Abstract
1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose (PGG) is a polyphenolic compound highly enriched in a number of medicinal herbals. Several in vitro and a handful of in vivo studies have shown that PGG exhibits multiple biological activities which implicate a great potential for PGG in the therapy and prevention of several major diseases including cancer and diabetes. Chemically and functionally, PGG appears to be distinct from its constituent gallic acid or tea polyphenols. For anti-cancer activity, three published in vivo preclinical cancer model studies with PGG support promising efficacy to selectively inhibit malignancy without host toxicity. Potential mechanisms include anti-angiogenesis, anti-proliferative actions through inhibition of DNA replicative synthesis and S-phase arrest and also G1 arrest, induction of apoptosis, anti-inflammation and anti-oxidation. Putative molecular targets include p53, Stat3, Cox-2, VEGFR1, AP-1, SP-1, Nrf-2 and MMP-9. For anti-diabetic activity, PGG and analogues appear to improve glucose uptake. However, very little is known about the absorption, pharmacokinetics and metabolism of PGG, nor its toxicity profile. The lack of large quantity of highly pure PGG has been a bottleneck limiting in vivo validation of cancer preventive and therapeutic efficacies in clinically relevant models.
Keywords: gallotannin, polyphenols, anti-cancer, anti-angiogenesis, anti-diabetes
1. Introduction
Medicinal herbals contain many novel natural compounds with attractive pharmacologic and physiologic activities. 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose (PGG) is a naturally occurring polyphenolic compound highly enriched in a number of medicinal herbals such as Rhus chinensis Mill and Paeonia suffruticosa (1-3). A number of in vitro and a handful of in vivo studies have shown that PGG exhibits multiple biological activities which implicate a great potential for PGG in the therapy and prevention of several major human diseases including cancer and diabetes. Here, we present a systematic review on these activities in the hope of stimulating research interests to test the health benefits of PGG and to build the case for its eventual bench to bedside translation.
2. Tannin, gallotannin and PGG
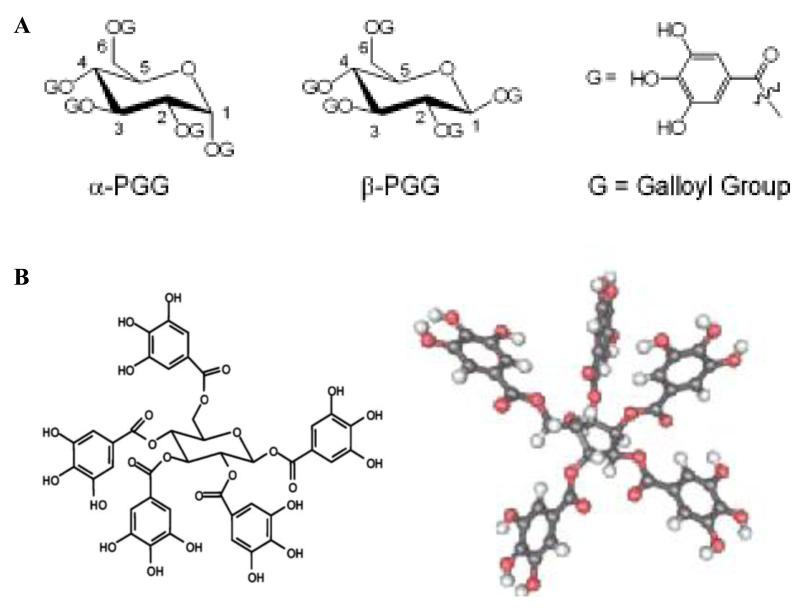
Tannins, secondary metabolites of plants, are mostly water-soluble phenolic compounds which can give the common phenolic reactions and can precipitate alkaloids, gelatin and other proteins(4). According to their structures, tannins are categorized as hydrolysable tannins, condensed tannins or complex tannins(5). Hydrolysable tannins are the esters of 3, 4, 5-trihydroxyl benzoic acid (gallic acid). Gallic acid molecules are esterified to a core polyol, and the galloyl groups may be further esterified or oxidatively cross-linked to form more complex structures. Gallotannins, the polygalloyl esters of glucose, are the simplest hydrolysable tannins. 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose (β-PGG) is a prototypical gallotannin and the central compound in the biosynthetic pathway of hydrolysable tannin. β-PGG has five ester bonds formed between carboxylic groups of gallic acids and aliphatic hydroxyl groups of the glucose core (Fig 1). Most of studies have been conducted with β-PGG due to its natural abundance, though the α-anomer (Anomers, Diastereoisomers of glycosides, hemiacetals or related cyclic forms of sugars, or related molecules differing in configuration only at C-1 of an aldose, C-2 of a 2-ketose, etc. by IUPAC Gold book) also exists naturally (6). For convenience, the terms PGG and β-PGG are used interchangeably in this review, unless noted otherwise.
Fig. 1.
A. Sterical configuration of α-PGG and β-PGG (ref. 3) B. Structure and 3D model of β-PGG (ref. 7)
3. Endogenous Metabolism of PGG in plants
As key members of an important family of plant secondary metabolites, PGG and tannins have been extensively studied in terms of their metabolism in plants. Interested readers are referred to an excellent review on this topic (7). PGG is synthesized from gallic acid and glucose by a series of strictly position-specific galloylation steps. Esterification of gallic acid and glucose to yield β-glucogallin (1-O-galloyl-β-D-glucose) is the first enzyme catalyzed reaction using UDP-glucose as activated substrate. Further substitution of glucose hydroxyls is not randomly distributed in these conversions but displays an unexpected extreme specificity, thus constituting the metabolic sequence β-glucogallin →1,6-digalloylglucose → 1,2,6-trigalloylglucose →1,2,3,6-tetragalloylglucose and finally 1,2,3,4,6-pentagalloylglucose(8-10). In those reactions, β-glucogallin exerts a dual role, functioning not only as an acyl acceptor but also as an efficient acyl donor.
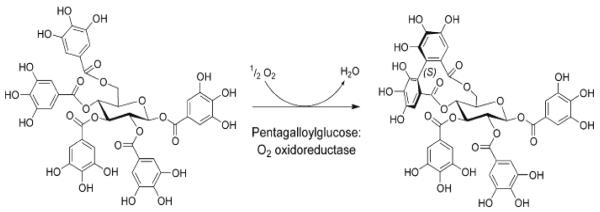
PGG is the common and immediate precursor of the two classes of hydrolyzable tannins, gallotannin and ellagitannin. Addition of further galloyl residues to PGG yields gallotannins such as hexa-, hepta- and octagalloylglucoses with their characteristic meta-depside groups(11, 12). This process could be regarded as a continuation of the esterification reactions of the preceding steps. However, esterification of gallic acid to phenolic hydroxyls yields depsides which are considerably less stable than the core ester linkages of PGG. Several gallotannin synthesizing β-glucogallin-dependent galloyltransferases (EC 2.3.1.-) with different substrate specificities have been identified. Intra or inter-molecular oxidative crosslinking between galloyl groups leads to the production of ellagitannins. Different oxidoreductases catalyze the intra- or inter-molecular oxidative reactions, respectively(13, 14) (Fig. 2).
Fig. 2.
An example of oxidoreductase-catalyzed the intra-molecular oxidative cross-linking reactions (ref 13).
4. Natural source of PGG and its preparation
4.1 Natural source
PGG is predominantly found in plants as the core structure of the higher galloyl glucoses, which comprise the commercial preparation known as tannic acid. The amount of free PGG varies among different plant species, but is present at sufficient levels to allow direct isolation from a number of Oriental herbs and other plants such as Rhus chinensis Mill(2), Paeonia suffruticosa(1), Paeonia lactiflora(15), Schinus terebinthifolius(16), Acer truncatum Bunge(17) and Terminalia chebula(18) by solvent extraction, liquid-liquid partition and chromatography separation. A recent review has summarized the distribution of PGG in 70 kinds of plants with both the scientific and popular names listed (3). The yield of PGG isolation from plants is variable (19). This has been a major bottleneck limiting the establishment of in vivo preventive or treatment efficacy of PGG against cancer or other diseases.
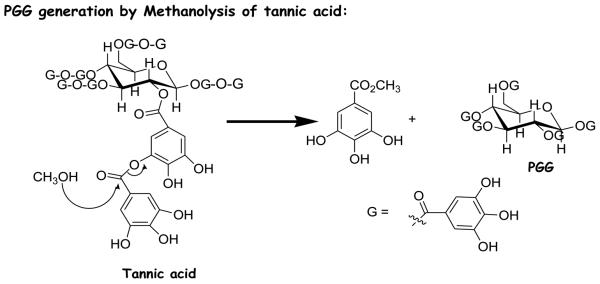
4.2 Preparation of PGG by methanolysis of tannic acid
The higher galloyl esters are abundant in the galls of many plants, and are commercially available as tannic acids. Tannic acids from various sources differ significantly in composition, with some preparations containing mainly tetragalloyl glucose and lower esters, and other preparations predominantly PGG and higher esters(20). Furthermore, the core polyol may be either glucose or in some cases other alcohols such as quinic acid. Hagerman and associates have prepared PGG by methanolysis of tannic acid (21) (Fig. 3). It is essential to confirm that the starting material has the correct core structure to yield the desired final product. In tannic acids, the depside bonds between galloyl groups are less stable than the aliphatic ester bonds between galloyl groups and glucose core, so mild methanolysis of tannic acids in weak acid (usually acetate buffer at pH 5.0) and methanol generates PGG and methyl gallate. Hydrolysis with strong inorganic acid leads to oxidative decomposition of the hydrolysis products. A scaled-up protocol has been refined to allow the reproducible isolation of multi-gram quantity of high purity crystalline PGG (>98%) from 100-g batches of tannic acid with a yield of 15% (A. Shaik, C. Xing and J. Lu, unpublished data).
Fig. 3.
Methanolysis of tannic acid to yield PGG under weak acidic condition (ref. 21).
4.3 Chemical synthesis of PGG and radio-labeled PGG
PGG can be chemically synthesized through Steglich esterification of glucose with gallic acids. Tri-O-benzylgallic acid, the protected form of gallic acid, was used in the esterification reaction and then de-protected to PGG (22). Epimerization at C-1 of the glucose ring occurs during the reaction, and the anomers (α and β) can be separated via normal phase chromatography. Recently, Binkley et al developed an anomeric-selective synthesis scheme for PGG using anomeric pure glucose as starting material (23). Hagerman group reported the synthesis of radio-labeled PGG from 14C-glucose (24), which has been used to study the bioavailability of PGG in a rodent model and in a Caco-2 cell culture model (25) (26).
5. Chemistry characteristics
The structure of PGG has been confirmed by UV, IR, MS, 1H-NMR and 13C-NMR(6, 22). It is worthy noting that the measured octanol/water partition ratio of PGG of 129 is much higher than that of many other polyphenol compounds such as gallic acid (7.76), green tea polyphenols epigallocatechin-3-gallate (EGCG) (12.1), epigallocatechin (EGC) (0.281) and hexagalloylglucose (1.51) (27). The data suggest that PGG is more hydrophobic than other polyphenolic compounds, probably due to the relative symmetric structure of PGG. Whether formation of intra-molecular hydrogen bonds or inter-molecular stacking are involved in accounting for this unexpected higher hydrophobicity should be examined. This unique characteristic may affect stability and in vivo bioavailability of PGG.
An interesting and unique distinction from other polyphenolic compounds is the lack of pro-oxidant activity of PGG in aqueous milieu (28). Some phenolic compounds such as gallic acid and EGCG are pro-oxidants through Fenton reaction in aqueous solution, leading to a rapid generation of measurable hydrogen peroxide within minutes (29, 30). Because PGG is a glucose ester of five gallic acid molecules, our group has compared the production of hydrogen peroxide in the cell-free culture medium by PGG with that of gallic acid in equal molar concentration of phenol groups (28). The results showed that gallic acid induced a significant production of hydrogen peroxide, peaking at 1 h, which was quenched by catalase added to the cell culture medium. PGG did not induce cell-free hydrogen peroxide production throughout 24 h. These data support PGG as a distinct entity from gallic acid and possibly other tea polyphenolics, in terms of possible tumor suppression effect or other activities.
