Summary
During development of the central nervous system, precise synaptic connections between pre- and postsynaptic neurons are formed. While significant progress has been made in our understanding of AMPA receptor trafficking during synaptic plasticity, less is known about the molecules that recruit AMPA receptors to nascent synapses during synaptogenesis. Here we identify a type II transmembrane protein (SynDIG1) that regulates AMPA receptor content at developing synapses in dissociated rat hippocampal neurons. SynDIG1 colocalizes with AMPA receptors at synapses and at extra-synaptic sites and associates with AMPA receptors in heterologous cells and brain. Altered levels of SynDIG1 in cultured neurons result in striking changes in excitatory synapse number and function. SynDIG1-mediated synapse development is dependent on association with AMPA receptors via its extracellular C-terminus. Intriguingly, SynDIG1 content in dendritic spines is regulated by neuronal activity. Altogether, we define SynDIG1 as an activity-regulated transmembrane protein that regulates excitatory synapse development.
Introduction
During development, excitatory synapse formation is directed by signaling between pre- and postsynaptic neurons and the expression of specific genes at the right time and place. Several classes of synaptogenic molecules serve as inductive signals that trigger the establishment of pre- and postsynaptic specializations [see (Dalva et al., 2007; McAllister, 2007; Scheiffele, 2003; Waites et al., 2005) for review]. Synaptic activity then directs whether synapses will be stabilized, eliminated or strengthened. Early events in synapse development include clustering of synaptic vesicles (SVs) to the presynaptic active zone, and N-methyl-D-aspartate (NMDA) receptors to the postsynaptic density (PSD) while later events include clustering of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors that might function to stabilize nascent synapses and mediate synaptic plasticity.
A number of studies were undertaken to dissect mechanisms of AMPA receptor trafficking during synaptic plasticity [reviewed in (Barry and Ziff, 2002; Bredt and Nicoll, 2003; Chen et al., 2006; Malenka, 2003; Nicoll et al., 2006; Sheng and Hyoung Lee, 2003)]; however, it is unclear if similar or distinct mechanisms underlie AMPA receptor targeting during the initial stages of synapse development. Known molecules that promote AMPA receptor clustering include NARP (O’Brien et al., 2002; O’Brien et al., 1998), EphB2 (Kayser et al., 2006), and SALM2 (Ko et al., 2006); however, the significance of these molecules in the targeting of AMPA receptors at developing synapses is not completely understood. For example, SALM2 overexpression did not change AMPA receptor or NMDA receptor synapse density even though direct aggregation of SALM2 can induce clustering of AMPA receptor and NMDA receptor subunits (Ko et al., 2006) while NARP’s clustering activity is restricted to glutamatergic synapses forming on inhibitory interneurons (Mi et al., 2002), suggesting that other molecules must exist to direct AMPA receptor recruitment to the majority of synapses during development.
A current topic of intense investigation is the identification of auxiliary subunits for AMPA receptors that influence excitatory synapse function. The transmembrane AMPA receptor regulatory proteins (TARPs) control both AMPA receptor trafficking and channel gating properties [reviewed in (Nicoll et al., 2006)]. TARPs function to facilitate AMPA receptor trafficking by a two-step process. First, TARPs mediate translocation from intracellular sites to the cell surface via direct interaction with AMPA receptor subunits. Second, TARPs subsequently deliver AMPA receptors to synapses via interaction with synaptic scaffolds such as PSD95 (Chen et al., 2000). In addition, a recent study identified the cornichon family of small transmembrane proteins as auxiliary subunits for AMPA receptors with similar activities as TARPs (Schwenk et al., 2009); however, the precise role played by cornichons in AMPA receptor-mediated synaptic transmission has yet to be reported.
To identify genes implicated in synapse development, a DNA microarray approach was applied to expression profile the cerebellum in wild type and mutant mouse lines with defects in neuronal differentiation (Diaz et al., 2002). One of the most highly differentially expressed genes [referred to here as Synapse Differentiation Induced Gene 1 (SynDIG1)] encodes a predicted transmembrane protein. In wild type cerebellum, SynDIG1 mRNA is upregulated during postnatal development; in contrast, SynDIG1 upregulation is defective in Lurcher (Lc) cerebellum (Diaz et al., 2002). In Lc mice, there is massive Purkinje cell death beginning at postnatal day 12 (P12) due to a point mutation in the δ2 glutamate receptor (Zuo et al., 1997), which is selectively expressed in cerebellar Purkinje neurons (Araki et al., 1993). However, at P10, prior to Purkinje cell death in Lc cerebellum, the rate of parallel fiber-Purkinje neuron synaptogenesis is decreased and synaptic ultrastructure is defective (Dumesnil-Bousez and Sotelo, 1992), suggesting that impaired synaptic maturation is provoked by the Lc mutation. SynDIG1 expression is reduced in Lc cerebellum prior to Purkinje cell death as determined by the difference in SynDIG1’s expression profile compared with Purkinje cell markers L7 and parvalbumin (Diaz et al., 2002), suggesting that SynDIG1 plays a role in synaptic differentiation of Purkinje neurons and potentially other neurons in which it is expressed.
Here we report evidence supporting a critical role for SynDIG1 in excitatory synapse development in dissociated rat hippocampal neurons. Specifically, SynDIG1 regulates AMPA receptor content at nascent synapses. SynDIG1 colocalizes with AMPA receptors at synapses and extra-synaptic sites and interacts with AMPA receptors in heterologous cells and brain extracts. Altered levels of SynDIG1 in cultured neurons result in significant changes in number and size of AMPA receptor containing synapses. Intriguingly, SynDIG1 content at synapses is regulated by neuronal activity, suggesting a role for SynDIG1 in activity-dependent synapse development and possibly synaptic plasticity. Thus, SynDIG1 represents an activity-regulated AMPA receptor interacting transmembrane protein that regulates development of excitatory synapses.
Results
SynDIG1 encodes a highly conserved transmembrane protein
The SynDIG1 cDNA sequence [originally identified as ‘Riken ZX00026N07’ in (Diaz et al., 2002)] is predicted to encode a protein with a calculated molecular mass of 28.5 kDa. The protein is not predicted to have any known domains besides two hydrophobic segments long enough to span the membrane (Figure 1A). SynDIG1 is highly conserved among vertebrates (Figure 1B). Sequence similarity to SynDIG1 was observed for three genes in the mouse genome with the highest degree of identity within the second half of the protein, including the two hydrophobic segments (Figure S1). The only related protein in mouse that has been characterized is referred to as “capucin” to reflect its predominant expression in caudate and putamen of the dorsolateral striatum (de Chaldee et al., 2006).
Figure 1. SynDIG1 encodes a conserved transmembrane protein.
(A) Hydropathy plot (Kyte-Doolittle, 13 amino acid window) of mouse SynDIG1 protein. Horizontal bars denote regions long enough to span the membrane.
(B) Alignment of amino acid sequences for SynDIG1 from different organisms. Abbreviations and Genbank accession numbers: Hs, Homo sapiens (NP_079169); Mm, Mus musculus (NP_001078990); Rn, Rattus norvegicus (NP_001020191); Gg, Gallus gallus (XP_415014); Dr, Danio rerio (XP_001332100). Identical amino acids are shaded in black, homologous amino acids are shaded in dark gray (high homology) or light gray (low homology). Asterisks mark the hydrophobic segments.
(C) SynDIG1 is a transmembrane protein. Extracts from HEK293 cells transfected with HA-SynDIG1 were probed with anti-HA antibody. SynDIG1 protein is enriched in membranes (lane 2) compared to cytosol (lane 1). SynDIG1 is not extracted from membranes by either high salt (lane 4, pellet; lane 7, extracted material) or high pH buffers (lane 5, pellet; lane 8, extracted material) and partially extracted by detergent containing buffer (lane 3, pellet; lane 6, extracted material). Molecular mass markers (kDa) are indicated.
See also Figures S1, S2.
When expressed in HEK293 cells, SynDIG1 is associated with the membrane fraction (Figure 1C, compare lanes 1–2). To test if SynDIG1 is an integral membrane protein, its extractability from membranes with high pH, high salt concentration or detergent containing buffers was determined. SynDIG1 protein is extracted from membranes only with detergent containing buffer (Figure 1C, compare lane 6 with lanes 7–8), confirming that SynDIG1 is an integral membrane protein. The absence of a signal sequence predicts that the N-terminus of SynDIG1 is intracellular. To test this possibility, COS cells were transfected with SynDIG1 constructs with an HA tag at the N-terminus (HA-SynDIG1) or the C-terminus (SynDIG1-HA) and live-labeled with anti-HA antibodies. As expected, anti-HA antibodies did not detect HA-SynDIG1 exposure to the extracellular environment (Figure S2A). In contrast, anti-HA antibodies detected SynDIG1-HA exposure to the extracellular environment (Figure S2A), suggesting that the C-terminal region of SynDIG1 is present at the outer surface of the plasma membrane. This result suggests that one hydrophobic segment spans the lipid bilayer of the plasma membrane while the other segment does not. To determine more precisely the topology of SynDIG1 protein, an additional construct was generated with three sequential HA-tags (to promote antigen recognition) between the two hydrophobic segments (SynDIG1-loop-HA). Live-labeling with anti-HA antibodies revealed exposure of SynDIG1-loop-HA to the extracellular environment (Figure S2A). All constructs were expressed efficiently in COS cells (Figure S2B). The topology of SynDIG1 protein is consistent with a type II transmembrane protein whereby the first hydrophobic segment spans the plasma membrane while the second hydrophobic segment is embedded in the outer region of the plasma membrane (Figure S2C). Interestingly, HA-SynDIG1 forms dimers resistant to SDS-PAGE and formation of dimers requires SynDIG1’s C-terminal extracellular hydrophobic segment (Figure S2D). Thus, an alternative possibility is that the second hydrophobic segment might be shielded from the hydrophilic environment upon SynDIG1 dimerization.
