SUMMARY
Changes of ocular dominance in the visual cortex can be induced by visual manipulations during a critical period in early life. However, the role of critical period plasticity in normal development is unknown. Here we show that at the onset of this time window, the preferred orientations of individual cortical cells in the mouse are mismatched through the two eyes and the mismatch decreases and reaches adult levels by the end of the period. Deprivation of visual experience during this period irreversibly blocks the binocular matching of orientation preference, but has no effect in adulthood. The critical period of binocular matching can be delayed by long-term visual deprivation from birth, like that of ocular dominance plasticity. These results demonstrate that activity-dependent changes induced by normal visual experience during the well-studied critical period serve to match eye-specific inputs in the cortex, thus revealing a physiological role for critical period plasticity during normal development.
INTRODUCTION
Optimal functioning of the nervous system requires selective wiring of neural circuits, the precision of which is achieved through experience-dependent refinement after birth (Katz and Shatz, 1996). The necessity of experience in neural systems development is often studied by depriving or manipulating sensory experiences (Feldman and Brecht, 2005; Hensch, 2004; Knudsen and Brainard, 1995). In the visual system, for example, following a period of monocular visual deprivation (MD) in juvenile animals, cortical neurons lose their responses to the deprived eye and become more responsive to the non-deprived eye (Wiesel and Hubel, 1963). Such MD-induced anatomical and physiological changes, referred to as ocular dominance (OD) plasticity, are largely restricted to a critical period in early life (Gordon and Stryker, 1996; Hubel and Wiesel, 1970; Issa et al., 1999; Wiesel and Hubel, 1965). Decades of studies have made OD plasticity and its critical period a classical model of experience-dependent neural development (Hensch, 2004). These studies, especially those after the mouse was established as a model system for OD plasticity (Gordon and Stryker, 1996), have provided important knowledge on the regulation of critical period timing and on synaptic changes induced by monocular deprivation (Hensch, 2005).
Despite these exciting advances, a fundamental question still remains unanswered: what purpose does this period of heightened plasticity serve during normal development? The critical period of OD plasticity overlaps with the normal maturation of visual acuity (Cancedda et al., 2004; Fagiolini et al., 1994; Movshon and Van Sluyters, 1981) and monocular visual deprivation was shown to decrease visual acuity (Boothe et al., 1985; Prusky and Douglas, 2003). However, the relationship between visual acuity increase in normal development and OD plasticity is unclear. This is because OD plasticity is only induced by an imbalance of inputs from the two eyes, a condition that does not exist in normal visual system development. In fact, the degree of cortical OD does not change during the critical period unless the system is manipulated experimentally (Sato and Stryker, 2008). In other words, while the critical period marks a period of increased cortical plasticity during development, functional cortical changes that normally take place during this time window are not known. Presumably, visual experience during the critical period induces synaptic changes that are important for normal cortical development. We set out to determine what cortical function is shaped by such normal vision-induced plasticity.
Two major transformations occur when visual information reaches the cortex. In addition to binocularity, cortical cells are also selective for stimulus orientation (Ferster and Miller, 2000; Hubel and Wiesel, 1962). Binocular cells in the cortex must then match their orientation tuning through the two eyes in order for the animal to perceive coherently. Indeed, in cats and primates, the preferred orientations of cortical neurons are similar through the two eyes (Bridge and Cumming, 2001; Ferster, 1981; Hubel and Wiesel, 1962; Nelson et al., 1977). How, then, is the binocularly matched orientation preference established? We hypothesize that the heightened plasticity during the critical period allows visual experience to drive the binocular matching of orientation preference during normal development. For this to be true, the following criteria have to be met. (1) The preferred orientations of individual cortical neurons should be mismatched between the two eyes in young animals. (2) The binocular similarity of orientation preference should improve and reach adult levels during the critical period. (3) Alterations in visual experience during the critical period, but not in adulthood, should disrupt the binocular matching. (4) Abnormal matching induced by visual deprivation in juvenile animals should not recover with subsequent visual experience. In this study, we have tested and confirmed each of these predictions in mice. Our results thus demonstrate that activity-dependent changes induced by normal visual experience during the critical period serve to match eye-specific inputs in the cortex. By ascribing a physiological role for critical period plasticity during normal development, our discovery therefore opens new areas of research in the study of experience-dependent visual system development.
RESULTS
Orientation tuning of cortical neurons are matched binocularly in adult mice
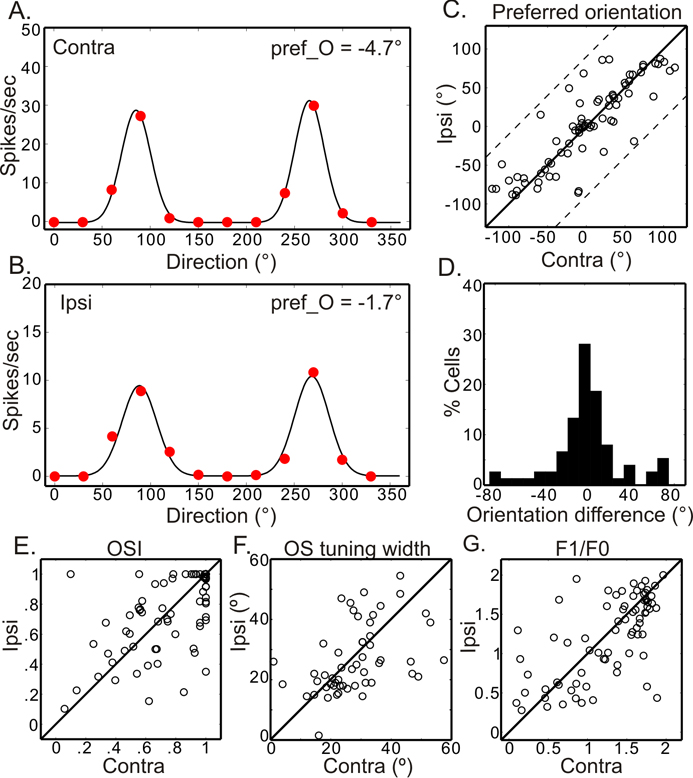
We first examined the binocular relationship of orientation preference in adult mice between P60 and P90, well after the critical period for OD plasticity. Single-unit recordings were made with microelectrodes in the binocular zone of the primary visual cortex (V1). The orientation tuning properties of each neuron were determined separately for each eye in response to drifting sinusoidal gratings of varying orientations. The neuron in Figure 1A and B, for example, was tuned to nearly identical orientations through the two eyes (only 3° difference). Such similarity in the preferred orientations between the two eyes was observed across the population (Fig. 1C, correlation coefficient r = 0.86 and P < 0.0001, n = 75). In adult mice, the difference in orientation preference between the two eyes (their absolute values are hereafter referred to as “ΔO”) was smaller than 20° in most cells (Fig. 1D), with a median of 10.4° and a mean of 19.7° ± 2.6° (Table 1). These results indicate that the orientation preference of individual cortical neurons is closely matched between the two eyes, consistent with studies in cats and primates (Bridge and Cumming, 2001; Ferster, 1981; Hubel and Wiesel, 1962; Nelson et al., 1977).
Figure 1. Binocularly matched orientation tuning in the visual cortex of adult mice.
(A and B) Orientation tuning curves of a binocular cell, which prefers nearly identical orientations through the contralateral (A) or ipsilateral (B) eye. The black lines are fitted curves of the response magnitudes (red dots). (C) Orientation preference is highly correlated binocularly (r = 0.86, P < 0.0001, n = 75 cells, 18 mice), with most points lying close to the unity line. The dotted lines bound the region in which the data points can lie. (D) Distribution of the difference in preferred orientations between the two eyes. (E–G) Correlation of orientation selectivity index (E, r = 0.60, P < 0.0001, n = 75), tuning width (F, r = 0.45, P < 0.001, n = 53), and F1/F0 ratio (G, r = 0.66, P < 0.0001, n = 75) through the two eyes. See also Figure S1.
Table 1.
