Abstract
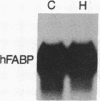
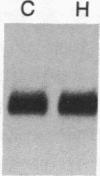
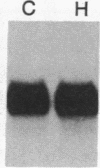
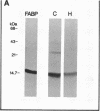
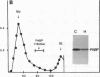
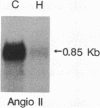
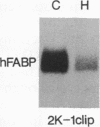
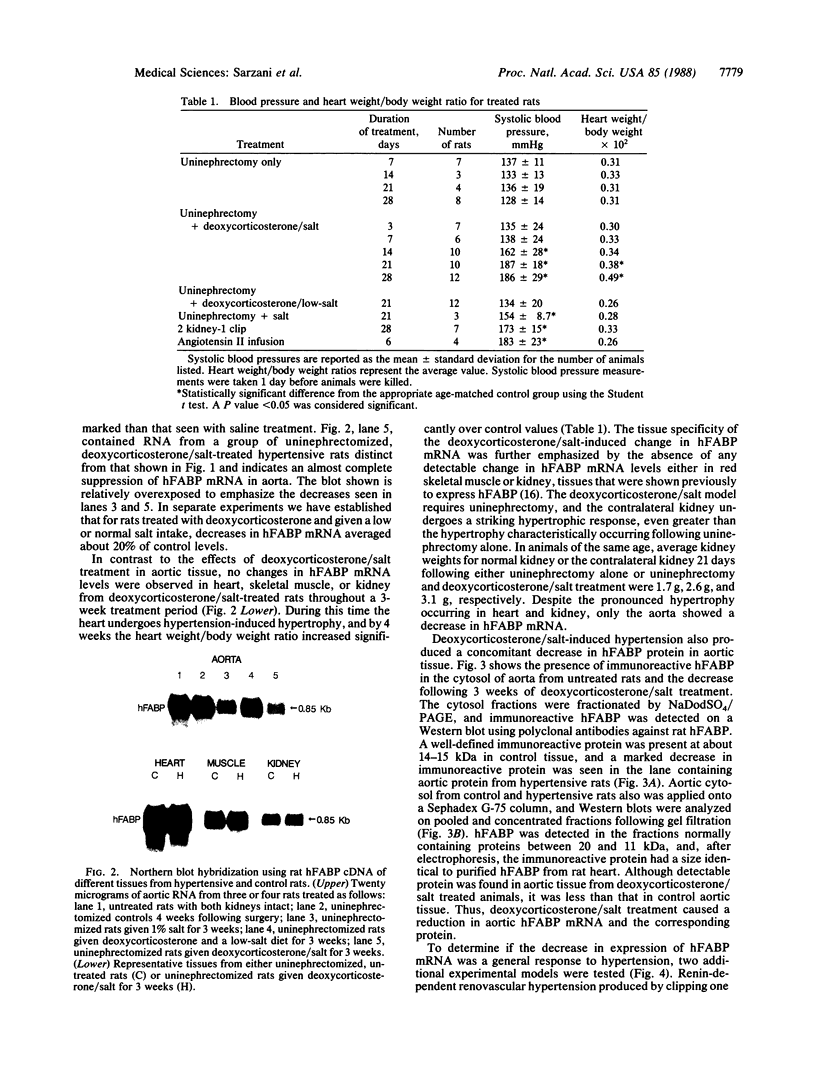
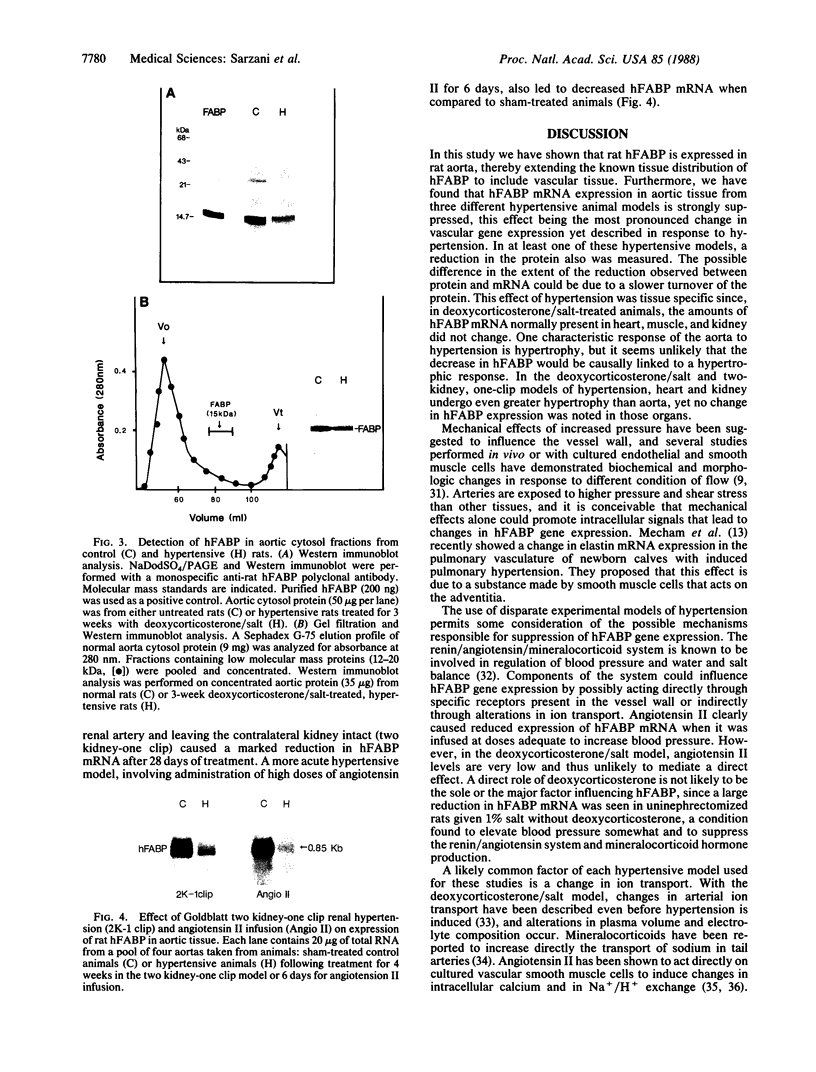
The effect of hypertension on the expression of a fatty acid binding protein localized in the rat aorta was studied. The presence of rat heart fatty acid binding protein (hFABP) was documented in aortic tissue by using a cDNA probe and polyclonal antibodies. Hypertension was induced in groups of rats by implantation of deoxycorticosterone acetate in conjunction with 1% salt in the drinking water (deoxycorticosterone/salt). By the third week of this treatment a marked reduction (by a factor of 20) in the expression of hFABP mRNA in aorta was found, concomitant with a reduction in immunologically detectable protein, suggesting transcriptional regulation. This effect was tissue specific, since no change in the normal amounts of hFABP mRNA in heart, skeletal muscle, or kidney was found. This reduction in aortic hFABP mRNA was also found in mildly hypertensive uninephrectomized rats given salt but no deoxycorticosterone and in normotensive rats given deoxycorticosterone but no excess salt intake. A marked decrease in aortic hFABP mRNA also was observed in the Goldblatt two kidney-one clip hypertensive model, and administration of angiotensin II for 6 days by osmotic minipump also caused a reduction. These findings suggest that hFABP is under complex regulation in aortic tissue and is suppressed by arterial hypertension.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Strauss A. W., Ockner R. K., Bass N. M., Gordon J. I. Cloning of a cDNA encoding rat intestinal fatty acid binding protein. Proc Natl Acad Sci U S A. 1984 Jan;81(2):313–317. doi: 10.1073/pnas.81.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassuk J. A., Tsichlis P. N., Sorof S. Liver fatty acid binding protein is the mitosis-associated polypeptide target of a carcinogen in rat hepatocytes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7547–7551. doi: 10.1073/pnas.84.21.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk B. C., Aronow M. S., Brock T. A., Cragoe E., Jr, Gimbrone M. A., Jr, Alexander R. W. Angiotensin II-stimulated Na+/H+ exchange in cultured vascular smooth muscle cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1987 Apr 15;262(11):5057–5064. [PubMed] [Google Scholar]
- Berk B. C., Brock T. A., Gimbrone M. A., Jr, Alexander R. W. Early agonist-mediated ionic events in cultured vascular smooth muscle cells. Calcium mobilization is associated with intracellular acidification. J Biol Chem. 1987 Apr 15;262(11):5065–5072. [PubMed] [Google Scholar]