Capucin localizes to the Golgi compartment in heterologous cells (de Chaldee et al., 2006). To determine if SynDIG1 also localizes to Golgi structures, the distribution of HA-SynDIG1 was analyzed in COS cells with immunocytochemistry. In addition to the cell surface (Figure S2A), SynDIG1 is also present in intracellular structures distributed throughout the cytoplasm (Figure S2E). These structures did not overlap extensively with the Golgi marker protein GM130 (Figure S2E), suggesting that in contrast to Capucin, SynDIG1 is not localized to Golgi complexes beyond its normal trafficking through the secretory pathway expected of an integral membrane protein. Furthermore, treatment of cells with Brefeldin A (BFA) to disrupt reversibly Golgi complexes (Ulmer and Palade, 1991) confirmed that SynDIG1 is not a Golgi resident protein (Figure S2E). Rather, the intracellular structures to which SynDIG1 localizes are early endosomes as determined by immunocychemistry with antibodies against the early-endosomal autoantigen EEA1 (Figure S2F), suggesting that SynDIG1 shuttles between the cell surface and early endosomes in heterologous cells.
SynDIG1 expression is spatially and developmentally regulated
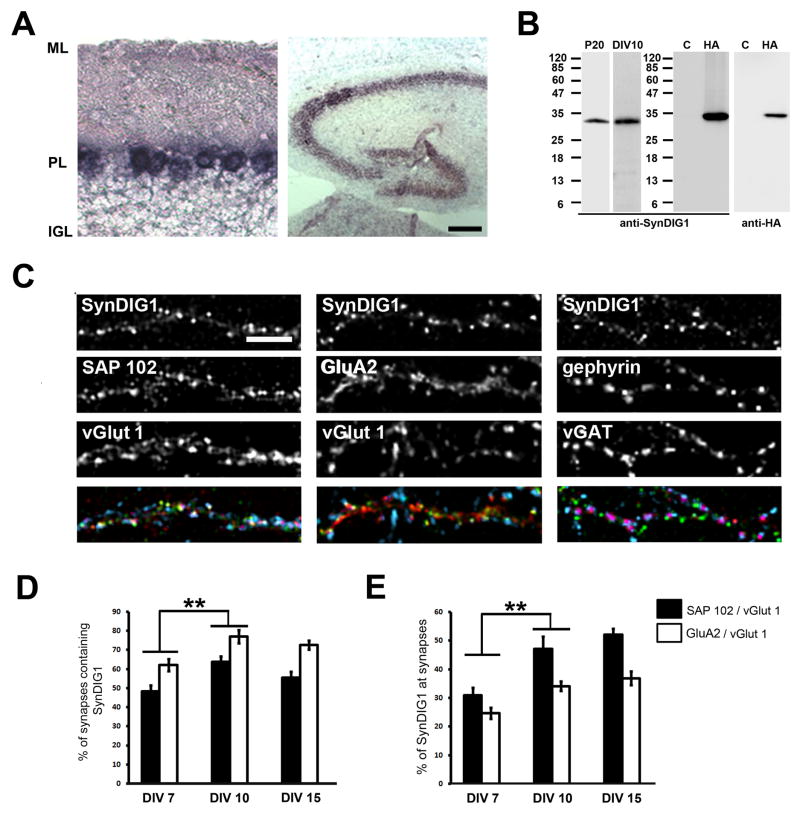
Analysis of the UniGene EST Profile Viewer database suggested that SynDIG1 mRNA is largely restricted to neural tissues. To examine the distribution of SynDIG1 mRNA in finer detail, in situ hybridization with digoxigenin-labeled riboprobes was performed with mouse brain sections. As expected, SynDIG1 mRNA is expressed in Purkinje neurons in cerebellum (Figure 2A). SynDIG1 is also detected in the hippocampus (Figure 2A).
Figure 2. SynDIG1 clusters localize to excitatory synapses.
(A) SynDIG1 mRNA distribution in postnatal hippocampus and cerebellum. Sagittal sections of mouse hippocampus and cerebellum at postnatal day 20 (P20) were hybridized in situ with digoxygenin-labeled antisense probe for SynDIG1. Abbreviations: ML, molecular layer; PL, Purkinje cell layer; IGL, internal granule layer. Scale bar, 25 μm.
(B) Specificity of anti-SynDIG1 monoclonal antibody (mAb). Left panels depict an immunoblot of protein extracts prepared from P20 brain or dissociated rat hippocampal neurons at 10 days in vitro (DIV) probed with anti-SynDIG1 mAb. Right panels depict an immunoblot of extracts from HEK293 cells transfected with HA-SynDIG1 (HA) or vector as control (C) probed with anti-SynDIG1 mAb (left) or anti-HA antibodies (right). The increased size of HA-SynDIG1 immunoreactivity relative to endogenous SynDIG1 is likely due to the additional mass contributed by the HA tag. Molecular mass markers (kDa) are indicated.
(C–E) SynDIG1 clusters are enriched at excitatory synapses. Immunostaining for SynDIG1 (green in merge), postsynaptic proteins SAP102 (left panel, red in merge), GluA2 (middle panel, red in merge) or gephyrin (right panel, red in merge), and presynaptic proteins vGlut1 (left and middle panels, blue in merge) or vGAT (right panel, blue in merge) in dissociated rat hippocampal neurons at 15 DIV. Scale bar, 5 μm. Graphs depict fraction of synapses [defined by overlap of vGlut1/SAP102 puncta (black bars) or vGlut1/GluA2 puncta (white bars)] that contain SynDIG1 (D) or the fraction of SynDIG1 puncta at synapses (E) in dissociated rat hippocampal neurons at 7, 10 and 15 DIV. vGlut1/SAP102 synapses: DIV 7, n = 16 cells, 50–200 synapses per cell; DIV 10, n = 12 cells, 300–700 synapses per cell; DIV 15, n = 13 cells, 500–1200 synapses per cell; vGlut1/GluA2 synapses: DIV 7, n = 24 cells, 50–200 synapses per cell; DIV 10, n = 12 cells, 300–700 synapses per cell; DIV 15, n = 14 cells, 500–1200 synapses per cell. Significance, p-value < 0.005 (**); Error bars, standard error of the mean (SEM).
See also Figures S3, S4A–C.
To characterize SynDIG1 distribution in neurons, a monoclonal antibody (mAb) was raised against the N-terminal region of the molecule. To demonstrate specificity of the mAb, immunoblot analysis of extracts from HEK293 cells transfected with HA-SynDIG1 compared with vector-transfected cells was performed (Figure 2B). Both anti-SynDIG1 mAb and anti-HA antibodies recognized a single immunoreactive band at ~32 kDa (Figure 2B), consistent with the calculated molecular mass of HA-SynDIG1. A single anti-SynDIG1 immunoreactive band of slightly lower molecular mass was also recognized in mouse brain extracts and dissociated rat hippocampal neurons (Figure 2B). COS cells transfected with HA-SynDIG1 revealed identical immunostaining patterns for anti-HA antibodies and anti-SynDIG1 mAb (Figure S3A). To begin to identify the epitope recognized by anti-SynDIG1 mAb, two HA-SynDIG1 deletion constructs were generated. Deletion of 33 amino acids of the C-terminus including the second hydrophobic domain (HA-SynDIG1ΔC33) had no effect on anti-SynDIG1 mAb recognition while deletion of 75 amino acids of the N-terminus (HA-SynDIG1ΔN75) resulted in complete loss of anti-SynDIG1 mAb immunoreactivity (Figure S3B). Importantly, anti-HA immunoreactivity was similar for all constructs (Figure S3B), demonstrating the presence of HA-tagged protein. SynDIG1 protein expression peaks during the second week of postnatal development (Figure S3C), the major period of synaptogenesis in rodents. Furthermore, SynDIG1 expression is restricted to brain (Figure S3D), consistent with the distribution of SynDIG1 mRNA in UniGene Database.
SynDIG1 clusters colocalize with AMPA receptors
Taking advantage of SynDIG1’s expression in hippocampus (Figure 2A), expression in dissociated rat hippocampal neurons was examined with anti-SynDIG1 mAb and anti-MAP2 antibodies. To determine the subcellular localization of SynDIG1, SynDIG1 expression in dissociated rat hippocampal neurons at different culture ages was examined by immunostaining with anti-SynDIG1 mAb and anti-MAP2 antibodies. In young cultures (2 DIV), SynDIG1 immunoreactivity was detected in the cell body and in neurites in a diffuse and punctate staining pattern (Figure S4A, top panels). At 8 DIV (Figure S4A, middle panels), SynDIG1 immunoreactivity continued to be apparent in the cell body and in dendrites as demonstrated by co-immunostaining with anti-MAP2 antibodies. In mature dendrites there seemed to be two types of immunoreactivity that developed over time (Figure S4A, bottom panels): 1) diffuse and punctate staining along the shafts of dendrites, especially apparent in thick primary dendrites; and 2) staining in protrusions along the dendrites. SynDIG1 clusters are enriched at excitatory synapses as defined by overlap with postsynaptic (SAP102) and presynaptic (vGlut1) markers compared with inhibitory synapses (Figure 2C). At 7 DIV, 48% of synapses (defined as overlap of vGlut1 and SAP102 clusters) contain SynDIG1 (Figure 2D). At 10 and 15 DIV, 64% and 56% of synapses, respectively, contain SynDIG1 (Figure 2D). The amount of SynDIG1 at synapses represents 31%, 47%, and 52% of total SynDIG1 puncta at 7, 10, and 15 DIV, respectively (Figure 2E), suggesting that as development proceeds, an increasing percentage of SynDIG1 becomes localized to excitatory synapses. To determine if SynDIG1 is present at the cell surface of excitatory synapses, neurons were transfected with SynDIG1-HA, live-labeled with anti-HA antibodies to stain surface HA epitopes, and fixed and stained with anti-PSD95 and anti-vGlut1 antibodies to label presynaptic specializations. A subset of SynDIG1 clusters overlap with colocalized pre- and postsynaptic clusters (Figure S4B), suggesting that SynDIG1 is present at the cell surface of excitatory synapses. Furthermore, a portion of SynDIG1 protein was enriched in PSD fractions from mouse brain (Figure S4C). Thus, SynDIG1 protein is localized to the postsynaptic cell in excitatory synapses both in dissociated rat hippocampal neurons and in mouse brain.
