Abstract
The piglet scaled cortical impact model creates a focal contusion using a skull-mounted, spring-loaded blunt indentation device scaled to achieve identical tissue strains in subjects with different brain sizes. Preliminary data showed that contusion size increased proportional to subject age. This study details the results from a new, larger series of subjects of three ages, and compares the effect of age and additional host and physiologic variables on injury response. Sixty-seven subjects, including infant (5- to 7-day-old), “toddler” (1-month-old), and early adolescent (4-month-old) swine underwent scaled cortical impact under strict anesthetic protocols. Serum glucose, testosterone, and 17β-estradiol levels were measured. Lesion size was measured at 1 week post injury, as the ratio of the lesion area over the area of the contralateral hemisphere. Adolescent subjects had lesions over eight times larger than infants (p < 0.0001). Lesion volumes were larger in toddlers than in infants, most significantly for males (p < 0.05). Adolescent subjects were warmer on average, but there was no correlation between temperature and lesion volume within any age group. Serum glucose did not differ among ages. Infant males had the highest levels of circulating sex steroids. In this model, age was the most robust predictor of lesion size. Temperature had an effect, but did not explain all the variability seen among age groups. There was an interaction among gender, hormone levels, and lesion size in younger subjects. Characterization of these variables allows use of this model for treatment trials for subjects at different stages of maturation.
Key words: brain, contusion, development, gender, head injury, swine
Introduction
The response of the immature brain to injury has been incompletely studied. Brain injury in infants and children can present with patterns unique for those populations, and distinctly different from their adult counterparts. Head injury remains the most common cause of death and disability in childhood, yet whether unique age-dependent responses occur that might inform specific treatment strategies remains unknown. The heterogeneous nature of clinical head trauma, as well as the complexity of the various stages of brain maturation, particularly in gyrencephalic animals, make standardization and analysis of age-specific differences challenging (Luerssen and Klauber, 1995). Large animal models of injury to the immature brain provide insights into these processes by offering a controlled environment that allows us to begin elucidating this complex interplay of many variables (Anderson et al., 2003; Povlishock et al., 1994; Bittigau et al., 1998; Shaver et al., 1996).
We have utilized a scaled cortical swine contusion model that accounts for the changes in mass and morphology of the brain as it matures. The model is designed to create a cortical contusion by rapid indentation of a volume measuring 1% of the total brain volume for subjects of that age, which is scaled in three dimensions for brain growth. Because of the rapid and forceful indentation of the brain surface, minor differences in mechanical properties of the brain are overcome in this model, which holds strain (deformation over time) constant through all ages (Duhaime et al., 2000). This type of scaling has been utilized because of the evidence that strain is the best predictor of resultant injury in biomechanical in-vivo models of brain trauma (Margulies et al., 1990; Morrison et al., 1998; Shreiber et al., 1999).
In a previous publication using this model in a small number of subjects, lesion size was found to increase significantly with subject age (Duhaime et al., 2000). However, subject numbers were too small to allow for analysis of specific physiological variables or host factors that might influence results. In addition, these experiments were performed using supplemental oxygen instead of room air, and thus did not mimic the conditions under which most clinical injuries occur. Some physiological variables might influence lesion size and be age-dependent; for instance, infants might be more susceptible to hypoglycemia and relative hypothermia, while the oldest subjects have been noted to have an increase in body temperature under stressful conditions. For these reasons, we present results from a new series using this model in which analysis of additional variables was made possible by larger subject numbers and improved physiological control.