Quantitative analysis of binocular matching and orientation tuning in different experimental groups.
| Mean ΔO | Median ΔO |
Mean OSI | Mean OS width | Number of Mice |
|||||
|---|---|---|---|---|---|---|---|---|---|
| All Cells | OSI > 0.33 | OSI > 0.50 | Contra | Ipsi | Contra | Ipsi | |||
| P20 – P23 | 30.1° ± 3.0° (n = 80) |
25.7° ± 3.3° (n = 59) |
24.7° ± 4.1° (n = 46) |
27.5° (n = 80) |
0.68 ± 0.04 (n = 80) |
0.66 ± 0.03 (n = 80) |
27.5° ± 1.8° (n = 39) |
28.6° ± 2.0° (n = 39) |
n = 16 |
| P31 – P36 | 17.0° ± 1.7° (n = 100) |
13.6° ± 1.5° (n = 73) |
11.6° ± 1.4° (n = 59) |
11.0° (n = 100) |
0.69 ± 0.03 (n = 100) |
0.70 ± 0.03 (n = 100) |
25.4° ± 1.9° (n = 49) |
27.5° ± 1.7° (n = 49) |
n = 15 |
| P60 – P90 | 19.7° ± 2.6° (n = 75) |
16.9° ± 2.6° (n = 67) |
14.2° ± 2.4° (n = 55) |
10.4° (n = 75) |
0.76 ± 0.03 (n = 75) |
0.75 ± 0.03 (n = 75) |
28.1° ± 1.6° (n = 53) |
26.7° ± 1.6° (n = 53) |
n = 18 |
|
DR (P24 to P31–36) |
29.6° ± 3.0° (n = 68) |
26.5° ± 3.3° (n = 50) |
24.8° ± 3.6° (n = 39) |
27.6° (n = 68) |
0.68 ± 0.04 (n = 68) |
0.68 ± 0.03 (n = 68) |
26.0° ± 1.9° (n = 31) |
27.7° ± 2.1° (n = 31) |
n = 7 |
| MD | 31.4° ± 3.5° (n = 56) |
30.2° ± 3.8° (n = 49) |
30.0° ± 4.1° (n = 40) |
24.3° (n = 56) |
0.76 ± 0.04 (n = 56) |
0.74 ± 0.04 (n = 56) |
30.5° ± 2.2° (n = 24) |
28.6° ± 2.4° (n = 24) |
n = 8 |
|
NMDAR Suppression |
28.6° ± 2.9° (n = 67) |
27.2° ± 3.1° (n = 57) |
25.6° ± 3.3° (n = 47) |
20.3° (n = 65) |
0.76 ± 0.03 (n = 67) |
0.74 ± 0.03 (n = 67) |
25.2° ± 1.9° (n = 33) |
23.7° ± 1.8° (n = 33) |
n = 8 |
|
Saline Control |
18.0° ± 2.2° (n = 65) |
17.4° ± 2.3° (n = 56) |
15.5° ± 2.5° (n = 47) |
12.6° (n = 65) |
0.77 ± 0.03 (n = 65) |
0.76 ± 0.03 (n = 65) |
25.2° ± 1.9° (n = 41) |
27.5° ± 2.1° (n = 41) |
n = 7 |
| MD Recovery | 29.0° ± 3.6° (n = 50) |
23.8° ± 3.7° (n = 39) |
22.6° ± 4.6° (n = 29) |
20.1° (n = 50) |
0.68 ± 0.04 (n = 50) |
0.66 ± 0.04 (n = 50) |
31.1° ± 2.1° (n = 28) |
26.8° ± 1.6° (n = 28) |
n = 6 |
| Adult MD | 20.5° ± 2.9° (n = 56) |
16.4° ± 2.6° (n = 42) |
12.7° ± 1.8° (n = 31) |
14.5° (n = 56) |
0.69 ± 0.04 (n = 56) |
0.77 ± 0.04 (n = 56) |
27.6° ± 2.0° (n = 30) |
26.6° ± 1.8° (n = 30) |
n = 8 |
|
DR (P0–P31) 0 Day |
38.6° ± 3.7° (n = 56) |
35.7° ± 4.1° (n = 47) |
37.0° ± 4.8° (n = 34) |
34.3° (n = 56) |
0.72 ± 0.03 (n = 56) |
0.68 ± 0.03 (n = 56) |
31.3° ± 2.2° (n = 23) |
34.1° ± 2.0° (n = 23) |
n = 6 |
|
DR (P0–P31) 6–7 Day |
21.0° ± 2.7° (n = 58) |
17.8° ± 2.8° (n = 49) |
14.6° ± 2.6° (n = 43) |
12.4° (n = 58) |
0.80 ± 0.03 (n = 58) |
0.77 ± 0.04 (n = 58) |
31.0° ± 1.9° (n = 35) |
27.2° ± 1.9° (n = 35) |
n = 4 |
We next quantified the degree of orientation selectivity of individual neurons by calculating an Orientation Selectivity Index (OSI, see Experimental Procedures for details). The vast majority of binocular neurons in adult mice are orientation selective. In fact, 89% of the cells (n = 67/75) had OSI greater than 0.33 for both eyes (a 2:1 response ratio for the preferred orientation over its orthogonal), and 73% (n = 55/75) had OSI greater than 0.5 (a 3:1 ratio). The mean OSI was equally high to the two eyes (contra: 0.76 ± 0.03, ipsi: 0.75 ± 0.03). These values are similar to those in a recent report of monocular neurons in adult mice (Niell and Stryker, 2008). Importantly, most cortical cells had similar OSI to the two eyes (Fig. 1E, r = 0.60 and P < 0.0001). We also calculated an Ocular Dominance Index (ODI, ranging from −1 to 1, where positive values indicate contralateral bias and negative values indicate ipsilateral bias). These cells had a mean ODI of 0.19 ± 0.03, confirming the contralateral bias in the binocular zone of the mouse visual cortex (Cang et al., 2005; Gordon and Stryker, 1996). Finally, we determined orientation tuning width, linearity, and preferred spatial frequency of these cells and found that they were all binocularly similar (Fig. 1F–G and Fig. S1).
Binocular matching of orientation preference during development
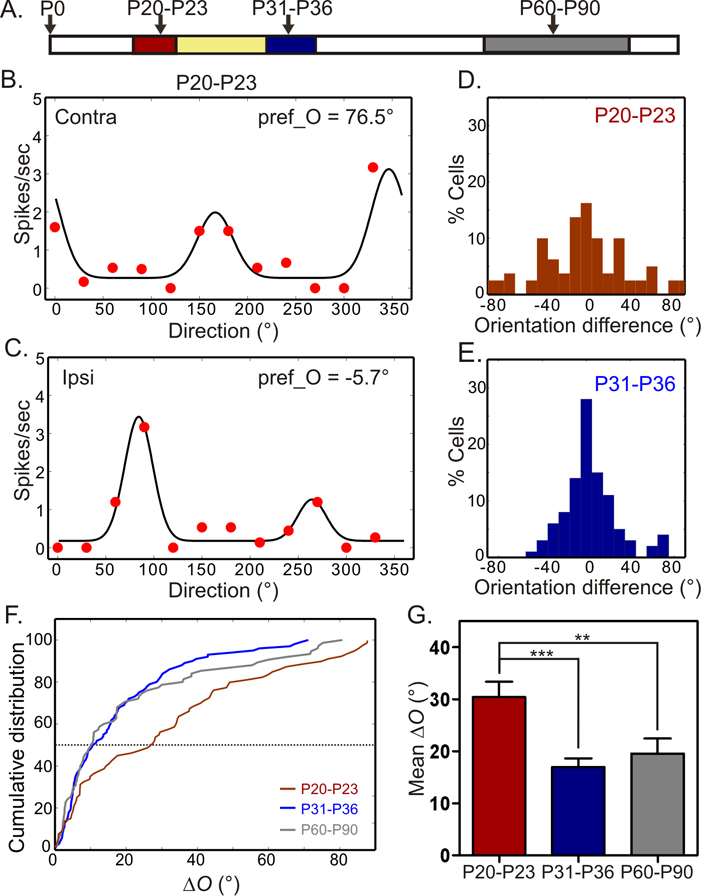
Although the orientation preferences are well matched binocularly in adulthood, we found that they are mismatched early during postnatal development. Two time periods were chosen in our study (Fig. 2A), P20–P23 and P31–P36, to correspond to the onset and offset of the critical period for OD plasticity (Gordon and Stryker, 1996). In mice between P20–P23, many cells were tuned to quite different orientations through the two eyes. For some cells (e.g. Fig. 2B–C), the preferred orientations through the two eyes were nearly 90° apart, the maximum possible difference. Overall, many more cells at this age had greater ΔO than in adult mice (compare Fig. 2D with Fig. 1D). At P20–P23, 50% of the cells had ΔO more than 27.5° (i.e., median = 27.5°, mean = 30.1° ± 3.0°, n = 80), significantly greater than in adult (median = 10.4°, mean = 19.7 ° ± 2.6°; P < 0.01; Fig. 2F–G), indicating that orientation preference is significantly mismatched binocularly in the young mice. The mismatch decreases with age (Fig. 2E–G and Fig. S2A) and reaches adult level by P31–P36 (median = 11.0°, mean = 17.0° ± 1.7°, n = 100; P < 0.001 compared to P20–P23, and P = 0.76 compared to adults).