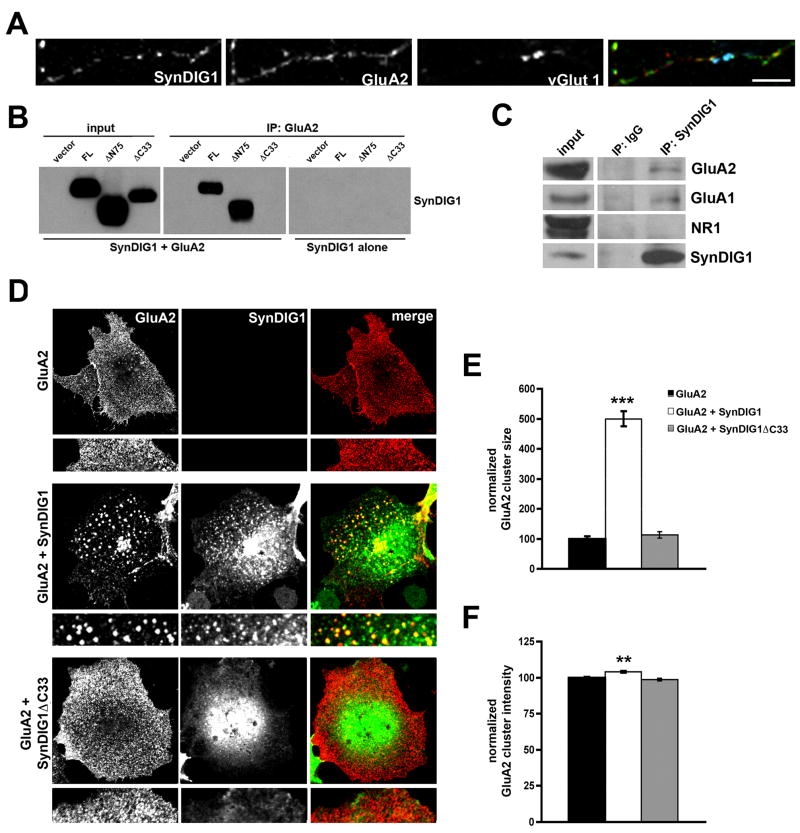
Next, the distribution of SynDIG1 and the AMPA receptor subunit GluA2 (Collingridge et al., 2008) at synapses was analyzed (defined as overlap of SynDIG1, GluA2, and vGlut1 puncta, Figure 2C). At 7 DIV, SynDIG1 is present at 62% of GluA2 containing synapses (Figure 2D). At 10 and 15 DIV, 77% and 73% of GluA2 positive synapses, respectively, contain SynDIG1 (Figure 2D). The amount of SynDIG1 at GluA2 containing synapses represents 25%, 34%, and 37% of total SynDIG1 puncta at 7, 10, and 15 DIV, respectively (Figure 2E). Thus, while a significant fraction of GluA2 positive synapses contain SynDIG1, a relatively smaller fraction of total SynDIG1 puncta overlap with GluA2 at synapses, suggesting that the majority of SynDIG1 clusters are found at non-synaptic sites. To test this possibility, young neurons (6 DIV) with low synapse density were examined (Figure 3A). Indeed, while 30% of GluA2 puncta and 25% of SynDIG1 puncta were found at synapses (defined as overlap of SynDIG1, GluA2, and vGlut1 puncta), a larger fraction of GluA2 (45%) and SynDIG1 (60%) overlapped at non-synaptic sites (defined as lack of overlap with vGlut1 puncta). Thus, the majority of SynDIG1 overlaps with GluA2 either at synapses or extra-synaptic sites, suggesting that SynDIG1 might associate with AMPA receptors.
Figure 3. SynDIG1 interacts with GluA2 in heterologous cells.
(A) SynDIG1 overlaps with GluA2 at synapses and non-synaptic sites. Immunostaining of SynDIG1 (green in merge), GluA2 (red in merge), and vGlut1 (blue in merge) in dissociated rat hippocampal neurons at 6 DIV. Scale bar, 5 μm.
(B) SynDIG1 and GluA2 interact in heterologous cells. COS cells were transfected with HA-SynDIG1, HA-SynDIG1ΔC33, or HA-SynDIG1ΔN75 and either HA-GluA2 or vector. Extracts were immunoprecipitated with anti-GluA2 antibodies and precipitates, along with input samples, were immunoblotted and probed with anti-HA antibodies to detect both HA-tagged constructs distinguished by their different electrophoretic mobility.
(C) SynDIG1 and AMPA receptors associate in brain. P14 mouse brain extracts were immunoprecipitated with anti-SynDIG1 antibodies and precipitates along with input samples were probed with anti-GluA1, anti-GluA2, anti-NR1 or anti-SynDIG1 antibodies.
(D–F) SynDIG1 promotes clustering of GluA2 in heterologous cells. COS cells were transfected with extracellular HA-tagged GluA2 in the presence or absence of intracellular HA-tagged full-length SynDIG1 or SynDIG1ΔC33. Cells were live-labeled with anti-HA antibodies to label surface GluA2 (C). After live-labeling, cells were fixed and stained with anti-SynDIG1 antibodies to asses distribution of SynDIG1. Graphs depict normalized surface-labeled GluA2 cluster area (E) or cluster fluorescence intensity (F) for cells transfected with HA-GluA2 alone (black bars), HA-GluA2 + HA- SynDIG1 (white bars), or HA-GluA2 + SynDIG1ΔC33 (gray bars). Data shown are the average of 10 cells per condition. Similar results were obtained in 3 independent experiments. Scale bar, 20 μm; Error bars, ± SEM. Significance, p-value < 0.001 (***).
See also Figure S4D.
SynDIG1 interacts with AMPA receptors
To test if SynDIG1 interacts with AMPA receptors, COS cells were transfected with HA-SynDIG1 alone or HA-SynDIG1 and HA-GluA2. Extracts were immunoprecipitated with anti-GluA2 antibodies and precipitates, along with input samples, were immunoblotted and probed with anti-HA antibodies to detect both HA-tagged constructs distinguished by their different electrophoretic mobility (Figure 3B). As expected, anti-GluA2 antibodies efficiently precipitate HA-GluA2 in extracts from COS cells expressing HA-GluA2 alone or coexpressing HA-GluA2 and HA-SynDIG1 (not shown). In addition, anti-GluA2 antibodies coprecipitated full-length HA-SynDIG1 or HA-SynDIG1ΔN75 (Figure 3B). In contrast, coimmunoprecipitation was not observed for HA-SynDIG1ΔC33 (Figure 3B). Input levels of all constructs were equivalent and anti-GluA2 antibodies failed to coprecipitate HA-SynDIG1 or HA-SynDIG1ΔN75 in the absence of HA-GluA2 (Figure 3B). Furthermore, anti-SynDIG1 antibodies coimmunoprecipitate GluA1 and GluA2 but not NR1 from mouse brain extracts (Figure 3C), suggesting that SynDIG1 associates with AMPA receptors in vivo.
To determine if SynDIG1 could alter HA-GluA2 distribution, COS cells transfected with HA-GluA2 and HA-SynDIG1, HA-SynDIG1ΔC33, or empty vector were live-labeled with anti-HA antibodies to examine surface GluA2 (Figure 3D). [Note: N-terminally HA-tagged SynDIG1 fails to yield any signal upon live-labeling with anti-HA antibodies (see Figure S2A)]. Subsequently, cells were fixed, permeabilized, and stained with anti-SynDIG1 mAb to assess distribution of SynDIG1 compared with GluA2 (Figure 3D). Full-length SynDIG1 changed the distribution of GluA2 such that the two proteins co-cluster (Figure 3D). Furthermore, surface-labeled HA-GluA2 clusters were increased upon coexpression of full-length SynDIG1 compared to control (Figure 3E). Mean GluA2 cluster intensity was also increased with full-length SynDIG1 compared with HA-GluA2 alone (Figure 3F). In contrast, coexpression of HA-SynDIG1ΔC33 failed to increase HA-GluA2 cluster size or intensity (Figure 3E–F), presumably due to its failure to interact with GluA2 (Figure 3B). Indeed, surface-labeled HA-GluA2 clusters (live-labeled with anti-HA antibodies) overlap exclusively with surface-labeled FLAG-tagged SynDIG1 (live-labeled with anti-FLAG antibodies; Figure S4D).
Reduced excitatory synapse development upon loss of SynDIG1
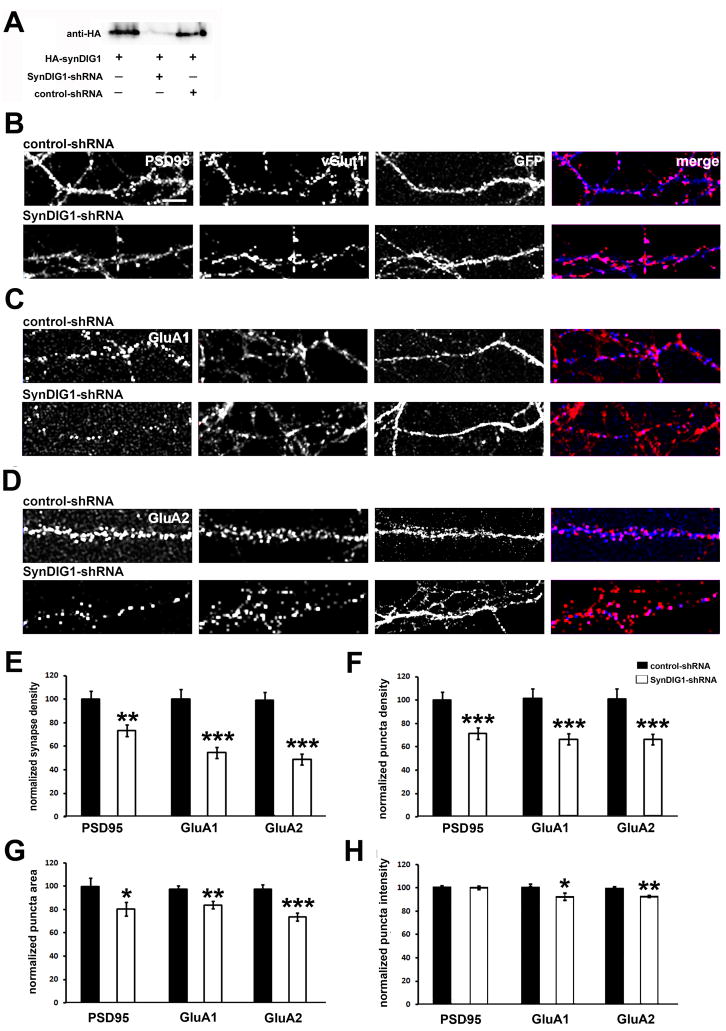
SynDIG1 association with GluA2 suggested that SynDIG1 might play an active role in synapse development. This possibility was tested with short hairpin RNA (shRNA)-mediated reduction of endogenous SynDIG1 in dissociated rat hippocampal neurons. Neurons were cotransfected by electroporation at the time of plating with EGFP (to identify transfected neurons) and an shRNA construct targeted against the SynDIG1 mRNA (SynDIG1-shRNA; see Experimental Procedures). SynDIG1-shRNA inhibited HA-SynDIG1 expression in HEK293 cells while a control-shRNA construct targeting the same region but containing an internal mismatch did not (Figure 4A). The knockdown efficiency of neurons transfected with SynDIG-shRNA compared with control-shRNA was analyzed (Figure S5A). The SynDIG1-shRNA construct decreased endogenous SynDIG1 protein by 70% in dissociated rat hippocampal neurons compared with control-shRNA (Figure S5B). Altering the levels of SynDIG1 in dissociated rat hippocampal neurons did not affect total neurite length (Figure S5C) or branching (Figure S5D) compared with control neurons.
