Abstract
Desert locusts (Schistocerca gregaria) can transform reversibly between the swarming gregarious phase and a solitarious phase, which avoids other locusts. This transformation entails dramatic changes in morphology, physiology, and behavior. We have used the lobula giant movement detector (LGMD) and its postsynaptic target, the descending contralateral movement detector (DCMD), which are visual interneurons that detect looming objects, to analyze how differences in the visual ecology of the two phases are served by altered neuronal function. Solitarious locusts had larger eyes and a greater degree of binocular overlap than those of gregarious locusts. The receptive field to looming stimuli had a large central region of nearly equal response spanning 120° × 60° in both phases. The DCMDs of gregarious locusts responded more strongly than solitarious locusts and had a small caudolateral focus of even further sensitivity. More peripherally, the response was reduced in both phases, particularly ventrally, with gregarious locusts showing greater proportional decrease. Gregarious locusts showed less habituation to repeated looming stimuli along the eye equator than did solitarious locusts. By contrast, in other parts of the receptive field the degree of habituation was similar in both phases. The receptive field organization to looming stimuli contrasts strongly with the receptive field organization of the same neurons to nonlooming local-motion stimuli, which show much more pronounced regional variation. The DCMDs of both gregarious and solitarious locusts are able to detect approaching objects from across a wide expanse of visual space, but phase-specific changes in the spatiotemporal receptive field are linked to lifestyle changes.
INTRODUCTION
The Desert Locust (Schistocerca gregaria Forskål) can reversibly transform between two forms or phases, depending on environmental conditions (Anstey et al. 2009; Simpson et al. 1999; Uvarov 1966, 1977). The two phases differ considerably in morphology, physiology, and particularly behavior, which lead to dramatic changes in their visual ecology (Matheson et al. 2004; Rogers et al. 2004, 2007). Gregarious locusts, which are notorious for their swarming, occur at high population densities (≤100,000/100 m3 in flight; Uvarov 1977). They are highly active and form cohesive groups so that, by definition, their visual environment is dominated by the presence of other locusts, many of which will be moving. Conversely, solitarious phase locusts actively shun each other and occur at correspondingly low population densities (<3/100 m2). Their cryptic behavior and green or brown coloration allows them to hide from potential predators.
Appropriate behavioral responses to visual stimuli are essential to the survival of most animals and the properties of photoreceptors and interneurons are specifically tuned to sense behaviorally relevant visual stimuli (Egelhaaf et al. 2002; Laughlin and Weckström 1993; O'Carroll et al. 1996; Sherk 1978). The dramatic change in lifestyle that locusts can undergo makes them a powerful model for analyzing how neuronal properties may alter in response to a changing visual environment. Collision avoidance and predator evasion in response to looming stimuli are important in a range of species across several phyla (Gabbiani et al. 1999; Hatsopoulos et al. 1995; Preuss et al. 2006; Rind and Simmons 1992, 1999; Sherk and Fowler 2001; Simmons and Rind 1992, 1999; Sun and Frost 1998; Yamamoto et al. 2003). A key element of the neuronal pathway underlying this behavior in locusts is a looming detector neuron, the lobula giant movement detector (LGMD) (Gabbiani et al. 2004; Judge and Rind 1997; Rind and Simmons 1992; Schlotterer 1977). The LGMD responds most vigorously to looming objects on a direct collision course with the locust, which expand approximately exponentially across the retina as they approach (Gabbiani et al. 1999, 2002; Hatsopoulos et al. 1995; Rind and Simmons 1992, 1997; Schlotterer 1977; Simmons and Rind 1992). The LGMD shows much weaker responses to nonlooming stimuli (Krapp and Gabbiani 2005; Peron and Gabbiani 2009; Simmons and Rind 1992) or to objects looming on a noncollision trajectory (Gray et al. 2001; Judge and Rind 1997). In gregarious locusts the LGMD makes a synaptic connection with a 1:1 spike transfer gain onto the descending contralateral movement detector (DCMD), which thus exactly copies the LGMD's spiking pattern (O'Shea and Williams 1974; Rind 1984). The DCMD conveys this information from the brain to the motor centers of the thorax (Pearson and Goodman 1979; Simmons 1980), where it may have a role in initiating flight-avoidance responses (Santer et al. 2005, 2006) and other escape or avoidance behaviors (Fotowat and Gabbiani 2007; Santer et al. 2008). The LGMD shows all the characteristics of habituation (as defined by Rankin et al. 2009). LGMD shows a progressive decrease in response to an asymptotic level following repeated stimulation, depending on the interstimulus interval. It also shows a recovery in response if a strong stimulus, either visual or nonvisual, is administered, i.e., it exhibits dishabituation (Bacon et al. 1995; Edwards 1982; Gray 2005; Matheson et al. 2004; Rind et al. 2008; Rowell 1971a).
The DCMDs of gregarious locusts display higher peak-firing rates and a greater resistance to habituation than those of solitarious locusts in the center of the receptive field (Matheson et al. 2004; Rogers et al. 2007). Analyses of the response properties of the DCMD using local-motion stimuli have indicated that its receptive fields cover most of the visual space around the locust (Rowell 1971b); however, no previous study has analyzed how the responsiveness of the DCMD to looming stimuli varies across the neurons' entire receptive field in either phase. In our study we systematically presented looming stimuli originating from many positions in space to both solitarious and gregarious locusts. We show that the two phases differ in eye size, position of the eyes on the head, and in binocular overlap and that there are phase-specific differences in response and susceptibility to habituation in certain parts of the receptive field. Nevertheless, both phases share a large region of nearly equal responsiveness spanning a large part of the receptive field.
This contrasts strongly with the receptive field of the LGMD–DCMD to purely local-motion stimuli (i.e., dots rotating around a small region of space) (Krapp and Gabbiani 2005), which shows a considerable spatial variation in response. We show how the response profile of the DCMD across their receptive field is strikingly different depending on the stimulus used.
METHODS
Animal rearing
Experiments were performed on Desert Locusts (Schistocerca gregaria Forskål) of both sexes 1–2 wk after they had molted to adulthood. Gregarious-phase locusts were obtained from a culture at the Department of Zoology, University of Cambridge, where they are maintained under high population densities (∼3,000/m3) and fed on seedling wheat and wheat bran flakes. These gregarious animals were maintained under a 12:12-h light:dark photoperiod, 37°C during the light period and 25°C during the dark period.
The solitarious phase animals were each reared in individual cages under visual, olfactory, and tactile isolation from other locusts (apart from breeding) for three generations, using husbandry procedures described by Roessingh et al. (1993). They had strongly solitarious morphological, physiological, and behavioral phenotypes, some of which take more than one generation of isolation to develop (Simpson et al. 1999). Solitarious locusts used for the eye measurements were obtained by isolating animals from the University of Cambridge gregarious colony for three generations under the same photoperiod and temperature regime. These solitarious and gregarious locusts were therefore genetically similar and differences in head morphology can thus be ascribed to phase and not strain differences.
The physiological experiments were performed earlier, at a time when the only source of solitarious locusts was from a colony maintained at the Department of Zoology, University of Oxford. These locusts were descended from a gregarious colony at Oxford. Our previous work established that there were no differences in DCMD responsiveness between the gregarious populations from Cambridge and Oxford (Matheson et al. 2004). The Oxford solitarious animals were reared in the same cages and using the husbandry procedures later adopted at Cambridge, under a 12:12-h photoperiod and a constant temperature of 30 ± 2°C.
Binocular overlap measurements
We measured the binocular overlap between the two compound eyes of five solitarious and six gregarious male locusts using a Zeiss goniometer. The locusts were immobilized by wrapping the body tightly in adhesive tape and their heads were fixed in a hole made in the center of a microscope slide using bees' wax. The slide and locust were positioned in the goniometer and the head was centered in the yaw axis. The pitch axis was centered on the eye equator. The deep pseudopupils of the eyes were examined under a Leica MZ16 dissecting microscope. The binocular overlap was measured as the yaw angle through which the locust had to be rotated until the center of one of its pseudopupils had reached the edge of the eye. The overlap was measured at elevations from +80 to −80°, at intervals of 5° in the range +60 to −60° and at 10° intervals outside this range (where 0° elevation corresponds to the eye equator). To compensate for small differences in the way individual locusts were positioned in the goniometer, data from some animals were shifted by up to ±5° (one measurement step) to minimize differences between animals (judged by obtaining the smallest sum of mean squares). This ensured the best alignment of results obtained from different individuals and reduced apparent changes in the binocular overlap produced through statistical variation in position.
