Abstract
The systemic administration of an agonist antibody against glucocorticoid-induced tumor necrosis factor receptor related (GITR) protein has been shown to be effective in overcoming immune tolerance and promoting tumor rejection in a variety of murine tumor models. However, little is known regarding the functional consequence of ligation of GITR with its natural ligand (GITR-L) in the context of regulatory T cell (Treg) suppression in vivo. To determine the mechanism of GITR-L action in vivo, we generated a panel of tumor cell clones that express varying levels of GITR-L. The ectopic expression of GITR-L on the tumor cell surface was sufficient to enhance anti-tumor immunity and delay tumor growth in syngeneic BALB/c mice. Within the range examined, the extent of anti-tumor activity in vivo did not correlate with the level of GITR-L expression, as all clones tested exhibited a similar delay in tumor growth. The localized expression of GITR-L on tumor cells led to a significant increase in CD8+ T cell infiltration compared to the levels seen in control tumors. The increased proportion of CD8+ T cells was only observed locally at the tumor site and was not seen in the tumor draining lymph node. Depletion studies showed that CD8+ T cells, but not CD4+ T cells, were required for GITR-L mediated protection against tumor growth. These studies demonstrate that signaling between GITR-L and GITR in the tumor microenvironment promotes the infiltration of CD8+ T cells, which are essential for controlling tumor growth.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-008-0622-2) contains supplementary material, which is available to authorized users.
Keywords: Rodent, T cells, T cells cytotoxic, Costimulation, Tumor immunity
Introduction
CD4+CD25+ regulatory T cells (Tregs) represent a subset of T cells with an essential role in controlling T cell responses to prevent over reactive immune responses [23]. The absence of Tregs can have deleterious effects and lead to the generation of organ-specific autoimmunity [24]. Conversely, the presence of Tregs can also be harmful by preventing the generation of anti-tumor immunity [27]. In a wide variety of human cancers, Tregs are present at high levels in the peripheral blood and in the tumor microenvironment of patients, suggesting that Tregs contribute to the negative regulation that limits anti-tumor immunity [11, 18, 36, 37]. Indeed, the increase in Treg cells in the peripheral blood and in the tumor of patients with cancer is correlated with poor prognosis and reduced survival [4, 25]. The depletion of Tregs prior to tumor challenge using an antibody targeting CD25 expressed on Tregs (PC61) is sufficient to promote the generation of tumor reactive T cells [21, 27] and enhance vaccine efficacy [32] in preclinical mouse models. However, administration of PC61 does not lead to regression of established tumors [21], indicating that overcoming Treg-mediated immune suppression in cancer after the expansion of tumor specific Tregs remains a critical obstacle to generating a protective anti-tumor response.
Glucocorticoid-induced TNF receptor related protein (GITR) is a member of the TNF receptor superfamily that is expressed at high levels on the surface of Tregs and is present at low levels on CD4+ and CD8+ T cells, macrophages, NK cells, and B cells [9, 12, 17, 22, 28, 29]. However, upon TCR activation, the expression of GITR is rapidly upregulated on CD4+ and CD8+ T cells [28]. The physiological ligand for GITR, GITR-L, is a type II transmembrane protein that has been detected on the surface of antigen presenting cells, including dendritic cells, B cells, and macrophages [13, 30, 33]. Activation of GITR on CD4+CD25− and CD8+ effector T cells using a GITR agonist antibody (DTA-1) or recombinant GITR-L has been shown to provide a potent costimulatory signal to promote their resistance to Treg suppression [22, 30, 33]. Additionally, signaling through GITR on Tregs has been shown to directly inhibit their ability to suppress T cell proliferation [28]. It is a matter of debate whether the mechanism responsible for breaking T cells out of Treg suppression functions through GITR signaling on Treg cells [28], CD4+ or CD8+ T cells [30], or possibly a combination of signals [22]. Nevertheless, systemic GITR stimulation in vivo using a GITR agonist antibody (DTA-1) has been effective in preventing the growth of advanced tumors [14], indicating that GITR stimulation in vivo is sufficient to overcome Treg-mediated tolerance in cancer.
To date, manipulation of GITR signaling for the treatment of cancer has been primarily achieved through the systemic administration of an agonist antibody specific for GITR. However, systemic GITR stimulation can affect many GITR expressing cells in vivo and has also been shown to exacerbate autoimmune responses [3, 15, 19, 28, 31, 35]. Interestingly, injection of anti-GITR directly into tumor masses can promote tumor regression at a much lower dose of antibody compared to systemic anti-GITR administration [14]. Furthermore, local anti-GITR administration results in a decreased level of autoantibody production compared to systemic anti-GITR administration [14]. Taken together, these results suggest that the manipulation of GITR signaling locally at the tumor site may be more effective at promoting anti-tumor immunity without deleterious autoimmunity. However, how local GITR signaling influences the cellular dynamics of tumor infiltrating lymphocytes is not clear.
To gain insight into the mechanisms of local GITR ligation in vivo, a panel of CT26 tumor cell clones expressing varying levels of GITR-L was generated. The localized expression of GITR-L in the tumor microenvironment was found to be sufficient for the development of an anti-tumor response that delayed tumor growth. Furthermore, the presence of GITR-L at the tumor site specifically promoted the local accumulation of CD8+ T cells which were essential for the observed anti-tumor activity.
Materials and methods
Mice
Six to 10-week-old female BALB/c mice were obtained from Taconic Farms Inc. (Germantown, New York). All experiments were performed according to the UCLA Chancellor’s Animal Research Committee (ARC; http://www.oprs.ucla.edu/) Animal Care and Use Training Manual.
EGFP-GITR-L fusion constructs
The full-length cDNA encoding murine GITR-L was amplified by PCR from pCR3.1 mGITR-L (a kind gift from Dr. B.S. Kwon, Ulsan, Korea) using the forward primer GCCTAGGATGGAGGAAATGCCTTTGAGAGAGTCAAGTC and the reverse primer CGGATCCCTAAGAGATGAATGGTAGATCAG. The plasmid pEGFP-C1 (Clontech, Mountain View, CA) was linearized by digesting with BglII and the overhangs filled in using Klenow. The blunt end PCR fragment was cloned into the linearized pEGFP-C1 to generate the plasmid pEGFP-GITR-L. Truncated pEGFP-ΔECD was generated by placing a premature stop codon after amino acid 48 of GITR-L, five amino acids downstream of the putative transmembrane domain. The pEGFP-ΔECD plasmid was amplified by PCR from the pEGFP-GITR-L plasmid with the forward primer AGTGAACCGTCAGATCGGCTAGCGCTACCGGT and the reverse primer GATCCGGATCCctaGACAGTTGGCTTGAGTGAAGTATAGATCAGTGTA containing a premature stop codon, shown underlined and in bold. The PCR fragment was digested with NheI and BamHI and inserted into pEGFP-GITR-L digested with NheI and BamHI. All amplification reactions were performed with 1 U of Pfu DNA polymerase (Stratagene, La Jolla, CA). PCR cycling began at 95°C for 5 min, followed by 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min, and ended with 10 min at 72°C.
Tumor cell lines
CT26 is a poorly immunogenic murine colorectal carcinoma cell line that is unable to elicit a detectable tumor specific CTL response when injected into syngeneic hosts [5]. CT26 cells were cultured in Iscoves Modified Dulbecco’s Medium (IMDM) supplemented with 5% bovine calf serum (CS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. The plasmid pEGFP-GITR-L or the control plasmid pEGFP-ΔECD was stably transfected into tumor cells using Lipofectin (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. G418 resistant cells were pooled and sorted for GFP expression on a FACSVantage SE cell sorter (Becton Dickson, San Jose, CA). GFP-positive cells were subsequently cloned by limiting dilution and analyzed again for GFP expression by flow cytometry. Three GITR-L expressing clones (Low, Med, Hi) and one non-functional GITR-L clone (ΔECD) were chosen for further analysis based on GFP expression. In vitro proliferation of tumor clones was measured using CellTiter 96 AQueous (MTS) solution (Promega, Madison, WI).