Tannins have the common characteristics of chelating metal ions, binding and/or precipitating proteins and quenching reactive oxygen species. Depending upon the concentration and target cells/organs examined, PGG exerts varied biological activities through multiple “mechanisms”. Some activities are shared with other tannins or polyphenols whereas others are unique to PGG itself due to its chemical structure.
6. Anti-cancer activities and mechanisms
PGG has been shown to exhibit in vivo anti-cancer effects against prostate cancer (28, 31), lung cancer (2), sarcoma (32) and breast cancer (unpublished data, HJ Lee, Lu and Kim). In vitro inhibitory effects on the growth and/or invasion of breast cancer, leukemia, melanoma and liver cancer have been reported (See details next). Mechanistic studies using cell culture indicated the involvement of pro-apoptosis, anti-proliferation, anti-angiogenesis, anti-metastasis, inhibition of P-glycoprotein, as well as other organ-specific pathways. However, whether the in vivo anti-cancer activities of PGG are mediated by these direct actions or through PGG metabolites or other indirect mechanisms remain unclear.
6.1 Prostate cancer
Collaborative work by authors Kim and Lu has recently shown the induction of G1- and S-phase arrests and caspase-mediated apoptosis in the human LNCaP cells (wild-type p53, androgen-dependent), and in DU145 cells (p53-mutant, androgen-independent) in concentration-dependent manner for up to 75μM PGG (28). In LNCaP cells, caspase-mediated apoptosis induced by PGG was mediated mainly by p53 activation. Intracellular reactive oxygen species production induced by PGG was found to be crucial for these molecular and cellular actions. In DU145 cells, which harbor constitutively active signal transducer and activator of transcription 3 (STAT3), caspase-mediated apoptosis induction by PGG was associated with an inhibition of STAT3 Tyr705 phosphorylation and the down-regulation of STAT3 transcriptional targets Bcl-XL and Mcl-1. Overexpression of Bcl-XL or knockdown of its binding partner Bak attenuated the apoptosis induction. Furthermore, we showed, for the first time, in vivo anti-cancer activity of PGG. Daily i.p. injection of 20 mg/kg PGG significantly suppressed the growth of DU145 xenograft in an athymic nude mouse model. This suppression was associated with decreased pSTAT3. The data strongly suggest PGG as a multi-targeting agent for chemoprevention and therapy of prostate cancer.
In a more recent publication, PGG was shown to suppresses the in vivo invasion and xenograft growth in nude mice with intra-tibially injected PC-3 human prostate cancer cells (31). At a dose of 25 mg per kg body weight, three times per week for 28 days, intraperitoneal injection of PGG suppressed PC-3 tumor weight from 4.2 g to 1 g. The authors also studied related mechanisms, focusing on epidermal growth factor (EGF), which can be generated from bone tissue to stimulate matrix metalloproteinase (MMP) secretions from metastatic prostate cancer cells. PGG inhibited the EGF-induced cell invasiveness and MMP-9 expression in a dose- and time-dependent manner by reducing the MMP-9 transcriptional activity. Their results showed that PGG suppressed the EGF-induced NF-kappaB nuclear translocation and also abrogated the EGF-induced activation of c-jun N-terminal kinase (JNK), an upstream modulator of NF-kappaB. Moreover, they showed that PGG reduced EGFR expression through the proteasomal pathway. Their in vitro and in vivo results suggest that PGG may be a therapeutic drug candidate for the treatment of advanced metastatic prostate cancer.
Earlier, Lee et al reported the inhibitory effect of PGG on androgen receptor (AR) signaling pathway in human prostate cancer LNCaP cells (33). Androgens play a critical role in regulating the growth, differentiation and survival of epithelial cells in androgen-responsive organs such as prostate. They found that PGG significantly reduced the growth of androgen-responsive LNCaP prostate cancer cells, suppressed the expression of the AR and lowered androgen induced prostate-specific antigen secretion and fatty acid synthase protein level. Additional studies indicated that PGG was a potent inhibitor of rat liver microsomal 5α-reductase (EC 1.3.99.5), which catalyzes the conversion of testosterone (T) to a more active androgen dihydrotestosterone (DHT) with an IC50 at 7.8μM. Kinetic studies showed that PGG was a competitive inhibitor for NADPH while a non-competitive inhibitor for testosterone. The mode of inhibition suggested that PGG may inhibit 5α-reductase by competing with NADPH binding site. However, a closer examination of the dose-response patterns for PGG and PSA suppression did not dissociate such an effect from PGG-induced apoptosis in the LNCaP cell model (28). The in vivo impact of PGG on PSA expression and AR signaling should be examined and verified.
The cell cycle effects of PGG in PCa cells have recently been evaluated by Lu and Kim groups (28, 34). The data show that treatment with sub-apoptotic doses of PGG induced S-arrest, whereas apoptotic doses of PGG induced not only S-arrest but also G1 arrest. Irrespective of the p53 functional status of the PCa cell lines, PGG exerted a rapid (within 2 h) and potent inhibition (IC50 ~ 6 μM) of BrdU incorporation into S phase cells. In isolated nuclei, PGG inhibited DNA replicative synthesis with superior efficacy than a known DNA polymerase-alpha inhibitor, aphidocolin. In addition to the S-arrest action, we have found a close association of down regulation of cyclin D1 with G1 arrest induced by PGG exposure in the pro-apoptotic range. Over-expressing this G1 cyclin abolished G1 arrest, but hastened the S-arrest induction by PGG. Together, our data indicate that PGG induced PCa S-arrest probably through DNA replicative blockage and induced G1-arrest via cyclin D1 down regulation to contribute to anti-cancer activity. The G1 and S arrest actions and the inhibition of DNA replication by PGG are also observed in breast cancer cell lines (Y. Chai and Lu, unpublished data). The data raise the hypothesis that PGG may be a novel inhibitor of DNA polymerases. Overall, the data further supported the potential role of PGG as a novel therapeutic or chemo-preventive agent for prostate cancer, but these effects need to be validated in vivo.
6.2 Lung cancer
Co-author Kim's group reported that i.p. injected PGG at once daily dosages of 4 and 20 mg/kg significantly inhibited the growth of the highly angiogenesis-dependent Lewis Lung Cancer (LLC) allograft by 57 and 91%, respectively (2). Immunohistochemistry staining revealed decreased microvessel density, suppressed expression of cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF). Reduced tumor cell proliferation and increased tumor cell apoptosis were associated with tumor suppression. In vitro, non-cytotoxic concentrations of PGG effectively inhibited the proliferation and tube formation in basic fibroblast growth factor (bFGF)-treated human umbilical vein endothelial cells (HUVECs), attenuated the expression of COX-2 and VEGF and reduced the secretion of VEGF and prostaglandin E2. PGG also disrupted the bFGF-induced neovascularization in the chick chorioallantoic membrane (CAM) model and in Matrigel plugs in the mice. These data strongly suggest anti-angiogenesis to play an important role in LLC inhibition by PGG and perhaps for cancers of other organ sites.
6.3 Breast cancer
The in vitro inhibitory effect of PGG on breast cancer cells was investigated by Chen et al (35). Growth of MCF-7 cells was decreased by 30 and 50μM PGG with a strong induction of G1 arrest. The levels of several G1 phase related cyclins and cyclin-dependent kinases did not change in these cells during a 24-hr treatment of PGG. However, the activity of cyclin E/CDK2 was decreased in a concentration- and time-dependent manner and the activity of cyclin D/CDK4 was inhibited when serum starvation synchronized cells were released from synchronization. In the mean time, PGG gradually increased the levels of p27 and p21, which were inhibitors of cyclin/CDK complexes in G1-phase.
In another study, Hua et al investigated the effect of PGG on estrogen receptor (ER) signaling pathway in the same model (36). They found PGG significantly suppressed the phosphorylation and protein level of ERα. Co-treatment with proteosome or lysosome inhibitors indicated that PGG depleted ERα through a lysosomal-dependent degradation mechanism. Down-regulation of ErbB family receptors such as epidermal growth factor receptor (EGFR), ErbB2 and ErbB3, as well as the estrogen-activated cyclin D1, were also observed. Those findings highlighted the potential role of PGG in the chemoprevention and treatment of breast cancer, especially ER positive breast cancers.
Recently accomplished studies with MDA-MB231 xenograft in nude mice indicated a remarkable in vivo efficacy of orally administered PGG (10 or 20 mg/kg) on the growth of this very aggressive triple negative (ER-, PR- and HER2-) breast cancer (HJ Lee, Lu and Kim, unpublished data). In comparison with paclitaxel, daily PGG gavage at 10 mg/kg was more efficacious at suppressing xenograft growth than weekly intraperitoneal injection of 10 mg/kg of this potent chemotherapy drug.
6.4 Leukemia
Pan et al reported that PGG induced caspase-mediated apoptosis in human promyelocytic leukemia HL-60 cells at a concentration range of 20 to 100μM (37). DNA fragmentation, PARP cleavage and activation of caspase-3 but not caspase-1 were observed. They also found that treatment with 50μM PGG caused a rapid loss of mitochondrial transmembrane potential, release of mitochondrial cytochrome c into cytosol, and subsequent induction of procaspase-9 processing. In human Jurkat T cells, PGG induced both G1 arrest and apoptosis. G1 arrest was accompanied by the induction of p21 and p27. Mechanistic studies indicated that turnover of those proteins was disrupted by PGG due to proteosome inhibition. This was further supported by a direct inhibition of 20 and 26S proteosome activity by PGG in the test tube (38). In vivo efficacy of PGG against leukemia has not been published to date.
6.5 Melanoma
Cancer metastasis is the major cause of mortality and cancer pain. Key molecules in the regulation of metastasis and invasion are matrix metalloproteinases (MMPs). Ho et al reported that up to 25μM PGG inhibited the invasion of highly metastatic mouse melanoma B16F10 cells in vitro(39). PGG treatment diminished the activity of MMP-9 more than that of MMP-2 at both protein and mRNA levels. Gel shift experiments showed that the transcription factor binding activities of activator protein-1 (AP-1) and Sp-1 sites were down-regulated by PGG in the concentration range of 5–15 μM, but the binding activities of nuclear factor κB (NF-κB), polioma enhancer activator 3 (PEA-3) and activator protein-2 (AP-2) were not significantly affected. A suppressing effect of PGG on MMP-9 promoter was also directly validated in a luciferase reporter system. The in vivo anti-metastatic efficacy of PGG against melanoma or other cancers needs to be demonstrated.
6.6 Liver cancer
Oh et al isolated PGG from the root of Paeonia suffruticosa ANDREWS and tested its in vitro effect on human hepatocellular carcinoma SK-HEP-1 cells (1). Up to 50μM PGG inhibited the growth of SK-HEP-1 cells in a dose-dependent fashion and 30μM PGG significantly induced G1 arrest. Gel shift experiments showed that 1h treatment with 30μM PGG inhibited not only basal but also TPA induced activation of NF-κB. However, neither apoptosis nor necrosis was observed in the cells treated with PGG. Their data suggested that PGG at the concentration studied was a cyto-static rather than cyto-toxic anticancer reagent in liver cells. In vivo efficacy has yet to be established.
6.7 Sarcoma
Miyamoto et al screened anti-tumor activity of sixty-three tannins and polyphenols on S180 sarcoma model in syngeneic mice (32). The chemicals were i.p. injected only once at 4 days before 105 tumor cells were i.p. inoculated. They found that 10 mg/kg PGG increased the life span of tumor bearing mice by 81.9%. Possible mechanism may be a potentiation of the immunity of the host animals. However, it remains to be determined whether PGG or metabolites may persist in the i.p. cavity to exert a direct effect on the tumor cells since the same route was used for drug delivery and tumor inoculation.
6.8 Anti-angiogenesis
Tumor angiogenesis is well accepted to be critical in the growth and metastasis of tumors. VEGF is one of the most important angiogenic factors mediating tumor-induced neovascularization. VEGF exerts its activities through binding to its receptor tyrosine kinase, KDR/Flk-1, expressed on the surface of endothelial cells. Lee et al showed that PGG strongly inhibited the binding of VEGF to KDR/Flk-1 and blocked VEGF-induced HUVEC proliferation and the growth of immortalized human microvascular endothelial HMEC-1 cells(40). In vitro functional study also showed that PGG blocked VEGF-induced capillary-like tube formation of endothelial cell on Matrigel. The involvement of anti-angiogenesis in the anti-cancer activities of PGG was further demonstrated by co-author Kim's group in the LLC model as described in section 5.2 (2). Whether PGG is indicated for in vivo treatment of other angiogenesis-related diseases needs further research.