Figure 2. Binocular matching of orientation preference during development.
(A) Schematic of the three age groups recorded under normal development, P20–P23 (red), P31–P36 (blue) and adult between P60–P90 (gray). Peak of the critical period for OD plasticity is shown in yellow. The same color code is followed in all figures. (B and C) Tuning curves of an orientation selective cell that has very different orientation preferences through the two eye (ΔO = 82.2°). (D–E) Distribution of difference in preferred orientation in P20–P23 (D, 80 cells, 16 mice) and P31–P36 mice (E, 100 cells, 15 mice). (F) Cumulative distribution of ΔO for all three age groups. (G) Mean ΔO decreases during the critical period and reaches adult level by P31–P36. See also Figure S2.
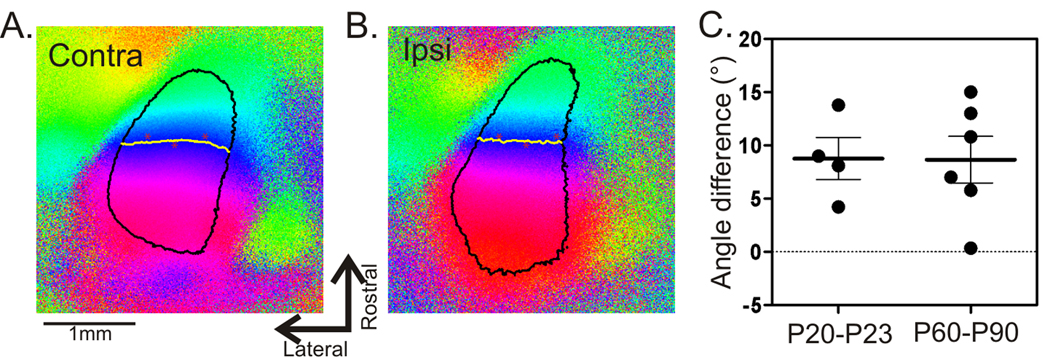
One important consideration is that the larger ΔO in younger animals may be due to a potential difference in eye alignment. The symmetrical, zero-centered, histograms of the difference in preferred orientations (Fig. 2D) suggested this unlikely, but they included multiple animals. We therefore compared the relative orientation between the two eyes in individual mice by optical imaging of cortical retinotopic maps (Fig. 3A–B). We determined the angle of rotation between the monocularly-obtained contralateral- and ipsilateral- maps. This angle, which reflects the relative rotation between the two eyes, was similar in juvenile and adult mice (Fig. 3C), ruling out the possibility of a different eye alignment in younger mice, whether a developmental phenomenon or an experimental artifact.
Figure 3. Alignment between the two eyes is similar in juvenile and adult mice.
(A and B) Examples of cortical elevation maps obtained through the two eyes. Black borders circle the responsive areas and the yellow lines mark the 0° contours. Red asterisks mark the same reference points on the cortex to help the comparison of the contour lines in the two maps. (C) Angle differences between the contralateral and ipsilateral maps are similar in juvenile and adult mice.
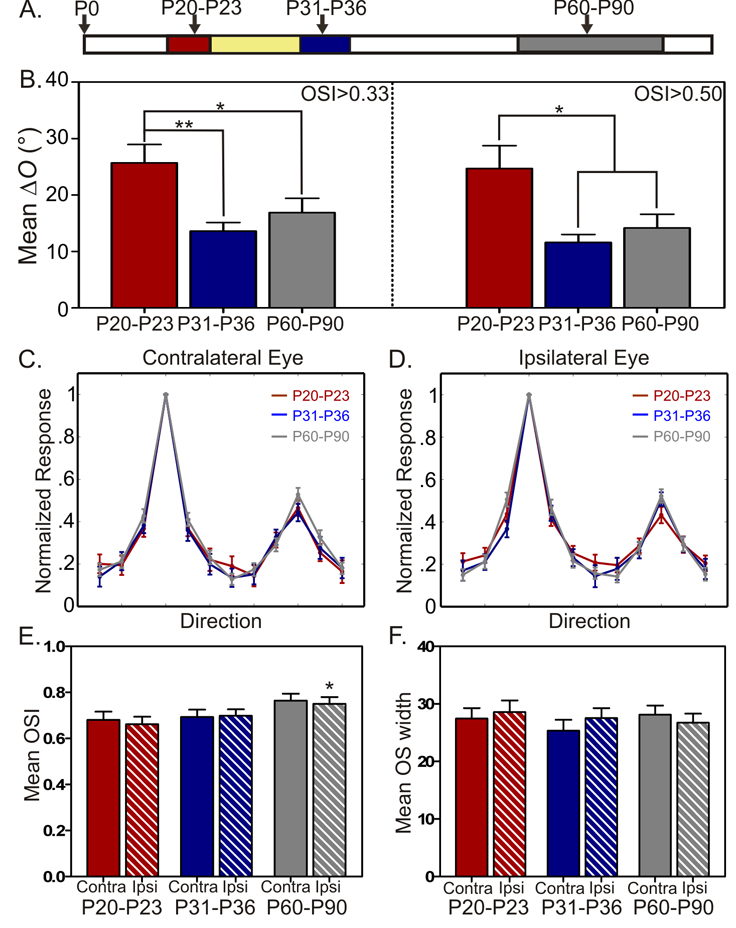
The development of binocular matching of orientation preference (i.e., decrease in ΔO) may be due to an increase in orientation selectivity, as less selective cells appeared to have slightly larger ΔO (Fig. S3). To address this possibility, we first limited our analysis of ΔO to orientation selective cells. When only selective (OSI > 0.33 to both eyes) or highly selective (OSI > 0.5) cells were included, the ΔO in P20–P23 mice was still significantly higher than in the older animals (Fig. 4B). These results indicate that in young mice, even cells that are highly orientation selective through both eyes are binocularly mismatched in their preferred orientations. Next, we examined directly the developmental profile of orientation tuning. Individual tuning curves of all cells were normalized and shifted to their max responses, and then averaged to be compared across development. These curves were indistinguishable across the three age groups (Fig. 4C–D). Furthermore, we also quantified the OSI and tuning width of individual cells and found that both of them were nearly identical between P20–P23 and P31–P36 (Fig. 4E–F, and Table 1). The OSI increased only slightly in adult mice (Fig. 4E). Together, these analyses indicate that by P20 cortical cells have acquired basic features of orientation selectivity monocularly, and the preferred orientations are then matched binocularly in a subsequent stage of visual system development.
Figure 4. Monocular features of orientation tuning develop before binocular matching of orientation preference.
(A) Schematic of the three age groups, same as in Figure 2. (B) Mean ΔO is significantly greater in P20–P23 mice than in both P31–P36 and P60–P90 mice for orientation selective cells (left, OSI > 0.33 to both eyes; 59 cells, 16 mice for P20–P23; 73 cells, 15 mice for P31–P36; 67 cells, 18 mice for P60–P90), and highly selective cells (right, OSI > 0.50; 46 cells, 16 mice for P20–P23; 59 cells, 15 mice for P31–P36; 55 cells, 18 mice for P60–P90). (C–D). Mean cortical tuning curves through contralateral (C) and ipsilateral (D) eyes for the three age groups. (E) Mean OSI is largely similar in the three age groups for both eyes, except between P20–P23 and P60–90 ipsilateral OSI (P = 0.048, t-test). (F) Mean orientation tuning width is similar for both eyes in all three age groups (t-test). In panels C–F, n = 80 cells, 16 mice for P20–P23; 100 cells, 15 mice for P31–P36; 75 cells, 18 mice for P60–P90. All bars represent mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001). See also Figure S3.