In vitro T cell proliferation assays
Splenocytes were harvested from naïve mice and depleted of red blood cells using ACK lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM EDTA, pH 7.3). Single-cell suspensions were prepared by passage of cells through a 70-μm filter (BD Biosciences, San Jose, CA). The splenocytes were subsequently labeled with 0.33 μM carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) according to the manufacturer’s instructions. Tumor cells were irradiated with 100 Gy in a Mark I 137Cs irradiator (JS Shepard and Associates, San Fernando, CA). CFSE labeled splenocytes were cultured in triplicate at a concentration of 1.3 × 106 cells/well in a 24 well tissue culture plate in the presence of irradiated CT26/ΔECD or CT26/GITR-L clones (1 x 105 cells/well) and stimulated with a sub-optimal concentration of anti-CD3 (clone 145-2C11) (BD Biosciences, San Jose, CA) (0.0125 or 0.05 μg/ml). Following incubation at 37°C for 60 h, the cells were stained with fluorescently conjugated antibodies specific for CD4 and CD8 and analyzed by flow cytometry. Proliferation of CD4+ or CD8+ T cells was evaluated by monitoring CFSE dilution by flow cytometry gating on either the CD4 positive or the CD8 positive population, respectively. Data were acquired in an LSR I flow cytometer (BD Biosciences) and analyzed using Flowjo software (Treestar, Ashland, OR).
In vivo tumor growth
To preserve cell surface molecules, tumor cells were harvested with PBS containing 1 mM EDTA, washed three times with Hanks balanced salt solution (HBSS), and resuspended at a concentration of 6.7 × 105 cells/ml. Eight mice per group were injected subcutaneously (s.c.) in the right flank with 105 tumor cells in 150 μl of HBSS and monitored three times a week for tumor growth using a caliper. Tumor size was followed in two perpendicular dimensions using calipers and described as the tumor cross-sectional area (mm2). The mice were sacrificed when the tumor diameter reached 15 mm.
Analysis of tumor infiltrating lymphocytes
To characterize tumor infiltrating lymphocytes, 105 tumor cells in 150 μl of HBSS were injected s.c. in the right flank as described above. Twelve days after tumor inoculation, tumors were removed and tumor infiltrating lymphocytes (TILs) liberated by mincing the tumor tissue into small pieces, followed by enzymatic digestion with 1 mg/ml collagenase (Sigma–Aldrich, St Louis, MO), 2.5 U/ml hyaluronidase (Sigma–Aldrich, St Louis, MO), and 0.1 mg/ml DNase (Sigma–Aldrich, St Louis, MO) for 2 h at 37°C with gentle agitation. Cell suspensions were layered onto Lympholyte-M (Cedarlane laboratories, Burlington, NC) according to the manufacturer’s instructions to remove dead cells and debris. Isolated lymphocytes were prepared for flow cytometric analysis as described below.
Flow cytometry
Cell surface antigens were characterized using the following mAbs: anti-CD4 (clone RM4-4), anti-CD8 (clone 53-6.7), anti-Foxp3 (clone FJK-16 s), and anti-CD3 (clone 145-2C11). The cells were incubated with FcBlock (BD Biosciences, San Jose, CA) in FACS staining buffer (PBS containing 1% CS and 0.02% azide), followed by incubation with the appropriate fluorochrome-conjugated mAbs for 20 min at room temperature, and then washed. For intracellular Foxp3 staining, cells were fixed and permeabilized with Foxp3 fixation/permeabilization buffer (eBiosciences, San Diego, CA) before intracellular staining with APC-conjugated anti-mouse Foxp3 (clone 2D5). All antibodies were purchased from BD Biosciences with the exception of anti-Foxp3, which was purchased from eBiosciences. For four-color fluorescence staining, 10,000 live events were collected on a cytofluorometer (LSR; BD Biosciences, San Jose, CA), and analyzed with Flowjo software (Treestar, Ashland, OR).
CD4+ and CD8+ T cell depletion in vivo
Rat anti-mouse CD4 (clone GK1.5) and rat anti-mouse CD8 (clone 2.43.7) were purified from hybridomas, a gift from Dr. Carrie Miceli (UCLA, Los Angeles, CA). The hybridomas were maintained in IMDM supplemented with 5% CS in a 37°C, 5% CO2 humidified incubator. The cells were expanded in roller bottles and the supernatants harvested and passed through a Sepharose-protein G column. Protein G bound GK1.5 or 2.43.7 was eluted using 0.1 M glycine at pH 2.5 and fractions were immediately neutralized with 2 M Tris–HCl pH 8.0.
Mice were depleted of CD4+ or CD8+ cells by i.p. injection with GK1.5 or 2.43.7, respectively. A total of 100 μg of antibody in PBS was injected every other day for a total of three injections. One day following the last injection, mice were injected s.c. with 105 CT26-ΔECD or CT26-Med tumor cells. To maintain T cell depletion, mice were injected i.p. with the same dose of antibodies every 6 days for the duration of the experiment. A set of control mice was sacrificed 1 day after the last injection and analyzed for depletion of CD4+ or CD8+ cells. Greater than 99% of circulating CD4+ and CD8+ cell subsets were depleted by this procedure as verified by flow cytometry using anti-mouse CD3 (clone 145-2C11), CD4 (clone RMA4-4), and CD8 (clone 53-6.7) (data not shown). The antibodies used for verification recognize a different epitope than the depleting antibody and did not interfere with their binding (data not shown). PBS alone was used as a control.
In vitro generation of cytotoxic T lymphocytes
Spleens were harvested from CT26-∆ECD and CT26-Med mice 14–15 days post-tumor inoculation. Cells were isolated as described above and resuspended at a concentration of 2.5 × 106 cells/ml in CTL medium (RPMI-1640 supplemented with 1% glutamine, 10% FBS, 50 μM 2-ME, 50 U/ml penicillin, and 50 μg/ml streptomycin). Splenocytes were cultured with gamma irradiated parental CT26 tumor cells (100 Gy) at a 20:1 ratio of splenocytes to target cells in 6-well plates at 37°C in a 5% CO2 air-humidified atmosphere for 5 days. Nonadherent cells were then harvested and used as effector cells at different effector to target ratios in a standard 4-h chromium-release cytotoxicity assay. Briefly, CT26 target cells were harvested using PBS containing 1 mM EDTA, washed twice with medium and labeled with 200 μCi 51Cr (Perkin Elmer, Waltham, Massachusetts) for 2 h at 37°C. Spontaneous Cr leakage was measured in the absence of splenic effector cells. Maximum Cr release was determined by addition of Triton X-100 to a final concentration of 1%. The percentage specific cytotoxicity was determined using the following equation: % specific cytotoxicity = (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100.
