Abstract
High-density lipoproteins (HDLs) are protein-lipid assemblies that remove excess cell cholesterol and prevent atherosclerosis. HDL are stabilized by kinetic barriers that decelerate protein dissociation and lipoprotein fusion. We propose that similar barriers modulate metabolic remodeling of plasma HDLs; hence, changes in particle composition that destabilize HDLs and accelerate their denaturation may accelerate their metabolic remodeling. To test this notion, we correlate existing reports on HDL-mediated cell cholesterol efflux and esterification, which are obligatory early steps in cholesterol removal, with our kinetic studies of HDL stability. The results support our hypothesis and show that factors accelerating cholesterol efflux and esterification in model discoidal lipoproteins (including reduced protein size, reduced fatty acyl chain length and/or increased cis-unsaturation) destabilize lipoproteins and accelerate their fusion and apolipoprotein dissociation. Oxidation studies of plasma spherical HDL show a similar trend: mild oxidation by Cu2+ or OCl- accelerates cell cholesterol efflux, protein dissociation and HDL fusion, while extensive oxidation inhibits these reactions. Consequently, moderate destabilization may benefit HDL functions by facilitating insertion of cholesterol and lipophilic enzymes, promoting dissociation of lipid-poor apolipoproteins, which are primary acceptors of cell cholesterol, and thereby accelerating HDL metabolism. Therefore, HDL stability must be delicately balanced to maintain structural integrity of the lipoprotein assembly and ensure structural specificity necessary for HDL interactions with its metabolic partners, while facilitating rapid HDL remodeling and turnover at key junctures of cholesterol transport. The inverse correlation between HDL stability and remodeling illustrates the functional importance of structural disorder in macromolecular assemblies stabilized by kinetic barriers.
Keywords: Kinetic stability, apolipoprotein dissociation, lipoprotein fusion, reverse cholesterol transport, structural disorder, atherosclerosis
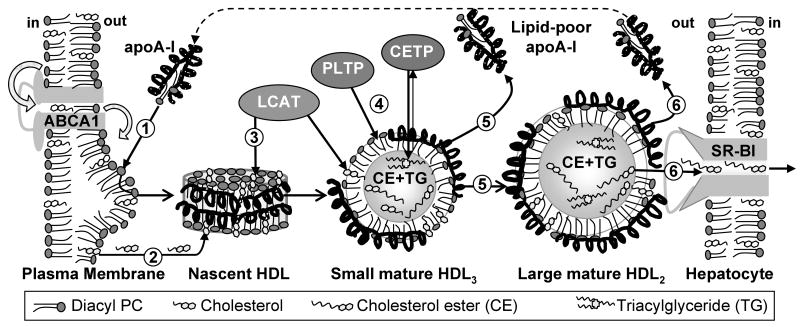
High-density lipoproteins (HDLs) are heterogeneous complexes 7-13 nm in diameter that are composed of several major proteins (termed apolipoproteins), many minor proteins, and variable amounts of lipid molecules. Plasma concentrations of HDL and its major protein, apoA-I (243 a.a.), correlate inversely with the risk of developing coronary artery disease (reviewed in (1, 2)). This cardioprotection results from the central role of HDLs in reverse cholesterol transport (RCT) and from their anti-oxidant and anti-inflammatory action (3). RCT is the sole pathway of cholesterol removal from peripheral tissues to the liver for excretion or to steroidogenic organs for hormone synthesis (1). During RCT, cell cholesterol is taken up by nascent discoidal HDLs or their lipid-poor precursors (4), esterified by lecithin:cholesterol acyltransferase (LCAT), and sequestered in the core of HDLs, thereby converting them to mature spherical particles (Fig. 1, steps 1-3). After further remodeling (steps 4, 5), large spherical HDLs deliver their cargo of cholesterol esters to the liver via the selective lipid uptake mediated by the scavenger receptor BI (SR-BI, step 6) (5). Throughout RCT, HDLs undergo dynamic remodeling by lipophilic enzymes, lipid transfer proteins (such as cholesterol ester transfer protein, CETP) and lipoprotein receptors (6). This may involve insertion of lipids (such as cholesterol) and proteins (such as LCAT and CETP), as well as apolipoprotein dissociation and HDL fusion that compensate for the imbalance between the particle core and surface upon remodeling. We propose that such remodeling is facilitated, in part, by the structural disorder in HDL surface.