Dissection and recording
The legs of locusts were removed and the wounds sealed with bees' wax. The locusts were then mounted ventral side uppermost in a plastic holder using wax and adhesive tape. Bees' wax was used to fix the position the head so that the long axis of the compound eye was vertical in both pitch and yaw and the cervical cuticle stretched. After exposing the ventral nerve cord, a pair of bipolar 50-μm-diameter silver hook electrodes were inserted under the right cervical connective and insulated using a 10:1 petroleum jelly:liquid paraffin mixture. DCMD spikes were recorded using an AC amplifier (×1,000 amplification; bandwidth: 50–10,000 Hz) designed and built at the University of Cambridge. The DCMD has the largest amplitude action potentials in extracellular recordings from a cervical connective and has characteristic response properties (Burrows and Rowell 1973), so it was thus readily identifiable in both solitarious and gregarious locusts (Matheson et al. 2004). There was occasionally some spike-amplitude attenuation during periods of rapid firing due to destructive interference between the waveforms of successive spikes as they passed each hook electrode. Thresholds of selection were chosen on a trial-by-trial basis to accommodate this variation in amplitude during an approach and the longer-term changes in recording quality that are inevitable during long recordings over the course of >3.5 h.
The locust in its holder was positioned on a stand so that the left eye was at the precise center of a semicircular metal meridian rack (50-cm diameter), which held a cathode ray tube (CRT) monitor that displayed the visual stimulus. The meridian rack could be moved around a vertical rotation axis through azimuths of 0–180° relative to the locust, where 0° was directly in front of the animal. The CRT mounted on the rack could be positioned at different elevations ranging from −70 to +75°, where 0° was horizontally level with the locust, thus permitting us to test the response to looming stimuli across almost the entire visual field of the eye.
Visual stimulation
The visual stimulation protocol was derived from that of Gabbiani et al. (1999). The Tektronix CRT (model 608) was fixed in the meridian rack such that its 100 × 120-mm screen was 68 mm from the center of the locust's head in all stimulus positions. The alignment of the locust's head with the center of the meridian rack meant that the screen was always oriented in parallel with a tangent plane defined by the normal to the surface of the right eye. The CRT had a refresh rate of 185 Hz and a spatial resolution of 0.48°/pixel at 60-mm distance, well above the temporal resolution (Howard 1981) and below the spatial resolution of the locust compound eyes (Krapp and Gabbiani 2005; Wilson 1975). The looming stimuli on the CRT were generated by a Picasso image synthesizer (Innisfree, Cambridge, MA), externally controlled by a computer via the digital–analog output of a Micro-1401 data acquisition system (Cambridge Electronic Design [CED], Cambridge, UK).
The stimulus simulated the last 5 s of approach before collision of a dark square object against a light background (52% contrast). The object appeared in the center of the screen, subtending an initial angle of 1.1°, and then expanded symmetrically as if it were approaching the locust. Because the rack limited how close the monitor could be positioned to the eye, the maximum visual angle subtended by the stimulus at its point of closest simulated approach was 50°, rather than the 80° used in our previous study (Matheson et al. 2004). This may have prevented the full activation of feedforward inhibition and thus slightly prolonged responses (Gabbiani et al. 2005). On reaching maximum extent the stimulus remained static for 500 ms then disappeared. We used a single stimulus with an l/|v| ratio of 30 ms, where l is the half size of the object and v is the approach velocity of the object, which by convention is negative for approaching objects (Gabbiani et al. 1999). This corresponds to, for example, a 120-mm-width object approaching at a velocity of 2 m/s or, conversely, a 50-mm object approaching at 0.83 m/s.
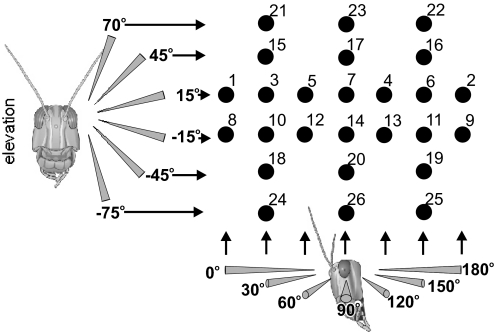
Locusts were stimulated with objects approaching from each of 26 positions ranging across 180° of azimuth and 145° of elevation (Fig. 1). Each locust was stimulated in each position in the same order, as indicated by the numbers in Fig. 1. Five approaching stimuli were presented at 40-s intervals at each position, before the CRT was repositioned. The stimulus interval was chosen following on from previous studies fully characterizing DCMD habituation in both phases to stimuli directed to the center of the eye (Matheson et al. 2004; Rogers et al. 2007; effect of interstimulus interval in gregarious locusts in Gray 2005). The 40-s interval was chosen as one that was likely to induce strong habituation in solitarious locusts, but only moderate habituation in gregarious locusts (which show little habituation at intervals >60 s). The aim was not to fully characterize habituation in this study (which would require ∼30 stimulus presentations at each location; Matheson et al. 2004), but to deliver enough stimuli to identify differences in the strength of habituation across the receptive field.
Fig. 1.
The array of 26 positions used to characterize the receptive field of the descending contralateral movement detector (DCMD). The diagrams of the locust heads show the directions of approach toward the eye in both elevation (left) and azimuth (below), ranging from directly in front of the locust's head (0° azimuth) to directly behind (180° azimuth) and from −75° elevation to +70° elevation, where 0° is at the eye equator. The numbers correspond to the stimulus presentation order.
There was a 5-min gap between the CRT being repositioned and the start of the next set of stimuli to allow the animal to dishabituate. The observed patterns of responsiveness bore no obvious relationship to the temporal order of stimulation and there was no evidence of a progressive change in responsiveness over the course of the experiment [repeated-measures ANOVA using stimulus order as a covariate; F(9,16) = 1.494, P = 0.232].
Data analysis
The analysis is based on data gathered from five gregarious and five solitarious phase locusts using an automated system programmed in Matlab (The MathWorks) and analyzed off-line using Spike2 (CED). DCMD spikes were isolated by applying a threshold to the extracellular recording traces and the number of spikes and their times relative to collision (which occurred at time = 0) were extracted. DCMD's firing rate was smoothed by convolving the spikes with a Gaussian filter (SD = 15 ms), which deemphasizes jitter in the instantaneous rate and gives a better indication of the underlying buildup of response to the stimuli (Gabbiani et al. 1999). Peak DCMD spike frequencies and the time of peak firing relative to collision were obtained from the convolved data.
Numerical data were analyzed statistically in SPSS (versions 10–16). Contour plots of the data were produced in Sigma Plot (version 11). Rates of decline in responsiveness following repeated stimulation were approximately linear for the first three stimuli presented in these experiments and therefore rates of habituation could be compared by fitting linear regressions to the data. Unless otherwise stated, all values quoted are means ± SE.
RESULTS
Solitarious and gregarious locust eyes differ in size, position, and binocular overlap
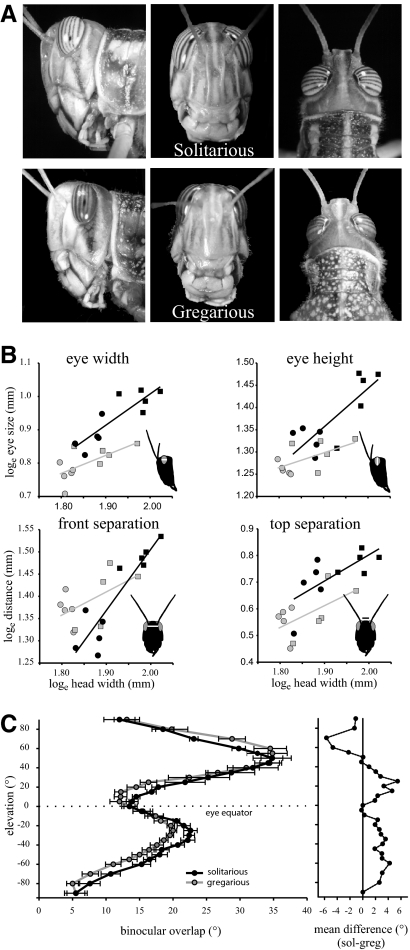
The size, position, and binocular overlap of the eyes of gregarious and solitarious locusts were compared since this could affect the receptive field properties of the LGMD. Locusts with larger heads had larger eyes, but solitarious locusts had larger eyes than those of gregarious locusts in both horizontal and vertical dimensions for any given head size (Fig. 2, A and B; Table 1). Solitarious locust eyes had an average dorsal–ventral height of 4 ± 0.08 mm and a rostral–caudal width of 2.5 ± 0.06 mm (n = 10), compared with an average eye height and width of 3.6 ± 0.04 and 2.2 ± 0.03 mm, respectively, in gregarious locusts (n = 10), a roughly 12% difference. Female locusts had larger eyes than those of males in both phases, but there was no difference in the size of the eyes between the sexes that could not be explained through differences in body size (Table 1). The eyes of solitarious locusts did not extend as far across the top of the head as they do in gregarious locusts, leading to a larger dorsal separation between them (distance between the top of the eyes: 2.08 ± 0.06 mm in solitarious and 1.78 ± 0.05 mm in gregarious locusts; Fig. 2B, Table 1). The anterior separation between the eyes at the level of the base of the antennae varied according to both phase and sex (sex × phase interaction term in Table 1). Gregarious locusts had a larger anterior gap between the eyes than that of solitarious locusts, but this difference was particularly pronounced in male gregarious locusts (Fig. 2B, Table 1). Male solitarious locusts had a frontal gap of 3.72 ± 0.07 mm, but in gregarious males the distance was 3.94 ± 0.06 mm, a 6% difference.
