Abstract
Odorants inhibit as well as excite olfactory receptor neurons (ORNs) in many species of animals. Cyclic nucleotide-dependent activation of canonical mammalian ORNs is well established but it is still unclear how odorants inhibit these cells. Here we further implicate phosphoinositide-3-kinase (PI3K), an indispensable element of PI signaling in many cellular processes, in olfactory transduction in rodent ORNs. We show that odorants rapidly and transiently activate PI3K in the olfactory cilia and in the olfactory epithelium in vitro. We implicate known G-protein–coupled isoforms of PI3K and show that they modulate not only the magnitude but also the onset kinetics of the electrophysiological response of ORNs to complex odorants. Finally, we show that the ability of a single odorant to inhibit another can be PI3K dependent. Our collective results provide compelling support for the idea that PI3K-dependent signaling mediates inhibitory odorant input to mammalian ORNs and at least in part contributes to the mixture suppression typically seen in the response of ORNs to complex natural odorants.
INTRODUCTION
It is increasingly clear that the mammalian sense of smell is organized into subsystems, including functional subsets of olfactory receptor neurons (ORNs) in the main olfactory epithelium (OE) itself (for reviews, see Breer et al. 2006; Ma 2007; Munger et al. 2009). Organizational complexity extends to individual ORNs since it has been long known that odorants can inhibit as well as excite canonical ORNs in the OE, as in most species of animals (for review, see Ache and Young 2005). Canonical ORNs are excited by the binding of odor molecules to their cognate odorant receptor (OR), which triggers a cyclic nucleotide signaling cascade that targets an olfactory cyclic nucleotide-gated (CNG) ion channel (Kaupp and Seifert 2002). The resulting Ca2+ influx into the ORN secondarily targets a Ca2+-activated chloride channel that further amplifies the output of the cell (Kleene 1993). Negative feedback from the elevated Ca2+ concentration causes a Ca2+/calmodulin-dependent decrease in the sensitivity of the CNG channel to cyclic adenosine monophosphate (cAMP) (Bradley et al. 2004, 2005). How odorants inhibit these cells, however, is still not understood.
A long-standing point of controversy has been whether phosphoinositide (PI) signaling plays a role in mammalian olfactory transduction (Gold 1999; Noe and Breer 1998; Schandar et al. 1998; Schild and Restrepo 1998). More recent evidence suggests the need to revisit the potential involvement of PI signaling in olfactory transduction. Some mammalian ORNs express TRPM5 (Lin et al. 2007) and transient receptor potential (TRP) channels are a common downstream target of PI signaling in other systems (Liu and Liman 2003; Nilius et al. 2007). Exogenous phosphatidylinositol (3,4,5)-trisphosphate (PIP3) negatively regulates the CNG channel (Zhainazarov et al. 2004) through complex interaction between PIP3 and Ca2+/calmodulin at the N-terminus of the channel (Brady et al. 2006). PI3K-mediated activity leading to the production of PIP3 can modulate odor-activated increases in intracellular Ca2+ in acutely dissociated rodent ORNs (Spehr et al. 2002). The latter two findings are particularly interesting in suggesting that PI3K-dependent signaling may mediate inhibitory odor input to mammalian ORNs.
Activation of PI3K, which generates 3′-phosphorylated inositol lipids, especially PIP3 in vivo, is an important signaling pathway through which cell-surface receptors regulate processes as diverse as proliferation, growth, survival, and intracellular trafficking (Fruman et al. 1998; Vanhaesebroeck et al. 2001), including the survival of mammalian ORNs (Moon et al. 2009). Thus it is critically important to establish the particular functional context in which PI3K-mediated signaling operates. If, as the emerging data could suggest, PI3K-dependent signaling mediates inhibitory input into rat ORNs, it should be possible to support several predictions. At least one isoform of PI3K known to couple through heteromeric G proteins, as do mammalian ORs (Jones and Reed 1989), should be expressed in mature ORNs. Odorants should rapidly and transiently activate PI3K. Finally, isoform-specific blockade of PI3K should effectively relieve inhibitory input in vivo, for example, by increasing the magnitude and/or the onset of the electrophysiological response of an ORN to an inhibitory odorant pairing.
We are now able to show that the β and γ isoforms of PI3K occur in membranes enriched in olfactory cilia, at least one of which can be localized to mature rat ORNs. We then show that odorants rapidly and transiently activate PI3K in rat olfactory cilia in vitro and in the dissociated OE. Finally, we show that β and γ isoform-specific inhibition of PI3K can increase the magnitude and onset of the electrophysiological response of ORNs to an odorant mixture of sufficient complexity to contain excitatory and inhibitory odorants, and that the ability of a single odorant to inhibit another is PI3K dependent. We conclude that PI3K-dependent signaling serves at least in part to mediate inhibitory odorant input to rat ORNs.
METHODS
Preparation of the intact olfactory epithelium
All experiments were performed on adult Sprague–Dawley rats ≥6 wk old. All procedures were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Florida. Rats were killed by inhalation of carbon dioxide and decapitated. The head was opened to keep the septum and the underlying olfactory turbinates intact. The septal olfactory epithelium (OE) was dissected free of the head and maintained in a petri dish filled with ice-cold modified artificial cerebrospinal fluid (ACSF) saturated with 95% O2-5% CO2 that contained (in mM): 120 NaCl, 25 NaHCO3, 5 KCl, 1.25 Na2HPO4, 1 MgSO4, 1 CaCl2, and 10 glucose (305 mOsm). A small piece of the septal OE was excised and transferred to a recording chamber (RC22, Warner Instruments) and secured with a custom-made holder for stability. Oxygenated ACSF was continuously superfused into the recording chamber at about 2 ml/min. All experiments were performed at the room temperature (20–22°C).
Electrophysiological recording from rat ORNs in situ
Extracellular electrical activity was recorded from the dendritic knobs of ORNs in the piece of septal epithelium using patch-clamp recording in the loose-patch configuration. The dendritic knobs were visualized using the infrared–differential interference contrast mode of an upright microscope (ZeissAxioskop 2 FS; Carl Zeiss Microimaging, Thornwood, NY) equipped with a ×40 water-immersion objective and a charge-coupled detector (CCD) camera. Recordings were made using patch pipettes pulled from a standard borosilicate glass (OD, 1.5 mm; Sutter BF-150) on a horizontal micropipette puller (P-87, Sutter Instrument) followed by fire polishing. Electrodes were filled with a normal Ringer solution containing (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, and 10 HEPES (pH 7.4, 305 mOsm). The typical seal resistance in the loose-patch configuration was 40–80 MΩ. Spontaneous and stimulation-evoked extracellular action potentials (spikes) were acquired at 10 kHz using an Axopatch 200A amplifier connected to an A/D signal converter interface (Digidata 1320) and controlled by Clampex 9.2 software (Molecular Devices, Sunnyvale, CA). Off-line analysis of electrophysiological recordings and spike sorting were performed using Clampfit 9.2 or IgorPro 4.09 (WaveMetrics, Lake Oswego, OR) software.
