Abstract
In prairie voles (Microtus ochrogaster), most virgin females are infanticidal. To determine the onset of maternal responsiveness, female prairie voles were tested for maternal behavior as virgins and at different times throughout pregnancy. Female voles that were infanticidal as virgins by and large remained infanticidal throughout pregnancy. In contrast, about 30% of voles that were maternal as virgins became infanticidal during pregnancy. To test whether events associated with parturition facilitate the onset of maternal behavior, females had their litters delivered by Caesarean section within a day of expected delivery or were allowed to give birth naturally with sham surgery occurring shortly before or after birth. Females that gave birth naturally were fully maternal and did not attack unrelated pups, but females subjected to artificial delivery remained infanticidal. This suggests that events closely related to parturition are crucial for full development of maternal behavior in female prairie voles.
Keywords: Infanticide, Caesarian section, Parental behavior, Rats, Postnatal
Introduction
Parental behaviors are a constellation of behaviors organized to provide nourishment, warmth, tactile stimulation and protection to the offspring. Parental strategies vary by species and can range from minimal investment in the survival of the young from one parent to long-term care from both parents (Clutton-Brock, 1991; Reynolds et al., 2002).
In rodents, reproductive events induce changes in maternal responsiveness (Wiesner and Sheard, 1933). Immediately upon appearance of the first born, parturient female rodents display a full complement of maternal behaviors, such as pup retrieval, grooming and crouching over the pups. In contrast, virgin female rodents often avoid contact with pups or attack them (Wiesner and Sheard, 1933; Rosenblatt, 1967; Fleming and Luebke, 1981; Jakubowski and Terkel, 1985). In rats, the appearance of maternal behaviors and a decrease in infanticidal behaviors usually occur within 24–48 h before the birth of the offspring (Wiesner and Sheard, 1933; Slotnick et al., 1973; Mayer and Rosenblatt, 1984). Hormones are an important factor in these changes because mimicking the hormonal changes characteristic of pregnancy induces maternal behaviors in naive female rats (Bridges, 1984; Moltz et al., 1970; Terkel and Rosenblatt, 1968; Novakov and Fleming, 2005). This suggests that, under natural circumstances, physiological changes associated with pregnancy prepare the brain for parental behavior.
The behavioral characteristics of prairie voles (Microtus ochrogaster) render it uniquely suited for the study of the induction of parental behavior. Both males and females take part in the care of the young. Likewise, reproductive experience produces dramatic shifts in parental responsiveness, especially in females. Reactions to pups depend on several factors: sex, age, and reproductive status. Adult male prairie voles are spontaneously paternal (Lonstein and de Vries, 1999), with an increase in paternal responsiveness noted after mating (Bamshad et al., 1994). In contrast, a high percentage of adult nulliparous females are infanticidal (Lonstein and de Vries, 1999). Although prepubescent females are often alloparental, parental responsiveness declines as the female matures (Roberts et al., 1996; Lonstein and de Vries, 2001; Bales et al., 2004b), reaching a plateau around the age of 90 days when most females show unresponsive or infanticidal reactions to pups (Lonstein and de Vries, 2001). As female voles are fully maternal after the birth of pups, events associated with pregnancy and possibly parturition appear to induce parental behavior. To determine when such events may take place, we measured parental responsiveness before and throughout pregnancy. Our data showed that infanticidal prairie voles remain infanticidal even through the last day of pregnancy. We tested, therefore, the hypothesis that events intimately related to parturition are essential for full development of parental behavior by comparing parental responsiveness in females subjected to artificial delivery and those allowed to give birth naturally.
Materials and methods
Subjects
Subjects were female prairie voles (M. ochrogaster) born and raised in our colony. The colony was established in 1996 at the University of Massachusetts, Amherst, from voles captured in 1994 from Urbana, IL by Betty McGuire (Smith College, Northampton, MA, USA) and Zuoxin Wang (Florida State University, Tallahassee, FL, USA) and outbred in 2000 with animals provided by Dr. S. Carter (University of Illinois, Chicago, IL, USA). The animals were approximately 90–130 days of age at the beginning of the experiment. The vivarium in which the voles were housed was temperature −(21 °C) and light-controlled (14 h light:10 h dark). The animals were housed in plastic cages (48×28×16 cm) containing wood chips, wood shavings, and substantial hay covering. The animals had ad libitum access to water and food (Purina rabbit chow, sunflower seeds, cracked corn, and whole oats). Litters were weaned 20 days after birth and sorted according to sex approximately 30 to 60 days later.
Male voles were used only for breeding and cohabitation. Three days before pairing males and females, the male voles were housed individually. One day prior to pairing, the female voles were exposed to bedding soiled by male voles to increase the likelihood that males and females mated on the day of pairing (e.g., decreased fighting and subsequent mortality upon pairing; Richmond and Conaway, 1969; Carter et al., 1987). Under these conditions, our animals gave birth within a 48-hour period (stretching over days 22–24, with day 0 being the day of pairing).
The experiments were conducted according to the standards set by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (DHEW Publication 80–23, revised 1985, Office of Science and Health Reports, DRR/NIH, Bethesda, MD) and the institutional guidelines of the University of Massachusetts.
Experimental design
All subjects had no previous encounters with pups except for their own littermates. Prior to cohabitation with a male, all females were tested for parental behavior and designated maternal or infanticidal (see below). In Experiment 1, infanticidal as well maternal females were retested either while still sexually naive or while pregnant. In Experiment 2, only mated infanticidal females were retested, this time after artificial or natural delivery of their offspring.
Experiment 1: maternal behavior across pregnancy
Females that had been designated maternal (n=63) or infanticidal (n=49) in the first test for parental behavior were mated with sexually naive male prairie voles (90–120 days of age). Females were randomly assigned to one of seven groups and retested for parental behavior at different stages of pregnancy: Days 4–6, Days 7–9, Days 10–12, Days 13–15, Days 16–18, and Days 19–21 (see Tables 1 and 2). Immediately after the test, prairie voles were sacrificed and the approximate extent of pregnancy was confirmed by the increased vascularization of uterine horns and presence of corpora lutea or the presence and size of embryos or fetuses. In fifteen cases (6 infanticidal, 9 maternal), no signs of pregnancy were detected. These animals were not included in the analysis. A smaller cohort of maternal (n=9) and infanticidal voles (n=15) were retested for parental behavior without having been mated with sexually naive male prairie voles.
