Abstract
One literature treats the hippocampus as a purely cognitive structure involved in memory; another treats it as a regulator of emotion whose dysfunction leads to psychopathology. We review behavioral, anatomical, and gene expression studies that together support a functional segmentation into 3 hippocampal compartments dorsal, intermediate and ventral. The dorsal hippocampus, which corresponds to the posterior hippocampus in primates, performs primarily cognitive functions. The ventral (anterior in primates) relates to stress, emotion and affect. Strikingly, gene expression in the dorsal hippocampus correlates with cortical regions involved in information processing, while genes expressed in the ventral hippocampus correlate with regions involved in emotion and stress (amygdala and hypothalamus).
Despite over 50 years of research, attention and debate, there is still controversy over the basic general function of the hippocampus. There is the cold cognitive hippocampus that stands as the gate to declarative memories, regardless of their emotional content or lack thereof. According to this view, hippocampal dysfunction leads to a “pure” amnesia. But the literature also shows another side to the hippocampus, a hot hippocampus that is intimately tied to emotion, regulates stress responses and whose dysfunction leads to affective disorders such as depression. The thesis of this brief review is that there is sufficient behavioral evidence indicating the existence of both functions within the hippocampus. However, gene expression and anatomical projections patterns that vary along the rostral/caudal-dorsal/ventral extent of the hippocampus suggest that it can be divided into separate structures or zones. We argue that the hippocampus can be thought of as a set of separate structures with a rostral/dorsal zone that serves the cold cognitive function and a caudal/ventral zone that corresponds to the hot/affective hippocampus. An intermediate region that has only partly overlapping characteristics with its neighbors separates the two. We review recently published data on CA1 (Dong et al., 2009) and CA3 (Thompson et al., 2009) and add a similar analysis of dentate gyrus. This approach allows us to provide a precise definition of these zones as an alternative to the more arbitrary reference to dorsal and ventral hippocampus that is common in the literature. Furthermore, this definition corresponds well with the available behavioral evidence. The coherence between gene expression, behavioral function and anatomical projections indicates that segmentation of the hippocampus along its rostral/caudal axis can guide future research toward a resolution of controversies surrounding the general function of the hippocampus.
Functions of the Hippocampus
Memory & Cognition
Since the groundbreaking case of H.M., who lost much of his memory when his medial temporal lobe was extirpated for the treatment of his intractable epilepsy, a vast amount of data has linked the hippocampus to memory in humans, other primates, rats and mice (Scoville & Milner, 1957; Squire, 1992). While the volumes of data on this subject are beyond the scope of any single review, it is important to point out that many of the specifics of amnesia following hippocampal loss in humans, such as temporally graded retrograde amnesia, are recapitulated in rodents, making these experimentally and genetically tractable species appropriate models (Kim & Fanselow, 1992; Squire et al., 2001). Additionally, the hippocampus is involved in some but not all types of memory. Certainly there is debate over how best to conceptualize the distinction over what makes memory hippocampus-dependent versus independent. However, there can be no argument that following removal of the hippocampus several forms of memory suffer (e.g., episodic memory, spatial learning, or contextual fear).
Most behavioral tests using rodents require some level of positive or negative emotion to motivate the animal to respond (e.g., hunger/food). For example, a common test to assess hippocampal function in rodents, contextual fear conditioning uses aversive electric shock. In the standard version of this task, rats or mice are placed in a chamber where they receive a mild electric shock signaled by a brief tone (Kim & Fanselow, 1992). When returned to the same chamber where it was shocked the rat freezes but there is no freezing when the animal is placed in a sufficiently different chamber. This shows that the animal has associated the shock with the training context. The rat will also freeze if the tone is presented and this tone test is typically done in an untrained chamber so a measure of the tone-shock association, in the absence of context fear, can be gained. Genetic, pharmacological and lesion manipulations of the hippocampus all produce a deficit in context but not tone fear. This selectivity to context suggests that the context fear deficit is caused by a failure in context processing and not by a general emotional deficit.
Contextual fear learning requires a period of exploration during which it is hypothesized that the many features of the context are integrated into a coherent representation of the context (Fanselow, 2000). If rats and mice are given insufficient time to explore the context prior to shock they show little or no context conditioning (Fanselow, 1986). Formation of the contextual representation can be temporally segregated from learning the context-shock association by giving context pre-exposure (without shock) on one day and giving shock shortly after placement in the chamber on another day (Fanselow, 1990). Without the pre-exposure rats will not learn context fear despite having the context-shock pairing. Using this context pre-exposure effect it has been found that NMDA antagonists and protein synthesis inhibitors directed at the hippocampus block contextual fear memories if given prior to the context pre-exposure but not when given prior to the context-shock pairing (Barrientos et al., 2002; Stote & Fanselow, 2004). Thus NMDA-mediated plasticity in the hippocampus is important for the more cognitive contextual integration and not the emotion based context-shock association. This corresponds well with the finding that place fields form in the hippocampus during exploration of an environment even in the absence of any explicit motivation (O’Keefe & Dostrovsky, 1971). Thus there is good evidence to believe that the hippocampus supports memory and cognitive functions that do not have an emotional/motivational component.
Emotion
Historically, the long-standing link between the hippocampus and emotion owes itself to this region’s prominent position in Papez’s limbic circuit and its hypothesized role in controlling emotion. Early, support for this view was taken from Kluver & Bucy’s (1937) classic finding that removal of the medial temporal lobe caused profound emotional disturbances in monkeys. Building upon such observations as well as Sokolov and Vinograda’s findings of hippocampal orienting responses to novelty and change, Gray suggested that the hippocampus is involved in “states of emotion, especially disappointment and frustration” (Gray, 1971, pp 201; Gray & McNaughton, 2000; Sokolov & Vinograda, 1975).
The hippocampus exerts strong regulatory control of the hypothalamic-pituitary-adrenal axis. Hippocampal lesions impair control of the hormonal stress response (Dedovic et al., 2009; Jacobson & Sapolsky, 1991). In turn, it is clear that elevations of stress hormones, lead to hippocampal dysfunction in both humans and rodents (McEwen et al., 1997; Herman et al., 2005). In humans decreased hippocampal volumes and hippocampal dysfunction are associated with psychological disorders with strong affective components such as post-traumatic stress disorder, bipolar disorder and depression (Bonne et al., 2008; Frey et al., 2007). Indeed, effective pharmacological treatments of these disorders target hippocampal function and physiology. Thus the linkage of the hippocampus with emotion and affect is as striking as its relationship with memory.
Anatomical Segregation of Hippocampal Function
In an influential review, Moser and Moser (1998) suggested that the hippocampus may not act as a unitary structure with the dorsal (septal pole) and ventral (temporal pole) portions taking on different roles. Their argument was based on 3 data sets. First, prior anatomical studies indicated that the input and output connections of the dorsal hippocampus (DH) and ventral hippocampus (VH) are distinct (Swanson & Cowan, 1977). Second, spatial memory appears to depend on DH not VH (Moser et al., 1995). Third, VH, but not DH, lesions alter stress responses and emotional behavior (Henke, 1990).
Behavioral tests of spatial navigation and memory have been particularly illuminating with regard to hippocampal function. An informative task for assessing spatial cognition in rodents is the Morris water maze, where animals must swim to a hidden location using landmarks placed outside the pool (Morris, 1981). This task clearly implicates the DH in spatial memory. Lesions restricted to as little as 25% of the DH impair acquisition on the water maze and additional damage to the ventral region does not exacerbate the deficit (Moser et al., 1995). Lesions restricted to the VH have no effect on this behavior. Consistent with the lesion data, there is a greater density of place fields in the DH as opposed to VH (Jung et al., 1994). Rats that learn the water maze show significant changes in expression of a large number of genes in the DH that is disproportionately greater (≈8-to 1) in the right than left DH (Klur et al., 2009). Again consistent is the finding that inactivation of the right but not left DH abolishes retrieval of this spatial memory (Klur et al., 2009). Similarly, when taxi drivers recall complex routes through a city the right but not left posterior hippocampus is differentially activated compared to the anterior hippocampus (Maguire et al., 1997). In primates, the posterior portions of the hippocampus correspond to the rodent DH, while the anterior portions are analogous to the VH. Recall, of verbal material also preferentially activates the human posterior over anterior hippocampus but now the left shows greater activation than the right (Greicius et al., 2003). Another fMRI study by Kumaran et al (2009) is particularly informative in this regard. They found that activity of the left posterior hippocampus tracks the emergence of new conceptual information. Conceptual information is typically thought of as the acquisition of rules that can guide behavior in novel situations. But it is also easy to see how such relational rules could guide the navigational behavior needed to find a safe platform when starting in a novel location.
Like the water maze, the radial arm maze tests spatial memory by requiring rodents to return to locations not previously visited to find food (Olton & Samuelson, 1976). Using the radial arm maze, Pothuizen et al (2004), found that while DH lesions caused a deficit in spatial memory, VH lesions did not. Returning to an arm previously associated with food is reduced by DH lesions and enhanced by VH lesions (Ferbinteanu & McDonald, 2001). That the same procedure shows opposite effects for DH and VH lesions provides strong support for the idea that dorsal and ventral zones support different functions. One can interpret these data as being consistent with the dorsal/spatial memory and ventral/emotion distinction. If DH lesions cause a loss of spatial information then the rats would be unable to return to the place associated with food. Rats with VH lesions necessarily had spatial information as they returned to the food-associated location. Rather, the enhancement in preference suggests an altered memory for the affective aspects of food.