Ca imaging recording from acutely dissociated rat ORNs
Acutely dissociated rat ORNs were obtained using a standard procedure. In brief, olfactory tissue was dissected and placed in low-Ca2+ (0.1 μM free Ca2+ buffered with EGTA) Ringer supplemented with 0.5 mg/ml papain (Sigma–Aldrich) and 10 units/ml DNAse (Sigma–Aldrich). The tissue was incubated with enzymes for 20 min at 37°C in a 5% CO2 incubator, washed gently with normal oxygenated ACSF several times, chopped with a razor blade, and accurately triturated with a fire-polished glass pipette. The resulting suspension was filtered through a 40-μm cell strainer (BD BioSciences) into a clean glass tube and stored at 4°C until needed. An aliquote of the suspension was mixed with 10 μM Fluo-3 AM containing 0.04% Pluronic F127 and placed on a glass coverslip coated with concanavalin A (Sigma–Aldrich) in a recording chamber (RC22, Warner Instruments). Dye loading was complete within 30 min at 37°C. The recording chamber was mounted on an inverted microscope (Axiovert 200, Carl Zeiss) and the cells were viewed using a ×10, NA 0.5 Fluar objective lens. Oxygenated ACSF was continuously superfused over the cells at 2 ml/min. The cells were illuminated at 500 nm (BP500 filter) using a rapid-wavelength switcher (Lambda DG-4, Sutter Instrument) at 100 ms per frame every 2 s. The excitation light was additionally attenuated 10-fold with a neutral-density filter to avoid photobleaching of the dye. The emitted light was collected at 530 nm (BP530 filter) by a 12-bit cooled CCD camera (SensiCam, PCO, Kelheim, Germany). Both the illumination and image acquisition were controlled by Imaging Workbench 5.2 software (INDEC BioSystems). Fluorescent signals were corrected for background and are presented as a fractional change in fluorescent light intensity: (F − F0)/F0, where F is the fluorescent light intensity at each pixel and F0 is the initial fluorescence at the beginning of the recording. Only functional ORNs activated by a mixture of 100 μM 3-isobutyl-1-methylxanthine (IBMX, a phosphodiesterase inhibitor) and 10 μM forskolin (a unique diterpene agonist of adenylate cyclases) or an odor (Henkel-100 [H100] at 10−4 dilution) were used in the experiments. Each cell was assigned a region of interest (ROI) and changes in fluorescence intensity within each ROI were analyzed using Imaging Workbench 5.2 and IgorPro 4.09 software.
Odorant stimulation
Odorants were delivered as solutions. H100, a complex odor mixture (Wetzel et al. 1999), was dissolved 1:1 in anhydrous dimethylsulfoxide (DMSO) as a stock. Citral and octanol (both from Sigma–Aldrich) were prepared as 0.5 M stock solutions. Final solution was prepared fresh daily from the stock by diluting in ACSF with extensive vortexing and sonication. Both dosage of H100 and concentration eliciting half-maximal response (EC50) used in this study are expressed as log units of the actual dilution. Average concentration of H100 odorants in the original undiluted mixture was estimated as 60 mM. ACSF supplemented with 0.1% DMSO as a carrier for odors served as the control solution. For electrophysiological recording, odor solutions were delivered to the dendritic knobs by a focal pressure injection from a seven-barrel pipette (FHC, Bowdoin, ME), with a tip size of 5 μm positioned approximately 100–200 μm upstream of the recording site. Pressure pulses were triggered by a Picospritzer (General Valve, Fairfield, NJ) under the control of Clampex software. Estimated delay of the odorant delivery did not exceed 100 ms, as measured for the responses evoked by pressure pulses of ACSF containing 45 mM KCl.
For Ca2+ imaging, odor solutions were delivered from independent gravity-fed supply lines connected to the perfusion chamber through a multichannel Teflon manifold (Warner Instruments). Odor or IBMX/forskolin solutions were applied for 5 s to provide sufficient volume to replace the solution in the recording chamber.
Western blotting and immunohistochemistry
Membrane fractions enriched in rat olfactory cilia were obtained as previously described (Washburn et al. 2002). Protein concentrations were determined using a Coomassie Plus Bradford assay (Pierce, Rockford, IL) and equal amounts were loaded per well for separation by SDS-PAGE (4–12% Bis-Tris NuPAGE; Invitrogen, Carlsbad, CA). The proteins were transferred to nitrocellulose membranes and the membranes were blocked with either 5% milk [anti-PI3Kpan (H-239) and anti-PI3Kβ (S-19), both from Santa Cruz Biotechnology (Santa Cruz, CA)] or 5% BSA [anti-PI3Kγ (4252; Cell Signaling, Danvers, MA)] in PBS-T (PBS, pH 7.4 with 0.1% Tween 20) for 1 h at room temperature prior to incubation with the primary antibodies. For immunostaining the nasal septum was dissected, embedded in OCT, and rapidly frozen in methylbutane cooled on dry ice. Coronal cryosections of the nasal septum (12 μm thick) were postfixed in acetone for 10 min at −20°C and air-dried. The sections were incubated with primary antibodies, the same anti-PI3Kβ used for Western blotting and goat polyclonal anti-olfactory marker protein (anti-OMP; Wako Chemicals), overnight at 4°C. Alternatively, cryosections were obtained from the rat nose dissected free of bones and fixed in 4% paraformaldehyde overnight at 4°C, cryoprotected in 30% sucrose (0.1 M PBS), and frozen in OCT. These sections were double labeled with mouse monoclonal antibody against p110β (gift from Dr. Emilio Hirsch; Ciraolo et al. 2008) and rabbit polyclonal antibody against adenylate cyclase 3 (Santa Cruz Biotechnology). Finally, sections were incubated consecutively with the respective secondary antibodies for 1 h at room temperature and examined with a confocal laser scanning microscope (Leica TCS SP2; Leica Microsystems) using a ×63, NA 1.2 water-immersion objective lens. Image processing and final rendering were done using Adobe Photoshop CS2 and CorelDraw12. All images were processed to maintain equally balanced brightness, contrast, and color.