Methods
Experimental model
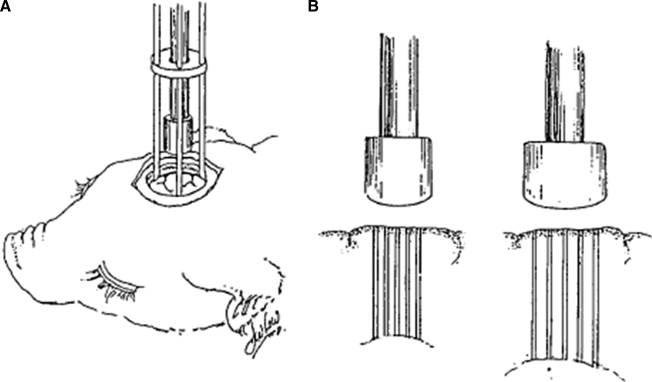
The model of traumatic brain injury used has been described in a prior publication (Duhaime et al., 2000) (Fig. 1). In brief, cortical indentation was created by a stainless steel spring-loaded device into which interchangeable blunt cylindrical stainless steel indentor tips can be secured. When fired, the tips depress the exposed brain surface over 400 msec. The indentor device is attached directly to the skull with three-point screw fixation, thereby allowing visible confirmation of the initial position of the indentor tip, ensuring a directly perpendicular indentation, and eliminating motion of the head relative to the indentor. Indentation volumes are scaled to displace 1% of the total brain volume for that age subject, and the dimensions of the indentor tip and depth of indentation are scaled such that comparable anatomical structures are displaced in all ages. Lesions are centered at the rostral gyrus of the frontal lobe to facilitate functional outcome comparisons among ages (Duhaime et al., 2003). Indentation depth extended approximately halfway between the cortical surface and the lateral ventricle. A 1-week survival time was chosen to minimize the possible effect of differences in timing of early injury evolution that might be seen with shorter survival times. This model produces reproducible, histologically visible lesions with an uncomplicated 7-day survival period.
FIG. 1.
(A) Schematic representation of the cortical injury device. (B) Illustration of the scaled indentor tips and their use in histological sampling. The tip diameter and indentation depth increase in proportion to increases in brain dimension with age, from youngest (left) to oldest (right), as described in the text. Histological samples were taken at the 0%, 25%, 50%, 75%, and 100% regions of tissue under the indentor tip for each subject (reprinted with permission from the Journal of Neurosurgery, www.thejns-net.org).
Subject population
Sixty-seven Yorkshire male and female domestic piglets were used in these studies. The animals were 5–7 days (n =26), 1 month (n = 20), or 4 months old (n = 21) at the time of injury. These ages have been shown to correspond with human infants, toddlers, and early adolescents, respectively, with regard to brain growth, myelination, and somatic development (Duhaime et al., 2000; Flynn, 1984; Pampiglione, 1971).
Anesthetic and surgical procedures
The study was approved by the Dartmouth Institutional Animal Care and Use committee in compliance with the Guide for Care and Use of Animals and all applicable laws and regulations. The animals were restricted from consuming solid food overnight prior to surgery, but were allowed access to formula (in the youngest subjects) and/or water. Anesthetic protocols utilized the same medications for all ages, with doses chosen for appropriate effect and to minimize side effects and toxicity at each age. Thus animals received medications prior to surgery that included midazolam (0.25 mg/kg for 5- to 7-day-old animals, 1 mg/kg for 1-month-old and 4-month-old animals), atropine (0.0035 mg/kg for 5- to 7-day-old animals, 0.015 mg/kg for 1-month-old and 4-month-old animals), and buprenorphine (0.005 for 5- to 7-day-old animals, 0.05 mg/kg for 1-month-old and 4-month-old animals). General anesthesia was induced using 1–2% isoflurane/21% oxygen administered by bag and mask ventilation initially during induction; the animals were then intubated and anesthesia was delivered via an endotracheal tube by a staff pediatric anesthesiologist. Continuous physiological monitoring was performed in order to maintain all physiological variables within the normal range at the time of injury and in the immediate post-injury period. Oxygen saturation was measured continuously with pulse oximetry and was kept above 95% by adjusting inspired oxygen and isoflurane levels, with all injuries occurring under conditions of room air. Core body temperature was kept at 37–39°C using heating blankets and forced warm-air devices. An intravenous line was placed in a dorsal ear vein or limb vein for administration of lactated Ringer's solution for treatment of hypotension as needed, and for administration of medications. Blood samples (1–2 mL) were drawn prior to surgery, and an additional blood sample was obtained 15 min after injury to measure serum markers as part of a separate study. Heart rate, blood pressure, end-tidal carbon dioxide, oxygen saturation, respiratory rate, and body temperature (rectal, and in a subset of subjects [n = 5] temporalis muscle) were recorded at baseline, 15 min prior to injury, at the time of injury, and immediately post-injury until recovery from anesthesia.