Figure 1.
Simplified diagram of reverse cholesterol transport. ① Lipid-poor apoA-I interacts with protrusions on the cell surface created by ABCA1 transporter. ② This active lipid transport leads to formation of nascent discoidal HDLs that accept additional cell cholesterol via such mechanisms as passive diffusion or interactions with ABCG1 transporter. ③ LCAT action leads to cholesterol ester (CE) accumulation in the core of nascent discoidal HDLs, converting them into mature spherical particles. ④ These small HDL3 are further remodeled by LCAT and other plasma factors, such as phospholipid and cholesterol ester transfer proteins, PLTP and CETP. ⑤ The resulting imbalance between the particle core and surface is compensated by HDL fusion and dissociation of lipid-poor apoA-I. ⑥ Interactions of HDL and hepatic scavenger receptor (SR-BI) lead to selective uptake of apolar core lipids and HDL disintegration; the dissociated apoA-I reenters RCT as lipid-poor or lipid-bound species.
To test this notion, we correlate existing functional data, including studies of cell cholesterol efflux and esterification using reconstituted or plasma HDLs of various compositions (7-13), with our kinetic stability studies of these lipoproteins (14-17) (Table S1, online supplement). Earlier studies, which were based on thermodynamic approach to HDL stability such as spectroscopic “end-point” measurements after incubation with denaturants ((8-12) and references therein), revealed a correlation between HDL functions and lipid fluidity (7- 9, 11) but showed no clear linkage with HDL stability. In contrast, our denaturation rate measurements in kinetic experiments (illustrated in Figs. 1S, online supplement) suggest an inverse correlation between HDL stability and metabolic remodeling in RCT (16, 17).
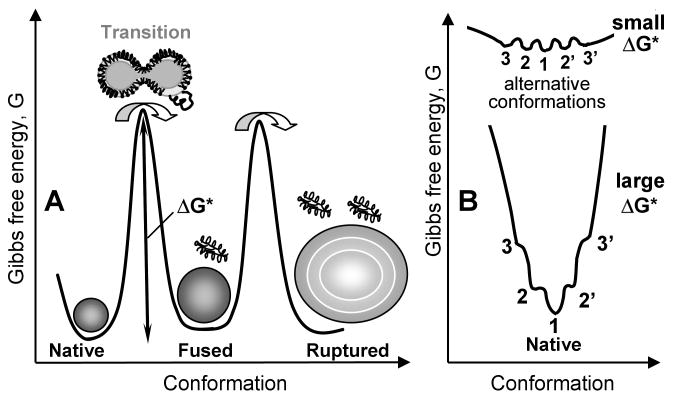
The kinetic approach to lipoprotein stability stems from the observation that HDL denaturation is a thermodynamically irreversible transition involving apolipoprotein unfolding, dissociation, and HDL fusion into larger particles that retain lipoprotein morphology (18-20). Upon further protein dissociation, spherical HDL disintegrate, rupture, and release their apolar core lipids that coalesce into large droplets (19, 20). The slow unfolding rate k(T) of HDL proteins reflects a high free energy barrier, ΔG* ∝ -RT ln k(T), that arises from transient disruption of protein and lipid interactions and transient solvent exposure of the apolar groups during protein dissociation and lipoprotein fusion and rupture. Unlike in the high-energy transition state, in native and fused HDL states most apolar groups are sequestered and polar groups are solvent-exposed, leading to the relatively low free energies of these states (Fig. 2A). Kinetic lipoprotein stability is defined by the free energy barriers separating native from fused and denatured states.
Figure 2.
