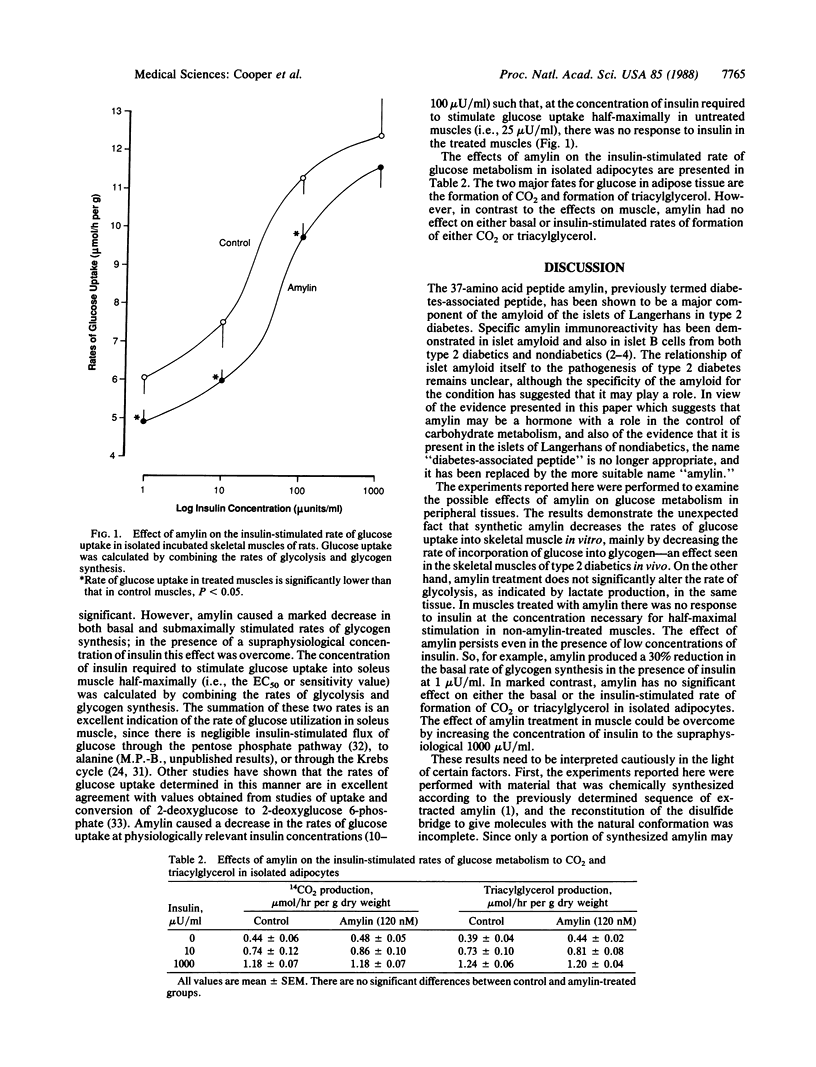
Abstract
Diabetes-associated peptide has recently been isolated and characterized from the amyloid of the islets of Langerhans in type 2 (non-insulin-dependent) diabetics, and immunoreactivity with antibodies to the peptide has been demonstrated in islet B cells of both normal and type 2 diabetic subjects. In view of the evidence presented in this paper that this 37-amino acid peptide may be a hormone present in normal individuals, we now propose the name "amylin" to replace "diabetes-associated peptide." Because increased amylin, deposited as amyloid within the islets of Langerhans, is characteristic of type 2 diabetes, the study below was performed to examine the possible effects of amylin on peripheral glucose metabolism. Whole amylin was synthesized by using solid-phase techniques, with formation of the disulfide linkage by oxidation in dilute aqueous solution and recovery of the peptide by lyophilization. The effects of amylin on glucose metabolism were studied in two preparations in vitro, isolated rat soleus muscle strips and isolated rat adipocytes. In skeletal muscle exposed to 120 nM amylin for 1 hr, there was a marked decrease in both basal and submaximally insulin-stimulated rates of glycogen synthesis, which resulted in significant reduction in the rates of insulin-stimulated glucose uptake. In muscles treated with amylin there was no response at the concentration of insulin required to stimulate glucose uptake half-maximally in untreated (control) muscles. In marked contrast, amylin had no effect on either basal or insulin-stimulated rates of glucose incorporation into either CO2 or triacylglycerol in isolated adipocytes. Therefore, amylin may be a factor in the etiology of the insulin resistance in type 2 diabetes mellitus, as both deposition of the peptide in islet amyloid and decreased rates of glucose uptake and glycogen synthesis in skeletal muscle are characteristic of this condition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berhanu P., Tsai P., Olefsky J. M. Insulin-stimulated glucose transport in cultured fibroblasts from normal and noninsulin-dependent (type II) diabetic human subjects. J Clin Endocrinol Metab. 1982 Dec;55(6):1226–1230. doi: 10.1210/jcem-55-6-1226. [DOI] [PubMed] [Google Scholar]
- Boden G., Ray T. K., Smith R. H., Owen O. E. Carbohydrate oxidation and storage in obese non-insulin-dependent diabetic patients. Effects of improving glycemic control. Diabetes. 1983 Nov;32(11):982–987. doi: 10.2337/diab.32.11.982. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Stone K., Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J Clin Invest. 1984 Apr;73(4):1185–1190. doi: 10.1172/JCI111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challiss R. A., Espinal J., Newsholme E. A. Insulin sensitivity of rates of glycolysis and glycogen synthesis in soleus, stripped soleus, epitrochlearis, and hemi-diaphragm muscles isolated from sedentary rats. Biosci Rep. 1983 Jul;3(7):675–679. doi: 10.1007/BF01172878. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Lozeman F. J., Leighton B., Newsholme E. A. Effects of the beta-adrenoceptor agonist isoprenaline on insulin-sensitivity in soleus muscle of the rat. Biochem J. 1986 Jan 15;233(2):377–381. doi: 10.1042/bj2330377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Clark A., Cooper G. J., Lewis C. E., Morris J. F., Willis A. C., Reid K. B., Turner R. C. Islet amyloid formed from diabetes-associated peptide may be pathogenic in type-2 diabetes. Lancet. 1987 Aug 1;2(8553):231–234. doi: 10.1016/s0140-6736(87)90825-7. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Willis A. C., Clark A., Turner R. C., Sim R. B., Reid K. B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. J., Willis A. C., Reid K. B., Clark A., Baker C. A., Turner R. C., Lewis C. E., Morris J. F., Howland K., Rothbard J. B. Diabetes-associated peptide. Lancet. 1987 Oct 24;2(8565):966–966. doi: 10.1016/s0140-6736(87)91444-9. [DOI] [PubMed] [Google Scholar]
- Crettaz M., Prentki M., Zaninetti D., Jeanrenaud B. Insulin resistance in soleus muscle from obese Zucker rats. Involvement of several defective sites. Biochem J. 1980 Feb 15;186(2):525–534. doi: 10.1042/bj1860525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuendet G. S., Loten E. G., Jeanrenaud B., Renold A. E. Decreased basal, noninsulin-stimulated glucose uptake and metabolism by skeletal soleus muscle isolated from obese-hyperglycemic (ob/ob) mice. J Clin Invest. 1976 Nov;58(5):1078–1088. doi: 10.1172/JCI108559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T. A., Klahr S., Tegtmeyer E. D., Osborne D. F., Howard T. L., Karl I. E. Glucose metabolism in epitrochlearis muscle of acutely exercised and trained rats. Am J Physiol. 1986 Feb;250(2 Pt 1):E137–E143. doi: 10.1152/ajpendo.1986.250.2.E137. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Gunnarsson R., Björkman O., Olsson M., Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985 Jul;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R., Deibert D., Hendler R., Felig P., Soman V. Insulin sensitivity and insulin binding to monocytes in maturity-onset diabetes. J Clin Invest. 1979 May;63(5):939–946. doi: 10.1172/JCI109394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Engel P. C., Jones J. B. Causes and elimination of erratic blanks in enzymatic metabolite assays involving the use of NAD+ in alkaline hydrazine buffers: improved conditions for the assay of L-glutamate, L-lactate, and other metabolites. Anal Biochem. 1978 Aug 1;88(2):475–484. doi: 10.1016/0003-2697(78)90447-5. [DOI] [PubMed] [Google Scholar]