Table 1.
Durations (seconds/10 min test) of behaviors (mean±SEM) of initially infanticidal voles at different stages during pregnancy (Days 4–6 to Days 19–21) or as virgins
| Days 4–6 | Days 7–9 | Days 10–12 | Days 13–15 | Days 16–18 | Days 19–21 | F test | Virgins | |
|---|---|---|---|---|---|---|---|---|
| Non-maternal behaviors | ||||||||
| No. of animals * | 6 | 8 | 7 | 8 | 7 | 7 | 15 | |
| Explore | 94.5±35.5 | 25.5±6.3 | 59.1±35.9 | 70.6±26.2 | 78.8±49.0 | 99.1±46.6 | 0.65 | 78.6±19.9 |
| Self-groom | 56.9±36.7a | 0b | 0 | 0b | 0.4±0.4 | 17.5±13.7 | 2.52† | 11.5±11.4 |
| Feeding | 0 | 0 | 1.9±1.9 | 0 | 0 | 10.33±7.4 | 1.87 | 0.2±0.2 |
| Inactivity | 69.7±39.9 | 4.7±1.2 | 24.0±13.7 | 19.2±8.0 | 46.5±33.3 | 79.5±47.9 | 1.22 | 13.2±5.3 |
| Total non-maternal activity‡ | 175.6±66.2 | 28.1±6.23 | 84.9±34.4 | 74.4±26.3 | 89.1±49.4 | 138.0±64.6 | 1.36 | 112.9±37.3 |
| Maternal behaviors | ||||||||
| No. of animals ** | 1 | 0 | 0 | 0 | 1 | 1 | 1 | |
| LickΔ | 282.5 | 0 | 0 | 0 | 341.2 | 191 | NT | 117.1 |
| RetrievalΔ | 1.4 | 0 | 0 | 0 | 0 | 0 | NT | 0 |
| CrouchingΔ | 181.2 | 0 | 0 | 0 | 34.5 | 117.1 | NT | 0 |
| Total maternal activity‡‡,Δ | 472.8 | 0 | 0 | 0 | 383.8 | 317.7 | NT | 119.3 |
| Attack latency | ||||||||
| No. of animals *** | 5 | 8 | 7 | 8 | 6 | 6 | 13 | |
| First test | 390.2±137.1 | 222.6±107.9 | 134.2±73.0 | 143.1±52.9 | 152.0±84.8 | 263.0±126.2 | 0.92 | 239.7±48.5 |
| Second test | 152.6±75.2 | 24.6±4.9 | 93.4±40.8 | 90.3±30.1 | 101.0±66.2 | 194.7±104.7 | 0.99 | 79.4±15.0 |
Total non-maternal activity consists of all active behaviors that do not contribute to the care of pups.
Total maternal activity includes all active maternal behaviors plus nesting and hovering quiescently over pups.
Comparisons of maternal behaviors were not made because of the low incidence of behavior among the groups.
p<0.05. Significant post hoc differences between groups are indicated by different letters (Tukey HSD, p<0.05). NT=not tested.
Total number of animals in group.
Total number of animals that were maternal in each group.
Total number of animals that attacked in each group.
Table 2.
Durations (seconds/10 min test) of behaviors (mean±SEM) of initially maternal voles at different stages of pregnancy (Days 4–6 to Days 19–21) or as virgins
| Days 4–6 | Days 7–9 | Days 10–12 | Days 13–15 | Days 16–18 | Days 19–21 | F test | Virgins | |
|---|---|---|---|---|---|---|---|---|
| Non-maternal behaviors | ||||||||
| No. of animals * | 9 | 10 | 9 | 10 | 10 | 9 | 9 | |
| Explore | 74.0±33.3 | 52.8±17.1 | 22.1±8.8 | 42.2±13.0 | 72.7±31.8 | 93.1±38.9 | 0.93 | 36.1±10.2 |
| Self-groom | 7.4±2.8 | 11.4±6.4 | 3.5±1.2 | 9.7±4.2 | 33.7±20.9 | 18.4±8.8 | 1.16 | 12.8±5.2 |
| Feeding | 0 | 0 | 3.1±3.1 | 0 | 17.0±9.7 | 13.7±13.7 | 1.28 | 1.1±1.0 |
| Inactivity | 39.4±28.5 | 5.5±2.5 | 11.4±8.4 | 9.9±6.9 | 10.6±5.0 | 6.4±1.8 | 1.06 | 6.4±4.4 |
| Total non-maternal activity‡ | 181.9±56.1 | 199.7±38.4 | 97.3±25.2 | 121.8±27.5 | 217.5±60.5 | 216.5±59.5 | 1.18 | 180.3±30.9 |
| Maternal behaviors | ||||||||
| No. of animals ** | 6 | 7 | 5 | 6 | 7 | 6 | 9 | |
| LickΔ | 171.2±53.7 | 167.8±40.4 | 124.0±43.2 | 184.8±57.8 | 157.9±43.6 | 148.2±51.3 | 0.13 | 148.6±30.9 |
| RetrievalΔ | 1.8±1.3 | 2.2±1.2 | 0.3±0.3 | 1.1±0.5 | 0.2±0.2 | 0.9±0.7 | 0.96 | 1.4±0.7 |
| CrouchingΔ | 63.9±27.9 | 57.9±16.0 | 141.9±51.2 | 92.0±40.2 | 105.2±37.6 | 71.1±31.1 | 0.80 | 205.2±37.2 |
| Total maternal activity‡‡ | 236.9±74.7 | 235.4±54.2 | 266.9±86.0 | 259.6±73.1 | 273.1±70.2 | 220.2±73.3 | 0.08 | 355.1±49.8 |
| Attack latency | ||||||||
| No. of animals *** | 3 | 3 | 4 | 4 | 3 | 3 | 1 | |
| First test | ||||||||
| Second test | 85.6±11.4 | 84.2±12.7 | 85.6±17.7 | 81.0±10.6 | 177.1±88.5 | 102.2±21.8 | 1 | 8.4 |
Total non-maternal activity consists of all active behaviors that do not contribute to the care of pups.