Binocular matching of orientation preference requires visual experience
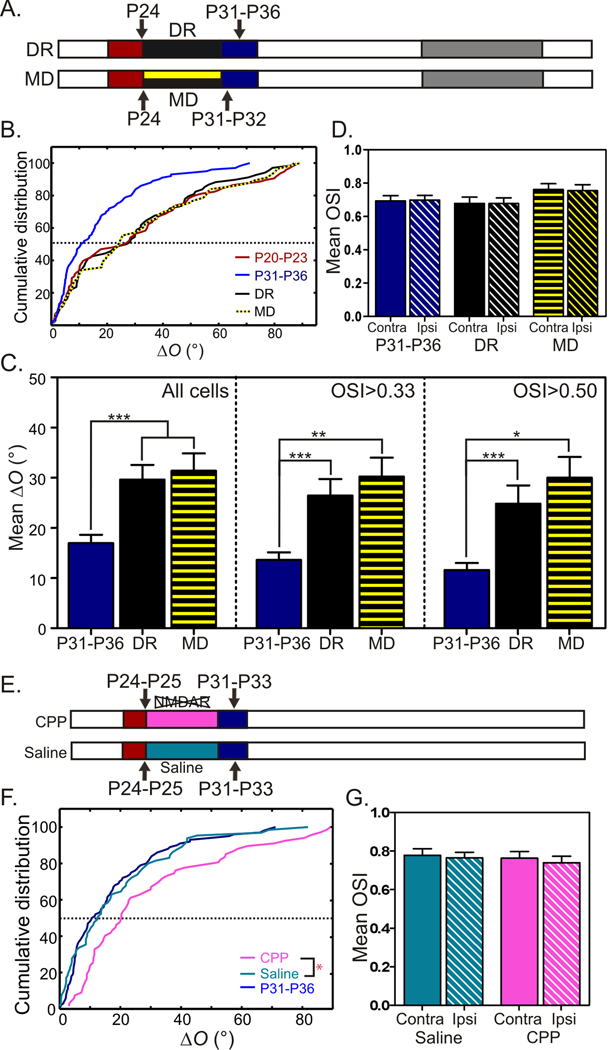
To determine whether the binocular matching we have just discovered requires normal visual experience, we used two types of manipulations: dark rearing and monocular deprivation (Fig. 5A). A short period of dark rearing (DR) during the critical period deprives the animal of any visual input, which is known to have no effect on ocular dominance. In contrast, monocular deprivation (MD) alters the balance of input between the two eyes and induces OD plasticity (Fagiolini et al., 1994; Gordon and Stryker, 1996; Issa et al., 1999; Wiesel and Hubel, 1963). For binocular matching, however, both manipulations blocked the developmental decrease of ΔO. The distribution of ΔO in both DR (from P24 to P31–P36, mean of 29.6° ± 3.0° and median of 27.6°) and MD (from P24 to P31/P32, mean of 31.4° ± 3.5° and median of 24.3°) groups were similar to P20–P23 mice (Fig. 5B), but greater than their age-matched controls (P31–36), This difference held true for analyses with all cells, selective and highly selective cells (Fig. 5C). Brief DR and MD resulted in no change in orientation selectivity (Fig. 5D). Therefore, normal visual experience between P24–P31 is required for the binocular matching of orientation preference. While a previous report showed that common input from the two eyes was not necessary for binocular matching of large-scale orientation columns (Godecke and Bonhoeffer, 1996), our results provide evidence for experience-dependent binocular matching at single cell level.
Figure 5. Binocular matching of orientation preference in juvenile mice requires visual experience and NMDA receptor activation.
(A) Visual deprivation by dark rearing (DR) from P24 to P31–P36 (upper diagram) or monocular deprivation (MD) from P24 to P31–P32 (lower diagram). (B) Distribution of ΔO in both DR (68 cells, 7 mice) and MD animals (56 cells, 8 mice) were higher than their age-matched controls, but similar to that of P20–P23. The ΔO distribution of P20–P23 and P31–P36 are reproduced from Figure 2F to help direct comparisons. (C) Mean ΔO of both DR and MD animals are significantly higher than P31–P36 controls. (D) Orientation selectivity of DR and MD animals remains similar to P31–P36 controls. (E) CPP solution (or saline) was injected in the critical period to suppress NMDA receptor activation. (F) Cumulative distribution of ΔO in the CPP-treated mice (67 cells, 8 mice), saline-treated controls (65 cells, 7 mice), and normal P31–P36 mice (P < 0.05, between CPP-treated and saline controls). (G) Mean OSI is similar between the CPP-treated and saline controls. All bars represent mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001).
Binocular matching of orientation preference requires NMDA receptor activation
The above experiments demonstrate that normal visual experience induces cortical changes to match binocular orientation preference. The binocular matching process is presumably driven by the correlated neuronal activity between the eye-specific inputs to individual cortical neurons. Because the NMDA receptor is known as a coincidence detector in synaptic plasticity (Bourne and Nicoll, 1993), we examined whether its activation is required for the binocular matching process.
Systemic administration of the competitive NMDA-receptor antagonist CPP was shown recently to block MD-induced OD changes in both juvenile and adult mice but have no effect on OD in normally-reared animals (Sato and Stryker, 2008). Following the established protocol, we injected CPP intraperitoneally to normally-reared mice starting from P24–P25 and repeated every ~24 hrs for 7–9 days (Fig. 5E). These mice were recorded about 24 hrs after the last injection. At P31–P33, their ΔO (28.6° ± 2.9°, n = 67) was still close to those of P20–P23 mice (Table 1) and significantly higher than the saline-injected controls (18.0° ± 2.2°, n = 65) (Fig. 5F). The difference held true for analyses with only selective and highly selective cells (Table 1, P < 0.05). Importantly, the pharmacological manipulation did not alter cortical orientation selectivity (both OSI and tuning width, see Fig. 5G and Table 1), consistent with the finding that these monocular tuning properties have already established by the onset of the critical period (Fig. 4). Together, these experiments indicate that NMDA-receptor-dependent synaptic plasticity, presumably driven by normal visual experience, take place in the critical period during normal development to match binocular orientation preference.
A critical period for binocular matching of orientation preference
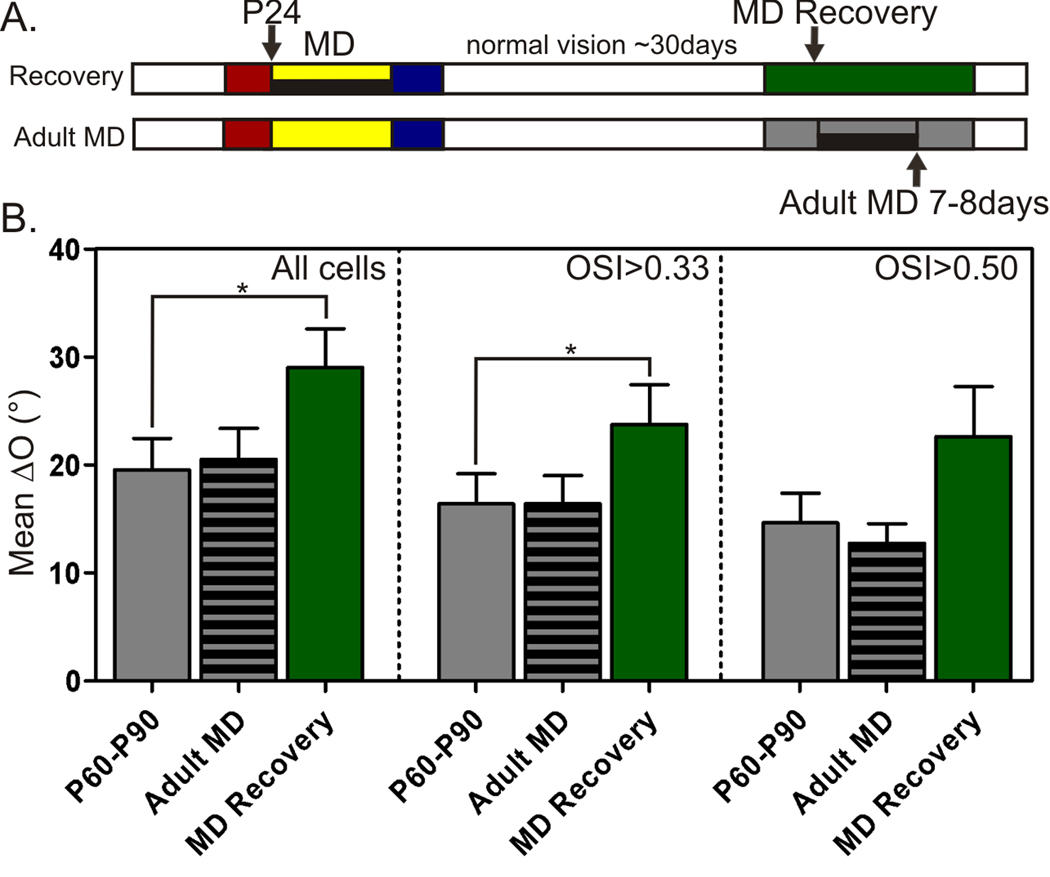
We next studied whether the period of heightened cortical plasticity, often revealed in the study of OD plasticity, is also “critical” for the binocular matching of orientation preference. If visual experience during this time window is truly crucial for the establishment of binocular matching, the disrupted matching induced by visual manipulation will not be able to recover, even after normal visual experience is restored after the critical period. To test this, we monocularly deprived mice at P24 and reopened the closed eye at P31–P32 (Fig. 6A). After approximately one month of normal visual experience following the MD, the ΔO in these mice (mean of 29.0° ± 3.6°) was still close to those immediately after MD (31.4° ± 3.5°), and significantly higher than in normal adult mice (19.7° ± 2.6°, P < 0.05; Fig. 6B and Table 1), indicating no recovery for the disrupted binocular matching of orientation preference.
