SUMMARY
Cell fusion is essential for fertilization, myotube formation, and inflammation. Macrophages fuse in various circumstances but the molecular signals involved in the distinct steps of their fusion are not fully characterized. Using null mice and derived cells, we show that the protease MT1-MMP is necessary for macrophage fusion during osteoclast and giant cell formation in vitro and in vivo. Specifically, MT1-MMP is required for lamellipodia formation and for proper cell morphology and motility of bone marrow myeloid progenitors prior to membrane fusion. These functions of MT1-MMP do not depend on MT1-MMP catalytic activity or downstream pro-MMP-2 activation. Instead, MT1-MMP-null cells show a decreased Rac1 activity and reduced membrane targeting of Rac1 and the adaptor protein p130Cas. Retroviral rescue experiments and protein binding assays delineate a signaling pathway in which MT1-MMP, via its cytosolic tail, contributes to macrophage migration and fusion by regulating Rac1 activity through an association with p130Cas.
INTRODUCTION
Cell fusion is fundamental in processes such as fertilization and vertebrate myogenesis (Chen et al., 2007), and may also be important in inflammation (Johansson et al., 2008; Nygren et al., 2008). Under certain circumstances, cells of the monocyte/macrophage lineage can fuse, giving rise to osteoclasts (OC) in bone or giant cells (GC) in inflamed soft tissues. These multinucleated derivatives acquire specialized functions in bone resorption and engulfment of pathogens and foreign bodies, respectively (Vignery, 2005). Several diseases of the adult skeleton are related to disturbances in OC function, either through increased activity (bone metastasis, osteoporosis, Paget’s disease) or decreased activity (osteopetrosis).
Molecules can contribute to cell fusion by either directly participating in membrane fusion or by affecting earlier steps in the process (Oren-Suissa and Podbilewicz, 2007; Primakoff and Myles, 2007). The signaling pathways involved in cell-cell fusion have mostly been characterized in yeast and invertebrates, and much less is known about the regulation of fusion by mammalian cells, and by macrophages in particular (Chen et al., 2007). For example, EFF-1 and AFF-1 act as direct membrane fusogens during C. elegans development (Mohler et al., 2002; Sapir et al., 2007), but the mechanisms by which proteins such as DC-STAMP, the d2 isoform v-ATPase and CD200 contribute to macrophage fusion remain undefined (Cui et al., 2007; Lee et al., 2006; Yagi et al., 2005). One central issue is how competent cells come into contact. In the case of myotubes, the myogenic precursors are already in close proximity, whereas sperm-egg fusion is dependent on sperm motility; however, the mechanisms by which OC and GC precursors achieve proximity are poorly understood.
MMPs are endopeptidases capable of degrading a variety of ECM components and of modulating the activity of several secreted and cell-surface proteins (Page-McCaw et al., 2007). MT1-MMP is a membrane-anchored collagenase that plays important roles in pathophysiological settings, including the development of skeletal, lung and adipose tissue, angiogenesis, and tumor invasion (Chun et al., 2006; Holmbeck et al., 1999; Oblander et al., 2005; Sabeh et al., 2004; Zhou et al.). Here we report that MT1-MMP, independently of its catalytic activity, regulates Rac1 signaling in myeloid cells, thereby contributing to their migration and fusion during osteoclastogenesis and GC formation in vitro and in vivo.
RESULTS
MT1-MMP-null myeloid cells are defective for OC multinucleation in vitro and in vivo
MT1-MMP participates in leukocyte migration (Matias-Roman et al., 2005; Yang et al., 2006), and we therefore analyzed hematopoietic development in MT1-MMP-null mice. These mice die two weeks after birth, so 8 day-old mice were used for all analyses. Flow cytometry showed that the percentage of cells doubly positive for Mac-1 (macrophage-1 antigen; CD11b/CD18; αMβ2 integrin; CR3)dull and c-fms (colony-stimulating factor-1 receptor) was significantly higher in bone marrow (BM) from MT1-MMP-null mice (2.5±1.4% versus 1.3±0.9%; p<0.0324; n=20). Since this population might be a common myeloid progenitor able to generate OC, and MT1-MMP-null mice display bone defects, we explored this point further.
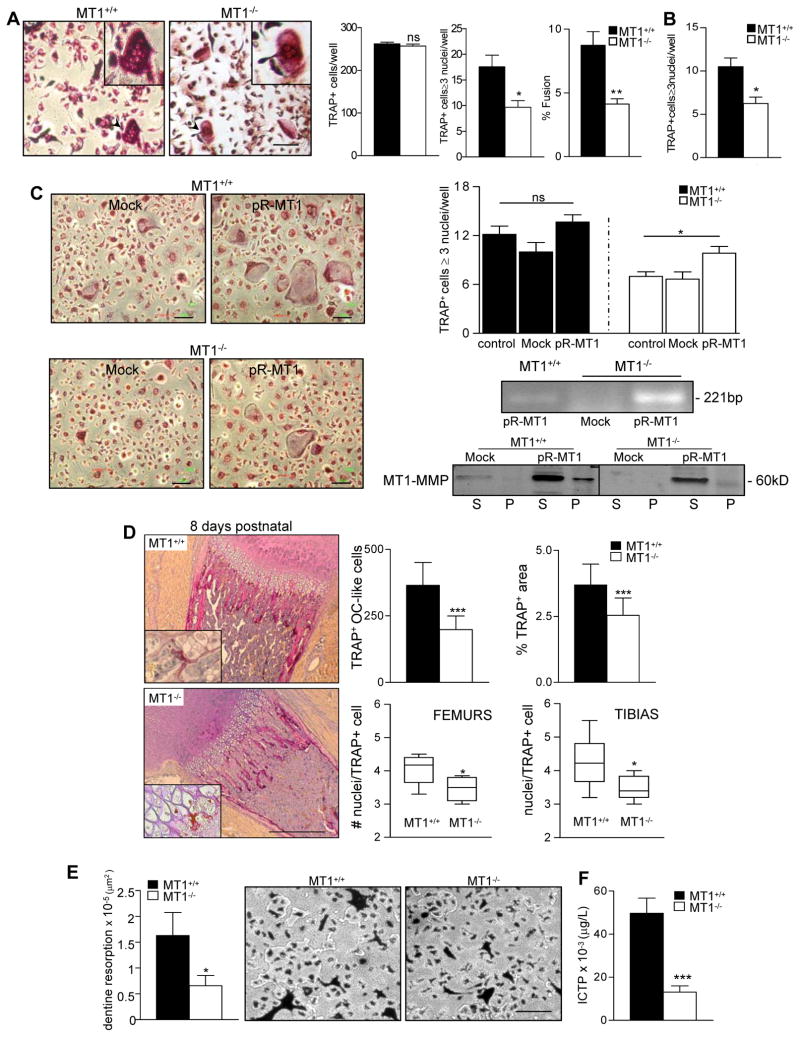
We exposed BM cultures to M-CSF (macrophage colony-stimulating factor) and RANKL (receptor activator for nuclear factor-κB ligand). These conditions differentiate progenitors towards OC, giving rise to typical multinucleated cells expressing characteristic phenotypic markers, including tartrate-resistant acid phosphatase (TRAP) (Yagi et al., 2005). The numbers of TRAP+ cells obtained from wild-type (WT) or MT1-MMP-null BM progenitors were similar; however, MT1-MMP-null progenitors generated fewer multinucleated TRAP+ cells (OC) and these were smaller and contained fewer nuclei (Figure 1A and Figure S1). No significant defects were found in multinucleated TRAP+ cells derived from heterozygous neonates (unpublished data). Similar results were obtained when MT1-MMP-null sorted Mac-1+ BM cells were differentiated (Figure 1B), indicating a direct contribution of MT1-MMP in the progenitors, although a role from stroma in vivo cannot be ruled out.
Figure 1.
MT1-MMP is required for OC multinucleation and function in vitro and in vivo. (A) Bone marrow (BM) progenitors from WT or MT1-MMP-null mice were differentiated to OC in vitro. Images of TRAP+ cells are shown. Bar, 100 m. Histograms show the total number of TRAP+ cells, the number of TRAP+ cells containing ≥3 nuclei (OC), and the percentage of cell fusion (TRAP+ cells containing ≥ 3 nuclei as a percentage of the total number of TRAP+ cells). Data are arithmetic means +/− S.E; n=20. (B) Mac1+ BM progenitors from WT or MT1-MMP-null mice were differentiated to OC as in (A). The histogram shows the number of TRAP+ cells containing ≥3 nuclei (n=4). (C) BM progenitors from WT or MT1-MMP-null mice were infected with retrovirus encoding human MT1-MMP and differentiated to OC. Images of TRAP+ cells are shown. Bar, 100 m. Data show the number of TRAP+ cells containing ≥3 nuclei (n=6) (top right). Human MT1-MMP expression was detected by RT-PCR and western blot (bottom right). Mock, infection with empty virus. (D) Bones from 8 day-old WT or MT1-MMP-null mice were stained for TRAP. Bar, 100 μm. Histograms show the number of TRAP+ OC-like cells and the relative TRAP area at the cartilage/bone interface (n=6). Floating bar graphs show distribution and numbers of nuclei per OC in the bone/cartilage interface area of WT or MT1-MMP-null mice (n=6). (E) BM progenitors from WT and MT1-MMP null mice were differentiated to OC on dentine slides. Data show the total area of dentine resorption (n=10). Images of TRAP-stained cultures on dentine are shown. Bar, 100 μm. (F) The COL I telopeptide ICTP was measured by RIA in serum. Histograms show ICTP levels in WT (n=8) and null mice (n=16). See also Figure S1, S2, S3 and S4.