Total maternal activity includes all shown behaviors plus nesting and all quiescent behaviors over pups.
Total number of animals in group.
Total number of animals that were maternal in each group.
Total number of animals that attacked in each group.
Experiment 2: maternal behavior after artificial delivery
Thirty-six pregnant females that were infanticidal when tested as virgins were used. Sham animals gave birth within a 48-hour period (stretching over days 22–24 with day 0 being the day of pairing). The pregnancies were monitored by palpating females from day 11 onward to determine the size of the conceptus and by following the development of teats and detecting the separation of the symphysis pubis. Based on these characteristics, we were able to predict the day of delivery reliably within approximately 24 h. Only then were animals randomly assigned to an experimental condition. Animals underwent artificial delivery by Caesarean section (C-Section; n=12) or sham surgery (n=14) on the expected day of delivery. The latter group gave birth 12–24 h after surgery. Eight animals, however, gave birth within hours before scheduled surgery. Those animals received sham surgery less than 3 h after delivery. Accuracy of the expected due dates for the C-Section group was confirmed by the delivery of pups that were able to breathe independently after minimal stimulation. Two females from the C-Section group were not included in the analysis because the artificial delivery occurred too soon (i.e., immature, non-viable pups). Testing for maternal behaviors occurred 3 h after artificial delivery or sham surgery following natural delivery. When sham surgery took place before parturition, testing occurred 0–3 h after natural delivery. An additional group of maternal virgin females (n=10) underwent sham surgery and was tested for maternal behaviors 3 h later.
Caesarean section
Pregnant females were anesthetized using an isoflurane/oxygen mixture on the expected day of delivery. After laparotomy and a transverse incision in each uterine horn, fetuses and placentas were expelled from the uterine horns. At the end of delivery, the horns, abdominal muscles and skin were sutured. Animals assigned to the sham surgery groups were subjected only to laparotomy and suturing of muscle and skin. Sham surgeries were done either before or within 3 h after parturition. The latter group was added to control for any possible confounds caused by discomfort after the 3-hour recovery time. For both sham groups, the amount of time sham animals were exposed to pups ranged from 0 to 3 h.
Parental behavior test
Subjects were placed in a clear cage containing wood chips, food, and a small amount of hay. After 30 min of habituation, two unrelated pups (0–2 days old) were placed in the corners opposite to the subject’s location. Behavioral responses were recorded continuously for 10 min using a Sony Handycam DC-TRV18. If subjects did not make contact with either pup during the 10-minute period, testing continued for an additional 10 min. During this time, the pups were moved closer to the subject every 3 min, each time reducing the distance between the subject and pup by half. The responses were scored using the Noldus Observer program 4.0 and Psion Walkabout hardware (Amsterdam, The Netherlands). In the event of an attack, pups were quickly removed from the cage and euthanized. To be considered parental, subjects had to engage for at least 100 s in maternal behaviors (e.g., licking, nesting, retrieving, and crouching), including at least 30 s of licking (cf. Lonstein et al., 2002). Animals that displayed these behaviors for shorter periods or made contact with the pups but showed no interest were classified as unresponsive. Other behaviors displayed that were considered nonresponsive were exploration of the cage, feeding, self-grooming, sniffing or moving pups, and inactivity.
Data analysis
Changes in behavioral responses during prescreening and pregnancy were analyzed using a McNemar Chi-square test (χ2). Differences in behavioral responses among the animals at different stages of pregnancy were analyzed using the Chi-square test (χ2). Changes in the duration of non-maternal and maternal behaviors and latency to attack and contact during pregnancy were analyzed using one-way analyses of variance (ANOVAs) with stage of pregnancy as the main factor. Post hoc analyses were done using Tukey HSD tests. Changes in latency to attack between the initial and final tests were analyzed using a two-way ANOVA (stage of pregnancy×tests) with repeated measures on tests (Experiment 1) or a dependent t-test (Experiment 2). Level of significance was set at p≤0.05.
Results
Experiment 1: maternal behavior across pregnancy
Overall, more prairie voles were infanticidal during pregnancy than in the initial test (χ2 =13.78, p<0.01). This change was mainly caused by maternal females becoming infanticidal (Fig. 1). There was no significant change in behavioral responsiveness among the virgin females when tested a second time (χ2 =0.33, p>0.05, Fig. 1).
Fig. 1.
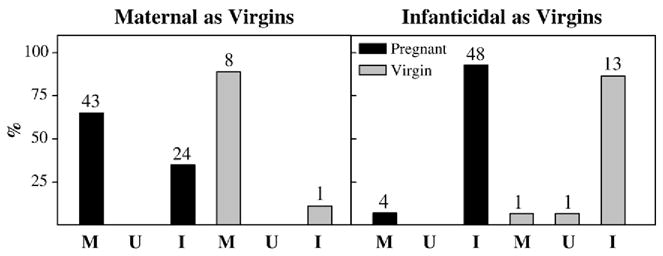
Percentage of animals displaying maternal or infanticidal behavior during the second of two tests. During the first test, all animals were virgins and were found to be maternal (left panel) or infanticidal (right panel). During the second test, females were pregnant (black bars) or remained virgins (gray bars) and displayed maternal (M), unresponsive (U), or infanticidal (I) behavior. Numbers above bars indicate number of animals per group. Note that a large percentage of initially maternal voles were infanticidal while pregnant whereas initially infanticidal voles were likely to remain so whether pregnant or not.