Figure 6. A critical period for binocular matching of orientation preference.
(A) MD recovery (upper diagram) where P24 mice were monocularly deprived for 7–8 days before reopened to allow approximately 30 days of normal vision. Adult MD (lower diagram) where mice between P60–P90 were monocularly deprived for 7–8 days before recording. (B) Mean ΔO of Adult MD animals (56 cells, 8 mice) is similar to P60–P90 controls (75 cells, 18 mice), while MD Recovery animals (50 cells, 6 mice) have significantly higher ΔO. All bars represent mean ± SEM (*P < 0.05).
A number of recent studies have shown that OD plasticity persists into adulthood in mice (Hofer et al., 2006; Pham et al., 2004; Sato and Stryker, 2008; Sawtell et al., 2003), questioning the concept of the critical period. We therefore investigated the effect of MD on binocular matching in adulthood and found that 7–8 days of MD in adult mice (Fig. 6A) had no effect on binocular matching of orientation preference, where ΔO (mean of 20.5° ± 2.9°, n = 56) remains similar to normal adult controls (Fig. 6B and Table 1; P = 0.16).
These two sets of experiments therefore demonstrate the existence of a critical period for binocular matching of orientation preference: visual experience in this period, but not after this period, is crucially needed for the normal development of cortical binocularity.
Long-term visual deprivation delays the critical period of binocular matching
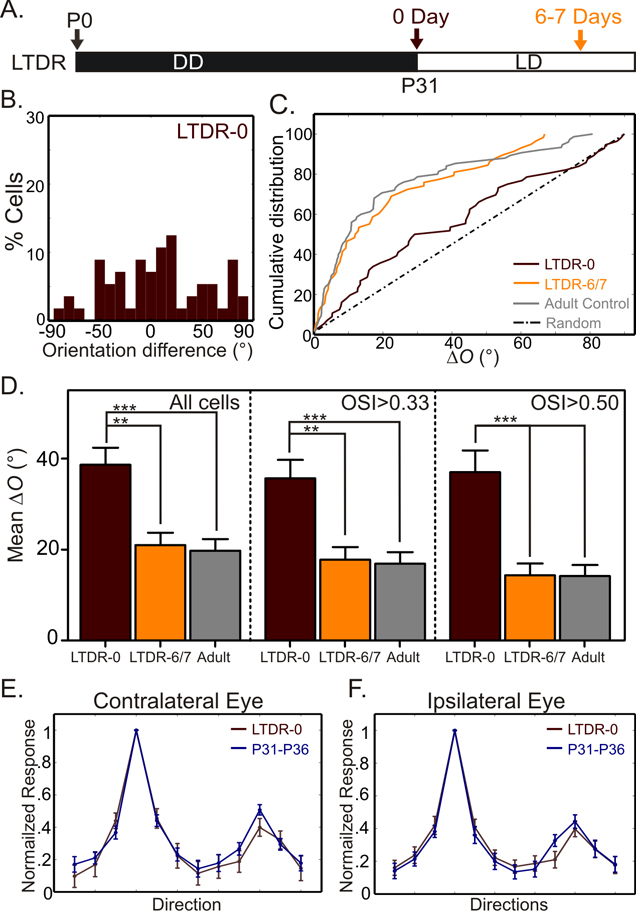
Dark-rearing from birth is known to delay the critical period of OD plasticity (Cynader and Mitchell, 1980; Fagiolini et al., 2003). We therefore studied whether the binocular matching of orientation preference can be similarly delayed by such long-term visual deprivation. The above experiments have demonstrated that the critical period of binocular matching normally closes by P31, as the disrupted matching can not recover with subsequent visual experience. We thus reared mice in constant darkness from birth to P31 and then switched to normal light-dark cycle to examine whether the binocular matching of orientation preference can still be achieved (Fig. 7A).
Figure 7. Dark rearing from birth delays the critical period of binocular matching.
(A) Mice were reared in constant darkness (DD) from P0 to P31 and then in normal light-dark (LD) cycle. They were recorded immediately or 6–7 days after the long-term dark rearing (LTDR-0 and LTDR-6/7, respectively). (B) Distribution of difference in preferred orientations through the two eyes in LTDR-0 mice. (C) Cumulative distribution of ΔO for LTDR-0 (56 cells, 6 mice) and LTDR-6/7 (58 cells, 4 mice). The ΔO distributions of normal adult control (solid gray line) and random matching (dotted gray line) are shown for comparison. (D) Mean ΔO of all groups, for all cells, selective and highly selective cells. (E–F) Mean tuning curves through contralateral (E) and ipsilateral (F) eyes for mice immediately after dark-rearing (LTDR-0) and age-matched controls (P31–P36).
Normal level of orientation tuning was observed immediately after dark-rearing, for OSI (Table 1), tuning width (Table 1), and mean tuning curves (Fig. 7E–F), consistent with a previous study in mice using dark-rearing of similar duration (Iwai et al., 2003). Importantly, this result provided an opportunity to determine the degree of binocular matching achieved in the absence of any visual experience, which is difficult to study in normal development because of the technical difficulty in recording from very young mice. Overall, cortical neurons were tuned to very different orientations through the two eyes immediately after dark-rearing (Fig. 7B; ΔO: mean of 38.6° ± 3.7° and median of 34.3°, n = 56). In fact, the distribution of ΔO (Fig. 7C) was not statistically different from random binocular matching, which would have been a uniform distribution (P = 0.07, one sample K–S test). This suggests that binocular matching of orientation preference is likely achieved entirely by experience-dependent processes. Remarkably, 6–7 days of vision after dark-rearing was able to decrease ΔO to the normal adult level (Fig. 7C–D and Table 1), indicating that the binocular matching can still take place after P31 when the critical period would have normally ended (Fig. 6). In other words, the critical period of the matching process is delayed by dark-rearing from birth, like that of OD plasticity. This suggests that the timing of the two processes is likely regulated by similar molecular and synaptic mechanisms.
DISCUSSION
In this study, we have shown that individual binocular neurons in the visual cortex match their orientation preference through the two eyes during a critical period in early life. At the onset of this time window, the preferred orientations are mismatched binocularly and the mismatch decreases and reaches adult levels by the end of the critical period. The binocular matching follows the maturation of monocular orientation tuning and requires normal visual experience. Deprivation of normal visual input during this period results in an irrecoverable disruption on the binocular matching. Once this period ends, however, binocular matching is unaffected by visual deprivation. Our findings thus reveal a functional purpose for critical period plasticity during normal development.