Anti-CD3 induced cell death
Single cell suspensions of splenocytes were isolated as described above. Splenocytes from naïve mice (1.3 × 106 cells) were incubated with irradiated (100 Gy) CT26/ΔECD or CT26/GITR-L clones (1 × 105 cells) and anti-CD3 (0.05 μg/ml) in a 24-well flat bottom plate. After 60 h of incubation, cells were harvested, blocked with FcBlock, stained with antibodies specific for CD4 and CD8, and assayed for apoptosis using Vybrant Apoptosis Assay Kit #2 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Histology and confocal microscopy
Mice were injected s.c. in the flank at day 0 with 105 CT26-∆ECD or CT26-Med tumor cells. At day 12, tumors were dissected and snap-frozen in OCT. Ten-micrometer sections were generated with a cryomicrotome, fixed in acetone, and stained with anti-CD4-PE, anti-CD8-APC, and anti-Foxp3-biotin followed by streptavidin-alexa 488. Samples were analyzed with a Leica Microsystems inverted confocal microscope with a 40× oil immersion objective. The images were analyzed with Leica Confocal Software and Adobe Photoshop (Adobe Systems, San Jose, CA).
Statistical analysis
The log-rank test was performed for survival analysis and Student’s t test was used for all other analyses. P values of <0.05 were considered significant.
Results
Generation of tumor cells expressing GITR-L
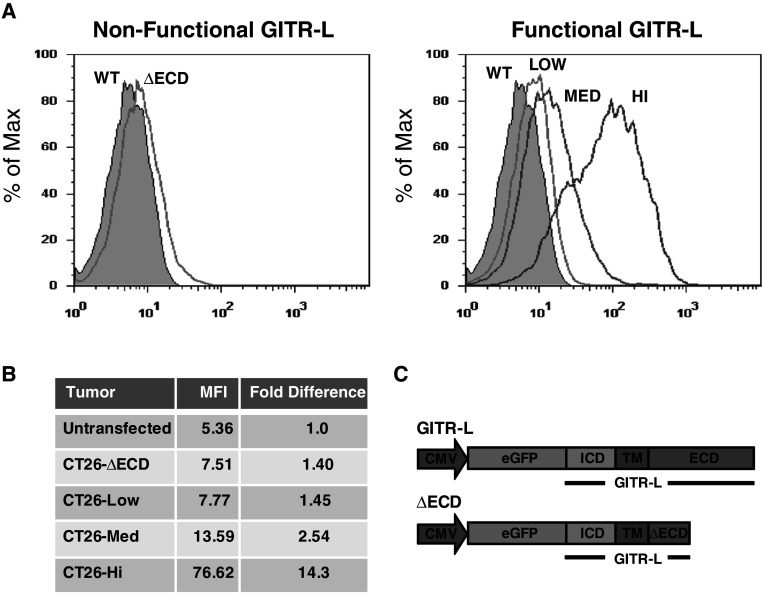
The growth of CT26 can be delayed or prevented if regulatory T cells are depleted prior to tumor challenge using a monoclonal antibody specific for CD25 expressed on Treg cells [7], suggesting that, with CT26, Tregs play a role in inhibiting anti-tumor immunity. In order to understand the functional outcome of GITR-L signaling in the context of Treg-mediated suppression in vivo, we generated CT26 tumor cell clones expressing varying levels of GITR-L. To monitor GITR-L expression, the sequence encoding full-length murine GITR-L was cloned inframe, downstream of enhanced (E) GFP (Fig. 1c). The resulting pEGFP-GITR-L vector was transfected into CT26 tumor cells and selected for stable expression using G418. A panel of GITR-L expressing clones was isolated from the stably transfected cells based on the level of GFP fluorescence (Low, Med, Hi) (Fig. 1a). A non-functional GITR-L clone generated by transfecting tumor cells with DNA encoding a GFP-GITR-L fusion with a truncated GITR-L extracellular domain (ΔECD) served as a control (Fig. 1a, c). The level of GFP expression in the CT26/GITR-L clones ranged from a 14-fold increase in mean fluorescence intensity (CT26-Hi) to a 1.4-fold increase (CT26-Low) compared to untransfected CT26 cells, which also correlated with an increase in GITR-L gene expression (Fig. 1b; supplemental Fig. 1). The level of expression of the control CT26-ΔECD was similar to the level of CT26-Low (Fig. 1b). Importantly, unlike many tumor cell lines of hematopoietic origin, CT26 does not express GITR [14] and the expression of GITR-L did not influence the growth of CT26 tumor cell in vitro (data not shown).
Fig. 1.
Expression of GITR-L in CT26 tumor clones. a Selected tumor clones were analyzed for expression of GITR-L fused to EGFP by flow cytometry. b Relative fluorescence intensity of tumor clones. Fold difference reflects the ratio in mean fluorescence intensity (MFI) compared to the untransfected parental CT26 tumor cells. c Schematic of plasmids (not to scale) used to transfect CT26 tumor cells. Since GITR-L is a type II transmembrane protein, the sequence encoding full-length murine GITR-L was cloned inframe, downstream of EGFP, to generate the GITR-L expression plasmid. The nonfunctional GITR-L tumor cell (ΔECD) was generated by transfection of a truncated form of GFP fused to GITR-L in which a stop codon was placed six amino acids downstream of the transmembrane domain of GITR-L. CMV cytomegalovirus promoter, ICD intracellular domain, TM transmembrane domain, ECD extracellular domain
GITR-L expressed on the tumors exhibits costimulatory activity in vitro
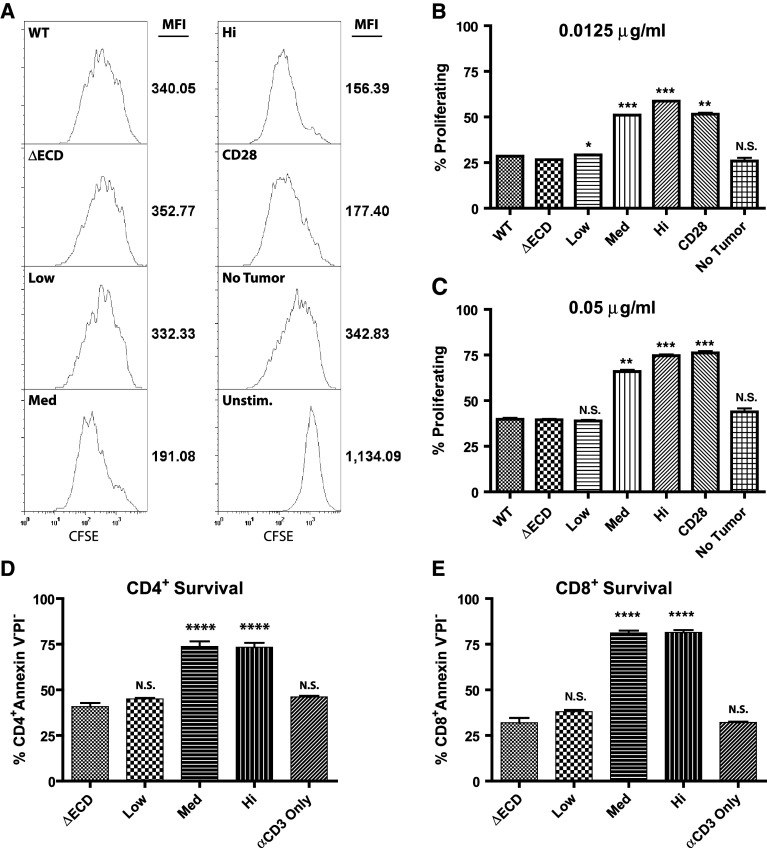
GITR signaling in T cells has been shown to enhance T cell proliferation by providing costimulatory signals [33]. To determine if GITR-L expressed on the surface of transfected tumor clones retains costimulatory activity, a T cell proliferation assay was performed. CFSE labeled splenocytes were stimulated with anti-CD3 in the presence of GITR-L expressing tumor cells and the proliferation of T cells measured by CFSE dilution. On a per cell basis, CD4+ T cells incubated with GITR-L expressing tumors underwent more rounds of cell division, as demonstrated by a decrease in CFSE mean fluorescence compared to control CT26-ΔECD tumors (Fig. 2a). The presence of CT26-Low, CT26-Med, and CT26-Hi tumors led to a 6, 46, and 56% decrease in CFSE mean fluorescence intensity of CD4+ T cells, respectively, compared to CT26-∆ECD tumors. Thus, the level of GFP expression of the tumor clones (Fig. 1b) closely correlates with GITR-L costimulatory activity. The costimulatory activity of GITR-L expressing tumors was evident at low concentrations of anti-CD3 (0.0125 or 0.05 μg/ml) (Fig. 2b, c, respectively), but not at higher concentrations (>0.5 μg/ml) (data not shown). CT26-Hi tumor cells exhibited a consistently higher costimulatory activity compared to anti-CD28 costimulation at the lower anti-CD3 concentration (0.0125 μg/ml) (P = 0.013), but at a higher anti-CD3 concentration (0.05 μg/ml) both CT26-Hi and anti-CD28 costimulation resulted in a similar enhancement in T cell proliferation. Similarly, the presence of CT26-Low, CT26-Med, and CT26-Hi tumors led to a 14, 34, and 39% decrease in CFSE mean fluorescence intensity of CD8+ T cells, respectively, compared to CT26-∆ECD tumors (supplemental Fig. 2). However, in vitro stimulated CD8+ T cells have been shown to proliferate more extensively than CD4+ T cells [6], which made it difficult to discriminate the extent of CD8+ T cell costimulation under these conditions.