Figure 4. Loss of SynDIG1 reduces excitatory synapse development.
(A) ShRNA-mediated reduction of HA-SynDIG1 expressed in heterologous cells. Extracts from HEK293 cells transfected with HA-SynDIG1 and either control-shRNA or SynDIG1-shRNA were blotted with anti-HA antibodies.
(B–D) Loss of SynDIG1 inhibits excitatory synapse development. Immunostaining of postsynaptic proteins (blue in merge) PSD95 (B), GluA1 (C), GluA2 (D) and presynaptic vGlut1 (red in merge) is shown. Dissociated rat hippocampal neurons were transfected by electroporation at the time of plating with EGFP (to identify transfected cells) and control-shRNA (top panels) or SynDIG1-shRNA (bottom panels) and analyzed at 8–10 DIV. Synapses are defined as overlap of pre- and postsynaptic clusters. For clarity, the green channel (EGFP) is not displayed in the merge images. Scale bar, 6.5 μm.
(E–H) Decreased SynDIG1 reduces excitatory synapse number. Graphs depict density of PSD95/vGlut1, GluA1/vGlut1, and GluA2/vGlut1 colocalized puncta (E), density of PSD95, surface-labeled GluA1, surface-labeled GluA2 puncta (F), area of PSD95, surface-labeled GluA1, and surface-labeled GluA2 puncta (G), and fluorescence intensity of PSD95, surface-labeled GluA1, surface-labeled GluA2 puncta (H) in dendrites of neurons transfected with EGFP and control-shRNA (black bars) or SynDIG1-shRNA (white bars). Normalized values relative to control-shRNA cells are shown for the average of 2–3 independent experiments. PSD95: control-shRNA, n = 46 cells, SynDIG1-shRNA, n = 50 cells; GluA1: control-shRNA, n = 90 cells, SynDIG1-shRNA, n = 82 cells; GluA2: control-shRNA, n = 58 cells, SynDIG1-shRNA, n = 61 cells. Error bars, ± SEM. Significance, p-value < 0.05 (*), < 0.005 (**), < 0.0001(***).
See also Figure S5.
Synapse development was examined by immunocytochemistry of 8–10 DIV neurons cotransfected at the time plating with EGFP (to identify transfected cells) and SynDIG1-shRNA or control-shRNA. To visualize synapses, cells were fixed and immunostained with antibodies against vGlut1 and PSD95, GluA1, or GluA2 (Figure 4B–D). Compared with control-shRNA transfected neurons, SynDIG1-shRNA resulted in a 27% decrease in synaptic PSD95 density (defined as overlap of PSD95 and vGlut1 puncta; Figure 4E) and a concomitant 29% decreased density of PSD95 puncta (Figure 4F). Mature AMPA receptor containing synapses were defined as the colocalization of surface-labeled GluA1 or GluA2 and vGlut1 puncta (Figure 4C–D). Neurons transfected with SynDIG1-shRNA exhibited 46% or 53% decreased density of surface-labeled GluA1 or GluA2 containing synapses, respectively, compared with control-shRNA (Figure 4E). Decreased GluA1 or GluA2 synapse density was also accompanied by a concomitant 35% decreased density of surface GluA1 or GluA2 puncta in SynDIG1-shRNA transfeced neurons (Figure 4F).
To determine if decreased SynDIG1 leads to a concomitant reduction in synapse size, the area and fluorescence intensity of GluA1 and GluA2 clusters were analyzed. A small but significant decrease in size of surface-labeled GluA1 and GluA2 clusters was observed in SynDIG1-shRNA transfected neurons compared with control-shRNA cells (Figure 4G). Similarly, the fluorescence intensity of surface-labeled GluA1 and GluA2 clusters was also significantly reduced (Figure 4H). Although loss of SynDIG1 led to a 20% decrease in area of PSD95 clusters (Figure 4G), no change in fluorescence intensity was observed (Figure 4H). These findings demonstrate that reduction of SynDIG1 leads to decreased number of mature synapses (as reflected in the decrease in GluA1 and GluA2 synapse density) as well as decreased size of mature synapses (as reflected in the decrease in GluA1 and GluA2 cluster area and intensity).
Averaging all experiments, the density of vGlut1 puncta in axons contacting SynDIG1-shRNA transfected neurons compared with control-shRNA transfected neurons was only slightly decreased (15%; p < 0.03). However, in contrast to PSD95, GluA1 and GluA2, this result was not reproduced in all individual experiments, supporting a primary role for SynDIG1 in postsynaptic development and maturation.
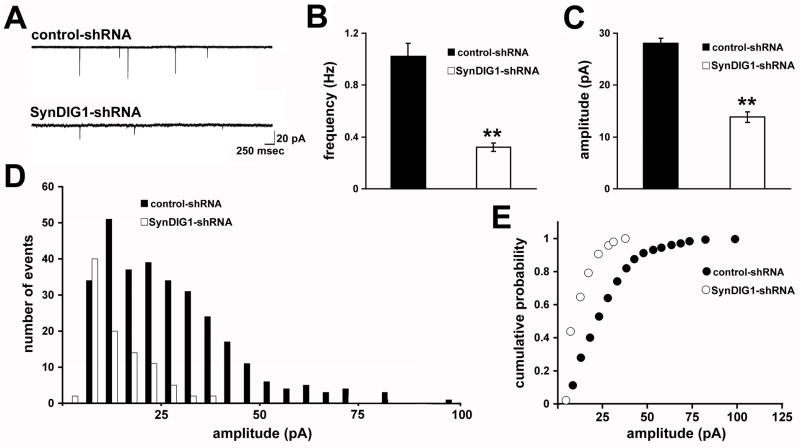
SynDIG1 regulates development of functional excitatory synapses
To assess the functional effect of decreased SynDIG1 on synapses, whole-cell patch-clamp recordings of miniature excitatory postsynaptic currents (mEPSCs) were analyzed. Neurons were cotransfected at the time of plating with EGFP (to identify transfected cells) and the shRNA constructs and mEPSCs were measured at 8 DIV (Figure 5A). Neurons transfected with SynDIG1-shRNA displayed 70% decreased mean mEPSC frequency (Figure 5B) and 50% decreased mean mEPSC amplitude (Figure 5C) compared with control cells. The histogram and cumulative probability distributions of mEPSC amplitudes were uniformly reduced upon decreased SynDIG1 compared with control neurons (Figure 5D–E), suggesting that SynDIG1 loss affects synapse development in a global manner.
Figure 5. Loss of SynDIG1 decreases functional excitatory synapses.
Miniature excitatory postsynaptic current (mEPSC) recordings from dissociated rat hippocampal neurons transfected with EGFP (to identify transfected cells) and either control-shRNA or SynDIG1-shRNA. Neurons were transfected at the time of plating with electroporation and mEPSCs were recorded at 8 DIV. Sample traces are shown in (A). Graphs depict mean frequency (B), mean amplitude (C), amplitude histogram (D), and amplitude cumulative probability plot (E) of mEPSC events from neurons transfected with control-shRNA (black bars) or SynDIG1-shRNA (white bars). Data are averaged from 2–3 independent experiments. Control-shRNA, n =10 cells; SynDIG1-shRNA, n = 10 cells.
Error bars, ± SEM. Significance, p-value < 0.005 (**).
See also Figure S6.
Because retraction of synapses and dendritic spines can be induced by off-target effects of a subset of shRNA sequences (Alvarez et al., 2006), three sets of control experiments were undertaken. First, the experiment in which SynDIG1 was knockdowned with shRNA was performed for a shorter time period. Neurons were cotransfected at 4 DIV with EGFP (to identify transfected cells) and the shRNA constructs and mEPSCs were measured at 8 DIV (Figure S6A). A similar reduction in mean frequency (Figure S6B) and mean amplitude (Figure S6C) of mEPSC events was observed in SynDIG1-shRNA transfected neurons compared with control-shRNA. The histogram and cumulative probability distributions of mEPSC amplitudes were also uniformly reduced upon decreased level of SynDIG1 for a shorter time period compared with control neurons (Figure S6D–E). Second, a rescue construct was generated based on the human SynDIG1 cDNA with three silent base pair changes in the region targeted by SynDIG1-shRNA. In contrast to mouse HA-SynDIG1, human HA-SynDIG1 is immune to SynDIG1-shRNA-mediated knockdown in heterologous cells (Figure S6F). Neurons were cotransfected at 4 DIV with EGFP (to identify transfected neurons) and control-shRNA or SynDIG1-shRNA in the presence or absence of human HA-SynDIG1 and analyzed by whole-cell patch-clamp to record mEPSCs (Figure S6A). Indeed, expression of human HA-SynDIG1 in dissociated rat hippocampal neurons rescues the SynDIG1-shRNA-mediated decrease in mean frequency (Figure S6B) and mean amplitude (Figure S6C) of mEPSCs compared with control-shRNA. Third, NMDA receptor mediated mEPSCs were recorded (Figure S6G) and no change in the NMDA receptor mediated mean mEPSC frequency (Figure S6H) or mean mEPSC amplitude (Figure S6I) was observed in SynDIG1-shRNA transfected neurons compared with control-shRNA. Taken together, these data demonstrate that the dramatic defects observed for excitatory synapse development with SynDIG1-shRNA are specifically due to the loss of SynDIG1 protein in dissociated rat hippocampal neurons and not due to off-target effects of SynDIG1-shRNA.