Ocular dominance plasticity and binocular matching in visual cortical development
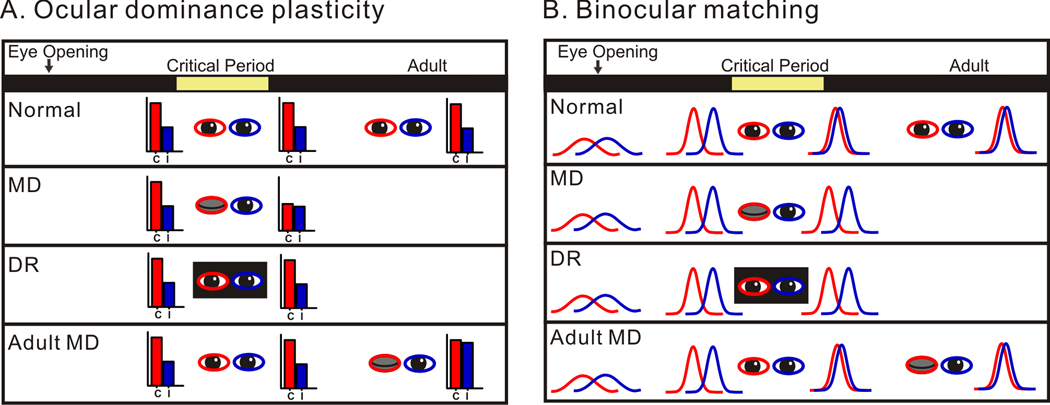
While both OD plasticity and binocular matching of orientation preference take place during a similar period of critical time in early life, important conceptual differences exist between them (Fig. 8). OD plasticity is a visual manipulation-induced plasticity, where cells lose responses to the deprived eye as an adaptation to pathological conditions. In contrast, binocular matching is a normal vision-induced plasticity and leads to a beneficial outcome, where cortical cells match their orientation preference to the two eyes. OD of cortical cells remains constant during the critical period in normal development (Sato and Stryker, 2008, Fig. 8A), while the preferred orientations of binocular cells are mismatched through the two eyes at the onset of this time period (Fig. 8B). Changes of OD are induced only by an imbalance between eye-specific inputs, such as in MD (Fig. 8A) or strabismus, but not by visual manipulations that deprive both eyes, such as dark rearing and binocular deprivation (Gordon and Stryker, 1996). On the other hand, binocular matching occurs during normal development and is blocked by any visual manipulation that disrupts binocular vision (Fig. 8B). Furthermore, MD in adulthood has no effect on binocular matching (Fig. 8B) even though it induces sub-threshold OD plasticity in mice (Morishita and Hensch, 2008), thus reestablishing the concept of critical period in visual cortical development.
Figure 8. Comparison between ocular dominance plasticity and binocular matching of orientation preference.
(A) OD remains the same throughout normal development (top panel). The colored bars represent response magnitude through the two eyes, with red for contralateral (C) and blue for ipsilateral eye (I). MD (second panel), but not DR (third panel), during the critical period induces a shift in OD. OD can also be shifted by MD in adult mice (bottom panel). The diagrams of MD-and Adult-MD-induced OD plasticity follow Sato and Stryker (2008). (B) Binocular matching of preferred orientations is established during the critical period under normal development (top panel). Binocular cells acquire basic properties of orientation tuning by the onset of critical period (top panel). The colored curves represent the orientation tuning of individual neurons. The orientation tuning properties are presumed to be immature at the time of eye-opening according to studies in other species. Both MD (second panel) and DR (third panel) during the critical period disrupt the binocular matching, while adult MD has no such effect on the matching (bottom panel).
Mechanistically, binocular matching and OD plasticity should also be different. OD plasticity is a competitive process where eye-specific inputs “fight” for postsynaptic targets based on their activity levels (Wiesel, 1982). Hebbian plasticity such as long-term potentiation and depression (Smith et al., 2009), and homeostatic plasticity (Kaneko et al., 2008; Mrsic-Flogel et al., 2007) have all been suggested to mediate components of OD plasticity. Binocular matching of orientation preference, on the other hand, is presumably mediated by correlation-based processes where the precise patterns of input activity instruct changes in eye-specific synaptic connections. According to the feed-forward model proposed originally by Hubel and Wiesel (Hubel and Wiesel, 1962), orientation selectivity arises from specific arrangement of geniculate inputs, and the preferred orientation of individual cortical cells is determined by the layout of the elongated ON and OFF subregions in their receptive field (Ferster et al., 1996; Reid and Alonso, 1995). In such a scenario, changes at the eye-specific geniculocortical synapses are required to align the receptive field subregions in order to match the preferred orientations binocularly. An alternative series of models, the feedback models, propose that orientation selectivity is an emergent property of intracortical circuitry (Adorjan et al., 1999; Ben-Yishai et al., 1997; Somers et al., 1995). In this case, binocular matching should be mediated largely by synaptic changes of intracortical connections. Regardless of the exact mechanisms of orientation selectivity, however, changes in synaptic connections must be under the precise and delicate control of the synchronized inputs from the two eyes evoked by normal visual experience. In contrast, the OD plasticity involves changes in the total strength of eye-specific inputs. As an initial attempt to probe the underlying molecular and synaptic mechanisms, we have shown in this study that the binocular matching requires NMDA receptor activation, which is also known to be involved in MD-induced OD plasticity (Kleinschmidt et al., 1987; Roberts et al., 1998; Sato and Stryker, 2008). In binocular matching, NMDA receptors likely detect the correlation between the two eyes’ inputs because this process is induced by normal binocular vision; whereas in OD plasticity the correlated activity is only within the inputs from the open eye. It is therefore of great importance to examine in future studies how NMDA receptor signaling and other plasticity processes that participate in OD plasticity mediate binocular matching of orientation preference.
OD plasticity has been assumed to be involved in the development of binocular disparity and stereo vision, but studies indicate that there is no systematic relationship between OD and binocular disparity (Kara and Boyd, 2009; Read and Cumming, 2004). On the other hand, cortical cells have to be tuned to similar orientations in the two eyes to encode binocular disparity of stimulus phase and position. In other words, binocular matching of orientation preference, which also does not correlate with OD (data not shown), is a requirement for certain aspects of binocular disparity. Future studies are needed to address how these two features of binocularity interact during development.
Critical period of binocular matching of orientation preference
One puzzling feature of the critical period for OD plasticity is that it does not start at the time of eye-opening in many species, when cortical circuits are generally more plastic (Gandhi et al., 2005). Although it is not yet known when exactly the binocular matching process starts, we show it takes place or at least continues after cortical cells acquire basic properties of orientation tuning. This finding provides a possible explanation for the timing of the critical period. In cats, ferrets and rodents, cortical orientation selectivity is immature or even almost absent at the time of eye-opening (Fregnac and Imbert, 1978; Blakemore and Van Sluyters, 1975; Chapman and Stryker, 1993; Fagiolini et al., 1994; but for cats see Hubel and Wiesel, 1963), and the critical period begins after 5–10 days of visual experience (Fagiolini et al., 1994; Gordon and Stryker, 1996; Hubel and Wiesel, 1970; Issa et al., 1999). Therefore, a functional significance of this “precritical period” (Feller and Scanziani, 2005) may be to allow cortical cells to become orientation selective before binocular matching (Fig. 8B).
We find that the critical period of binocular matching can be delayed by dark-rearing from birth, like that of OD plasticity (Cynader and Mitchell, 1980; Fagiolini et al., 2003; Mower, 1991). This suggests that the timing of the two processes is likely regulated by similar molecular and synaptic mechanisms. It is known that the timing of the critical period of OD plasticity is under the control of local inhibitory circuits (Hensch, 2005). The critical period can be advanced or delayed, respectively, by enhancing or reducing GABAergic transmission in the cortex (Fagiolini and Hensch, 2000; Hanover et al., 1999; Hensch et al., 1998; Huang et al., 1999; Iwai et al., 2003). Long-term dark rearing has been shown to delay the maturation of inhibitory circuits (Morales et al., 2002), possibly leading to the delay in the critical period. The relationship among critical period timing, cortical inhibition, and orientation selectivity, each of which is already under extensive studies, should now be studied in the context of the newly discovered binocular matching of orientation preference. Such studies will likely shed new light on the functional development of the visual cortex.
Implications for experience-dependent neural development
Why is experience-dependent plasticity needed in neural systems development? Experience-dependent processes give the developing brain the ability to adapt, both to various external environments and to differing intrinsic features. For example, a difference in head size or facial features between individuals requires subtle but important differences in the neural circuits for binocular vision. Matching binocular orientation preference through experience-dependent processes, instead of being determined entirely by genetic programs, enables the cortical circuits to adjust to individual anatomical differences in order to form a coherent visual perception. Similarly, in the auditory system, which is capable of localizing sound with extraordinary precision using various sets of cues, an on-going experience-dependent recalibration of the system during development is especially important (Brainard and Knudsen, 1998). Such experience-dependent matching of different streams of information has been studied for sound localization in barn owls, where the associations between auditory cues and visual locations are made during early development (Brainard and Knudsen, 1998). Binocular matching, where cells integrate two streams of information within the same modality, may share similar mechanisms as in the development of multimodal integration. Given the extensive knowledge on the visual system and the great power of mouse genetics, the model system we have established here presents an exciting opportunity to study the mechanisms underlying experience-dependent development.