Fig. 2.
Costimulatory activity of CT26/GITR-L tumor cells. Proliferation assays were performed by stimulating naïve splenocytes labeled with CFSE with a sub-optimal concentration of anti-CD3 (0.0125 or 0.05 μg/ml) in the presence of gamma irradiated (100 Gy) tumor cells or 2 μg/ml anti-CD28. a CFSE labeled splenocytes stimulated with 0.0125 μg/ml anti-CD3 and the other indicated stimulants for 60 h were harvested and stained with anti-CD4. No tumor represents anti-CD3 alone and unstimulated represents proliferation in the absence of anti-CD3 stimulation. Histograms represent CFSE fluorescence intensity of CD4+ cells as analyzed by flow cytometry. CFSE mean fluorescence intensity is shown to the right of the graphs. b, c Graphs represent the percent of CD4+ cells that have undergone at least one round of cell division, as indicated by a decrease in CFSE fluorescence compared to unstimulated controls, when stimulated for 60 h with b 0.0125 μg/ml or c 0.05 μg/ml anti-CD3 and the other indicated stimulants. Anti-CD3 induced apoptosis of d CD4+ and (E) CD8+ apoptosis was measured by incubating gamma irradiated (100 Gy) tumor cells with naïve splenocytes and stimulating T cells with anti-CD3 (0.05 μg/ml) and the other indicated stimulants. After 60 h of incubation, cells were harvested and stained for CD4, CD8, propidium iodide, and annexin V and analyzed by flow cytometry. Data are representative of 3 experiments performed in duplicate. Statistics represent differences compared to ∆ECD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
GITR, like many costimulatory molecules, has been shown to protect T cells from anti-CD3-induced apoptosis [20]. Thus, to determine whether tumors expressing GITR-L enhance the survival of CD8+ T cells, apoptosis was measured by annexin V and propidium iodide staining at 60 h after anti-CD3 stimulation. Incubation with CT26-Med and CT26-Hi tumors significantly enhanced the survival of anti-CD3 stimulated CD4+ T cells (Fig. 2d) and CD8+ T cells (Fig. 2e) compared to controls. Under these conditions, the CT26-Low tumors did not demonstrate GITR-L activity and did not protect T cells from anti-CD3-induced apoptosis.
These results confirm that the GFP-GITR-L fusion protein expressed by the tumor cells retains GITR-L activity and can provide a potent costimulatory signal for both CD4+ and CD8+ T cells. Additionally, these experiments also demonstrate that GITR-L costimulatory activity with low levels of anti-CD3 stimulation in vitro correlates with the level of GFP expression, and presumably the level of GITR-L expression.
Localized expression of GITR-L inhibits tumor development in vivo
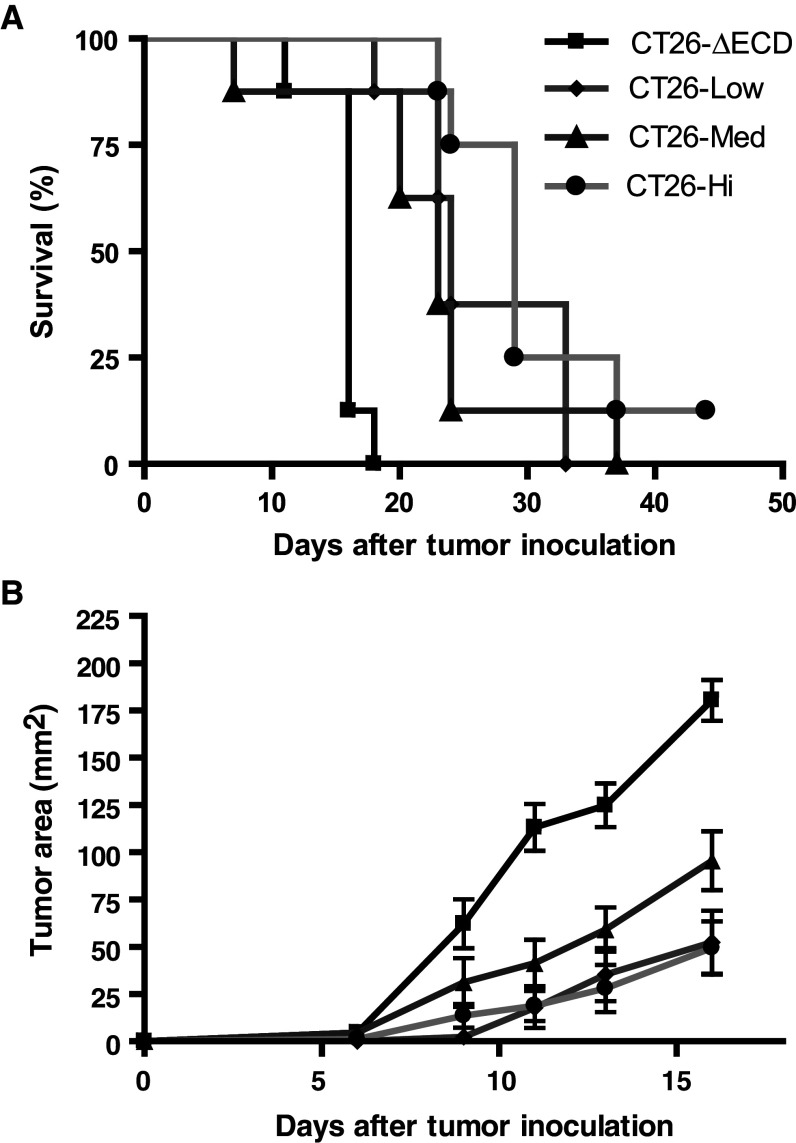
To investigate if GITR-L expression on the tumors was sufficient to alter tumor growth kinetics in vivo, CT26/GITR-L tumor cells were injected s.c. into syngeneic BALB/c mice. Irrespective of the level of GITR-L expression, the presence of GITR-L on the tumor cells significantly enhanced the survival of mice challenged with the tumor compared to the control CT26-ΔECD tumors (P < 0.001, log-rank test) (Fig. 3a). The average tumor size of the CT26/GITR-L tumors was ~50–70% smaller than that of the CT26-ΔECD control tumors at day 16 (P < 0.01, Student’s t test), when the tumor diameter of the mice injected with CT26-ΔECD control tumor cells reached 1.5 cm and required euthanization (Fig. 3b). Despite the correlation between the level of GITR-L expression and GITR-L costimulatory activity observed in vitro (Fig. 2b), the average growth rate of the tumors and the overall survival of mice did not correlate with GITR-L expression (Fig. 3a, b). The CT26-Low tumors, which demonstrated minimal costimulatory activity in vitro (Fig. 2), exhibited a similar growth and survival rate compared to CT26-Med and CT26-Hi tumors. Importantly, ectopic expression of EGFP in the CT26 tumor cells did not alter their in vivo growth rate, as CT26-ΔECD tumor cells grew at a rate similar to that of untransfected parental CT26 tumor cells (data not shown). Taken together, localized GITR-L expression in tumor cells can result in significantly delayed tumor growth in vivo, but within the range analyzed, the rate of tumor growth did not appear to correlate with the level of GITR-L expression. Although the tumors expressing GITR-L grew more slowly, virtually all of the mice eventually succumbed to the tumor. Since the level of GITR-L expression was not predictive of overall survival, the remaining experiments were performed using CT26-Med tumors.