The numbers of viable cells in cultures derived from WT or MT1-MMP-null BM were similar (Figure S2A). Furthermore, WT and MT1-MMP-null BM cells or derived OC showed similar relative mRNA expression of the OC genes PU.1, c-fms, RANK, TRAP, NFATc1, and calcitonin receptor (CTR) (Figure S2B). This suggests that commitment and early differentiation of OC were not affected by the absence of MT1-MMP. Consistently, serum levels of TRAP, a measure of early commitment to the OC lineage, were similar in MT1-MMP-null mice and control littermates (MT1-MMP+/+ 8.4±2 U/l and MT1-MMP−/− 8.6±5 U/l; n=8).
These data indicate a requirement for MT1-MMP at later stages of OC differentiation. Accordingly, MT1-MMP mRNA expression was upregulated by day 4 of OC differentiation in WT cells (Figure S3), coinciding with the reported initiation of cell fusion (Saginario et al., 1995).
Retroviral re-expression of human MT1-MMP in deficient BM progenitors resulted in increased numbers of multinucleated TRAP+ cells (OC) (Figure 1C), confirming the requirement for MT1-MMP. Moreover, mixed culture of WT and MT1-MMP-null progenitors rescued the multinucleation defect (% fusion=5.5 for WT, 2.6 for null and 5.5 for mixed) and showed that WT progenitors can fuse with deficient ones (Figure S4); these data indicate that the presence of MT1-MMP in one partner allows efficient multinucleation.
To directly assess the impact of MT1-MMP deficiency on OC multinucleation in vivo, we analyzed TRAP expression in sections of femur and tibia from 8 day-old MT1-MMP-null and WT mice (Figure 1D, left panel). MT1-MMP-null bones contained significantly fewer TRAP+ OC-like cells and a smaller relative TRAP+ area at the cartilage:bone interface (Figure 1D, top right panel). The number of nuclei per TRAP+ cell at this interface was also significantly lower, confirming that decreased multinuclearity occurs in vivo (Figure 1D, bottom right panel).
MT1-MMP deficiency results in decreased OC function in vitro and in vivo
Since multinucleation correlates with OC functionality (Chen et al., 2007), we next tested the function of MT1-MMP-null OC. Dentine resorption was significantly impaired in OC differentiated from MT1-MMP-null BM progenitors (Figure 1E). These OC contained fewer nuclei than WT counterparts (unpublished data), indicating that the defect is matrix independent. Dentine resorption was rescued by re-expression of MT1-MMP in the null progenitors (mock=2×105and pR-MT1=6.3×105 μ2; n=3 p=0.011).
To evaluate bone resorption in vivo we measured serum levels of the C-terminal COL I telopeptide ICTP (Kiviranta et al., 2005). Serum ICTP levels were significantly decreased in MT1-MMP-null mice compared with WT (Figure 1F), showing that bone resorption by OC is less efficient in neonate MT1-MMP-null mice.
MT1-MMP is required for macrophage fusion during GC formation in vitro and in vivo
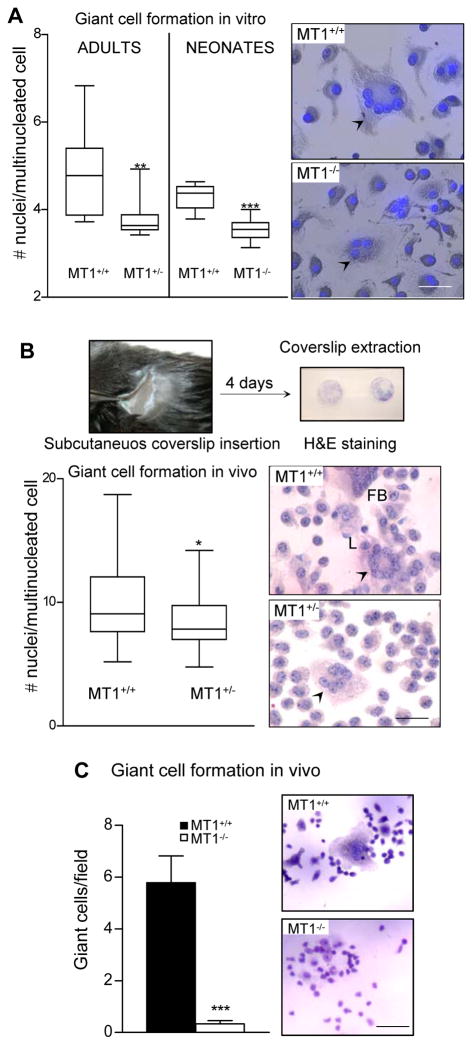
To evaluate the generality of the requirement for MT1-MMP in monocyte/macrophage fusion, we investigated the effect of MT1-MMP deficiency on GC formation. Formation of multinucleated GC in vitro was induced by stimulating BM progenitors with IL4 (Helming and Gordon, 2007). The average number of nuclei per GC was significantly lower in multinucleated cells derived from progenitors from adult heterozygous mice or MT1-MMP-null neonates (Figure 2A).
Figure 2.
MT1-MMP promotes giant cell formation in vitro and in vivo. (A) GC formation by adult (WT and MT1-MMP heterozygotes) and neonate (WT and MT1-MMP-null) BM progenitors was induced in vitro (see Experimental procedures). The graphic shows the distribution and numbers of nuclei per GC (n=7 for adults and n=9 for neonates; on average 25 cells were counted per condition). Images of the GC obtained are shown. Bars, 50 μm. (B) Foreign body reaction was induced in WT and MT1-MMP heterozygous mice (see Experimental procedures). The graphic shows the distribution and numbers of nuclei per GC (n=14; on average 25 cells were counted per mouse). Pictures show GC in WT and heterozygous mice (L and FB for Langhans and Foreign Body type GC, respectively). Bar, 50 μm. (C) Foreign body reaction was induced in WT and MT1-MMP-null neonate mice. The graphic shows the number of GC per field (n=8). Pictures show GC formation in WT and null mice. Bar, 100 μm.
Foreign body reactions were induced in vivo by subcutaneous implantation of glass coverslips in WT and MT1-MMP heterozygous adult mice. Although number of cells and fusion efficiency were similar on coverslips from both genotypes, the average number of nuclei per GC was significantly lower in MT1-MMP heterozygous mice (Figure 2B), indicating a role of MT1-MMP in GC formation in vivo. These findings were confirmed in similar experiments performed with neonatal MT1-MMP-deficient mice, in which the formation of GC in response to foreign body was almost abolished (Figure 2C).
MT1-MMP-null OC progenitors display impaired chemotaxis, motility and morphology
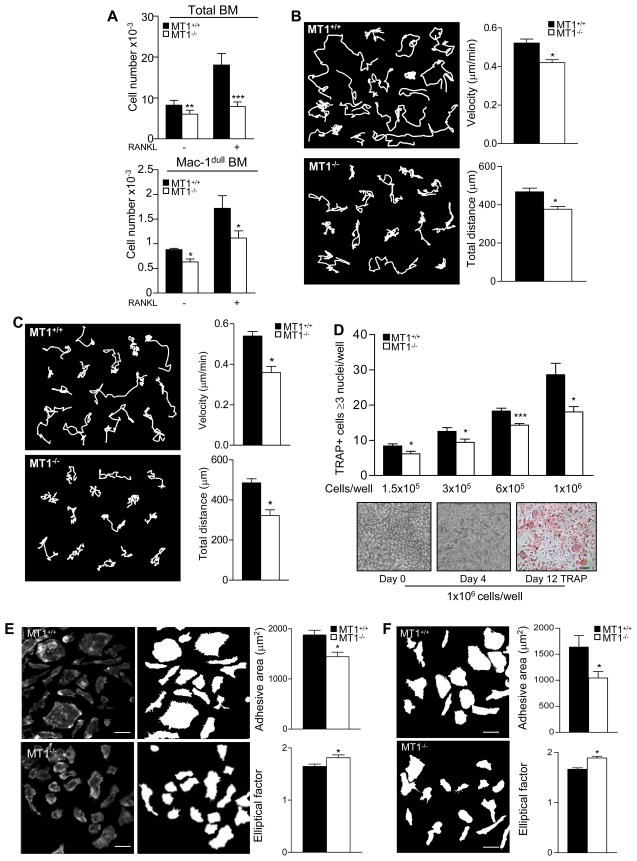
Progenitors within the OC differentiation niche must come into contact in order to fuse. Chemotactic cues involved include the cytokine RANKL and the chemokine MCP-1/CCL2 (Henriksen et al., 2003; Kim et al., 2005). We previously showed that MT1-MMP is involved in human monocyte migration towards MCP-1/CCL2 (Matias-Roman et al., 2005). Here, we analyzed the chemotactic response of mouse MT1-MMP-null BM cells to RANKL across activated endothelial cell monolayers to model the BM stromal niche. Compared with WT counterparts, MT1-MMP-null BM cells (total cells or the progenitor-rich Mac-1dull subpopulation) showed significantly diminished endothelial transmigration in response to RANKL (Figure 3A).