SynDIG1 overexpression increases excitatory synapse development
To gain insight into the mechanism of SynDIG1 function, the effect of HA-SynDIG1 overexpression on morphological synapses was examined with immunocytochemistry. Neurons were transfected at 4 DIV and examined at 8 DIV. Neurons were stained with anti-HA antibodies to identify transfected cells and with antibodies against vGlut1 and PSD95 to visualize excitatory synapses (Figure 6A). Compared with non-transfected neurons within the same experiment, HA-SynDIG1 overexpression caused a significant increase in synapse density (defined as overlap of PSD95 and vGlut1 puncta; Figure 6D). This effect was in part due to an increased density of PSD95 puncta in HA-SynDIG1 transfected neurons compared with untransfected neurons (Figure 6E).
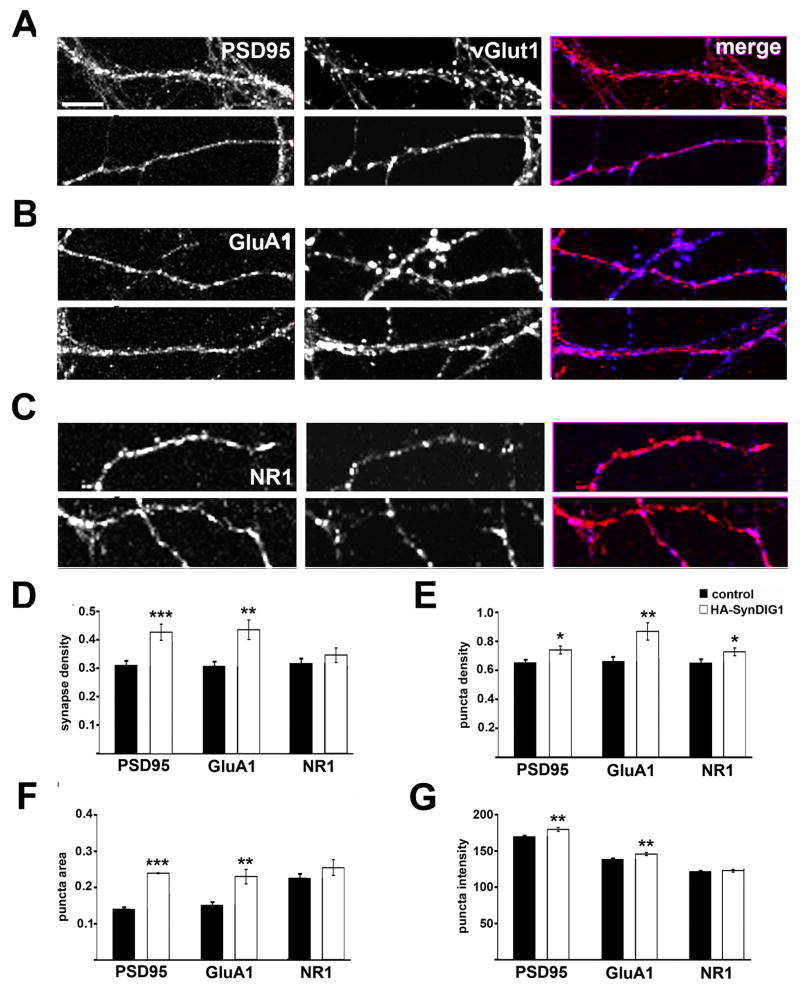
Figure 6. SynDIG1 overexpression promotes excitatory synapse development.
Immunostaining of postsynaptic proteins (red in merge) PSD95 (A), GluA1 (B), or NR1 (C) and presynaptic vGlut1 (blue in merge) puncta in dissociated rat hippocampal neurons transfected with HA-SynDIG1. Neurons were transfected at 4 DIV and analyzed at 8 DIV. Synapses are defined as overlap of pre- and postsynaptic clusters. Graphs depict density of PSD95/vGlut1, GluA1/vGlut1, and NR1/vGlut1 colocalized puncta (D), density of PSD95, total GluA1, total NR1 puncta (E), area of PSD95, total GluA1, and total NR1 puncta (F), and fluorescence intensity of PSD95, total GluA1, and total NR1 puncta (G) in dissociated rat hippocampal neurons transfected with HA-SynDIG1 (white bars) compared with untransfected neurons within the same experiment (black bars). PSD95: control, n = 62, HA-SynDIG1, n = 31 cells; GluA1: control, n = 60, HA-SynDIG1, n = 30 cells; NR1: control, n = 54, HA-SynDIG1, n = 28 cells. Data are averaged from two independent experiments.
Scale bar, 10 μm. Error bars, ± SEM. Significance, p-value < 0.05 (*), < 0.005 (**), < 0.0001(***) relative to untransfected neurons.
Composition of HA-SynDIG1-induced synapses was examined in further detail. AMPA receptor and NMDA receptor containing synapses were defined as overlap of vGlut1 and GluA1 clusters or vGlut1 and NR1 clusters, respectively (Figure 6B–C). To avoid potential clustering artifacts of live-labeling, neurons were fixed, permeabilized and stained with anti-vGlut1 antibodies and anti-GluA1 or anti-NR1 antibodies to label total protein. Neurons transfected with HA-SynDIG1 exhibited increased density of GluA1 containing synapses compared with non-transfected control neurons (Figure 6D). The increased GluA1 synapse density was accompanied with an increased density of total GluA1 puncta (Figure 6E). While HA-SynDIG1 overexpression led to a small but significant increase in the density of total NR1 clusters (Figure 6E), the increased NR1 cluster density did not reflect an increase in NR1 containing synapses (Figure 6D), suggesting that SynDIG1 is selective for AMPA receptors.
A significant increase in GluA1 cluster size was observed in HA-SynDIG1 transfected neurons compared with untransfected neurons (Figure 6F). In addition, a small but significant increase in fluorescence intensity of GluA1 clusters in HA-SynDIG1 transfected neurons compared with untransfected neurons was observed (Figure 6G). HA-SynDIG1 overexpression also led to increased area and fluorescence intensity of PSD95 clusters (Figure 6F–G). However, HA-SynDIG1 did not influence NR1 cluster area or fluorescence intensity (Figure 6F–G). Nor were the density, area or fluorescence intensity of vGlut1 clusters changed in axons contacting HA-SynDIG1 transfected neurons compared with untransfected neurons (not shown). These findings suggest a primary role for SynDIG1 in promoting postsynaptic maturation via increased level of AMPA receptors at synapses.
SynDIG1 promotes functional excitatory synapse development
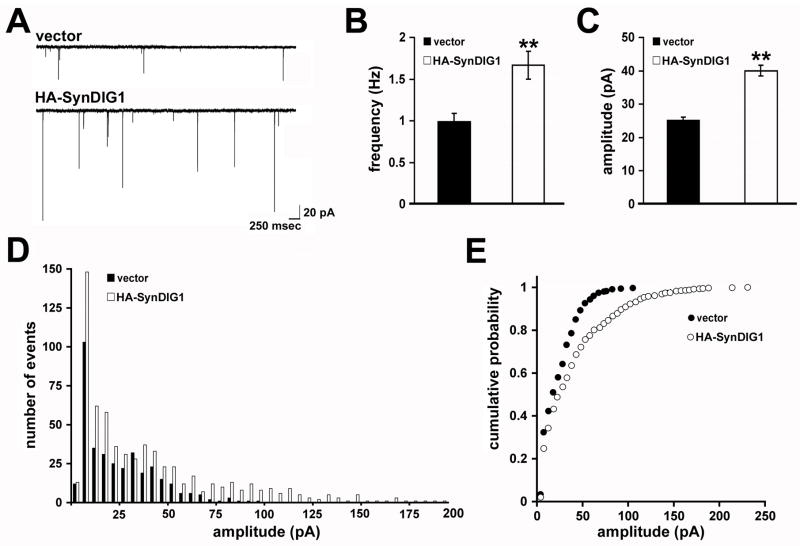
To determine if HA-SynDIG1 overexpression increases functional synapses, neurons were cotransfected at the time of plating with EGFP (to identify transfected cells) and HA-SynDIG1 or vector and mEPSCs were recorded on 8 DIV (Figure 7A). HA-SynDIG1 overexpression led to a significant 67% increase in mean mEPSC frequency compared with vector-transfected cells (Figure 7B). A significant 60% increase in mean mEPSC amplitude was also observed in HA-SynDIG1 transfected neurons compared with vector-transfected cells (Figure 7C). The histogram and cumulative probability distribution of mEPSC amplitudes were uniformly increased upon HA-SynDIG1 overexpression compared with control cells (Figure 7D–E).
Figure 7. SynDIG1 overexpression induces functional excitatory synapses.
mEPSC recordings from dissociated rat hippocampal neurons transfected with EGFP (to identify transfected cells) and either empty pHM6 vector or HA-SynDIG1. Neurons were transfected at the time of plating and mEPSCs were recorded at 8 DIV. Sample traces are shown in (A). Graphs depict mean frequency (B), mean amplitude (C), amplitude histogram (D), and amplitude cumulative probability (E) of mEPSC events from neurons transfected with empty vector (black bars) or HA-SynDIG1 (white bars). Data shown are the average of 2–3 independent experiments. Vector, n = 12 cells; HA-SynDIG1, n = 12 cells. Error bars, ± SEM. Significance, p-value < 0.005 (**) relative to vector.
See also Figure S7.