Overlay dot blot and ELISA assay
Olfactory ciliary membranes or intact cells dissociated from the OE were incubated with H100 (10−3 dilution) for different time periods. To block PI3K activity the cells were preincubated with 10 μM 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002; Calbiochem, San Diego, CA) for 10 min before adding H100. Enzyme reactions were stopped by immediate freezing of samples in liquid N2 followed by the addition of ice-cold 0.5 M trichloroacetic acid. Membrane fractions were centrifuged 5 min at 1,500 rpm. The pellet (or dissociated cells) was further treated with MeOH:CHCl3:HCl (80:40:1) to extract lipids. The aqueous phase was dried in a vacuum dryer (800 rpm, Savant Speedvac Plus SC 110A). Dried lipids were resuspended in 120 μl PIP3 buffer and placed in multiwell plates provided by Echelon (PIP3 Mass ELISA Kit, Echelon Biosciences). 3′-Phosphorylated phospholipids were detected using a protein lipid overlay assay (PIP3 Mass Strip Kit) or by using a PIP3 Mass ELISA Kit, following the manufacturer's protocols (Echelon Biosciences). The plates were read on a plate reader (Bio-Rad Laboratories, Hercules, CA) at 450 nm. The data were quantified with standards (0.625 to 20 pmol of PIP3 per well) and analyzed with Microplate Manager 4.0 software (Bio-Rad Laboratories).
Data analysis
All data are expressed as means ± SE. Statistical significance was assessed using Student's unpaired t-test using GraphPad Prism. For the concentration-dependence functions, data were fitted to the Hill equation yielding the Hill coefficient (kH) and EC50 value using built-in function in IgorPro.
RESULTS
PI3K is expressed in the olfactory cilia of mature rat ORNs
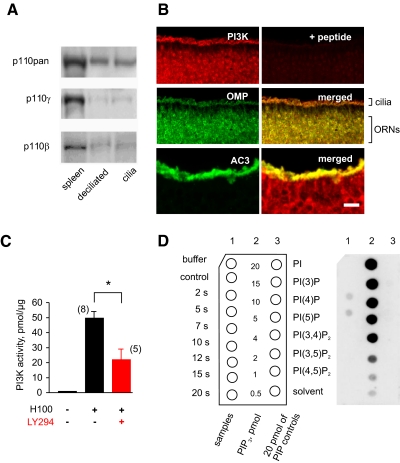
We focused attention on the β and γ isoforms of PI3K known to couple through G proteins in other systems (Maier et al. 1999; Murga et al. 2000; Yart et al. 2002). A band of appropriate size was detected on a Western blot of a ciliary-enriched membrane fraction of the rat OE by pan-specific, p110β-specific, and p110γ-specific polyclonal antibodies (Fig. 1A). These antibodies are highly specific for p110 protein detection in Western blots in mice (Ciraolo et al. 2008; Guillermet-Guibert et al. 2008; Murga et al. 2000). A 110-kD protein band was not detected in the blots where the primary antibodies were omitted (unpublished data). Rat spleen, known to be enriched in PI3K (Li et al. 2000), served as a positive control for p110 proteins. Since ≤2–5% of the total protein in the ciliary-enriched membrane fraction of the OE can be from cell types other than ORNs (Mayer et al. 2008), we attempted to localize p110β immunoreactivity within the rat OE. p110β was present in many, if not most, ORNs in OE (Fig. 1B, top left), as could be confirmed by its ability to colocalize with olfactory marker protein (OMP), a signature protein for mature ORNs (Fig. 1B, middle right). The immunoreactivity was significantly less in the apical zone of the OE between the ciliary layer and cell bodies of ORNs where the sustentacular and microvillar cells reside (Fig. 1B, middle right). Also p110β immunoreactivity could be confirmed in the ciliary layer by its ability to colocalize with adenylate cyclase 3, a specific marker for olfactory cilia (Bakalyar and Reed 1990) (Fig. 1B, bottom right). The broad distribution of p110β across the rat OE argues against its expression being confined to a special subpopulation of ORNs. Localization of p110γ immunoreactivity within the OE was not possible due to the lack of a p110γ-specific antibody suitable for immunohistochemistry. However, very strong evidence of broad tissue distribution of p110γ in the OE comes from a p110γ/LacZ transgenic mouse (Hirsch et al. 2000), where the LacZ reporter is expressed throughout the OE, suggesting that p110γ, like p110β, is expressed in most, if not all, ORNs in rodents (Brunert et al., unpublished data).
Fig. 1.
Phosphoinositide-3-kinase (PI3K) localizes to the olfactory cilia and can be activated by odorants. A: Western blot analysis of the catalytic p110 subunit using a pan-specific PI3K antibody (p110pan) and antibodies against p110β and p110γ in rat spleen extract, deciliated olfactory receptor neuron (ORN) membranes, and olfactory cilia-enriched membranes. Data are representative of ≥3 independent experiments. B: immunostaining of rat olfactory epithelium (OE) for p110β (red, top left) and olfactory marker protein (OMP), which stains mature ORNs (green, middle left). Double labeling (merged, middle right) shows p110β is abundantly present in mature ORNs. Preabsorption of the p110β antibody with the respective blocking peptide eliminated the staining to the background level (top right). p110β is colocalized in olfactory cilia with adenylate cyclase 3 (green, bottom left; merged, bottom right). Scale bars: 50 μm (top and middle), 25 μm (bottom). C: total PI3K activity measured as pmol of 3′-phosphorylated inositol lipids per 50 μg of protein with an enzyme-linked immunosorbant assay (ELISA) assay in membranes isolated from the cells dissociated from OE and incubated with Henkel-100 (H100, 10−3 dilution) for 5 s. The level of PI3K activity in the absence of odorant (left bar) was below the detection threshold. 2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002, 10 μM; red bar) significantly reduced the odorant-dependent increase in PI3K activity (t-test: *P < 0.05). D: direct detection of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) using an overlay dot blot. Left: schematic of the actual blot shown on the right. Data shown are representative of 4 independent experiments. Incubating olfactory ciliary membranes with H100 (10−3 dilution) for the indicated period of time transiently elevated levels of PIP3 at 2 and 5 s (lane 1). PIP3 standards (lane 2) were used to estimate the absolute concentration generated. The inability of the probe to detect different from PIP3 phospholipids (lane 3) controlled for the specificity of the method.
Odors rapidly and transiently activate PI3K in rat ORNs and olfactory cilia
We then determined whether the PI3K in rat ORNs indeed is activated by odorants. From essentially undetectable basal levels Henkel-100 (H100, 10−3 dilution) substantially raised the PI3K activity in the OE measured by ELISA (Fig. 1C, n = 8). At this dilution each component of the 100 components in H100 is approximately at 60 μM concentration, equal to or exceeding the EC50 at which odorants typically activate mammalian ORNs under physiological conditions (Araneda et al. 2004; Duchamp-Viret et al. 1999; Ma et al. 1999; Peterlin et al. 2005). LY294002 (10 μM), a pan-specific inhibitor of PI3K (Vlahos et al. 1994), significantly reduced the level of odor-stimulated PI3K activity by 2.5-fold (Fig. 1C, n = 5). Because the PI3K activity was not necessarily localized to the ciliary (transduction) compartment in this dissociated OE cell assay, we further measured the ability of H100 to stimulate PI3K in an enriched olfactory ciliary membrane preparation. H100 (10−3 dilution) consistently raised the concentration of PIP3 as measured with a protein overlay assay within 10 s of odor stimulation over undetectable levels of PIP3 prior to odorant stimulation (Fig. 1D, n = 4). The fastest increase in the level of PIP3 occurred within 2 s of odor stimulation and was transient, in agreement with an earlier report from our lab (Klasen et al. 2010).