In a study that clearly manipulated stress over cognition, Henke (1990) reported that VH but not DH lesions enhanced cold/restraint stress ulcers. Furthermore, Kjelstrup et al (2002) reported that lesions of the most ventral quarter of the hippocampus increased entry into the open (unprotected) arms of an elevated plus maze and decreased defecation in a brightly lit chamber, both of which are consistent with a reduction in anxiety. The VH lesioned animals also showed less of an increase in corticosterone in response to confinement in the brightly lit chamber.
Fear conditioning tasks offer a test of spatial (context fear) and nonspatial (cued fear) memory where performance is motivated by emotion. For the DH the data are clear that dorsal lesions cause an impairment in retention of contextual as opposed to cued fear (Kim & Fanselow, 1992) and this contextual deficit may be more related to dorsal CA1 than CA3 (Hunsaker & Kesner, 2008). As pointed out earlier, the contextual pre-exposure effect described above offers a way of separating the contextual and emotional learning components of contextual fear conditioning, and pharmacological manipulations aimed at DH are highly effective during the pre-exposure period.
While the effects of VH manipulations on fear conditioning tasks are a bit less straightforward they suggest if anything the deficits are more pronounced and more general. As in DH (Quinn et al., 2005) NMDA antagonists infused into the VH block the acquisition of context fear but not fear to a tone that accurately signals shock (Zhang et al, 2001). However, VH lesions or infusions of muscimol (which temporarily inactivates neurons) block tone fear and produce less consistent effects on context fear (Hunsaker & Kesner, 2008; Maren & Holt, 2004; Rogers and Kesner, 2006). The greater, or at least more consistent, effects of VH lesions on tone than context fear cannot be attributed to sensory modality. Contexts usually contain an olfactory component and Hunsaker et al. (2008) using a temporal-order discrimination task found that VH lesions had more pronounced effects when olfactory cues as opposed to visual or spatial cues were used. The opposite was true for DH lesions. This role of the VH in Pavlovian fear is consistent with the suggestions of the Moser group, that the hippocampus regulates emotion, and Anagnostaras et al (2002) that VH manipulations alter fear conditioning by depriving the amygdala of both dorsal and ventral hippocampal information. The amygdala has a very general role in mediating fear memory and only receives direct hippocampal input via the VH (Maren & Fanselow, 1995).
However, the idea that the VH plays no role in spatial memory is not ubiquitous. Ferbinteanu et al (2003) using a “match-to-position” version of the water maze found a perfect parallel in the deficits in spatial memory produced by just DH or just VH lesions, both slowed acquisition and the deficit was overcome by repeated training. Additionally, Rudy & Matus-Amat (2005) challenged the idea that the VH has no role in context processing using the context pre-exposure design to isolate context learning from emotional learning. They found that inactivating the VH before and blocking protein synthesis immediately after context pre-exposure attenuated the benefits of pre-exposure. Since no shock is given during the pre-exposure these VH effects seem unlikely to be through affective processing. To further support this argument, infusion of the protein synthesis inhibitor immediately after context-shock pairing had no effect on subsequent fear memory even though this is the period during which an affective memory should be consolidated.
Conclusions
There is substantial data supporting the Moser theory that the dorsal or septal pole of the hippocampus, which corresponds to the human posterior hippocampus, is specifically involved in memory function and the ventral or temporal pole of the hippocampus, which corresponds to the anterior hippocampus in humans, modulates emotional and affective processes. Consistent with Gray’s (1971) original idea that the hippocampus is involved in negative affect such as frustration and anxiety, VH manipulations tend to decrease fear and anxiety (Kjelstrup et al., 2002; Maren & Holt, 2004), and increase motivation for food (Ferbinteanu & McDonald, 2001). However, there are several pieces of data that do not fit easily into this distinction. One potential explanation of the discrepancies is that the field has not adopted a single definition of what exactly is the DH vs VH, that is based on a set of independent and objective criteria. Bannerman et al (1999) suggested that DH be defined as 50% of the total hippocampus starting at the septal pole, with VH as the remaining half. This is an arbitrary definition as it relies on no independent objective attributes. Studies from the Moser group are clearest in separating function when lesions are restricted to 25% of hippocampal volume starting at either the septal pole (spatial tasks-Moser et al., 1995) or temporal pole (emotion-Kjelstrup et al., 2002). Studies that implicated the VH in spatial learning have had drug infusion sites or lesioned regions that extended dorsally to at least the intermediate hippocampus. Therefore, the next section of this paper uses newly available gene expression data to try to help define DH and VH.
Molecular and functional domains of the hippocampus
The basic cytoarchitectonic scheme of the hippocampus was established originally by Ramón Y Cajal (1901) and Lorente de Nó (1934). Their pioneering work illustrated the distinct morphological properties of small pyramidal neurons in CA1 (region superior of Cajal), and large pyramidals in CA3 (region inferior of Cajal, with mossy fibers) and CA2 (without mossy fibers). Indeed, Cajal (1901–1902) was the first to notice differences in the hippocampus across the dorsal-to-ventral axis. He originally distinguished two perforant paths from the entorhinal cortex, “superior” and “inferior,” that target what was later referred to on connectional grounds (Gloor, 1997; Swanson & Cowan, 1975) as the “dorsal” and “ventral” hippocampus, respectively. Lorente de Nó (1934) also divided the “Ammonic system” into three main segments along its longitudinal axis according to their different afferent inputs. He stated that while there is no sharp boundary, each of these segments has special structural features, although he did not give detailed descriptions of their borders.
Two recent reports based on the systematic, high-resolution analysis of a comprehensive, genome-wide digital gene expression library—the Allen Brain Atlas (ABA, www.brain-map.org) revealed that pyramidal neurons in both CA1 and CA3 display clear regional and laminar specificities in C57Bl/6 mice. Using these robust gene markers, both of these fields were parceled into multiple, spatially distinct molecular domains and subdomains (Dong et al., 2009; Thompson et al., 2008). This genomic-anatomic evidence, together with our careful re-evaluation of the hippocampal cytoarchitecture, as well as the literature of numerous neuronal connectivity and functional studies in the last three decades, leads us to provide a testable hippocampal structural-functional model for understanding the heterogeneity of the DH and VH.
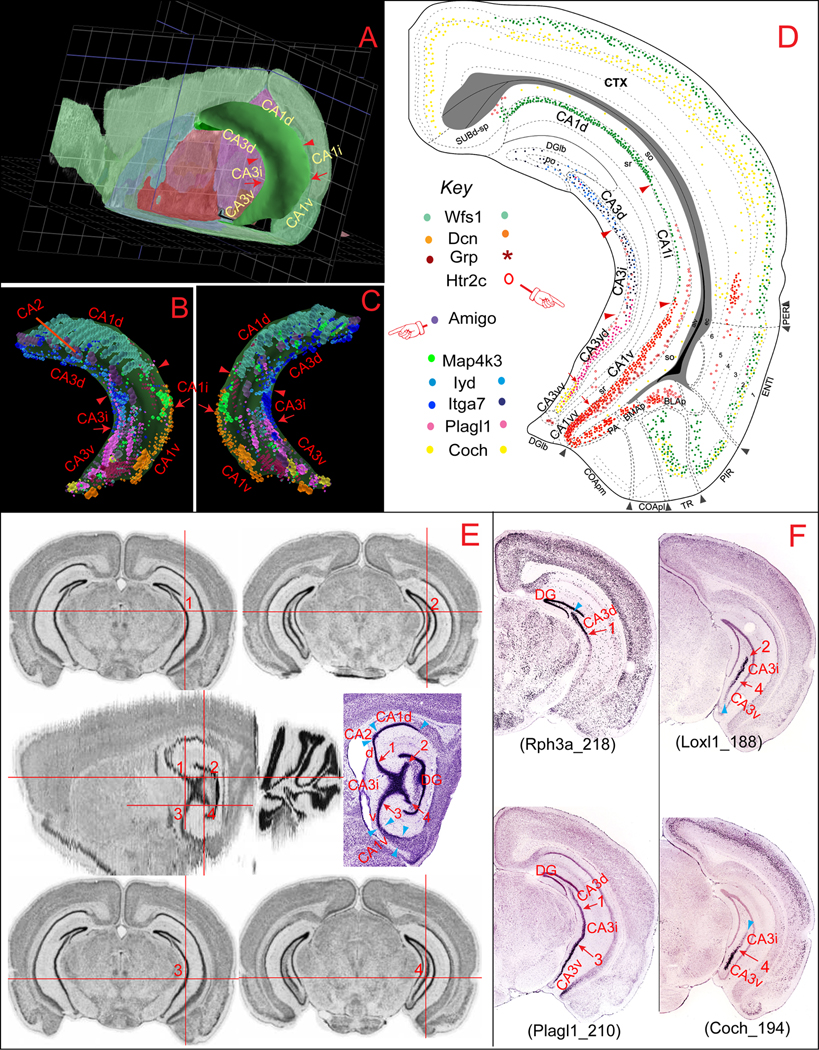
Our model suggests that both CA1 and CA3—the Ammon’s horn as a whole—are divided respectively into three major molecular domains: dorsal (CA1d and CA3d), intermediate (CA1i and CA3i), and ventral (CA1v and CA3v) (Dong et al., 2009). The complex geographic topology of these three domains is better appreciated in the three-dimensional context of the mouse brain (Fig. 1A), in which the entire Ammon’s horn appears to be an elongated C-shaped cylinder. Its two free ends compose the major proportions of the dorsal (CA1d and CA3d) or ventral (CA1v and CA3v) domains respectively, arching rostromedially, while the intermediate domains of the CA1 (CA1i) and CA3 (CA3i) defined here occupy the intermediate one-third, primarily the vertical part of the “C”. Our dorsal, ventral, and intermediate domains correspond approximately to the septal, temporal, and caudal poles of Swanson and Cowan (1977), although they did not give clear rationale for how these boundaries were drawn. At one sagittal level of the C57/Black/6J mouse brain atlas (~ 2.494 mm lateral to midline) showing the maximal extension of the hippocampus (where the dorsal and ventral parts merge into one unit), the CA3 pyramidal neurons cluster together and appear as one dark “X-shaped pyramidal pool” (Fig. 1E, 2nd row). The geographic scope within the four corners of this “X-shaped-pyramidal pool” (indicated by 1, 2, 3, or 4 in Fig. 1E) corresponds to the CA3i defined here. It is located right in the middle (or intermediate) portion of the hippocampus and appears to be the most obvious landmark between the DH and VH. Starting from this point rostrally and medially, the hippocampus is separated into two individual dorsal and ventral parts. Caudally/laterally, these two parts appear as one entity in which the CA3i, CA2 caudal portion, and CA1i contiguously occupy the vertical portion of the “C” shaped hippocampus progressively towards the more lateral side of the brain on sagittal planes.