To control for possible non-specific effects due to the length of HA-SynDIG1 overexpression, the experiment was repeated with a shorter period of overexpression. Neurons were cotransfected at 4 DIV with EGFP (to identify transfected cells) and HA-SynDIG1 or vector as control and mEPSCs were measured at 8 DIV (Figure S7A). A similar increase in mean frequency (Figure S7B) and mean amplitude (Figure S7C) of mEPSC events was observed in HA-SynDIG1 transfected neurons compared with control neurons. The histogram and cumulative probability distributions of mEPSC amplitudes were also uniformly increased upon overexpression of HA-SynDIG1 for 4 days compared with control neurons (Figure S7D–E). Furthermore, overexpression of human HA-SynDIG1 led to a similar increased mean frequency and amplitude of mEPSC events (Figure S7A–E), demonstrating the functional conservation between mouse and human SynDIG1. NMDA receptor mediated mEPSCs were recorded (Figure S7F) and no change in the NMDA receptor mediated mean mEPSC frequency (Figure S7G) or mean mEPSC amplitude (Figure S7H) was observed in HA-SynDIG1 transfected neurons compared with vector only, suggesting that SynDIG1 promotes selectively AMPA receptor content at developing synapses. Importantly, SynDIG1-mediated increase in excitatory synapse development required the C-terminal 33 amino acids (Figure S7I–K), suggesting that SynDIG1-mediated excitatory synapse development requires interaction with AMPA receptors.
SynDIG1 distribution at excitatory synapses is activity regulated
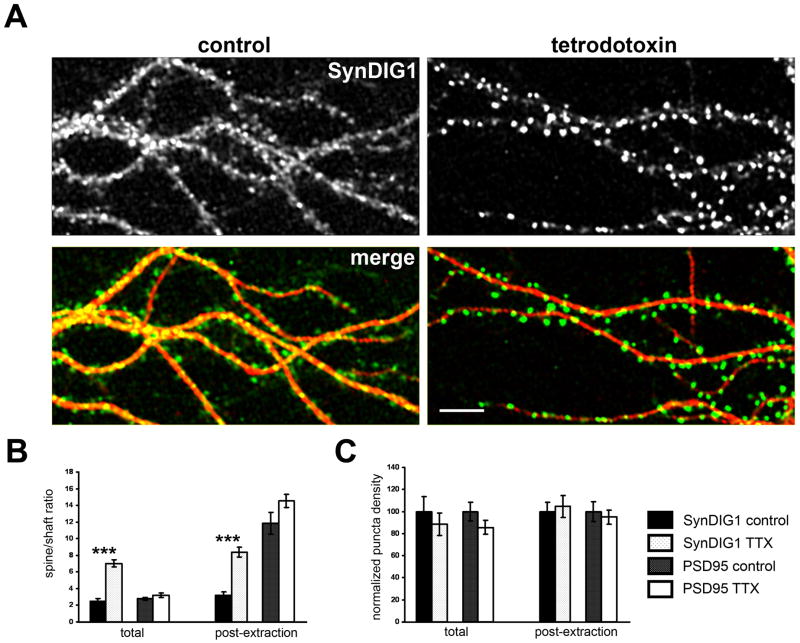
Because AMPA receptor content at synapses is regulated by synaptic activity [reviewed in (Barry and Ziff, 2002; Bredt and Nicoll, 2003; Chen et al., 2006; Malenka, 2003; Nicoll et al., 2006; Sheng and Hyoung Lee, 2003)], SynDIG1 distribution in response to changes in activity levels was examined. Sodium channel-dependent action potentials in hippocampal neurons were blocked by addition of tetrodotoxin (TTX) at 10 DIV. Upon activity blockade for two to four days, SynDIG1 immunoreactivity redistributed from diffuse and punctate staining in dendrite shafts to bright clusters, presumably spines, protruding from dendrites (Figure 8A). The overall level of SynDIG1 protein did not change in neurons treated with TTX compared with vehicle as assessed by immunobloting with anti-SynDIG1 mAb (Figure S8A). Under control conditions, SynDIG1 is enriched 2.5-fold in spines relative to shafts; that enrichment increases significantly to 7.0-fold after TTX treatment (Figure 8B). In contrast, SynDIG1 puncta density in the presence or absence of TTX does not change (Figure 8C). Thus, SynDIG1 distribution but not synthesis is regulated by synaptic activity in hippocampal neurons.
Figure 8. SynDIG1 distribution at excitatory synapses is activity regulated.
(A) Immunostaining for SynDIG1 (top panels, green in merge) and MAP2 (red in merge) in dissociated rat hippocampal neurons at 10 DIV treated with 1 μM TTX (lower panel) or vehicle (dH20, upper panel) for four days prior to fixation. Graphs depict the enrichment of SynDIG1 or PSD95 in spines relative to shafts (B) or the density of SynDIG1 or PSD95 puncta in dendrites (C) in dissociated rat hippocampal neurons upon treatment with TTX or vehicle (n = 10 cells for each condition). Permeabilization and immunostaining was performed on fixed neurons (left bars) or neurons treated with detergent prior to fixation (right bars) to stain selectively proteins imbedded in the PSD. Scale bar, 8 μm. Error bars, ± SEM. Significance, p-value < 0.001.
See also Figure S8.
Activity blockade might lead to an overall change in spine volume, thereby leading to increased level of all postsynaptic proteins in spines. Therefore, to test if this effect is specific to SynDIG1, the distribution of PSD95, an abundant postsynaptic protein, was analyzed under identical conditions (Figure S8B). In contrast to SynDIG1, analysis of PSD95 revealed no significant change in enrichment at spines relative to shafts in TTX treated neurons compared with control neurons (p-value = 0.20). The density of PSD95 puncta also did not change upon activity blockade (p-value = 0.19). Because PSD95 is a cytoplasmic protein, this type of analysis might be difficult to interpret compared with the transmembrane protein SynDIG1. Thus, neurons were treated with detergent prior to fixation to extract proteins not embedded in the PSD matrix according to published protocols [(Sharma et al., 2006); Figure S8C]. In this manner, only SynDIG1 and PSD95 protein embedded in the PSD should be preserved. This treatment caused an expected increase in the ratio of spine to shaft signal for PSD95 compared with total PSD95 puncta; however, no significant change in enrichment of PSD95 in spines compared with shafts upon TTX treatment was observed (p-value = 0.1, Figure 8C). In contrast, this treatment resulted in an overall increase in SynDIG1 enrichment in spines relative to shafts in control conditions or activity blockade conditions (Figure 8B), suggesting that SynDIG1 becomes more resistant to Triton extraction, and thus, more highly embedded in the PSD, following TTX treatment (see Figure S8C for representative images). Indeed, upon activity block, SynDIG1 clusters are enriched at excitatory but not inhibitory synapses as measured by colocalization with synaptic markers (Figure S8D). The number of synapses (defined by overlap of vGlut1 and SAP102 puncta) that contain SynDIG1 increases significantly from 55% to 77% upon activity blockade (vehicle, n = 10 cells; TTX, n = 13 cells; p-value < 0.0001; Figure S8E) and the percentage of total SynDIG1 puncta present at synapses increases significantly from 52% to 67% upon activity blockade (p-value < 0.0001; Figure S8F). However, the total synapse density did not change upon activity blockade compared with control neurons (p-value = 0.4).
AMPA receptors redistribute to excitatory synapses upon similar activity blockade in a variety of cultured neurons including hippocampal neurons (Lissin et al., 1998), spinal neurons (O’Brien et al., 1998), and neocortical neurons (Wierenga et al., 2005). Indeed, a significant increase in GluA1 enrichment in spines compared with shafts upon activity block was observed (vehicle, 1.2-fold, n = 18; TTX, 1.7-fold, n = 17, p-value < 0.01). In contrast, GluA1 puncta density did not change significantly (control = 0.47 GluA1 puncta/μm, n = 18; TTX = 0.38 GluA1 puncta/μm, n = 17; p-value > 0.05). These data suggest that SynDIG1 content at excitatory synapses is correlated with AMPA receptor content in response to changes in activity levels, suggesting that SynDIG1 might also play a role in synaptic plasticity.
Discussion
Here we report the identification and characterization of an activity-regulated AMPA receptor interacting type II transmembrane protein that we have named SynDIG1. Biochemical, immunocytochemical and electrophysiological evidence are provided to conclude that SynDIG1 plays a critical function in the development of AMPA receptor containing synapses in dissociated rat hippocampal neurons. While significant progress has been made in our understanding of pre- and postsynaptic differentiation including SV clustering and recruitment of scaffolds and NMDA receptors, less is known about the molecules that regulate AMPA receptor delivery to nascent synapses. A large body of work exists documenting mechanisms of AMPA receptor trafficking during synaptic plasticity [reviewed in (Barry and Ziff, 2002; Bredt and Nicoll, 2003; Chen et al., 2006; Malenka, 2003; Nicoll et al., 2006; Sheng and Hyoung Lee, 2003)]; however, whether similar or distinct mechanisms underlie AMPA receptor targeting during the initial stages of synapse development is a current topic of investigation. Thus, SynDIG1 represents a unique mechanism underlying the development of AMPA receptor containing synapses and addresses a major gap in the field of excitatory synapse development.
SynDIG1 regulates development of AMPA receptor containing synapses
How does SynDIG1 regulate development of AMPA receptor containing synapses? One possibility is that SynDIG1 promotes delivery of AMPA receptors to existing synapses. Indeed, SV clustering represents an early stage of synapse development and a consistent effect on the density or size of vGlut1 puncta upon changes in the level of SynDIG1 was not observed. Furthermore, SynDIG1 did not influence the density of NMDA receptor containing synapses (Figure 6D) or NMDA receptor-mediated mEPSCs (Figures S10G–I, S11F–H), providing strong support for the conclusion that SynDIG1 regulates specifically AMPA receptor content at existing nascent synapses.
An alternative possibility is that SynDIG1 promotes development of AMPA receptor only containing synapses. Indeed, HA-SynDIG1 overexpression displayed a trend towards an increase in overall GluA1 synapse density compared with the overall NR1 synapse density (Figure 6D), suggesting that under certain conditions SynDIG1 might be capable of forming AMPA receptor only containing synapses. Furthermore, decreased or increased SynDIG1 resulted in a corresponding change in PSD95 containing synapses (defined as overlap of vGlut1 and PSD95 puncta, Figures 4E, 6D), suggesting that SynDIG1 regulates overall synapse number. Because it is established that PSD95 regulates synaptic AMPA receptors (Elias et al., 2006) through interaction with Stargazin (Bats et al., 2007) and that PSD95 controls AMPA receptor incorporation during synaptic plasticity (Ehrlich and Malinow, 2004), the SynDIG1-dependent effect on PSD95 defined synapses is likely mediated through AMPA receptor interaction with a TARP family member expressed in the hippocampus since TARPs bind PSD95 [reviewed in (Nicoll et al., 2006)]. Thus, we favor the model that SynDIG1 regulates AMPA receptor content at existing synapses during development.