6.9 P-glycoprotein inhibition
Drug resistance is one of the major challenges in cancer chemotherapy. Over-expression of P-glycoprotein (P-gp), a membrane protein which extrudes therapeutic drugs out of cancer cells, plays an important role in drug resistance. Kitagawa et al studied the effect of tannic acid and PGG on the function of P-gp in the multi-drug resistant oral cancer KB-C2 cells (41). They found both compounds dramatically increased the intra-cellular levels of P-gp drug substrates. Further study indicated that inhibition of ATPase activity of P-gp by PGG was associated with the functional inhibition. However, they did not provide any kinetic studies of ATPase inhibition mechanics. Their data suggested that PGG may reverse drug resistance in cancer chemotherapy via P-glycoprotein inhibition. The in vivo efficacy needs to be established for PGG as a combination modality to enhance drug efficacy and reverse drug resistance.
In summary, PGG has been demonstrated to have promising in vitro inhibitory effects against cancer cells from different organs through multiple mechanisms. However, its in vivo efficacy has been established in a very limited number of organ sites, including Lewis Lung cancer allograft and prostate and breast cancer xenografts. More clinically relevant in vivo therapeutic and chemo-preventive studies of PGG against prostate and breast cancer are ongoing in the authors' labs.
7. Insulin-mimicking and anti-adipogenesis activities
Liu et al first reported that tannic acid stimulated glucose transport and inhibited adipogenesis in 3T3-L1 cells (42). Later on, Li et al showed that both β-PGG and its anomer α-PGG possessed insulin-mimicking activity in the absence of insulin, and that α-PGG was more potent than β-PGG (43). α-PGG itself stimulated glucose uptake in 3T3-L1 adipocytes. However, α-PGG weakened the activity of insulin if treated together. Mechanistic studies indicated that the insulin receptor (IR) was involved in the insulin-mimicking activity of α-PGG. α-PGG induced phosphorylation of the IR, PI 3-kinase and AKT and stimulated membrane translocation of GLUT4. Receptor binding studies indicate that α-PGG bound to the IR in a competitive manner. Treatment with cross-linking reagent Sulfo-SANPAH resulted in an up-shift of IR α-subunit band detected by Western blot. Such data supported the direct binding between IR and α-PGG. In vivo studies demonstrated that α-PGG reduced resting blood glucose levels and improved glucose tolerance in diabetic and obese mice. Their results suggest that PGGs may serve as lead compounds for the development of new types of therapeutics for diabetes and metabolic syndrome.
8. Other biological activities
8.1 Anti-oxidative and anti-mutagenic activities
8.1.1 Direct anti-oxidative effect
Tannins are good direct antioxidants. Even the tannin-protein complex can act as radical scavengers and radical sinks (44). The anti-oxidant potency of PGG has been evaluated in several models (45). PGG showed an EC50 of scavenging 1, 1-diphenyl-2-picrylhydrazil (DPPH) free radical at about 1μg/mL (1.1 μM) in test tubes, which was more potent than vitamin E. In another cell based model, lipid peroxidation was induced by hydrogen peroxide in human leukemia K562 cells. Pretreatment with PGG or gallic acid significantly inhibited lipid peroxidation. It was also very interesting to note that a concentration of 100μg/mL (i.e. 106 μM) PGG was more effective than higher concentrations of 200 and 400μg/mL (i.e. 212 and 424 μM). Whether the higher levels produce a pro-oxidant action as do some other anti-oxidants remains a possibility.
Okubo et al reported that 10 μM PGG significantly scavenged the superoxide and hydroxyl radical generated by phenylhydroquinone (PHQ) and tert-butylhydroquinone (TBHQ) in test tubes. Much higher levels 100 to 1000 μM PGG almost totally inhibited PHQ and TBHQ induced oxidative cleavage of DNA (pUC18 plasmid) (46). In this system, PGG was more potent than other polyphenolic compounds such as paeonol, catechin and galloylpaeoniflorin. Park et al reported that pretreatment with 3 to 50μM PGG significantly protected primary rat hepatocytes from necrosis/apoptosis induced by tert-butyl hydroperoxide (tBH) (47). Since oxidative stress is one of the mechanisms of the toxicity of tBH, anti-oxidative effect of PGG may be involved in the hepato-protection.
8.1.2 Anti-mutagenic activity
Okuda et al reported that PGG exerted remarkably strong inhibition of mutagenicity of 3-hydroxyamino-1-methyl-5H-pyrido[4,3-b]indole on Salmonella typhimurium in the absence of S9 mix. On equal weight base, PGG was more potent than gallic acid, ellagic acid, ECG and EGCG(48). In another study, the SOS chromotest was used to test the anti-mutagenic effect of PGG on aflatoxin B1 (AFB1: indirect acting mutagen and a known liver carcinogen) or nifuroxazide- (direct acting mutagen) induced genotoxicity. Mutagenicity induced by either of these compounds could be totally blocked by PGG. Interestingly, in the case of nifuroxazide, gallic acid was more effective than PGG on equal weight basis (45). In vivo mutagenicity test in models such as Big Blue rats should be more relevant and informative and needs to be carried out.
8.1.3 PGG as an indirect antioxidant
Some antioxidants can exert their function indirectly (49), i.e. induction of cyto-protective proteins such as Glutathione S-transferase (GST), NAD(P)H dehydrogenase quinine (NQO) and Heme oxygenase-1 (HO). Besides directly scavenging free radicals, PGG can also act as an indirect antioxidant. Choi et al examined the effect of PGG on the expression of neuronal heme oxygenase-1 (HO-1), an inducible stress protein that degrades heme to generate the anti-oxidant, biliverdin (50). Exposure of Neuro 2A cells to PGG (10–50 μM) resulted in a concentration- and time-dependent induction of HO-1 mRNA and protein expressions and heme oxygenase activity. Interestingly, pretreatment of the neuronal cells with PGG resulted in enhanced cellular resistance to hydrogen peroxide. This cyto-protective effect was reversed by zinc protoporphyrin IX, an inhibitor of HO-1. This study showed that PGG could protect neuronal cells from oxidative stress via the induction of HO-1.
Pae et al studied the hepato-protective effect of PGG in HepG2 cells treated with tBH and the role of cyto-protective proteins in this process (51). They also found that 5-20μM PGG up-regulated HO-1 expression without causing obvious toxicity to cells. Pretreatment with PGG significantly protected cells against oxidative injury induced by tBH. Knockdown of HO-1 expression by siRNA abolished the protective effect of PGG whereas over-expression of HO-1 itself made the cells more resistant to tBH. Thus, induction of HO-1 expression could play an important role in the hepato-protective activity of PGG. Mechanistic studies indicated that nuclear translocation of Nrf2, an important upstream transcription factor of HO-1 expression, was stimulated after 30min treatment by 20 μM PGG. Nrf2 siRNA treatment not only reduced intracellular HO-1 level but also attenuated the cyto-protective effect of PGG. Further data showed that phosphorylation of ERK-1/2 was induced by PGG; co-treatment with 10 μM ERK inhibitor U0123 abolished all the activities of PGG on HO-1 expression, Nrf-2 nuclear translocation and cyto-protection. Taken together, those data indicated that PGG up-regulated HO-1 expression by stimulating Nrf2 nuclear translocation in an ERK-dependent fashion.
8.1.4 PGG can reduce intracellular oxidative stress induced by carcinogen
Bhimani et al studied the effect of tamoxifen and several natural products on intracellular hydrogen peroxide and DNA adduct levels in HeLa cells treated with 12-O-tetradecanoylphorbol-13-acetate (TPA). They found that PGG could significantly reduce intracellular hydrogen peroxide level and reduce the formation of DNA adducts 8-hydroxyl-2′-deoxyguanosine (8-OHdG) and 5-hydroxymethyl-2′-deoxyuridine (HMdU) with IC50 at 5, 1 and 1μM, respectively. In this system, the potency of PGG was comparable with tamoxifen. These data further suggest that PGG could be an effective cancer chemo-preventive agent through inhibition of DNA mutagenesis(52).
8.2 Inflammation related activities
8.2.1 Induction of the secretion of TNFα and IL-1β from peripheral blood mononuclear cells
As mentioned in section 5.1.7, PGG given once by injection 4 days prior to tumor inoculation inhibited the in vivo growth of S-180 sarcoma. Other polyphenolic compounds were also tested and showed varying activities. Feldman et al hypothesized that the induction of endogenous cytokines may contribute to the anti-cancer activity of those compounds (53). They treated human peripheral blood mononuclear cells (PBMCs) with PGG and other polyphenols and measured the secreted TNFα and IL-1β levels, with LPS as positive control. Both TNFα and IL-1β secretion were significantly stimulated by PGG with a stronger effect on IL-1β. The potency of stimulating TNFα secretion by PGG and other polyphenols correlated well with their in vivo anti-cancer activity on S-180. Their data suggest that induction of endogenous cytokines may be one of the mechanisms of the anti-cancer activity of PGG and other polyphenols.
8.2.2 Anti-inflammation activity
Though PGG itself stimulated the secretion of TNFα and IL-1β, which are pro-inflammatory cytokines, it attenuated the stimulating effect of LPS when treated together. This effect was tested in both in vitro and in vivo models (54). PBMCs harvested from three healthy human subjects were stimulated with 5μg/mL LPS in the absence or presence of PGG. There was a pronounced suppression of LPS-stimulated TNFα release by PGG. Maximum inhibition of TNFα output was achieved at 5 μM of PGG. In an in vivo model, LPS and PGG were administrated intravenously to chronically catheterized, conscious, unrestrained rats (250 g each). A bolus of PGG (10 mg) was administered to rats, followed by continuous infusion of 7 mg over 90 min. All animals were treated with 0.25 mg of LPS at 10 min after the initial bolus PGG treatment. Blood samples were collected at 90 min for cytokines assay. Pre-treatment with PGG significantly reduced serum levels of TNFα but not IL-1β compared to LPS alone. This may be due to the stronger IL-1β stimulating effect of PGG itself. Recently, Wu et al also reported that PGG inhibited the secretion of TNFα and IL-6 by IL-1β treated rat synoviocytes (55).
In addition, PGG has been shown to protect mice from a lethal challenge by LPS (56). In order to look for an alternative therapeutic strategy for sepsis, they screened lipid A binding components from the aqueous extracts of 42 traditional Chinese herbs by an affinity biosensor technology. They identified PGG as a lipid A binding molecule with a Kd of 32μM. In vitro, PGG inhibited LPS induced Limulus Amebocyte Lysate (LAL) reaction and significantly repressed TNFα and IL-6 secretion by PMBCs stimulated with 100ng/mL LPS. In vivo, mice challenged with 20 mg/kg LPS by i.v. injection all died within 24hrs, but pretreatment with 40 and 80 mg/kg PGG by i.v. injection resulted in 7-days survival rates of 45 and 70%, respectively. Accordingly, serum endotoxin and TNFα levels of endotoxemic animals were significantly reduced by PGG.
More experiments in different models supported the anti-inflammation effect of PGG. Oh et al reported that PGG inhibited IL-8 mRNA expression and secretion in human monocytic U937 cells stimulated with phorbol 12-myristate 13-acetate (PMA) or TNFα (57). Lee et al showed that 10 μg/mL(10.6μM) PGG strongly suppressed PMA and calcium ionophore A23187 induced expression of TNFα, IL-1β and IL-6 in human mast cells at both mRNA and proteins level (58). In macrophages RAW 264.7 cells stimulated by LPS, PGG significantly inhibited the COX-2 and inducible nitric oxide synthase (iNOS) activity, as well as NO production (59). Kang et al reported that incubation of human umbilical vein endothelial cells (HUVECs) with 0.1-10μM PGG increased the production of cGMP in a dose-dependent manner. Moreover, PGG suppressed the expression levels of adhesion molecules including intracellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) induced by TNF-α. TNF-α-induced monocyte chemoattractant protein-1 (MCP-1) expression was also attenuated by PGG. PGG treatment also inhibited cellular adhesion of U937 cells onto human umbilical vein endothelial cells induced by TNF-α(60). Those data strongly suggest the potential for PGG to suppress the vascular inflammatory process and may be useful as an anti-arteriosclerosis agent.