Fig. 3.
GITR-L expression in CT26 tumor cells delays tumor development. BALB/c mice were injected s.c. with 1 × 105 CT26-∆ECD tumor cells or CT26/GITR-L tumor cells and monitored for tumor progression. a Kaplan–Meier survival curves of CT26-∆ECD and CT26/GITR-L tumors are shown. b Average tumor growth of CT26-∆ECD and CT26/GITR-L tumor cells are shown. Each data point represents the mean tumor area ± SEM of eight mice and is representative of 3 independent experiments. The P values of survival of mice bearing CT26-Low, CT26-Med, CT26-Hi tumor cells compared to CT26-∆ECD are P < 0.001, P < 0.01, and P < 0.001, respectively, as determined by log-rank analysis
Ectopic GITR-L expression promotes the local accumulation of CD8+ T cells at the tumor site
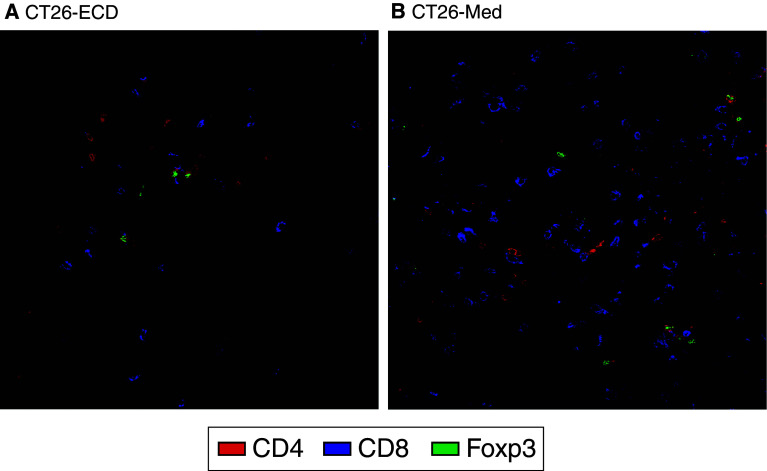
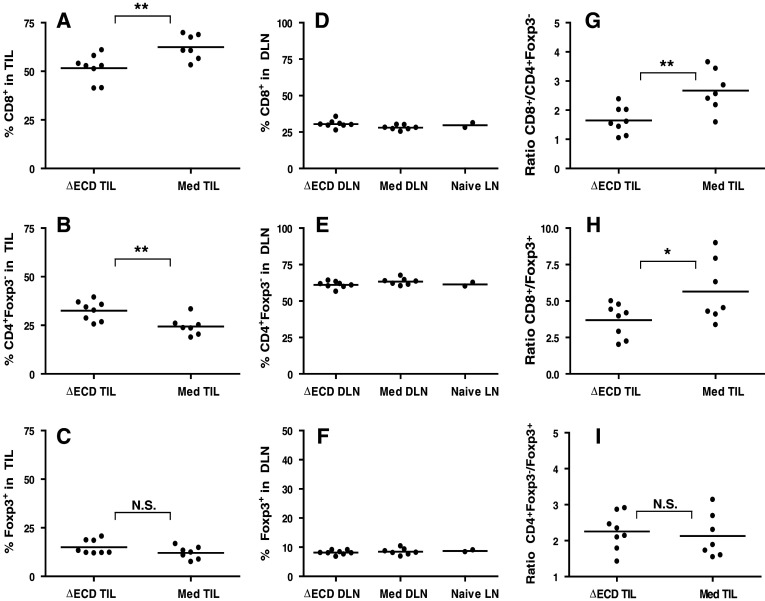
To elucidate the mechanisms responsible for the slower tumor growth of tumors expressing GITR-L, the cellular dynamics of tumor infiltrating lymphocytes were examined. Immunohistochemical characterization of CT26-ΔECD tumors at day 12 revealed the presence of tumor infiltrating CD4+ T cells, Foxp3+ Treg cells, and CD8+ T cells. Within the CT26-Med tumors, the number of tumor infiltrating CD4+ T cells and to Treg cells was similar to that seen in CT26-∆ECD tumors, but there was a dramatic increase in the number of infiltrating CD8+ T cells (Fig. 4). To quantify the differences in the T cell populations of tumor associated lymphocytes, tumors and draining lymph nodes were harvested at day 12 post-tumor challenge and analyzed by flow cytometry. Consistent with the immunohistochemical data, the majority of T cells infiltrating the CT26-Med tumor were CD8+ T cells (Fig. 5a; supplemental Fig. 3). The GITR-L expressing CT26-Med tumors exhibited an increase in the proportion of tumor infiltrating CD8+ T cells compared to the control CT26-ΔECD tumor (P = 0.008) (Fig. 5a), while the proportion CD4+Foxp3− conventional T cells was decreased (P = 0.007) (Fig. 5b). No significant differences were observed in the proportion of Tregs between CT26-∆ECD and CT26-Med tumors (Fig. 5c). The tumor infiltrating CD8+ T cells from CT26-Med tumors also exhibited a modest but consistent increase in the level of granzyme B compared to CT26-∆ECD tumors as determined by flow cytometry (supplemental Fig. 4). The relative increase in CD8+ T cells appeared to be specific to the microenvironment of the tumors expressing GITR-L, as no differences were observed in the proportion of T cells compared to naïve mice in the lymph nodes draining either GITR-L expressing or non-expressing tumors (Fig. 5d–f). Furthermore, no changes were seen in the proportion of T cells in the draining lymph node at an earlier time point (day 6, data not shown). The increase in CD8+ T cell accumulation resulted in an increase in the ratio of CD8+ T cells to CD4+Foxp3− cells (Fig. 5g) and an increase in the ratio of CD8+ T cells to Treg cells (Fig. 5h), whereas the ratio of CD4+Foxp3− to Treg cells remained unchanged (Fig. 5i). Taken together, the data suggest that ectopic GITR-L expression on CT26 tumors promotes the accumulation of CD8+ T cells with increased effector function within the tumor.
Fig. 4.
GITR-L mediated T cell infiltration. Immunofluorescence microscopy of 10 μm thick sections from 12-day-old tumors from (a) CT26-∆ECD mice and (b) CT26-Med mice. Tumor sections were fixed in acetone and stained with anti-CD4-PE (red), anti-CD8-APC (blue), and anti-Foxp3-biotin followed by streptavidin-Alexa 488 (green). Samples were analyzed with a Leica Microsystems inverted confocal microscope with a 40× oil immersion objective. Data are representative of three independent experiments with four mice per group
Fig. 5.