Fig. 1.
Molecular domains of the hippocampal CA1 and CA3. A shows a three dimensional (3D) model of Ammon's horn, which appears as a “C” shaped cylinder with its dorsal and ventral ends towards rostral and medial directions of brain. CA1 occupies the area dorsal, lateral, and caudal to the CA3. B (lateral view) and C (medial view) display heterogenic spatial distribution patterns of several representative marker genes expressed specifically in CA1 (Wfs1, Dcn, Grp, and Htr2c), CA2 (Amigo), or CA3 (Map4k3, Iyd, Itga7, Plagl1, and Coch). Expression of these genes in the Ammon’s horn reveals clear segregation between the dorsal (including CA1d, CA2, CA3d), intermediate (CA1i and CA3i), and ventral (CA1v and CA3v) areas. Expression of these genes were plotted onto representative coronal planes of the Allen Reference Atlas (Dong, 2007) as shown in D, which reveals clear boundaries between these molecular domains in CA1 and CA3. The 3D model and gene expression in Ammon’s horn were generated in BrainExplore (Lau et al, 2008), a 3D application of the Allen Reference Atlas (www.brain-map.org). E illustrates the spatial definition of the CA3i, which appears as an “X”-shaped pyramidal neuronal pool on one particular “re-sliced” sagittal plane of the Allen Reference Atlas (in the middle panel, ~2.494 mm from the middle line). The detailed Nissl-stained cytoarchitecture of the hippocampus is shown side by side. Numbers 1–4 indicate four corners of the “X” shaped pyramidal pool in domain CA3i at this sagittal plane and their corresponding spatial positions on the coronal planes (shown in the dorsal and ventral panels), which indicate the boundaries between the CA3d and CA3i (number 1; number 2 represents the dorsal end of the CA3 at the most caudal level) and between CA3i and CA3v (number 3 at more rostral and 4 more caudal). These images were generated with the AGEA application of the ABA. F shows four representative genes that are expressed preferentially in both domain CA3d and CA3i (Rph3a), CA3i (Loxl1), and CA3v (Plagl1 and Coch). Numbers 1–4 indicate corresponding anatomic locations in E. These gene expression digital images were downloaded from the ABA
On coronal planes (Fig. 1D-F), the CA3i, which includes regions 5 (characterized by gene Serpinf1) and 4 (the caudal-dorsal end of the CA3 characterized by gene Col15a1 and Ccdc3) of Thompson et al (2008), first appear at the levels where the orientation of the hippocampus sweeps from the transverse (pyramidal neurons are aligned along the medial-to-lateral direction) to vertical (pyramidal neurons are “stacked” along the dorsal-to-ventral direction), and the DH and VH are merging as one unit. The CA3d is defined as the CA3 portion dorsal/rostral to the CA3i towards its septal end. The CA3d can be further subdivided into three subdomains: dorsal-medial (CA3dm, towards the dentate gyrus), dorsal-intermediate (CA3di), and dorsal-lateral (CA3dl; towards the CA2). These three subdomains correspond respectively to regions 1, 2, and 3 of Thompson et al. (2008), and at least partially overlap with the CA3c, CA3b, and CA3a of Lorente de Nó (1934), which we believe referred mostly to different parts of Ammon’s horn along the horizontal (rostral-to-caudal) and transverse (medial-to-lateral), but not longitudinal (dorsal-to-ventral) axis. The CA3v refers to the portion of CA3 ventral to the CA3i and can also be subdivided into at least two subdomains, CA3 ventral-dorsal (CA3vd) and CA3 ventral-ventral (CA3vv), which correspond respectively to regions 6 (characterized by gene Plagl1) and 7 (ventral tip of the CA3, characterized by gene Coch) of Thompson et al (2008).
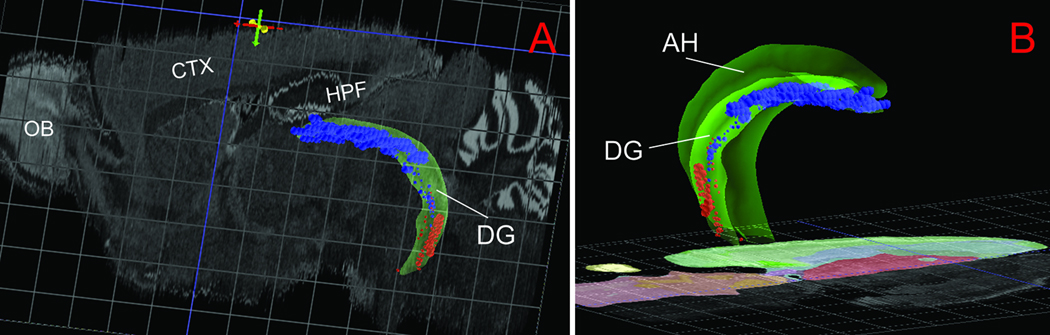
The CA2 (characterized by Amigo), which is clearly located between the CA1d and CA3d at the rostral one-third of the hippocampus (Fig. 1B, C), should be included in the dorsal domain of the Ammon’s horn. Nevertheless, a number of gene markers in the ABA database, including Map4k3 and Adcy4, reveal that CA2’s caudal portion at the levels where the DH and VH merge, overlap partially with the rostral portion of CA1i that is sandwiched between CA1d and CA1v (depending on the cutting angels of brain sections). Finally, it is worthy noting that gene expression in the dentate gyrus also displays distinct regional specificity. As shown in Figure 2, Lct is preferentially expressed in the dorsal/septal/rostal part of the dentate gyrus, which runs in parallel with the CA1d and CA3d. In contrast, Trhr is expressed specifically in its ventral/temporal/caudal part, while the intermediate portion contains only sparse signal for these two genes. This suggests that the entire hippocampal region, including both the Ammon’s horn and dentate gyrus, may be composed of three distinct molecular domains, dorsal, intermediate, and ventral.
Fig. 2.
Three dimensional model of the dentate gyrus in the context of the whole mouse brain (A, lateral view) and its spatial relationship with Ammon’s horn (dark green in B, medial view). Two genes, Lct (blue) and Trhr (red), are expressed preferentially in the dorsal/septal one third or ventral/temporal one third of the dentate gyrus respectively. These images were generated in BrainExplorer, one three dimensional version of the ABA (Dong, 2007). Abbreviations: AH, Ammon’s horn; CTX, cerebral cortex; DG, dentate gyrus; HPF, hippocampal formation; OB, olfactory bulb.
Of equal importance, gene expression in pyramidal neurons of both CA1 and CA3 also display clear laminar specificities (Dong, et al., 2009; Thompson et al., 2008). Accordingly, Dong et al (2009) subdivided the CA1 pyramidal layer into 2–3 sublayers, which show distinct cytoarchitectonic and gene expression specificities in different domains and subdomains along the longitudinal axis. Domain CA1d pyramidal layer consists of two very distinctive sublayers: the darkly stained, tightly arranged superficial layer (CA1d-sps) and the loosely arranged deep layer (CA1d-spd). These morphological properties become progressively less distinctive towards the ventral (temporal) direction, although the thickness of pyramidal layer (especially the deep layer) increases incrementally. In two dorsally located subdomains of the CA1v (CA1vd and CA1vid), one more sub-layer (the middle sublayer) appears between the superficial and deep layers. Nevertheless, towards the more ventral area, especially in the CA1vv (the most ventral tip of the CA1), all pyramidal neurons appear to have similar morphology and form a uniformed single layer with pyramidal neurons arranged in 7–8 parallel rows. In fact, Lorente de Nó noticed the difference between these types of pyramidal neurons in superficial and deep layers of CA1. According to him, the deep pyramids correspond more or less to what Cajal calls ‘pirámides dislocadas’ (luxated pyramids), which are less numerous in lower mammals (mouse, rabbit, dog, cat) than in the primates (monkey, man). Another important fact is that these two types of pyramidal neurons have a different relation to the basket cells. The superficial pyramids are in contact with the end arborizations of the pyramidal, horizontal and polygonal basket cells, while the deep pyramids are chiefly in contact with the polygonal basket cells, and the deepest have almost no contact with the basket plexus. This distinction is very important considering that basket neurons play a key role in regulating activity of pyramidal neurons.