CONCLUSION
We have discovered a new form of experience-dependent plasticity during postnatal development, when normal visual experience induces cortical changes to match eye-specific inputs to individual binocular neurons. The experience-dependent nature of matching multiple streams of information during development enables cortical circuits to adapt to environmental changes and individual differences. Because most studies on cortical plasticity are focused on changes induced by depriving or manipulating sensory experience, our discovery opens new areas of research in the study of neural systems development.
EXPERIMENTAL PROCEDURES
Animals
Wild type C57BL/6 mice of different ages, P20–23, P31–P36, and P60–P90, were used in our experiments. All animals were used in accordance with protocols approved by Northwestern University Institutional Animal Care and Use Committee.
Visual deprivation and CPP injections
For short term dark rearing, mice (n = 7) were placed in complete darkness for 7 to 12 days beginning at P24, before physiological recording at P31–P36. For long term dark rearing, mice (n = 10) were raised in constant darkness from P0 to P31. These mice were either recorded immediately after removing from the darkness (n = 6) or allowed 6–7 days of normal visual experience before recording (n = 4). During the procedure of monocular deprivation, mice of P24 (n = 8) or between P60–P90 (n = 8) were anesthetized with 2–3% isofluorane in oxygen. The right eyes of all the animals were sutured shut with three mattress sutures, according to published protocols (Cang et al., 2005; Gordon and Stryker, 1996). In all cases, MD was maintained for 7–8 days before physiological recording. For MD recovery (n = 6), the closed eyes were re-opened after 7–8 days of deprivation and then the animals were given ~30 days of normal visual experience before physiological recordings. Mice in which the eyes were not fully closed during MD or had any indication of corneal damage or cataract were excluded from the study.
The competitive NMDA receptor antagonist (R,S)-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP, Tocris Bioscience) was dissolved in saline at a concentration of 1.5 mg/ml. Its solution or the same volume of saline was injected intraperitoneally at a dose of 15 mg/kg every ~24 h (Frenkel et al., 2006; Sato and Stryker, 2008) starting from P24 or P25. The injections were repeated for 7–9 days before physiological recording which was about 24 hours after the last injections.
In vivo physiology
Mice of various ages were anesthetized using urethane (1.2–1.3g/kg in 10% saline solution, i.p.) and supplemented by the sedative chlorprothixene (10mg/kg, i.m.) as described previously (Cang et al., 2008). Atropine (0.3 mg/kg) and dexamethasone (2.0 mg/kg) were injected subcutaneously. Additional urethane (0.2–0.3g/kg) was administered as needed. A tracheotomy was performed and electrocardiograph leads were attached across the skin to monitor the heart rate continuously throughout the experiment. The animal was placed in a stereotaxic apparatus on a heating pad. The animal’s temperature was monitored with a rectal thermoprobe and maintained at 37°C through a feedback heater control module (Frederick Haer Company, Bowdoinham, ME). Silicon oil was applied on both eyes to prevent from drying.
A small craniotomy (~2 mm2) was then performed at the left hemisphere to expose the cortex for recording. 5–10 MΩ tungsten microelectrodes (FHC, Bowdoinham, ME) were penetrated perpendicular to the pial surface in the binocular zone of V1 (3.0–3.3 mm lateral from the midline and 0.5–0.8 mm anterior from the lambda suture). In each animal, 2–6 penetrations were made with minimum spacing of 50 µm and cells recorded across all layers were included in our analysis. Electrical signals, both spikes (filtered between 0.3 and 5 KHz and sampled at 25 KHz) and field potentials (filtered between 10 and 300 Hz and sampled at 800 Hz), were acquired using a System 3 workstation (Tucker Davis Technologies, FL) and the spike waveforms were further sorted offline into single units using OpenSorter (Tucker Davis Technologies, FL). The animals were euthanized at the end of recording.
Visual stimuli and analysis of receptive field properties
Visual stimuli were generated with Matlab programs developed originally by Dr. Cris Niell (Niell and Stryker, 2008) using the Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997). A ViewSonic video monitor (40 × 30cm, 60 Hz refresh rate, ~35 cd/m2 mean luminance), was placed at 25 cm in front of the animal to display the stimuli, with its midline aligned with the animal.
Sinusoidal gratings drifting perpendicular to their orientations were used to determine V1 neurons’ orientation selectivity and spatial tuning. The drifting direction (θ) and spatial frequency of the gratings (full contrast and temporal frequency of 2 Hz) were varied between 0° – 360° (12 steps at 30° spacing), and 0.01 – 0.32 cycle/degree (6 logarithmic steps) in a pseudorandom order. The average spike rate during the 1.5sec presentation of each condition that was repeated 4–6 times, subtracted with the spontaneous rate determined by blank condition presentation, was calculated as the response magnitude (R). The preferred direction was determined as the one that gave maximum response (Rpref), averaging across all spatial frequencies. The preferred spatial frequency (pref_SF) was the one that gave peak response at this direction. Responses across all directions at the preferred spatial frequency, R(θ), were used to calculate the preferred orientation, orientation selectivity index (OSI), and tuning width.
Half of the complex phase of ΣR(θ)*e2i*θ/ΣR(θ) was calculated (Niell and Stryker, 2008), which is essentially a weighted mean of θ across a 180° cycle. This value was further converted to the preferred orientation (pref_O) by subtracting 90° because θ was expressed in stimulus direction. The difference in preferred orientation between the two eyes was calculated by subtracting ipsilateral pref_O from contralateral pref_O along the 180° cycle (−90° to 90°). The absolute values of these differences (ΔO) were used in all quantifications. In a number of cells (n = 52 for adults; n = 41 for P20–P23), we used drifting gratings of 24 directions at 15° spacing and analyzed the pref_O using either all 24 directions or only 12 directions at 30° spacing from the same data. The two ways of analyses gave nearly identical pref_O in the same cells in both age groups (Fig. S2B). In addition, for CPP-treated mice and their saline controls, 24-direction drifting gratings (15° spacing) at 4 spatial frequencies (0.01, 0.02, 0.04, and 0.08 cpd) were used. The saline controls had nearly identical ΔO distribution as P31–P36 mice for which 12-direction (30° spacing) gratings were used (Fig. 5F). In another set of control experiments, 2 series of 12-direction, 6-spatial frequency stimuli were conducted to compare the calculated pref_O. The difference was similarly small in both P20–P23 and adult mice (Fig. S2C).
OSI was calculated as the ratio of (Rpref − Rorth)/(Rpref + Rorth), where Rorth was the mean response of the two directions orthogonal to the preferred direction. Orientation tuning width was determined by obtaining the half-width at half-maximum response after fitting the tuning curve as the sum of two Gaussians (Niell and Stryker, 2008). Note that only cells that were well fitted were included in the analysis of tuning width. Spatial tuning and linearity of response were also analyzed following the published procedure (Niell and Stryker, 2008).
The ocular dominance index (ODI) for each cell was calculated as (C − I)/(C + I), where C and I represent the maximum response magnitude (Rpref) for the contralateral and ipsilateral eyes, respectively (Cang et al., 2005). The ODI ranges from −1 to 1, where positive values indicate contralateral bias and negative values indicate ipsilateral bias. Because only binocular cells were included in our analysis, the mean ODI value was an underestimation in normal and dark-reared mice that have more cells driven only by the contralateral eye than ipsilateral-only cells (Gordon and Stryker, 1996; Mrsic-Flogel et al., 2007).
Functional imaging and analysis of retinotopic maps
Following the same surgical procedure described above, cortical retinotopic maps were obtained independently for each eye using published methods (Cang et al., 2008; Kalatsky and Stryker, 2003). In particular, the stimulus monitor was placed the same way as in electrophysiology, and the animal was presented with horizontal light bars (2° wide and full screen long) drifting along the dorsoventral axis in order to stimulate the constant lines of elevation. The spatial frequency of drifting bar was 1 cycle/100°, and temporal frequency was 1 cycle/8 s.
Within the more responsive regions of each map (above 30% of the peak response), we computed a contour of 0° elevation, which corresponds to the horizontal midline of the stimulus monitor. The contours were fitted to straight lines in both contralateral and ipsilateral maps and the angle between the two lines was used to quantify the relative difference in the orientations of the two eyes.
Statistical analysis
All values were presented as mean ± SEM. Differences between different groups were tested for significance using the Kolmogorov-Smirnov test (KS-test), unless otherwise indicated. Statistic analyses and graphing were done with Prism (GraphPad Software Inc) and Matlab (Mathworks). In the figures, *P < 0.05, **P < 0.01, and ***P < 0.001.