Blocking PI3K modulates the rapid electrophysiological output of rat ORNs
We then determined whether blocking PI3K could modulate the electrophysiological output of ORNs in situ. In the absence of odor stimulation, ORNs generated random action potentials at an average frequency between 0.03 and 7 Hz (1.1 ± 1.8 Hz, n = 18) as recorded with loose-patch electrodes attached to the dendritic knob in the semi-intact preparation of OE (Fig. 2A, top traces). In all, 55% of the ORNs stimulated with H100 (0.5 s, n = 51 cells) responded with a transient burst of action potentials in a concentration-dependent manner (Fig. 2A, left). The data from individual cells were fit to the Hill equation. The average EC50 value of the response to H100 was 10−4.3 dilution. This dilution translates to about 3 μM for each component in the mixture.
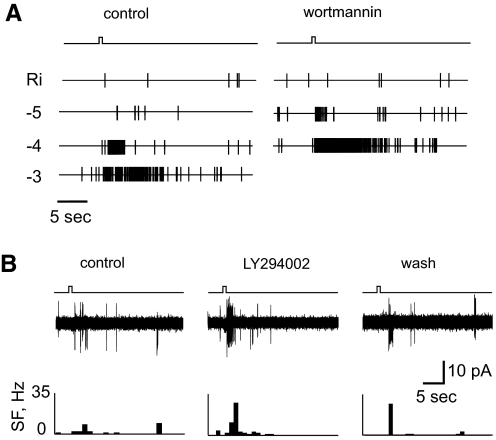
Fig. 2.
Pan-specific inhibition of PI3K increases the rapid electrophysiological response of some rat ORNs in situ. A: raster plots of the response of one cell to increasing concentrations of H100 before (left) and after (right) exposure to wortmannin (100 nM). The log dilution of H100 is indicated at the left of each raster plot of spike discharge. Note: blocking PI3K does not affect control response to Ringer (Ri) solution, whereas it increases the response to H100 by an order of magnitude in a concentration-dependent manner. The complete reversal of the inhibitor's effect allowed us to perform these measurements. B: representative recordings from the dendritic knob of a different ORN before (left), during (middle), and after (right) exposure to LY294002 (10 μM), another pan-specific PI3K inhibitor. Plotted below each trace is a histogram of the discharge frequency in 1-s bins as a function of time. Note that, like wortmannin, LY294002 also reversibly increased the peak magnitude and onset of the response.
Preincubating the OE with the pan-specific PI3K inhibitor wortmannin (100 nM; Arcaro and Wymann 1993) for 3 min prior to stimulation with H100 increased the response in 3/30 (10%) of the ORNs tested at the lowest concentration of H100 (10−5 dilution; Fig. 2A, left vs. right). In most cases the effect of PI3K inhibitors was reversed after washout, as with the Ca2+ responses shown in the following text. The effect held across a range of odorant concentrations, as could be tested in one exceptionally stable cell (Fig. 2A), suggesting that wortmannin effectively left-shifted the dose–response function to the odorant. Another pan-specific PI3K inhibitor, LY294002 (10 μM), similarly increased the response to H100 compared with the pretreatment response in 4/27 (15%) of the ORNs tested (Fig. 2B). Although the reported half-maximal inhibitory concentration (IC50) of LY294002 is about 500-fold higher than that of wortmannin, LY294002 is widely used in cell biology as a specific PI3K inhibitor because it is much more stable than wortmannin in aqueous solution. LY303511, an inactive form of LY294002, had no effect on the response to H100 in any of 126 ORNs tested in electrophysiological and Ca2+ imaging experiments (data not shown).
It is important to note that blocking PI3K specifically increased the overall onset (i.e., the early phase) of the response as reflected in an earlier onset and increased peak frequency of discharge. This can be clearly seen in one cell at an expanded sweep speed (Fig. 3, A and B). Data from a total of four cells recorded under the same conditions and showing an increase of the odorant response induced by wortmannin or LY294002 were pooled and analyzed. Blocking PI3K increased the peak number of action potentials roughly threefold from 17 ± 3 to 48 ± 10 spikes (P = 0.014, n = 6) (Fig. 3C) and decreased the latency of the evoked discharge from 1.65 ± 0.26 to 0.96 ± 0.08 s (P = 0.031, n = 6) (Fig. 3D).
Fig. 3.
Blocking PI3K modulates the rapid electrophysiological output of rat ORNs. A: early phase of the response to H100 (10−4 dilution) from the same ORN shown in Fig. 2A at higher sweep speed. B: plot of the instantaneous spike frequency (IF) of the same 2 responses as a function of time from the pressure pulse trigger (vertical dotted line in A). First point of the IF plot corresponds to the first spike. Note: wortmannin reduces the latency and increases the peak magnitude and rate of rise of the response (black trace) compared with control (gray trace). C: plot of the peak magnitude of the response measured as a total number of action potentials within the 20-s interval following stimulation with H100 (10−4 dilution) before (control) and following exposure to wortmannin or LY294002 (inhib). D: plot of the latency of the responses before (control) and after exposure to the inhibitors (inhib) measured as the time between the trigger of the odorant pulse and the first evoked action potential. Measurements (n) were done in 4 cells and were significant (t-test: *P < 0.05; **P < 0.01).
Known G-protein–coupled isoforms of PI3K can be implicated in modulating the output of ORNs
We then determined whether specific blockers of isoforms of PI3K known to couple through G proteins in other systems had the same effect as the pan-specific blockers tested earlier. Like the pan-specific inhibitors, PI3Kγ inhibitor-1 (1 μM, original name AS-605240), a PI3Kγ-selective inhibitor (Camps et al. 2005), could also increase the magnitude and decrease the latency of the electrophysiological response to H100 (Fig. 4A) and did so with a similar incidence (3/19 cells, 16%) to the pan-specific inhibitors. The incidence of the effect measured electrophysiologically could be verified in a larger population of cells with Ca2+ imaging. Only cells in good physiological condition and confirmed as canonical ORNs by their response to 100 μM IBMX and 10 μM forskolin were used in this analysis. Another highly PI3Kγ selective inhibitor, AS252424 (IC50 of 35 nM for PI3Kγ compared with >20,000 nM for PI3Kβ; Condliffe et al. 2005), reversibly increased the Ca2+ response to H100 (10−5 dilution) in 34/347 (9.8%) of the cells tested (Fig. 4B). Also like the pan-specific inhibitors, TGX-221 (200 nM), which is highly specific for PI3Kβ at 200 nM (IC50 of 7 nM for PI3Kβ compared with 3,500 nM for PI3Kγ; Condliffe et al. 2005), increased the Ca2+ response to H100 (Fig. 4C) and did so in 32/308 (10.4%) of the cells tested. Due to differences in the sensitivity of individual cells it is impossible to directly determine the effect of blocking PI3K activity. Therefore to quantitatively measure the extent of the effect we measured the concentration dependence before and after treatment of cells with the isoform-specific PI3K inhibitors. Results similar to those shown in Fig. 4, B and C were obtained from a total of 9 cells using the PI3Kγ blocker and another 10 cells using the PI3Kβ blocker. The data were normalized for each cell to the saturating response evoked by application of 100 μM IBMX and 10 μM forskolin (Fig. 4, B and C). The derived concentration dependence of the odorant responses (R) was fitted by the Hill equation, R = Rmax/{1 + (EC50/C)kH}, where EC50 is the concentration of H100 (C) eliciting half-maximal response and kH is the Hill coefficient. AS-252424 left-shifted the dose–response function by −0.62 ± 0.11 log unit (n = 10, P < 0.005) (Fig. 4D), whereas TGX-221 similarly shifted the dose–response function by −0.62 ± 0.08 log unit (n = 9, P < 0.005) (Fig. 4E), suggesting that both isoforms are equipotent. Interestingly, both isoform-selective inhibitors could act on the same ORN. All of 12 cells that showed an effect to one inhibitor and on which the other was tested also showed the same effect to the other inhibitor (Fig. 4F).
