer Va septal cells show recovery of the PW and the three strongest surround whiskers despite overall cortical activity blockade. Of the 4 subdivisions of layer V, the SRF is affected least in Va septal cells. C: layer Vb barrel cells exhibit recovery of the principal whisker but show a profound loss of surround whisker responses. Occasionally a single surround whisker is present (gray bars). D: layer Vb septal cells also show significantly reduced surround responses, but they are not affected as strongly as layer Vb barrel cells (*P < 0.05; NS, not significant).
Fig. 8.
Reactivation during muscimol blockade can restore surround whisker responses in some cases. A: multiwhisker reactivation in the case of a cell located in layer Vb of the delta barrel column that initially responded strongly to 3 main whiskers and weakly to 2 others (control, top row). The principal whisker evoked the shortest latency response before (11 ms) and after treatment (13 ms). When reactivated the cell responded to the same three whiskers as before and showed a greater time-locked responses to the 2 other whiskers (E1 and E2). B: an example of a cell located in layer Va of the gamma barrel column that initially responded to 2 main whiskers and more weakly to 2 others (C1 and delta). The principal whisker evoked the shortest latency response before (9 ms) and after treatment (8 ms). In this case reactivation caused recovery of 2 whisker responses in the receptive field, which responded more strongly than before. The cell no longer responded to the other whiskers in the receptive field. Inset numbers show the number of spikes produced in reply to 50 stimuli minus spontaneous activity. Bin width = 1 ms.
Neurons reactivated in this way had principal whisker response magnitudes that were overall significantly larger than control values [F(1,1) = 22.1, P < 0.0001, total df = 198] (Fig. 9). The average reactivated principal whisker response magnitude was 204% of the control average for Va barrel, 192% for Va septal, and 172% for Vb septal cells. The one exception to this rule occurred for cells in layer Vb barrel-column locations, which showed no significant overactivation, but did return the principal whisker response to control levels [119% of control; t(37) = 0.77, P = 0.38] (Fig. 9). This finding implies that layer Vb barrel cells are subject to less endogenous inhibitory control from GABAA receptors than are other subregions of layer V. In general, the fact that the reactivated principal whisker responses returned to at least control values and usually far greater than control values, whereas the surround receptive fields were absent, implies that the lack of a surround receptive field component was not due to the recorded neuron being unexcitable.
SURROUND RECEPTIVE FIELD PROPERTIES.
There was a marked contrast in the effect of local reactivation on center receptive field (CRF) and surround receptive field (SRF) components of layer V cells. The CRF responses always exceeded or returned to control values with local reactivation (Fig. 9), whereas the SRFs most commonly comprised only one or two whiskers, rather than the average four or five found in control animals (see Tables 1 and 2). Although only 15.0% of the layer V cells studied had single whisker receptive fields under normal conditions, in the absence of intracortical activity this value increased to 68.1%. These results suggest that intracortical activity contributes significantly to the SRF of layer V cells.
Table 2.
Reactivated receptive field (RF) parameters recorded in animals with global muscimol blockade and local relief from muscimol with BMC iontophoresis
| Reactivated (BMC With Muscimol) | Cells (n) | PW Magnitude, spikes/50 stimuli | PW Latency, time/ms | RF Size, Responding Whiskers | Spontaneous Activity, Hz |
|---|---|---|---|---|---|
| Va barrel | 30 | 73.6 ± 10.0 | 11.4 ± 1.0 | 1.5 ± 0.21 | 0.11 ± 0.07 |
| Va septum | 12 | 68.5 ± 14.3 | 17.1 ± 1.7 | 2.3 ± 0.48 | 0.59 ± 0.32 |
| Vb barrel | 14 | 52.1 ± 10.6 | 14.5 ± 1.6 | 1.1 ± 0.10 | 0.21 ± 0.12 |
| Vb septum | 14 | 61.4 ± 17.3 | 12.7 ± 2.0 | 1.9 ± 0.40 | 2.57 ± 1.10 |
Values are means ± SE. Only cells directly inactivated by diffusion to the site of the recording are included.
We analyzed the receptive fields of reactivated cells dependent on their location relative to barrel and septal boundaries and layer Va and layer Vb sublaminae. In general, we found a higher occurrence of single whisker receptive fields (i.e., the principal whisker only and no surround receptive field component) in layers Va and Vb beneath the layer IV barrels, whereas cells showing larger reactivated surround receptive fields were most common in septal columns of layer Va and layer Vb (Fig. 10).
Fig. 10.