The percentage of females that were infanticidal when pregnant did not vary significantly across pregnancy both in animals that were infanticidal (χ2(5)=3.74, p>0.05; Fig. 2A) or maternal (χ2(5)=0.70, p>0.05; Fig. 2B) when tested as virgins. Although the latency to attack did not differ significantly across pregnancy either (F(5,34)=0.99, p>0.05), subjects took about half the time to attack during pregnancy than when tested as virgins (F(1,34)=8.64, p <0.01, Table 1). Similarly, the virgin females that were tested a second time as virgins showed a shorter attack latency in the second test (79.4±15.0 s versus 271.2±51.2 s; t(12)=3.87, p<0.01). Generally, the stage of pregnancy did not affect other behaviors measured, except for self-grooming (Tables 1 and 2). Among the females that were infanticidal during the initial tests, those tested on Days 4–6 spent more time self-grooming than did females tested at any other time during pregnancy (F(6,40)=2.46, p<0.05; Table 1).
Fig. 2.
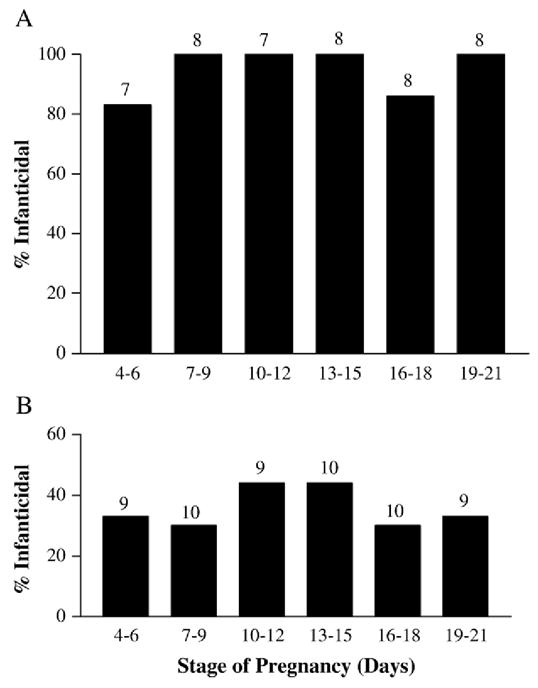
Percentage of females deemed infanticidal (A) or maternal (B) when tested as virgins that displayed infanticidal behavior at different stages during pregnancy. Numbers above bars indicate number of animals per group.
Experiment 2: maternal behavior after artificial delivery
Eight out of the 36 females gave birth within hours of the planned C-Section. Animals that were given sham surgery before birth gave birth 12–24 h after surgery. Generally, the type of behavioral response to pups depended upon type of delivery. While all of the sham-operated dams that delivered naturally were maternal, those subjected to artificial delivery continued to be infanticidal (9/12: χ2 =22.44, p≤0.001; Fig. 3).
Fig. 3.
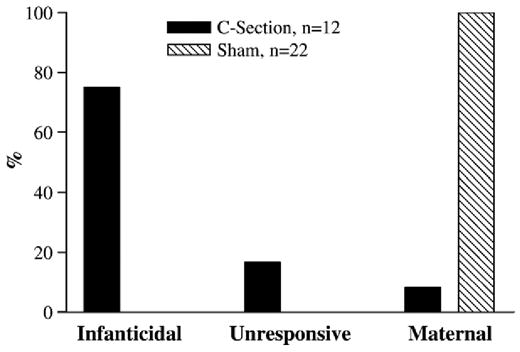
Percentage of voles that were infanticidal, unresponsive or maternal towards unrelated pups after artificial (C-Section) or natural (Sham) delivery.
The dams in the C-Section group took significantly more time to attack than when tested as virgins (444.6±122.1 versus 84.7±23.0 s; t(10)=−2.87, p<0.02). This difference is also reflected in the time it took C-Section animals to make contact with the pups, which was significantly longer than the contact latencies for the sham groups (F(2,31)=9.22, p<0.001; Table 3). However, the C-Sectioned dams did not spent less time displaying non-maternal behaviors than the sham groups, but they spent more time in an inactive state (F(2,31)=4.73, p<0.02; Table 3).
Table 3.
Durations (seconds/10 min test) of behaviors (mean±SEM) of initially infanticidal voles after being subjected to artificial delivery (C-Section) or sham surgery before (Sham before) or after (Sham after) parturition, and an additional group of virgins after sham surgery (Virgin)
| C-Section | Sham Before | Sham after | F test | Virgins | |
|---|---|---|---|---|---|
| Non-maternal behaviors | |||||
| No. of animals * | 12 | 14 | 8 | 10 | |
| Explore | 225.7±37.9 | 191.3±24.0 | 170.3±31.4 | 0.71 | 188.1±35.3 |
| Self-groom | 47.1±23.9 | 21.9±8.2 | 19.7±13.8 | 0.83 | 98.5±26.3 |
| Feeding | 3.0±2.0 | 4.5±3.3 | 0 | 0.63 | 32.1±21.9 |
| Inactivity | 80.3±24.4a | 15.2±10.7b | 18.3±9.3a,b | 4.73† | 41.3±11.4 |
| Total non-maternal activity‡ | 286.1±50.9 | 222.4±28.2 | 197.2±35.3 | 1.24 | 442.0±39.6 |
| Maternal behaviors | |||||
| No. of animals ** | 1 | 14 | 8 | 10## | |
| LickΔ | 147.8 | 187.3±25.4 | 193.1±33.6 | 0.02 | 128.9±29.6 |
| RetrievalΔ | 2.6 | 4.4±1.3 | 1.9±0.8 | 1.81 | 2.4±1.4 |
| CrouchingΔ | 0 | 133.0±29.0 | 125.3±50.8 | 0.02 | 27.6±17.2 |
| Total maternal activity‡‡,Δ | 150.4 | 342.6±33.7 | 341.9±49.6 | 0.3 | 159.0±39.6 |
| Contact Latency | 296.0±75.9a | 47.1±16.5b | 43.6±11.9b | 9.22†† | 280.5±80.8 |
| Attack latency | |||||
| No. of animals *** | 12 | 14 | 8 | 0 | |
| First test | 84.7±23.0 | 152.4±66.0 | 75.6±38.4 | 0.69 | |
| No. of animals*** | 9 | 14 | 8 | 0 | |
| Second test | 444.6±122.1 | ||||
| t test | −2.87††† | ||||
Total non-maternal activity consists of all active behaviors (e.g., ambiguous) that do not contribute to the care of pups.