The vertex of the head was clipped and prepped with chlorhexidine solution. Local anesthetic (1% lidocaine with epinephrine 1:100,000) was administered. A C-shaped incision was fashioned centered over the sagittal and right coronal sutures. A craniectomy was performed beginning with a 1.1-cm burr hole just anterior to the junction of the right coronal and sagittal sutures. The burr hole was then enlarged using rongeurs to a diameter 1 cm larger than the indentor tip sized for that subject's age (2–3 cm). Following the craniectomy, the dura was opened in a cruciate fashion to expose the underlying cortical surface. The cortical indentation device was secured to the bone opening, and the appropriately sized indentor tip for each age group was threaded securely into the indentor collar. The spring-loaded device was fired with a time course of 400 msec and then removed. After injury, the lesion site was gently irrigated with sterile saline to ensure hemostasis. The lesion site was scored in 59 of the 67 subjects by the surgeon on a four-point scale with respect to visible surface subarachnoid hemorrhage (none, minimal, moderate, or marked). The dura was reapproximated over the lesion and the skin was closed with running suture and skin sealant. The animals were allowed to recover from general anesthesia and were monitored until they could ambulate. The subjects were observed for a period of 7 days post-injury, and examined daily for any changes in their neurological or behavioral status. Blood samples were collected on postoperative days 4 and 7 from all subjects, using brief sedation with 2% isoflurane gas.
Histology protocol
At 7 days post-injury, the animals were anesthetized as noted previously and underwent MRI imaging (results to be reported separately), and were then administered sodium pentobarbital (50 mg/kg IM) to achieve deep surgical anesthesia. Intracardiac perfusion was performed using a right thoracotomy incision with 0.9% normal saline solution followed by 10% buffered formalin. The brain was removed and further fixed in 10% buffered formalin for a period of 3–5 days. Three parallel 5-mm-thick coronal sections centered over the lesion site were blocked, processed, and embedded in paraffin for histological analysis, as previously described (Grate et al., 2003). These 5-mm slabs allowed for equal penetration of fixative despite brains of different sizes. Once the tissue was fixed and embedded, serial coronal sections 10 μm thick were taken every 0.25 mm and mounted onto 3-aminopropylethylsilane- or poly-L-lysine-coated slides and stained with hematoxylin and eosin (H&E). Adjacent sections were saved for additional studies. To compare relative lesion size, five brain slices were analyzed per animal, corresponding to the 0%, 25%, 50%, 75% and 100% regions of tissue equally spaced and extending through the entire thickness of the brain directly under the indentor tip for each animal age group. In this way, five coronal sections throughout the area of deformation were obtained for each subject, which were also scaled for the size of the brain at each age, in order to sample comparable regions of the injured zone. This enabled equivalent scaled sampling of the lesioned area, and encompassed both the center of the lesion and the penumbra at the depth of the lesion for subjects of each age.
Lesion analysis
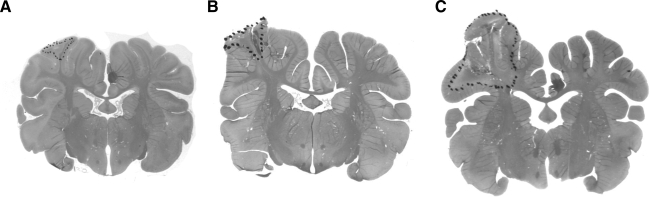
Brain slices were reviewed by a single neuropathologist (B.H.) without knowledge of the age of the animal. Lesions were quantified as previously described (Duhaime et al., 2000). In brief, areas of brain injury, as defined by the presence of necrosis with or without hemorrhage, neuronal dropout, or damage, and/or reactive gliosis, were identified by light microscopy and outlined on the slides (Fig. 2). In order to compare relative lesion dimensions among age groups, the area of the lesion and the area of the contralateral hemisphere were computed using an image analysis system (MCID; Imaging Research, Inc., St. Catharines, Ontario, Canada). For each animal, the mean ratio of lesion area to the area of the corresponding contralateral hemisphere for the five slides examined was calculated.
FIG. 2.
Photographs of H&E-stained coronal sections that demonstrate cortical surface contusion injury with dots outlining a portion of the injury on the rostral gyrus in a brain from a 7-day-old pig (infant: A), a 1-month-old pig (toddler: B), and a 4-month-old pig (early adolescent: C) collected 7 days after scaled cortical impact.