In summary, although laminar and regional specificities of pyramidal neurons in the isocortex have been studied extensively, surprisingly very little is known about different phenotypes of pyramidal neurons in the hippocampus. Pyramidal neurons within the CA1 or CA3 display both regional and laminar specificities in different molecular domains. Distinctively expressed gene markers will provide an extremely powerful tool for understanding the functional roles of specific neuronal groups in anatomic, physiological, and genetic studies.
Anatomic connectivity
Neuronal connectivity of the hippocampus has been studied extensively in the last three decades using modern tract tracing methods in rats, cats, and monkeys (Burwell, 2000; Swanson, 1987; Witter & Amaral, 2004). One critical question that remains to be clarified is how these connectivity data correlate with the molecular domains of the hippocampus defined in C57Bl/6 mice as discussed in the last section (see also Dong et a., 2009; Thompson et al., 2008). Ultimately, it would be necessary to map expression of these marker genes in rats, monkeys, and even humans, to provide novel molecular insight underlying the abundant anatomic, physiological, behavioral, and functional data collected in these species. It is also necessary to systematically examine and validate the neuronal connectivity of the hippocampus in the C57Bl/6 mouse, which has become the most frequently used animal model because of the availability of powerful genetic tools. Nevertheless, it is well accepted that the fundamental organization of hippocampal connectivity, both intrinsic and extrinsic, is very consistent in rats, cats, monkeys and humans (Burwell, 2000; Swanson, 1987; Witter & Amaral, 2004). Thus, it is very likely that hippocampal connectivity in mice also follows the same principle, although this remains to be confirmed, hopefully in the near future.
Accumulated evidence reviewed below suggests that different parts of the hippocampus display distinctive, topographically arranged, neuronal connectivity patterns, which coincide well with the gene-expression based model in mice (Dong et al., 2009). For the sake of clarity, it is worth noting that the dorsal (septal), intermediate, and ventral (temporal) parts of the hippocampus in rats, as originally illustrated in Swanson and Cowan (1977), at least partially overlap with our dorsal, intermediate, and ventral molecular domains of the hippocampal formation. The dorsal and ventral subiculum were also arbitrarily defined as the parts that are dorsomedial and ventromedial to CA1, while the intermediate part was considered the portion that is caudal (behind) the caudal end of CA1 (Kishi et al., 2000). In addition, Swanson and his colleagues (Cenquizca & Swanson, 2007; Petrovich et al., 2001; Swanson, 2004) also divided the entire hippocampus into five functional domains on a flattened map along the longitudinal axis, although the exact boundaries of these domains on the coronal planes are yet to be clearly defined. Based on our own observation of gene expression and neuronal connectivity data, it appears that our domain CA1d in mice corresponds to the dorsal half of their domain 1, and domain CA1i to the ventral half of their domain 1, while our domain CA1v relates to their domain 2–5 as whole.
Intra-hippocampal connectivity
In general, the fundamental organization of the hippocampal formation as a whole can be succinctly described as a series of parallel cortical strips that are interrelated by a series of transverse association (and commissural) pathways (Swanson, 1987). The entire entorhinal cortex can be divided into three relatively independent, rostrocaudally oriented, parallel band-like zones: the caudolateral, intermediate, and rostromedial zones, which may represent three distinct functional units because their neuronal inputs are different and direct connections between these three zones are very sparse (Burwell, 2000; Dolorfo & Amaral, 1998; Insausti et al., 1997;). In general, the caudolateral band receives the most visuospatial information (mostly via adjacent perirhinal and postrhinal cortex), and in turn, projects specifically to the dorsal/septal (caudal in monkey) hippocampal region. The medial band, which receives primarily olfactory, visceral, and gustatory inputs, projects specifically to the ventral/temporal (anterior in monkey) hippocampus; while the intermediate band seems to receive even more widespread inputs and projects primarily to the intermediate parts of the hippocampus. This topographically ordered, at least partly non-overlapping manner of dorsal-to-dorsal, intermediate-to-intermediate, and ventral-to-ventral projection patterns are repeated at each step of the classic “trisynaptic” circuits (dentate gyrus >CA3>CA1>the subiculum). This fundamental organization is conserved in rats (Cenquizca & Swanson, 2007; Dolorfo & Amaral, 1998; Insausti et al., 1997; Ishizuka et al., 1990), cats (Witter & Groenewegen, 1984), and monkeys (Chrobak & Amaral, 2007; Suzuki & Amaral, 1990; Witter & Amaral, 1991). Additionally, more extensive serial and parallel intrahippocampal circuits have been well characterized. It is clear that the entorhinal cortex innervates all of the hippocampal components, and both the CA1 and subiculum send direct projections back to the entorhinal area, which correspond to their reciprocal projections from the entorhinal cortex to the CA1 and subiculum that follow the same topographic patterns along the longitudinal axis (Cenquizca & Swanson, 2007; Kloosterman et al., 2003; Naber et al., 2001; Tamamaki & Nojyo, 1995; van Groen et al., 1986).
In the next section, we review projections from the CA1 and subiculum, which represent the “ending points” of the “trisynaptic circuit” and primary sources of “extrinsic” hippocampal-subicular projections.
Neuronal connectivity of the dorsal hippocampus
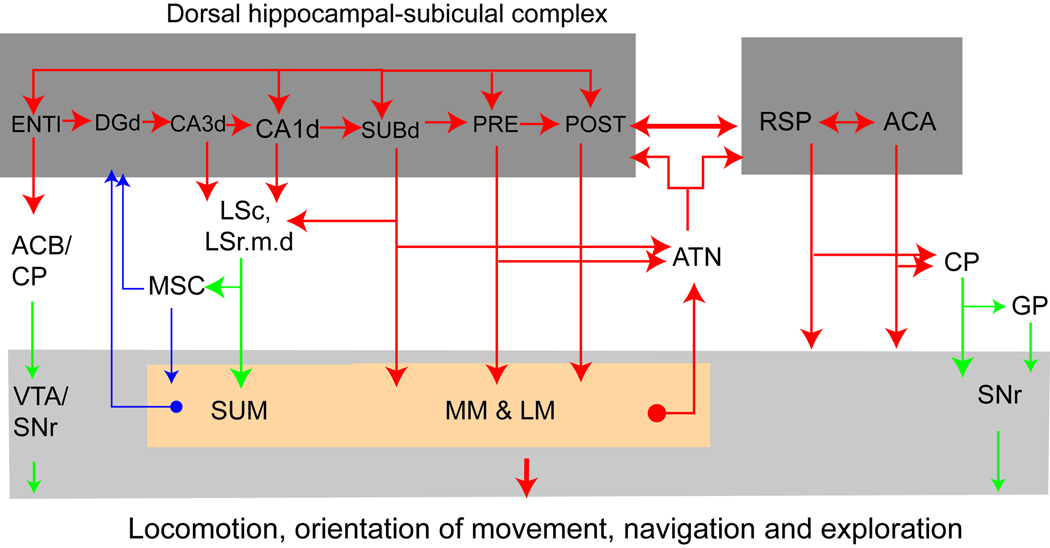
The dorsal (septal, caudal in primates) CA1, which contains the greatest density and selectivity of place cells coding spatial location (Jung et al., 1994; Muller et al., 1996), sends massive sequential, multi-synaptic, and presumably feed-forward excitatory projections to the dorsal parts of the subiculum, presubiculum, and postsubiculum (Figure 3; Amaral et al., 1991; Swanson & Cowan, 1977;van Groen & Wyss, 1990; Witter & Amaral, 2004; Witter & Groenewegen, 1990). The dorsal parts of the subicular complex contain the most ‘head direction’ or ‘compass’ cells for coding head position in space (Taube et al., 1990; 2007).
Fig. 3.
Schematic overview showing the organization of the dorsal hippocampal network Abbreviations: ACA, anterior cingulated area; ACB, nucleus accumbens; ATN, anterior thalamic complex; CP, caudoputamen; DGd, dorsal domain of the dentate gyrus; ENTl, the caudolateral band of the entorhinal cortex; GP, globus pallidus; LM, lateral mammilary nucleus; LSc, the caudal part of the lateral septal nucleus; MM, medial mammilary nucleus; MSC, medial septal complex; PRE, presubiculum; POST, postsubiculum; RSP, retrosplenial cortex; SNr, reticular part of the substantial nigra; SUBd, dorsal subiculum; SUM, supramammillary nucleus; VTA, ventral tegmental area.
The most prominent cortical projections from the dorsal CA1 and the dorsal parts of the subicular complex are to the retrosplenial and anterior cingulated cortices in rats (Cenquizca & Swanson, 2007; Risold et al., 1997; van Groen & Wyss, 2003; Vogt & Miller, 1983) and monkeys (Kobayashi & Amaral, 2007; Parvizi et al., 2006; Roberts et al., 2007) — two cortical regions involved primarily in the cognitive processing of visuospatial information and memory processing (Frankland et al., 2004; Han et al., 2003; Jones & Wilson, 2005; Lavenex et al., 2006) and environmental exploration (spatial navigation) in rats (Harker & Whishaw, 2004), monkeys (Lavenex et al., 2007) and humans (Maguire et al., 2006; Spiers & Maguire, 2006). Meanwhile, the dorsal (but not ventral) parts of this subicular complex send massive parallel projections through the postcommissural fornix to the medial and lateral mammillary nuclei and the anterior thalamic complex (Ishizuka, 2001; Kishi et al., 2000; Swanson & Cowan, 1975) — two structures containing the most navigation-related neurons (Taube, 2007). In turn, these subcortical structures send their projections back to the DH and retrosplennial cortex (Risold et al., 1997). It is apparent that this neural network, composed of the dorsal CA1-dorsal subicular complex-mammillary body—anterior thalamic nuclei, provides the most important interface to register a cognitive map for the navigation/direction system, thus, enabling animals to properly orient and execute behaviors in a learned environment (Muller et al., 1996; Jeffery, 2007; Taube et al., 1990; 2007).
