Abstract
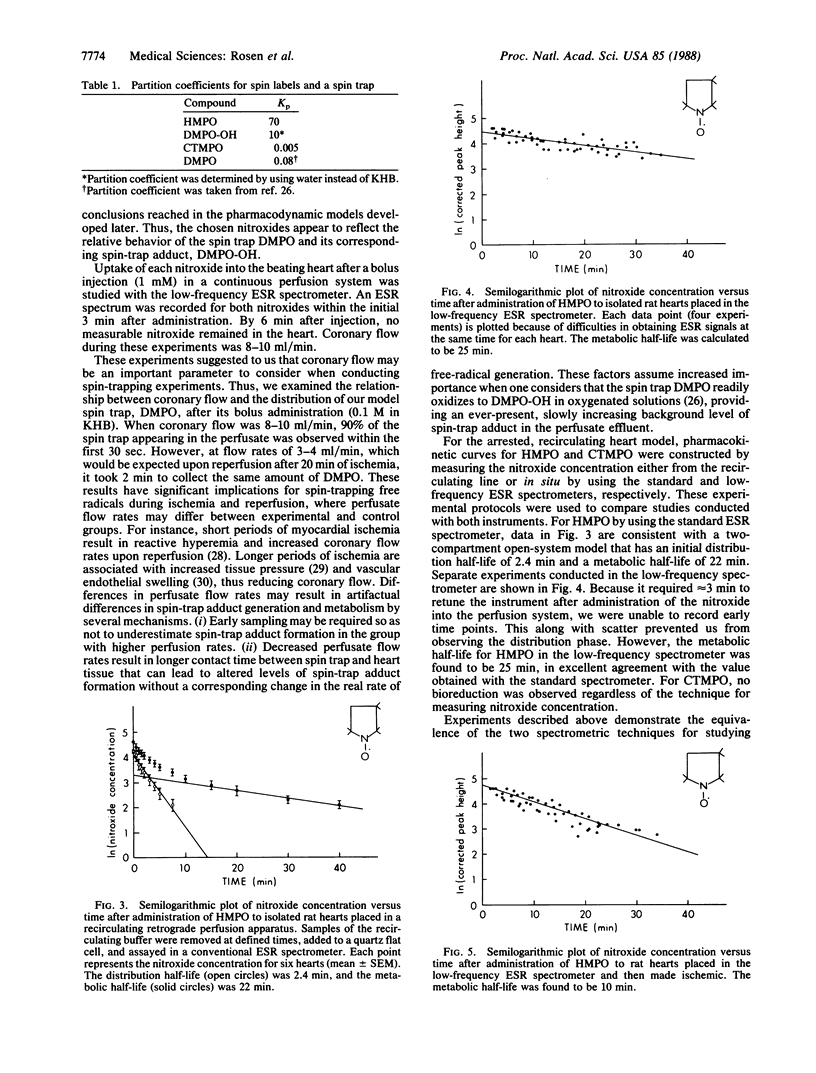
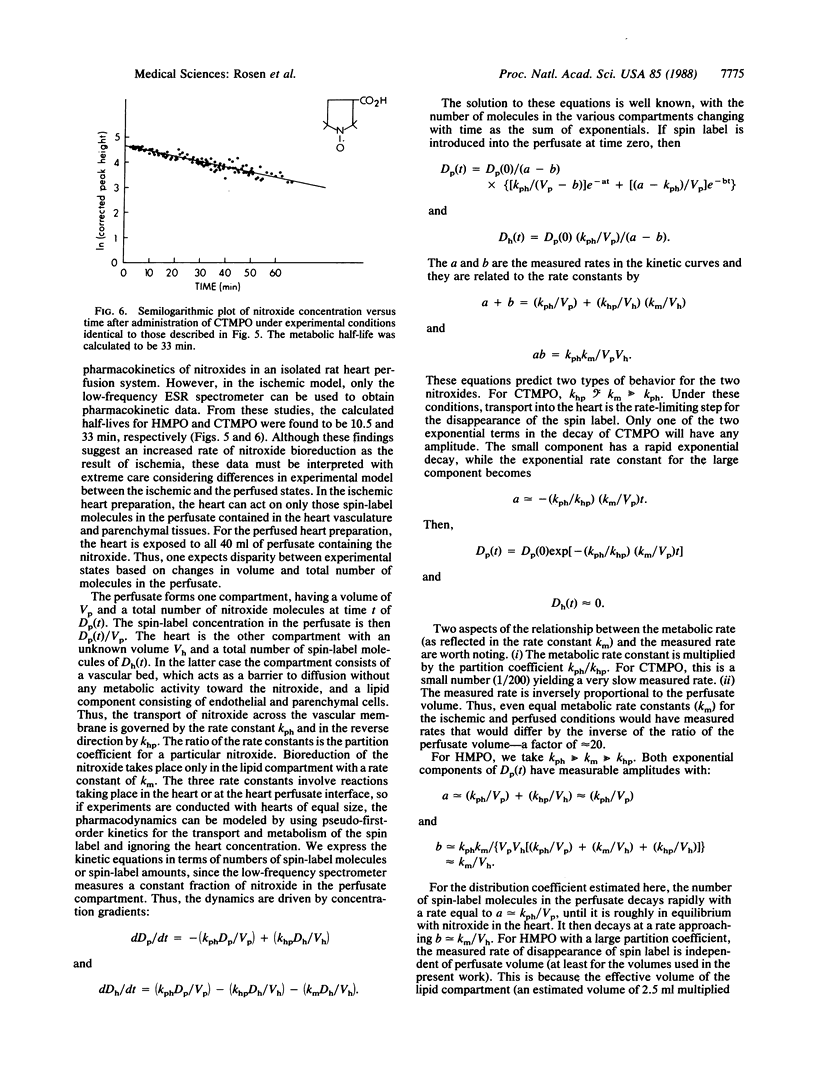
The pharmacokinetics of two nitroxides were investigated in isolated rat hearts situated in a low-frequency electron spin resonance spectrometer. The spin labels 2,2,3,3,5,5-hexamethyl-1-pyrrolidinyloxy and 3-carboxy-2,2,5,5-tetramethyl-1-pyrrolidinyloxy were chosen for their physiochemical analogy to the spin trap 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and its corresponding spin-trapped adduct, 2-hydroxy-5,5-dimethyl-1-pyrrolidinyloxy (DMPO-OH). The bioreductive rates of the two nitroxides were measured during constant perfusion as well as during ischemia and are discussed in terms of a two-compartment pharmacokinetic model. These data provide information necessary to the design and application of spin traps to detect oxy radicals during reperfusion of ischemic tissue and suggest the feasibility of monitoring free-radical processes in intact, functioning mammalian tissues by using a low-frequency electron spin resonance spectrometer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akizuki S., Yoshida S., Chambers D. E., Eddy L. J., Parmley L. F., Yellon D. M., Downey J. M. Infarct size limitation by the xanthine oxidase inhibitor, allopurinol, in closed-chest dogs with small infarcts. Cardiovasc Res. 1985 Nov;19(11):686–692. doi: 10.1093/cvr/19.11.686. [DOI] [PubMed] [Google Scholar]
- Arroyo C. M., Kramer J. H., Dickens B. F., Weglicki W. B. Identification of free radicals in myocardial ischemia/reperfusion by spin trapping with nitrone DMPO. FEBS Lett. 1987 Aug 31;221(1):101–104. doi: 10.1016/0014-5793(87)80360-5. [DOI] [PubMed] [Google Scholar]
- Atalla S. L., Toledo-Pereyra L. H., MacKenzie G. H., Cederna J. P. Influence of oxygen-derived free radical scavengers on ischemic livers. Transplantation. 1985 Dec;40(6):584–590. doi: 10.1097/00007890-198512000-00002. [DOI] [PubMed] [Google Scholar]
- Baker G. L., Corry R. J., Autor A. P. Oxygen free radical induced damage in kidneys subjected to warm ischemia and reperfusion. Protective effect of superoxide dismutase. Ann Surg. 1985 Nov;202(5):628–641. doi: 10.1097/00000658-198511000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M. J., Rosen G. M., Siegel L. M., Rauckman E. J. Evidence for formation of superoxide and formate radicals in Methanobacterium formicicum. J Bacteriol. 1983 Mar;153(3):1282–1286. doi: 10.1128/jb.153.3.1282-1286.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne R. M., Rubio R. Regulation of coronary blood flow. Adv Cardiol. 