Plasma glucose, testosterone, and estradiol analysis
Blood was collected in EDTA-coated tubes and centrifuged within 15 min of collection at 3000 × g, and plasma was stored at −80°C until analysis using enzyme-linked immunosorbent assays per the manufacturers' instructions for glucose, testosterone, and 17β-estradiol (Cayman Chemical, Ann Arbor, MI). Samples were run in duplicate. For glucose, the intra-assay coefficient of variation (CV) was 4.6%, and the inter-assay CV was 4.3%. For 17β-estradiol, the intra-assay CV was 10.3% and was run in a single assay. For the testosterone assay, samples were run undiluted or at a 1:2 dilution, and the intra-assay CV was 9.3%, and the inter-assay CV was 6.7%.
Statistical analysis
The main effects of age and gender and their interactions with other variables were determined by one-way ANOVA for lesion size, and two-way ANOVA for lesion size, mean body temperature (at the time of injury and 15 min after injury), mean end-tidal carbon dioxide, mean oxygen saturation, mean heart rate, and concentrations of 17β-estradiol, testosterone, and glucose. Differences among means were determined using the least-squared-means procedure of the SAS software statistical package (version 9.1.4; SAS Institute, Cary, NC). The effect of age on lesion size with temperature as a covariate was determined using analysis of covariance with age, gender, and temperature as the main effects, followed by means comparisons using contrasts. Pearson correlations between lesion size and mean body temperature, concentrations of 17β-estradiol, and testosterone were performed both among age groups and within age groups. p Values < 0.05 were considered significant. Differences in the proportions of categories of subarachnoid hemorrhage among 5- to 7-day-old, 1-month-old, and 4-month-old subjects, and between males versus females were tested using the chi-square test of homogeneity. Correlations between the degree of subarachnoid hemorrhage and lesion size were performed using Spearman's rank correlation. All statistical calculations were performed using SAS. Data are presented as means ± standard error of the mean (SEM).
Results
Subject distribution and physical parameters
The 67 subjects tested across the three age groups included 30 males and 37 females, with each age group having similar numbers of each gender. Body weight increased with age (2.5 ± 0.06 kg for infants, 9.6 ± 0.6 kg for toddlers, and 58.6 ± 1.2 kg for adolescents). There were no differences in body weight between genders at any age.
Histology
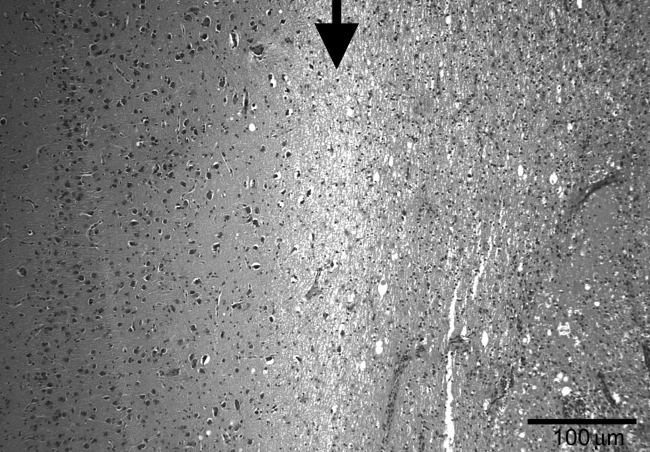
Lesions were characterized by discrete areas of contusion injury characterized by variable histopathological changes of necrosis, inflammation, hemorrhage, tissue rarefaction, and gliosis with clear boundaries (Fig. 3). They varied in size from no detectable lesion to 46.4% of the area of the corresponding contralateral hemisphere among all ages combined; lesions within each age group showed less variability (as described in more detail below). There were variable degrees of intraparenchymal hemorrhage, although most lesions did not contain marked solid hematoma.
FIG. 3.
Intermediate-power view showing the border between normal cortex (left) and area of the lesion (right) surrounded by an area of edematous white matter (middle). The border is marked by the arrow (H&E stain, original magnification 100 ×; scale bar = 100 μm).
Effects of visible surface hemorrhage
There was no correlation between the degree of visible surface subarachnoid hemorrhage and lesion size as determined by histology (r = −0.09, p = 0.69). There was no difference in the proportions of the degree of surface hemorrhage among ages or between genders, and therefore with ages and genders combined, 8.5% (5/59) had no visible hemorrhage, 49.1% (29/59) had mild hemorrhage, 35% (21/59) had moderate hemorrhage, and 6.8% (4/59) had marked hemorrhage.