Free energy diagram illustrating global kinetic stability and local structural disorder. A – Kinetic stability of native HDL (discoidal or spherical) is defined by the free energy barrier ΔG* = Gtrans – Gnative that decelerates protein dissociation and lipoprotein fusion (shown by double arrow); Gtrans refers to the high-energy transition state (16, 21). In spherical HDL, the second kinetic step involves further protein dissociation, particle rupture and release of apolar core lipids (19, 20). The order of magnitude of these barriers is ∼20 kcal/mol (16, 18). B - Linkage between the global structural stability and local disorder shown by zooming in on the free energy profile of the native state. If ΔG* is small (low global stability, top), then the alternative conformations in the native state (marked 1-3) have comparable occupancies since they have similar free energies, G1 ≅ G2 ≅ G3…, and are separated by low energy barriers (comparable to the free energy of thermal motion, RT∼0.6 kcal/mol). If ΔG* is large (high global stability, bottom), then G1 < G2 < G3, hence only state 1 is occupied with high probability while states 2, 3, etc. have significantly lower occupancies. Thus, low global stability facilitates structural flexibility (top) while high global stability hampers it (bottom).
Since both denaturation and metabolic remodeling of HDLs involve apolipoprotein dissociation and lipoprotein fusion, we proposed that these transitions are modulated by similar kinetic barriers (19, 20). Furthermore, since HDL rupture, which involves lipoprotein disintegration and release of the apolar lipids, occurs both at the final stage of denaturation and at the final stage of RCT during SR-BI-mediated lipid uptake (5, 21), these in vitro and in vivo transitions may involve similar kinetic barriers. If our hypothesis is correct, then less stable HDLs will tend to undergo faster metabolic remodeling during RCT. To test this notion, we correlate functional and kinetic stability studies of HDLs varying in protein and lipid composition. Below, we briefly describe the key steps in RCT and outline the role of structural disorder in HDL remodeling at these steps (summarized in Table S1).
Structural disorder facilitates lipid efflux from plasma membrane to apolipoproteins
ApoA-I and other exchangeable (water-soluble) apolipoproteins are secreted from the liver and gut in a lipid-free or lipid-poor form; lipid-poor apolipoproteins are also formed upon metabolic remodeling of HDLs and other lipoproteins (4, 21). These physiologically important species are rapidly converted to the lipid-bound state by binding to the existing lipoproteins or by taking up cell cholesterol and phospholipids from plasma membranes. This active lipid efflux is mediated by ATP-binding cassette (ABC) transporter A1 (ABCA1), a transmembrane protein that moves lipids from the inner to the outer leaflet, thereby bending the membrane and creating protrusions that facilitate apolipoprotein insertion (22, 23) (Fig. 1, step 1). Lipid-poor apolipoproteins, which are thought to comprise one protein and several lipid molecules, are the primary lipid acceptors in this process (4, 21). Structural stability of these transient species is probably comparable to the low thermodynamic stability of lipid-free apolipoproteins at near-physiologic conditions, ΔG=Gunfolded–Gnative<2.5 kcal/mol. This and other properties of apolipoproteins in solution (such as compact globular shape with substantial secondary but loosely folded tertiary structure, low-cooperativity unfolding with low effective enthalpy and low heat capacity increment, high aggregating propensity, binding of small apolar ligands, etc.) are characteristic of the molten globular state (24). Tertiary structure flexibility may reflect the lack of polar or charged residues in the large apolar faces of the apolipoprotein α-helices (25); hence, these helices cannot form buried H-bonds or salt bridges that confer specificity and stability to the structures of globular proteins. This tertiary structural plasticity may facilitate conformational changes necessary for apolipoprotein binding to lipid surface (24, 26). In summary, structural disorder in the lipid acceptor (apolipoproteins) and the donor (plasma membrane distorted by ABCA1) facilitates the first step in RCT.
Local disorder in nascent discoidal HDL facilitates cholesterol insertion and esterification
Efflux of phospholipids and cholesterol to lipid-poor or lipid-free apolipoproteins results in the formation of nascent discoidal HDLs. These particles are comprised of the cholesterol-containing phospholipid bilayer and apolipoproteins wrapped around the disk perimeter in a belt-like α-helical conformation (27, 28). Nascent HDLs acquire additional cell cholesterol via passive diffusion (Fig. 1, step 2) or active transport via ABCG1 (reviewed in (29)), and form preferred substrates for LCAT.