8.2.3 Mechanisms of anti-inflammation effect of PGG
Mechanistic studies showed that inhibition of nuclear factor-κB (NF-κB) played an important role in the anti-inflammation activity of PGG. Pan et al reported that PGG inhibited IκB kinase (IKK) activity in activated macrophages RAW 264.7 cells activated by LPS (61). Furthermore, PGG blocked phosphorylation of IκB from the cytosolic fraction, inhibited nuclear factor-κB (NFκB) activity, and inhibited increases in inducible nitric oxide synthase (NOS) levels in activated macrophages. These results suggest that PGG may exert its anti-inflammatory actions by suppressing the activation of NF-κB via inhibition of IKK activity. Data by Oh et al also indicated that inhibition of NF-κB was involved in PGG inhibited IL-8 expression in U937 cells stimulated with PMA (57). Yokozawa et al reported that treatment of BALB/c mice peritoneal macrophages with 50μg/mL (i.e. 53μM) of PGG substantially inhibited LPS-induced intracellular iNOS and NADPH-diaphorase activities and NO release. The cytotoxicity of LPS was also attenuated (62).
8.3 Anti-allergy activity
Cavalher-Machado et al isolated PGG from the ethyl acetate fraction of Schinus terebinthifolius and tested its anti-allergic effect (16). PGG (100 μg/mL, i.e. 106μM) inhibited by 50-80% of histamine secretion in rat peritoneal mast cells challenged in vitro with allergen. In vivo, oral pre-treatment with the crude extract (100 mg/kg) significantly inhibited paw edema induced by compound 48/80 (100 ng/paw). This effect was related to the inhibition of CCL11/eotaxin and CCL5/RANTES in pleural lavage fluid. Possible mechanisms include an inhibition of pro-inflammatory mechanisms triggered via histamine and direct action on mast cell membrane.
8.4 Hypo-cholestrolemic effect
Park et al reported that PGG isolated from Paeonia moutan significantly inhibited rat microsomal squalene synthase activity with IC50 around 1 μM (63). Squalene synthase is a key enzyme in cholesterol synthesis. The in vitro cholesterol biosynthesis from [1-14C] acetate was decreased by PGG. They further tested the hypocholestrolemic effect of PGG in vivo. Oral administration of 100 mg PGG/kg per day to hamster for 10 days significantly decreased serum total cholesterol level by 22.9% but did not affect total triglyceride and lipoprotein levels. When intraperitoneally administrated, 10 mg PGG/kg per day for only 3 days significantly lowered both serum total cholesterol and triglyceride levels. The authors did not mention effect on body weight or other health parameters. The results supported PGG as a potential hypocholestrolemic agent and suggested limited oral bioavailability of PGG.
8.5 Inhibition of acid secretion
Ono et al purified PGG from Paeoniae radix, which has been used for the treatment of gastritis and peptic ulcers in traditional Chinese medicine, and showed a strong inhibitory effect on H+, K+- ATPase with IC50 of 0.166μM (64). Kinetic study indicated that the inhibition was noncompetitive with respect to K+. Functional studies showed that PGG inhibited the accumulation of [14C]aminopyrine in guinea pig stomach parietal cells stimulated by histamine or dbc-AMP. Their results suggested that PGG could be responsible for the inhibition of acid secretion by Paeoniae radix.
8.6 Stabilization of elastin and collagen
Maintaining the integrity of arterial elastin is vital for the prevention of abdominal aortic aneurysm (AAA) development. To search for novel agents to intervene in AAA development, Isenburg et al studied the effect of PGG on stabilization of aortic elastin(65). In vivo efficacy of PGG was evaluated within a well-established AAA model in rats on the basis of CaCl2-mediated aortic injury. One-time periadventitial delivery of noncytotoxic levels of PGG (0.6 to 3 mg/mL, i.e. 640 to 3200 μM) inhibited elastin degeneration, attenuated aneurysmal expansion and hindered AAA development in rats without interfering with the pathogenic mechanisms typical of this model such as inflammation, calcification, and high metalloproteinase activities. PGG bound specifically to arterial elastin and preserved the integrity of elastic lamellae despite the presence of high levels of proteinases derived from inflammatory cells. Their results suggested that stabilization of aortic elastin in aneurysm-prone arterial segments by PGG offered great potential toward the development of safe and effective therapies for AAAs.
In a similar study, stabilizing effect of PGG on collagen was investigated by Tedder et al (66). The ideal scaffolds for heart valve must function immediately after implantation but also need to tolerate cell infiltration and gradual remodeling. For this purpose, they treated collagen scaffolds prepared from decellularized porcine pericardium with 0.3% PGG and then evaluated its properties. They found that PGG-treated collagen was initially resistant to collagenase and degraded gradually, which indicated a partially stabilizing effect. In addition, PGG-treated collagen exhibited excellent biaxial mechanical properties, did not calcify in vivo, and supported infiltration by host fibroblasts and the subsequent matrix remodeling. Those findings highlight the potential use of PGG in tissue engineering.
8.7 Cardiovascular protective and anti-coagulation activities
Goto et al were first to report the vasodilatory effect of PGG (67). They found that PGG isolated from the roots of Paeonia lactiflora Pallas relaxed prostaglandin F2-precontracted aortic ring preparations from isolated rat aorta that contained endothelium. This activity was further studied by Kang et al (60). They found that 1 to 30 μM PGG induced a concentration-dependent relaxation of the phenylephrine pre-contracted rat aorta. This effect depended on the presence of functional endothelium. Pretreatment of the aortic tissues with either nitric oxide (NO) synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME) or guanylyl cyclase inhibitor 1H-[1,2,4]-oxadiazole-[4,3-α]-quinoxalin-1-one (ODQ) inhibited the relaxation. These results suggested that PGG-induced vascular relaxation was closely related to activation of a NO-cGMP pathway. Further mechanistic studies found that 0.1 to 10μM PGG blocked NF-κB translocation in HUVEC cells in a dose-dependent manner, which was consistent with other reports about the role of NF-κB in the anti-inflammation activities of PGG(57, 61).
Dong et al purified PGG from the MeOH extract of the whole plant of Geum japonicum. They found that PGG showed potent anticoagulant activity by significantly prolonging the clotting of rabbit plasma. Kinetic studies indicated that PGG was a noncompetitive inhibitor of thrombin but a competitive inhibitor of Factor Xa. Synthesized permethylated derivative of PGG and Penta-O-benzoyl-β-D-glucopyranoside did not show inhibitory effect on thrombin. So the phenolic hydroxyl groups of PGG could play an important role in the inhibition of clotting (68).
Liu et al reported the anti-hypertensive effects of tannins isolated from traditional Chinese herbs as inhibitors of angiontensin converting enzyme (ACE) (69). Their data showed that PGG and 1, 2, 3, 6-tetra-O-galloyl-β-D-glucose (4GG) inhibited ACE in test tubes. Presence of 25μg/mL BSA in the reaction abolished the inhibitory activity of PGG but not 1,2,3,6-Tetra-O-galloyl- β -D-glucose, suggesting a non-specific inhibitory mechanism of PGG through protein binding. In vivo study with spontaneously hypertensive rats showed that 1, 2, 3, 6-Tetra-O-galloyl-β-D-glucose had a strong inhibitory effect on angiotensin I induced blood pressure elevation.
8.8 Anti-convulsion (epilepsy) activity
To investigate the mechanism of anticonvulsant activity of peony root Paeoniae radix, Sugaya et al studied the effect of selected chemicals isolated from peony root on EEG power spectrum and extra-cellular calcium and potassium levels in rat cerebral cortex (70). They found that orally administrated PGG (10 mg/kg) completely inhibited the EEG power spectrum changes induced by pentylenetetrazol as well as the extracellular calcium and potassium concentration changes related to seizure. Their data suggest that PGG contributes to the anticonvulsant activity of peony root.
8.9 Anti-kidney stone formation
Calcium oxalate monohydrate (COM) crystals bind avidly to the surface of proliferating and migrating renal endothelial cells, and oxalate-induced peroxidative injury can promote crystal attachment to renal epithelial cells. Co-author Kim and collaborators recently showed that PGG significantly decreased COM crystal adhesion to cultured MDCK I cells at a low concentration (10 μM) which was not cytotoxic (71). PGG exerted anti-adhesion effects whether cells or crystals were pre-coated. PGG also inhibited cell migration in scrape-wounding test of confluent monolayer, decreased subsequent adhesion of crystals to proliferating and migrating cells, and decreased expression of the crystal binding molecule hyaluronan. These findings suggest that PGG represents a potential urolithiasis prevention compound through multifaceted actions involving crystal coating by PGG, decreased cell migration and the associated surface expression of hyaluronan. The latter represents a novel mechanism of nephrolithiasis prevention(71).
8.10 Antivirus and antibacterial activities
Based on an activity-guided separation scheme, Lee et al purified PGG from the root of Paeonia lactiflora PALL and validated its anti-hepatitis B virus (HBV) activity in an HBV-producing HepG2.2.15 cell culture system (15). They showed that after 8 days of treatment, PGG decreased the level of extracellular HBV (IC50, 1.0 μg/ml, i.e. 1.1μM)) in a dose-dependent manner and also reduced the HBsAg level by 25% at a concentration of 4 μg/ml (4.2 μM). Gallic acid showed a much weaker inhibitory effect, demonstrating a structure-activity relationship requiring polygalloylation rather than isolated galloyl groups to achieve inhibition of HBV DNA replication.
Others reported the inhibition by PGG of several key enzymes in the virus life circle. Ahn et al isolated several compounds containing galloyl moiety from Terminalia chebula Retz fruits including PGG, 1, 3, 6-tri-O-galloyl-β-D-glucose (3GG) and gallic acid (18). PGG showed a stronger inhibitory effect on HIV integrase and reverse transcriptase than the other two compounds. Duan et al purified 1, 2, 6-tri-O-galloyl-β-D-glucose (3GG), 1, 2, 3, 6-tetra-O-galloyl-β-D-glucose (4GG) and PGG (5GG) from the ethyl acetate extract fraction of the traditional Chinese medicine Galla chinensis. These compounds inhibited HCV NS3 protease with IC50 of 1.9, 0.8, and 1.6 μM, respectively (72). Molecular docking of those compounds onto the active site of HCV NS3 protease indicated five potential hydrogen bond interactions between the compounds and the enzyme at D81, K136, A157, R155, C159 and a potential hydrophobic interaction between the glucose ring and V158. 1, 2, 3, 6-tetra-O-galloyl-β-D-glucose (4GG) and the enzyme had the best alignment, which was consistent with the highest inhibitory effect of this compound.
PGG has been shown to have anti-virus activities against HSV (herpes simplex rirus), HIV, HBV and HCV. Takechi et al tested the anti-HSV activity of PGG and structural related hydrolysable tannins in an in vitro FL cells culture model. The data showed that PGG had an anti-HSV ED50 of 12μM whereas its IC50 to host cells was 117μM. Structure-activity relationship studies indicated that anti-virus activity was positively correlated with the numbers of phenolic groups. The cytotoxicities to host cells of tannins paralleled their antiviral activities, which suggested that the activities could be based on the interaction with the proteins of FL cell surfaces(73). Nakashima et al evaluated the anti-HIV activity of sulfated pentagalloylglucose (Y-ART-3). In vitro, Y-ART-3 inhibited both HIV-1 and HIV-2 through inhibition of viral adsorption to CD4-positive cells. For in vivo study, 4 mg/kg Y-ART-3 was daily administrated to hu-PBL-SCID mice that had been infected with HIV via i.p. injection. Human Ig- and CD4-positive cells were detected at similar levels in Y-ART-3-treated mice compared with mice that were not infected with HIV. They concluded that Y-ART-3 may be considered to be a potent and effective anti-HIV compound(74).