- Espinal J., Dohm G. L., Newsholme E. A. Sensitivity to insulin of glycolysis and glycogen synthesis of isolated soleus-muscle strips from sedentary, exercised and exercise-trained rats. Biochem J. 1983 May 15;212(2):453–458. doi: 10.1042/bj2120453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A., Newsholme E. A. Sensitivity of glucose uptake and lipolysis of white adipocytes of the rat to insulin and effects of some metabolites. Biochem J. 1979 May 15;180(2):365–370. doi: 10.1042/bj1800365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Landau B. R. Contribution of the pentose cycle to glucose metabolism in muscle. Arch Biochem Biophys. 1965 Sep;111(3):569–575. doi: 10.1016/0003-9861(65)90236-5. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Howard B. V., Hidaka H., Ishibashi F., Fields R. M., Bennett P. H. Type II diabetes and insulin resistance. Evidence of lack of inherent cellular defects in insulin sensitivity. Diabetes. 1981 Jul;30(7):562–567. doi: 10.2337/diab.30.7.562. [DOI] [PubMed] [Google Scholar]
- Howard C. F., Jr Diabetes in Macaca nigra: metabolic and histologic changes. Diabetologia. 1974 Nov;10 (Suppl):671–677. doi: 10.1007/BF01222003. [DOI] [PubMed] [Google Scholar]
- Howard C. F., Jr Longitudinal studies on the development of diabetes in individual Macaca nigra. Diabetologia. 1986 May;29(5):301–306. doi: 10.1007/BF00452067. [DOI] [PubMed] [Google Scholar]
- Johnson K. H., Stevens J. B. Light and electron microscopic studies of islet amyloid in diabetic cats. Diabetes. 1973 Feb;22(2):81–90. doi: 10.2337/diab.22.2.81. [DOI] [PubMed] [Google Scholar]
- Katz L. D., Glickman M. G., Rapoport S., Ferrannini E., DeFronzo R. A. Splanchnic and peripheral disposal of oral glucose in man. Diabetes. 1983 Jul;32(7):675–679. doi: 10.2337/diab.32.7.675. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton B., Budohoski L., Lozeman F. J., Challiss R. A., Newsholme E. A. The effect of prostaglandins E1, E2 and F2 alpha and indomethacin on the sensitivity of glycolysis and glycogen synthesis to insulin in stripped soleus muscles of the rat. Biochem J. 1985 Apr 1;227(1):337–340. doi: 10.1042/bj2270337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton B., Challiss R. A., Lozeman F. J., Newsholme E. A. Effects of dexamethasone treatment on insulin-stimulated rates of glycolysis and glycogen synthesis in isolated incubated skeletal muscles of the rat. Biochem J. 1987 Sep 1;246(2):551–554. doi: 10.1042/bj2460551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy A. L., Longnecker D. S., Greenberg E. R. The relation of islet amyloid to the clinical type of diabetes. Hum Pathol. 1981 Oct;12(10):917–922. doi: 10.1016/s0046-8177(81)80197-9. [DOI] [PubMed] [Google Scholar]
- Meyer H. U., Curchod B., Maeder E., Pahud P., Jequier E., Felber J. P. Modifications of glucose storage and oxidation in nonobese diabetics, measured by continuous indirect calorimetry. Diabetes. 1980 Sep;29(9):752–756. doi: 10.2337/diab.29.9.752. [DOI] [PubMed] [Google Scholar]
- Wajngot A., Roovete A., Vranić M., Luft R., Efendić S. Insulin resistance and decreased insulin response to glucose in lean type 2 diabetics. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4432–4436. doi: 10.1073/pnas.79.14.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Hayden D. W., O'Brien T. D., Johnson K. H. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]