What structural and stability properties of nascent HDLs facilitate cholesterol insertion and esterification? This was explored by using reconstituted discoidal HDLs of controlled composition, which provide excellent models for nascent HDLs. Davidson and colleagues studied binary complexes of apoA-I with diacyl PCs containing 14 to 18-carbon chains that were fully saturated or contained one or two cis-double bonds (oleic or linoleic) in sn-2 position; the results revealed that shorter-chain and cis-unsaturated PCs form more efficient acceptor particles for cell cholesterol (11). Our kinetic stability studies of apolipoprotein complexes with these lipids showed that shorter-chain and cis-unsaturated PCs form less stable disks: thus, a decrease in length by two CH2 groups in fully saturated acyl chains or the presence of a cis-double bonds lowers kinetic stability of apoC-I: PC disks by δΔG*(37°C) ≅ 1.4 kcal/mol (16). A similar trend is observed in apoA-I:PC disks ((16) and Fig. 2S, 3S in the supplement). This suggests an inverse correlation between kinetic stability of discoidal HDLs and their ability to accept cholesterol. Studies of lipoproteins varying in protein composition further support this notion: binary complexes of DMPC with smaller and less helical proteins tend to be less stable (14, 15) and form more efficient cholesterol acceptors (12). Since the cells used in the functional studies contained little ABCA1 or ABCG1 (10, 11), cell cholesterol efflux in these studies occurred mainly via passive diffusion to discoidal HDLs. Thus, the inverse correlation between the stability of discoidal HDLs and their ability to accept cholesterol suggests that global destabilization of a lipoprotein increases local disorder in its surface (Fig. 2B), thereby accelerating cholesterol insertion into this surface.
Disorder in the lipid bilayer may also be important for PC lipolyisis by the surface-active proteins, LCAT and phospholipase A2 (PLA2), whose function requires their insertion into the lipid surface. Hydrophobic packing defects in the lipid bilayer play key role in insertion and activation of PLA2 (30, 31). Since PLA2 reaction is the first step in LCAT reaction (which hydrolyses sn-2 acyl chain from PC prior to transferring it to cholesterol to form cholesterol ester), LCAT activity is also expected to be enhanced by disorder in the HDL surface. Our results, together with studies of Pownall et al., support this notion: decrease in acyl chain length from 18 to 12 carbons or increase in the number of cis-double bonds from 0 to 1 or 2 in diacyl PC complexes with apoA-I or apoC-I destabilizes these complexes ((16) and Figs. S2, S3) and augments their ability to activate LCAT (7).
This differs from the effect of bilayer thickness on integral membrane proteins such as rhodopsins, hexose transporter, diacylclycerol kinase, ATPases, etc. The activity of these proteins (defined by the rate of photoactivation, transport, catalysis, etc.) is usually maximal for 18-carbon acyl chains, which are the predominant species in biological membranes, as a result of optimized hydrophobic match between the protein and lipid bilayer (reviewed in (32)). In contrast, PLA2 and LCAT have higher activity with shorter-chain PCs (7, 30), probably due to increased local disorder in a thinner bilayer (33) facilitating enzyme insertion that is necessary for lipolysis. In summary, moderate destabilization of nascent discodial HDLs and the ensuing increase in surface disorder promote the insertion of cholesterol and LCAT, thereby facilitating efflux and esterification of cholesterol at early steps of RCT (Fig. 1, steps 2 and 3).
Destabilization of spherical HDLs promotes their metabolic remodeling and fusion
Cholesterol esters produced by LCAT move from the lipoprotein surface to its core, leading to formation of mature spherical HDLs. Further remodeling of these small particles by plasma factors creates an imbalance between the apolar core and the amphipathic surface, which may lead to apolipoprotein dissociation and HDL fusion. For example, LCAT action on small spherical HDL3 (d=7-9 nm, two apoA-I per particle) depletes surface cholesterol and phospholipids and produces cholesterol esters that accumulate in the core; this surface depletion and core expansion are compensated by fusion of HDL3 into larger HDL2 particles (d=10-13 nm, three-to-four apoA-I) that may be accompanied by dissociation of lipid-poor apoA-I (4). Another example is CETP, which exchanges triacylglycerides from low- and very low-density lipoproteins for cholesterol esters in HDL (34). This equimolar exchange shrinks the core, since depletion of cholesterol esters exceeds enrichment with triacylglycerides that are hydrolyzed by hepatic lipase; the ensuing imbalance between HDL core and surface induces dissociation of lipid-poor apoA-I and HDL fusion (6, 35). Furthermore, phospholipid transfer protein, which transfers phospholipids from chylomicrons and very low-density lipoproteins to HDL, also creates an imbalance between the particle core and surface, leading to HDL fusion and dissociation of lipid-poor apoA-I (36) (Fig. 1, steps 4, 5). Finally, selective uptake of HDL core lipids by SR-BI at the last step of RCT generates excess surface material, including lipid-poor apolipoproteins that re-enter RCT (5, 21) (Fig. 1, step 6); this HDL disassembly resembles rupture and release of core lipids and dissociated proteins upon denaturation (19, 20). Thus, HDL fusion and rupture accompanied by apolipoprotein dissociation occur during metabolic remodeling by several plasma factors (4-6).