Fig. 2.
The eyes of gregarious and solitarious locusts differ in size, position, and binocular overlap. A: lateral, frontal, and dorsal views of the heads of male solitarious (top) and gregarious (bottom) locusts. Solitarious locusts have larger eyes than those of gregarious locusts. B: eye size and placement parameters plotted against maximum head width (gena–gena) in male (circle) and female (square), solitarious (black) and gregarious (gray) locusts. See Table 1 for analysis. Lines are linear regressions fitted to the data. C: binocular overlap plotted against elevation for solitarious (black) and gregarious (gray) locusts, where 0° elevation corresponds to the eye equator. The mean difference between phases (solitarious − gregarious) is shown to the right. Data are means ± SE, n = 5 locusts solitarious and 6 gregarious.
Table 1.
Results of general linear models analyzing eye size and position parameters depending on head size, phase (solitarious or gregarious), and sex
| Dependent Variable | Head Width | Phase | Sex | Sex × Phase |
|---|---|---|---|---|
| Eye width | F(1,15) = 43.03 | F(1,15) = 9.08 | F(1,15) = 0.12 | F(1,15) = 0.40 |
| P < 0.001 | P = 0.009 | P = 0.737 | P = 0.537 | |
| Eye height | F(1,15) = 67.51 | F(1,15) = 11.99 | F(1,15) = 0.74 | F(1,15) = 0.19 |
| P < 0.001 | P = 0.003 | P = 0.413 | P = 0.669 | |
| Dorsal separation | F(1,15) = 31.75 | F(1,15) = 6.53 | F(1,15) = 1.75 | F(1,15) = 0.06 |
| P < 0.001 | P = 0.022 | P = 0.206 | P = 0.808 | |
| Anterior separation | F(1,15) = 35.03 | F(1,15) = 6.47 | F(1,15) = 0.136 | F(1,15) = 11.41 |
| P < 0.001 | P = 0.023 | P = 0.717 | P = 0.004 |
Significant results (α < 0.05) are shown in bold type.
The pattern of binocular overlap at different elevations (Fig. 2C) was broadly similar in locusts of both phases, but nevertheless differed significantly in detail [repeated-measures ANOVA based on mean binocular overlap across the entire elevation range, effect of phase; F(1,9) = 5.27, P = 0.047]. Binocular overlap was similar in both phases directly in front of the animal (0° elevation; mean overlap 13.5 ± 0.82° in solitarious, 13.5 ± 1.15° in gregarious). The binocular overlap increased ventrally, reaching a maximum at −25° elevation in both phases, where solitarious locusts displayed a 10% greater overlap than that of gregarious locusts (22.6 ± 1.0° in solitarious and 20 ± 0.56° in gregarious; t-test, t9 = 2.38, P = 0.041). Below −25° elevation, the binocular overlap decreased again, with solitarious locusts having greater overlap than that of gregarious locusts at the same elevation (Fig. 2C). At elevations above the eye equator in the range of +10 to 25° the binocular overlap increased in both phases, with greater values again found in solitarious locusts (a 28% difference at +15°, overlap 16.8 ± 0.7° in solitarious and 12.2 ± 0.92° in gregarious; t-test, t9 = 3.83, P = 0.004). The binocular overlap increased substantially up to a maximum at +50°, where the overlap was 34.8 ± 2.8° in solitarious and 34.8 ± 1.7° in gregarious locusts. Both phases showed similar binocular overlaps in the range +35 to 55° elevation. At higher elevations the degree of binocular overlap decreased in both phases, but in this region gregarious locusts showed greater overlaps than those of solitarious locusts, possibly reflecting the smaller distance between the eyes of gregarious locusts on the top of the head (at +70°, there was a 21% difference between phases; overlap was 23.1 ± 0.64° in solitarious, 28.8 ± 2.2° in gregarious; t-test, t8 = −2.49, P = 0.037).
Receptive field of DCMD to looming stimuli
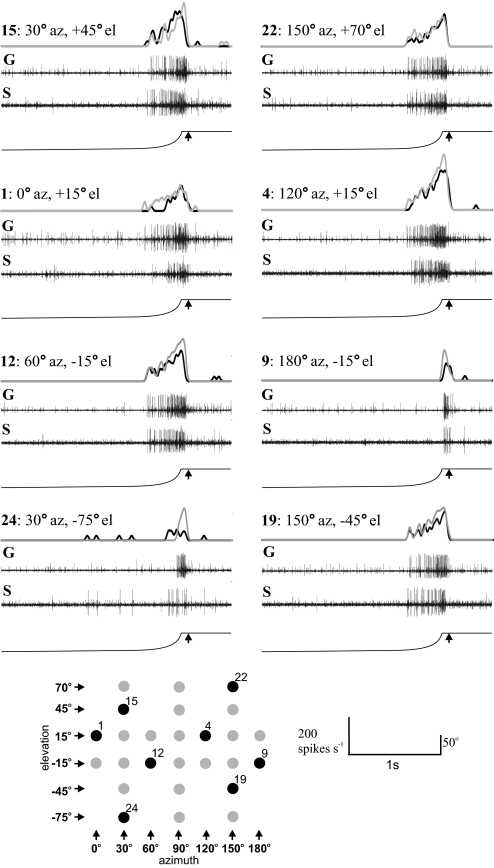
When a looming object approached a locust, the DCMD responded by producing a burst of spikes with an escalating frequency at all locations across the eye (Fig. 3). The receptive field of the DCMD was broadly similar in form for both phases, but differed quantitatively. Numbers of spikes elicited by the looming stimulus and the peak firing rate differed significantly with approach direction, repeated stimulation, and between phases [repeated-measures ANOVA for number of spikes: position of stimulus: F(9.04,361.4) = 125, P < 0.001; for repeated stimulation: F(4,40) = 8.30, P < 0.001; and between phases: F(1,40) = 11.56, P = 0.002. For peak firing rate: position of stimulus: F(7.9,369.4) = 14.29, P < 0.001; for repeated stimulation: F(4,40) = 4.59, P = 0.004; and between phases: F(1,40) = 22.10, P < 0.001]. In most regions of the receptive field, DCMDs of gregarious locusts produced more spikes and had greater peak-firing rates than did those of solitarious locusts, particularly in the more central parts of the receptive field.
Fig. 3.
Representative responses of DCMD to looming stimuli presented across visual space, shown by the black positions in the array beneath. At each position, the bottom trace shows the angular extent of the visual stimulus, with the simulated time of collision marked by an arrow. Above this are recordings taken from a single solitarious locust (S) and a single gregarious locust (G) showing the spiking response of DCMD. The top trace shows the spiking responses convolved with a Gaussian filter from both the solitarious (black) and gregarious (gray) locusts. These have been overlaid to allow comparison of the response in the 2 phases. Each position is indicated in both azimuth (az) and elevation (el).