A popular model posits that synapses develop via an NMDA receptor only intermediate (so-called ‘silent synapses’) with subsequent conversion of silent synapses upon NMDA receptor activation to mature synapses containing AMPA receptors (Liao et al., 1999; Petralia et al., 1999). Indeed, blockade of NMDA receptors increases NMDA receptor only synapses while AMPA receptor inhibition decreases NMDA receptor only synapses due to the appearance of AMPA receptors at silent synapses (Liao et al., 1999). Thus, a prediction of this model is that blocking NMDA receptor activation might inhibit HA-SynDIG1’s ability to increase AMPA receptor content at developing synapses. Conversely, blocking AMPA receptor activation upon SynDIG1-shRNA knockdown might increase NMDA receptor only synapses due to the inability of AMPA receptors to be delivered to silent synapses. These studies will provide further evidence that SynDIG1 regulates AMPA receptor content at existing synapses.
Mechanism of SynDIG1-regulated AMPA receptor content at synapses
How might SynDIG1 influence AMPA receptor content at existing synapses? SynDIG1 interacts with AMPA receptors in heterologous cells (Figure 3B) and in brain (Figure 3C). Furthermore, HA-SynDIG1ΔC33, which is unable to interact with AMPA receptors (Figure 3B), fails to increase AMPA receptor content at developing synapses (Figure S7I–K), suggesting that AMPA receptor association is required for SynDIG1 function. One possibility is that SynDIG1 facilitates AMPA receptor trafficking through the secretory pathway and ultimately the PSD. Indeed, a larger fraction of GluA2 and SynDIG1 overlapped at non-synaptic sites compared with synaptic sites, suggesting that SynDIG1 and AMPA receptors might traffic together to synapses. Live-cell imaging of fluorescently tagged GluA2 and SynDIG1 fusion proteins will be necessary to test this possibility directly. Furthermore, loss of SynDIG1 resulted in decreased density of surface-labeled GluA1 and GluA2 clusters (Figure 4F), suggesting that SynDIG1 is required for surface expression of AMPA receptors. Biotinylation studies of surface AMPA receptors will provide further evidence if SynDIG1 influences AMPA receptor trafficking through the secretory pathway.
An alternative possibility is that SynDIG1 might capture and stabilize AMPA receptors brought to the PSD via other mechanisms. Interestingly, SynDIG1 influences AMPA receptor clustering in heterologous cells and this activity requires SynDIG1’s C-terminal 33 amino acids (Figure 3D–F). This region is important for both AMPA receptor interaction (Figure 3B) and SynDIG1 dimerization (Figure S2D), suggesting that AMPA receptor clustering might be coupled to SynDIG1 dimerization. It is particularly interesting that in heterologous cells, SynDIG1 cycles between the plasma membrane and endosomes (Figures S2A, S2F), suggesting the intriguing possibility that SynDIG1 facilitates clustering of AMPA receptors and delivery to synapses via an endosomal trafficking pathway. Indeed, endocytic trafficking maintains a pool of mobile surface AMPA receptors important for synaptic plasticity (Petrini et al., 2009), suggesting that trafficking through endosomes might underlie SynDIG1-regulated AMPA receptor content at developing synapses. Other studies support a role for lateral movement and exocytosis of AMPA receptors during synaptic plasticity (Makino and Malinow, 2009). Thus, SynDIG1 might influence AMPA receptor content at developing synapse via multiple mechanisms. However, these speculations are tempered by limitations of using heterologous cells. Thus, an important future direction is to determine if SynDIG1 cycles between the plasma membrane and endosomes in neurons and if so, what role it plays in SynDIG1-regulated AMPA receptor content at synapses.
Is SynDIG1 an AMPA receptor auxiliary subunit?
Epitope tagging experiments predict that SynDIG1’s second hydrophobic segment does not span the membrane (Figure S2A). The second hydrophobic segment might be embedded into the plasma membrane from the extracellular side or shielded from the aqueous environment within SynDIG1’s tertiary structure or via protein-protein interaction. Interestingly, membrane embedded hydrophobic regions in ion channel proteins such as the AMPA receptor form the pore of the channel although in the case of the AMPA receptor the pore-forming domain dips into the membrane from the cytosolic side [reviewed in (Bredt and Nicoll, 2003)]. Coupled with the observation that SynDIG1 appears to form dimers, it is possible that this region of SynDIG1 might form a pore within the plasma membrane or perhaps acts as an auxiliary pore-forming subunit for known channels. For example, TARPs control both AMPA receptor trafficking and channel gating properties [reviewed in (Nicoll et al., 2006)] and similar activities have been attributed to the recent identification of the cornichon family (Schwenk et al., 2009). Coincidentally, SynDIGs, TARPs, and cornichons are all relatively small proteins (~15–35 kDa). However, it is unknown if either of the TARP or cornichon protein families contribute to the pore-forming region of AMPA receptors nor do we have evidence as yet to suggest a role for SynDIG1 in regulating AMPA receptor channel gating properties. AMPA receptor interaction with TARPs and cornichons appear to be mutually exclusive (Schwenk et al., 2009); thus, it will be very interesting to determine the relationship between SynDIG1 interaction and TARP and cornichon associated AMPA receptors.
SynDIG1 content at excitatory synapses is regulated by activity
A number of AMPA receptor interacting proteins are important for AMPA receptor trafficking during synaptic plasticity [reviewed in (Barry and Ziff, 2002; Bredt and Nicoll, 2003; Chen et al., 2006; Malenka, 2003; Nicoll et al., 2006; Sheng and Hyoung Lee, 2003)]. Interestingly, upon global activity blockade with TTX, SynDIG1 enrichment in spines relative to shafts increases compared with control neurons. Intriguingly, AMPA receptors redistribute to excitatory synapses upon similar activity blockade protocols in multiple types of cultured neurons including hippocampal neurons (Lissin et al., 1998), spinal neurons (O’Brien et al., 1998), and neocortical neurons (Wierenga et al., 2005). Such redistribution is thought to represent a mechanism underlying homeostatic plasticity [reviewed in (Turrigiano and Nelson, 2004)]. These facts together with the observation that SynDIG1 regulates AMPA receptor content at developing synapses make it tempting to speculate that SynDIG1 might be involved in regulation of synaptic scaling. A prediction of this model is that concurrent treatment of TTX and SynDIG1-shRNA-mediated reduction of SynDIG1 will inhibit synaptic scaling compared with control-shRNA.
SynDIG family members and synapse development
SynDIG defines a family of four genes in the mouse genome none of which have been well characterized. SynDIG4 was reported to be present in purified PSD fractions from rodent brains using mass spectrometry (Jordan et al., 2004), suggesting that other SynDIG family members might also be present at synapses. Furthermore, the highest level of identity between SynDIG family members includes the C-terminal region, suggesting the intriguing possibility that other SynDIG family members might interact with AMPA receptors and/or form heterodimers. Detailed biochemical studies will be necessary to address this possibility. Finally, Capucin is down-regulated prior to striatal cell death in rodent models of Huntington’s disease (de Chaldee et al., 2006), reminiscent of the observation that SynDIG1 is down-regulated prior to Purkinje cell death in Lc cerebellum (Diaz et al., 2002). Thus, it is tempting to speculate that synaptic defects might precede neuronal death in Huntington’s disease.
In summary, our data support a model in which SynDIG1 regulates AMPA receptor content at developing synapses. A logical extension of these studies in dissociated rat hippocampal neurons in culture will be to determine the role of SynDIG1 in vivo. Analysis of transgenic mice with a targeted conditional deletion of the SynDIG1 gene will permit an analysis of the role of SynDIG1 in synapse development in vivo. For example, because SynDIG1 is expressed in cerebellar Purkinje neurons, one possibility is that SynDIG1 knockout mice will be ataxic like Lc mice due to defects in synapse development.
Experimental Procedures
Animals
Timed pregnant rats were purchased from Charles River. CD-1 mice were bred in house and maintained in the animal facility at UC Davis. The use and maintenance of animals were according to the guidelines set forth by UC Davis, NIH, and AALAC.
Antibodies
Anti-SynDIG1 mAb L42/17 was generated using standard procedures (Bekele-Arcuri et al., 1996) from a Balb/c mouse immunized with a GST fusion protein containing amino acids 1–183 of mouse SynDIG1. L42/17 is available from NeuroMab (http://www.neuromab.org/), a cooperative venture between UC Davis, NINDS and NIMH for generation and distribution of mouse monoclonal antibodies. Other antibodies: rat anti-HA (Roche); mouse anti-PSD95, mouse anti-SAP102 (NeuroMab); rabbit anti-GFP, mouse anti-GFP, rabbit anti-GluA1, mouse anti-GluA2, guinea pig anti-vGlut1, mouse antigephyrin, rabbit anti-vGat (all from Chemicon); mouse anti-NMDAR1 (BD Biosciences); rabbit anti-GluA1 (CalBiochem); Alexa 488-conjugated secondary antibodies (Molecular Probes), Cy3- or Cy5-conjugated secondary antibodies (Jackson ImmunoResearch), HRP-conjugated secondary antibodies (Invitrogen).
Constructs
SynDIG1 is annotated in the GenBank database as “tmem90b”. Full length and truncated versions of mouse SynDIG1 coding sequence were amplified by PCR from the RIKEN AV149920 cDNA clone and inserted into pHM6 (Roche) to create an in-frame HA tag at the N-terminus. Constructs for SynDIG1 knockdown were created with the pSuper vector (Oligoengine) system to contain an 18-nucleotide target sequence (GCT GTG GCC AAA GGA GAC) or control sequence (GCT GTG GAC AAA GGA GAC) with a single nucleotide mismatch for the mouse and rat SynDIG1 genes.
In situ hybridization
In situ hybridization on frozen sections using digoxigenin-labeled riboprobes was performed as described (Diaz et al., 2002).
Immunoprecipitation
Mouse brain membranes or COS cells transfected with the appropriate plasmids were homogenized in cell lysis buffer (50 mM Tris-Cl, 150 mM NaCl, 1% Triton X-100) containing protease inhibitors 48 hr after transfection and cleared by centrifugation at 37,000 × g for 15 min. Solubilized lysates were immunoprecipitated with anti-GluA2 antibody for 16 hr at 4°C. Protein A/G Sepharose (Amersham) was added for 1 hr at 4°C. The resultant resin was washed five times with cell lysis buffer. Bound proteins were eluted with SDS sample buffer and separated by SDS-PAGE. Input lanes represent 5% of input sample.