Figure 3.
MT1-MMP-null BM cells show impaired chemotaxis, motility and morphology. (A) Transmigration of total and Mac-1dull BM cells towards RANKL through activated human endothelial monolayers was analyzed. Data show the total number of cells in the lower chamber. Experiments were run in triplicate (n=6). (B) Single cell-track analysis was performed on time-lapse microscopy recordings from WT and MT1-MMP-null BM cells cultured with M-CSF and RANKL for 4 days. Individual cell-track analysis was done on at least 100 cells for each genotype, in 7 independent experiments from individual mice. Representative trajectories of WT and MT1-MMP- null cells are shown (left). Histograms show velocities and total migrated distances, (right). (C) Mac1+ BM cells from WT and MT1-MMP-null mice were cultured and analyzed as in (B) (n=4). (D) OC differentiation cultures were established from WT or MT1-MMP-null BM progenitors at the standard cell density of 1.5 × 105 cells per well or at 3 × 105, 6 × 105, and 1 × 106 cells per well. The histogram shows the numbers of TRAP+ cells containing ≥3 nuclei (n=8) (top). Statistical significance is indicated for MT1-MMP-null cells versus WT for each condition. Pictures show MT1-MMP-null BM cells on different days after plating at 1 × 106 cells per well (bottom). Bar, 100 μm. (E) F-actin stained images were analyzed with Metamorph software. Pictures and mask images for each genotype are shown (left). Bar, 20 μm. Histograms shows average adhesive area (top) and average EF (bottom) for WT and MT1-MMP-null BM OC progenitors. A total of 200 cells for each genotype were analyzed (n=10). (F) Mac1+ BM cells from WT and MT1-MMP-null mice were cultured and analyzed as in (E). Histograms show data for average adhesive area and EF for WT and MT1-MMP-null BM OC progenitors. A total of 200 cells for each genotype were analyzed (n=4). See also Movie S1, S2, S3 and S4.
The effect of MT1-MMP deficiency on the behavior of individual OC progenitors was next investigated by time lapse microscopy of BM cells cultured in the presence of M-CSF and RANKL for 4 days, just before cell fusion starts (Movies S1 and S2). MT1-MMP-null cells moved more slowly than WT (Figure 3B, left panel), and whereas WT cells migrated in various directions over relatively long distances, MT1-MMP-null cells mostly migrated over shorter distances, correlating with a to-and-fro movement pattern in the movies. Quantification of velocity and migrated distance showed that these differences were significant (Figure 3B, right panel). Similar defects were observed with MT1-MMP-deficient Mac1+ BM cells (Figure 3C and Movies S3 and S4).
We then explored whether this defect in progenitor migration could account for the defect in OC multinucleation by MT1-MMP-null cells. The multinucleation efficiency of MT1-MMP-null progenitors increased with increasing plating density but never matched that of WT, even when cells were plated in contact (Figure 3D). Detailed analysis of movies showed that MT1-MMP-null cells had a weaker membrane protrusive activity. Accordingly, MT1-MMP-null OC progenitors had a significantly smaller average adhesive area and a high elliptical factor (EF), an index of cellular elongation (Figure 3E). These findings demonstrate that MT1-MMP modulates not only the locomotion of BM OC progenitors but also other migration-associated parameters that contribute to fusion such as morphology, spreading and lamellipodia formation. Equivalent phenotypes (decreased adhesive area and increased EF) were observed in similarly treated MT1-MMP-deficient Mac-1+ BM cells (Figure 3F).
MT1-MMP catalytic activity is not essential for its regulation of myeloid cell migration and fusion
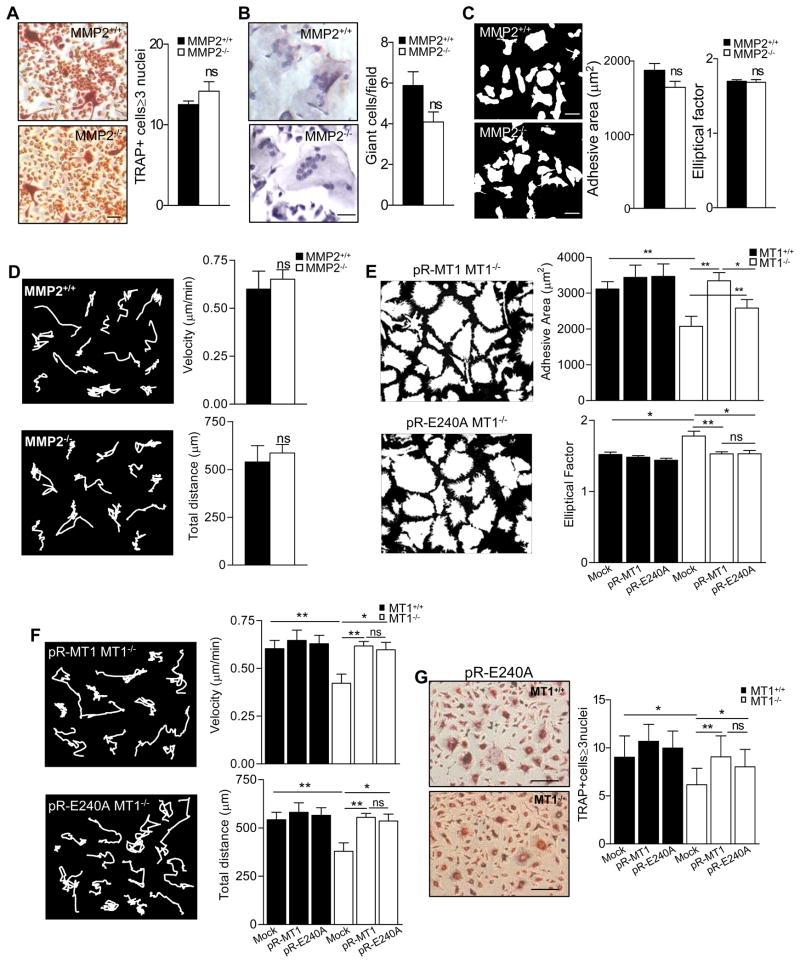
One of the main downstream targets of MT1-MMP is pro-MMP-2, which is activated by MT1-MMP-mediated proteolysis (Sato et al., 1994). Genetic evidence indicates that MMP-2 is involved in bone biology (Martignetti et al., 2001; Mosig et al., 2007), and we therefore investigated whether the lack of MMP-2 activation might contribute to the defects observed in MT1-MMP-null cells. MMP-2-null neonate mice and derived cells did not differ from WT in the numbers of BM-derived TRAP+ OC in vitro or in the formation of GC in vivo (Figures 4A and B). Similarly, MMP-2- null BM myeloid progenitors showed no differences from WT equivalents in adhesive area, EF or motility after culture with M-CSF and RANKL (Figures 4C and D). Therefore the phenotypes observed in MT1-MMP-null myeloid progenitors appear to be unrelated to the lack of active MMP-2.
Figure 4.