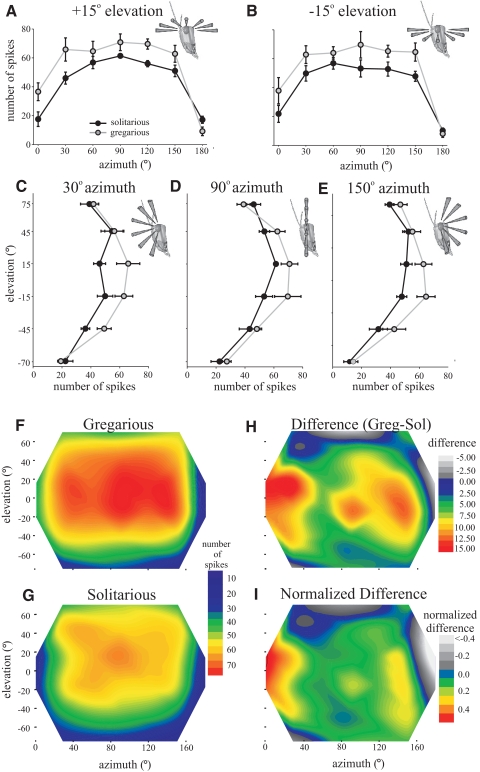
Number of evoked spikes
The number of spikes elicited per approach was similar across a large part of the receptive field in both phases. Near the eye equator (elevations of −15 and +15°) there were no significant differences in numbers of spikes evoked by approaches from 30 to 150° azimuth for either phase (Fig. 4, A and B; means ± SE plotted from 0 to 180 ° azimuth at +15 and −15° elevations), producing a flat region of similar response. Solitarious locusts produced on average 19.7% fewer spikes than did gregarious locusts across this region, with 53 ± 1.3 and 66 ± 1.8 spikes, respectively, being elicited during the first approach (Fig. 4, A and B; t8 = 2.7, P = 0.028). Contour plots show the mean numbers of spikes in gregarious (Fig. 4F) and solitarious locusts (Fig. 4G) across the entire receptive field, whereas the adjacent contour plots show the absolute (Fig. 4H) and relative (Fig. 4I) differences between gregarious and solitarious locusts. In solitarious locusts the mean sensitivity across all azimuths at +45° elevation was the same as that at the eye equator, with 53 ± 4.1 spikes being evoked. In gregarious locusts, however, there was a 12% decrease in response at +45° elevation compared with the eye equator, with the stimulus eliciting a mean 58 ± 5.1 spikes (statistically similar to that of solitarious locusts at the same elevation, t8 = 0.69, P = 0.513; Fig. 4, C–E, means ± SE across three planes of azimuth; Fig. 4, F–I).
Fig. 4.
A–E: sections through the receptive field of DCMD showing the total number of spikes evoked by looming stimuli across visual space in gregarious (gray) and solitarious (black) locusts. A: horizontal section through 0 to180° azimuth at +15° elevation. B: horizontal section at −15° elevation. C: vertical section from −70 to +75° elevation at 30° azimuth. D: vertical section at 90° azimuth. E: vertical section at 150° azimuth. F–I: contour maps of the receptive field (color coded such that red regions indicate the highest spike numbers) in gregarious (F) and solitarious (G) locusts, showing the total numbers of spikes evoked by looming stimuli in each position. H: the difference between the number of spikes evoked in gregarious and solitarious locusts (gregarious number of spikes − solitarious number of spikes). I: the normalized difference between phases (gregarious response normalized to 1).
Numbers of evoked spikes were much lower directly in front of the locust (0° azimuth) than for more lateral azimuths (from 30 to 150°; Fig. 4A). This frontal region showed the greatest relative difference in sensitivity between phases, with solitarious locusts showing 47% less activity than of gregarious locusts [20 ± 4.1 spikes in solitarious and 37 ± 6.4 spikes in gregarious locusts (averaged across both +15 and −15° elevation at 0° azimuth); Fig. 4, H and I; t-test, t8 = 2.31, P = 0.05]. Responsiveness also fell off sharply at 180° azimuth, directly behind the locust, when only 14 ± 2.0 spikes in solitarious and 8.6 ± 1.91 spikes in gregarious spikes were evoked by the stimulus (t8 = 1.84, P = 0.103, not significantly different).
At the highest elevation tested, +75°, the sensitivity was lower than that at the equator in both phases and were almost identical to each other (42 ± 3.7 spikes in solitarious compared with 42 ± 3.8 spikes in gregarious, averaged across all azimuths; t8 = 0.20, P = 0.846).
DCMD was generally less sensitive to stimuli presented from below the eye equator than from above. Stimuli approaching from −45° elevations elicited 30% fewer spikes in both phases relative to the eye equator (37 ± 3.3 and 47 ± 3.1 spikes in solitarious and gregarious locusts, respectively; Fig. 4, C–I). Responsiveness decreased even further when stimuli were presented from −70° and was similar between both phases with a total of only about 20 action potentials being elicited from this elevation along the entire azimuth range (Fig. 4, C–I). At the most posterior and ventral parts of the receptive field, solitarious locusts showed equally strong or even stronger responses to looming stimuli than gregarious locusts, even though the total number of spikes evoked was low in both phases (Fig. 3, gray regions in Fig. 4, H and I).
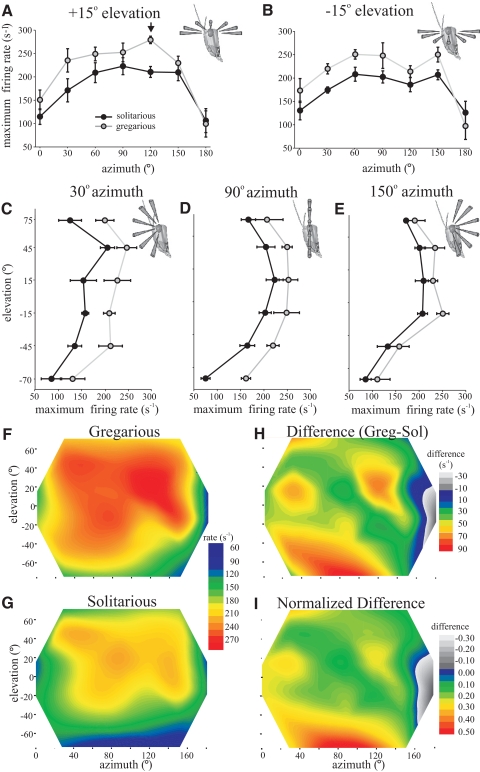
Peak-firing frequency
The receptive field organization characterized by peak-firing rates was broadly similar to that shown for the total number of spikes (Fig. 5) and there was a high degree of correspondence between total number of evoked spikes and peak-firing rate across the entire receptive field [general linear model (GLM) of peak-firing rate against number of evoked spikes using averages of each position for each phase, F(1,39) = 271.6, P < 0.001, R2 = 0.93]. The spatial maps of peak-firing rate [Fig. 5, F (gregarious) and G (solitarious)] reveal a broad region of approximately similar response amplitude, spanning from 30 to 150° azimuth and from −15 to +45° elevation, which showed even less variation than the numbers of spikes evoked by the looming stimuli [Fig. 5, A–E, means ± SE for both phases plotted at ±15° elevation (A, B) and at 30° (C), 90° (D), and 120° (E) azimuth; cf. Fig. 4]. In this region there was an 18% difference between phases with peak-firing rates reaching 232 ± 9.52 spikes s−1 in gregarious and 197 ± 6.1 spikes s−1 in solitarious locusts (t8 = 3.13, P = 0.014). In gregarious locusts only there was a single exception to this isotopic receptive field with a distinct peak of responsiveness occurring at +15° elevation and 120° azimuth [Fig. 5, A, arrow; F and H (absolute); and I (relative) differences between phases, yellow-red region in caudal receptive field]. This small region produced the highest overall firing rates, at 279 ± 8 spikes s−1, and was significantly different from values of adjacent sample points [ANOVA, F(2,12) = 4.97, P = 0.027].
Fig. 5.
Sections through the receptive field of DCMD showing the peak-firing rate evoked by looming stimuli across visual space in gregarious (gray) and solitarious (black) locusts. A: horizontal section through 0 to 180° azimuth at +15° elevation. The arrow indicates the foci of local increased responsiveness found in gregarious locusts. B: horizontal section at −15° elevation. C: vertical section from −70 to +75° elevation at 30° azimuth. D: vertical section at 90° azimuth. E: vertical section at 150° azimuth. F–I: contour maps of the receptive field (color coded such that red regions indicate the highest mean peak-firing rate) in gregarious (F) and solitarious (G) locusts showing the peak-firing rate evoked by looming stimuli in each position. H: the difference between the peak-firing rate in gregarious and solitarious locusts (gregarious rate − solitarious rate). I: the normalized difference between phases (gregarious response normalized to 1).
The peripheral visual field was less sensitive in all directions relative to this broad lateral region. At +75° elevation there was a 22% decrease in peak-firing rate in solitarious locusts, whereas in gregarious locusts there was only an 11.5% decrease (Fig. 5, C–E and I). The responsiveness showed a more marked decline below the eye equator. At −45° elevation, solitarious locust DCMD responses were 24% lower than those in the central region and gregarious locust responses were 16% lower. Below this, at −70° elevation, peak-firing frequency had nearly halved (to 54%) in solitarious locusts to 90 ± 9.3 spikes s−1 and in gregarious locusts it had decreased by 39% to 141 ± 15.8 spikes s−1. DCMD was 37% less sensitive in solitarious locusts to stimuli approaching from directly in front (0° azimuth) compared with more lateral approaches toward the eye equator (producing 123 ± 13.3 spike s−1) and 30% less sensitive in gregarious locusts (producing 162 ± 17.9 spikes s−1) (Fig. 5, A, B, and F–I).