Fig. 4.
p110γ- and p110β-specific inhibition of PI3K enhances the odorant response in rat ORNs. A: PI3Kγ inhibitor-1 (1 μM) significantly increased the rapid electrophysiological response of rat ORNs in situ. Representative recording from the dendritic knob of one ORN stimulated by H100 (0.5 s, 10−4 dilution). Inhibiting PI3K significantly increased the peak magnitude and the rate of rise, while decreasing the latency of the response, mimicking the effect of a pan-specific blockade (Fig. 2). The average spike frequency (SF) plotted as a histogram of the number of spikes in 1-s bins is shown under each trace. B and C: representative Ca2+ responses of 2 other dissociated rat ORNs to H100 (10−5 dilution) applied for 5 s at 100-s intervals (arrowheads). B: AS-252424 (424, 200 nM, black bar), another highly selective inhibitor of PI3Kγ, increased the Ca2+ response to H100. C: TGX-211 (TGX, 200 nM, black bar), a highly selective inhibitor of PI3Kβ, can also increase the Ca2+ response to H100. The ability of the cells to respond to 100 μM 3-isobutyl-1-methylxanthine (IBMX)/10 μM forskolin (IF) denotes them as canonical ORNs. The cells retain the ability to respond to a higher concentration of H100 (H-4, 10−4 dilution). The inhibitors alone did not induce a response. D and E: plots showing the normalized peak magnitude of the Ca2+ signal evoked by H100 before (solid symbols) and after (open symbols) inhibition of PI3K with the PI3Kγ inhibitor AS-252424 (200 nM) and the PI3Kβ inhibitor TGX-221 (200 nM). Responses were normalized to the response evoked by IBMX/forskolin. Solid lines represent the best fit of the pooled data with the Hill equation with the following parameters: (D) control kH = 0.98, concentration eliciting half-maximal response (EC50) = 10−5.01 dilution, AS-252424, kH = 1.3, EC50 = 10−5.56 dilution; (E) control kH = 1.4, EC50 = 10−4.55 dilution, TGX-221, kH = 1.6, EC50 = 10−5.16 dilution. F: representative Ca2+ response of another dissociated rat ORN to H100 (10−5 dilution) applied for 5 s at 100-s intervals (arrowheads) before and following blockade with AS-252424 (424, 200 nM) and TGX-221 (TGX, 200 nM). The cell retains the ability to respond to a higher concentration of H100 (H-4, 10−4 dilution) and to IBMX/forskolin (IF). Similar effect was found in a different 11 cells. Arrowheads indicate time of test odorant application.
Inhibition of a single odorant by another can be PI3K dependent
The PI3K-dependent enhancement of the response to complex odorants suggests that PI3K activity may control inhibition induced by one or more odorants in the odorant mixture. If so, it should be possible to show that the inhibition of one single odorant by another one is PI3K dependent. Since identifying a pair of odorants showing inhibition in any particular cell could be inherently difficult, we focused on testing binary odorant mixtures already shown to display antagonistic interaction, such as octanol and citral (Peterlin et al. 2005). Canonical ORNs activated by octanol (50 μM) were subsequently tested with citral (100 μM). Surprisingly, 13 of 91 (14.3%) cells tested showed a consistent and significant reduction in the response to the stronger agonist when both odorants were coapplied (Fig. 5, A and B). Responses in each individual cell were normalized to the saturating response induced by H100 (10−4 dilution) and the data were pooled. Citral itself was not an effective ligand for these ORNs compared with octanol (Fig. 5B). In all 13 of these cells, LY294002 (10 μM) relieved the inhibition in the same manner observed in cells showing PI3K-dependent enhancement of the response to the complex odorant H100. To control for the specificity of the effect we tested the PI3K isoform-selective inhibitors, AS-252424 and TGX-221 (200 nM), and the inactive analog of LY294002, LY303511 (10 μM). As expected, AS-252424 and TGX-221 also relieved the citral-induced inhibition of the response to octanol, whereas LY303511 was completely ineffective (n = 3, data not shown). Coapplying both odorants failed to reduce the Ca2+ response evoked by the stronger agonist in the remaining 78 of 91 (85.7%) of the canonical ORNs; in these cells LY294002 (10 μM) had no effect on the response to octanol/citral (Fig. 5B). Understanding the mechanism of this interaction—i.e., whether blocking PI3K controls the agonistic properties of citral and/or octanol—awaits future research.
Fig. 5.
Inhibition of one single odorant on another is PI3K-dependent. A: representative recording of the Ca2+ response from one of 13 dissociated rat ORNs activated by octanol (OOL, 50 μM) in which citral (CIT, 100 μM) evoked little or no Ca2+ response but substantially inhibited an almost saturating response to OOL. Preapplication of LY294002 (LY294, 10 μM) for 10 s completely relieved the CIT-dependent inhibition in all 13 cells. B: responses in each individual cell were normalized to the response induced by a common concentration of H100 (H-4, 10−4 dilution) and the data pooled. Note that citral was not an effective agonist for these ORNs, yet potently inhibited the response to octanol (t-test: ***P < 0.001; n, number of measurements). C: representative recording of the Ca2+ response from one of 78 other cells activated by OOL (50 μM), CIT (100 μM), and their mixture (OOL/CIT). CIT did not change the magnitude of the response to OOL, nor did LY294002 (LY294, 10 μM) change the response to OOL/CIT. Control responses to a higher concentration of H100 (H-4, 10−4 dilution) and 100 μM IBMX/10 μM forskolin (IF) denote the cell as canonical ORN. Arrowheads indicate time of odorant application.