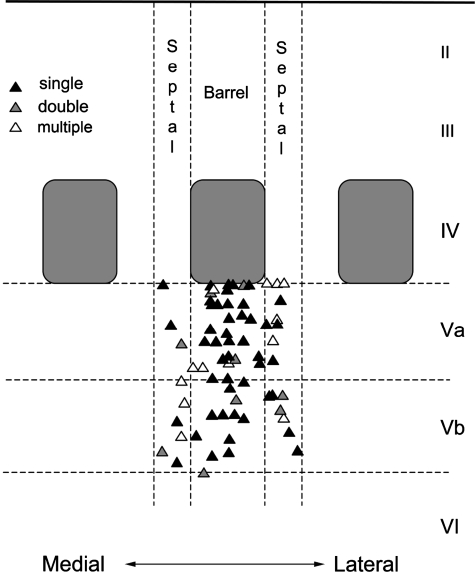
Receptive field size of layer V cells in the absence of global intracortical activity. A schematic representation of the reactivated receptive field sizes of layer V cells with respect to location. Cells were reactivated by BMC only after direct inactivation by muscimol diffusion from the surface of the cortex. Triangles mark the recording positions. Black triangles indicate a single whisker receptive field. Gray triangles indicate a two whisker and white triangles a multiple (>2) whisker receptive field. Histological localization was performed by making marker lesions following recording and identifying the lesion location in postmortem coronal sections. Note that the septum is divided in 2 halves between neighboring barrels.
In barrel locations, 79.5% of cells showed single whisker receptive fields compared with 48.0% in septal locations. A comparison of the incidence of single, double, and multiwhisker responses with respect to recording location revealed a highly significant difference between barrel and septal distributions (χ2 = 6.8, P < 0.03, df = 2) and no significant difference between sublaminae (χ2 = 1.6, P = 0.45, df = 2). We tested the possibility that some of the cells near the Va/Vb border may have been misattributed to the wrong sublamina, thereby inadvertently obscuring a real sublamina difference, by excluding all cells from the analysis that lay within 100 μm of the border (28/69 excluded). Again we found that there was a significant difference between barrel and septal areas (χ2 = 7.4, P < 0.03, df = 2), but not between Va and Vb (χ2 = 1.1, P = 0.56, df = 2), supporting the original conclusion.
Surround receptive field amplitudes were strongly affected by global cortical inhibition. A three-way ANOVA for the response magnitude of the SRF whiskers against muscimol/BMC treatment, sublaminar location, and septal/barrel location revealed that the global inhibition of barrel cortex affected the response level of the locally reactivated cells for S1 and S2 whiskers [for S1: F(1,1) = 5.09, P < 0.0001; for S2: F(1,1) = 6.19, P < 0.001]. We also found interactions between global inhibition and both the sublaminar location [for S1: F(1,1) = 2.3, P < 0.025; for S2: F(1,1) = 2.65, P < 0.01] and the septal barrel subdivisions of the cells studied [for S1: F(1,1) = 2.6, P < 0.01; for S2: F(1,1) = 2.3, P < 0.025].
Post hoc t-tests showed that the interactions arose because SRF responses were significantly smaller in the presence of global barrel cortex inhibition in all barrel locations and Vb septal locations, but that S1–S3 were not significantly affected in Va septal locations (Fig. 9) and had responses that were not different from control levels (α = 0.05, Q = 1.97). This implies that a subcortical input generates the first three most powerful SRF inputs to layer Va septal cells, whereas the same inputs are derived intracortically for all other layer V locations.
We tested the possibility that cells that were reactivated more powerfully by BMC might have larger SRF responses by cross-correlating the magnitude of the principal whisker response (which always returned with BMC application), with that of the S1 whisker (Supplemental Fig. S1).1 We found that there was a significant correlation between the size of the principal whisker response and S1 whisker response for cells located in layer Va septal locations (R = 0.594, P < 0.01), but not for any other subdivision of layer V (α = 0.05). This result would be expected if the Va septal cells received a significant CRF and SRF from a subcortical source. The lack of correlation for the other layer V sublocations suggests that in these cases CRF and SRF inputs come from different sources, which given the global activity blockade by muscimol is likely to be subcortical for the principal whisker and intracortical for the surround whiskers.
The next three surround receptive field inputs (S4–S6) were significantly different from control levels in all layer V locations including layer Va septal cells (Fig. 9), again suggesting a cortical origin. The two smallest SRF inputs (S7 and S8) were significantly different for barrel locations, but not at all for Vb septal locations, and S8, not for Va septal locations. It is likely that these responses were in any case too small in control conditions to be sure of a clear effect.