MT1-MMP catalytic activity is not required for regulation of myeloid cell morphology, motility and fusion. (A) BM progenitors from WT or MMP-2-null mice were differentiated to OC as in Figure 1A. Images of TRAP+ cells are shown. Bar, 50 μm. The histogram shows the number of TRAP+ cells containing ≥3 nuclei (n=5 for WT and n=13 for null mice). (B) Foreign body reaction was induced in WT and MMP-2-null neonates as in Figure 2C. Pictures show GC formation. Bar, 50 μm. The histogram shows the number of GC per field (n=4 for WT and n=7 for null mice). (C) BM cells from WT and MMP-2-null were cultured and analyzed as in Figure 3E. Histograms show average adhesive area and EF for WT and MMP-2-null BM OC progenitors. A total of 100 cells were analyzed for each genotype (n=5 for WT and n=13 for null mice). (D) BM cells from WT and MMP-2-null were cultured and analyzed as in Figure 3B. Histograms show velocity and total distance traveled for WT and MMP-2-null BM OC progenitors. A total of 100 cells were analyzed for each genotype (n=5 for WT and n=8 for null mice). (E) BM progenitors from WT or MT1-MMP-null mice were infected with retrovirus encoding human MT1-MMP (pR-MT1) or a catalytically inactive MT1-MMP mutant (pR-E240A) and cultured and analyzed as in Figure 3E. Histograms show average adhesive area (top) and EF (bottom). A total of 200 cells was analyzed per genotype (n=4 for WT and n=6 for null mice). (F) BM cells were infected as in (E), and analyzed as in Figure 3B. At least 100 cells were analyzed per genotype (n=4). Representative trajectories are shown of WT and MT1-MMP-null cells. Histograms show average velocities (top) and total migrated distances (bottom) for infected WT and MT1-MMP-null cells. (G) BM cells were infected as in (E) and differentiated to OC. Images of TRAP+ cells are shown. Bar, 20 μm. Data show the number of TRAP+ cells containing ≥3 nuclei (n=6). See also Movie S5, S6 and S7.
We directly assessed the requirement of MT1-MMP catalytic activity by re-expressing WT and catalytically inactive MT1-MMP in null myeloid progenitors. Retrovirally re-expressed wild-type MT1-MMP increased the adhesive area and membrane protrusive activity while decreasing the EF (Figure 4E); moreover, null cells with restored MT1-MMP expression also migrated at higher speed, over longer distances and with more varied trajectories than mock-infected MT1-MMP-null cells (Figure 4F and Movies S6 and S7). Remarkably, the adhesive area, EF, membrane protrusive activity, and motility phenotypes were similarly rescued by re-expression of a catalytically dead version of human MT1-MMP, harboring the E240A mutation (Figures 4E and 4F and Movie S8). Re-expression of the inactive MT1-MMP mutant also significantly increased OC formation (Figure 4G), confirming that catalytic activity is dispensable for the contribution of MT1-MMP to these events.
MT1-MMP contributes to the migration and fusion of BM myeloid progenitors by regulating Rac1 activity
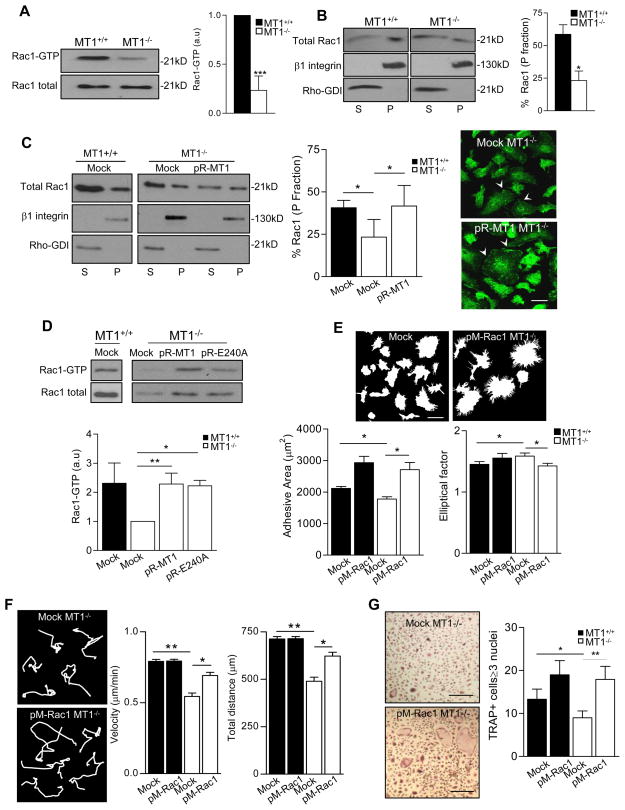
The small GTPase Rac1 regulates macrophage cell spreading, membrane ruffling, morphology and migration, and is implicated in distinct cell fusion scenarios (Jay et al., 2007; Pajcini et al., 2008; Wang et al., 2008; Wells et al., 2004). Rac1 activity and membrane targeting were significantly reduced in MT1-MMP-null BM progenitors (Figure 5A and 5B). Accordingly, Rac1 nicely decorated the lamellipodia of WT progenitors, colocalizing with F-actin, in contrast to reduced Rac1 presence at these sites in MT1-MMP-null cells (Figure S5A). This correlates with reduced numbers of focal complexes at the perimeter of MT1-MMP-null progenitors (Figure S5B).
Figure 5.
MT1-MMP regulates Rac1 activity in BM OC progenitors. (A) BM cells from WT or MT1-MMP-null mice were cultured with M-CSF and RANKL for 4 days and lysed for pull-down with GST-PBD. A representative blot is shown. The histogram shows the average fold induction of active Rac1 quantified by densitometric analysis (n=4 independent experiments from cells pooled from a total of 20 mice). (B) Cells treated as in (A) were fractionated, and particulate/membranous (P) and soluble/cytosolic (S) fractions analyzed by western blot; a representative blot is shown. The histogram shows the percentage of Rac1 in the particulate fraction, quantified by densitometric analysis (n=3 independent experiments from 15 mice) (left). (C) BM progenitors from WT or MT1-MMP-null mice were infected with retrovirus encoding human MT1-MMP (pR-MT1) and cultured as in (A). Cells were fractionated, and particulate (P) and soluble (S) fractions analyzed by western blot; a representative blot is shown. The histogram shows the percentage of Rac1 in the particulate fraction, quantified by densitometric analysis (n=5 independent experiments from 15 mice). Images of cells stained for Rac1 (green) are shown. Bar, 20 μm. (D) Cells were infected as in (C) or with the inactive MT1-MMP mutant (pR-E240A) and lysed for pull-down with GST-PBD. A representative blot is shown. The histogram shows the average fold induction of active Rac1 quantified by densitometric analysis (n=3 independent experiments from 9 mice). (E) BM progenitors were infected with retrovirus encoding constitutively active Rac1 (pM-Rac1) and cultured as in (A). Morphological parameters were analyzed as in Figure 3E. A total of 100 cells was analyzed per genotype (n=4). (F) Motility parameters for BM cells infected with pM-Rac1 were measured as in Figure 3B for at least 100 cells per genotype (n=4). (G) BM cells were infected as in (D) and cultured in osteoclastogenic conditions for 12 days. Images of TRAP+ cells are shown. Bar, 100 μm. Histogram shows the numbers of TRAP+ cells containing ≥3 nuclei (n=6). See also Figure S5.
Retroviral re-expression of MT1-MMP in null BM progenitors significantly increased Rac1 membrane targeting and activity (Figures 5C and 5D), demonstrating that MT1-MMP is required for proper Rac1 activity in these cells. Rac1 activity in null progenitors was also increased by re-expression of the inactive mutant MT1-MMPE240A, indicating that the catalytic activity of MT1-MMP is dispensable for its regulation of Rac1 (Figure 5D).
To assess whether this impaired Rac1 activity contributed to the observed migration and fusion defects, we expressed constitutively active Rac1 (Guo and Zheng, 2004) in MT1-MMP-null myeloid progenitors. Retroviral expression of active Rac1 in MT1-MMP-null cells replicated the effects of MT1-MMP re-expression on cell spreading, morphology, membrane protrusive activity, and motility (Figures 5E and 5F); accordingly, active Rac1 expression rescued the OC multinucleation defect of MT1-MMP-null progenitors (Figure 5G).