DISCUSSION
PI3K signaling can play a role in numerous constitutive cellular functions, including the survival of mammalian ORNs (Moon et al. 2009), so establishing the time course of activation of PI3K is critical to implicate PI3K in a rapid cellular function such as olfactory transduction. Finding that inhibition of PI3K increases the peak magnitude and onset of the electrophysiological response to H100 and that H100 rapidly elevates levels of PIP3 not only in olfactory cilia in vitro (Klasen et al. 2010) but also in dissociated ORNs within several seconds argue not only that the enzyme is functional and can be activated by odorants, but also that it can be activated sufficiently fast to play a role in transduction. Although PI3K activity rise time of 2 s is still relatively slow compared with the <1-s latency of the electrophysiological response of cells to odor stimulation, it is within an acceptable range, considering the level of PIP3 that could be measured by either assay was just above the threshold of detection. Such rapid activation of PI3K by odorants is consistent with evidence that PI3K-dependent translocation of PIP3 triggered by activation of G-protein–coupled receptors (GPCRs) occurs within seconds in Dictyostelium (Dormann et al. 2004) and mouse leukocytes (Condliffe et al. 2005).
We conclude, as assumed, the pharmacological blockade targeted the transduction compartment. We never observed, for example, any effect of blocking PI3K on Ca2+ responses evoked by KCl-dependent membrane depolarization (data not shown). Although LY294002 can act directly on other targets, including voltage-gated K+ and Ca2+ channels (El-Kholy et al. 2003; Welling et al. 2005), members of the type II AAA ATPase family, and other proteins unrelated to PI3K (Gharbi et al. 2007), we could reproduce the same odorant-dependent effects with wortmannin, a structurally dissimilar yet highly potent specific PI3K blocker, in agreement with earlier findings from our lab (Spehr et al. 2002). Another possible confound is that L-type Ca2+ channels (Cav1.2) can be directly potentiated by PIP3 (Le Blanc et al. 2004) and L-type Ca2+ channels are abundantly expressed in mammalian ORNs (Trombley and Westbrook 1991), where they are primarily responsible for the odorant-induced Ca2+ influx used as a readout in our Ca2+ imaging experiments. The caveat here is that PI3K blockade reduces PIP3 levels and would be expected to decrease the odorant-induced Ca2+ signal if it were acting on L-type Ca2+ channels, the opposite effect to what we actually observed in our experiments. Finally, we could show the same effect using blockers shown to be highly isoform selective inhibitors of PI3K in other systems (Camps et al. 2005; Chaussade et al. 2007; Ciraolo et al. 2008; Guillermet-Guibert et al. 2008).
The direction of the effect of PI3K blockade on the electrophysiological response to H100, like the effect on the Ca2+ response reported earlier (Spehr et al. 2002), was always to increase the magnitude of the response, suggesting that blockade of PI3K could be relieving inhibition from one or more inhibitory odorants in the mixture. Our ability to now show blockade of PI3K relieves inhibition of a single odorant on another strongly supports that interpretation. Our data also indicate that the magnitude of the effect can be significant. The PI3K-dependent increase in both the electrophysiological discharge and the Ca2+ response of the cells translated into a left-shift of the dose–response curve of about 0.6 log unit. This shift correlates to that evoked by exogenous PIP3 on the native olfactory CNG channel, which right-shifted the cAMP-dependent activation of the channel by 0.78 log unit (Zhainazarov et al. 2004). Although the ability of PIP3 to suppress the sensitivity of the olfactory CNG channel to cAMP could potentially account for the electrophysiological effect we report here, we have yet to understand the detailed mechanism of action of PI3K-dependent inhibition in vivo.
We conclude that PI3K signaling functions in many ORNs rather than in a specialized subset of cells. Many, if not most, cells in the ORN layer were immunopositive for PI3Kβ, an isoform of PI3K that could be implicated functionally. Although localization of PI3Kγ in rat tissue was not possible for technical reasons, results from PI3Kγ transgenic mice (Brunert et al., unpublished data) indicate that this isoform is equally abundant in the OE of rodents. Ubiquitous expression would also be consistent with our pharmacological evidence that isoform-specific blockade of both PI3Kβ and PI3Kγ can modulate the output of the same ORNs. The functional significance of having both the β and γ isoforms of PI3K in the same cell is unclear and beyond the scope of the present study, although the β and γ isoforms of PI3K function in a redundant manner in human neutrophils, mouse fibroblasts, and macrophages (Condliffe et al. 2005; Guillermet-Guibert et al. 2008) and could do so in ORNs as well.
We conclude that PI3K-dependent signaling occurs in mature canonical ORNs. PI3Kβ could be colocalized with OMP and adenylate cyclase 3, markers for mature functional ORNs. Cells showing PI3K-dependent modulation could be recorded by patch-clamping dendritic knobs projecting at the surface of the OE, typically assumed to extend from mature, functional ORNs. Finally, cells showing PI3K-dependent, including PI3Kβ-dependent, modulation responded to IBMX/forskolin, hallmark probes for the cyclic nucleotide signaling pathway in mature canonical ORNs. The presence of PI3K signaling in many mature ORNs and not just a specialized subset of cells is consistent with the idea that PI3K-dependent signaling serves a fundamental role in canonical ORNs such as mediating inhibitory input.
Finding PI3K in many or most mature ORNs would seem to conflict with the observation that only 10–15% of the cells activated by H100 showed PI3K-dependent response enhancement. We interpret the incidence of the effect to be constrained by the odorant. H100 was used as an odorant to increase the possibility of having at least one excitatory and one inhibitory ligand for a reasonable percentage of ORNs. Even an odorant like H100, however, contains only a small fraction of the potential ligands for the nearly 1,500 ORs predicted in mouse and rat (Godfrey et al. 2004; Quignon et al. 2005). Accordingly, one would predict the incidence of seeing a physiological effect even in response to H100 would be relatively low. Our ability to show that the inhibition of one single odorant on another is PI3K dependent supports this interpretation. The incidence rate of the effect could also depend on the concentration of individual odorants. For example, in H100 used in experiments each odorant was present at about 0.6 μM, close to the absolute sensitivity threshold. However, in the experiment with a binary mixture both octanol and citral were at 50 and 100 μM, probably having a better chance to activate PI3K signaling and thus to increase the probability of detecting the effect.