Zhang et al studied the anti-bacterial activity of Acer truncatum Bunge extract and found that PGG inhibited several bacteria strains such as Staphylococcus aureus, Staphylococcus epidermidis, E.coli and Pseudomonas aeruginosa with MIC (Minimal Inhibitory Concentration) at 0.25, 0.06, 0.25 and 0.125 mg/mL (266, 64, 266 and 133μM), respectively (17). The inhibitory mechanism proposed was related to an inhibition of the bacterial type II fatty acid synthesis system. Their data showed that PGG potently inhibited the β-oxoacyl-ACP reductase (FabG) with the IC50 value of 0.9μM. Kinetic studies indicated that PGG was a mixed-type inhibitor of FabG with respect to NADPH. In another study, Cannell et al isolated a isomer of PGG, 3-O-digalloyl- 1,2,6-trigalloylglucose, from the freshwater green alga Spirogyra varians, and found that this compound irreversibly inhibited α-glucosidase and was more potent than PGG, 1 ,2, 3, 6-Tetra-O-galloylglucose or 1 ,2, 6-Tri-O-galloylglucose (75). The PGG isomer was proposed as the antibacterial agent in the methanol extract of Spirogyra varians.
8.11 Radio-protective activity of PGG-rich extract
To search for safe and efficacious radio-protective agents, Park et al tested the effect of Elaeocarpus sylvestris var. ellipticus extract, which was rich in PGG, in protecting mice from radiation injury by single whole-body irradiation (76). The extract was i.p. administrated at 25 mg/kg on day 1, 3, 6 and 9 after 9 Gy lethal γ-ray radiation. The mean life span of mice receiving the extract was significantly increased by more than 30%. Moreover, endogenous splenocytes colonies and proliferation of splenocytes from irradiated mice were also stimulated by the i.p. injected extract. These data suggested that the PGG-rich extract exerted the radio-protective activity by intensifying hematopoietic repair capacities. Since only the crude extract was tested in the experiments, PGG may or not account for the biological activities seen.
8.12 Inhibition of mitochondria respiration
Adachi et al investigated the effects of PGG on mitochondrial respiration in test tube assays (77). PGG (20μM) decreased the respiratory control ratio (RCR) of highly coupled rat liver mitochondria by half, whereas PGG barely affected the adenosine-5′-diphosphate/oxygen (ADP/O) ratio. When more than 30μM PGG was used, neither RCR nor ADP/O ratio could be measured. A similar effect was observed with tannic acid. These findings suggested a bi-phasic mechanism of inhibition of mitochondrial respiration by PGG. At lower concentrations, PGG only inhibited the electron transport system to decrease RCR, while at higher concentrations, it damaged the structural integrity of the mitochondrial membrane and directly inhibited the exposed electron transport system. In order to identify the inhibitory sites of PGG on electron transport system, they tested the inhibitory effect of PGG on enzyme activities of sub-mitochondrial particles (SMP). PGG strongly inhibited succinate dehydrogenase in a competitive manner with respect to succinate and inhibited NADH dehydrogenase and ubiquinol-1 oxidase in a noncompetitive manner. The data suggested that PGG was a potential respiratory chain inhibitor with multiple targets. Whether such activities mediate the reactive oxygen generation found in PGG-treated prostate cancer cells(28) remains an intriguing postulate.
8.13 PGG as enzyme inhibitors and its kinetic characteristics
PGG has been reported as an enzyme inhibitor for microsomal 5α-reductase (33), CDK2, CDK4 (35), proteosome (38), ATPase activity of P-glycoprotein (41), HIV integrase and reverse transcriptase (18), HCV NS3 protease (72), β-oxoacyl-ACP reductase (17), α-glucosidase (75, 78), angiontensin converting enzyme (ACE) (69) and H+, K+- ATPase (64) PGG has also been reported to inhibit fatty acid synthase (FASN) (79), xanthine oxidase (80), aminopeptidase N, neutral endopeptidase (81) human salivary α-amylase (82) and tyrosinase (83, 84).
Detailed inhibitory kinetic mechanisms of PGG are not available on most enzymes. Of the few with the kinetic characterization, PGG inhibited succinate dehydrogenase in submitochondrial particles with respect to succinate and microsomal 5α-reductase with respect to NADPH in a competitive manner, whereas it inhibited other enzymes such as NADH dehydrogenase, ubiquinol-1 oxidase, human salivary α-amylase and H+, K+- ATPase in a noncompetitive manner. This suggests that PGG could affect the availability of enzymes, cofactors or substrates as well as directly compete for substrate binding.
8.14 Binding to biomolecules
Tannins have the ability to bind and precipitate alkaloids, gelatins and proteins. Binding to a wide range of molecules is the characteristic bioactivity of all the compounds classified as tannins, and the binding activities of PGG reflect this typical activity. Takechi et al quantitatively studied the binding potency of PGG to various proteins, lipids, nucleic acids, sugars and cultured human amniotic cells (FL cells)(85). The results showed that PGG bound less to acidic proteins than to neutral and basic proteins, but more to basic phospholipids than to the other lipids. In general, PGG did not strongly bind to nucleic acids and sugars. Using bovine serum albumin (BSA) as model protein, Hagerman et al. demonstrated that PGG-protein interactions are largely due to hydrophobic interactions(27). The stoichiometry of soluble PGG-serum albumin complexes was established as ranging from one to four PGG per protein (21). The mechanisms of the interactions between PGG and biological molecules were further studied by He et al(86). Their data suggested that the hydrophobic galloyl groups of PGG interacted with aliphatic side chains of amino acids such as glycine, alanine, proline and leucine. PGG -protein and PGG-phospholipids interactions were mediated by cooperative effects of hydrogen bonds and hydrophobic interactions, whereas hydrogen bonds contributed mainly to the interactions between PGG and sugars. In another study with human salivary α-amylase (82), surface plasmon resonance (SPR) binding experiments and saturation transfer difference (STD) experiment by NMR showed that the aromatic rings of PGG may be involved in the interaction, so stacking of the aromatic rings of PGG with the aromatic amino acids side chains of amylase could be the possible binding and enzyme inhibition mechanism. Replacement of the aromatic amino acids with aliphatic ones significantly decreased the amylase inhibitory effect of PGG, which supported the hypothesized mechanism.
Tannins bind to protein in a selective fashion, with particularly high affinities for proline-rich proteins which have open structures and form strong peptide-phenolic hydrogen bonds(87). In accordance with this generalization, PGG has higher affinity for the proline-rich protein gelatin than for other globular proteins such as serum albumin(88). Detailed NMR studies of the interaction between PGG and proline rich peptides suggest a stacking interaction between adjacent galloyl rings on the polyphenolic and proline residues of the peptide(89). The high affinity of PGG and other tannins for proline-rich proteins may impact bioavailability of ingested tannins, since human saliva is rich in proline-rich proteins(90).
Under oxidizing conditions, PGG and other polyphenolics form quinones at basic pH or polymers at acidic pH, via semiquinone radical intermediates. Oxidation in the presence of protein prevents quinone or polymer formation, but promotes formation of covalently stabilized protein-PGG adducts(91).
Overall, binding between tannins and other molecules is likely to occur in almost all biological systems. The challenge remains in defining how binding characteristics contribute to the biological activities of specific polyphenols such as PGG.
9. Pharmacokinetics and Stability Issues
Pharmacokinetic studies including absorption, distribution, metabolism and excretion (ADME) are very crucial to define the mechanisms underlying biological activities. Ren et al commented that α-PGG was orally bioavailable because it was detectable in blood 1 h after gavage into mice (92). However, the original data were not presented (92).
Cai et al studied the transport of PGG across cultured epithelial cells using a human intestinal epithelial Caco-2 cell monolayer model (26, 93). According to their data, transport of PGG could occur either from the apical into the basolateral compartment of the Caco-2 monolayer or the reverse direction. However, the apparent permeability coefficient (Papp) data suggested a relative poor bioavailability for PGG. HPLC-MS analysis revealed that a large proportion of PGG was degraded during the transport process across Caco-2 cells. At least part of this degradation occurred early in the transcellular transport in both apical and basolateral directions as evidenced by the presence of tri- and tetragalloyl glucose in the loading solution at the end of incubation. The identification of tri- and tetragalloyl glucose suggested the presence of esterase on both the apical and basolateral sides of the cells. The transport from the apical into the basolateral side was measured at PGG concentrations varying from 5 to 90 μM. Within the tested concentration ranges, Papp decreased with increasing PGG concentration, which suggested the transport was saturable. When serum was added to the receiving solution, transport of PGG was increased. This could be due to the presence of polyphenol binding proteins in serum since such binding would reduce free PGG and its metabolites in the receiving side, thus creating a sink for PGG and shifting the balance. Interestingly, Papp for basolateral outflow was four-fold higher than Papp for apical uptake. The difference in Papp suggests that the cells have an effective mechanism for elimination of PGG and its metabolites from the basolateral compartment. Verapamil, an inhibitor of P-glycoprotein did not decrease basolateral to apical transport of PGG at concentrations of 50 and 75 μM. In contrast, an inhibitor of multidrug resistance-associated protein MK571, when present at the concentration of 50 and 75 μM, caused a 51% and 73% reduction in Papp of PGG and its metabolites, respectively. Phlorizin, a specific inhibitor of the sodium-dependent glucose transporter SGLT-1, inhibited transport of PGG or its metabolites in a concentration dependent manner, indicating a role for this transporter in apical to basolateral transport of PGG or its metabolites across Caco-2 cells. Addition of 40 μM PGG to the basolateral compartment of the Caco-2 monolayer caused transepithelial electric resistance (TEER) to decrease below 350Ω/cm2, which indicated that the cell layer was no longer intact. The compromised monolayer integrity therefore complicates the interpretation of the results. Taken at face value, it is reasonable to speculate that PGG metabolites are generated at the gastrointestinal tract level.
Phenol groups on polyphenolic compounds are susceptible to oxidation. However, our data indicated no cell-free generation of hydrogen peroxide by PGG in cell culture medium, which suggested a lack of oxidation of PGG in Fenton-driven systems(28). Cai et al also reported that in their Caco-2 cells monolayer system, HPLC analysis of PGG solutions that had been incubated in the absence of cells for 3 hrs demonstrated only a prominent peak with the same elution position as PGG (26), which indicated that PGG was stable in solution during conditions of the cell transport experiments. Co-author Hagerman's group has found that PGG is quite stable in pure water or saline for days, but not in phosphate-buffer saline or in alkaline condition.
Tannase (tannin acyl hydrolase, EC, 3.1.1.20) can hydrolyze PGG to gallic acid (94). Though humans have not been reported to produce tannase yet, tannase activity has been found in foods such as wine and preserved vegetable (Korean kimichi)(95, 96). Tannase-producing microbes also have been isolated from human fecal samples(97). Some of them were correlated with colon cancer(98). Whether PGG can be de-activated by tannase in food and GI tract or can be converted to even more active forms by those microbes should be considered, especially if PGG is going to be administrated orally or used as an active component of health-promoting foods.
There is an urgent need to establish the in vivo PK data and identify the PGG metabolites for improving the mechanistic understanding of the biological activities and for pharmaceutical development.
10. Toxicology
Tannins are usually considered safe or even beneficial at low dietary levels. Tannins diminish protein digestibility when present in high levels in diets of low protein content(90). Some animals have mechanisms to accommodate diets with high levels of tannin including production of “salivary proline-rich proteins (PRPs)” which appear to bind to tannin immediately after ingestion and thus neutralize its detrimental effects(99). PRPs are constitutively expressed in human saliva and can be induced by PGG in rats. For example, when rats were fed with high-PGG (3%) diet, they lost weight in the first couple of days, but once the PRPs were induced, their food intake and body weight gain returned to the normal level (25).
A few studies report on the acute toxicity of PGG in animals. Feldman et al reported that infusion of 50-60 mg PGG per rat (~200 g body weight) resulted in a precipitous and lethal drop in blood pressure within 30 minutes, whereas 30 mg per rat did not affect blood pressure or blood glucose levels(54). Nishizawa et al found that i.p administration of 1 ~ 5mg PGG per rat caused a significant reduction in blood urea nitrogen level by 18-25% compared with control (100). Other gallotannin such as tri-, tetra, hexa-, hepta-, octa-, nona- and decagalloylglucose had similar effects to PGG. Therefore, the long-term safety of PGG for cancer chemoprevention still needs to be rigorously established. Since PGG is astringent(88) and inhibits human salivary α-amylase(82), potential negative effect on starch digestion and food taste should be considered during preclinical or clinical studies.