Even though absolute sensitivity decreased in both solitarious and gregarious locusts toward the periphery of the receptive field, the greatest relative differences between phases were in the anterior and ventral-most regions, which showed a 30–50% relative difference (Fig. 5I). Across the main part of the visual field, there was a relatively constant 10–20% difference between phases (average 16%), excluding the peak of sensitivity at +15° elevation, 120° azimuth in gregarious locusts, where there was a 25% difference between phases.
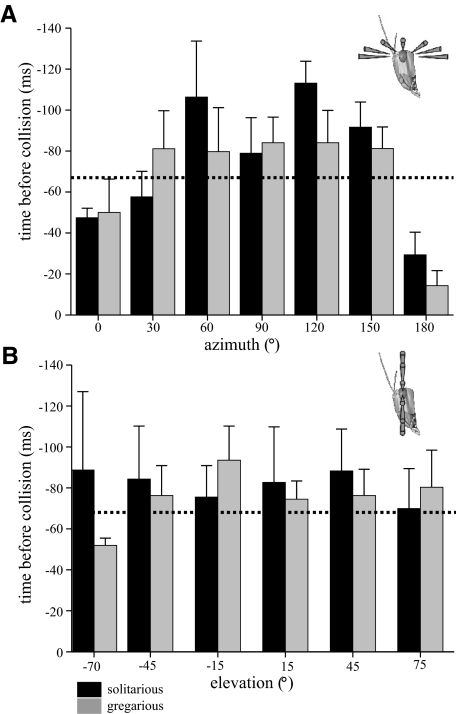
Time of peak-firing rate during looming approaches
For the stimulus we used, the times of peak firing in the DCMD preceded collision were constant across most of the receptive field and did not differ between phases. Figure 6 shows the horizontal profile across all azimuths at +15° elevation (A) and vertically for all elevations at 90° azimuth (Fig. 6B) [for elevation +15°, ANOVA, F(6,54) = 2.136, P = 0.189; for azimuth 90°, ANOVA, F(6,54) = 0.289, P = 0.901]. This uniformity of time of peak firing concurs with previous analyses of looming stimuli presented from a more limited range of angles along the azimuth to the eye equator (Gabbiani et al. 2001) and our previous comparison between phases (Matheson et al. 2004). Peak firing occurred later in the approach at the extreme periphery of the receptive field, particularly when objects approached from directly in front or behind (0 and 180° azimuths, Fig. 6A). These late peaks of firing occurred after the object had reached 50° visual subtenses and stopped expanding, suggesting that the DCMD showed persistent firing to objects approaching from these extreme edges of the receptive field, which outlasted the expansion of the stimulus.
Fig. 6.
The time of peak DCMD firing prior to collision was similar across the receptive field in both gregarious (gray bars) and solitarious (black bars) locusts. A: a section at elevation +15° across all values of azimuth. B: a section at azimuth 90° across all values of elevation. Maximum expansion of the stimulus occurred 67 ms prior to collision (dotted line), so smaller values indicate that the peak firing occurred after the end of object expansion.
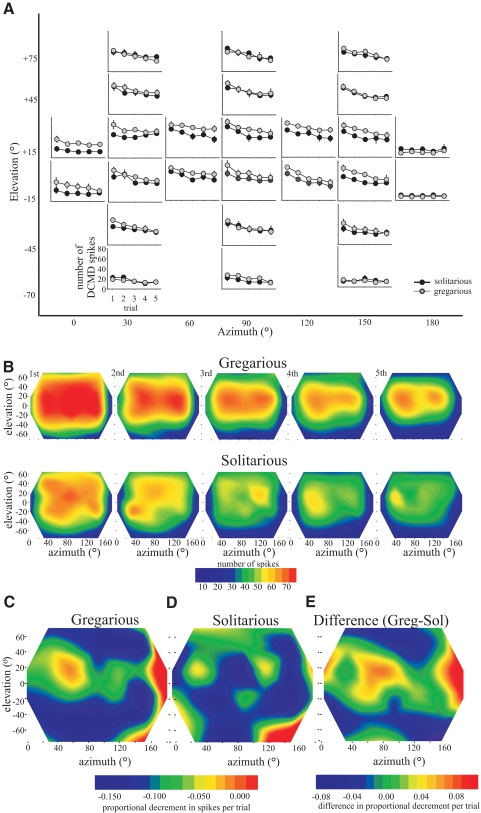
Differences in the degree of habituation across the receptive field
The repeated presentation of visual stimuli from the same position at 40-s intervals led to a marked habituation of the DCMD in both phases (Fig. 7). Within a location and phase, the degree of habituation was similar regardless of whether it was measured by comparing the decreases in peak firing frequency or the decrease in the total number of evoked spikes (relationship between peak firing frequency and number of spikes, r2 = 0.717). Only the change in the number of spikes is shown (Fig. 7). There were significant differences in the amount of habituation shown by DCMD in different locations in the visual field [interaction between number of spikes and order of stimulus presentation, repeated-measures ANOVA, F(25,1175) = 3.59, P = 0.001] and also in the degree of habituation between phases (interaction between number of spikes and phase, repeated-measures ANOVA, F25,1175 = 4.2, P < 0.001). The same difference in the extent of habituation across the receptive field and between phases was also seen in peak DCMD firing rate [interaction between location and peak-firing rate, repeated-measures ANOVA, F(9.3,419.5) = 2.48, P = 0.008; between phases, ANOVA, F(9.3,419.5) = 3.13, P = 0.001; degrees of freedom are adjusted to compensate for the nonsphericity of the data]. The differences in the degree of habituation across the receptive field could not be explained simply by differences in the initial strength of response evoked in different regions since areas of similar initial sensitivity underwent differential habituation. In gregarious locusts, for example, the habituation at +15° elevation was less marked, i.e., DCMD response declined less on repeated stimulus presentation, than that at +45° (Fig. 7B), even though the strength of response was initially similar at both elevations.
Fig. 7.
The effect of repeated stimulation by looming stimuli on the habituation of DCMD in different regions of the receptive field. A: graphs showing the means ± SE number of spikes elicited by looming stimuli presented 5 times at 40-s intervals. Each graph represents data from a different position in visual space, as indicated by the large axes marking azimuth (x) and elevation (y). B: contour maps of the receptive field (color coded such that red regions indicate the highest mean peak-firing rate) in gregarious (top) and solitarious (bottom) locusts showing the total numbers of spikes evoked by looming stimuli in each position on each subsequent presentation. C and D: gradients of linear regressions of the strength of habituation plotted across visual space in gregarious (C) and solitarious (D) locusts. E: the difference in gradient of habituation between gregarious and solitarious locusts. Gregarious locusts showed reduced habituation compared with that of solitarious locusts in a zone stretching across +15° elevation.
To compare habituation across the receptive field and between phases more directly, the data were normalized such that the initial response was set at 1 and subsequent responses expressed as a proportion of the initial response. During the early stages of habituation, such as occurred during the first three presentations of the stimulus, the decline of the response strength was approximately linear. We therefore fitted linear regressions to these data and plotted the gradients of these regressions across the visual field [Fig. 7, C (gregarious) and D (solitarious)].
In the most caudal part of the receptive field (180° azimuth), where the initial response was weak (<20 spikes evoked), there was little or no habituation in locusts of either phase (Fig. 7, C and D). Conversely, in the most ventral region of the receptive field, where the initial strength of response was similarly weak, the habituation was more pronounced. The response after five approaches was only 40–50% of the strength of the first approach (Fig. 7, C and D). In gregarious locusts there was a region of reduced habituation at +15° elevation extending across nearly the entire azimuth range, where a strong response to repeated stimulation was maintained. (In Fig. 7, A and B, the red-yellow band in the center of the receptive field remains, even though there is marked habituation, shown as an increase in green and blue above and below this zone. The small gradient of decline is shown in Fig. 7C as a green-yellow band.) This led to a marked difference in the degree of habituation in this region between phases (green-orange regions in Fig. 7E). In solitarious locusts, relative habituation was similar over the greater part of the receptive field, leading to a more steady decrease in response (Fig. 7, D and E). Since the central region of the receptive field showed the strongest initial responses, this region maintained the greatest absolute level of responses during habituation. Habituation appeared to be least strong in the most dorsal part of the receptive field, in contrast to the pattern seen in gregarious locusts (green in Fig. 7D).