We conclude that PI3K is activated through G proteins. AS-605240 and AS-252424, both well-characterized inhibitors with high selectivity for PI3Kγ, mimicked the effect of the pan-specific inhibitors on both the electrophysiological and the Ca2+ responses of the cells. PI3Kγ is well established to couple through GPCRs in other systems (Graness et al. 1998; Maier et al. 1999). TGX-221, a selective inhibitor of PI3Kβ, also mimicked the effect of the pan-specific inhibitors on the odorant-evoked response and is increasingly recognized to also couple through GPCRs in other systems (Condliffe et al. 2005; Guillermet-Guibert et al. 2008; Murga et al. 2000; Yart et al. 2002). Having odorants activate PI3K in a G-protein–dependent manner is consistent with our understanding that known mammalian ORs are GPCRs (Buck 1992). Our data do not address the interesting possibility that this could be a G protein other than Golf, the excitatory G protein in canonical ORNs. Certainly other receptors signal in a ligand-specific manner through multiple G proteins, including the β2-adrenergic receptor (Xiao 2001), the corticotropin-releasing factor receptor (Blank et al. 2003), and the dopamine D1 receptor (Mannoury la Cour et al. 2007), and multiple G proteins in addition to Gs/Golf, including Gi/o and Gq, occur in olfactory cilia (Gorojankina et al. 2007; Mayer et al. 2008; Schandar et al. 1998).
Collectively, our results provide compelling support for the idea that PI3K-dependent signaling mediates inhibitory odorant input to mammalian ORNs and at least in part contributes to the mixture suppression typically seen in the response of ORNs to complex natural odorants. Knowing which odorants activate PI3K, the nature of the receptor coupling, and the possible interaction with PLC-dependent signaling (Klasen et al. 2010) will be important next steps to more fully understand the detailed role of phosphoinositide signaling in mammalian olfactory transduction.
GRANTS
This work was supported by the National Institute on Deafness and Other Communication Disorders Grants DC-05512 to B. W. Ache and DC-009730 to E. A. Corey, a Deutsche Forschungsgemeinschaft Postdoctoral Fellowship to K. Klasen, and a Feodor Lynen Research Fellowship from the Alexander von Humboldt Foundation to D. Brunert.
ACKNOWLEDGMENTS
We thank Dr. Ming-Hong Ma laboratory members for detailed technical advice and Drs. Emilio Hirsch, Bart Vanhaesbroeck, and Yuriy Bobkov for conceptual input. Mouse monoclonal p110β antibody was a generous gift from Dr. Emilio Hirsch and Henkel-100 was a gift from Dr. Hanns Hatt.
REFERENCES
- Ache BW, Young JM. Olfaction: diverse species, conserved principles. Neuron 48: 417–430, 2005 [DOI] [PubMed] [Google Scholar]
- Araneda RC, Peterlin Z, Zhang X, Chesler A, Firestein S. A pharmacological profile of the aldehyde receptor repertoire in rat olfactory epithelium. J Physiol 555: 743–756, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J 296: 297–301, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalyar HA, Reed RR. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science 250: 1403–1406, 1990 [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci 23: 700–707, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J, Bonigk W, Yau KW, Frings S. Calmodulin permanently associates with rat olfactory CNG channels under native conditions. Nat Neurosci 7: 705–710, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J, Reisert J, Frings S. Regulation of cyclic nucleotide-gated channels. Curr Opin Neurobiol 15: 343–349, 2005 [DOI] [PubMed] [Google Scholar]
- Brady JD, Rich ED, Martens JR, Karpen JW, Varnum MD, Brown RL. Interplay between PIP3 and calmodulin regulation of olfactory cyclic nucleotide-gated channels. Proc Natl Acad Sci USA 103: 15635–15640, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breer H, Fleischer J, Strotmann J. The sense of smell: multiple olfactory subsystems. Cell Mol Life Sci 63: 1465–1475, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck LB. The olfactory multigene family. Curr Opin Neurobiol 2: 282–288, 1992 [DOI] [PubMed] [Google Scholar]
- Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Francon B, Martin T, Gretener D, Perrin D, Leroy D, Vitte PA, Hirsch E, Wymann MP, Cirillo R, Schwarz MK, Rommel C. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 11: 936–943, 2005 [DOI] [PubMed] [Google Scholar]
- Chaussade C, Rewcastle GW, Kendall JD, Denny WA, Cho K, Gronning LM, Chong ML, Anagnostou SH, Jackson SP, Daniele N, Shepherd PR. Evidence for functional redundancy of class IA PI3K isoforms in insulin signalling. Biochem J 404: 449–458, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C, Wu H, Dastru W, Martin EL, Silengo L, Altruda F, Turco E, Lanzetti L, Musiani P, Ruckle T, Rommel C, Backer JM, Forni G, Wymann MP, Hirsch E. Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Sci Signal 1: ra3, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condliffe AM, Davidson K, Anderson KE, Ellson CD, Crabbe T, Okkenhaug K, Vanhaesebroeck B, Turner M, Webb L, Wymann MP, Hirsch E, Ruckle T, Camps M, Rommel C, Jackson SP, Chilvers ER, Stephens LR, Hawkins PT. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood 106: 1432–1440, 2005 [DOI] [PubMed] [Google Scholar]
- Dormann D, Weijer G, Dowler S, Weijer CJ. In vivo analysis of 3-phosphoinositide dynamics during Dictyostelium phagocytosis and chemotaxis. J Cell Sci 117: 6497–6509, 2004 [DOI] [PubMed] [Google Scholar]
- Duchamp-Viret P, Chaput MA, Duchamp A. Odor response properties of rat olfactory receptor neurons. Science 284: 2171–2174, 1999 [DOI] [PubMed] [Google Scholar]
- El-Kholy W, Macdonald PE, Lin JH, Wang J, Fox JM, Light PE, Wang Q, Tsushima RG, Wheeler MB. The phosphatidylinositol 3-kinase inhibitor LY294002 potently blocks K(V) currents via a direct mechanism. FASEB J 17: 720–722, 2003 [DOI] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem 67: 481–507, 1998 [DOI] [PubMed] [Google Scholar]
- Gharbi SI, Zvelebil MJ, Shuttleworth SJ, Hancox T, Saghir N, Timms JF, Waterfield MD. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J 404: 15–21, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey PA, Malnic B, Buck LB. The mouse olfactory receptor gene family. Proc Natl Acad Sci USA 101: 2156–2161, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold GH. Controversial issues in vertebrate olfactory transduction. Annu Rev Physiol 61: 857–871, 1999 [DOI] [PubMed] [Google Scholar]
- Gorojankina T, Grebert D, Salesse R, Tanfin Z, Caillol M. Study of orexins signal transduction pathways in rat olfactory mucosa and in olfactory sensory neurons-derived cell line Odora: multiple orexin signalling pathways. Regul Pept 141: 73–85, 2007 [DOI] [PubMed] [Google Scholar]