Since both denaturation and metabolic remodeling of mature spherical HDL involve protein dissociation and lipoprotein fusion, these in vitro and in vivo HDL transitions may be modulated by similar kinetic barriers (19). Several lines of evidence support this notion (Table S1). First is the role of apoA-II, a second-major HDL protein with unclear function (37). The consensus is that apoA-II on spherical HDLs i) stabilizes lipoproteins against chemical or thermal denaturation (38) and ii) counteracts the anti-atherogenic effects of apoA-I, apparently by impeding dissociation of lipid-poor apoA-I and decelerating HDL metabolism (37, 39). This suggests that apoA-II decelerates both denaturation and metabolic remodeling of spherical HDL, which supports our central hypothesis. Second is the effect of oxidation on HDL stability and functions. Recent studies of human plasma HDLs oxidized by copper or hypochlorite showed that mild oxidation (which involves modification of Met and certain aromatic residues in the apolar helical faces and apolipoprotein cross-linking into dimers and trimers) accelerates protein dissociation and HDL fusion and improves HDL ability to accept cell cholesterol via the ABCA1-mediated transport to dissociated apoA-I; this may result from reduced affinity of mildly oxidized apolipoproteins for the lipid surface, which facilitates protein dissociation (13, 17). In contrast, advanced oxidation (which leads to massive protein cross-linking and lipolysis) prevents protein dissociation and lipoprotein fusion and reduces HDL ability to accept cholesterol (13, 17); this may result, in part, from lipolysis of core lipids (17).
Taken together, these studies suggest that the inverse correlation between HDL stability and ability to undergo functional remodeling is a general trend that applies to both discoidal and spherical HDL. Since moderate destabilization accelerates HDL remodeling at key junctures of RCT, it may benefit HDL functions in cholesterol removal. Importantly, this general trend may not always apply to specific steps in RCT that may be modulated by multiple factors. For example, LCAT reaction requires not only disorder in HDL surface, but also specific interactions between the partners (such as apoA-I, LCAT, PCs and cholesterol). Since optimal conditions for such specificity may differ from those which optimize surface disorder (8), structural disorder and specificity must be delicately balanced for optimal HDL functions.
Functional role of structural disorder in proteins and their complexes
Even though insertion of cholesterol and LCAT into HDL surface at early steps of RCT may not involve a global structural transition from intact HDL to fused particles and dissociated lipid-poor proteins, reduction in the free energy barrier for such a transition may promote local structural fluctuations in HDL surface, thereby facilitating this insertion (Fig. 2B). Similarly, global protein transitions are often coupled to local structural fluctuations that modulate ligand binding (40). These and other types of intrinsic disorder have been found in an increasing number of proteins (41-44), including those involved in cardiovascular disease (24, 26, 44). Intrinsic disorder in these proteins is thought to be functionally important for binding to phospholipid surface, high specificity / low affinity binding, specific binding to multiple targets, rapid protein turnover, allosteric regulation, and signal transduction (24, 40-44). We propose that intrinsic disorder is also important for functions of macromolecular assemblies that are stabilized by kinetic barriers, such as lipoproteins.