Association of the MT1-MMP cytosolic tail with p130Cas is involved in its regulation of cell morphology, motility and fusion in myeloid progenitors
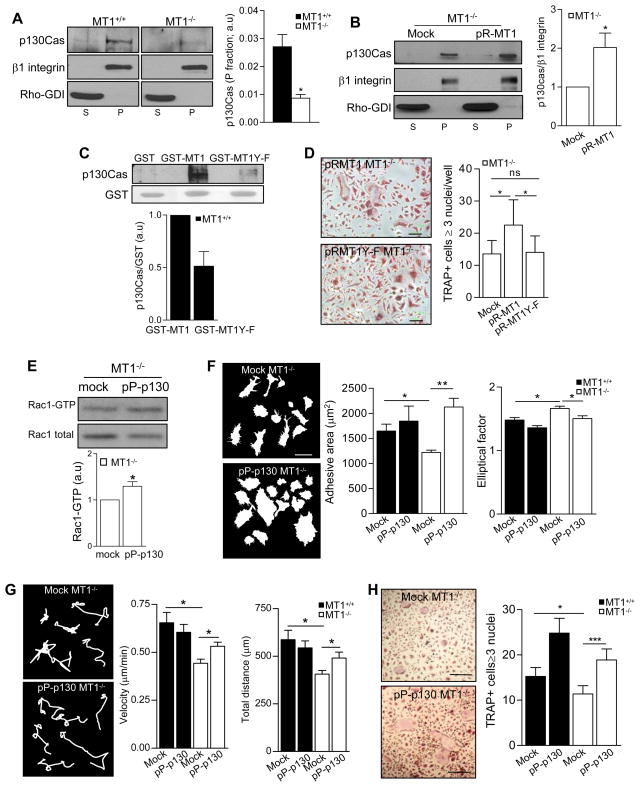
MT1-MMP can interact with the focal adhesion protein p130Crk-associated substrate (CAS) in endothelial cells (Gingras et al., 2008), and the p130Cas-Crk complex participates in Rac1 membrane targeting in certain cell types (Albert et al., 2000). The amount of p130Cas in the membrane fraction was significantly decreased in MT1-MMP-null progenitors (Figure 6A). In addition, colocalization of p130Cas with the leukocyte membrane marker MHCII was barely detectable by immunofluorescence in MT1-MMP-null progenitors, whereas it was observed in WT cells (Figure S6). Retroviral re-expression of MT1-MMP significantly increased the amount of p130Cas in the membrane fraction of null myeloid progenitors (Figure 6B). These data suggest that MT1-MMP is required for proper p130Cas localization at the cell membrane of these cells.
Figure 6.
Association of the cytosolic tail of MT1-MMP with p130Cas in myeloid progenitors contributes to its roles in Rac1 activity, cell morphology, motility and fusion. (A) p130Cas expression in WT and MT1-MMP null myeloid progenitors was analyzed by cell fractionation as in Figure 5B. The histogram shows densitometric quantification of the p130Cas/β1 integrin ratio in the particulate fraction (n=4 experiments from 20 mice). (B) BM progenitors from WT or MT1-MMP-null mice were infected with retrovirus encoding human MT1-MMP (pR-MT1) and cultured with M-CSF and RANKL for 4 days. Cells were fractionated, and particulate (P) and soluble (S) fractions analyzed by western blot for p130Cas; a representative blot is shown. The histogram shows densitometric quantification of the p130Cas/β1 integrin ratio in the particulate fraction (n=5 independent experiments from 15 mice). (C) GST fusion proteins of the MT1-MMP cytosolic tail (GST-MT1) and a mutated version in which Y573 is replaced by F (GST-MT1Y-F) were used in pull-down assays with WT BM cells treated for 4 days with RANKL+M-CSF. A representative blot of p130Cas is shown. The histogram shows densitometric quantification of the p130Cas/GST ratio normalized to MT1-GST values (n=3). (D) MT1-MMP-null BM progenitors were infected with retrovirus encoding non-mutated or Y573F mutated human MT1-MMP (pR-MT1 or pR-MT1Y-F) and cultured under osteoclastogenic conditions for 12 days. Images of TRAP+ cells are shown. Bar, 100 μm. Histogram shows the number of TRAP+ cells containing ≥3 nuclei (n=10). (E) MT1-MMP-null BM progenitors were infected with retrovirus encoding p130Cas (pP-p130Cas), cultured with M-CSF and RANKL for 4 days, and lysed for pull-down assays with GST-PBD. A representative blot is shown. The histogram shows the average fold induction of active Rac1 quantified by densitometric analysis (n=3 independent experiments from 9 mice). (F) BM progenitors from WT or MT1-MMP-null mice were infected as in (E). Morphological parameters were analyzed as in Figure 3E. A total of 100 cells were analyzed per genotype (n=5). Histograms show the average adhesive area and EF. (G) Cells were infected and cultured as in (E) and motility parameters were measured as in Figure 3B for at least 100 cells per genotype (n=4). Histograms show the velocity and total distance traveled in the different conditions (n=4). (H) BM cells infected with pP-p130Cas were cultured in osteoclastogenic conditions for 12 days. Images of TRAP+ cells are shown. Bar, 100 μm. Histogram shows the number of TRAP+ cells containing >3 nuclei (n=8). See also Figure S6.
Pull-down assays in myeloid progenitors showed that p130Cas can bind the cytosolic domain of MT1-MMP; moreover, the mutant MT1-MMPY573F bound p130Cas less efficiently, showing that MT1-MMP Tyr 573 participates in the association (Figure 6C). Retroviral expression of MT1-MMPY573F did not rescue the OC multinucleation phenotype of MT1-MMP-null cells (Figure 6D), indicating that interaction between MT1-MMP and p130Cas is required for this event.
The role of p130Cas in the phenotype of MT1-MMP-null myeloid progenitors was analyzed by retroviral infection with p130Cas, which increases p130Cas expression in both the cytosolic and membrane fractions (unpublished data). Overexpression of p130Cas increased Rac1 activity in MT1-MMP-null myeloid cells, suggesting that p130Cas is a key intermediate between MT1-MMP and Rac1 in these cells (Figure 6E). Consistently, p130Cas overexpression increased the adhesive area of MT1-MMP-null cells and decreased their EF (Figure 6F), increased their velocity and migrated distance (Figure 6G), and rescued their OC multinucleation defect (Figure 6H), mimicking the effect of re-expressed MT1-MMP or active Rac1.
DISCUSSION
In this report we identify a new function of MT1-MMP, independent of its catalytic activity, in the formation of multinucleated OC and GC from myeloid progenitors. This function is mediated by regulation of Rac1 via a novel signaling pathway involving association of the MT1-MMP cytosolic tail with p130Cas. MT1-MMP thus regulates the morphology, motility and fusion of BM myeloid progenitors.
Macrophage cell fusion occurs in distinct pathophysiological settings (Vignery, 2005). CD44, CD47 and its ligand MFR/SIRP1α, DC-STAMP, CD200, and the d2 isoform of v-ATPase are implicated in macrophage fusion, but the underlying molecular mechanisms are not well defined (Cui et al., 2007; Cui et al., 2006; Lee et al., 2006; Yagi et al., 2005). Within the metzincin protease superfamily, members of the ADAM subfamily were suggested to participate in cell fusion events, although this remains controversial (Primakoff and Myles, 2007); and MMP-9 participates in GC formation (Maclauchlan et al., 2009). The demonstration that MT1-MMP contributes to macrophage multinucleation in distinct cell contexts identifies a novel participant in this process and a new function for this protein.
The physiological relevance of these findings is highlighted by the decreased bone resorption in newborn MT1-MMP-null mice, as indicated by lower amounts of serum ICTP. However, this impaired OC function does not result in increased bone mass, in contrast to other mouse models of defective OC fusion such as DC-STAMP and d2 v-ATPase null mice (Lee et al., 2006; Yagi et al., 2005). Osteoblast function is also compromised in MT1-MMP null mice, and this might contribute to the complex OC phenotype observed, possibly suggesting a dual role for MT1-MMP in bone development, with actions in OC-mediated bone resorption and also in osteoblast bone deposition. It will be interesting to dissect this dual role in the future by conditional MT1-MMP deletion in OC and osteoblasts, once a floxed allele is available. Furthermore, the drastic reduction of GC formation in MT1-MMP null neonates and the impairment of this process in heterozygotes suggest that a minimum amount of MT1-MMP is required for an effective inflammatory response to foreign bodies, reinforcing the importance of MT1-MMP in this inflammatory situation. Hence, cell-type specific modulation of macrophage MT1-MMP might have potential in the treatment of disorders involving either increased OC-mediated bone resorption (such as osteoporosis and bone metastasis) or chronic formation of GC (such as granulomatous disease). Whether MT1-MMP participates in heterotypic fusion events relevant to inflammation also deserves further work (Johansson et al., 2008; Nygren et al., 2008).
Cell fusion is a complex process in which several sequential steps must be finely orchestrated. It is unlikely that MT1-MMP acts as a direct fusogen (Oren-Suissa and Podbilewicz, 2007), and we therefore explored alternative mechanisms by which MT1-MMP might contribute to macrophage fusion, such as the modulation of membrane receptors. MT1-MMP can process the hyaluronan receptor CD44, which has diverse roles in OC development and macrophage fusion (Cui et al., 2006; de Vries et al., 2005; Kajita et al., 2001). However, we found no link between impaired CD44 shedding in the absence of MT1-MMP and the multinucleation defect (unpublished data).
MT1-MMP participates in human monocyte migration and transmigration (Matias-Roman et al., 2005), and efficient migration is required for proper macrophage fusion. Our data showing defective chemotaxis and motility of MT1-MMP-null BM cells and the partial rescue of the multinucleation phenotype in high density cultures indicate that impaired locomotion contributes to the fusion defect in the absence of MT1-MMP. However, the incomplete rescue even at high cell densities indicates that other MT1-MMP actions are involved, including regulation of cell spreading and lamellipodia formation, important events in the fusion process.