Layer V sensitivity to bicuculline iontophoresis
The reactivation studies described earlier suggest differences between receptive field origins for cells in septal and barrel locations and in particular a difference for layer Va septal cells. To determine whether any of the regional differences might be attributable to differential sensitivity to the GABAA antagonist BMC, we iontophoresed BMC in normal animals in the absence of muscimol. We found that BMC increased surround receptive field whisker responses in both barrel and septal regions (Fig. 11). Principal whisker responses were elevated to values similar to those seen when BMC was iontophoresed in muscimol-treated cortex (compare Tables 2 and 3) except for layer Va septum, where responses increased above levels seen in muscimol-treated cortex. Compared with control levels before BMC application, principal whisker response magnitudes increased to 182.6% in Va barrel, 283.2% in Va septal, 98.5% in Vb barrel, and 179.9% in Vb septal areas. This suggests that layer Va septal regions are most sensitive to the GABAA antagonist and may well be under greater inhibitory control than other layer V subdivisions. Conversely, in Vb barrel locations, the principal whisker and the first two surround whisker responses (S1–S2) showed an increase in response to BMC application up to, but not significantly beyond, control values [PW: t(33) = 0.11, P = 0.91; S1: t(33) = 0.44, P = 0.66; S2: t(33) = 1.8, P = 0.08], indicating reduced sensitivity to BMC and little control from inhibitory connections. This is not to say, however, that the layer Vb cells lack GABAA receptors because their spontaneous activity and sensory responses could be antagonized by muscimol application and reactivated by BMC. In all but one case we noted an increase in spontaneous activity toward the end of the 10-min application of BMC (1/9), so it was clear from spontaneous activity of the cell and the increase in peripheral receptive field whisker responses that the BMC was administered at an effective dose.
Fig. 11.
Effect of BMC on normal otherwise untreated layer V cells. A: responses of cells located in layer Va barrel columns to stimulation of the principal whisker (PW) and surround whiskers (S1–S7) in the presence of bicuculline (BMC, gray bars) or without treatment (control, black bars). B: PW and surround whisker responses increase strongly in the presence of BMC for layer Va septal cells. C: the PW and S1–S2 are not affected by BMC application, although the weaker surround receptive field whiskers are significantly increased (S3–S7). D: layer Vb septal cells are strongly enhanced by BMC treatment. (*P < 0.05, **P < 0.01, t-test). Identical applications were used across all regions, yet cells in Vb barrel regions appear less sensitive to γ-aminobutyric acid type A (GABAA) antagonism than other regions of layer V.
Table 3.
Effect of BMC on cortical receptive field (RF) parameters measured in control untreated animals
| BMC (Muscimol Absent) | Cells (n) | PW Magnitude, spikes/50 stimuli | PW Latency, time/ms | RF Size, Responding Whiskers | Spontaneous Activity, Hz |
|---|---|---|---|---|---|
| Va barrel | 18 | 66.3 ± 8.6 | 13.6 ± 0.4 | 5.7 ± 0.4 | 0.97 ± 0.16 |
| Va septum | 15 | 101.0 ± 14.7 | 12.1 ± 0.4 | 6.1 ± 0.5 | 3.35 ± 0.77 |
| Vb barrel | 9 | 43.0 ± 6.1 | 13.4 ± 0.5 | 5.8 ± 0.4 | 2.41 ± 0.42 |
| Vb septum | 7 | 61.0 ± 9.4 | 12.3 ± 0.6 | 5.9 ± 0.5 | 4.60 ± 1.10 |
Values are means ± SE.
Layer Va septal cells showed the largest reactivation of SRFs under muscimol inhibition and it is therefore possible that some of the recovery of the SRF in muscimol-treated cortex was due to greater relief of inhibition in this subregion. However, the similarity between the reactivated surround receptive fields of layer Va and layer Vb barrel cells (Fig. 9) occurred despite the relative lack of sensitivity of layer Vb barrel cells to BMC compared with Va barrel cells (Fig. 11). Therefore despite the difference in inhibition, layer Vb barrel cells still showed small receptive fields on reactivation when intracortical activity was blocked, suggesting an intracortical origin for their SRFs.
DISCUSSION
One of the main findings of this study is that despite the overtly similar receptive field size, latency, and magnitude of response of cells situated in barrel and septal locations, the two subdivisions receive multiwhisker input from different sources. In general, layer V cells located in any subdivision of layer V have similar receptive fields, comprising seven or eight whiskers. For example, the receptive field size of septal and barrel located cells are the same. Similar receptive field sizes have been found in previous experiments using extracellular (Armstrong-James and Fox 1987) and intracellular recordings (Moore and Nelson 1998; Zhu and Connors 1999). However, although the surround receptive fields are generated almost entirely intracortically for cells beneath the barrel, we found that additional multiwhisker input from subcortical sources contribute to the surround receptive fields in septal locations.