Total maternal activity includes all shown behaviors plus nesting and all quiescent behaviors over pups.
p<0.02.
p<0.001. Significant post hoc differences between groups are indicated by different letters (Tukey HSD, p<0.01).
Other comparisons were made only between the Sham groups because of the low incidence of behavior among the C-Sectioned females.
Prescreen latencies were much shorter than after C-Section, p<0.02. Latencies from maternal females were excluded from this analysis.
This number includes data from those animals that did not meet criteria for designation as maternal.
Total number of animals in group.
Total number of animals that were maternal in each group.
Total number of animals that attacked in each group.
Three out of twelve C-Sectioned dams did not attack the pups; two of these were unresponsive in their responses to pups and one was maternal. The unresponsive animals spent only minimal time in contact with the pups (5.3 and 31.6 s out of 600 s) and did not display any maternal behaviors. The C-Sectioned dam that was maternal spent a total of 150.4 s caring for the pups, which was the shortest amount of time engaged in maternal behavior among all of the maternal females in this study.
When allowed to give birth naturally, dams were maternal regardless of whether the sham surgery took place before or after birth or whether the pups were removed immediately after birth or up to 3 h later (22/22; χ2 =15.75, p<0.01; Fig. 3). There were no differences in time spent engaged in maternal behaviors between animals that received sham surgery before or after parturition (see Table 3).
Sham surgery did not increase the incidence of infanticide among females that were maternal before surgery (0/10; Table 3). Four out of ten females did not meet criteria for being considered maternal (i.e., having engaged in maternal behaviors for at least 100 s, including at least 30 s of licking). However, of those four animals, only one female did not display any maternal behaviors. There was an increase in the latency to make contact with pups (test before surgery 77.0±22.3 s; test after surgery 280.5±80.8 s; t(9)=−2.94, p<0.05).
Discussion
The results from the present study suggest that parity (i.e., the condition of having given birth) rather than stage of pregnancy predicts the level of maternal responsiveness in female prairie voles. The transition from virgin to primigravid did not alter how the infanticidal females responded to pups. Females that were infanticidal as virgins remained infanticidal throughout pregnancy. In contrast, over 30% of voles that were maternal as virgins were infanticidal when pregnant. Events closely associated with natural parturition were responsible for the change in behavior of infanticidal females because females whose pups were delivered by Caesarean section remained infanticidal.
Artificial delivery occurred within 24 h of estimated time of natural delivery. At the time of surgery, some of the animals had given birth. Animals that were still pregnant but received sham surgery gave birth 12–24 h later. As these animals were randomly chosen from the same group that underwent Caesarian section, pups from the latter group would probably have been born approximately 12–24 h later as well. Therefore, maternal responsiveness appears to emerge in infanticidal females during the last hours of pregnancy. Following this interpretation, the three females that were not infanticidal after artificial delivery might have experienced aspects of parturition that inhibit infanticide and trigger the appearance of maternal behavior.
In rats, the last days of pregnancy are accompanied by changes in levels of hormones, e.g., progesterone, prolactin and oxytocin, which may stimulate maternal behaviors irrespective of any physical changes associated with parturition (for a review, see Rosenblatt et al., 1988; Numan and Insel, 2003). Indeed, artificially subjecting virgin female rats to hormonal changes characteristic of pregnancy stimulates maternal responsiveness (Bridges, 1984; Fleming, 1986). However, because voles continued to be infanticidal during the last few days of pregnancy (Experiment 1) and after artificial delivery (Experiment 2), our results suggest that, in prairie voles, physical and hormonal changes that accompany pregnancy are not sufficient to induce full display of maternal behavior.
Although the type of behavioral response in infanticidal voles remained consistent before and during pregnancy, females attacked pups sooner when pregnant than as virgins. Although aggressive behavior among prairie voles increases after mating (Getz et al., 1981; Villalba et al., 1997), mating and/or cohabitation with males cannot explain the shorter attack latencies as infanticidal females that were tested twice as virgins also attacked sooner in the second test. The shortened latency may therefore simply reflect an attack priming effect, in which animals show shorter latencies to attack during subsequent tests (Lagerspetz, 1969; Tellegen and Horn, 1972). Similar priming effects on attack latency have been found in aggressive behavior tests in other rodents (Potegal and Popken, 1985; Potegal, 1992).
In contrast, females tested after artificial delivery took much longer to attack than they did as virgins. Such a response could have resulted from the surgical procedure and/or the dramatic change in physiology resulting from the abrupt changes in fluid and hormone levels after artificial delivery of the pups. If so, these same changes may also have increased the likelihood that these animals show infanticidal behavior. However, sham surgery did not make maternal virgin animals more likely to attack. Interestingly, however, after surgery these animals did take longer to contact pups, similar to the Caesarian sectioned females. This delay reduced quantity but not quality (i.e., types of behaviors displayed) of care. In contrast, dams subjected to sham surgery did not show a change in contact latency, suggesting that their maternal drive may have masked any negative effects of the surgery.