- Graness A, Adomeit A, Heinze R, Wetzker R, Liebmann C. A novel mitogenic signaling pathway of bradykinin in the human colon carcinoma cell line SW-480 involves sequential activation of a Gq/11 protein, phosphatidylinositol 3-kinase beta, and protein kinase Cepsilon. J Biol Chem 273: 32016–32022, 1998 [DOI] [PubMed] [Google Scholar]
- Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A, Meek S, Smith AJ, Okkenhaug K, Vanhaesebroeck B. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc Natl Acad Sci USA 105: 8292–8297, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 287: 1049–1053, 2000 [DOI] [PubMed] [Google Scholar]
- Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science 244: 790–795, 1989 [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev 82: 769–824, 2002 [DOI] [PubMed] [Google Scholar]
- Klasen K, Corey EA, Kuck F, Wetzel CH, Hatt H, Ache BW. Odorant-stimulated phosphoinositide signaling in mammalian olfactory receptor neurons. Cell Signal 22: 150–157, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene SJ. Origin of the chloride current in olfactory transduction. Neuron 11: 123–132, 1993 [DOI] [PubMed] [Google Scholar]
- Le Blanc C, Mironneau C, Barbot C, Henaff M, Bondeva T, Wetzker R, Macrez N. Regulation of vascular L-type Ca2+ channels by phosphatidylinositol 3,4,5-trisphosphate. Circ Res 95: 300–307, 2004 [DOI] [PubMed] [Google Scholar]
- Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science 287: 1046–1049, 2000 [DOI] [PubMed] [Google Scholar]
- Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci USA 104: 2471–2476, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA 100: 15160–15165, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M. Encoding olfactory signals via multiple chemosensory systems. Crit Rev Biochem Mol Biol 42: 463–480, 2007 [DOI] [PubMed] [Google Scholar]
- Ma M, Chen WR, Shepherd GM. Electrophysiological characterization of rat and mouse olfactory receptor neurons from an intact epithelial preparation. J Neurosci Methods 92: 31–40, 1999 [DOI] [PubMed] [Google Scholar]
- Maier U, Babich A, Nurnberg B. Roles of non-catalytic subunits in gbetagamma-induced activation of class I phosphoinositide 3-kinase isoforms beta and gamma. J Biol Chem 274: 29311–29317, 1999 [DOI] [PubMed] [Google Scholar]
- Mannoury la Cour C, Vidal S, Pasteau V, Cussac D, Millan MJ. Dopamine D1 receptor coupling to Gs/olf and Gq in rat striatum and cortex: a scintillation proximity assay (SPA)/antibody-capture characterization of benzazepine agonists. Neuropharmacology 52: 1003–1014, 2007 [DOI] [PubMed] [Google Scholar]
- Mayer U, Ungerer N, Klimmeck D, Warnken U, Schnolzer M, Frings S, Mohrlen F. Proteomic analysis of a membrane preparation from rat olfactory sensory cilia. Chem Senses 33: 145–162, 2008 [DOI] [PubMed] [Google Scholar]
- Moon C, Liu BQ, Kim SY, Kim EJ, Park YJ, Yoo JY, Han HS, Bae YC, Ronnett GV. Leukemia inhibitory factor promotes olfactory sensory neuronal survival via phosphoinositide 3-kinase pathway activation and Bcl-2. J Neurosci Res 87: 1098–1106, 2009 [DOI] [PubMed] [Google Scholar]
- Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Annu Rev Physiol 71: 115–140, 2009 [DOI] [PubMed] [Google Scholar]
- Murga C, Fukuhara S, Gutkind JS. A novel role for phosphatidylinositol 3-kinase beta in signaling from G protein-coupled receptors to Akt. J Biol Chem 275: 12069–12073, 2000 [DOI] [PubMed] [Google Scholar]
- Nilius B, Mahieu F, Karashima Y, Voets T. Regulation of TRP channels: a voltage-lipid connection. Biochem Soc Trans 35: 105–108, 2007 [DOI] [PubMed] [Google Scholar]
- Noe J, Breer H. Functional and molecular characterization of individual olfactory neurons. J Neurochem 71: 2286–2293, 1998 [DOI] [PubMed] [Google Scholar]
- Peterlin Z, Ishizawa Y, Araneda R, Eckenhoff R, Firestein S. Selective activation of G-protein coupled receptors by volatile anesthetics. Mol Cell Neurosci 30: 506–512, 2005 [DOI] [PubMed] [Google Scholar]
- Quignon P, Giraud M, Rimbault M, Lavigne P, Tacher S, Morin E, Retout E, Valin AS, Lindblad-Toh K, Nicolas J, Galibert F. The dog and rat olfactory receptor repertoires. Genome Biol 6: R83, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schandar M, Laugwitz KL, Boekhoff I, Kroner C, Gudermann T, Schultz G, Breer H. Odorants selectively activate distinct G protein subtypes in olfactory cilia. J Biol Chem 273: 16669–16677, 1998 [DOI] [PubMed] [Google Scholar]
- Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol Rev 78: 429–466, 1998 [DOI] [PubMed] [Google Scholar]
- Spehr M, Wetzel CH, Hatt H, Ache BW. 3-Phosphoinositides modulate cyclic nucleotide signaling in olfactory receptor neurons. Neuron 33: 731–739, 2002 [DOI] [PubMed] [Google Scholar]
- Trombley PQ, Westbrook GL. Voltage-gated currents in identified rat olfactory receptor neurons. J Neurosci 11: 435–444, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem 70: 535–602, 2001 [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269: 5241–5248, 1994 [PubMed] [Google Scholar]
- Washburn KB, Turner TJ, Talamo BR. Comparison of mechanical agitation and calcium shock methods for preparation of a membrane fraction enriched in olfactory cilia. Chem Senses 27: 635–642, 2002 [DOI] [PubMed] [Google Scholar]
- Welling A, Hofmann F, Wegener JW. Inhibition of L-type Cav1.2 Ca2+ channels by 2,(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) and 2-[1-(3-dimethyl-aminopropyl)-5-methoxyindol-3-yl]-3-(1H-indol-3-yl) maleimide (Go6983). Mol Pharmacol 67: 541–544, 2005 [DOI] [PubMed] [Google Scholar]
- Wetzel CH, Oles M, Wellerdieck C, Kuczkowiak M, Gisselmann G, Hatt H. Specificity and sensitivity of a human olfactory receptor functionally expressed in human embryonic kidney 293 cells and Xenopus laevis oocytes. J Neurosci 19: 7426–7433, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao RP. Beta-adrenergic signaling in the heart: dual coupling of the beta2-adrenergic receptor to G(s) and G(i) proteins. Sci STKE 2001: RE15, 2001 [DOI] [PubMed] [Google Scholar]
- Yart A, Roche S, Wetzker R, Laffargue M, Tonks N, Mayeux P, Chap H, Raynal P. A function for phosphoinositide 3-kinase beta lipid products in coupling beta gamma to Ras activation in response to lysophosphatidic acid. J Biol Chem 277: 21167–21178, 2002 [DOI] [PubMed] [Google Scholar]
- Zhainazarov AB, Spehr M, Wetzel CH, Hatt H, Ache BW. Modulation of the olfactory CNG channel by Ptdlns(3,4,5)P3. J Membr Biol 201: 51–57, 2004 [DOI] [PubMed] [Google Scholar]