The response of DCMD in gregarious locusts habituates less than that of solitarious locusts to stimuli directed perpendicular to the eye equator (0° elevation, 90° azimuth) (Matheson et al. 2004). Subtracting the habituation gradients of solitarious locusts from those of gregarious locusts reveals that gregarious and solitarious locusts show a similar degree of habituation across much of the receptive field (blue regions; Fig. 7E). It is only in the central region about the eye equator (shown in green- orange) that gregarious locusts are notably more resistant to habituation than solitarious locusts.
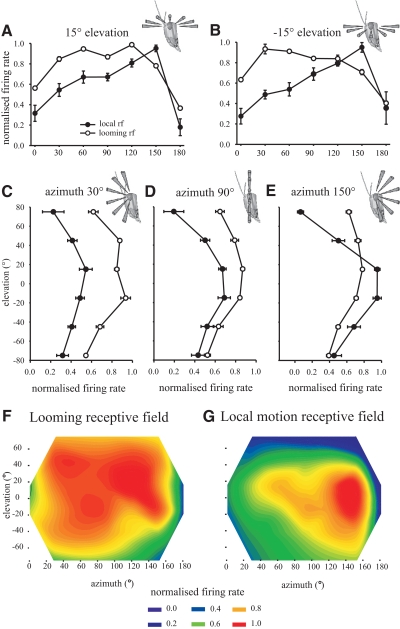
Difference between the receptive fields to looming objects versus local-motion sensitivity
A previous study by Krapp and Gabbiani (2005) analyzed the response of DCMD to local-motion stimuli in the same gregarious population of locusts as used in this study (from the culture at Cambridge University), using the same electrophysiological recording techniques. We therefore asked how the receptive field organization of the LGMD–DCMD in gregarious locusts obtained with looming objects in this study differed from the previously reported receptive field organization using local-motion stimuli (Fig. 8). Although looming objects induce responses of similar strength within a large region of the receptive field (as described earlier), responses to local-motion stimuli show much more spatial variation (Fig. 8; Krapp and Gabbiani 2005). Since peak-firing frequencies to looming objects are always higher than those to nonlooming objects, all the data in Fig. 8 have been normalized to the maximum response obtained from each animal (where maximum response = 1) and expressed as a proportion of this maximum response to compare relative spatial responsiveness. Values for the local-motion receptive field were normalized from the original raw data of Krapp and Gabbiani (2005). The receptive field of the LGMD–DCMD obtained with local-motion stimuli showed a marked and progressive increase in responsiveness from 0 to 150° azimuth before falling off sharply at 180° azimuth (Fig. 8, A and B, means ± SE from 0 to 180° azimuth at ±15° elevations; G contour plot of entire local-motion receptive field). By contrast the highest response to looming stimuli occurred more anteriorly at 120° azimuth at the end of a plateau of near similar response strength spanning from 30° to 120° azimuth [Fig. 8, A–D and F contour plot of entire looming receptive field; repeated-measures ANOVA, interaction between stimulus type and location for +15° elevation, F(6,54) = 6.81, P < 0.001; for −15° elevation, F(6,54) = 5.94, P < 0.001]. The response to local motion decreases progressively and sharply at higher elevations [Fig. 8, C–E, means ± SE plotted across all elevations at azimuths of 30° (Fig. 8C), 90° (Fig. 8D), and 120° (Fig. 8E); also see Fig. 8G], but the response to looming stimuli is much stronger in the dorsal part of the receptive field and shows much less fall off in maximum firing rate (Fig. 8, C–F). Only in the ventral receptive field do the relative strengths of response to local-motion and looming stimuli decrease in a similar manner and resemble each other (Fig. 8, C–G). Thus although the decline in response to local motion above and below the eye equator is approximately symmetrical, it is strongly asymmetric for looming stimuli, with stronger responses in the dorsal than in the ventral receptive field.
Fig. 8.
The receptive field of DCMD to looming stimuli differs in spatial responsiveness from that to local-motion stimuli in gregarious locusts. Data were normalized to the maximum firing rate for each individual (maximum = 1) and show means ± SE for (A) a horizontal section through 0 to 180° azimuth at +15° elevation. B: horizontal section at −15° elevation. C: vertical section from −75 to +70° elevation at 30° azimuth. D: vertical section at 90° azimuth. E: vertical section at 150° azimuth. F and G: contour plots showing the normalized strength of response to (F) looming and (G) local-motion stimuli in the range 0 to 180° azimuth and −70 to +75° elevation.
DISCUSSION
We have analyzed the full spatial and temporal characteristics of the receptive field of the DCMD in both solitarious and gregarious locusts to their most effective stimulus: a looming object on collision course. The DCMD had a high sensitivity to looming stimuli across a broad region of the visual field in both phases, extending from 30 to 150° azimuth and from −15 to +45° elevation. In both phases, peak-firing rates and numbers of spikes evoked by looming stimuli were remarkably similar throughout this central region. The only exception was that gregarious locusts had a small region of higher peak-firing rate at +120° azimuth and +15° elevation, where the response was 16% higher than that in the immediately surrounding area. Gregarious locust DCMD produced more spikes and had greater peak-firing rates than those of solitarious locusts throughout the central region, although both measures of response were similar between phases above, below, and behind this region. Gregarious locusts therefore had a more curved receptive field profile, which fell away more sharply at the periphery than in solitarious locusts.
Previous studies have analyzed the responses of the DCMD to individual looming stimuli approaching from a range of different azimuth positions in a horizontal plane (Gabbiani et al. 2001) and to two objects approaching simultaneously from widely separate azimuths (Gray 2005; Guest and Gray 2006). Gray et al. (2001) also investigated the differences in the response of the DCMD to looming stimuli across a 14° range of azimuths and elevations, centered on the frontal receptive field (i.e., only to objects approaching a flying locust from within 14° of head-on), but no previous study has systematically analyzed the looming receptive field.
Difference in looming versus local-motion receptive fields
The optical axis density of the locust eye is highly anisotropic and does not positively correlate with the receptive field of the LGMD for either local-motion (Krapp and Gabbiani 2005) or looming stimuli. The optical axis density is greatest frontally, where LGMD shows some of its weakest visual sensitivity, but LGMD is most sensitive to both local-motion and looming stimuli in the less optically dense lateral and caudolateral visual field. This disparity can be partially explained by the dendritic branching pattern and electrotonically extensive nature of LGMD (Peron et al. 2006). Inputs to the LGMD from the optically dense anterior retina are attenuated by the greater distance they must propagate to reach the spike-initiating zone compared with inputs from the less-dense posterior retina (Krapp and Gabbiani 2005). Peron et al. (2006) suggested, however, that these properties of the LGMD could not completely account for the observed receptive field to local motion and that additional neuronal mechanisms must shape the receptive field, particularly along elevation. Recent work has suggested that active conductances located in the dendrites of the LGMD may affect the propagation of signals from dorsal and ventral dendrites (Gabbiani and Krapp 2006). Other mechanisms such as variable synaptic strength of inputs onto the LGMD and/or a presynaptic spreading of the signals from individual ommatidia to adjacent input synapses could also be responsible for the difference in the LGMD's receptive field properties from those predicted by a passive compartmental model (Peron et al. 2006). Recently Peron and Gabbiani (2009) have shown how spike-frequency adaptation in the LGMD significantly contributes to establish its selectivity to looming stimuli. There are obvious differences between these two stimulus paradigms. Local-motion stimuli presented at a given position in the visual field will activate an approximately constant number of local excitatory and inhibitory inputs. Looming stimuli, however, are highly dynamic with respect to object size and object edge velocity. As they approach they expand to cover an ever greater proportion of the eye. This expansion comes with a nearly exponential increase of the number of inputs during looming stimuli that may induce active mechanisms within the LGMD dendrites that act to produce an equally strong response to looming stimuli across much of the neuron's receptive field. Whether the same active mechanisms in the LGMD that shape the local-motion receptive field are also responsible for generating the large region of the nearly equally strong response in the looming receptive field is at present unknown.
Peak-firing rates in the LGMD occur at a fixed time after the approaching object subtends a threshold angle across the retina (Gabbiani et al. 1999, 2002; Hatsopolous et al. 1995; Matheson et al. 2004; but for an alternative analysis and data interpretation see Rind and Bramwell 1996; Rind and Simmons 1997, 1998, 1999; Rind et al. 2008). Our previous comparative work on both phases suggests that the threshold angle is 25° on average, ranging from 7 to 37° (Matheson et al. 2004). The critical angle therefore occurs at a time during the approach when much of the retina is still unstimulated by the looming stimulus (it is smaller, e.g., than the 30° steps we used to map out visual space). The more homogeneous receptive field to looming as opposed to local stimuli cannot simply be explained as a simple summing process across local responses to different dendritic input regions of the LGMD as the approaching object expands across the retina.