A further finding concerned the origin of center receptive fields of layer V cells. The principal whisker could not easily be predicted from the whisker evoking the largest response because the surround receptive field whiskers usually evoked responses of comparable magnitude, although the principal whisker could be predicted from the whisker evoking the shortest latency response in most cases (70% over all subdivisions of layer V). Short-latency responses (of <10 ms) most probably originate from direct thalamic activation via VPm, rather than intracortical relay (Armstrong-James and Fox 1987). This conclusion is consistent with the finding that most cells showed single whisker receptive fields in the absence of intracortical activity (68%). These findings therefore imply that the center receptive field is at least partly generated from subcortical sources.
A completely unexpected finding concerned the relative paucity of phasic inhibition evoked in the layer Vb cells beneath the barrels compared with cells in layer Va or in septal subdivisions. Iontophoresis of BMC barely affected the responses of layer Vb barrel cells to sensory stimulation of all but the most peripheral aspects of the receptive field (Fig. 11). Similarly, BMC only returned the principal whisker response to control values during muscimol treatment, rather than overactivate it, as occurred for all of layer Va and layer Vb septal cells (Fig. 9). The surround receptive fields of layer V cells have been noted to be relatively less sensitive to BMC application in other studies, although a great deal of variation had been found between cells (Kyriazi et al. 1998). One possibility is that the heterogeneity arises in part from the difference between layer Va and layer Vb. Inhibitory circuits certainly differ between sublaminae because layer Vb pyramidal cells receive input from parvalbumin-positive basket cells lacking cannabinoid receptors, whereas layer Va cells receive input from cholecystokinin (CCK)-positive basket cells with cannabinoid presynaptic receptors (Bodor et al. 2005). A second possibility is that the heterogeneity arises from intrinsic burster (IB) and regular-spiking (RS) cell differences, given that a relative lack of inhibition has previously been noted for IB cells compared with RS cells (Schubert et al. 2001). It remains to be determined whether either of these features is responsible for the lack of phasic inhibition in layer Vb barrel cells and/or the heterogeneity present in layer V.
Subcortical sources of receptive field input to layer V
VPm and POm are the most likely subcortical sources for creating layer V receptive fields in the absence of intracortical activity. VPm and POm preferentially target barrel and septal subdivisions of layer IV, respectively (Koralek et al. 1988; Olavarria et al. 1984). In layer IV, anatomical factors such as the topographic precision of the thalamic axon terminations and the orientation of the stellate cell dendrites away from the barrel wall restrict receptive field size (Jensen and Killackey 1987; Simons and Woolsey 1984; Woolsey et al. 1975), as does the requirement of synchrony for thalamocortical inputs to activate the layer IV neurons (Bruno and Sakmann 2006; Jensen and Killackey 1987; Simons and Woolsey 1984; Woolsey et al. 1975).
The projection to the septal areas arises from POm cells that have multiwhisker receptive fields (Diamond et al. 1992), which might appear to explain why many layer IV septal cells also have multiwhisker receptive fields in the absence of intracortical activity. However, two factors mitigate against this interpretation, at least for this study. First, POm input is stronger in layer Va than it is in layer Vb (Bureau et al. 2006) and yet we found little difference in the receptive fields or origin of receptive fields for cells in these two sublaminae. The second is that POm tends to be relatively quiet in urethane-anesthetized animals (Armstrong-James and Fox 1987). Although POm receives a projection from the brain stem interpolaris nucleus (Jacquin et al. 1989; Veinante et al. 2000) it tends not to respond because GABAergic zona incerta neurons inhibit it (Trageser and Keller 2004). Instead, POm derives much of its input from cortex in the anesthetized preparation (Diamond et al. 1992) and the cortex was of course silenced by muscimol in this study.
This leaves VPm itself as a possible source of the two distinctly different receptive field types in septal and barrel locations. One possibility is that the convergence of thalamic input from different VPm barreloids is greater at the junction of the cortical columns. Although this might explain the double receptive fields seen occasionally, it is unlikely to explain the receptive fields of five or so whiskers that could be reactivated in the absence of global cortical activity. Another possibility is that neurons in the ventrolateral subdivision of VPm (VPMvl) preferentially relay multiwhisker input to the septal cells. The VPMvl cells lie at the tail of the barreloids and receive multiwhisker input from the interpolaris nucleus of the brain stem trigeminal nuclei (Bokor et al. 2008; Pierret et al. 2000). They tend to project preferentially to the septal regions of the barrel cortex, unlike the majority of VPm cells lying in the main core of the barreloids (Bokor et al. 2008), and could therefore provide multiwhisker input in the presence of global cortical inactivation.