In summary, moderate structural disorder accelerates metabolic remodeling of HDLs, which may benefit HDL functions in cholesterol removal. Therefore, HDL stability must be delicately balanced to maintain the structural integrity of the lipoprotein assembly and ensure structural specificity necessary for HDL interactions with its metabolic partners, yet enable efficient efflux and processing of cell cholesterol and rapid HDL remodeling and turnover at key junctures of RCT.
Supplementary Material
Acknowledgments
We are indebted to Dr. Donald M. Small for reading the manuscript prior to submission, to Dr. Sangeeta Benjwal for many useful discussions, and to David Plotkin for editorial assistance.
This work was supported by the National Institutes of Health grants RO1 GM 067260 and HL 026355.
Abbreviations
- HDL
high-density lipoprotein
- apo
apolipoprotein
- RCT
reverse cholesterol transport
- LCAT
lecithin:cholesterol acyltransferase
- ABC
ATP-binding cassette transporter
- SR-BI
scavenger receptor BI
- CETP
cholesterol ester transfer protein
- PLA2
phospholipase A2
- PC
phosphatidylcholine
Footnotes
Supporting Information Available Table S1 lists references that support the correlation between structural stability and functions of HDL. Figures 1S-3S show thermal denaturation data of apoA-I complexes with PCs. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.von Eckardstein A, Nofer JR, Assman G. High density lipoproteins and arteriosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:13–27. doi: 10.1161/01.atv.21.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Barter PJ, Rye KA. Relationship between the concentration and antiatherogenic activity of high-density lipoproteins. Curr Opin Lipidol. 2006;17(4):399–403. doi: 10.1097/01.mol.0000236365.40969.af. [DOI] [PubMed] [Google Scholar]
- 3.Barter PJ, Puranik R, Rye KA. New insights into the role of HDL as an anti-inflammatory agent in the prevention of cardiovascular disease. Curr Cardiol Rep. 2007;9(6):493–498. doi: 10.1007/BF02938394. [DOI] [PubMed] [Google Scholar]
- 4.Rye KA, Barter PJ. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 2004;24(3):421–428. doi: 10.1161/01.ATV.0000104029.74961.f5. [DOI] [PubMed] [Google Scholar]
- 5.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271(5248):518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 6.Barter PJ. Hugh Sinclair Lecture: The regulation and remodeling of HDL by plasma factors. Atheroscler Suppl. 2002;3(4):39–47. doi: 10.1016/s1567-5688(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 7.Pownall HJ, Pao Q, Massey JB. Isolation and specificity of rat lecithin: cholesterol acyltransferase: comparison with the human enzyme using reassembled high-density lipoproteins containing ether analogs of phosphatidylcholine. Biochim Biophys Acta. 1985;833(3):456–462. doi: 10.1016/0005-2760(85)90103-1. [DOI] [PubMed] [Google Scholar]
- 8.Parks JS, Gebre AK. Long-chain polyunsaturated fatty acids in the sn-2 position of phosphatidylcholine decrease the stability of recombinant high density lipoprotein apolipoprotein A-I and the activation energy of the lecithin:cholesterol acyltransferase reaction. J Lipid Res. 1997;38(2):266–275. [PubMed] [Google Scholar]
- 9.Parks JS, Huggins KW, Gebre AK, Burleson ER. Phosphatidylcholine fluidity and structure affect lecithin:cholesterol acyltransferase activity. J Lipid Res. 2000;41(4):546–553. [PubMed] [Google Scholar]
- 10.Davidson WS, Lund-Katz S, Johnson WJ, Anantharamaiah GM, Palgunachari MN, Segrest JP, Rothblat GH, Phillips MC. The influence of apolipoprotein structure on the efflux of cellular free cholesterol to high density lipoprotein. J Biol Chem. 1994;269(37):22975–22982. [PubMed] [Google Scholar]