11. Taste property of PGG
It is widely believed that the oral sensation of astringency is a consequence of interactions between tannins and salivary proline-rich proteins or mucous tissues. Hoffman et al studied the astringency threshold and dose-response of PGG using the half-tongue test within a trained human panel (88). The taste threshold concentration for the astringent sensation induced by PGG in aqueous solution (pH 4.5) was 1.8 μM. Protein binding experiments suggested that PGG selectively bound soluble proteins and only associated with membranes when presented at high concentrations, resulting in a relatively high taste threshold. This was further supported by the steep taste dose/response curve for PGG. Whether this property can lead to food avoidance in dietary supplementation experiments for cancer chemoprevention should be considered.
12. Structure-activity relationship
Detailed understanding of the structure-activity relationship of PGG will be very helpful to elucidating mechanisms related to the various activities covered in this review. Among a limited number of studies, most of the researchers simply focused on how the number of galloyl groups affects the biological activities of galloyl-glucoses and analogues. In general, fully or almost fully esterified form of glucose, i.e. PGG (5GG) or 1, 2, 3, 6-tetragalloylglucose (4GG), had the best biological activities, including anti-cancer effect on S-100 sarcoma, inhibition of acid secretion (64), inhibition of HIV integrase and reverse transcriptase (18) and inhibition of HCV protease (72). These have been discussed in the above sections.
In simple chemical assays, e.g. radical scavenging, PGG is often noted to be five times more active on a molar basis than methyl gallate, suggesting that its chemical activity is simply the sum of the activities of the individual phenolic moieties(101). In more complex biological assays both in vitro and in vivo, PGG has activities that are clearly distinct from those of the monomeric phenolic compounds. In a study of the inhibitory effect of PGG on rat intestinal α-glucosidase the activities of PGG, monogallyolglucose and gallic acid were compared. On the basis of equimolar concentrations of phenol groups, PGG was much more active than monogallyolglucose or gallic acid(78). Isenburg et al also reported that PGG but not gallic acid or glucose stabilized vascular elastin (102).
Ren et al synthesized a series of PGG analogues and tested their stimulatory activity on glucose transport (92). In their study, they compared the functional importance of each individual galloyl group, the glucose core, peripheral position of the galloyl groups and the stereochemical arrangement of the galloyl groups. Their data showed that both the carbohydrate core and the galloyl substituents with their three free phenolic hydroxyl groups were essential for activity. α-PGG and β-PGG were equally potent in this biological system. In both anomers, the glucose core was required as a scaffold to present galloyl groups in a certain spatial arrangement to interact with targets and express the activity. The removal of a galloyl group from position 1, 2, 3, or 4 of glucose resulted in a complete loss of glucose transport stimulation. However, activity was retained when a galloyl group linked to position 6 was removed and the R configuration at C-1 was present (α-PGG anomer). These results suggested that the galloyl groups linked to position 1, 2, 3, or 4 of glucose in α-PGG were functionally important, while the galloyl group bound to C-6 was not. On the other hand, β-PGG required all five galloyl groups to exert its activity. Their observation suggests the possibility to achieve better activity of α-PGG by further modification of the groups bound to C-6 of the glucopyranose. Whether this structure-activity relationship holds true in other systems is still unknown.
13. Challenges and questions
Though the work reviewed above supports PGG as a very promising candidate for therapy and prevention of cancer, diabetes or other diseases, many questions remain. First, what is its in vivo bioavailability, especially when administrated orally? A handful of oral gavage studies with PGG have shown in vivo anti-cancer and other activities, suggesting that PGG and/or its degradation products and metabolites must be bioavailabe to mediate the biological activities. Second, what metabolites of PGG are produced in vivo? The Caco-2 cell study by Cai et al (26) suggests generation of PGG degradation products at the gastrointestinal tract level. The speciation of in vivo metabolites will be facilitated with isotopically labeled PGG. The bioavailability and metabolite questions have important implications for further mechanistic investigation and pharmaceutical development. These include the structure-activity relationship for PGG and its metabolites to define the essential chemical moieties (pharmacophore) to allow further derivatives to be developed and tested. Third, PGG seems more stable than other polyphenolic compounds in aqueous milieu. What is the structural basis? Finally, PGG is capable of binding to lots of biological molecules. How does this binding characteristic contribute to its biological properties? Understanding the molecular basis for binding is essential to using PGG for selective bioactivation or inhibition in light of its avidity for so many biological molecules.
14. Concluding remarks
The systematic review supports PGG as a very promising and novel drug candidate for the therapy and prevention of cancer, diabetes or other diseases. Rigorous preclinical studies are needed to establish the efficacy of PGG for these applications and are now feasible due to the optimization of PGG preparation in highly pure crystalline form and in multiple-gram quantities from the readily available tannic acid. Chemically and functionally, PGG appears to be distinct from its constituent gallic acid and from tea polyphenols. For anti-cancer activity, potential mechanisms include anti-angiogenesis, anti-proliferative actions through inhibition of DNA replicative synthesis and S-phase arrest and also G1 arrest, induction of apoptosis, anti-inflammation and anti-oxidation. Putative molecular targets include p53, Stat3, Cox-2, VEGFR1, AP-1, SP-1, Nrf-2 and MMP-9. For anti-diabetic activity, PGG and analogues appear to improve glucose uptake. However, very little is known about the absorption, pharmacokinetics and metabolism of PGG, nor its toxicity profile. Serious efforts are needed urgently to address these issues to provide critical information for the identification and validation of the in vivo active chemical species, molecular targets and for further pharmaceutical development.
Acknowledgments
Grant support:
This work was supported, in parts, by The Hormel Foundation, NIH grant CA136953 and by MRC grant (No. 2009-0063466) from Korea Ministry of Education, Science and Technology.
References
- 1.Oh GS, Pae HO, Oh H, et al. In vitro anti-proliferative effect of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose on human hepatocellular carcinoma cell line, SK-HEP-1 cells. Cancer Lett. 2001;174(1):17–24. doi: 10.1016/s0304-3835(01)00680-2. [DOI] [PubMed] [Google Scholar]
- 2.Huh JE, Lee EO, Kim MS, et al. Penta-O-galloyl-beta-D-glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: roles of cyclooxygenase-2 and mitogen-activated protein kinase pathways. Carcinogenesis. 2005;26(8):1436–45. doi: 10.1093/carcin/bgi097. [DOI] [PubMed] [Google Scholar]
- 3.Ren YM, Chen XZ. Distribution, Bioactivities and Therapeutical Potentials of Pentagalloylglucopyranose. Current Bioactive Compounds. 2007;3:81–9. [Google Scholar]
- 4.Swain TB-S, E. C. Flavonoid compounds. In: Mason MFHS, Mason Howard S., editors. Comp Biochem Marcel Florkin. Academic Press; 1962. pp. 755–809. [Google Scholar]
- 5.Yoshida TH, Tsutomu, Hideyuki Ito. High molecular weight plant polyphenols (tannins): Prospective functions. Recent Advances in Phytochemistry. 2005:163–90. [Google Scholar]
- 6.Nishizawa M, Yamagishi T, Nonaka G, Nishioka I, Bando H. Novel Hydrolyzable Tannins from Nuphar-Japonicum Dc. Chemical & Pharmaceutical Bulletin. 1982;30(3):1094–7. [Google Scholar]
- 7.Niemetz R, Gross GG. Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry. 2005;66(17):2001–11. doi: 10.1016/j.phytochem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Cammann J, Denzel K, Schilling G, Gross GG. Biosynthesis of gallotannins: beta-glucogallin-dependent formation of 1,2,3,4,6-pentagalloylglucose by enzymatic galloylation of 1,2,3,6-tetragalloylglucose. Arch Biochem Biophys. 1989;273(1):58–63. doi: 10.1016/0003-9861(89)90161-6. [DOI] [PubMed] [Google Scholar]
- 9.Grundhofer P, Niemetz R, Schilling G, Gross GG. Biosynthesis and subcellular distribution of hydrolyzable tannins. Phytochemistry. 2001;57(6):915–27. doi: 10.1016/s0031-9422(01)00099-1. [DOI] [PubMed] [Google Scholar]
- 10.Niemetz R, Gross GG. Gallotannin biosynthesis: Purification of beta-glucogallin: 1,2,3,4,6-pentagalloyl-beta-D-glucose galloyltransferase from sumac leaves. Phytochemistry. 1998;49(2):327–32. doi: 10.1016/s0031-9422(01)00300-4. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann AS, Gross GG. Biosynthesis of gallotannins: formation of polygalloylglucoses by enzymatic acylation of 1,2,3,4,6-penta-O-galloylglucose. Arch Biochem Biophys. 1990;283(2):530–2. doi: 10.1016/0003-9861(90)90678-r. [DOI] [PubMed] [Google Scholar]
- 12.Frohlich B, Niemetz R, Gross GG. Gallotannin biosynthesis: two new galloyltransferases from Rhus typhina leaves preferentially acylating hexa- and heptagalloylglucoses. Planta. 2002;216(1):168–72. doi: 10.1007/s00425-002-0877-3. [DOI] [PubMed] [Google Scholar]
- 13.Niemetz R, Gross GG. Oxidation of pentagalloylglucose to the ellagitannin, tellimagrandin II, by a phenol oxidase from Tellima grandiflora leaves. Phytochemistry. 2003;62(3):301–6. doi: 10.1016/s0031-9422(02)00557-5. [DOI] [PubMed] [Google Scholar]
- 14.Niemetz R, Schilling G, Gross GG. Biosynthesis of the dimeric ellagitannin, cornusiin E, in Tellima grandiflora. Phytochemistry. 2003;64(1):109–14. doi: 10.1016/s0031-9422(03)00280-2. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Lee HK, Jung MK, Mar W. In vitro antiviral activity of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose against hepatitis B virus. Biol Pharm Bull. 2006;29(10):2131–4. doi: 10.1248/bpb.29.2131. [DOI] [PubMed] [Google Scholar]
- 16.Cavalher-Machado SC, Rosas EC, Brito Fde A, et al. The anti-allergic activity of the acetate fraction of Schinus terebinthifolius leaves in IgE induced mice paw edema and pleurisy. Int Immunopharmacol. 2008;8(11):1552–60. doi: 10.1016/j.intimp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Luo SY, Ye YB, et al. The antibacterial efficacy of an aceraceous plant [Shantung maple (Acer truncatum Bunge)] may be related to inhibition of bacterial beta-oxoacyl-acyl carrier protein reductase (FabG) Biotechnol Appl Biochem. 2008;51(Pt 2):73–8. doi: 10.1042/BA20070255. [DOI] [PubMed] [Google Scholar]