Intracortical sources of receptive field input to layer V
Thalamic input is thought to synapse on the basal dendrites of layer V cells (Lu and Lin 1993), although synapses have also been observed on apical dendrites in layer IV (White and Hersch 1982), and it has recently been shown that VPm has a strong input onto the apical dendrite of layer V cells as they run though layer IV (Petreanu et al. 2009). However, the fact that muscimol often blocked center receptive fields before superficially applied muscimol had had time to diffuse to the depth of layer IV, or the basal dendrites in layer V, suggests that a strong intracortical component of excitation is also required in addition to the subcortical component for even the center receptive field to reach threshold. It is possible that the early inhibition of layer V cells by muscimol in superficial layers was partly due to inhibition of the apical dendrites, clamping them at the GABAA reversal potential of approximately −62 mV. However, the estimate that roughly 30% of layer II/III cells within the column provide input to layer V cells supports the idea that synaptic input to layer V from layer II/III is a significant factor (Schubert et al. 2001). The input to layer V from layer II/III cells terminates on both the apical and basal dendrites, but about fivefold as much projects to the basal dendrites (Petreanu et al. 2009). Both sources of input would have been inhibited by muscimol in superficial layers of the cortex.
In the case of global cortical inhibition with muscimol, a response could be generated in layer V by local GABA antagonism with BMC in the absence of layer II/III input. This suggests that the thalamic input to layer V is below threshold unless the local inhibitory circuits are antagonized with BMC. This is consistent with evidence that local inhibitory inputs to layer V cells come from layers IV and V in rat barrel cortex (Schubert et al. 2006). In the normal case, combined thalamic and layer II/III input converge to create a suprathreshold input to the cell.
It may be useful to keep in mind that muscimol can affect GABAB receptors (Yamauchi et al. 2000), which are known to be presynaptic on the thalamocortical afferents in developing (P10–P23) mouse barrel cortex (Porter and Nieves 2004). If these receptors are present on thalamocortical afferents of adult rat cortex, then the thalamic afferents in superficial layers of cortex may also have been inhibited to some extent, which would have led us to underestimate the thalamocortical input to the layer V cells and to overestimate the superficial layer input. Similarly, if the muscimol generally lowers the input resistance of the cells by opening GABA receptors along the dendrites, then the threshold for the thalamic afferents to depolarize the cell would increase, again leading to an underestimate of the thalamocortical input. It is difficult to gauge the extent to which this would be a significant factor because bicuculline iontophoresis would close the GABA receptors in the vicinity of the soma during the measurement of whisker input to the cell.
Layer V cells receive strong connections from neighboring barrels that are different for the two laminar subdivisions. Layer Va cells receive horizontal connections from both layer IV and other layer Va cells (Schubert et al. 2006). Layer Vb cells receive strong input from all layers of the neighboring barrels and especially layer VI (Schubert et al. 2006). In addition, intrinsic burster (IB) cells tend to receive transcolumnar input that is stronger from layers IV and VI than it is for regular-spiking (RS) cells (Schubert et al. 2001). There is therefore ample evidence for the connections by which the intracortical surround receptive fields are generated.
Layer V cells have basal dendrites that can extend into surrounding barrels and thereby form connections with cells in neighboring barrels. Previous studies using intracellular recordings have shown that dendrites of cells located on the edges of barrels that potentially form connections in this way reach into two or three barrels, but that the receptive fields of layer V cells are much larger than this (on average 4.3 whiskers in Ito 1992; 4.97 in this study). This suggests that longer range intracortical connections (i.e., from other barrel columns) are required for creating responses of barrel-located neurons to other whiskers for nonadjacent barrels.