MT1-MMP is a pericellular collagenase also involved in the proteolytic activation of pro-MMP-2 (Sato et al., 1994). Similar to its role in lung and submandibular gland development (Oblander et al., 2005), the contribution of MT1-MMP to OC and GC formation was found to be independent of pro-MMP-2 activation since the absence of MMP-2 did not impair these processes or alter the morphology and motility of myeloid progenitors. More importantly, the demonstration that retroviral re-expression of an inactive MT1-MMP mutant rescues the morphology, motility and fusion phenotypes of null progenitors provides convincing evidence in support of important pathophysiological functions of MT1-MMP unrelated to its proteolytic activity (Sakamoto and Seiki, 2009).
Signaling pathways involved in cell-cell fusion have mainly been analyzed in mating yeast and myoblast fusion in D. melanogaster. The Drosophila Duf-Ants-Mbc-Rac-Scar pathway and in mammals the guanine-nucleotide exchange factors (GEFs) Brag2 and Dock180 (Mbc mammalian homolog), which respectively act on ADP-ribosylation factor 6 (ARF6) and Rac1, are important for myoblast fusion (Chen et al., 2007; Pajcini et al., 2008). The Dock180/Rac1 pathway also participates in the formation of multinucleated GC (Pajcini et al., 2008). MT1-MMP is identified as an upstream regulator of Rac1 activity in BM progenitors that might act at a similar hierarchical level to Duf in Drosophila myoblast fusion. Our findings also support the participation of Rac1 in macrophage fusion during OC and GC formation in vivo. Rac1 might contribute to myeloid cell and myoblast fusion through distinct mechanisms; for example Rac1 regulates migration and actin assembly in myeloid pre-OC but regulates post-migration events during myoblast fusion (Wang et al., 2008; Vasyutina et al., 2009). MT1-MMP-null mice also display myoblast fusion defects and therefore, similarly to Rac1, the contribution of MT1-MMP may be different in these two distinct fusion scenarios (this report and Ohtake et al., 2006).
MT1-MMP acts as a positive regulator of Rac1 in primary BM progenitors, with Rac1 activity in MT1-MMP-null OC progenitors less than 25% that in WT cells. This would place MT1-MMP-null progenitors between stages I and II on the Pankov scale, with very low Rac activity and almost no migration (Pankov et al., 2005). This low Rac1 activity might explain most of the phenotypes of MT1-MMP-null progenitors, including small size, elongated morphology, low lamellipodia activity and impaired migration (Wang et al., 2008; Wells et al., 2004); and in fact the low lamellipodia activity might contribute, together with impaired migration, to the defective cell fusion observed in the absence of MT1-MMP (Jay et al., 2007). The chemotactic defects in BM cells might, however, be explained by impaired Cdc42 activity (Allen et al., 1998), which was observed in MT1-MMP-null progenitors (unpublished data). Rac1 might also regulate BM progenitor motility by modulating focal complex formation, consistent with the reduction in vinculin-positive structures at the lamellipodia of MT1-MMP-null progenitors. This pattern might indicate an action of MT1-MMP in the recruitment of focal adhesion components or in the modulation of integrin binding or recycling at these sites. This idea is supported by the suggestion that MT1-MMP can participate in focal adhesion turnover (Takino et al., 2006). In cell contexts in which Rac1 activity is deregulated or dispensable, it may be that the requirement of MT1-MMP for migration on 2D-matrices can be circumvented (Sabeh et al., 2004).
Rac1 activity can be regulated at the levels of GTP loading and cell membrane targeting; and Rac1 membrane targeting is in fact reduced in the absence of MT1-MMP. The primary event triggering Rac1 membrane targeting is integrin binding to the extracellular matrix (del Pozo et al., 2002). MT1-MMP can associate with αvβ3 and β1 integrins in distinct cell contexts, and MT1-MMP localizes to adhesion sites in response to integrin ligation through a Rab8-mediated mechanism (Bravo-Cordero et al., 2007; Galvez et al., 2002). It is possible that MT1-MMP acts as an integrin co-receptor, modulating Rac1 targeting. Consistent with this, both MT1-MMP and Rac1 are localized at similar lipid-rich membrane microdomains (del Pozo et al., 2004; Galvez et al., 2004), but it is not known if MT1-MMP influences integrin-mediated Rac1 targeting at these domains.
The independence of MT1-MMP-mediated Rac1 regulation from proteolytic activity indicates a mechanism not involving the extracellular catalytic domain. The p130Cas-Crk adaptor complex is another promoter of Rac1 membrane targeting (Cho and Klemke, 2002), and the MT1-MMP cytosolic tail can interact with p130Cas in endothelial cells (Gingras et al., 2008). Our data confirm the MT1-MMP–p130Cas interaction and show that MT1-MMP Tyr 573, in the cytosolic tail, plays a role in this association. MT1-MMP participates in the recruitment of p130Cas to the myeloid progenitor cell membrane, as shown by the decrease in p130Cas membrane targeting in MT1-MMP-null progenitors and the rescue of this targeting by re-expression of MT1-MMP in null cells. Since the p130Cas-Crk complex can act upstream of Cdc42 (Liu et al., 2007), the altered membrane localization of p130Cas might also contribute to the impaired Cdc42 activity found in MT1-MMP-null progenitors (unpublished data). The importance of the interaction of Tyr 573 with p130Cas for myeloid cell fusion is demonstrated by the inability of the MT1-MMPY573F mutant to rescue the multinucleation phenotype. Moreover, the increased Rac1 activity in MT1-MMP-null cells overexpressing p130Cas indicates that p130Cas is a signaling intermediate between MT1-MMP and Rac1. However, additional mechanisms cannot be ruled out, such as regulation by the MT1-MMP cytosolic tail of Rac1 GEFs; in this regard, the MT1-MMP cytosolic tail can interact with p27RF-Rho, a regulator of RhoA activation (Hoshino et al., 2009).
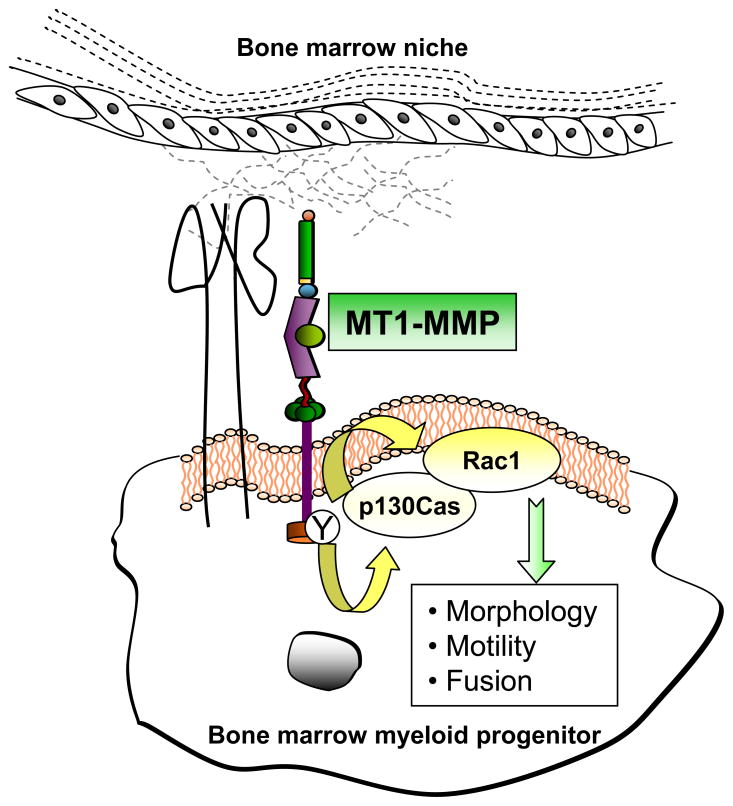
Our results suggest a model in which MT1-MMP at the membrane of BM myeloid progenitors contributes to the efficient recruitment and activation of the p130Cas-Rac1 complex at lamellipodia (Figure 7). Impairment of this signaling pathway in the absence of MT1-MMP would decrease lamellipodia activity and cell migration, resulting in inefficient cell fusion.
Figure 7.
MT1-MMP contributes to myeloid cell fusion by regulating Rac1 signaling. MT1-MMP is expressed in BM myeloid progenitors and is required for optimal Rac1 activity in these cells. The functional impact of this regulation is underlined by the defects in morphology, motility and cell fusion observed in the absence of MT1-MMP and that can be rescued by constitutively active Rac1. The role of MT1-MMP in this process does not require its catalytic activity. Instead, the MT1-MMP cytosolic tail can bind the adaptor protein p130Cas, and MT1-MMP Tyr573 seems to play a role in this association. p130Cas acts as an intermediate modulator of Rac1 activity in this setting since overexpression of p130Cas also rescues Rac1 activity and the multinucleation phenotype in MT1-MMP-null progenitors.