- 18.Ahn MJ, Kim CY, Lee JS, et al. Inhibition of HIV-1 integrase by galloyl glucoses from Terminalia chebula and flavonol glycoside gallates from Euphorbia pekinensis. Planta Med. 2002;68(5):457–9. doi: 10.1055/s-2002-32070. [DOI] [PubMed] [Google Scholar]
- 19.Wang WC, Wang C, Song XY, Zhao WH, Wang Q. Determination of 1, 2, 3, 4, 6-penta-O-galloyl-D-glucose in forty four kinds of Chinese traditional medicines by HPLC. Zhongguo Zhong Yao Za Zhi. 2008;33(6):656–9. [PubMed] [Google Scholar]
- 20.Hagerman AE, Robbins CT, Weerasuriya Y, Wilson TC, McArthur C. Tannin Chemistry in Relation to Digestion. Journal of Range Management. 1992;45:57–62. [Google Scholar]
- 21.Chen Y, Hagerman AE. Characterization of soluble non-covalent complexes between bovine serum albumin and beta-1,2,3,4,6-penta-O-galloyl-D-glucopyranose by MALDI-TOF MS. Journal of agricultural and food chemistry. 2004;52(12):4008–11. doi: 10.1021/jf035536t. [DOI] [PubMed] [Google Scholar]
- 22.Khanbabaee K, Lotzerich K. Efficient total synthesis of the natural products 2,3,4,6-tetra-O-galloyl-d-glucopyranose, 1,2,3,4,6-penta-O-galloyl-beta-D-glucopyranose and the unnatural 1,2,3,4,6-penta-O-galloyl-alpha-D-glucopyranose. Tetrahedron. 1997;53(31):10725–32. [Google Scholar]
- 23.Binkley RC, Ziepfel JC, Himmeldirk KB. Anomeric selectivity in the synthesis of galloyl esters of D-glucose. Carbohydr Res. 2009;344(2):237–9. doi: 10.1016/j.carres.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Chen YM, Hagerman AE, Minto RE. Preparation of 1,2,3,4,6-penta-O-galloyl-[U-C-14]-D-glucopyranose. Journal of Labelled Compounds & Radiopharmaceuticals. 2003;46(1):99–105. [Google Scholar]
- 25.Skopec MM, Hagerman AE, Karasov WH. Do salivary proline-rich proteins counteract dietary hydrolyzable tannin in laboratory rats? J Chem Ecol. 2004;30(9):1679–92. doi: 10.1023/b:joec.0000042395.31307.be. [DOI] [PubMed] [Google Scholar]
- 26.Cai K, Hagerman AE, Minto RE, Bennick A. Decreased polyphenol transport across cultured intestinal cells by a salivary proline-rich protein. Biochem Pharmacol. 2006;71(11):1570–80. doi: 10.1016/j.bcp.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Hagerman AE, Rice ME, Ritchard NT. Mechanisms of protein precipitation for two tannins, pentagalloyl glucose and epicatechin(16) (4 -> 8) catechin (procyanidin) Journal of agricultural and food chemistry. 1998;46(7):2590–5. [Google Scholar]
- 28.Hu H, Lee HJ, Jiang C, et al. Penta-1,2,3,4,6-O-galloyl-beta-D-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Mol Cancer Ther. 2008;7(9):2681–91. doi: 10.1158/1535-7163.MCT-08-0456. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa H, Hasumi K, Woo JT, Nagai K, Wachi M. Generation of hydrogen peroxide primarily contributes to the induction of Fe(II)-dependent apoptosis in Jurkat cells by (−)-epigallocatechin gallate. Carcinogenesis. 2004;25(9):1567–74. doi: 10.1093/carcin/bgh168. [DOI] [PubMed] [Google Scholar]
- 30.Lee KW, Hur HJ, Lee HJ, Lee CY. Antiproliferative effects of dietary phenolic substances and hydrogen peroxide. Journal of agricultural and food chemistry. 2005;53(6):1990–5. doi: 10.1021/jf0486040. [DOI] [PubMed] [Google Scholar]
- 31.Kuo PT, Lin TP, Liu LC, et al. Penta-O-galloyl-beta-D-glucose suppresses prostate cancer bone metastasis by transcriptionally repressing EGF-induced MMP-9 expression. Journal of agricultural and food chemistry. 2009;57(8):3331–9. doi: 10.1021/jf803725h. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto K, Kishi N, Koshiura R, Yoshida T, Hatano T, Okuda T. Relationship between the Structures and the Antitumor Activities of Tannins. Chemical & Pharmaceutical Bulletin. 1987;35(2):814–22. doi: 10.1248/cpb.35.814. [DOI] [PubMed] [Google Scholar]
- 33.Lee HH, Ho CT, Lin JK. Theaflavin-3,3′-digallate and penta-O-galloyl-beta-D-glucose inhibit rat liver microsomal 5alpha-reductase activity and the expression of androgen receptor in LNCaP prostate cancer cells. Carcinogenesis. 2004;25(7):1109–18. doi: 10.1093/carcin/bgh106. [DOI] [PubMed] [Google Scholar]
- 34.Hu H, Zhang J, Lee HJ, Kim SH, Lu J. Penta-O-galloyl-beta-D-glucose induces S- and G(1)-cell cycle arrests in prostate cancer cells targeting DNA replication and cyclin D1. Carcinogenesis. 2009;30(5):818–23. doi: 10.1093/carcin/bgp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen WJ, Chang CY, Lin JK. Induction of G1 phase arrest in MCF human breast cancer cells by pentagalloylglucose through the down-regulation of CDK4 and CDK2 activities and up-regulation of the CDK inhibitors p27(Kip) and p21(Cip) Biochem Pharmacol. 2003;65(11):1777–85. doi: 10.1016/s0006-2952(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 36.Hua KT, Way TD, Lin JK. Pentagalloylglucose inhibits estrogen receptor alpha by lysosome-dependent depletion and modulates ErbB/PI3K/Akt pathway in human breast cancer MCF-7 cells. Mol Carcinog. 2006;45(8):551–60. doi: 10.1002/mc.20226. [DOI] [PubMed] [Google Scholar]
- 37.Pan MH, Lin JH, Lin-Shiau SY, Lin JK. Induction of apoptosis by penta-O-galloyl-beta-D-glucose through activation of caspase-3 in human leukemia HL-60 cells. Eur J Pharmacol. 1999;381(23):171–83. doi: 10.1016/s0014-2999(99)00549-x. [DOI] [PubMed] [Google Scholar]
- 38.Chen WJ, Lin JK. Induction of G1 arrest and apoptosis in human jurkat T cells by pentagalloylglucose through inhibiting proteasome activity and elevating p27Kip1, p21Cip1/WAF1, and Bax proteins. J Biol Chem. 2004;279(14):13496–505. doi: 10.1074/jbc.M212390200. [DOI] [PubMed] [Google Scholar]
- 39.Ho LL, Chen WJ, Lin-Shiau SY, Lin JK. Penta-O-galloyl-beta-D-glucose inhibits the invasion of mouse melanoma by suppressing metalloproteinase-9 through down-regulation of activator protein-1. Eur J Pharmacol. 2002;453(23):149–58. doi: 10.1016/s0014-2999(02)02340-3. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Lee HM, Ji ST, Lee SR, Mar W, Gho YS. 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose blocks endothelial cell growth and tube formation through inhibition of VEGF binding to VEGF receptor. Cancer Lett. 2004;208(1):89–94. doi: 10.1016/j.canlet.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Kitagawa S, Nabekura T, Nakamura Y, Takahashi T, Kashiwada Y. Inhibition of P-glycoprotein function by tannic acid and pentagalloylglucose. J Pharm Pharmacol. 2007;59(7):965–9. doi: 10.1211/jpp.59.7.0008. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Kim JK, Li Y, Li J, Liu F, Chen X. Tannic acid stimulates glucose transport and inhibits adipocyte differentiation in 3T3-L1 cells. J Nutr. 2005;135(2):165–71. doi: 10.1093/jn/135.2.165. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Kim J, Li J, et al. Natural anti-diabetic compound 1,2,3,4,6-penta-O-galloyl-D-glucopyranose binds to insulin receptor and activates insulin-mediated glucose transport signaling pathway. Biochem Biophys Res Commun. 2005;336(2):430–7. doi: 10.1016/j.bbrc.2005.08.103. [DOI] [PubMed] [Google Scholar]
- 44.Riedl KM, Hagerman AE. Tannin-protein complexes as radical scavengers and radical sinks. Journal of agricultural and food chemistry. 2001;49(10):4917–23. doi: 10.1021/jf010683h. [DOI] [PubMed] [Google Scholar]
- 45.Abdelwahed A, Bouhlel I, Skandrani I, et al. Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus. Confirmation by microarray expression profiling. Chem Biol Interact. 2007;165(1):1–13. doi: 10.1016/j.cbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Okubo T, Nagai F, Seto T, Satoh K, Ushiyama K, Kano I. The inhibition of phenylhydroquinone-induced oxidative DNA cleavage by constituents of Moutan Cortex and Paeoniae Radix. Biol Pharm Bull. 2000;23(2):199–203. doi: 10.1248/bpb.23.199. [DOI] [PubMed] [Google Scholar]
- 47.Park EJ, Zhao YZ, An RB, Kim YC, Sohn DH. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose from Galla Rhois protects primary rat hepatocytes from necrosis and apoptosis. Planta Med. 2008;74(11):1380–3. doi: 10.1055/s-2008-1081300. [DOI] [PubMed] [Google Scholar]
- 48.Okuda T, Mori K, Hayatsu H. Inhibitory Effect of Tannins on Direct-Acting Mutagens. Chemical & Pharmaceutical Bulletin. 1984;32(9):3755–8. doi: 10.1248/cpb.32.3755. [DOI] [PubMed] [Google Scholar]
- 49.Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res. 2008;52(Suppl 1):S128–38. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- 50.Choi BM, Kim HJ, Oh GS, et al. 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose protects rat neuronal cells (Neuro 2A) from hydrogen peroxide-mediated cell death via the induction of heme oxygenase-1. Neurosci Lett. 2002;328(2):185–9. doi: 10.1016/s0304-3940(02)00513-x. [DOI] [PubMed] [Google Scholar]
- 51.Pae HO, Oh GS, Jeong SO, et al. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose up-regulates heme oxygenase-1 expression by stimulating Nrf2 nuclear translocation in an extracellular signal-regulated kinase-dependent manner in HepG2 cells. World J Gastroenterol. 2006;12(2):214–21. doi: 10.3748/wjg.v12.i2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhimani RS, Troll W, Grunberger D, Frenkel K. Inhibition of Oxidative Stress in Hela-Cells by Chemopreventive Agents. Cancer Research. 1993;53(19):4528–33. [PubMed] [Google Scholar]
- 53.Feldman KS, Sahasrabudhe K, Smith RS, Scheuchenzuber WJ. Immunostimulation by plant polyphenols: a relationship between tumor necrosis factor-alpha production and tannin structure. Bioorg Med Chem Lett. 1999;9(7):985–90. doi: 10.1016/s0960-894x(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 54.Feldman KS, Sahasrabudhe K, Lawlor MD, Wilson SL, Lang CH, Scheuchenzuber WJ. In vitro and In vivo inhibition of LPS-stimulated tumor necrosis factor-alpha secretion by the gallotannin beta-D-pentagalloylglucose. Bioorg Med Chem Lett. 2001;11(14):1813–5. doi: 10.1016/s0960-894x(01)00332-8. [DOI] [PubMed] [Google Scholar]
- 55.Wu M, Gu Z. Screening of bioactive compounds from moutan cortex and their anti-inflammatory activities in rat synoviocytes. Evid Based Complement Alternat Med. 2009;6(1):57–63. doi: 10.1093/ecam/nem066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Genfa L, Jiang Z, Hong Z, et al. The screening and isolation of an effective anti-endotoxin monomer from Radix Paeoniae Rubra using affinity biosensor technology. Int Immunopharmacol. 2005;5(6):1007–17. doi: 10.1016/j.intimp.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Oh GS, Pae HO, Choi BM, et al. Penta-O-galloyl-beta-D-glucose inhibits phorbol myristate acetate-induced interleukin-8 [correction of intereukin-8] gene expression in human monocytic U937 cells through its inactivation of nuclear factor-kappaB. Int Immunopharmacol. 2004;4(3):377–86. doi: 10.1016/j.intimp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 58.Lee SH, Park HH, Kim JE, et al. Allose gallates suppress expression of pro-inflammatory cytokines through attenuation of NF-kappaB in human mast cells. Planta Med. 2007;73(8):769–73. doi: 10.1055/s-2007-981553. [DOI] [PubMed] [Google Scholar]
- 59.Lee SJ, Lee IS, Mar W. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 activity by 1,2,3,4,6-penta-O-galloyl-beta-D-glucose in murine macrophage cells. Arch Pharm Res. 2003;26(10):832–9. doi: 10.1007/BF02980029. [DOI] [PubMed] [Google Scholar]