Layer Va and layer Vb subcircuits and cortical receptive field properties
Layer Va and layer Vb project to different subcortical targets and therefore might be expected to process different types of information within the cortex. Layer Va cells project to the striatum, whereas layer Vb cells project back to the thalamus and to the pons (Mercier et al. 1990). In the mouse barrel cortex, there is evidence that VPm tends to project to layer Vb cells, whereas POm projects to layer Va cells (Bureau et al. 2006). However, in the rat cortex the distinction is not as clear and anatomical studies suggest that POm projects to both layer Va and layer Vb (Lu and Lin 1993). Layer Vb neurons have the opportunity to contact VPm axons. The core and tail components of VPm project to the top of layer VI, where they might contact descending layer Vb basal dendrites (Pierret et al. 2000). In spite of these facts, we were unable to distinguish any major differences between the subcortical receptive field components in the two sublayers of layer V. Layer Va and layer Vb cells had small subcortically generated receptive fields if they lay below the barrel and multiwhisker subcortically generated receptive fields if they lay below the septae. It is conceivable that our depth measurements were not accurate enough to distinguish cells close to the Va/Vb border and that inclusion of such cells tended to blur any differences that might have been present. However, when we specifically tested this possibility by excluding cells within 100 μm of the border we still saw no difference between sublaminar receptive field properties. Previous studies have been able to distinguish a smaller receptive field size in layer Va than that in layer Vb neurons (de Kock et al. 2007; Manns et al. 2004). The latter studies were performed on younger animals (25–31 days) than those used here (42–91 days) and it is conceivable that the layer Va receptive fields increase in size during this period of development.
Although the general size of receptive fields were the same in layer Va and in layer Vb, temporal receptive field properties may differ substantially between the layers. For example, during active whisking layer Va cells show a substantial increase in latency to whisker stimulation between the first and third whisk cycles, whereas layer Vb cells’ response latencies remain constant, in common with layer IV (Ahissar et al. 2001). Furthermore, layer Va neurons show a frequency-dependent phase lag at steady state, which could allow them to operate as a phase-locked loop and decode whisker position (Ahissar et al. 2001). The different dynamic properties of layer Va versus layer Vb have been attributed to the different major sources of their input from POm and VPm, respectively, and their different roles in whisker movement versus whisker touch (Alloway 2008; Yu et al. 2006). It is possible that these layer properties are more extreme between barrel and septal locations than those between one layer and another, which is why we are able to detect differing levels of subcortical input to septal barrel than to sublayers of layer V. Recent anatomical studies suggest that the whisker-movement circuits that communicate bilaterally and with primary motor cortex are more closely associated with septal circuits than with a particular sublamina of layer V (Alloway 2008; Chakrabarti and Alloway 2006).
Evidence for parallel channels within barrel cortex
The inputs and connectivity of septal and barrel subcircuits differ. Septal cells connect widely with one another over several barrels, whereas the barrel connectivity is largely confined to a single barrel, with some sparse interbarrel connectivity (Hoeflinger et al. 1995; Kim and Ebner 1999; Schubert et al. 2001, 2003). Cells in a barrel derive their surround receptive fields from within the cortex, whereas septal cells receive some multiwhisker input from subcortical sources (Fox et al. 2003). Columnar projections from layer IV project to layers II/III and thereby appear to continue the septal barrel distinctions (Brecht et al. 2003), albeit blurred by overlapping axon projections (Bureau et al. 2004). The present studies suggest that the septal/barrel subdivision of circuitry then continues further into layer V. There are two direct columnar pathways that might accomplish this, one from layer II/III to layer V and one from layer IV to layer V. Consistent with this idea the layer V responses rely on layer II/III input and layer II/III cells have different properties depending on their septal or barrel location (Brecht et al. 2003). Similarly, the layer IV projection to layer Va (Feldmeyer et al. 2005; Schubert et al. 2006) would convey intracortically generated surround whisker information from layer IV and subcortical multiwhisker information from the septum (Fox et al. 2003). Projections from layer V to other cortical areas continue the separate channels of information. Septal columns preferentially connect with motor cortex and project bilaterally, whereas barrel columns do not (Alloway et al. 2004; Olavarria et al. 1984). The present studies therefore provide evidence that a considerable degree of multiwhisker input of subcortical origin is processed in septally located layer V cells and subsequently communicated with motor cortex, SII and SI bilaterally (Alloway et al. 2004). The barrel-located layer V cells also provide their targets in SII (Chakrabarti and Alloway 2006) and subcortical locations (Jacquin et al. 1990) with multiwhisker information, but as we show in this study, the difference with the septally located cells is that the surround receptive field of barrel circuit cells is almost entirely generated from intracortical rather than subcortical processing.
GRANTS
This work was supported by the Medical Research Council, United Kingdom, and the Silvio A. Conte Center at Johns Hopkins University School of Medicine, funded by the National Institute of Mental Health.