CT26/GITR-L TILs contain a high frequency of CD8+ T cells. CD3 expressing cells of the tumor infiltrating lymphocytes (a–c) and tumor draining lymph nodes (d–f) were analyzed by flow cytometry for the expression of CD8, CD4, and Foxp3. The proportion of a, d CD8+ T cells, b, e CD4+Foxp3− T cells, and c, f Foxp3+ Treg cells are shown. The ratios of g CD8+ T cells/CD4+Foxp3− T cells, h CD8+ T cells/Treg cells, and i CD4+Foxp3− T cells/Treg cells are also shown. Data are representative of four independent experiments with 6–8 mice per group. *P < 0.05, **P < 0.01, NS not significant
CD8+ T cells are critical for GITR-L mediated delayed tumor growth
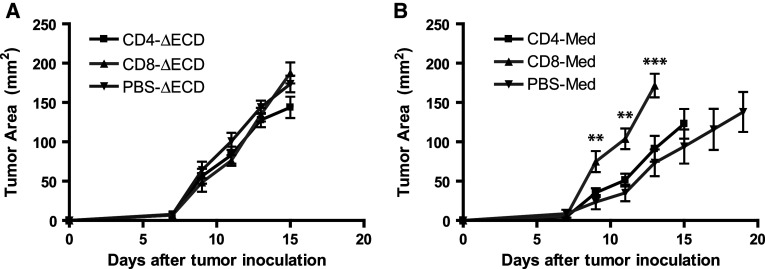
To determine if CD8+ T cells are the critical effectors in GITR-L mediated protection against tumor growth, CD4+ or CD8+ T cells were depleted in mice using monoclonal antibodies specific for each cell type. Mice depleted of CD4+ or CD8+ T cells were injected s.c. with 105 CT26-ΔECD or CT26-Med tumor cells and tumor growth monitored. Whereas the depletion of CD4+ or CD8+ T cells did not influence the growth rate of CT26-ΔECD tumors compared to PBS treated controls (Fig. 6a), the depletion of CD8+ T cell completely abrogated the delayed tumor growth observed for CT26-Med tumors (Fig. 6b). The absence of CD4+ T cells did not alter the growth rate of CT26-Med tumors compared to PBS treated mice (P > 0.05) (Fig. 6b), suggesting that CD4+ T cell costimulation and/or loss of Treg function did not make a significant contribution to the delayed growth of GITR-L expressing tumors. These results suggest that the generation of functional CD8+ T cells is the primary mechanism of GITR-L mediated inhibition of tumor growth.
Fig. 6.
Contribution of CD4+ and CD8+ T cells to GITR-L mediated tumor protection. Average tumor growth following T cell depletion. a Control CT26-∆ECD tumor cells or b GITR-L expressing CT26-Med tumor cells were injected into mice depleted of CD4+ T cells, CD8+ T cells, or injected with PBS alone and tumor growth monitored. Mice were euthanized when the tumor reached 1.5 cm in diameter. Data are representative of two independent experiments with eight mice per group. **P < 0.01, ***P < 0.001
GITR-L expression by tumor cells can lead to the generation of tumor specific CTLs
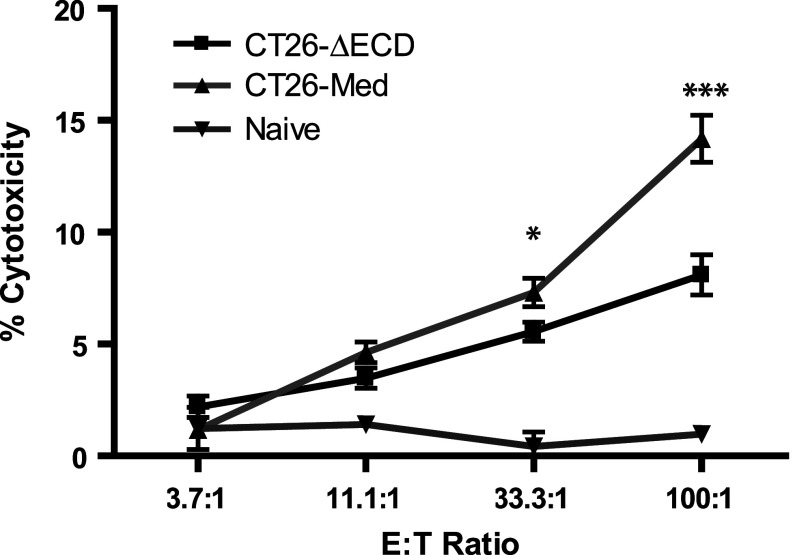
To determine if the enhanced CD8+ T cell infiltration observed in GITR-L expressing tumors correlated with the generation of cytotoxic CD8+ T cells specific to CT26 tumor antigens, splenocytes and draining lymph nodes harvested at day 14 from mice challenged with CT26-∆ECD or CT26-Med tumors were used to generate CTLs in vitro using parental CT26 tumor cells as stimulators. CTLs from mice bearing GITR-L expressing CT26-Med tumors consistently showed increased CTL activity compared to CTLs from mice bearing CT26-∆ECD tumors (Fig. 7). Thus, localized GITR-L expression within the tumor can lead to the generation of peripheral cytotoxic T cell precursors with tumoricidal activity in vitro.
Fig. 7.
CTL activity of splenocytes from CT26/GITR-L mice. Splenocytes and draining lymph nodes from CT26-∆ECD mice or CT26-Med mice were collected at day 14 post-tumor challenge and cultured for 5 days in the presence of untransfected CT26 cells and tested for cytotoxicity against CT26 in a standard 4 hour 51Cr-release assay. The specificity was confirmed by blocking with anti-CD3 (clone 145-2C11) (data not shown). Data are representative of three independent experiments with 4 mice per group. *P < 0.05, ***P < 0.001
Discussion
The expression of GITR on many types of immune cells has made it difficult to ascertain the functional outcome of GITR signaling in different contexts in vivo. In this study, we have used the poorly immunogenic CT26 colon adenocarcinoma as an in vivo model to analyze the impact of GITR signaling in the tumor microenvironment. We have demonstrated that localized GITR signaling in the tumor microenvironment is sufficient to promote an anti-tumor response through a CD8+ T cell dependent mechanism. Within the range tested, the level of GITR-L expression at the tumor site did not appear to influence the extent of in vivo anti-tumor activity. The presence of CD4+ T cells, which would include both the CD4+Foxp3− and the Treg cells, was not required for GITR-L mediated protection from tumor growth, suggesting that the generation of functional CD8+ T cells was the primary mechanism of GITR-L mediated protection against tumor growth.
Our data support a model in which the local expression of GITR-L promotes the accumulation of CD8+ T cells at the tumor site and these CD8+ T cells are necessary to control tumor growth. Contrary to the expansion of T cells in the tumor draining lymph node observed upon systemic GITR stimulation in tumor bearing mice [39], the lack of changes in proportion of T cell populations in the draining lymph node (Fig. 5d–f) suggests that GITR-L is acting locally by directly providing costimulatory signals to the CD8+ T cells infiltrating the tumor. Additional support for this is provided by the observation that the generation of CD8+ T cell dependent anti-tumor immunity did not require CD4+ T cell help as a similar enhancement of anti-tumor immunity was observed by GITR-L expressing tumors even after CD4+ T cell depletion (Fig. 6b). However, the possibility that tumor specific CD8+ T cells are activated by antigen presenting cells and receive further costimulation at the tumor site cannot be excluded. Although we did not directly assess if GITR signaling on non-T cells plays a role in delaying tumor growth, the complete loss of protection observed following CD8+ T cell depletion suggests that they are the primary effector cell type (Fig. 6b). Interestingly, a recent study in which an agonist antibody was used for the systemic stimulation of GITR has shown an essential role for CD4+ T cells in the resulting anti-tumor immunity [39]. Thus, the context of GITR activation in vivo, whether through systemic GITR activation or through local GITR activation of tumor infiltrating lymphocytes, can lead to significantly different functional outcomes.