- 60.Kang DG, Moon MK, Choi DH, Lee JK, Kwon TO, Lee HS. Vasodilatory and anti-inflammatory effects of the 1,2,3,4,6-penta-O-galloyl-beta-D-glucose (PGG) via a nitric oxide-cGMP pathway. Eur J Pharmacol. 2005;524(13):111–9. doi: 10.1016/j.ejphar.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 61.Pan MH, Lin-Shiau SY, Ho CT, Lin JH, Lin JK. Suppression of lipopolysaccharide-induced nuclear factor-kappaB activity by theaflavin-3,3′-digallate from black tea and other polyphenols through down-regulation of IkappaB kinase activity in macrophages. Biochem Pharmacol. 2000;59(4):357–67. doi: 10.1016/s0006-2952(99)00335-4. [DOI] [PubMed] [Google Scholar]
- 62.Yokozawa T, Chen CP, Tanaka T, Kitani K. A study on the nitric oxide production-suppressing activity of Sanguisorbae Radix components. Biological & Pharmaceutical Bulletin. 2000;23(6):717–22. doi: 10.1248/bpb.23.717. [DOI] [PubMed] [Google Scholar]
- 63.Park JK, Cho HJ, Lim Y, Cho YH, Lee CH. Hypocholestrolemic effect of CJ90002 in hamsters: A potent inhibitor for squalene synthase from Paeonia moutan. Journal of Microbiology and Biotechnology. 2002;12(2):222–7. [Google Scholar]
- 64.Ono K, Sawada T, Murata Y, et al. Pentagalloylglucose, an antisecretory component of Paeoniae radix, inhibits gastric H+, K(+)-ATPase. Clin Chim Acta. 2000;290(2):159–67. doi: 10.1016/s0009-8981(99)00184-9. [DOI] [PubMed] [Google Scholar]
- 65.Isenburg JC, Simionescu DT, Starcher BC, Vyavahare NR. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation. 2007;115(13):1729–37. doi: 10.1161/CIRCULATIONAHA.106.672873. [DOI] [PubMed] [Google Scholar]
- 66.Tedder ME, Liao J, Weed B, et al. Stabilized Collagen Scaffolds for Heart Valve Tissue Engineering. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2008.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goto H, Shimada Y, Akechi Y, Kohta K, Hattori M, Terasawa K. Endothelium-dependent vasodilator effect of extract prepared from the roots of Paeonia lactiflora on isolated rat aorta. Planta Med. 1996;62(5):436–9. doi: 10.1055/s-2006-957934. [DOI] [PubMed] [Google Scholar]
- 68.Dong H, Chen SX, Kini RM, Xu HX. Effects of tannins from Geum japonicum on the catalytic activity of thrombin and factor Xa of blood coagulation cascade. Journal of Natural Products. 1998;61(11):1356–60. doi: 10.1021/np9801458. [DOI] [PubMed] [Google Scholar]
- 69.Liu JC, Hsu FL, Tsai JC, et al. Antihypertensive effects of tannins isolated from traditional Chinese herbs as non-specific inhibitors of angiontensin converting enzyme. Life Sci. 2003;73(12):1543–55. doi: 10.1016/s0024-3205(03)00481-8. [DOI] [PubMed] [Google Scholar]
- 70.Sugaya A, Suzuki T, Sugaya E, Yuyama N, Yasuda K, Tsuda T. Inhibitory effect of peony root extract on pentylenetetrazol-induced EEG power spectrum changes and extracellular calcium concentration changes in rat cerebral cortex. J Ethnopharmacol. 1991;33(12):159–67. doi: 10.1016/0378-8741(91)90174-c. [DOI] [PubMed] [Google Scholar]
- 71.Lee JH, Yehl M, Ahn KS, Kim SH, Lieske JC. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose attenuates renal cell migration, hyaluronan expression, and crystal adhesion. European Journal of Pharmacology. 2009;606(13):32–7. doi: 10.1016/j.ejphar.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 72.Duan D, Li Z, Luo H, Zhang W, Chen L, Xu X. Antiviral compounds from traditional Chinese medicines Galla Chinese as inhibitors of HCV NS3 protease. Bioorg Med Chem Lett. 2004;14(24):6041–4. doi: 10.1016/j.bmcl.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 73.Takechi M, Tanaka Y, Takehara M, Nonaka G, Nishioka I. Structure and Antiherpetic Activity among the Tannins. Phytochemistry. 1985;24(10):2245–50. [Google Scholar]
- 74.Nakashima H, Ichiyama K, Hirayama F, et al. Sulfated pentagalloyl glucose (Y-ART-3) inhibits HIV replication and cytopathic effects in vitro, and reduces HIV infection in hu-PBL-SCID mice. Antiviral Research. 1996;30(23):95–108. doi: 10.1016/0166-3542(95)00903-5. [DOI] [PubMed] [Google Scholar]
- 75.Cannell RJ, Farmer P, Walker JM. Purification and characterization of pentagalloylglucose, and alpha-glucosidase inhibitor/antibiotic from the freshwater green alga Spirogyra varians. Biochem J. 1988;255(3):937–41. doi: 10.1042/bj2550937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park E, Lee NH, Baik JS, Jee Y. Elaeocarpus sylvestris modulates gamma-ray-induced immunosuppression in mice: implications in radioprotection. Phytother Res. 2008;22(8):1046–51. doi: 10.1002/ptr.2430. [DOI] [PubMed] [Google Scholar]
- 77.Adachi H, Konishi K, Horikoshi I. The effects of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose on rat liver mitochondrial respiration. Chem Pharm Bull (Tokyo) 1989;37(5):1341–4. doi: 10.1248/cpb.37.1341. [DOI] [PubMed] [Google Scholar]
- 78.Toda M, Kawabata J, Kasai T. Inhibitory effects of ellagi- and gallotannins on rat intestinal alpha-glucosidase complexes. Biosci Biotechnol Biochem. 2001;65(3):542–7. doi: 10.1271/bbb.65.542. [DOI] [PubMed] [Google Scholar]
- 79.Li XC, Joshi AS, ElSohly HN, et al. Fatty acid synthase inhibitors from plants: isolation, structure elucidation, and SAR studies. J Nat Prod. 2002;65(12):1909–14. doi: 10.1021/np020289t. [DOI] [PubMed] [Google Scholar]
- 80.Hayashi T, Nagayama K, Arisawa M, et al. Pentagalloylglucose, a xanthine oxidase inhibitor from a Paraguayan crude drug, “Molle-i” (Schinus terebinthifolius) J Nat Prod. 1989;52(1):210–1. doi: 10.1021/np50061a035. [DOI] [PubMed] [Google Scholar]
- 81.Kiss AK, Derwinska M, Dawidowska A, Naruszewicz M. Novel biological properties of Oenothera paradoxa defatted seed extracts: effects on metallopeptidase activity. Journal of agricultural and food chemistry. 2008;56(17):7845–52. doi: 10.1021/jf801372h. [DOI] [PubMed] [Google Scholar]
- 82.Gyemant G, Zajacz A, Becsi B, et al. Evidence for pentagalloyl glucose binding to human salivary alpha-amylase through aromatic amino acid residues. Biochim Biophys Acta. 2009;1794(2):291–6. doi: 10.1016/j.bbapap.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 83.Kim YJ, Uyama H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci. 2005;62(15):1707–23. doi: 10.1007/s00018-005-5054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nithitanakool S, Pithayanukul P, Bavovada R, Saparpakorn P. Molecular docking studies and anti-tyrosinase activity of Thai mango seed kernel extract. Molecules. 2009;14(1):257–65. doi: 10.3390/molecules14010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takechi M, Tanaka Y. Binding of 1,2,3,4,6-Pentagalloylglucose to Proteins, Lipids, Nucleic-Acids and Sugars. Phytochemistry. 1987;26(1):95–7. [Google Scholar]
- 86.He Q, Shi B, Yao K. Interactions of gallotannins with proteins, amino acids, phospholipids and sugars. Food Chemistry. 2006;95(2):250–4. [Google Scholar]
- 87.Hagerman AE, Butler LG. The Specificity of Proanthocyanidin-Protein Interactions. Journal of Biological Chemistry. 1981;256(9):4494–7. [PubMed] [Google Scholar]
- 88.Hofmann T, Glabasnia A, Schwarz B, Wisman KN, Gangwer KA, Hagerman AE. Protein binding and astringent taste of a polymeric procyanidin, 1,2,3,4,6-penta-O-galloyl-beta-D-glucopyranose, castalagin, and grandinin. Journal of agricultural and food chemistry. 2006;54(25):9503–9. doi: 10.1021/jf062272c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baxter NJ, Lilley TH, Haslam E, Williamson MP. Multiple interactions between polyphenols and a salivary proline-rich protein repeat result in complexation and precipitation. Biochemistry. 1997;36(18):5566–77. doi: 10.1021/bi9700328. [DOI] [PubMed] [Google Scholar]
- 90.Mehansho HB, Larry G, Carlson, Don M. Dietary tannins and salivary proline-rich proteins: interactions, induction, and defense mechanisms. Annual Review of Nutrition. 1987;7:423–40. doi: 10.1146/annurev.nu.07.070187.002231. [DOI] [PubMed] [Google Scholar]
- 91.Chen YM, Hagerman AE. Reaction pH and protein affect the oxidation products of beta-pentagalloyl glucose. Free Radical Research. 2005;39(2):117–24. doi: 10.1080/10715760400013789. [DOI] [PubMed] [Google Scholar]
- 92.Ren Y, Himmeldirk K, Chen X. Synthesis and structure-activity relationship study of antidiabetic penta-O-galloyl-D-glucopyranose and its analogues. J Med Chem. 2006;49(9):2829–37. doi: 10.1021/jm060087k. [DOI] [PubMed] [Google Scholar]
- 93.Cai K, Bennick A. Effect of salivary proteins on the transport of tannin and quercetin across intestinal epithelial cells in culture. Biochem Pharmacol. 2006;72(8):974–80. doi: 10.1016/j.bcp.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 94.Aguilar CN, Gutierrez-Sanchez G. Review: Sources, properties, applications and potential uses of tannin acyl hydrolase. Food Science and Technology International. 2001;7(5):373–82. [Google Scholar]
- 95.Vaquero I, Marcobal A, Munoz R. Tannase activity by lactic acid bacteria isolated from grape must and wine. International Journal of Food Microbiology. 2004;96(2):199–204. doi: 10.1016/j.ijfoodmicro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 96.Kwon T, Shim S, Lee J. Characterization of Lactobacilli with Tannase Activity Isolated from Kimchi. Food Science and Biotechnology. 2008;17(6):1322–6. [Google Scholar]
- 97.Nishitani Y, Sasaki E, Fujisawaz T, Osawa R. Genotypic analyses of lactobacilli with a range of tannase activities isolated from human feces and fermented foods. Systematic and Applied Microbiology. 2004;27(1):109–17. doi: 10.1078/0723-2020-00262. [DOI] [PubMed] [Google Scholar]
- 98.Noguchi N, Ohashi T, Shiratori T, et al. Association of tannase-prodncing Staphylococcus lugdunensis with colon cancer and characterization of a novel tannase gene. Journal of Gastroenterology. 2007;42(5):346–51. doi: 10.1007/s00535-007-2012-5. [DOI] [PubMed] [Google Scholar]
- 99.Mehansho H, Hagerman A, Clements S, Butler L, Rogler J, Carlson DM. Modulation of Proline-Rich Protein-Biosynthesis in Rat Parotid-Glands by Sorghums with High Tannin Levels. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences. 1983;80(13):3948–52. doi: 10.1073/pnas.80.13.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nishizawa M, Yamagishi T, Nonaka G, Nishioka I, Nagasawa T, Oura H. Tannins and related compounds. XII. Isolation and characterization of galloylglucoses from Paeoniae Radix and their effects on urea-nitrogen concentration in rat serum. Chem Pharm Bull (Tokyo) 1983;31(8):2593–600. doi: 10.1248/cpb.31.2593. [DOI] [PubMed] [Google Scholar]
- 101.Riedl KMC S, Alessio HM, McCarthy M*, Hagerman AE. Antioxidant Activity of Tannins and Tannin-Protein Complexes: Assessment in vitro and in vivo. In: Morello MaS F, editor. Free Radicals in Foods: Chemistry, Nutrition and Health American Chemical Society. Washington, DC: 2002. pp. 188–200. [Google Scholar]
- 102.Isenburg JC, Karamchandani NV, Simionescu DT, Vyavahare NR. Structural requirements for stabilization of vascular elastin by polyphenolic tannins. Biomaterials. 2006;27(19):3645–51. doi: 10.1016/j.biomaterials.2006.02.016. [DOI] [PubMed] [Google Scholar]