Supplementary Material
ACKNOWLEDGMENTS
We thank V. Jacob for critically reading the manuscript and helping with aspects of the analysis.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Ahissar E, Sosnik R, Bagdasarian K, Haidarliu S. Temporal frequency of whisker movement. II. Laminar organization of cortical representations. J Neurophysiol 86: 354–367, 2001 [DOI] [PubMed] [Google Scholar]
- Alloway KD. Information processing streams in rodent barrel cortex: the differential functions of barrel and septal circuits. Cereb Cortex 18: 979–989, 2008 [DOI] [PubMed] [Google Scholar]
- Alloway KD, Zhang M, Chakrabarti S. Septal columns in rodent barrel cortex: functional circuits for modulating whisking behavior. J Comp Neurol 480: 299–309, 2004 [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol 263: 265–281, 1987 [DOI] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci 25: 6845–6856, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokor H, Acsady L, Deschênes M. Vibrissal responses of thalamic cells that project to the septal columns of the barrel cortex and to the second somatosensory area. J Neurosci 28: 5169–5177, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Roth A, Sakmann B. Dynamic receptive fields of reconstructed pyramidal cells in layers 3 and 2 of rat somatosensory barrel cortex. J Physiol 553: 243–265, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312: 1622–1627, 2006 [DOI] [PubMed] [Google Scholar]
- Bureau I, Shepherd GM, Svoboda K. Precise development of functional and anatomical columns in the neocortex. Neuron 42: 789–801, 2004 [DOI] [PubMed] [Google Scholar]
- Bureau I, von Saint Paul F, Svoboda K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol 4: e382, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Alloway KD. Differential origin of projections from SI barrel cortex to the whisker representations in SII and MI. J Comp Neurol 498: 624–636, 2006 [DOI] [PubMed] [Google Scholar]
- de Kock CP, Bruno RM, Spors H, Sakmann B. Layer- and cell-type-specific suprathreshold stimulus representation in rat primary somatosensory cortex. J Physiol 581: 139–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. J Comp Neurol 318: 462–476, 1992 [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Roth A, Sakmann B. Monosynaptic connections between pairs of spiny stellate cells in layer 4 and pyramidal cells in layer 5A indicate that lemniscal and paralemniscal afferent pathways converge in the infragranular somatosensory cortex. J Neurosci 25: 3423–3431, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. The cortical component of experience-dependent synaptic plasticity in the rat barrel cortex. J Neurosci 14: 7665–7679, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Armstrong-James M. The role of the anterior intralaminar nuclei and N-methyl D-aspartate receptors in the generation of spontaneous bursts in rat neocortical neurones. Exp Brain Res 63: 505–518, 1986 [DOI] [PubMed] [Google Scholar]
- Fox K, Wright N, Wallace H, Glazewski S. The origin of cortical surround receptive fields studied in the barrel cortex. J Neurosci 23: 8380–8391, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol 81: 2243–2252, 1999 [DOI] [PubMed] [Google Scholar]
- Hoeflinger BF, Bennett-Clarke CA, Chiaia NL, Killackey HP, Rhoades RW. Patterning of local intracortical projections within the vibrissae representation of rat primary somatosensory cortex. J Comp Neurol 354: 551–563, 1995 [DOI] [PubMed] [Google Scholar]
- Hoffer ZS, Arantes HB, Roth RL, Alloway KD. Functional circuits mediating sensorimotor integration: quantitative comparisons of projections from rodent barrel cortex to primary motor cortex, neostriatum, superior colliculus, and the pons. J Comp Neurol 488: 82–100, 2005 [DOI] [PubMed] [Google Scholar]
- Ito M. Simultaneous visualization of cortical barrels and horseradish peroxidase-injected layer 5b vibrissa neurones in the rat. J Physiol 454: 247–265, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin MF, Barcia M, Rhoades RW. Structure–function relationships in rat brainstem subnucleus interpolaris: IV. Projection neurons. J Comp Neurol 282: 45–62, 1989 [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Wiegand MR, Renehan WE. Structure–function relationships in rat brain stem subnucleus interpolaris. VIII. Cortical inputs. J Neurophysiol 64: 3–27, 1990 [DOI] [PubMed] [Google Scholar]
- Jensen KF, Killackey HP. Terminal arbors of axons projecting to the somatosensory cortex of the adult rat. I. The normal morphology of specific thalamocortical afferents. J Neurosci 7: 3529–3543, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, Ebner FF. Barrels and septa: separate circuits in rat barrels field cortex. J Comp Neurol 408: 489–505, 1999 [PubMed] [Google Scholar]
- Koralek KA, Jensen KF, Killackey HP. Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex. Brain Res 463: 346–351, 1988 [DOI] [PubMed] [Google Scholar]