Objects approaching from the periphery of the receptive field
Sensitivity at 0° azimuth, directly in front of the locust, was low in both phases with peak-firing rates and numbers of spikes only 50–66% of the values found at 30° azimuth (see also Gray et al. 2001; Rowell 1971; Schlotterer 1977). This frontal region also had the greatest relative differences in sensitivity between phases. Both gregarious and solitarious locusts have a similar 13.5° binocular overlap at the eye equator, so any object approaching from the front will activate both left and right LGMDs (Gabbiani et al. 2001; Gray 2005; Gray et al. 2001). Each DCMD descends down the nerve cord contralateral to the eye it receives its input from and makes a number of both ipsilateral and contralateral connections to postsynaptic neurons in the thoracic ganglia (Burrows and Rowell 1973; Pearson and Goodman 1979; Simmons 1980). Weak sensitivity in the frontal receptive field may be compensated for in part by some postsynaptic neurons receiving summed inputs from both DCMDs. Even though there were significant differences in the binocular overlap at different elevations between phases, the differences were small, with solitarious locusts having at maximum a roughly 5° greater overlap in the ventral receptive field. It is thus unlikely that solitarious locusts can overcome their particularly poor frontal responsiveness by integrating binocular information from a much greater area. Locusts in the migratory gregarious phase may have greater sensitivity in the frontal DCMD receptive field to better avoid frontal collision when flying or marching in a swarm.
An object approaching from the periphery of the visual field will appear to expand asymmetrically since one edge of the image will reach the edge of the retina and can expand no further, possibly compromising the neural computation performed by LGMD. Only an object approaching from a direction offset by ≳15° from the edge of the eye will reach the critical angle that elicits the peak-firing rate while still expanding symmetrically. The shape and curvature of the eye make a precise estimation difficult, but it is likely that the objects in our experiments approaching from −75° elevation were near the edge of the visual field and may have expanded to the edge of the retina before the critical angle was reached. Objects approaching from high elevations at +70°, however, should fall well within the angular extent of the eye, which reaches the top of the head at +90° elevation.
Differential habituation between phases and across the receptive field
The DCMD response of gregarious locusts shows substantially less habituation than that of solitarious locusts on repeated presentations of the same stimulus (Matheson et al. 2004). Looming objects approaching at 0° elevation and 90° azimuth at 1-min intervals induced only a 15% decrease in sensitivity in gregarious locusts compared with a decrease of >60% in solitarious locusts, over the course of 30 stimulus presentations (Matheson et al. 2004). In the present study the interval between successive presentations of the stimulus was only 40 s and this led to an increase in the degree of habituation in both phases. Previous studies have shown that habituation in LGMD is a local process and the neuron can habituate to stimuli in one part of the receptive field, but still respond vigorously to stimuli originating from a different region in visual space (Bacon et al. 1995; Gray 2005; Judge and Rind 1997; Rowell 1971) or after being given a nonvisual dishabituating stimulus (Rind et al. 2008). Our analysis here suggests that different parts of the receptive field of DCMD may have different intrinsic susceptibilities to habituation. Solitarious locusts showed little variation in relative habituation across the entire receptive field except in the most anterior and posterior regions, where numbers of evoked spikes were initially low and showed little further decrease. Gregarious locusts had a degree of habituation similar to that of solitarious locusts across much of the receptive field, except for a zone near the eye equator, which showed reduced habituation compared with the same region in solitarious locusts, consistent with our previous work. It is likely that reduced habituation in gregarious locusts is an adaptation to living in a visual environment that is by definition dominated by the presence of other locusts. The observation that only part of the receptive field of the DCMD in gregarious locusts is more resistant to habituation than that in solitarious locusts suggests that the intrinsic structure of flying swarms or marching hopper bands may subject the locusts in these swarms to looming stimuli coming disproportionately from a range of directions near 0° elevation.
Biological significance of DCMD receptive field organization
The broad response profile of the DCMD receptive field suggests that the neurons are tuned to detect objects approaching from a wide range of directions. On the basis of the low frontal sensitivity, Gray et al. (2001) suggested that LGMD–DCMD is more specifically tuned toward detecting objects approaching the locust rather than for allowing the locust to avoid fixed objects while walking or flying toward them, which will mostly seem to loom from in front. The data in this study support that suggestion.
The reduced response of the DCMD to objects approaching below the horizon can at least in part be explained by the position of the eyes on the head, but it suggests that the most behaviorally relevant stimuli arrive from above or from the side. For locusts on the ground, objects cannot approach from low elevations, but for a flying locust this would be possible. Locusts will often perch vertically on plant stems, however, and will slide around the stem to shield themselves from approaching objects such as potential predators (Hassenstein and Hustert 1999). A locust will already be partially concealed by its perch from objects approaching from low elevations (relative to the locust) and will require less movement to fully hide itself. Conversely, an object approaching from above or the sides will require a more extensive response. An anomalous feature of the generally flat looming receptive field in gregarious-phase locusts was the highly localized region of strong response at 120° azimuth and +15° elevation, slightly above and to the back of the locust. Solitarious locusts are more likely to make crepuscular or nocturnal flights than gregarious locusts, which are strongly diurnal (Uvarov 1977). The two phases may therefore be subject to different predators with different approach strategies, which may thus account for the small focus of increased response in the looming receptive field of DCMD in gregarious, but not solitarious, locusts. The receptive field of DCMD in gregarious locusts, although showing strong similarities to those of solitarious locusts, has functional specializations in the zone of reduced habituation and increased responsiveness, which adapt it to the radically different visual environment that occurs when living in a swarm.
GRANTS
This work was supported by a Biotechnology and Biological Sciences Research Council (UK) grant to M. Burrows and T. Matheson.
ACKNOWLEDGMENTS
We thank P. Simmons for reading and commenting on the manuscript.
REFERENCES
- Anstey ML, Rogers SM, Ott SR, Burrows M, Simpson SJ. Serotonin mediates behavioral gregarization underlying swarm formation in Desert Locusts. Science 323: 627–630, 2009 [DOI] [PubMed] [Google Scholar]
- Bacon J, Thompson KSJ, Stern M. Identified octopaminergic neurons provide an arousal mechanism in the locust brain. J Neurophysiol 74: 2739–2743, 1995 [DOI] [PubMed] [Google Scholar]
- Burrows M, Rowell CHF. Connections between descending visual interneurons and metathoracic motoneurons in the locust. J Comp Physiol 85: 221–234, 1973 [Google Scholar]
- Edwards DH. The cockroach dcmd neurone. II. Dynamics of response habituation and convergence of spectral inputs. J. Exp Biol 99: 91–107, 1982 [Google Scholar]
- Egelhaaf M, Kern R, Krapp HG, Kretzberg J, Kurtz R, Warzecha AK. Neural encoding of behaviourally relevant visual-motion information in the fly. Trends Neurosci 25: 96–102, 2002 [DOI] [PubMed] [Google Scholar]
- Fotowat H, Gabbiani F. Relationship between the phases of sensory and motor activity during a looming-evoked multistage escape behaviour. J Neurosci 27: 10047–10059, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F, Cohen I, Laurent G. Time-dependent activation of feed-forward inhibition in a looming-sensitive neuron. J Neurophysiol 94: 2150–2161, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F, Krapp HG. Spike-frequency adaptation and intrinsic properties of an identified, looming-sensitive neuron. J Neurophysiol 96: 2951–2962, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F, Krapp HG, Hatsopoulos N, Mo CH, Koch C, Laurent G. Multiplication and stimulus invariance in a looming-sensitive neuron. J Physiol (Paris) 98: 19–34, 2004 [DOI] [PubMed] [Google Scholar]