EXPERIMENTAL PROCEDURES
Mice and cells
Mmp14-deficient mice (MT1-MMP−/−) in the C57BL/6 background were generated as described (Zhou et al., 2000). Mmp2-deficient mice (Itoh et al., 1997) were kindly provided by S.J. Weiss (University of Michigan, Ann Arbor, MI). Mice were handled under pathogen-free conditions in accordance with institutional guidelines; the animal protocols were approved by the institutional committee. Experiments were performed with 8 day-old MT1-MMP−/− or WT littermates unless otherwise indicated. Single cell suspensions were obtained from bone marrow (BM) and spleen; only data from BM cells are presented. Serum was obtained from peripheral blood incubated for 1 h at 37°C. Human umbilical vein endothelial cells (HUVEC) were cultured as described (Galvez et al., 2002).
Osteoclastogenesis and dentine resorption assays
OC were generated from BM or spleen progenitors. Cells were seeded at 1.5×105 per well in 96-well plates in triplicate and incubated in α-MEM supplemented with 10% FBS, 25 ng/ml M-CSF and 25 ng/ml RANKL (PeproTech). After washing on day 3, M-CSF was decreased to 10 ng/ml and RANKL kept at 25 ng/ml. Cultures were stained with tartrate resistant acid phosphatase (TRAP; Sigma) on day 12. Percentage of fusion was calculated from the number of TRAP+ cells containing ≥ 3 nuclei with respect to the total number of TRAP+ cells. Resorption was assayed by culturing progenitors on dentine plates (Biocoat, OAAS™) for 16 days. Areas of dentine resorption were measured with Laserpix software. DiO and DiI probes were from Molecular Probes.
Giant cell formation
GC formation in vivo was induced as described (Mariano and Spector, 1974). Glass coverslips (5 or 12 mm diameter) were implanted subcutaneously in the backs of mice, and after 4 days the coverslips were removed and stained with hematoxylin & eosin (H&E). GC formation was also induced in vitro (Helming and Gordon, 2007). BM progenitors were cultured in the presence of 25 ng/ml M-CSF. After 3 days, cells were plated at 1.5×105 cells/well in 96-well plates with 50 ng/ml IL4 (PeproTech) and cultured for a further 4 days. Cells were stained with H&E and Hoechst 33342 (Sigma). The number of nuclei per multinucleated cell was counted by two blinded observers; at least 20 cells were counted per condition.
GTP-ase pull-down assay, subcellular fractionation, and western blot analysis
Rac1 activity was determined by GST-PBD (Pak1 p21-binding domain) pull-down as described (del Pozo et al., 2002). Particulate and soluble fractions were obtained from cultured OC progenitors (del Pozo et al., 2002), and equal protein amounts were analyzed by western blot; densitometry analysis was performed with Quantity-one or Image J software. The percentage of Rac1 and the amount of p130Cas in the particulate fraction (normalized to β1 integrin levels) were calculated. Anti-Rac was from Upstate Biotechnology, and anti-p130Cas mAb was from Pharmingen.
Time-lapse microscopy imaging
BM cells were cultured with M-CSF and RANKL for 4 days, and phase-contrast images were collected with a LEICA microscope with a 10× objective. Images were analyzed with MetaMorph software (Universal Imaging Corp). To determine cell trajectories in phase-contrast image series (images captured at 15-min intervals over 16 h), the centroids of the cell nuclei were tracked. Tracking analysis was quantified in 12 cells from n independent movies using the track object function.
Quantification of cell adhesive area and elliptical factor (EF)
F-actin-stained OC progenitors (after 4-day culture with M-CSF and RANKL) were outlined using the Kirsch edge detection algorithm included in the MetaMorph software. Outlines were checked and corrected by hand if necessary. Using MetaMorph’s integrated morphometry analysis function, cell EF (length/breadth) was also determined as a measure of elongation.
Statistical analysis
Data are shown as arithmetic means +/− S.E. for the independent experiments performed for each condition determined with Prism 3.0 (GraphPad Software). Statistical differences among experimental groups were evaluated by Student’s t-test; differences were considered statistically significant at *p ≤0.05, **p ≤0.01, and ***p ≤0.001.
Supplementary Material
Acknowledgments
We thank Dr. K. Tryggvason for the generation of MT1-MMP-null mice, Dr. X. Bustelo for providing the Rac1 retroviral construct, J.C. Ramírez for help with retrovirus production, and S. Bartlett for editing. This work was supported by National Institutes of Health Grant AR47074 (to SSA), the Fundación Ramón Areces and the Spanish Fondo de Investigación Sanitaria grant RD06/0014/1016 (to AGA), the Spanish Ministry of Science and Innovation, through grants SAF2008-02100 to MAdP and SAF2008-02104 to AGA, and by EUROHORCS (European Heads Of Research Councils) and the European Science Foundation (ESF) through a EURYI (European Young Investigator) award to MadP. PG was funded by the ‘Juan de la Cierva’ program and the Fondo de Investigación Sanitaria. MCG and MVHR are supported by fellowships BES-2006-13204 and 12790 from the Spanish Ministry of Science and Innovation. The CNIC is supported by the Spanish Ministry of Science and Innovation and the Pro-CNIC Foundation.
Footnotes
Author contributions: P.G. designed and performed research; M.C.G., M.V.H.R., A.P., A.G.G., A.V., and J.R performed research; R.A.B., C.A., R.C., J.T., and S.S.A. contributed reagents and analytic tools; M.A.P. contributed reagents and discussion; and A.G.A. designed the research and wrote the paper.
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert ML, Kim JI, Birge RB. alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol. 2000;2:899–905. doi: 10.1038/35046549. [DOI] [PubMed] [Google Scholar]
- Allen WE, Zicha D, Ridley AJ, Jones GE. A role for Cdc42 in macrophage chemotaxis. J Cell Biol. 1998;141:1147–1157. doi: 10.1083/jcb.141.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Marrero-Diaz R, Megias D, Genis L, Garcia-Grande A, Garcia MA, Arroyo AG, Montoya MC. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 2007;26:1499–1510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Cuartas E, Ke J, Zhang Q, Einarsson HB, Sedgwick JD, Li J, Vignery A. CD200 and its receptor, CD200R, modulate bone mass via the differentiation of osteoclasts. Proc Natl Acad Sci U S A. 2007;104:14436–14441. doi: 10.1073/pnas.0702811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Ke JZ, Zhang Q, Ke HZ, Chalouni C, Vignery A. The intracellular domain of CD44 promotes the fusion of macrophages. Blood. 2006;107:796–805. doi: 10.1182/blood-2005-05-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EH, Grote E, Mohler W, Vignery A. Cell-cell fusion. FEBS Lett. 2007;581:2181–2193. doi: 10.1016/j.febslet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- Cho SY, Klemke RL. Purification of pseudopodia from polarized cells reveals redistribution and activation of Rac through assembly of a CAS/Crk scaffold. J Cell Biol. 2002;156:725–736. doi: 10.1083/jcb.200111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- de Vries TJ, Schoenmaker T, Beertsen W, van der Neut R, Everts V. Effect of CD44 deficiency on in vitro and in vivo osteoclast formation. J Cell Biochem. 2005;94:954–966. doi: 10.1002/jcb.20326. [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Kiosses WB, Alderson NB, Meller N, Hahn KM, Schwartz MA. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol. 2002;4:232–239. doi: 10.1038/ncb759. [DOI] [PubMed] [Google Scholar]
- Galvez BG, Matias-Roman S, Yanez-Mo M, Sanchez-Madrid F, Arroyo AG. ECM regulates MT1-MMP localization with beta1 or alphavbeta3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J Cell Biol. 2002;159:509–521. doi: 10.1083/jcb.200205026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez BG, Matias-Roman S, Yanez-Mo M, Vicente-Manzanares M, Sanchez-Madrid F, Arroyo AG. Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol Biol Cell. 2004;15:678–687. doi: 10.1091/mbc.E03-07-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras D, Michaud M, Di Tomasso G, Beliveau E, Nyalendo C, Beliveau R. Sphingosine-1-phosphate induces the association of membrane-type 1 matrix metalloproteinase with p130Cas in endothelial cells. FEBS Lett. 2008;582:399–404. doi: 10.1016/j.febslet.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Guo F, Zheng Y. Rho family GTPases cooperate with p53 deletion to promote primary mouse embryonic fibroblast cell invasion. Oncogene. 2004;23:5577–5585. doi: 10.1038/sj.onc.1207752. [DOI] [PubMed] [Google Scholar]