The differences in contact latency between voles subjected to sham surgery or Caesarian section may also reflect a change in emotional state as contact latencies can depend on an animal’s level of anxiety, depression, or fear (for a review, see Blanchard and Blanchard, 1989). In rats, parturition is accompanied by a reduction in anxiety, which may stimulate the display of maternal behaviors (Fleming et al., 1989; Fleming and Luebke, 1981; Neuman et al., 1998a,b). In rats, this reduction in anxiety occurs relatively independent of parturition as it also occurs with artificial delivery (Moltz et al., 1966; Lonstein, 2005). However, an abrupt termination of pregnancy can be anxiogenic (Bitran and Smith, 2005). Quite possibly, the increase in attack latency in female voles subjected to artificial delivery resulted from heightened anxiety as demonstrated by the increased latency to make contact with the pups. Moreover, female prairie voles that are infanticidal are more likely to show signs of anxiety than those that are maternal (Olazabal and Young, 2005). Because vaginocervical stimulation produces many autonomic, physiological and behavioral responses (Erskine, 1992, 1995; Rodriguez-Sierra et al., 1977; Robbins and Sato, 1991; Sansone et al., 1997; Cueva-Rolon et al., 1996; Blaustein and Erskine, 2002), vaginocervical stimulation related to parturition may directly influence factors such as anxiety in voles thereby reducing infanticide and facilitating the display of maternal behaviors.
A drop in maternal responsiveness shown by pregnant animals in Experiment 1 has also been reported for rats and mice. Mayer and Rosenblatt (1984) reported that, approximately 2 to 5 days before parturition, primigravid rats were more likely to attack pups than virgin rats. In addition, Noirot and Goyens (1971) reported that mice were more likely to attack pups when tested in the first 2 weeks of pregnancy. Although parental responsiveness did not change across pregnancy in prairie voles, the drop in maternal responsiveness during pregnancy suggests that changes associated with pregnancy influence maternal behavior. It is possible that pregnancy, acting as a stressor, induced infanticide in these females. However, this remains to be determined since the neuroendrocine and behavioral profile of prairie voles during pregnancy has yet to be established.
Regardless of whether infanticide appeared before or during pregnancy, infanticidal tendencies were replaced fully with maternal behaviors only after natural delivery of the pups (Experiment 2). Although it is possible that the time spent with pups after natural delivery (maximum of 3 h) may enhance maternal reactions, this did not seem to be an issue in the current experiment as even sham-operated females that had their pups removed immediately upon delivery were fully maternal. The persistence of infanticidal behavior after Caesarian section suggests that voles differ from rats in the importance of parturition for maternal behavior. Primiparous rats that are subjected to Caesarean section a few days before the expected delivery are fully maternal (Moltz et al., 1966; Rosenblatt and Lehrman, 1963; Bridges and Feder, 1978; Rosenblatt and Siegel, 1975; Wiesner and Sheard, 1933). Even for strains of rats in which virgins are typically infanticidal (e.g., Long–Evans), pregnancy alone facilitates the expression of maternal behaviors (Stern, 1985; Peters and Kristal, 1983; Slotnick et al., 1973). However, in such infanticidal rats, parturition is necessary for all of the females to become maternal (Stern, 1985). Perhaps in such strains, the mechanisms that trigger maternal behavior resemble those of voles.
The role of parturition in triggering of maternal behaviors in infanticidal prairie voles resembles that of species such as sheep. In sheep, parentally naive females respond aggressively towards young conspecifics whether virgin or pregnant (Le Neindre et al., 1979; Keverne et al., 1983; Kendrick and Keverne, 1991). In this species, distension of the birth canal triggers maternal behaviors (Keverne et al., 1983; Krehbiel et al., 1987; Kendrick et al., 1991). The same may be true for prairie voles as preliminary evidence suggests that voles are more likely to be parental if the offspring is allowed to descend into the birth canal (Hayes and de Vries, 2005). Therefore, the differences in infanticidal and parental behavior between voles whose pups were delivered naturally or artificially suggest that feedback from the uterus, cervix, and possibly vagina may affect emotional state and parental behavior.
The results of this study illustrate the sexually dimorphic nature of control of parental behavior in voles. In contrast to virgin females, the majority of virgin males are not infanticidal (Lonstein and de Vries, 1999). In addition, whereas females showed no change in parental responsiveness during pregnancy in the present study, males show a gradual increase in parental responsiveness that begins within days after mating and reaches maximal levels days before pups are born (Bamshad et al., 1994). It may be that the denser vasopressin innervation found in male versus female voles (Bamshad et al., 1993) contributes to this sex difference as intracranial vasopressin injections promote paternal behavior whereas injections of vasopressin antagonists inhibit it (Wang et al., 1994; Bales et al., 2004a). Having a denser vasopressin innervation may therefore make virgin males more likely to show parental behavior than virgin females and may help males reach full parental responsiveness after mating much earlier than females even though they do not get pregnant, let alone ever experience parturition. This sex difference may prove useful in unraveling the mechanisms that trigger parental behavior in females. Understanding the factors that promote maternal behavior and the role of parturition in this process is clinically relevant. Type of delivery may contribute to feelings ranging from dysphoria to depression during the postpartum period (Jacobsen, 1999; Lovejoy et al., 2000; Martins and Gaffan, 2000), which may influence how mothers interact with their babies (Murray et al., 1999; Corter and Fleming, 2002).
Acknowledgments
The authors would like to thank Rosa Yip and Michael DiMarzio for their assistance. This research was funded by NIMH grants RO1 MH47538 and KO2 MH01497.