Additionally, the dorsal CA1 and dorsal CA3 project rather selectively to the caudal part (LSc) and tiny dorsal region of the medial zone of the rostral part (LSr.m.d) of the lateral septal nucleus, which in turn projects to the medial septal complex and supramammillary nucleus (Risold & Swanson, 1996, 1997) — two structures that generate and control the hippocampal theta rhythm activated during voluntary locomotion (Kocsis & Vertes, 1997; Stewart & Fox, 1990). Furthermore, the dorsal subiculum and lateral band of the lateral and medial entorhinal cortex send massive projections to the rostrolateral part of the nucleus accumbens and rostral caudoputamen (Groenewegen et al., 1996; Naber & Witter, 1998; Swanson & Kohler, 1986), both of which send descending projections either directly, or indirectly via the substantia innominata (ventral pallidum) or globus pallidus (dorsal pallidum), to innervate the ventral tegmental area and/or reticular part of the substantial nigra (SNr) (Groenewegen & Russchen, 1984; Groenewegen et al., 1996; Mogenson et al., 1983). The ventral tegmental area plays a critical role in locomotion (Swanson & Kalivas, 2000), while the SNr mediates in orienting movements of the eyes, head, neck and even upper limbs, via its massive projection to the deeper layers of the superior colliculus (Hikosaka & Wurtz, 1983; Werner et al., 1997). Accordingly, Swanson (2000) proposed that these structures, together with the immediately adjacent mammillary body in the caudal hypothalamus, compose a “caudal behavior control column” underlying expression of exploratory or foraging behavior. Together each of these three structures are involved in three essential aspects of exploration: locomotion (the ventral tegmental area), orientation of movements (SNr), and spatial direction (mammillary body).
In short, the dorsal hippocampal-subiculum complex forms a critical cortical network with the retrosplenial and anterior cingulate cortical areas that mediate cognitive process such as learning, memory, navigation, and exploration.
Neuronal connectivity of the ventral hippocampus
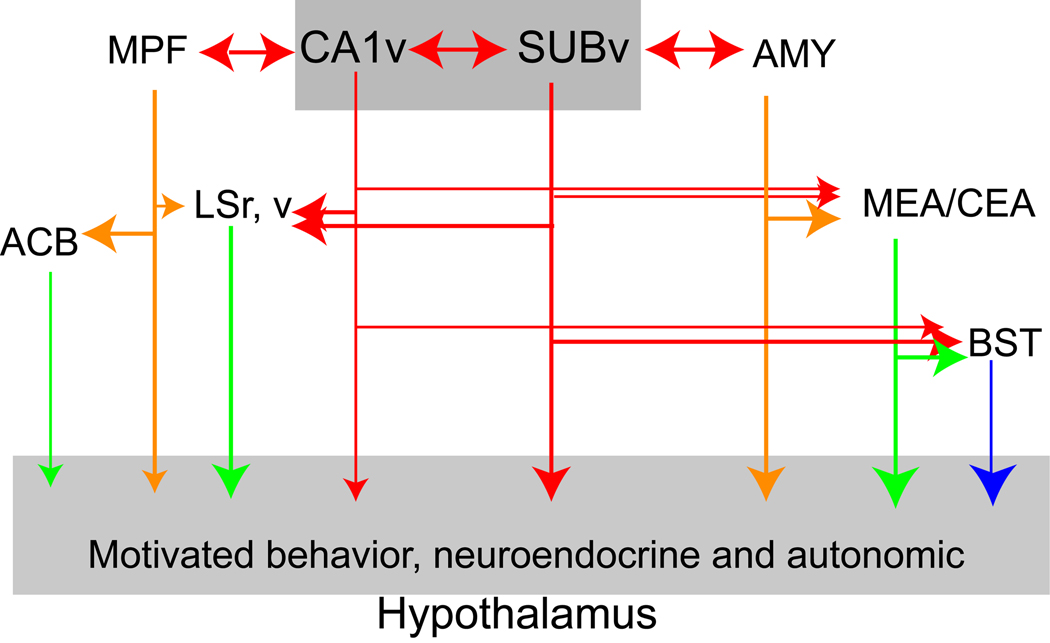
The first distinct connectivity of ventral CA1 from that of dorsal CA1 is in its direct projection to the olfactory bulb (with significantly denser terminals in the accessory olfactory bulb) and several other primary olfactory cortical areas, including the anterior olfactory nucleus, piriform cortex, and endopiriform nucleus in rats (Cenquizca & Swanson, 2007;) and monkeys (Roberts et al., 2007). Such projections may play a role in the depression-like symptoms that follow loss of the olfactory bulb that are reversed by antidepressants and cannot be attributed to a loss in olfaction (Song & Leonard, 2005; Wang et al., 2007). Next, the ventral CA1 and ventral subiculum share massive bi-directional connectivity with amygdalar nuclei that receive main and accessory olfactory sensory inputs, including the posterior amygdalar, posteromedial cortical amygdalar, posterior basomedial amygdalar nuclei, postpiriform transition area, and medial amygdalar nuclei (Cenquizca & Swanson, 2007; Kishi et al., 2000; Petrovich et al., 2001; Pitkanen et al., 2000; Saunders et al., 1988; Witter & Amaral, 2004). Additionally, the ventral CA1/subiculum and these amygdalar nuclei also share intimate bi-directional connectivity with the infralimbic, prelimbic and agranular insular cortices (Chiba, 2000; Hoover & Vertes, 2007; Jones & Wilson, 2005; Roberts et al., 2007; Thierry et al., 2000). Figure 4 shows that these ventral hippocampal/subicular-amygdalar-medial prefrontal cortical structures form a series of parallel, segregated descending projections, either directly or indirectly through the lateral septum (rostral and ventral parts), the medial and central amygdalar nuclei, and bed nuclei of the stria terminalis (BST), to innervate the periventricular and medial zones of the hypothalamus — the primary structure involved in the control of neuroendocrine, autonomic, and somatic motor activities associated with three basic classes of motivated behaviors having strong emotional components: ingestion (feeding and drinking), reproduction (sexual and parental), and defense (Dong et al., 2001a; Dong & Swanson, 2006; Herman et al., 2005; Kishi et al., 2000; Petrovich et al., 2001).
Fig. 4.
Schematic diagram to illustrate the major neuronal connectivity of the ventral hippocampus. Abbreviations: ACB, nucleus accumbens; AMY, cortical-like amygdalar areas (nuclei); BST, bed nuclei of the stria terminalis; CEA, central amygdalar nucleus; LSr, v, the rostral and ventral parts of the lateral septal nucleus; MEA, medial amygdalar nucleus; MPF, medial prefrontal cortex; SUBv, the ventral subiculum.
Two subsets of this ventral hippocampal network deserve more attention. First, the most ventral tips of the CA1 and subiculum (domain CA1vv in C57Bl/6 mice as defined here and domain 5 in rats of Swanson, 2004), as well as their immediately adjacent the posterior amygdalar nucleus, presumably form one unique cortical network in the medial temporal lobe specifically for controlling neuroendocrine activities, via their strong projections to the ventral part of the lateral septum (LSv) and anteromedial nuclei of the BST (Canteras et al., 1992; Dong et al., 2001a; Risold & Swanson, 1996), two cerebral nuclei that send massive projections to the hypothalamic neuroendocrine motor neuron pool (Dong et al., 2001b; Dong & Swanson, 2006; Risold & Swanson, 1996). Projections from the VH to the anteromedial group of the BST may be critical for understanding neuroendocrine dysfunctions associated with psychiatric disorders (such as depression, anxiety, and PTSD), because the latter is the only known cerebral structure that sends direct projections to innervate CRH neuroendocrine neurons in the hypothalamic paraventricular nucleus (PVH) (Cullinan et al., 1993; Dong et al., 2001b; Dong & Swanson, 2006). The BST is one critical relay station for the hippocampal regulation of the hypothalamic-pituitary-adrenal response to psychological stress (Cullinan et al., 1993; Choi et al., 2008; Choi et al., 2007) and plays an important role in anxiety (Walker et al., 2009).
Second, both the ventral CA1 and subiculum send direct projections to the central amygdalar nucleus, especially its capsular part (CEAc) (Cenquizca & Swanson, 2007; Kishi et al., 2006), which may have the potential to mediate the VH contribution to fear learning (Maren & Holt, 2004). The CEAc receives dense projections from the external-lateral part of the parabrachial nucleus, which is specifically involved in processing and relaying aversive sensory information and is necessary for taste aversion learning (Clark & Bernstein, 2009, Bernard et al., 1993; Tokita et al., 2007). Therefore, the connections between VH and CEAc may support the newly discovered role of the VH in long-delay taste aversion learning (Koh et al., 2009). It is important to recall that the ventral CA1 and subiculum also receive substantial inputs from the lateral amygdalar and basolateral amygdalar nuclei (Petrovich et al., 2001; Pitkanen et al., 2000), which, together with the central nucleus, are essential components of Pavlovian fear conditioning (Fanselow & Poulos, 2005; McGaugh, 2004; Rodrigues et al., 2009). These circuits provide a firm foundation for further investigation of the role of the hippocampus in expression of anxiety and other neuropsychiatric disorders (Herman et al., 2005; McEwen et al., 1997; Rodrigues et al., 2009).