Primary culture of dissociated rat hippocampal neurons
The protocol for culturing hippocampal neurons from E18 rat embryos was based on the Banker method (Goslin et al., 1998). Briefly, neurons were plated on poly-lysine coated coverslips at a density of 30,000/cm2 and maintained over an astroglia feeder layer in serum-free media [MEM with N2 (Invitrogen) and 0.1% ovalbumin (Calbiochem)]. At 3 DIV, B27 (invitrogen) and 1–5 μM cytosine arabinoside (Sigma) was added to prevent proliferation of non-neuronal cells. Neurons were maintained for up to 21 DIV in a humidified incubator (5% CO2 at 37°C). For activity blockade experiments, 1 μM TTX (EMD Biosciences) or vehicle (water) was added to cultures two to four days prior to immunocytochemistry. For transfection experiments, dissociated hippocampal neurons were transfected by electroporation (Nucleofector, Amaxa, Inc.) on the day of plating or by calcium phosphate precipitation (Invitrogen) at 4 DIV. Surface receptor staining was performed as described in (Wyszynski et al., 2002).
Immunocytochemistry
Dissociated hippocampal neurons were fixed in either 100% methanol for 10 min at −20°C or 4% paraformaldehyde in 1 X PBS for 10 min at RT. After fixation, coverslips were rinsed in PBS, permeabilized for 10 min at RT with 0.1% Triton X-100 in PBS and blocked with 5% nonfat milk powder in 1 X PBS (blocking solution) for 30 min. After incubation with primary antibodies and washes, coverslips were incubated with secondary antibodies diluted in blocking solution. Following rinses in PBS, coverslips were mounted on microscope slides with Gel Mount (Biomedia), sealed with clear nail polish, and examined using an epifluorescence microscope (Zeiss Axioplan2) with the appropriate filters. For live-labeling, cells were incubated with appropriate primary antibody diluted in culture medium, rinsed with warm culture medium, and incubated with appropriate secondary antibody diluted in culture medium, and incubated for 30 min at 37°C. After final rinses with warm culture medium, neurons were fixed and labeled with additional primary antibodies as described above.
For quantitative analyses only pyramidal neurons were selected for imaging. Images were acquired using a Zeiss LSM510 scanning confocal microscope with identical settings for laser power, photomultiplier gain and offset. Images were imported into image analysis software (Zeiss AxioVision4.4) to determine abundance and distribution of synaptic clusters. Numbers of immunostained structures were determined after thresholds established such that all recognizable punctate structures were included in the analysis. The analyzer was blinded as to the identification of each image. For colocalization, regions were created around thresholded puncta in one channel and overlaid as a mask on the second channel. Thresholded puncta defined by at least partially overlapping regions were considered colocalizing. For quantification of SynDIG1 localization in synapses the cell body was excluded from the quantification. For quantification of synapse density upon SynDIG1 knockdown or overexpression, at least five dendritic stretches per cell were selected for quantification. For quantifications of spine versus shaft enrichment in TTX and control treated neurons, regions of spine and shaft were selected manually and the average fluorescent intensity inside the regions was measured. SynDIG1, GluA1, and PSD95 puncta in control and TTX treated neurons were quantified manually in selected dendritic stretches. Student’s t-tests were used to assess statistical significant of pairwise comparisons between experimental and control datasets.
Electrophysiology
In dissociated rat hippocampal cultures, mEPSC recordings were performed by whole-cell patch-clamp technique. Pipettes were pulled from borosilicate glass capillaries and fire-polished at the tips to yield resistance of 1–2 MΩ when filled with pipette solution. After obtaining the whole-cell mode, neurons were held at a membrane potential of −70 mV and mEPSCs recorded. The extracellular buffer contained (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 Glucose, pH 7.3. Recordings were performed with 0.3 μM TTX, and 10 μM Bicuculline in the extracellular buffer and the pipette buffer contained (in mM): 140 Potassium Gluconate, 5 NaCl, 2 CaCl2, 1 MgCl2, 10 EGTA, 3 Mg-ATP, 0.2 Na-GTP, 10 HEPES, pH 7.3. Miniature events were analyzed using IGOR Pro software.
Supplementary Material
Acknowledgments
We thank A.K. McAllister, P. Scheiffele, D. Speca, and K. Zito for helpful comments on the manuscript, Q. Gong, A.K.M., and M. Ferns for sharing of reagents, H. Misono for help establishing primary neuronal cultures in the Diaz lab, K. Misono, L. Guy, and K. Brinckmann for assistance in generating anti-SynDIG1 mAb, D. Lee for assistance in generating the HA-SynDIG1 expression construct, S. Azizi for assistance during early stages of the image analysis presented in Figure 4, and J.W. Hell for help with the culturing of hippocampal neurons using NS21 media.
This work was supported by grants to E.D. from the Alfred P. Sloan Research Foundation, the Whitehall Foundation, and the National Science Foundation (0542281; 0842724), to J.S.T from the NIH-NINDS (NS42225), and to D.P.M. from start-up funds from the University of Iowa Office of the Vice President of Research. Confocal image acquisition was conducted in a facility supported by Research Facilities Improvement Program Grant Number C06 RR-12088-01 from the National Center for Research Resources, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez VA, Ridenour DA, Sabatini BL. Retraction of synapses and dendritic spines induced by off-target effects of RNA interference. J Neurosci. 2006;26:7820–7825. doi: 10.1523/JNEUROSCI.1957-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M. Selective expression of the glutamate receptor channel delta 2 subunit in cerebellar Purkinje cells. Biochem Biophys Res Commun. 1993;197:1267–1276. doi: 10.1006/bbrc.1993.2614. [DOI] [PubMed] [Google Scholar]
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Bekele-Arcuri Z, Matos MF, Manganas L, Strassle BW, Monaghan MM, Rhodes KJ, Trimmer JS. Generation and characterization of subtype-specific monoclonal antibodies to K+ channel alpha- and beta-subunit polypeptides. Neuropharmacology. 1996;35:851–865. doi: 10.1016/0028-3908(96)00128-1. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Chen L, Tracy T, Nam CI. Dynamics of postsynaptic glutamate receptor targeting. Curr Opin Neurobiol. 2006 doi: 10.1016/j.conb.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2008;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chaldee M, Brochier C, Van de Vel A, Caudy N, Luthi-Carter R, Gaillard MC, Elalouf JM. Capucin: a novel striatal marker down-regulated in rodent models of Huntington disease. Genomics. 2006;87:200–207. doi: 10.1016/j.ygeno.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Diaz E, Ge Y, Yang YH, Loh KC, Serafini TA, Okazaki Y, Hayashizaki Y, Speed TP, Ngai J, Scheiffele P. Molecular analysis of gene expression in the developing pontocerebellar projection system. Neuron. 2002;36:417–434. doi: 10.1016/s0896-6273(02)01016-4. [DOI] [PubMed] [Google Scholar]
- Dumesnil-Bousez N, Sotelo C. Early development of the Lurcher cerebellum: Purkinje cell alterations and impairment of synaptogenesis. J Neurocytol. 1992;21:506–529. doi: 10.1007/BF01186954. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Goslin K, Asmussen H, Banker G. Rat hippocampal neurons in low-density culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. Cambridge: MIT Press; 1998. pp. 339–370. [Google Scholar]
- Jordan BA, Fernholz BD, Boussac M, Xu C, Grigorean G, Ziff EB, Neubert TA. Identification and verification of novel rodent postsynaptic density proteins. Mol Cell Proteomics. 2004;3:857–871. doi: 10.1074/mcp.M400045-MCP200. [DOI] [PubMed] [Google Scholar]
- Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci. 2006;26:12152–12164. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Kim S, Chung HS, Kim K, Han K, Kim H, Jun H, Kaang BK, Kim E. SALM synaptic cell adhesion-like molecules regulate the differentiation of excitatory synapses. Neuron. 2006;50:233–245. doi: 10.1016/j.neuron.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Liao D, Zhang X, O’Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci. 1999;2:37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- Lissin DV, Gomperts SN, Carroll RC, Christine CW, Kalman D, Kitamura M, Hardy S, Nicoll RA, Malenka RC, von Zastrow M. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc Natl Acad Sci U S A. 1998;95:7097–7102. doi: 10.1073/pnas.95.12.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi R, Tang X, Sutter R, Xu D, Worley P, O’Brien RJ. Differing mechanisms for glutamate receptor aggregation on dendritic spines and shafts in cultured hippocampal neurons. J Neurosci. 2002;22:7606–7616. doi: 10.1523/JNEUROSCI.22-17-07606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- O’Brien R, Xu D, Mi R, Tang X, Hopf C, Worley P. Synaptically targeted narp plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J Neurosci. 2002;22:4487–4498. doi: 10.1523/JNEUROSCI.22-11-04487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63:92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, Klocker N. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323:1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- Sharma K, Fong DK, Craig AM. Postsynaptic protein mobility in dendritic spines: long-term regulation by synaptic NMDA receptor activation. Molecular and cellular neurosciences. 2006;31:702–712. doi: 10.1016/j.mcn.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hyoung Lee S. AMPA receptor trafficking and synaptic plasticity: major unanswered questions. Neurosci Res. 2003;46:127–134. doi: 10.1016/s0168-0102(03)00040-3. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Ulmer JB, Palade GE. Effects of Brefeldin A on the Golgi complex, endoplasmic reticulum and viral envelope glycoproteins in murine erythroleukemia cells. European journal of cell biology. 1991;54:38–54. [PubMed] [Google Scholar]
- Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M, Kim E, Dunah AW, Passafaro M, Valtschanoff JG, Serra-Pages C, Streuli M, Weinberg RJ, Sheng M. Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron. 2002;34:39–52. doi: 10.1016/s0896-6273(02)00640-2. [DOI] [PubMed] [Google Scholar]
- Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. Neurodegeneration in Lurcher mice caused by mutation in delta2 glutamate receptor gene. Nature. 1997;388:769–773. doi: 10.1038/42009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.