Effects of age
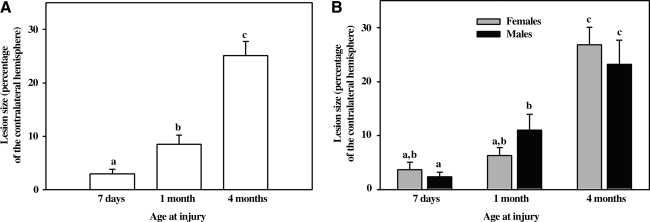
When analyzed with a one-way ANOVA with the main effect of age, lesion sizes (ratio of the injured area divided by the area of contralateral hemisphere averaged over five equally-spaced sections under the indentor tip) 1 week after injury increased as a function of age (p < 0.0001). Infants had smaller lesion sizes than toddlers (3.0 ± 0.8 versus 8.5 ± 1.6; p = 0.04) and toddlers had smaller lesion sizes than adolescents (8.5 ± 1.6 versus 25.1 ± 2.7; p < 0.0001) (Fig. 4).
FIG. 4.
Lesion size as a percentage of the contralateral hemisphere 7 days after scaled cortical impact of the rostral gyrus in 5- to 7-day-old, 1-month-old, and 4-month-old pigs. The letters a, b, c are used to label the groups and indicate when the difference among groups is statistically significant (p < 0.05). Groups are different when they do not share a common letter (e.g., a is different from b, but there no significant difference between a and a,b, or b and a,b). (A) Lesion size in 5- to 7-day-old (n = 26), 1-month-old (n = 19), and 4-month-old (n = 22) pigs. Lesion size increased correspondent with age. (B) The same subjects shown in A, but separated by gender. Lesion size in 5- to 7-day-old females (n = 13) and males (n = 13), 1-month-old females (n = 10) and males (n = 9), and 4-month-old females (n = 12) and males (n = 10). Lesion size was greater in 5- to 7-day-old than in 1-month-old males, but not females of the corresponding ages.
Effects of gender
There was no difference in lesion size between male and female subjects within any of the three age groups. However, there was an effect of gender across ages. While there was a significant increase in lesion size between the infant and toddler age groups overall, when the effects of both age and gender were analyzed, toddler males had larger lesions than infant males (p = 0.02), while in females lesion size was numerically larger in toddlers compared to infants, but this did not reach significance (Fig. 4).
Circulating sex steroids
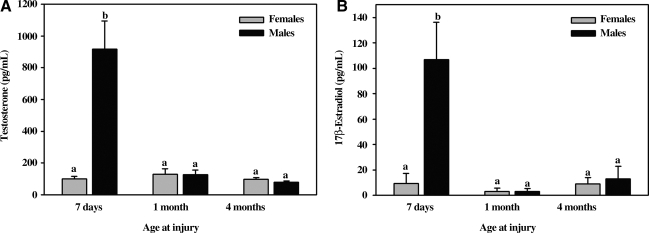
To determine if the effect of gender on lesion size was associated with changes in sex steroids, concentrations of testosterone and 17β-estradiol were measured in plasma collected following anesthetic induction, prior to scaled cortical impact. Both steroids were elevated in infant males compared to female infants, male and female toddlers, and male and female adolescents. Comparing all age groups, concentrations of testosterone were inversely correlated with lesion size (r = −.330, p = 0.02), while there was no relationship between concentrations of 17β-estradiol and lesion size (r = −0.09, p = 0.62) (Fig. 5).
FIG. 5.
Concentrations of testosterone (A) and 17β-estradiol (B) in venous plasma collected post-anesthetic induction, prior to scaled cortical impact in male and female pigs 5–7 days, 1 month, or 4 months old. a,bMeans ± SEM with different letters differ significantly (p < 0.05). Infant males (5–7 days old) had significantly higher (p < 0.0001) concentrations of testosterone and 17β-estradiol than their female counterparts and 1-month-old and 4-month-old subjects.
Physiological parameters
Oxygen saturation, end-tidal carbon dioxide, heart rate, and blood pressure
There were no differences in mean oxygen saturation or end-tidal carbon dioxide among groups. As expected, heart rate decreased and mean arterial pressure increased with increasing subject age (p < 0.0001); these physiological effects were not gender-dependent (Table 1).
Table 1.