It is worth noting that the ventral CA1, along with the ventral subiculum and medial band of the lateral and medial entorhinal cortical areas, also gives rise to direct projections to the caudomedial (shell) nucleus accumbens (but not the rostral and lateral parts) (Groenewegen et al., 1996; Naber & Witter, 1998), which plays a critical role in reward processing (Wassum et al., 2009) and motivation of feeding behavior (Kelley et al., 2005a, b). Finally, axonal terminals of the ventral CA1 and ventral subiculum overlap with the circadian-rhythm related inputs from the suprachiasmatic nucleus of the hypothalamic subparaventricular zone and dorsomedial hypothalamic nucleus (Cenquizca & Swanson, 2007; Kishi et al., 2000; Watts et al., 1987) — two brain structures recently shown to control the sleep-wake circadian circle (Saper et al., 2005). The latter two structures may provide a critical interface for the hippocampal inputs to influence general behavioral states and affect. For example, depression and sleep disturbances are highly co-morbid and are sensitive to similar pharmacological treatments (Holshoe, 2009; Pandi-Perumal et al., 2009).
In summary, the connectivity of the VH places it in an ideal situation to regulate the impact of emotional experiences and to control general affective states.
Neuronal connectivity of the intermediate hippocampus
The intermediate dentate gyrus and hippocampus proper receive input preferentially from the intermediate band of the lateral and medial entorhinal cortex, which receives widespread intermixed cortical inputs (Burwell, 2000). Two recent studies using the sensitive PHAL anterograde tracing method found that projections from the intermediate CA1 (Cenquizca & Swanson, 2007) and subiculum (Kishi et al., 2000) display distinctive extrinsic projection patterns. First, unlike that of the dorsal CA1, the intermediate CA1 does not send direct projections to the retrosplenial area; instead, it generates moderate to light direct projections to two primary olfactory cortical areas (the anterior olfactory nucleus and dorsal tenia tecta) and the infralimbic and prelimbic areas of the medial prefrontal cortex (Cenquizca & Swanson, 2007), all of which receive denser inputs from the ventral CA1 as reviewed above. On the other hand, unlike that of the ventral CA1v, the intermediate CA1 does not generate direct projections to the amygdala, BST, or hypothalamus (Cenquizca & Swanson, 2007). However, the intermediate part of the subiculum, which is heavily innervated by the intermediate CA1, sends substantial inputs to several amygdalar nuclei, including the lateral, basolateral (both anterior and posterior parts), and basomedial (both anterior and posterior) amygdalar nuclei (Kishi et al., 2006; Pitkanen et al., 2000). In turn, these amygdalar nuclei send substantial projections back to the intermediate part of the subiculum and, to a lesser degree, to the intermediate CA1 and CA3 (which corresponds to the ventral half of domain 1 of Swanson (2004; Petrovich et al., 2001; Pitkanen et al., 2000). Additionally, it appears that neuronal inputs from several amygdalar nuclei, especially the ventromedial region of the lateral, posterior basomedial, and posterior basolateral amygdalar nuclei, terminate heavily in the intermediate region of the lateral entorhinal cortex (Petrovich et al., 2001; Pitkanen et al., 2000), by which they subsequently reach the intermediate parts of the hippocampus proper and subiculum.
Similar to that of the dorsal subiculum, hypothalamic projections arising from the intermediate subiculum predominantly run through the postcommissural fornix pathway, but not the medial corticohypothalamic tract (Kishi et al., 2000). These projections generate a cluster of terminal fields specifically in the part of the perifornical region that lies between the fornix and the posterior part of the anterior hypothalamic and anterior part of the dorsomedial hypothalamic nuclei. However, this projection’s connectivity and functional significance are poorly understood. Additionally, the different parts of the intermediate subiculum also generate differential input to the anterior hypothalamic, supramammillary, and medial mammillary nuclei (Kishi et al., 2000). Alternatively, the intermediate CA1 (Swanson & Cowan, 1978) gives rise to two distinct terminal fields in the rostral and caudal parts of the lateral septum, which in turn sends dense projections to the anterior hypothalamic and supramammillary nucleus (Risold and Swanson, 1997). Nevertheless, the specific connectivity pattern of the intermediate hippocampus and subiculum remain to be further characterized. And very little is known about its specific functions.
Interactions between hippocampal zones
As reviewed above, the dorsal, intermediate, and ventral parts of the hippocampus display distinctive patterns of connectivity. However, it should also be recognized that these 3 areas are not completely isolated from each other. Instead, they can interact via several routes. The perirhinal and postrhinal cortical areas provide one potential interface for these interactions. These two cortical areas projecti to almost the entire entorhinal cortex, with its strongest inputs to the lateral (DH-projecting) band with substantially weaker inputs to the medial (VH-projecting) band, in addition to their direct projections to the dorsal CA1 and subiculum (Burwell, 2000; Shi & Cassell, 1999; Witter & Amaral, 2004). Interestingly, the ventral CA1, but not dorsal CA1, sends substantial projections to the perirhinal and postrhinal cortical areas (Cenquizca & Swanson, 2007). Information from the ventral CA1 can also reach the perirhinal and postrhinal cortical areas indirectly through the ventromedial portion of the lateral amygdala and posterior basomedial amygdalar nuclei. These two amygdalar nuclei share bi-directional connectivity with the ventral (but not dorsal) CA1 and subiculum (Burwell, 2000; Petrovich et al., 2001; Pitkanen et al., 2000). Apparently, the perirhinal and postrhinal cortical areas provide a critical interface for ongoing information from the VH to be dynamically integrated with complex multi-modal inputs from other cortical areas (e.g., visual/spatial and olfactory information), medial prefrontal cortex, and amygdalar nuclei, before it reaches the DH. This interaction may provide critical support for the ability of emotion to enhance memory consolidation in general (Malin & McGaugh, 2006). Additionally, perirhinal and postrhinal cortices are critical for long-term retention of contextual fear memories (Bucci et al., 2000; Burwell et al., 2004).
The rostral part of the reuniens nucleus of the midline thalamus may serve as another critical juncture for the VH network to affect the DH network, via several potential multi-synaptic cortico-subcortico-cortical loops. This thalamic nucleus receives massive inputs from all three components of hypothalamic defensive behavioral control network (anterior hypothalamic, dorsomedial part of the ventromedial hypothalamic, and dorsal premammillary nuclei), all of which are innervated by the ventral CA1 and subiculum (Risold & Swanson, 1996; Risold et al., 1997). In turn, the reuniens nucleus sends massive projections to the entire CA1 and subiculum, as well as to the entorhinal, perirhinal and postrhinal cortical areas (Risold et al., 1997; Vertes et al., 2007). Furthermore, the reunion thalamic nucleus serves to gate the flow of information from the medial prefrontal cortex to the hippocampus (Vertes et al., 2007). Thus, these long “feedback” projection pathways may dynamically coordinate and synchronize ongoing goal-orientated motivated behavior regulated by the VH network, with orientation/navigation/direction controlled by the DH network.
On the other hand, the DH network can also affect the VH. The most obvious route is through the dorsal zone’s projections to the medial septal complex and supramammillary nucleus, because both of these structures send widespread projections back to the entire hippocampus (Gaykema et al., 1991; Haglund et al., 1984; Vertes & Kocsis, 1997). In this way, the flow of information associated with navigation/direction can dynamically modulate DH output to the hypothalamic neuronal network controlling goal-oriented motivated behavior (such as fighting, mating, and feeding).
Conclusions
Differences in the connectivity of the dorsal and ventral portions of the hippocampus first lead anatomists to speculate that these two regions may serve different functions. The septal pole being better situated to communicate with brain regions associated with cognition and the temporal pole better situated to contribute to emotional reactions. Gradually, behavioral data has accumulated that is generally consistent with this segregation, although there were some exceptions. Recent detailed gene expression analysis unequivocally supports a segregation of all the major hippocampal subfields (CA1, CA3, and dentate gyrus) into dorsal, intermediate and ventral zones. Each of the 3 zones possesses very distinct neuronal connectivity patterns. The genetic data not only support the segregation suggested by the anatomical connection data and behavioral results, it much more clearly demarks these regions. By clarifying the boundaries, inconsistencies in the behavioral findings appear to dissolve. For example, rodent studies that suggested DH and VH support similar cognitive functions appear to have targeted what we call the intermediate rather than VH (Ferbinteanu et al., 2003; Rudy & Matus-Amat, 2005).
One issue confronting a functional segregation of the hippocampus is that the obvious similarities between DH and VH should not be overlooked. The intrinsic wiring throughout the longitudinal axis of the hippocampus still revolves around the tri-synaptic circuit, whose major characteristics are preserved in both dorsal and ventral zones. There are place fields throughout the hippocampus although the size of the fields increases dramatically as the hippocampus is traversed from the dorsal to ventral zones (Kjelstrup et al., 2008). If the DH and VH serves such different biological functions why is their circuitry so similar? We speculate that the topography of the circuitry reflects a common set of calculations. When Gray and McNaughton (2000) theorized about how the hippocampal formation processes emotion they suggested that the computations were based on a series of comparators that compared multiple goals and initiated corrective actions. These sorts of operations are exactly what need to occur for navigation; current position needs to be compared with current course and goal and then course adjustments must be made. It should also be noted that the place field size in the ventral pole of the rat’s hippocampus is so large (e.g., 10 m, Kjelstrup et al., 2008) that it may be better suited to conveying the emotional or motivational significance of a large area rather than navigation between two points.