One challenge is to use anti-GITR or GITR-L to manipulate the T cell compartment and develop an immune response to malignancy without promoting autoimmunity. Under certain conditions, systemic administration of anti-GITR has been shown to break self-tolerance and elicit autoimmune disease [3, 28]. Although no signs of autoimmunity were seen in the present study, it must be noted that the experiments were of short duration given the rapid growth of the tumors and it remains possible that the manipulation of tumor infiltrating CD8+ T cells could lead to autoimmunity. However, since only CD8+ T cells at the tumor site and not in the draining lymph node appeared to be affected by GITR-L expression on the tumor, it is unlikely that the level of CD8+ T cell activation is sufficient to promote systemic autoimmunity.
As GITR is expressed constitutively on Treg cells and is upregulated on conventional CD4+Foxp3− T cells, the observation that the absence of CD4+ T cells did not influence GITR-L mediated protection was surprising (Fig. 6b). Studies by Calmels et al., have demonstrated that the injection of adenovirus expressing recombinant GITR-L into palpable B16 tumors promotes CD4+ and CD8+ T cell infiltration [2]. However, we observed no discernable increase in CD4+ T cell infiltration into tumors constitutively expressing GITR-L (Fig. 4b). This difference may reflect the kinetics or level of GITR-L expression or secondary effects of adenovirus mediated immune stimulation [10]. There may be several other reasons why localized expression of GITR-L does not appear to modulate the activity of CD4+Foxp3− cells or Treg cells in our system. CT26, a tumor of non-hematopoeitic origin, does not express MHC class II which presents antigen to CD4+ T cells. In the absence of antigen specific stimulation of CD4+ T cells by the GITR-L expressing tumor cells, GITR-L mediated costimulation is likely to be ineffective. Support for this hypothesis comes from studies by Shimizu et al., demonstrating that abrogation of Treg suppression using an agonist anti-GITR is only effective when provided at the time of TCR stimulation [28]. Alternatively, GITR stimulation may provide qualitatively different signals to tumor infiltrating CD4+ T cells and CD8+ T cells. Muriglan et al. have shown that whereas anti-GITR stimulation of allogenic CD8+ T cells enhances graft-versus-host disease, allogenic CD4+ T cells are inhibited by anti-GITR stimulation [19].
While differences in the level of GITR-L expression by CT26 tumor cells resulted in different levels of in vitro costimulatory activity, there was an apparent lack of influence of the level of GITR-L expression in promoting anti-tumor immunity. Even the CT26-Low tumor clone that exhibited minimal GITR-L activity in the in vitro assays grew with delayed kinetics in vivo. This difference may reflect the lack of sensitivity of the in vitro assays. Alternatively, it is possible that the level of GITR-L expression by CT26-Low is equal or greater than the level of GITR-L expression during an immune response. The difference may also be attributed to the temporal kinetics of GITR stimulation. Studies using anti-GITR in combination with xenogeneic DNA vaccination have shown that the timing of GITR signaling is crucial for the effective generation of tumor specific CD8+ T cells [3]. In our model, we have observed no loss of GITR-L expression from freshly isolated tumor cells (data not shown), indicating that GITR-L is constitutively expressed on the tumor cell surface and thus would provide continuous costimulation during exposure to antigen.
It has become increasingly clear that the presence of tumor associated regulatory T cells can significantly impact overall survival. For example, the removal of tumor infiltrating Tregs by the local intratumoral depletion of CD4+ T cells is sufficient to promote the rejection of established tumors [38], suggesting that altering the balance of Treg cells to effector T cells in the tumor microenvironment may overcome Treg suppression. Indeed, in ovarian cancer, a high ratio of intraepithelial CD8+ T cells to CD4+Foxp3− and Tregs cells is associated with improved survival [26]. In our studies, the ectopic expression of GITR-L on the tumor led to a shift in the balance of tumor infiltrating lymphocytes, preferentially promoting the accumulation of CD8+ T cells at the tumor site with an increase in the ratios of CD8+ to T cells to CD4+Foxp3− and to Treg cells (Fig. 5g, h). This shift in T cell populations correlated with the generation of peripheral CTLs with cytolytic activity (Fig. 7) and significantly delayed tumor growth (Fig. 3). Thus, increasing the proportion of tumor infiltrating CD8+ T cells using GITR-L may be a useful strategy for the treatment of human cancer patients, as it has been demonstrated that human GITR-L (huGITR-L) can provide a costimulatory signal for CD4+ and CD8+ T cells [16, 34]. Although huGITR-L has been shown to be ineffective in abrogating Treg suppression in coculture assays in vitro using PBMCs [16, 34], the over-expression of huGITR-L in human monocyte-derived dendritic cells enhances their capacity to prime antigen specific CD8+ T cells [34] and may also enhance tumor specific CD8+ T cells in the appropriate context.
Interestingly, it has recently been reported that several human tumor cell lines and some primary human tumors express substantial levels of GITR-L which diminishes the activity of natural killer (NK) cells against the tumor in vitro [1]. However, it should be noted that plasmacytoid dendritic cells activated with CpG can promote NK-cell activity through GITR-L mediated mechanisms [9], suggesting that GITR/GITR-L interactions can have significantly differing outcomes depending on the context of signaling. Importantly, we observed no expression of GITR-L on untransfected CT26 tumor cells (supplemental Fig. 1) and it remains to be seen whether similar mechanisms of NK cell suppression can be observed in vivo.
Recently, it has been shown that reverse signaling through GITR-L appears to promote immunosuppression through the upregulation of TGF-β on human cancer cells [1] or indolamine 2,3-dioxygenase (IDO) on plasmacytoid dendritic cells [8]. These observations suggest that GITR-L mediated reverse signaling may provide a means of negative feedback to prevent excessive costimulation through GITR. However, the role of reverse signaling through GITR-L was not assessed in our studies as little is known regarding the molecular basis of GITR-L mediated reverse signaling.
In previous studies, it has not been clear which cell subpopulation is influenced by manipulating GITR/GITR-L interactions as systemic administration of the agonist antibody DTA-1 results in an increase in the activation state of many GITR expressing cells [39]. In the present study, GITR-L is expressed at high levels by the tumor cells which resulted in an increased accumulation of CD8+ T cells at the tumor site. The lack of changes in T cell populations in the draining lymph nodes further supports the hypothesis that the effect of localized GITR-L expression is within the microenvironment of the tumor, although additional interactions between T cells and other GITR-L expressing cells cannot be excluded. Although within the range investigated, the level of GITR-L expression did not influence the extent of tumor specific immunity, it is not possible to correlate the level of GITR-L expression on tumor cells with the levels present on antigen presenting cells during an immune response. However, these studies suggest that GITR ligation of tumor infiltrating CD8+ T cells may provide a useful means to expand tumor specific CTLs and, when used in combination with other strategies designed to enhance CD4+ Th cell help, inhibit Treg function, or both, may provide a means to generate effective anti-tumor immunity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Koteswara R. Chintalacharuvu, Caiyun Xuan, and Sepideh Afshar for critically reading the manuscript. We are also grateful to Letitia Wims for help with protein purification and Dr. B. S. Kwon for generously providing the cDNA of GITR-L. Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility that is supported by National Institutes of Health awards CA-16042 and AI-28697, and by the JCCC, the UCLA AIDS Institute, the David Geffen School of Medicine at UCLA, and the UCLA Chancellor’s Office. J. S. Cho was supported in part by NIH Tumor Immunology training grant 5 T32 CA009120: 32.