- 11.Davidson WS, Gillotte KL, Lund-Katz S, Johnson WJ, Rothblat GH, Phillips MC. The effect of high density lipoprotein phospholipid acyl chain composition on the efflux of cellular free cholesterol. J Biol Chem. 1995;270(11):5882–5890. doi: 10.1074/jbc.270.11.5882. [DOI] [PubMed] [Google Scholar]
- 12.Boucher J, Ramsamy TA, Braschi S, Sahoo D, Neville TA, Sparks DL. Apolipoprotein A-II regulates HDL stability and affects hepatic lipase association and activity. J Lipid Res. 2004;45(5):849–858. doi: 10.1194/jlr.M300431-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Pirillo A, Uboldi P, Pappalardo G, Kuhn H, Catapano AL. Modification of HDL3 by mild oxidative stress increases ATP-binding cassette transporter 1-mediated cholesterol efflux. Cardiovasc Res. 2007;75(3):566–574. doi: 10.1016/j.cardiores.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Jayaraman S, Gantz DL, Gursky O. Kinetic stabilization and fusion of discoidal lipoproteins containing human apoA-2 and DMPC: Comparison with apoA-1 and apoC-1. Biophys J. 2005;88:2907–2918. doi: 10.1529/biophysj.104.055921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjwal S, Jayaraman S, Gursky O. Role of secondary structure in protein-phospholipid surface interactions: reconstitution and denaturation of apolipoprotein C-I:DMPC complexes. Biochemistry. 2007;46(13):4184–4194. doi: 10.1021/bi062175c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guha M, Gantz DL, Gursky O. Effects of acyl chain length, unsaturation, and pH on thermal stability of model discoidal HDLs. J Lipid Res. 2008;49(8):1752–1761. doi: 10.1194/jlr.M800106-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao X, Jayaraman S, Gursky O. Mild oxidation promotes and advanced oxidation impairs remodeling of human high-density lipoprotein in vitro. J Mol Biol. 2008;376(4):997–1007. doi: 10.1016/j.jmb.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gursky O, Ranjana, Gantz DL. Complex of human apolipoprotein C-1 with phospholipid: thermodynamic or kinetic stability? Biochemistry. 2002;41:7373–7384. doi: 10.1021/bi025588w. [DOI] [PubMed] [Google Scholar]
- 19.Mehta R, Gantz DL, Gursky O. Human plasma high-density lipoproteins are stabilized by kinetic factors. J Mol Biol. 2003;328(1):183–192. doi: 10.1016/s0022-2836(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 20.Jayaraman S, Gantz DL, Gursky O. Effects of salt on thermal stability of human plasma high-density lipoproteins. Biochemistry. 2006;45(14):4620–4628. doi: 10.1021/bi0524565. [DOI] [PubMed] [Google Scholar]
- 21.Duong PT, Weibel GL, Lund-Katz S, Rothblat GH, Phillips MC. Characterization and properties of pre beta-HDL particles formed by ABCA1-mediated cellular lipid efflux to apoA-I. J Lipid Res. 2008;49(5):1006–1014. doi: 10.1194/jlr.M700506-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85(4):1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 23.Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem. 2007;282(34):25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 24.Gursky O, Atkinson D. Thermal unfolding of human high-density apolipoprotein A-1: implications for a lipid-free molten globular state. Proc Natl Acad Sci USA. 1996;93(7):2991–2995. doi: 10.1073/pnas.93.7.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segrest JP, Jones MK, De Loof H, Brouillette CG, Venkatachalapathi YV, Anantharamaiah GM. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J Lipid Res. 1992;33(2):141–166. [PubMed] [Google Scholar]
- 26.Morrow JA, Hatters DM, Lu B, Hochtl P, Oberg KA, Rupp B, Weisgraber KH. Apolipoprotein E4 forms a molten globule. A potential basis for its association with disease. J Biol Chem. 2002;277(52):50380–50385. doi: 10.1074/jbc.M204898200. [DOI] [PubMed] [Google Scholar]
- 27.Lund-Katz S, Liu L, Thuahnai ST, Phillips MC. High density lipoprotein structure. Front Biosci. 2003;8:d1044–d1054. doi: 10.2741/1077. [DOI] [PubMed] [Google Scholar]