Although the profound significance underlying the intimate correlation between gene expression patterns and the topography of neuronal connectivity in the CA1’s molecular domains remains to be determined, it is obvious that the DH and VH are genetically wired independently in a way that allows for different functional capabilities. It is clear that the DH is primarily involved in the cognitive process of learning and memory associated with navigation, exploration, and locomotion, whereas the ventral hippocampus is the part of the temporal lobe associated with motivational and emotional behavior. The nature of the intermediate zone suggests involvement in translating cognitive and spatial knowledge into motivation and action critical for survival (Bast, 2007; Bast et al., 2009). Researchers should probably approach these three zones as separate structures. But the genetic information is likely to do far more than help classify these regions. It should open doors to many new tools that will provide keys that further unlock the function of these regions.
Acknowledgements
Supported by National Institute of Mental Health grant number MH62122 to M.S.F. and MH083180 to H.W.D. We thank B. Knowlton for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG, Dolorfo C, Alvarez-Royo P. Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat. Hippocampus. 1991;1:415–435. doi: 10.1002/hipo.450010410. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. The hippocampus and Pavlovian fear conditioning: reply to Bast et al. Hippocampus. 2002;12:561–565. doi: 10.1002/hipo.10071. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113:1170–1188. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, O'Reilly RC, Rudy JW. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav Brain Res. 2002;134:299–306. doi: 10.1016/s0166-4328(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Bast T. Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev Neurosci. 2007;18:253–281. doi: 10.1515/revneuro.2007.18.3-4.253. [DOI] [PubMed] [Google Scholar]
- Bast T, Wilson IA, Witter MP, Morris RG. From rapid place learning to behavioral performance: a key role for the intermediate hippocampus. PLoS Biol. 2009;7:e1000089. doi: 10.1371/journal.pbio.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Bonne O, Vythilingam M, Inagaki M, Wood S, Neumeister A, Nugent AC, Snow J, Luckenbaugh DA, Bain EE, Drevets WC, Charney DS. Reduced posterior hippocampal volume in posttraumatic stress disorder. J Clin Psychiatry. 2008;69:1087–1091. doi: 10.4088/jcp.v69n0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Phillips RG, Burwell RD. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behav Neurosci. 2000;114:882–894. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Cajal SR. Significación probable de las células de axón corto. Trab Lab Investig Biol. 1901–1902;1:151–157. [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Connections of the posterior nucleus of the amygdala. J Comp Neurol. 1992;324:143–179. doi: 10.1002/cne.903240203. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J Comp Neurol. 2006;497:101–114. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T. Collateral projection from the amygdalo--hippocampal transition area and CA1 to the hypothalamus and medial prefrontal cortex in the rat. Neurosci Res. 2000;38:373–383. doi: 10.1016/s0168-0102(00)00183-8. [DOI] [PubMed] [Google Scholar]
- Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP. The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinol. 2008;149:818–826. doi: 10.1210/en.2007-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Amaral DG. Entorhinal cortex of the monkey: VII. intrinsic connections. J Comp Neurol. 2007;500:612–633. doi: 10.1002/cne.21200. [DOI] [PubMed] [Google Scholar]
- Clark EW, Bernstein IL. Establishing aversive, but not safe, taste memories requires lateralized pontine-cortical connections. Behav Brain Res. 2009;197:356–363. doi: 10.1016/j.bbr.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: organization of intrinsic connections. J Comp Neurol. 1998;398:49–82. doi: 10.1002/(sici)1096-9861(19980817)398:1<49::aid-cne4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Dong HW. The Allen Reference Atlas: A Digital Color Brain Atlas of the C57BL/6J Male Mouse. Hoboken: Wiley; 2008. [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001b;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006;494:142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW, Chen L, Fanselow MS, Toga AW. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0812608106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Associative vs. topographical accounts of the immediate shock freezing deficit in rats: Implications for the response selection rules governing species specific defensive reactions. Learn Motiv. 1986;17:16–39. [Google Scholar]
- Fanselow MS. Factors governing one trial contextual conditioning. An Learn Behav. 1990;18:264–270. [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, McDonald RJ. Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus. 2001;11:187–200. doi: 10.1002/hipo.1036. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Ray C, McDonald RJ. Both dorsal and ventral hippocampus contribute to spatial learning in Long-Evans rats. Neurosci Lett. 2003;345:131–135. doi: 10.1016/s0304-3940(03)00473-7. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Nery FG, Martins MR, Quevedo J, Soares JC, Kapczinski F. The role of hippocampus in the pathophysiology of bipolar disorder. Behav Pharmacol. 2007;18:419–430. doi: 10.1097/FBP.0b013e3282df3cde. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, van der Kuil J, Hersh LB, Luiten PG. Patterns of direct projections from the hippocampus to the medial septum-diagonal band complex: anterograde tracing with Phaseolus vulgaris leucoagglutinin combined with immunohistochemistry of choline acetyltransferase. Neuroscience. 1991;43:349–360. doi: 10.1016/0306-4522(91)90299-4. [DOI] [PubMed] [Google Scholar]
- Gloor P. The Temporal Lobe and Limbic System. New York: Oxford University Press; 1997. [Google Scholar]
- Gray J, Jeffrey A. World University Library. New York, NY, England: Mcgraw-Hill; 1971. The psychology of fear and stress. [Google Scholar]
- Gray J, McNaughton N. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. 2nd ed. Oxford: Oxford University Press; 2000. [Google Scholar]
- Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13:164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223:347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- Haglund L, Swanson LW, Kohler C. The projection of the supramammillary nucleus to the hippocampal formation: an immunohistochemical and anterograde transport study with the lectin PHA-L in the rat. J Comp Neurol. 1984;229:171–185. doi: 10.1002/cne.902290204. [DOI] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ. Impaired place navigation in place and matching-to-place swimming pool tasks follows both retrosplenial cortex lesions and cingulum bundle lesions in rats. Hippocampus. 2004;14:224–231. doi: 10.1002/hipo.10159. [DOI] [PubMed] [Google Scholar]
- Henke PG. Hippocampal pathway to the amygdala and stress ulcer development. Brain Res Bull. 1990;25:691–695. doi: 10.1016/0361-9230(90)90044-z. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 1983;49:1285–1301. doi: 10.1152/jn.1983.49.5.1285. [DOI] [PubMed] [Google Scholar]
- Holshoe JM. Antidepressants and sleep: a review. Perspect Psychiatr Care. 2009;45:191–197. doi: 10.1111/j.1744-6163.2009.00221.x. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol Learn Mem. 2008;89:61–69. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Herrero MT, Witter MP. Entorhinal cortex of the rat: cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus. 1997;7:146–183. doi: 10.1002/(SICI)1098-1063(1997)7:2<146::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Ishizuka N. Laminar organization of the pyramidal cell layer of the subiculum in the rat. J Comp Neurol. 2001;435:89–110. doi: 10.1002/cne.1195. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ. Integration of the sensory inputs to place cells: what, where, why, and how? Hippocampus. 2007;17:775–785. doi: 10.1002/hipo.20322. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005a;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005b;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Ono K, Yokota S, Ishino H, Yasui Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. J Comp Neurol. 2000;419:205–222. doi: 10.1002/(sici)1096-9861(20000403)419:2<205::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Yokota S, Yasui Y. Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. J Comp Neurol. 2006;496:349–368. doi: 10.1002/cne.20919. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Kloosterman F, Van Haeften T, Witter MP, Lopes Da Silva FH. Electrophysiological characterization of interlaminar entorhinal connections: an essential link for re-entrance in the hippocampal-entorhinal system. Eur J Neurosci. 2003;18:3037–3052. doi: 10.1111/j.1460-9568.2003.03046.x. [DOI] [PubMed] [Google Scholar]
- Klur S, Muller C, Pereira de Vasconcelos A, Ballard T, Lopez J, Galani R, Certa U, Cassel JC. Hippocampal-dependent spatial memory functions might be lateralized in rats: An approach combining gene expression profiling and reversible inactivation. Hippocampus. 2009;19:800–816. doi: 10.1002/hipo.20562. [DOI] [PubMed] [Google Scholar]
- Klüver H, Bucy PC. “Psychic blindness” and other symptoms following bilateral temporal lobectomy in Rhesus monkeys. Am J Physiol. 1937;119:352–353. [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. J Comp Neurol. 2007;502:810–833. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Vertes RP. Phase relations of rhythmic neuronal firing in the supramammillary nucleus and mammillary body to the hippocampal theta activity in urethane anesthetized rats. Hippocampus. 1997;7:204–214. doi: 10.1002/(SICI)1098-1063(1997)7:2<204::AID-HIPO7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Koh MT, Wheeler DS, Gallagher M. Hippocampal lesions interfere with long-trace taste aversion conditioning. Physiol Behav. 2009;98:103–107. doi: 10.1016/j.physbeh.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Summerfield JJ, Hassabis D, Maguire EA. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009;63:889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Lavenex PB, Amaral DG. Spatial relational learning persists following neonatal hippocampal lesions in macaque monkeys. Nat Neurosci. 2007;10:234–239. doi: 10.1038/nn1820. [DOI] [PubMed] [Google Scholar]