- Helming L, Gordon S. Macrophage fusion induced by IL-4 alternative activation is a multistage process involving multiple target molecules. Eur J Immunol. 2007;37:33–42. doi: 10.1002/eji.200636788. [DOI] [PubMed] [Google Scholar]
- Henriksen K, Karsdal M, Delaisse JM, Engsig MT. RANKL and vascular endothelial growth factor (VEGF) induce osteoclast chemotaxis through an ERK1/2-dependent mechanism. J Biol Chem. 2003;278:48745–48753. doi: 10.1074/jbc.M309193200. [DOI] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Hoshino D, Tomari T, Nagano M, Koshikawa N, Seiki M. A novel protein associated with MT1-MMP binds p27kip1, and regulates RhoA activation, actin remodelling and matrigel invasion. J Biol Chem. 2009;284:27315–27326. doi: 10.1074/jbc.M109.041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997;272:22389–22392. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- Jay SM, Skokos E, Laiwalla F, Krady MM, Kyriakides TR. Foreign body giant cell formation is preceded by lamellipodia formation and can be attenuated by inhibition of Rac1 activation. Am J Pathol. 2007;171:632–640. doi: 10.2353/ajpath.2007.061213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Youssef S, Koleckar K, Holbrook C, Doyonnas R, Corbel SY, Steinman L, Rossi FM, Blau HM. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol. 2008;10:575–583. doi: 10.1038/ncb1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Day CJ, Morrison NA. MCP-1 is induced by receptor activator of nuclear factor-{kappa}B ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J Biol Chem. 2005;280:16163–16169. doi: 10.1074/jbc.M412713200. [DOI] [PubMed] [Google Scholar]
- Kiviranta R, Morko J, Alatalo SL, NicAmhlaoibh R, Risteli J, Laitala-Leinonen T, Vuorio E. Impaired bone resorption in cathepsin K-deficient mice is partially compensated for by enhanced osteoclastogenesis and increased expression of other proteases via an increased RANKL/OPG ratio. Bone. 2005;36:159–172. doi: 10.1016/j.bone.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, Kang JS, Miyamoto T, Suda T, Lee SK, et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med. 2006;12:1403–1409. doi: 10.1038/nm1514. [DOI] [PubMed] [Google Scholar]
- Liu G, Li W, Gao X, Li X, Jurgensen C, Park HT, Shin NY, Yu J, He ML, Hanks SK, et al. p130CAS is required for netrin signaling and commissural axon guidance. J Neurosci. 2007;27:957–968. doi: 10.1523/JNEUROSCI.4616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclauchlan S, Skokos EA, Meznarich N, Zhu DH, Raoof S, Shipley JM, Senior RM, Bornstein P, Kyriakides TR. Macrophage fusion, giant cell formation, and the foreign body response require matrix metalloproteinase 9. J Leukoc Biol. 2009;85:617–626. doi: 10.1189/jlb.1008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano M, Spector WG. The formation and properties of macrophage polykaryons (inflammatory giant cells) J Pathol. 1974;113:1–19. doi: 10.1002/path.1711130102. [DOI] [PubMed] [Google Scholar]
- Martignetti JA, Aqeel AA, Sewairi WA, Boumah CE, Kambouris M, Mayouf SA, Sheth KV, Eid WA, Dowling O, Harris J, et al. Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat Genet. 2001;28:261–265. doi: 10.1038/90100. [DOI] [PubMed] [Google Scholar]
- Matias-Roman S, Galvez BG, Genis L, Yanez-Mo M, de la Rosa G, Sanchez-Mateos P, Sanchez-Madrid F, Arroyo AG. Membrane type 1-matrix metalloproteinase is involved in migration of human monocytes and is regulated through their interaction with fibronectin or endothelium. Blood. 2005;105:3956–3964. doi: 10.1182/blood-2004-06-2382. [DOI] [PubMed] [Google Scholar]
- Mohler WA, Shemer G, del Campo JJ, Valansi C, Opoku-Serebuoh E, Scranton V, Assaf N, White JG, Podbilewicz B. The type I membrane protein EFF-1 is essential for developmental cell fusion. Dev Cell. 2002;2:355–362. doi: 10.1016/s1534-5807(02)00129-6. [DOI] [PubMed] [Google Scholar]
- Mosig RA, Dowling O, Difeo A, Ramirez MC, Parker IC, Abe E, Diouri J, Aqeel AA, Wylie JD, Oblander SA, et al. Loss of MMP-2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth. Hum Mol Genet. 2007;16:1113–1123. doi: 10.1093/hmg/ddm060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren JM, Liuba K, Breitbach M, Stott S, Thoren L, Roell W, Geisen C, Sasse P, Kirik D, Bjorklund A, et al. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat Cell Biol. 2008;10:584–592. doi: 10.1038/ncb1721. [DOI] [PubMed] [Google Scholar]
- Oblander SA, Zhou Z, Galvez BG, Starcher B, Shannon JM, Durbeej M, Arroyo AG, Tryggvason K, Apte SS. Distinctive functions of membrane type 1 matrix-metalloprotease (MT1-MMP or MMP-14) in lung and submandibular gland development are independent of its role in pro-MMP-2 activation. Dev Biol. 2005;277:255–269. doi: 10.1016/j.ydbio.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Ohtake Y, Tojo H, Seiki M. Multifunctional roles of MT1-MMP in myofiber formation and morphostatic maintenance of skeletal muscle. J Cell Sci. 2006;119:3822–3832. doi: 10.1242/jcs.03158. [DOI] [PubMed] [Google Scholar]
- Oren-Suissa M, Podbilewicz B. Cell fusion during development. Trends Cell Biol. 2007;17:537–546. doi: 10.1016/j.tcb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajcini KV, Pomerantz JH, Alkan O, Doyonnas R, Blau HM. Myoblasts and macrophages share molecular components that contribute to cell-cell fusion. J Cell Biol. 2008;180:1005–1019. doi: 10.1083/jcb.200707191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov R, Endo Y, Even-Ram S, Araki M, Clark K, Cukierman E, Matsumoto K, Yamada KM. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P, Myles DG. Cell-cell membrane fusion during mammalian fertilization. FEBS Lett. 2007;581:2174–2180. doi: 10.1016/j.febslet.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saginario C, Qian HY, Vignery A. Identification of an inducible surface molecule specific to fusing macrophages. Proc Natl Acad Sci U S A. 1995;92:12210–12214. doi: 10.1073/pnas.92.26.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Seiki M. Cytoplasmic tail of MT1-MMP regulates macrophage motility independently from its protease activity. Genes Cells. 2009;14:617–626. doi: 10.1111/j.1365-2443.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- Sapir A, Choi J, Leikina E, Avinoam O, Valansi C, Chernomordik LV, Newman AP, Podbilewicz B. AFF-1, a FOS-1-regulated fusogen, mediates fusion of the anchor cell in C. elegans. Dev Cell. 2007;12:683–698. doi: 10.1016/j.devcel.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Takino T, Watanabe Y, Matsui M, Miyamori H, Kudo T, Seiki M, Sato H. Membrane-type 1 matrix metalloproteinase modulates focal adhesion stability and cell migration. Exp Cell Res. 2006;312:1381–1389. doi: 10.1016/j.yexcr.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Vasyutina E, Martarelli B, Brakebusch C, Wende H, Birchmeier C. The small G-proteins Rac1 and Cdc42 are essential for myoblast fusion in the mouse. Proc Natl Acad Sci U S A. 2009;106:8935–8940. doi: 10.1073/pnas.0902501106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignery A. Macrophage fusion: the making of osteoclasts and giant cells. J Exp Med. 2005;202:337–340. doi: 10.1084/jem.20051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lebowitz D, Sun C, Thang H, Grynpas MD, Glogauer M. Identifying the relative contributions of Rac1 and Rac2 to osteoclastogenesis. J Bone Miner Res. 2008;23:260–270. doi: 10.1359/jbmr.071013. [DOI] [PubMed] [Google Scholar]
- Wells CM, Walmsley M, Ooi S, Tybulewicz V, Ridley AJ. Rac1-deficient macrophages exhibit defects in cell spreading and membrane ruffling but not migration. J Cell Sci. 2004;117:1259–1268. doi: 10.1242/jcs.00997. [DOI] [PubMed] [Google Scholar]
- Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202:345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MX, Qu X, Kong BH, Lam QL, Shao QQ, Deng BP, Ko KH, Lu L. Membrane type 1-matrix metalloproteinase is involved in the migration of human monocyte-derived dendritic cells. Immunol Cell Biol. 2006;84:557–562. doi: 10.1111/j.1440-1711.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.