- 28.Davidson WS, Thompson TB. The structure of apolipoprotein A-I in high density lipoproteins. J Biol Chem. 2007;282(31):22249–22253. doi: 10.1074/jbc.R700014200. [DOI] [PubMed] [Google Scholar]
- 29.Baldán A, Tarr P, Lee R, Edwards PA. ATP-binding cassette transporter G1 and lipid homeostasis. Curr Opin Lipidol. 2006;17(3):227–232. doi: 10.1097/01.mol.0000226113.89812.bb. [DOI] [PubMed] [Google Scholar]
- 30.Jorgensen K, Davidsen J, Mouritsen OG. Biophysical mechanisms of phospholipase A2 activation and their use in liposome-based drug delivery. FEBS Letters. 2002;531(1):23–27. doi: 10.1016/s0014-5793(02)03408-7. [DOI] [PubMed] [Google Scholar]
- 31.Ray S, Scott JL, Tatulian SA. Effects of lipid phase transition and membrane surface charge on the interfacial activation of phospholipase A2. Biochemistry. 2007;46(45):13089–13100. doi: 10.1021/bi7015102. [DOI] [PubMed] [Google Scholar]
- 32.Lee AG. How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta. 2004;1666(1-2):62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Hønger T, Jørgensen K, Biltonen RL, Mouritsen OG. Systematic relationship between phospholipase A2 activity and dynamic lipid bilayer microheterogeneity. Biochemistry. 1996;35(28):9003–9006. doi: 10.1021/bi960866a. [DOI] [PubMed] [Google Scholar]
- 34.Qiu X, Mistry A, Ammirati MJ, Chrunyk BA, Clark RW, et al. Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat Struct Mol Biol. 2007;14(2):106–113. doi: 10.1038/nsmb1197. [DOI] [PubMed] [Google Scholar]
- 35.Rye KA, Hime NJ, Barter PJ. Evidence that cholesteryl ester transfer protein-mediated reductions in reconstituted high density lipoprotein size involve particle fusion. J Biol Chem. 1997;272(7):3953–3960. doi: 10.1074/jbc.272.7.3953. [DOI] [PubMed] [Google Scholar]
- 36.Settasatian N, Duong M, Curtiss LK, Ehnholm C, Jauhiainen M, Huuskonen J, Rye KA. The mechanism of the remodeling of high density lipoproteins by phospholipid transfer protein. J Biol Chem. 2001;276(29):26898–26905. doi: 10.1074/jbc.M010708200. [DOI] [PubMed] [Google Scholar]
- 37.Blanco-Vaca F, Escolà-Gil JC, Martín-Campos JM, Julve J. Role of apoA-II in lipid metabolism and atherosclerosis: advances in the study of an enigmatic protein. J Lipid Res. 2001;42(11):1727–1739. [PubMed] [Google Scholar]
- 38.Tall AR, Deckelbaum RJ, Small DM, Shipley GG. Thermal behavior of human plasma high density lipoprotein. Biochim Biophys Acta. 1977;487(1):145–153. doi: 10.1016/0005-2760(77)90051-0. [DOI] [PubMed] [Google Scholar]
- 39.Rye KA, Wee K, Curtiss LK, Bonnet DJ, Barter PJ. Apolipoprotein A-II inhibits high density lipoprotein remodeling and lipid-poor apolipoprotein A-I formation. J Biol Chem. 2003;278(25):22530–22536. doi: 10.1074/jbc.M213250200. [DOI] [PubMed] [Google Scholar]
- 40.Whitten ST, García-Moreno EB, Hilser VJ. Local conformational fluctuations can modulate the coupling between proton binding and global structural transitions in proteins. Proc Natl Acad Sci USA. 2005;102(12):4282–4287. doi: 10.1073/pnas.0407499102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright PE, Dyson HJ. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol. 1999;293(2):321–331. doi: 10.1006/jmbi.1999.3110. [DOI] [PubMed] [Google Scholar]
- 42.Fink AL. Natively unfolded proteins. Curr Opin Struct Biol. 2005;15(1):35–41. doi: 10.1016/j.sbi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Hilser VJ, Thompson EB. Intrinsic disorder as a mechanism to optimize allosteric coupling in proteins. Proc Natl Acad Sci USA. 2007;104(20):8311–8315. doi: 10.1073/pnas.0700329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng Y, LeGall T, Oldfield CJ, Dunker AK, Uversky VN. Abundance of intrinsic disorder in protein associated with cardiovascular disease. Biochemistry. 2006;45(35):10448–10460. doi: 10.1021/bi060981d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.