References
- Bales KL, Kim AJ, Lewis-Reese AD, Carter CS. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm Behav. 2004a;45:354–361. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bales KL, Pfeifer LA, Carter CS. Sex differences and developmental effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Dev Psychobiol. 2004b;44:123–131. doi: 10.1002/dev.10165. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster, and meadow voles, Microtus pennsylvanicus. J Neuroendocrinol. 1993;5:247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, de Vries GJ. Cohabitation alters vasopressin innervation and paternal behavior in prairie voles (Microtus ochrogaster) Physiol Behav. 1994;56:751–758. doi: 10.1016/0031-9384(94)90238-0. [DOI] [PubMed] [Google Scholar]
- Bitran D, Smith SS. Termination of pseudopregnancy in the rat produces an anxiogenic-like response that is associated with an increase in benzodiazepine receptor binding density and a decrease in GABA-stimulated chloride influx in the hippocampus. Brain Res Bull. 2005;64:511–518. doi: 10.1016/j.brainresbull.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Attack and defense in rodents as ethoexperimental models for the study of emotion. Prog Neuropsycho-pharmacol Biol Psychiatry. 1989;13(Suppl):S3–S14. doi: 10.1016/0278-5846(89)90105-x. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS. Feminine sexual behavior: cellular integration of hormonal and afferent information in the rodent forebrain. In: Pfaff DW, Arnold AR, Etgen AM, Farbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press: Amsterdam; 2002. pp. 139–214. [Google Scholar]
- Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Feder HH. Inhibitory effects of various progestins and deoxycorticosterone on the rapid onset of maternal behavior induced by ovariectomy–hysterectomy during late pregnancy in rats. Horm Behav. 1978;10:30–39. doi: 10.1016/0018-506x(78)90022-3. [DOI] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Horm Behav. 1987;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH. The Evolution of Parental Care. Princeton University Press: Princeton; 1991. [Google Scholar]
- Corter CM, Fleming AS. Psychobiology of maternal behavior in human beings. In: Bornstein MH, editor. Handbook of Parenting. Erlbaum; Mahwah, NJ: 2002. [Google Scholar]
- Cueva-Rolon R, Sansone G, Bianca R, Gomez LE, Beyer C, Whipple B, Komisaruk BR. Vagotomy blocks responses to vaginocervical stimulation after genitospinal neurectomy in rats. Physiol Behav. 1996;60:19–24. doi: 10.1016/0031-9384(95)02245-7. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Pelvic and pudendal nerves influence the display of paced mating behavior in response to estrogen and progesterone in the female rat. Behav Neurosci. 1992;106:690–697. doi: 10.1037//0735-7044.106.4.690. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Prolactin release after mating and genitosensory stimulation in females. Endocr Rev. 1995;16:508–528. doi: 10.1210/edrv-16-4-508. [DOI] [PubMed] [Google Scholar]
- Fleming AS. Psychobiology of rat maternal behavior: how and where hormones act to promote maternal behavior at parturition. Ann N Y Acad Sci. 1986;474:234–251. doi: 10.1111/j.1749-6632.1986.tb28015.x. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Luebke C. Timidity prevents the virgin female rat from being a good mother: emotionality differences between nulliparous and parturient females. Physiol Behav. 1981;27:863–868. doi: 10.1016/0031-9384(81)90054-8. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Cheung U, Myhal N, Kessler Z. Effects of maternal hormones on “timidity” and attraction to pup-related odors in female rats. Physiol Behav. 1989:449–453. doi: 10.1016/0031-9384(89)90019-x. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carterr CS, Gavish L. The mating system of prairie voles, Microtus ochrogaster. Field and laboratory evidence for pair-bonding. Behav Ecol Sociobiol. 1981;8:189–194. [Google Scholar]
- Hayes UL, de Vries GJ. Role of labor on the induction of maternal behavior in infanticidal female prairie voles. Abstr - Soc Neurosci. 2005;419:2. [Google Scholar]
- Jacobsen T. Effects of postpartum disorders on parenting and on offspring. In: Miller LJ, editor. Postpartum Mood Disorders. American Psychiatric Press Inc; Washington, DC: 1999. pp. 119–139. [Google Scholar]
- Jakubowski M, Terkel J. Transition from pup killing to parental behavior in male and virgin female albino rats. Physiol Behav. 1985;34:683–686. doi: 10.1016/0031-9384(85)90364-6. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB. Importance of progesterone and estrogen priming for the induction of maternal behavior by vaginocervical stimulation in sheep: effects of maternal experience. Physiol Behav. 1991;49:747–750. doi: 10.1016/0031-9384(91)90313-d. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Levy F, Keverne EB. Importance of vaginocervical stimulation for the formation of maternal bonding in primiparous and multiparous parturient ewes. Physiol Behav. 1991;50:595–600. doi: 10.1016/0031-9384(91)90551-x. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Levy F, Poindron P, Lindsay DR. Vaginal stimulation: an important determinant of maternal bonding in sheep. Science. 1983;219:81–83. doi: 10.1126/science.6849123. [DOI] [PubMed] [Google Scholar]
- Krehbiel D, Poindron P, Levy F, Prud’Homme MJ. Peridural anesthesia disturbs maternal behavior in primiparous and multiparous parturient ewes. Physiol Behav. 1987;40:463–472. doi: 10.1016/0031-9384(87)90031-x. [DOI] [PubMed] [Google Scholar]
- Lagerspetz K. Aggression and aggressiveness in laboratory mice. In: Garattini S, Sigg EB, editors. Aggressive Behaviour. Wiley; New York: 1969. [Google Scholar]