Abbreviations
- GITR
Glucocorticoid-induced TNF receptor related
- GITR-L
GITR ligand
- Tregs
Regulatory T cells
- ECD
Extracellular domain
References
- 1.Baltz KM, Krusch M, Bringmann A, Brossart P, Mayer F, Kloss M, Baessler T, Kumbier I, Peterfi A, Kupka S, Kroeber S, Menzel D, Radsak MP, Rammensee HG, Salih HR. Cancer immunoediting by GITR (glucocorticoid-induced TNF-related protein) ligand in humans: NK cell/tumor cell interactions. FASEB J. 2007;21(10):2442–2454. doi: 10.1096/fj.06-7724com. [DOI] [PubMed] [Google Scholar]
- 2.Calmels B, Paul S, Futin N, Ledoux C, Stoeckel F, Acres B. Bypassing tumor-associated immune suppression with recombinant adenovirus constructs expressing membrane bound or secreted GITR-L. Cancer Gene Ther. 2005;12(2):198–205. doi: 10.1038/sj.cgt.7700781. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AD, Diab A, Perales MA, Wolchok JD, Rizzuto G, Merghoub T, Huggins D, Liu C, Turk MJ, Restifo NP, Sakaguchi S, Houghton AN. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer Res. 2006;66(9):4904–4912. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 5.Fearon ER, Itaya T, Hunt B, Vogelstein B, Frost P. Induction in a murine tumor of immunogenic tumor variants by transfection with a foreign gene. Cancer Res. 1988;48(11):2975–2980. [PubMed] [Google Scholar]
- 6.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168(4):1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 7.Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32(11):3267–3275. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, Orabona C, Belladonna ML, Ayroldi E, Nocentini G, Boon L, Bistoni F, Fioretti MC, Romani L, Riccardi C, Puccetti P. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13(5):579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 9.Hanabuchi S, Watanabe N, Wang YH, Wang YH, Ito T, Shaw J, Cao W, Qin FX, Liu YJ. Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL) Blood. 2006;107(9):3617–3623. doi: 10.1182/blood-2005-08-3419. [DOI] [PubMed] [Google Scholar]
- 10.Hartman ZC, Appledorn DM, Amalfitano A. Adenovirus vector induced innate immune responses: Impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132:1–14. doi: 10.1016/j.virusres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9(12):4404–4408. [PubMed] [Google Scholar]
- 12.Ji HB, Liao G, Faubion WA, Abadia-Molina AC, Cozzo C, Laroux FS, Caton A, Terhorst C. Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression. J Immunol. 2004;172(10):5823–5827. doi: 10.4049/jimmunol.172.10.5823. [DOI] [PubMed] [Google Scholar]
- 13.Kim JD, Choi BK, Bae JS, Lee UH, Han IS, Lee HW, Youn BS, Vinay DS, Kwon BS. Cloning and characterization of GITR ligand. Genes Immun. 2003;4(8):564–569. doi: 10.1038/sj.gene.6364026. [DOI] [PubMed] [Google Scholar]
- 14.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T, Sakaguchi S. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3 + CD25 + CD4 + regulatory T cells. J Exp Med. 2005;202(7):885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohm AP, Williams JS, Miller SD. Cutting edge: ligation of the glucocorticoid-induced TNF receptor enhances autoreactive CD4 + T cell activation and experimental autoimmune encephalomyelitis. J Immunol. 2004;172(8):4686–4690. doi: 10.4049/jimmunol.172.8.4686. [DOI] [PubMed] [Google Scholar]
- 16.Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, Roncarolo MG. Human CD25 + CD4 + T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med. 2002;196(10):1335–1346. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Mahesh SP, Kim BJ, Buggage RR, Nussenblatt RB. Expression of glucocorticoid induced TNF receptor family related protein (GITR) on peripheral T cells from normal human donors and patients with non-infectious uveitis. J Autoimmun. 2003;21(1):83–92. doi: 10.1016/S0896-8411(03)00085-4. [DOI] [PubMed] [Google Scholar]
- 18.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169(5):2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 19.Muriglan SJ, Ramirez-Montagut T, Alpdogan O, Van Huystee TW, Eng JM, Hubbard VM, Kochman AA, Tjoe KH, Riccardi C, Pandolfi PP, Sakaguchi S, Houghton AN, Van Den Brink MR. GITR activation induces an opposite effect on alloreactive CD4(+) and CD8(+) T cells in graft-versus-host disease. J Exp Med. 2004;200(2):149–157. doi: 10.1084/jem.20040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, Migliorati G, Riccardi C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci USA. 1997;94(12):6216–6221. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59(13):3128–3133. [PubMed] [Google Scholar]
- 22.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34(3):613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 23.Sakaguchi S. Naturally arising CD4 + regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;16(1):72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4 + CD25 + regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98(5):1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 26.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8 + tumor-infiltrating lymphocytes and a high CD8 +/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+ CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163(10):5211–5218. [PubMed] [Google Scholar]
- 28.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3(2):135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 29.Shin HH, Lee MH, Kim SG, Lee YH, Kwon BS, Choi HS. Recombinant glucocorticoid induced tumor necrosis factor receptor (rGITR) induces NOS in murine macrophage. FEBS Lett. 2002;514(2–3):275–280. doi: 10.1016/S0014-5793(02)02379-7. [DOI] [PubMed] [Google Scholar]
- 30.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4 + CD25 + T cells. J Immunol. 2004;173(8):5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 31.Suri A, Shimizu J, Katz JD, Sakaguchi S, Unanue ER, Kanagawa O. Regulation of autoimmune diabetes by non-islet-specific T cells—a role for the glucocorticoid-induced TNF receptor. Eur J Immunol. 2004;34(2):447–454. doi: 10.1002/eji.200324599. [DOI] [PubMed] [Google Scholar]
- 32.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP, Waldmann H. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci USA. 2003;100(25):15059–15064. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuyaerts S, Van Meirvenne S, Bonehill A, Heirman C, Corthals J, Waldmann H, Breckpot K, Thielemans K, Aerts JL. Expression of human GITRL on myeloid dendritic cells enhances their immunostimulatory function but does not abrogate the suppressive effect of CD4+ CD25+ regulatory T cells. J Leukoc Biol. 2007;82(1):93–105. doi: 10.1189/jlb.0906568. [DOI] [PubMed] [Google Scholar]
- 35.Uraushihara K, Kanai T, Ko K, Totsuka T, Makita S, Iiyama R, Nakamura T, Watanabe M. Regulation of murine inflammatory bowel disease by CD25+ and CD25− CD4+ glucocorticoid-induced TNF receptor family-related gene + regulatory T cells. J Immunol. 2003;171(2):708–716. doi: 10.4049/jimmunol.171.2.708. [DOI] [PubMed] [Google Scholar]
- 36.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9(2):606–612. [PubMed] [Google Scholar]
- 37.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61(12):4766–4772. [PubMed] [Google Scholar]
- 38.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, Fu YX. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201(5):779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou P, L’Italien L, Hodges D, Schebye XM. Pivotal roles of CD4+ effector T cells in mediating agonistic anti-GITR mAb-induced-immune activation and tumor immunity in CT26 tumors. J Immunol. 2007;179(11):7365–7375. doi: 10.4049/jimmunol.179.11.7365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.