Abstract
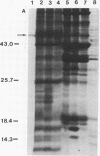
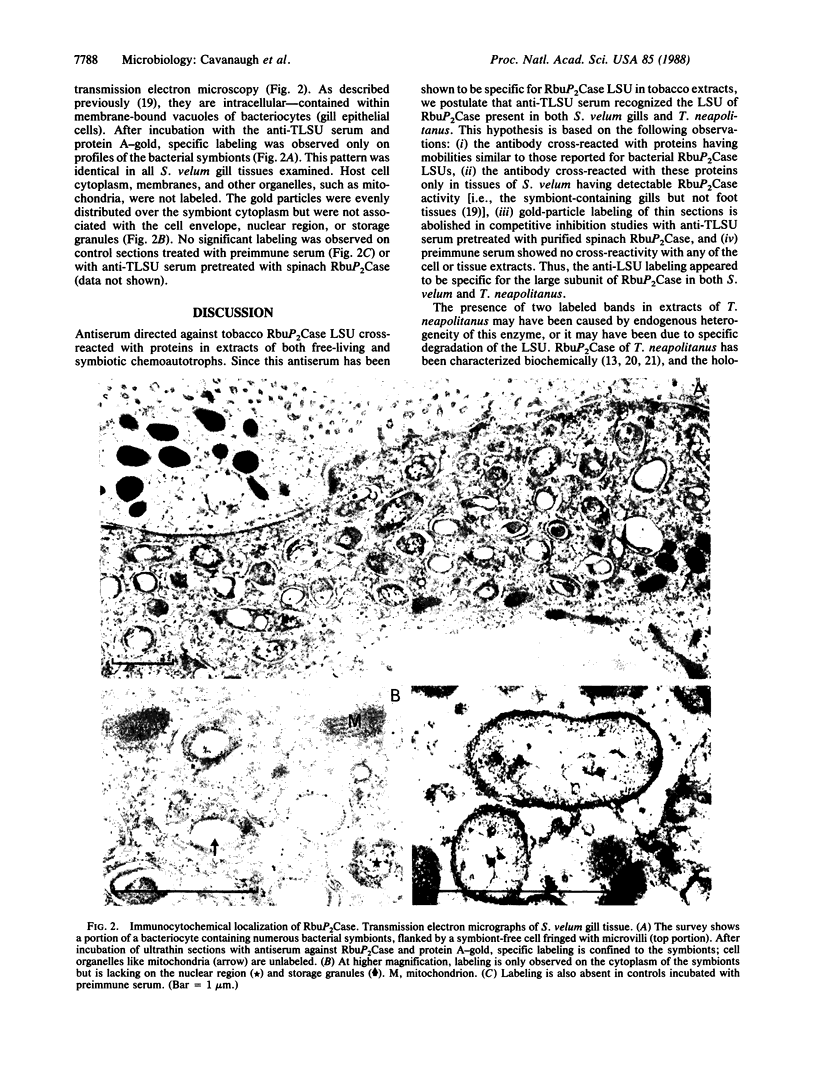
The distribution of the Calvin cycle enzyme ribulose-1,5-bisphosphate carboxylase (RbuP2Case; EC 4.1.1.39) was examined by using two immunological methods in tissues of Solemya velum, an Atlantic coast bivalve containing putative chemoautotrophic symbionts. Antibodies elicited by the purified large subunit of RbuP2Case from tobacco (Nicotiana tabacum) cross-reacted on immunoblots with a protein of similar molecular mass occurring in extracts of the symbiont-containing gill tissue of S. velum. No cross-reactivity was detected in symbiont-free tissue extracts. The antiserum also cross-reacted in immunoblots with proteins of Thiobacillus neapolitanus, a free-living sulfuroxidizing chemoautotroph whose RbuP2Case has been well characterized. In protein A-gold immunoelectron microscopy studies, this antiserum consistently labeled the symbionts but not surrounding host gill tissue, indicating that the symbionts are responsible for the RbuP2Case activity.
Keywords: chemoautotroph, sulfur-oxidizing bacteria, deep-sea hydrothermal vents, symbiosis
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K., Caton J. Sequence analysis of the Alcaligenes eutrophus chromosomally encoded ribulose bisphosphate carboxylase large and small subunit genes and their gene products. J Bacteriol. 1987 Oct;169(10):4547–4558. doi: 10.1128/jb.169.10.4547-4558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman F. C., Stringer C. D., Lee E. H. Complete primary structure of ribulosebisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. Arch Biochem Biophys. 1984 Jul;232(1):280–295. doi: 10.1016/0003-9861(84)90544-7. [DOI] [PubMed] [Google Scholar]
- Kuenen J. G., Beudeker R. F. Microbiology of thiobacilli and other sulphur-oxidizing autotrophs, mixotrophs and heterotrophs. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 13;298(1093):473–497. doi: 10.1098/rstb.1982.0093. [DOI] [PubMed] [Google Scholar]
- Lacoste-Royal G., Gibbs S. P. Immunocytochemical Localization of Ribulose-1,5-Bisphosphate Carboxylase in the Pyrenoid and Thylakoid Region of the Chloroplast of Chlamydomonas reinhardtii. Plant Physiol. 1987 Mar;83(3):602–606. doi: 10.1104/pp.83.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]