- Lavenex PB, Amaral DG, Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J Neurosci. 2006;26:4546–4558. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Ng L, Thompson C, Pathak S, Kuan L, Jones A, Hawrylycz M. Exploration and visualization of gene expression with neuroanatomy in the adult mouse brain. BMC Bioinformatics. 2008;9:153. doi: 10.1186/1471-2105-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de Nó R. Studies of the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol. 1934;46:113–177. [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Nannery R, Spiers HJ. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain. 2006;129:2894–2907. doi: 10.1093/brain/awl286. [DOI] [PubMed] [Google Scholar]
- Malin EL, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proc Natl Acad Sci U S A. 2006;103:1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav Neurosci. 2004;118:97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory reconsolidation hypothesis revived but restrained: theoretical comment on Biedenkapp and Rudy (2004) Behav Neurosci. 2004;118:1140–1142. doi: 10.1037/0735-7044.118.5.1140. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Conrad CD, Kuroda Y, Frankfurt M, Magarinos AM, McKittrick C. Prevention of stress-induced morphological and cognitive consequences. Eur Neuropsychopharmacol. 1997;7 Suppl 3:S323–S328. doi: 10.1016/s0924-977x(97)00064-3. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Swanson LW, Wu M. Neural projections from nucleus accumbens to globus pallidus, substantia innominata, and lateral preoptic-lateral hypothalamic area: an anatomical and electrophysiological investigation in the rat. J Neurosci. 1983;3:189–202. doi: 10.1523/JNEUROSCI.03-01-00189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Stead M, Pach J. The hippocampus as a cognitive graph. J Gen Physiol. 1996;107:663–694. doi: 10.1085/jgp.107.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber PA, Lopes da Silva FH, Witter MP. Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus. 2001;11:99–104. doi: 10.1002/hipo.1028. [DOI] [PubMed] [Google Scholar]
- Naber PA, Witter MP. Subicular efferents are organized mostly as parallel projections: a double-labeling, retrograde-tracing study in the rat. J Comp Neurol. 1998;393:284–297. [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Olton DS, Samuelson RJ. Remembrance of places passed: Spatial memory in rats. J Exper Psychol: An Behav Proc. 1976;2:97–116. [Google Scholar]
- Pandi-Perumal SR, Moscovitch A, Srinivasan V, Spence DW, Cardinali DP, Brown GM. Bidirectional communication between sleep and circadian rhythms and its implications for depression: lessons from agomelatine. Prog Neurobiol. 2009;88:264–271. doi: 10.1016/j.pneurobio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci U S A. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Zhang WN, Jongen-Relo AL, Feldon J, Yee BK. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within-subject, within-task comparison of reference and working spatial memory. Eur J Neurosci. 2004;19:705–712. doi: 10.1111/j.0953-816x.2004.03170.x. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Science. 1996;272:1484–1486. doi: 10.1126/science.272.5267.1484. [DOI] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Brain Res Rev. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Tomic DL, Parkinson CH, Roeling TA, Cutter DJ, Robbins TW, Everitt BJ. Forebrain connectivity of the prefrontal cortex in the marmoset monkey (Callithrix jacchus): an anterograde and retrograde tract-tracing study. J Comp Neurol. 2007;502:86–112. doi: 10.1002/cne.21300. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Kesner RP. Lesions of the dorsal hippocampus or parietal cortex differentially affect spatial information processing. Behav Neurosci. 2006;120:852–860. doi: 10.1037/0735-7044.120.4.852. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Matus-Amat P. The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behav Neurosci. 2005;119:154–163. doi: 10.1037/0735-7044.119.1.154. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Rosene DL, Van Hoesen GW. Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: II. Reciprocal and non-reciprocal connections. J Comp Neurol. 1988;271:185–207. doi: 10.1002/cne.902710203. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Perirhinal cortex projections to the amygdaloid complex and hippocampal formation in the rat. J Comp Neurol. 1999;406:299–328. doi: 10.1002/(sici)1096-9861(19990412)406:3<299::aid-cne2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Sokolov EN, Vinograda OS. Neuronal mechanisms of the orienting reflex. New Jersey: Erlbaum; 1975. [Google Scholar]
- Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage. 2006;31:1826–1840. doi: 10.1016/j.neuroimage.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Clark RE, Knowlton BJ. Retrograde amnesia. Hippocampus. 2001;11:50–55. doi: 10.1002/1098-1063(2001)11:1<50::AID-HIPO1019>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Steffenach HA, Witter M, Moser MB, Moser EI. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005;45:301–313. doi: 10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Stewart M, Fox SE. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci. 1990;13:163–168. doi: 10.1016/0166-2236(90)90040-h. [DOI] [PubMed] [Google Scholar]
- Stote DL, Fanselow MS. NMDA receptor modulation of incidental learning in Pavlovian context conditioning. Behavioral Neuroscience. 2004;118:253–257. doi: 10.1037/0735-7044.118.1.253. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Cortical inputs to the CA1 field of the monkey hippocampus originate from the perirhinal and parahippocampal cortex but not from area TE. Neurosci Lett. 1990;115:43–48. doi: 10.1016/0304-3940(90)90515-b. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Kalivas PW. Regulation of locomotor activity by metabotropic glutamate receptors in the nucleus accumbens and ventral tegmental area. J Pharmacol Exp Ther. 2000;292:406–414. [PubMed] [Google Scholar]
- Swanson LW. Brain Maps III: Structure of the Rat Brain. Third ed. Amsterdam: Elsevier; 2004. [Google Scholar]
- Swanson LW, Kohler C, Bjorklund A. The limbic region. I: The septohippocampal system. Vol. 5. Elsevier; 1987. [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. Hippocampo-hypothalamic connections: origin in subicular cortex, not ammon's horn. Science. 1975;189:303–304. doi: 10.1126/science.49928. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:681–715. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kohler C. Anatomical evidence for direct projections from the entorhinal area to the entire cortical mantle in the rat. J Neurosci. 1986;6:3010–3023. doi: 10.1523/JNEUROSCI.06-10-03010.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:681–715. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Nojyo Y. Preservation of topography in the connections between the subiculum, field CA1, and the entorhinal cortex in rats. J Comp Neurol. 1995;353:379–390. doi: 10.1002/cne.903530306. [DOI] [PubMed] [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Degenetais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Pathak SD, Jeromin A, Ng LL, MacPherson CR, Mortrud MT, Cusick A, Riley ZL, Sunkin SM, Bernard A, Puchalski RB, Gage FH, Jones AR, Bajic VB, Hawrylycz MJ, Lein ES. Genomic anatomy of the hippocampus. Neuron. 2008;26:1010–1021. doi: 10.1016/j.neuron.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Tokita K, Shimura T, Nakamura S, Inoue T, Yamamoto T. Involvement of forebrain in parabrachial neuronal activation induced by aversively conditioned taste stimuli in the rat. Brain Res. 2007;1141:188–196. doi: 10.1016/j.brainres.2007.01.023. [DOI] [PubMed] [Google Scholar]
- van Groen T, van Haren FJ, Witter MP, Groenewegen HJ. The organization of the reciprocal connections between the subiculum and the entorhinal cortex in the cat: I. A neuroanatomical tracing study. J Comp Neurol. 1986;250:485–497. doi: 10.1002/cne.902500407. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. The connections of presubiculum and parasubiculum in the rat. Brain Res. 1990;518:227–243. doi: 10.1016/0006-8993(90)90976-i. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol. 2003;463:249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Miller MW. Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J Comp Neurol. 1983;216:192–210. doi: 10.1002/cne.902160207. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009 doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Noda Y, Tsunekawa H, Zhou Y, Miyazaki M, Senzaki K, Nabeshima T. Behavioural and neurochemical features of olfactory bulbectomized rats resembling depression with comorbid anxiety. Behav Brain Res. 2007;178:262–273. doi: 10.1016/j.bbr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT, Balleine BW. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci U S A. 2009;106:12512–12517. doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- Werner W, Hoffmann KP, Dannenberg S. Anatomical distribution of arm-movement-related neurons in the primate superior colliculus and underlying reticular formation in comparison with visual and saccadic cells. Exp Brain Res. 1997;115:206–216. doi: 10.1007/pl00005691. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J Comp Neurol. 1991;307:437–459. doi: 10.1002/cne.903070308. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Hippocampal Formation. In: Paxinos G, editor. The Rat Nervous System. Amsterdam: Elsevier; 2004. pp. 635–704. [Google Scholar]
- Witter MP, Groenewegen HJ. The subiculum: cytoarchitectonically a simple structure, but hodologically complex. Prog Brain Res. 1990;83:47–58. doi: 10.1016/s0079-6123(08)61240-6. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ. Laminar origin and septotemporal distribution of entorhinal and perirhinal projections to the hippocampus in the cat. J Comp Neurol. 1984;224:371–385. doi: 10.1002/cne.902240305. [DOI] [PubMed] [Google Scholar]
- Witter MP, Van Hoesen GW, Amaral DG. Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci. 1989;9:216–228. doi: 10.1523/JNEUROSCI.09-01-00216.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss JM, Van Groen T. Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus. 1992;2:1–11. doi: 10.1002/hipo.450020102. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav Brain Res. 2001;126:159–174. doi: 10.1016/s0166-4328(01)00256-x. [DOI] [PubMed] [Google Scholar]