- Gabbiani F, Krapp HG, Koch C, Laurent G. Multiplicative computation in a visual neuron sensitive to looming. Nature 420: 320–324, 2002 [DOI] [PubMed] [Google Scholar]
- Gabbiani F, Krapp HG, Laurent G. Computation of object approach by a wide-field, motion-sensitive neuron. J Neurosci 19: 1122–1141, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F, Mo CH, Laurent G. Invariance of angular threshold computation in a wide-field looming-sensitive neuron. J Neurosci 21: 314–329, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR. Habituated visual neurons in locusts remain sensitive to novel looming objects. J Exp Biol 208: 2515–2532, 2005 [DOI] [PubMed] [Google Scholar]
- Gray JR, Lee JK, Robertson RM. Activity of descending contralateral movement detector neurons and collision avoidance behaviour in response to head-on visual stimuli in locusts. J Comp Physiol A Sens Neural Behav Physiol 187: 115–129, 2001 [DOI] [PubMed] [Google Scholar]
- Guest BB, Gray JR. Responses of a looming-sensitive neuron to compound and paired object approaches. J Neurophysiol 95: 1428–1441, 2006 [DOI] [PubMed] [Google Scholar]
- Hassenstein B, Hustert R. Hiding responses of locusts to approaching objects. J Exp Biol 202: 1701–1710, 1999 [DOI] [PubMed] [Google Scholar]
- Hatsopoulos N, Gabbiani F, Laurent G. Elementary computation of object approach by a wide-field visual neuron. Science 270: 1000–1003, 1995 [DOI] [PubMed] [Google Scholar]
- Horridge GA. The separation of visual axes in apposition compound eyes. Philos Trans R Soc Lond B Biol Sci 285: 1–59, 1978 [DOI] [PubMed] [Google Scholar]
- Howard J. Temporal resolving power of the photoreceptors of Locusta migratoria. J Comp Physiol 144: 61–66, 1981 [Google Scholar]
- Judge SJ, Rind FC. The locust DCMD, a movement-detecting neurone tightly tuned to collision trajectories. J Exp Biol 200: 2209–2216, 1997 [DOI] [PubMed] [Google Scholar]
- Krapp HG, Gabbiani F. Spatial distribution of inputs and local receptive field properties of a wide-field, looming sensitive neuron. J Neurophysiol 93: 2240–2253, 2005 [DOI] [PubMed] [Google Scholar]
- Laughlin SB, Weckström M. Fast and slow photoreceptors: a comparative study of the functional diversity of coding and conductances in the diptera. J Comp Physiol A Sens Neural Behav Physiol 172: 593–609, 1993 [Google Scholar]
- Matheson T, Rogers SM, Krapp HG. Plasticity in the visual system is correlated with a change in lifestyle of solitarious and gregarious locusts. J Neurophysiol 91: 1–12, 2004 [DOI] [PubMed] [Google Scholar]
- O'Carroll DC, Bidwell NJ, Laughlin SB, Warrant EJ. Insect motion detectors matched to visual ecology. Nature 382: 63–66, 1996 [DOI] [PubMed] [Google Scholar]
- O'Shea M, Williams JLD. The anatomy and output connection of a locust visual interneurone; the lobula giant movement detector (LGMD) neurone. J Comp Physiol 91: 257–266, 1974 [Google Scholar]
- Pearson KG, Goodman CS. Correlation of variability in structure with variability in synaptic connections of an identified interneurone in locusts. J Comp Neurol 184: 141–166, 1979 [DOI] [PubMed] [Google Scholar]
- Peron SP, Gabbiani F. Spike frequency adaptation mediates looming stimulus selectivity in a collision detecting neuron. Nat Neurosci 12: 318–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peron SP, Krapp HG, Gabbiani F. Influence of electrotonic structure and synaptic mapping on the receptive field properties of a collision-detecting neuron. J Neurophysiol 97: 159–177, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss T, Osei-Bonsu PE, Weiss SA, Wang C, Faber DS. Neural representation of object approach in a decision-making motor circuit. J Neurosci 26: 3454–3464, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wum C-F, Thompson RF. Habituation revisited: an updated and revised description of the behavioural characteristics of habituation. Neurobiol Learn Mem 92: 135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rind FC. A chemical synapse between two motion detecting neurones in the locust brain. J Exp Biol 110: 143–167, 1984 [DOI] [PubMed] [Google Scholar]
- Rind FC, Bramwell DI. Neural network based on the input organization of an identified neuron signaling impending collision. J Neurophysiol 75: 967–985, 1996 [DOI] [PubMed] [Google Scholar]
- Rind FC, Santer RD, Wright GA. Arousal facilitates collision avoidance mediated by a looming sensitive visual neuron in a flying locust. J Neurophysiol 100: 670–680, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rind FC, Simmons PJ. Orthopteran DCMD neuron: a reevaluation of responses to moving objects. I. Selective responses to approaching objects. J Neurophysiol 68: 1654–1666, 1992 [DOI] [PubMed] [Google Scholar]
- Rind FC, Simmons PJ. Signaling of object approach by the DCMD neuron of the locust. J Neurophysiol 77: 1029–1033, 1997 [DOI] [PubMed] [Google Scholar]
- Rind FC, Simmons PJ. Local circuit for the computation of object approach by an identified visual neuron in the locust. J. Comp Neurol 395: 405–415, 1998 [PubMed] [Google Scholar]
- Rind FC, Simmons PJ. The many ways of building collision-sensitive neurons. Reply (Letter). Trends Neurosci 22: 438, 1999 [DOI] [PubMed] [Google Scholar]
- Roessingh P, Simpson SJ, James S. Analysis of phase-related changes in behavior of desert locust nymphs. Proc R Soc Lond B Biol Sci 252: 43–49, 1993 [Google Scholar]
- Rogers SM, Burrows M, Krapp HG, Matheson T. Compensatory plasticity at an identified synapse tunes a visuomotor pathway. J Neurosci 27: 4621–4633, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SM, Matheson T, Sasaki K, Kendrick K, Simpson SJ, Burrows M. Substantial changes in central nervous system neurotransmitters and neuromodulators accompany phase change in the locust. J. Exp Biol 207: 3607–3617, 2004 [DOI] [PubMed] [Google Scholar]
- Rowell CHF. Variable responsiveness of a visual interneurone in the free-moving locust, and its relation to behaviour and arousal. J Exp Biol 55: 727–747, 1971a [Google Scholar]
- Rowell CHF. The orthopteran descending movement detector (DMD) neurones: a characterisation and review. Z Vergl Physiol 73: 167–194, 1971b [Google Scholar]
- Santer RD, Rind FC, Stafford R, Simmons PJ. Role of an identified looming-sensitive neuron in triggering a flying locust's escape. J Neurophysiol 95: 3391–3400, 2006 [DOI] [PubMed] [Google Scholar]
- Santer RD, Simmons PJ, Rind FC. Gliding behaviour elicited by lateral looming stimuli in flying locusts. J Comp Physiol A Sens Neural Behav Physiol 191: 61–73, 2005 [DOI] [PubMed] [Google Scholar]
- Santer RD, Yamawaki Y, Rind FC, Simmons PJ. Preparing for escape: an examination of the role of the DCMD neuron in locust escape jumps. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 19: 69–77, 2008 [DOI] [PubMed] [Google Scholar]
- Schlotterer GR. Response of the locust descending movement detector neuron to rapidly approaching and withdrawing visual stimuli. Can J Zool 55: 1372–1376, 1977 [Google Scholar]
- Sherk H, Fowler GA. Visual analysis and image motion in locomoting cats. Eur J Neurosci 13: 1239–1248, 2001 [DOI] [PubMed] [Google Scholar]
- Sherk TE. Development of the compound eyes of dragonflies (Odonata). IV. Development of the adult compound eyes. J Exp Zool 203: 183–200, 1978 [DOI] [PubMed] [Google Scholar]
- Simmons PJ. Connexions between a movement-detecting visual interneurone and flight motoneurones of a locust. J Exp Biol 86: 87–97, 1980 [Google Scholar]
- Simmons PJ, Rind FC. Orthopteran DCMD neuron: a reevaluation of responses to moving objects. II. Critical cues for detecting approaching objects. J Neurophysiol 68: 1667–1682, 1992 [DOI] [PubMed] [Google Scholar]
- Simmons PJ, Rind FC. Seeing what is coming: building collision sensitive neurones. Trends Neurosci 22: 215–220, 1999 [DOI] [PubMed] [Google Scholar]
- Simpson SJ, McCaffery AR, Hagele BF. A behavioural analysis of phase change in the desert locust. Biol Rev 74: 461–480, 1999 [Google Scholar]
- Sun HJ, Frost BJ. Computation of different optical variables of looming objects in pigeon nucleus rotundus neurons. Nat Neurosci 1: 296–303, 1998 [DOI] [PubMed] [Google Scholar]
- Uvarov BP. Grasshoppers and Locusts: A Handbook of General Acridology: Volume 1. Anatomy, Physiology, Development, Phase Polymorphism, Introduction to Taxonomy Cambridge, UK: Cambridge Univ. Press, 1966 [Google Scholar]
- Uvarov BP. Grasshoppers and Locusts: A Handbook of General Acridology: Volume 2. Behaviour, Ecology, Biogeography, Population Dynamics London: Centre for Overseas Pest Research, 1977 [Google Scholar]
- Wilson M. Angular sensitivity of light and dark adapted locust retinula cells. J Comp Physiol 97: 323–328, 1975 [Google Scholar]
- Yamamoto K, Nakata M, Nakagawa H. Input and output characteristics of collision avoidance behavior in the frog, Rana catesbeiana. Brain Behav Evol 62: 201–211, 2003 [DOI] [PubMed] [Google Scholar]