- Le Neindre P, Poindron P, Delouis C. Hormonal induction of maternal behavior in non-pregnant ewes. Physiol Behav. 1979;22:731–734. doi: 10.1016/0031-9384(79)90239-7. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Reduced anxiety in postpartum rats requires recent physical interactions with pups, but is independent of suckling and peripheral sources of hormones. Horm Behav. 2005;47:241–255. doi: 10.1016/j.yhbeh.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, de Vries GJ. Sex differences in the parental behavior of adult virgin prairie voles: independence from gonadal hormones and vasopressin. J Neuroendocrinol. 1999;11:441–449. doi: 10.1046/j.1365-2826.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, de Vries GJ. Social influences on parental and nonparental responses toward pups in virgin female prairie voles (Microtus ochrogaster) J Comp Psychol. 2001;115:53–61. doi: 10.1037/0735-7036.115.1.53. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Rood BD, de Vries GJ. Parental responsiveness is feminized after neonatal castration in virgin male prairie voles, but not masculinized by perinatal testosterone in virgin females. Horm Behav. 2002;41:80–87. doi: 10.1006/hbeh.2001.1740. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Martins C, Gaffan EA. Effects of early maternal depression on patterns of infant–mother attachment: a meta-analytic investigation. J Child Psychol Psychiatry. 2000;41:737–746. [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. Prepartum changes in maternal responsiveness and nest defense in Rattus norvegicus. J Comp Psychol. 1984;98:177–188. [PubMed] [Google Scholar]
- Moltz H, Robbins D, Parks M. Caesarean delivery and maternal behavior of primiparous and multiparous rats. J Comp Physiol Psychol. 1966;61:455–460. doi: 10.1037/h0023263. [DOI] [PubMed] [Google Scholar]
- Moltz H, Luin M, Leon M, Numan M. Hormonal induction of maternal behavior in the ovariectomized nulliparous rat. Physiol Behav. 1970;5:1373–1377. doi: 10.1016/0031-9384(70)90122-8. [DOI] [PubMed] [Google Scholar]
- Murray L, Sinclair D, Cooper P, Ducournau P, Turner P, Stein A. The socioemotional development of 5-year-old children of postnatally depressed mothers. J Child Psychol Psychiatry. 1999;40:1259–1271. [PubMed] [Google Scholar]
- Neuman ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophyseal changes. J Physiol. 1998a;508:289–300. doi: 10.1111/j.1469-7793.1998.289br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman ID, Wigger A, Liebsch G, Holsboer F, Landgraf R. Increased basal activity of the hypothalamo–pituitary–adrenal axis during pregnancy in rats bred for high anxiety-related behavior. Psychneuroendocrinology. 1998b;23:449–463. doi: 10.1016/s0306-4530(98)00023-7. [DOI] [PubMed] [Google Scholar]
- Noirot E, Goyens J. Changes in maternal behavior during gestation in the mouse. Horm Behav. 1971;2:207–215. [Google Scholar]
- Novakov M, Fleming AS. The effects of early rearing environment on the hormonal induction of maternal behavior in virgin rats. Horm Behav. 2005;48:528–536. doi: 10.1016/j.yhbeh.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. Springer; New York: 2003. [Google Scholar]
- Olazabal DE, Young LJ. Variability in “spontaneous” maternal behavior is associated with anxiety-like behavior and affiliation in naive juvenile and adult female prairie voles (Microtus ochrogaster) Dev Psychobiol. 2005;47:166–178. doi: 10.1002/dev.20077. [DOI] [PubMed] [Google Scholar]
- Peters LC, Kristal MB. Suppression of infanticide in mother rats. J Comp Psychol. 1983;97:167–177. [PubMed] [Google Scholar]
- Potegal M. Time course of aggressive arousal in female hamsters and male rats. Behav Neural Biol. 1992;58:120–124. doi: 10.1016/0163-1047(92)90339-6. [DOI] [PubMed] [Google Scholar]
- Potegal M, Popken J. The time course of attack priming effects in female golden hamsters. Behav Processes. 1985;11:199–208. doi: 10.1016/0376-6357(85)90061-0. [DOI] [PubMed] [Google Scholar]
- Reynolds JD, Goodwin NB, Freckleton RP. Evolutionary transitions in parental care and live bearing in vertebrates. Philos Trans R Soc Lond, Ser B Biol Sci. 2002;357:269–281. doi: 10.1098/rstb.2001.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M, Conaway CH. Induced ovulation and oestrus in Microtus ochrogaster. J Reprod Fertil. 1969;(Suppl 6):357–376. [Google Scholar]
- Robbins A, Sato Y. Cardiovascular changes in response to uterine stimulation. J Auton Nerv Syst. 1991;33:55–63. doi: 10.1016/0165-1838(91)90018-x. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Zullo A, Gustafson EA, Carter CS. Perinatal steroid treatments alter alloparental and affiliative behavior in prairie voles. Horm Behav. 1996;30:576–582. doi: 10.1006/hbeh.1996.0060. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sierra JF, Crowley WR, Komisaruk BR. Vaginal stimulation in rats induces prolonged lordosis responsiveness and sexual receptivity. J Comp Physiol Psychol. 1977;89:79–85. doi: 10.1037/h0076442. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–1514. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Lehrman DS. Maternal behavior of the laboratory rat. In: Rheingold HL, editor. Maternal Behavior in Mammals. John Wiley & Sons; New York: 1963. pp. 8–57. [Google Scholar]
- Rosenblatt JS, Siegel HI. Hysterectomy-induced maternal behavior during pregnancy in the rat. J Comp Physiol Psychol. 1975;89:685–700. doi: 10.1037/h0077052. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Mayer AD, Giordano AL. Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:29–46. doi: 10.1016/0306-4530(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sansone GR, Bianca R, Cueva-Rolon R, Gomez LE, Komisaruk BR. Cardiovascular responses to vaginocervical stimulation in the spinal cord-transected rat. Am J Physiol. 1997;273:R1361–R1366. doi: 10.1152/ajpregu.1997.273.4.R1361. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Carpenter ML, Fusco R. Initiation of maternal behavior in pregnant nulliparous rats. Horm Behav. 1973;4:53–59. [Google Scholar]
- Stern JM. Parturition influences initial pup preferences at later onset of maternal behavior in primiparous rats. Physiol Behav. 1985;35:25–31. doi: 10.1016/0031-9384(85)90167-2. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Horn JM. Primary aggressive motivation in three inbred strains of mice. J Comp Physiol Psychol. 1972;78:207–304. doi: 10.1037/h0032192. [DOI] [PubMed] [Google Scholar]
- Terkel J, Rosenblatt JS. Maternal behavior induced by maternal blood plasma injected into virgin rats. J Comp Physiol Psychol. 1968;65:479–482. doi: 10.1037/h0025817. [DOI] [PubMed] [Google Scholar]
- Villalba C, Boyle PA, Caliguri EJ, de Vries GJ. Effects of the selective serotonin reuptake inhibitor fluoxetine on social behaviors in male and female prairie voles (Microtus ochrogaster) Horm Behav. 1997;32:184–191. doi: 10.1006/hbeh.1997.1420. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Ferris CF, De Vries GJ. The role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci U S A. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner BP, Sheard NM. Maternal Behaviour in the Rat. Oliver and Boyd; Edinburgh: 1933. [Google Scholar]