1974;12(0):303–317. doi: 10.1159/000395474. [DOI] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys. 1980 Mar;200(1):1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Lewis C. A., Wong P. K. High-pressure liquid chromatography--electrochemical detection of oxygen free radicals. Methods Enzymol. 1984;105:231–237. doi: 10.1016/s0076-6879(84)05030-8. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Höllwarth M. E., Parks D. A. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986;548:47–63. [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Hearse D. J., Garlick P. B., Humphrey S. M. Ischemic contracture of the myocardium: mechanisms and prevention. Am J Cardiol. 1977 Jun;39(7):986–993. doi: 10.1016/s0002-9149(77)80212-9. [DOI] [PubMed] [Google Scholar]
- Leaf A. Cell swelling. A factor in ischemic tissue injury. Circulation. 1973 Sep;48(3):455–458. doi: 10.1161/01.cir.48.3.455. [DOI] [PubMed] [Google Scholar]
- Mansbach C. M., 2nd, Rosen G. M., Rahn C. A., Strauss K. E. Detection of free radicals as a consequence of rat intestinal cellular drug metabolism. Biochim Biophys Acta. 1986 Aug 29;888(1):1–9. doi: 10.1016/0167-4889(86)90063-7. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hoidal J. R., Ferris T. F. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984 Oct;74(4):1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N., Bulkley G. B., Shah A. K. Soybean trypsin inhibitor attenuates ischemic injury to the feline small intestine. Gastroenterology. 1985 Jul;89(1):6–12. doi: 10.1016/0016-5085(85)90738-3. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Cheronis J. C., Rodell T. C., Linas S. L., Patt A. Pulmonary oxygen toxicity and ischemia-reperfusion injury. A mechanism in common involving xanthine oxidase and neutrophils. Am Rev Respir Dis. 1987 Aug;136(2):483–485. doi: 10.1164/ajrccm/136.2.483. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Kunkel S. L., Abrams G. D., Schork M. A., Lucchesi B. R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. R., Blackwell W. H., Crute S. L., Loughlin V., Greenfield L. J., Hess M. L. Inhibition of surgically induced ischemia/reperfusion injury by oxygen free radical scavengers. J Thorac Cardiovasc Surg. 1983 Aug;86(2):262–272. [PubMed] [Google Scholar]
- Turner M. J., 3rd, Rosen G. M. Spin trapping of superoxide and hydroxyl radicals with substituted pyrroline 1-oxides. J Med Chem. 1986 Dec;29(12):2439–2444. doi: 10.1021/jm00162a004. [DOI] [PubMed] [Google Scholar]
- Vasko K. A., DeWall R. A., Riley A. M. Effect of allopurinol in renal ischemia. Surgery. 1972 May;71(5):787–790. [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988 Jan 25;263(3):1353–1357. [PubMed] [Google Scholar]