Effect of Age and Gender on Mean Oxygen Saturation, Mean End-Tidal Carbon Dioxide, Mean Heart Rate, Mean Arterial Pressure (MAP), Glucose Concentration, and Mean Body Temperature Prior to Scaled Cortical Impact
| |
7 Days at injury |
1 Month at injury |
4 Months at injury |
|||
|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | |
| Oxygen saturation (%) | 95.7 ± 1.6 | 97.7 ± 0.6 | 95.1 ± 1.1 | 97.1 ± 0.6 | 95.3 ± 0.9 | 95.0 ± 0.8 |
| End-tidal carbon dioxide pressure (mm Hg) | 43.1 ± 2.3 | 38.9 ± 2.0 | 39.1 ± 3.3 | 37.7 ± 1.7 | 39.4 ± 1.4 | 36.9 ± 2.7 |
| Heart rate (beats/min) | 159.2 ± 6.9a | 157.1 ± 8.4a | 118.1 ± 3.9b | 111.3 ± 7.7b | 97.7 ± 5.9c | 98.3 ± 3.9c |
| MAP (mm Hg) | 40.1 ± 1.6a | 41.2 ± 2.6a | 50.2 ± 2.2b | 48.7 ± 3.1b | 51.1 ± 2.1b | 60.8 ± 2.7c |
| Glucose (mg/dL) | 115.8 ± 13.5 | 89.2 ± 9.0 | 108.7 ± 11.7 | 109.8 ± 6.3 | 81.7 ± 14.3 | 83.6 ± 7.0 |
| Mean body temperature (°C) | 37.39 ± 0.48a | 37.40 ± 0.30a | 37.21 ± 0.35a | 37.42 ± 0.20a | 39.18 ± 0.09b | 38.69 ± 0.13b |
Means ± SEM with different letters within a row differ significantly (p < 0.05).
Mean body temperature values are also shown for each animal group. Temperature was measured by a rectal probe in the 15 min prior to injury, at the time of injury, and in the 15 min after injury for each subject, and averaged for the 5- to 7-day-old, 1-month-old, and 4-month-old male and female subjects.
Temperature
Despite immediate warming using forced-warm-air blankets after induction of anesthesia, body temperature was lower at the time of injury and 15 min after injury in infant and toddler subjects compared to adolescent subjects, resulting in an average difference of 1.6°C between the oldest animals and the two younger groups (p < 0.0001). While loss of surface temperature resulting in a decrease in core body temperature is expected in all individuals, young pigs have greater heat loss due to lack of significant body hair and larger surface area-to-volume ratio, which results in difficulty in maintaining body temperature (Miller and Blith, 1958; Mount and Rowell, 1960; English et al., 1991). There was no significant difference between rectal temperatures and temporalis temperatures in the subset of subjects who had both types of temperature measurement. While the 4-month-old subjects had the highest temperatures and the largest lesions, mean body temperature was not correlated with lesion size among individual subjects within any age group (infants r = −1.2, p = 0.54; toddlers r = 0.003, p = 0.99; adolescents r = −0.1, p = 0.64). Temperatures were no different in the two youngest age groups despite differences in lesion size. Lesion size increased as a function of age, even when temperature was included as a covariate (infant versus toddler, p = 0.04; infant versus adolescent, p < 0.0001; toddler versus adolescent, p < 0.0001).
Serum glucose
Serum glucose collected just prior to injury did not differ among groups, and no group appeared to be hypo- or hyperglycemic prior to injury (Table 1).
Discussion
Effects of age and gender
Consistent with our prior work, age was found to be the strongest predictor of lesion size at 7 days post-injury in this scaled model (Duhaime et al., 2000). This suggests an age-dependent difference in injury response, with younger age conferring relative protection, or increased age conferring increased vulnerability to injury. Because this result was unexpected in our initially reported series, we wanted to perform a larger series under more strict physiological control in order to ascertain whether confounders were contributing to these findings. In addition, lesion size in large animal models is generally more variable than that found in rodent models, so we wanted to confirm our prior findings in a larger series.
In the current series, despite attempts at strict control of physiological variables among all groups, temperature did differ according to age because of the rapid cooling of the younger animals under general anesthesia. However, even with temperature included in the analysis as a covariate, age still influenced lesion size, with the oldest animals sustaining lesions that were, on average, eight times larger than those sustained by the youngest subjects.