Supplementary Material
ACKOWLEDGEMENTS
We thank Cris Niell for help with visual stimulation and data analyses; and Martha Vitaterna for providing space for dark-rearing mice. We also thank Xiaorong Liu, Tom Bozza, David Ferster, Indira Raman, Garth Fowler, and members of the Cang laboratory for discussions and comments on the manuscript. This work was supported by a US National Institutes of Health (NIH) grant (EY018621), a Sloan Research Fellowship, a Klingenstein Fellowship Award in Neurosciences, and a Brain Research Foundation Seed Grant to J.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adorjan P, Levitt JB, Lund JS, Obermayer K. A model for the intracortical origin of orientation preference and tuning in macaque striate cortex. Vis Neurosci. 1999;16:303–318. doi: 10.1017/s0952523899162114. [DOI] [PubMed] [Google Scholar]
- Ben-Yishai R, Hansel D, Sompolinsky H. Traveling waves and the processing of weakly tuned inputs in a cortical network module. J Comput Neurosci. 1997;4:57–77. doi: 10.1023/a:1008816611284. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Van Sluyters RC. Innate and environmental factors in the development of the kitten's visual cortex. The Journal of physiology. 1975;248:663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothe RG, Kiorpes L, Carlson MR. Studies of strabismus and amblyopia in infant monkeys. Journal of pediatric ophthalmology and strabismus. 1985;22:206–212. doi: 10.3928/0191-3913-19850901-10. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Nicoll R. Molecular machines integrate coincident synaptic signals. Cell. 1993;72 Suppl:65–75. doi: 10.1016/s0092-8674(05)80029-7. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Brainard MS, Knudsen EI. Sensitive periods for visual calibration of the auditory space map in the barn owl optic tectum. J Neurosci. 1998;18:3929–3942. doi: 10.1523/JNEUROSCI.18-10-03929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge H, Cumming BG. Responses of macaque V1 neurons to binocular orientation differences. J Neurosci. 2001;21:7293–7302. doi: 10.1523/JNEUROSCI.21-18-07293.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda L, Putignano E, Sale A, Viegi A, Berardi N, Maffei L. Acceleration of visual system development by environmental enrichment. J Neurosci. 2004;24:4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Kalatsky VA, Lowel S, Stryker MP. Optical imaging of the intrinsic signal as a measure of cortical plasticity in the mouse. Vis Neurosci. 2005;22:685–691. doi: 10.1017/S0952523805225178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Niell CM, Liu X, Pfeiffenberger C, Feldheim DA, Stryker MP. Selective disruption of one Cartesian axis of cortical maps and receptive fields by deficiency in ephrin-As and structured activity. Neuron. 2008;57:511–523. doi: 10.1016/j.neuron.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Stryker MP. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J Neurosci. 1993;13:5251–5262. doi: 10.1523/JNEUROSCI.13-12-05251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M, Mitchell DE. Prolonged sensitivity to monocular deprivation in dark-reared cats. Journal of neurophysiology. 1980;43:1026–1040. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, Hensch TK. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc Natl Acad Sci U S A. 2003;100:2854–2859. doi: 10.1073/pnas.0536089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science (New York, N.Y) 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Feller MB, Scanziani M. A precritical period for plasticity in visual cortex. Current opinion in neurobiology. 2005;15:94–100. doi: 10.1016/j.conb.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Ferster D. A comparison of binocular depth mechanisms in areas 17 and 18 of the cat visual cortex. The Journal of physiology. 1981;311:623–655. doi: 10.1113/jphysiol.1981.sp013608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D, Chung S, Wheat H. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature. 1996;380:249–252. doi: 10.1038/380249a0. [DOI] [PubMed] [Google Scholar]
- Ferster D, Miller KD. Neural mechanisms of orientation selectivity in the visual cortex. Annual review of neuroscience. 2000;23:441–471. doi: 10.1146/annurev.neuro.23.1.441. [DOI] [PubMed] [Google Scholar]
- Fregnac Y, Imbert M. Early development of visual cortical cells in normal and dark-reared kittens: relationship between orientation selectivity and ocular dominance. The Journal of physiology. 1978;278:27–44. doi: 10.1113/jphysiol.1978.sp012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, Sawtell NB, Diogo AC, Yoon B, Neve RL, Bear MF. Instructive effect of visual experience in mouse visual cortex. Neuron. 2006;51:339–349. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Cang J, Stryker MP. An eye-opening experience. Nature neuroscience. 2005;8:9–10. doi: 10.1038/nn0105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godecke I, Bonhoeffer T. Development of identical orientation maps for two eyes without common visual experience. Nature. 1996;379:251–254. doi: 10.1038/379251a0. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19:RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annual review of neuroscience. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science (New York, N.Y) 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Prior experience enhances plasticity in adult visual cortex. Nature neuroscience. 2006;9:127–132. doi: 10.1038/nn1610. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. The Journal of physiology. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive Fields of Cells in Striate Cortex of Very Young, Visually Inexperienced Kittens. Journal of neurophysiology. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. The Journal of physiology. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in the Ferret's visual cortex. J Neurosci. 1999;19:6965–6978. doi: 10.1523/JNEUROSCI.19-16-06965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y, Fagiolini M, Obata K, Hensch TK. Rapid critical period induction by tonic inhibition in visual cortex. J Neurosci. 2003;23:6695–6702. doi: 10.1523/JNEUROSCI.23-17-06695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalatsky VA, Stryker MP. New paradigm for optical imaging: temporally encoded maps of intrinsic signal. Neuron. 2003;38:529–545. doi: 10.1016/s0896-6273(03)00286-1. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara P, Boyd JD. A micro-architecture for binocular disparity and ocular dominance in visual cortex. Nature. 2009;458:627–631. doi: 10.1038/nature07721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science (New York, N.Y.) 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Bear MF, Singer W. Blockade of "NMDA" receptors disrupts experience-dependent plasticity of kitten striate cortex. Science (New York, N.Y.) 1987;238:355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Brainard MS. Creating a unified representation of visual and auditory space in the brain. Annual review of neuroscience. 1995;18:19–43. doi: 10.1146/annurev.ne.18.030195.000315. [DOI] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Hensch TK. Critical period revisited: impact on vision. Current opinion in neurobiology. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Van Sluyters RC. Visual neural development. Annual review of psychology. 1981;32:477–522. doi: 10.1146/annurev.ps.32.020181.002401. [DOI] [PubMed] [Google Scholar]
- Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Brain research. 1991;58:151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Nelson JI, Kato H, Bishop PO. Discrimination of orientation and position disparities by binocularly activated neurons in cat straite cortex. Journal of neurophysiology. 1977;40:260–283. doi: 10.1152/jn.1977.40.2.260. [DOI] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The Video Toolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Pham TA, Graham SJ, Suzuki S, Barco A, Kandel ER, Gordon B, Lickey ME. A semi-persistent adult ocular dominance plasticity in visual cortex is stabilized by activated CREB. Learn Mem. 2004;11:738–747. doi: 10.1101/lm.75304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. Developmental plasticity of mouse visual acuity. Eur J Neurosci. 2003;17:167–173. doi: 10.1046/j.1460-9568.2003.02420.x. [DOI] [PubMed] [Google Scholar]
- Read JC, Cumming BG. Ocular dominance predicts neither strength nor class of disparity selectivity with random-dot stimuli in primate V1. Journal of neurophysiology. 2004;91:1271–1281. doi: 10.1152/jn.00588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RC, Alonso JM. Specificity of monosynaptic connections from thalamus to visual cortex. Nature. 1995;378:281–284. doi: 10.1038/378281a0. [DOI] [PubMed] [Google Scholar]
- Roberts EB, Meredith MA, Ramoa AS. Suppression of NMDA receptor function using antisense DNA block ocular dominance plasticity while preserving visual responses. Journal of neurophysiology. 1998;80:1021–1032. doi: 10.1152/jn.1998.80.3.1021. [DOI] [PubMed] [Google Scholar]
- Sato M, Stryker MP. Distinctive features of adult ocular dominance plasticity. J Neurosci. 2008;28:10278–10286. doi: 10.1523/JNEUROSCI.2451-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Smith GB, Heynen AJ, Bear MF. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philosophical transactions of the Royal Society of London. 2009;364:357–367. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DC, Nelson SB, Sur M. An emergent model of orientation selectivity in cat visual cortical simple cells. J Neurosci. 1995;15:5448–5465. doi: 10.1523/JNEUROSCI.15-08-05448.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cat's lateral geniculate body. J. Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. Journal of neurophysiology. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.