- Bevan R. D., van Marthens E., Bevan J. A. Hyperplasia of vascular smooth muscle in experimental hypertension in the rabbit. Circ Res. 1976 Jun;38(6 Suppl 2):58–62. doi: 10.1161/01.res.38.6.58. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Hamlyn J. M. Sodium transport inhibition, cell calcium, and hypertension. The natriuretic hormone/Na+-Ca2+ exchange/hypertension hypothesis. Am J Med. 1984 Oct 5;77(4A):45–59. doi: 10.1016/s0002-9343(84)80037-6. [DOI] [PubMed] [Google Scholar]
- Bond J. F., Farmer S. R. Regulation of tubulin and actin mRNA production in rat brain: expression of a new beta-tubulin mRNA with development. Mol Cell Biol. 1983 Aug;3(8):1333–1342. doi: 10.1128/mcb.3.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecher P., Chan C. T., Franzblau C., Faris B., Chobanian A. V. Effects of hypertension and its reversal on aortic metabolism in the rat. Circ Res. 1978 Oct;43(4):561–569. doi: 10.1161/01.res.43.4.561. [DOI] [PubMed] [Google Scholar]
- Böhmer F. D., Kraft R., Otto A., Wernstedt C., Hellman U., Kurtz A., Müller T., Rohde K., Etzold G., Lehmann W. Identification of a polypeptide growth inhibitor from bovine mammary gland. Sequence homology to fatty acid- and retinoid-binding proteins. J Biol Chem. 1987 Nov 5;262(31):15137–15143. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chobanian A. V. The influence of hypertension and other hemodynamic factors in atherogenesis. Prog Cardiovasc Dis. 1983 Nov-Dec;26(3):177–196. doi: 10.1016/0033-0620(83)90005-1. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claffey K. P., Herrera V. L., Brecher P., Ruiz-Opazo N. Cloning and tissue distribution of rat heart fatty acid binding protein mRNA: identical forms in heart and skeletal muscle. Biochemistry. 1987 Dec 1;26(24):7900–7904. doi: 10.1021/bi00398a054. [DOI] [PubMed] [Google Scholar]
- Crisman T. S., Claffey K. P., Saouaf R., Hanspal J., Brecher P. Measurement of rat heart fatty acid binding protein by ELISA. Tissue distribution, developmental changes and subcellular distribution. J Mol Cell Cardiol. 1987 May;19(5):423–431. doi: 10.1016/s0022-2828(87)80394-2. [DOI] [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982 Apr;62(2):347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- Garwitz E. T., Jones A. W. Altered arterial ion transport and its reversal in aldosterone hypertensive rat. Am J Physiol. 1982 Dec;243(6):H927–H933. doi: 10.1152/ajpheart.1982.243.6.H927. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Glatz J. F., Veerkamp J. H. Intracellular fatty acid-binding proteins. Int J Biochem. 1985;17(1):13–22. doi: 10.1016/0020-711x(85)90080-1. [DOI] [PubMed] [Google Scholar]