Gender-specific differences were noted when comparing the infant and toddler subjects. The increase in lesion sizes in toddler males compared to infant males coincided with a decrease in circulating concentrations of testosterone and 17β-estradiol. Elevated testicular production of these steroids and concomitant sexual differentiation of the brain in male pigs that begins during gestation and continues into early postnatal life has been described (Ford, 1990; Ford, 1983; Ford et al., 1980; Colenbrander et al., 1978). It is conceivable that the decrease in these steroids occurring in the fourth week of life contributed to a withdrawal effect in the male subjects at 1 month of age and led to an increase in lesion size after brain trauma (Ford, 1983). There are reports that elevated levels of sex steroids could provide protection against necrotic damage and behavioral abnormalities following traumatic brain injury (Shear et al., 2002; Hurn and Macrae, 2000; Hurn et al., 2005; Emerson et al., 1993; Bramlett and Dietrich, 2001; Stein, 2001; Roof and Hall, 2000a, 2000b). However, it is unlikely that high sex steroid levels alone confer a protective effect, given the lack of difference in lesion sizes between males and females in the youngest animals despite their very different levels of circulating sex steroids. The inverse correlation between testosterone and lesion size in our overall series likely reflects the fact that testosterone was only elevated in the infants, who had the smallest lesions, and that the largest lesions were found in the oldest subjects. Thus age, rather than hormonal status, appeared to be the major factor in lesion size, though testosterone may play some role in males early in life. Concentrations of testosterone and estradiol were similar between males and females in the adolescent subjects, an age that corresponds to early adolescence in humans from a brain maturational point of view (Dobbing and Sands, 1979; Pond et al., 2000). While humans have a prolonged increase in hormones over several years during adolescence, it has previously been established that female swine exhibit an increase in concentrations of 17β-estradiol only a week prior to the first ovulation (Esbenshade et al., 1982; Allrich et al., 1982). Because hormonal factors have been reported to influence head injury outcomes in small animal models and in humans, with some studies showing deleterious or protective effects of estrogens or progesterone, species differences in these parameters at various developmental stages need to be taken into account when generalizing from large-animal models to the human corollary. Thus while 4 months was chosen as the age in swine corresponding to early adolescence from the point of view of brain growth and myelination, it may be that slightly older animals would better reflect the hormonal milieu seen in human adolescents.
Physiologic parameters
Temperature did appear to affect lesion size in the oldest subjects, whose temperatures on average were 1.6°C higher than those of the two younger groups. Temperature has been shown to be an important determinant of lesion size in other trauma models and in stroke (Dietrich et al., 1996; Natale et al., 2000; Castillo et al., 1999; Kim et al., 1996). In the present series, temperature cannot explain the differences between the youngest two age groups, nor did temperature correlate with lesion size in any individual age group. The elevated temperature in the oldest subjects in part reflects the normal temperature elevation seen in swine compared to humans, and is not considered hyperthermic for this species. The mean body temperature of young (10- to 14-week-old) swine is 38.8°C (Ingram and Legge, 1970).
The differences in temperature seen in this series highlights some of the difficulties in using large-animal models to study age-dependent effects on traumatic brain injury. As seen in human infants and young children, rapid cooling occurs under general anesthesia due to changes in blood flow from core to periphery, which is accentuated in the smallest subjects. Despite the great care taken to keep subjects passively warmed throughout all aspects of the procedure, and to minimize the time from induction to active air-blanket warming in this series, which typically took place in under 5 min, the youngest animals still cooled rapidly. Data from other series in our lab utilizing chronically implanted temperature probes suggest that the baseline temperatures in these subjects are similar across ages (unpublished data). Therefore rapid cooling, rather than different baseline temperature starting points, appears to be the cause of the differences observed. Pre-warming of the younger subjects could be undertaken, but would significantly affect the duration of anesthetic seen in these groups. Thus these variables need to be considered when utilizing large-animal models to study age effects.
Limitations
These results reflect a single outcome measure, lesion size, ascertained at a single time point after injury. It is possible that lesions might evolve over different time courses in subjects of different ages, ultimately reaching similar sizes among different ages. However, in other studies using this model, subjects surviving 1 month post-injury showed similar differences, with smaller lesions (as measured by hemispheric volume) in younger animals (Duhaime et al., 2000).
It should be kept in mind as well that this model mimics a particular type of head injury, namely direct cortical deformation causing focal contusion. The model was designed specifically to isolate impact forces and minimize inertial forces. Thus conclusions cannot be generalized to age-dependent vulnerability to injuries with a greater inertial component. In fact, some authors have found that immaturity may increase vulnerability to pure inertial loading, although precise scaling for brain mass has not been undertaken in that model (Raghupathi et al., 2004).