- Kyriazi H, Carvell GE, Brumberg JC, Simons DJ. Laminar differences in bicuculline methiodide's effects on cortical neurons in the rat whisker/barrel system. Somatosens Mot Res 15: 146–156, 1998 [DOI] [PubMed] [Google Scholar]
- Leergaard TB, Alloway KD, Mutic JJ, Bjaalie JG. Three-dimensional topography of corticopontine projections from rat barrel cortex: correlations with corticostriatal organization. J Neurosci 20: 8474–8484, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SM, Lin RC. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res 10: 1–16, 1993 [DOI] [PubMed] [Google Scholar]
- Manns ID, Sakmann B, Brecht M. Sub- and suprathreshold receptive field properties of pyramidal neurones in layers 5A and 5B of rat somatosensory barrel cortex. J Physiol 556: 601–622, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier BE, Legg CR, Glickstein M. Basal ganglia and cerebellum receive different somatosensory information in rats. Proc Natl Acad Sci USA 87: 4388–4392, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CI, Nelson SB. Spatiotemporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J Neurophysiol 80: 2882–2892, 1998 [DOI] [PubMed] [Google Scholar]
- Olavarria J, Van Sluyters RC, Killackey HP. Evidence for the complementary organization of callosal and thalamic connections within rat somatosensory cortex. Brain Res 291: 364–368, 1984 [DOI] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature 457: 1142–1145, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierret T, Lavallee P, Deschênes M. Parallel streams for the relay of vibrissal information through thalamic barreloids. J Neurosci 20: 7455–7462, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, Nieves D. Presynaptic GABAB receptors modulate thalamic excitation of inhibitory and excitatory neurons in the mouse barrel cortex. J Neurophysiol 92: 2762–2770, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Kotter R, Luhmann HJ, Staiger JF. Morphology, electrophysiology and functional input connectivity of pyramidal neurons characterizes a genuine layer Va in the primary somatosensory cortex. Cereb Cortex 16: 223–236, 2006 [DOI] [PubMed] [Google Scholar]
- Schubert D, Kotter R, Zilles K, Luhmann HJ, Staiger JF. Cell type-specific circuits of cortical layer IV spiny neurons. J Neurosci 23: 2961–2970, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Staiger JF, Cho N, Kotter R, Zilles K, Luhmann HJ. Layer-specific intracolumnar and transcolumnar functional connectivity of layer V pyramidal cells in rat barrel cortex. J Neurosci 21: 3580–3592, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Woolsey TA. Morphology of Golgi-Cox-impregnated barrel neurons in rat SmI cortex. J Comp Neurol 230: 119–132, 1984 [DOI] [PubMed] [Google Scholar]
- Trageser JC, Keller A. Reducing the uncertainty: gating of peripheral inputs by zona incerta. J Neurosci 24: 8911–8915, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Lavallee P, Deschênes M. Corticothalamic projections from layer 5 of the vibrissal barrel cortex in the rat. J Comp Neurol 424: 197–204, 2000 [DOI] [PubMed] [Google Scholar]
- Welker E, Hoogland PV, Van der Loos H. Organization of feedback and feedforward projections of the barrel cortex: a PHA-L study in the mouse. Exp Brain Res 73: 411–435, 1988 [DOI] [PubMed] [Google Scholar]
- White EL, Hersch SM. A quantitative study of thalamocortical and other synapses involving the apical dendrites of corticothalamic projection cells in mouse SmI cortex. J Neurocytol 11: 137–157, 1982 [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res 171: 11–28, 1979 [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Dierker ML, Wann DF. Mouse SmI cortex: qualitative and quantitative classification of Golgi-impregnated barrel neurons. Proc Natl Acad Sci USA 72: 2165–2169, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AK, Norrie L, Arbuthnott GW. Corticofugal axons from adjacent “barrel” columns of rat somatosensory cortex: cortical and thalamic terminal patterns. J Anat 196: 379–390, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AK, Norrie L, Ingham CA, Hutton EA, Arbuthnott GW. Double anterograde tracing of outputs from adjacent “barrel columns” of rat somatosensory cortex. Neostriatal projection patterns and terminal ultrastructure. Neuroscience 88: 119–133, 1999 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Hori T, Takahashi T. Presynaptic inhibition by muscimol through GABAB receptors. Eur J Neurosci 12: 3433–3436, 2000 [DOI] [PubMed] [Google Scholar]
- Yu C, Derdikman D, Haidarliu S, Ahissar E. Parallel thalamic pathways for whisking and touch signals in the rat. PLoS Biol 4: e124, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Connors BW. Intrinsic firing patterns and whisker-evoked synaptic responses of neurons in the rat barrel cortex. J Neurophysiol 81: 1171–1183, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.