- Haudenschild C. C., Prescott M. F., Chobanian A. V. Effects of hypertension and its reversal on aortic intima lesions of the rat. Hypertension. 1980 Jan-Feb;2(1):33–44. doi: 10.1161/01.hyp.2.1.33. [DOI] [PubMed] [Google Scholar]
- Heuckeroth R. O., Birkenmeier E. H., Levin M. S., Gordon J. I. Analysis of the tissue-specific expression, developmental regulation, and linkage relationships of a rodent gene encoding heart fatty acid binding protein. J Biol Chem. 1987 Jul 15;262(20):9709–9717. [PubMed] [Google Scholar]
- Hunt C. R., Ro J. H., Dobson D. E., Min H. Y., Spiegelman B. M. Adipocyte P2 gene: developmental expression and homology of 5'-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3786–3790. doi: 10.1073/pnas.83.11.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McMahon E. G., Paul R. J. Metabolic and mechanical properties of aortas from aldosterone-salt hypertensive rats. Circ Res. 1984 Sep;55(3):349–357. doi: 10.1161/01.res.55.3.349. [DOI] [PubMed] [Google Scholar]
- Mecham R. P., Whitehouse L. A., Wrenn D. S., Parks W. C., Griffin G. L., Senior R. M., Crouch E. C., Stenmark K. R., Voelkel N. F. Smooth muscle-mediated connective tissue remodeling in pulmonary hypertension. Science. 1987 Jul 24;237(4813):423–426. doi: 10.1126/science.3603030. [DOI] [PubMed] [Google Scholar]
- Menard M. R., Friedman S. M. Direct measurement of sodium influx in vascular smooth muscle. Stimulation by aldosterone. Hypertension. 1985 Nov-Dec;7(6 Pt 1):873–878. doi: 10.1161/01.hyp.7.6.873. [DOI] [PubMed] [Google Scholar]
- Offner G. D., Troxler R. F., Brecher P. Characterization of a fatty acid-binding protein from rat heart. J Biol Chem. 1986 Apr 25;261(12):5584–5589. [PubMed] [Google Scholar]
- Olesen S. P., Clapham D. E., Davies P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988 Jan 14;331(6152):168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Schwartz S. M. Vascular smooth muscle cell hypertrophy and hyperploidy in the Goldblatt hypertensive rat. Circ Res. 1983 Oct;53(4):491–501. doi: 10.1161/01.res.53.4.491. [DOI] [PubMed] [Google Scholar]
- Seidel C. L., Strong R. Metabolic characteristics of aorta from spontaneously hypertensive and renal and deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 1986 Feb;8(2):103–108. doi: 10.1161/01.hyp.8.2.103. [DOI] [PubMed] [Google Scholar]
- Sweetser D. A., Heuckeroth R. O., Gordon J. I. The metabolic significance of mammalian fatty-acid-binding proteins: abundant proteins in search of a function. Annu Rev Nutr. 1987;7:337–359. doi: 10.1146/annurev.nu.07.070187.002005. [DOI] [PubMed] [Google Scholar]
- Wolinsky H. Long-term effects of hypertension on the rat aortic wall and their relation to concurrent aging changes. Morphological and chemical studies. Circ Res. 1972 Mar;30(3):301–309. doi: 10.1161/01.res.30.3.301. [DOI] [PubMed] [Google Scholar]