Exactly how to scale injury input in a brain injury model so that it is comparable across ages is challenging. The model used in these studies was specifically developed to scale for those factors most predictive of equivalent injury response, which in this case is strain experienced by the tissue; however, other factors by which one might consider scaling, such as force, pressure, and energy delivered, are also very similar across ages in this model (Duhaime et al., 2000). The model also has been used to investigate functional effects of injury and repair processes. Sensory function in the rostral gyrus, as measured by functional magnetic resonance imaging and somatosensory evoked potentials, is similarly disrupted early after injury in all three age groups; thus the early functional consequence of the injury appears to be similar across ages (Duhaime et al., 2003). In addition, subjects at all ages sustain MRI changes indicative of significant focal injury, although the extent and time course of recovery varies among ages. Of interest, the toddler group sustains the largest MRI lesions when viewed at 24 h post-injury, despite the fact that the adolescent group sustains the largest histological lesions when analyzed at 7 days post-injury (Duhaime et al., 2003).
For these reasons, it appears that differences seen in outcome with this model in subjects of different ages reflect differences in host response rather than differences in mechanical loading conditions. Ongoing studies utilizing other measures of acute injury response, including serum markers, may provide additional characterization of injury response among ages.
Why the brain should be more resistant to injury earlier in development is not entirely clear. Different processes may be in play at different time points during development, which might explain why, for instance, brain swelling is most pronounced in the toddler age group. As in human children, this corresponds to the age of maximal cerebral blood flow, and this may influence specific pathophysiological cascades at this age (Duhaime et al., 2003). Ongoing studies of early repair processes using this model have not to date suggested major differences in visible cellular repair. At present, based on these data and results of early MRI studies, we suspect that these age-dependent differences most likely represent resistance to processes that can lead to a cascade of cell death in older subjects, but more work is needed to elucidate these advantages more completely. Understanding these processes may provide insights that could be applied to develop interventions to similarly enhance resistance in older subjects.
Conclusions
The results of this study support earlier findings of an age-dependent response to focal cortical contusion injury using this model, and confirm that these effects are present when injury occurs under room air conditions, and further elucidate additional host factors that may influence injury severity. Age was the most robust predictor of lesion size among the three different animal groups, with younger age conferring a progressive advantage with respect to histological lesion size at 1 week post-injury. Higher body temperature contributed to increased lesion size in the older subjects, but did not fully account for the age and gender effects that were identified across all ages. Age- and gender-dependent differences in circulating hormones were identified, and a decline in hormone levels corresponded to an increase in lesion volume in the youngest male subjects.
The mechanisms by which younger age confers advantage after injury are not fully known, but prior and ongoing studies utilizing this model have suggested that different pathophysiological cascades are likely important at different stages of development. Improved understanding of relevant host factors and other covariables has now made this scaled piglet cortical impact model feasible for treatment trials to test strategies for head injury management in children of different ages.
Acknowledgments
We would like to acknowledge Rachel Curtis for her assistance with this project. This work was supported by National Institute of Child Health and Human Development grant no. R01HD045364 to Ann-Christine Duhaime.
Author Disclosure Statement
The authors do not report any conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- Allrich R. Christenson R. Ford J. Zimmerman D. Pubertal development of the boar: Testosterone, estradiol-17ß, cortisol and LH concentrations before and after castration at various ages. J. Anim. Sci. 1982;55:1139–1146. doi: 10.2527/jas1982.5551139x. [DOI] [PubMed] [Google Scholar]
- Anderson R. Brown C. Blumbergs P. McLean A. Jones N. Impact mechanics and axonal injury in a sheep model. J. Neurotrauma. 2003;20:961–974. doi: 10.1089/089771503770195812. [DOI] [PubMed] [Google Scholar]
- Bittigau P. Pohl D. Sifringer M. Shimizu H. Ikeda M. Ishimaru M. Stadhaus D. Fuhr S. Dikranian K. Olney J. Ikonomidou C. Modeling pediatric head trauma: Mechanisms of degeneration and potential strategies for neuroprotection. Restor. Neurol. Neurosci. 1998;13:11–23. [PubMed] [Google Scholar]
- Bramlett D. Dietrich D. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J. Neurotrauma. 2001;18:891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- Castillo J. Davalos A. Noya M. Aggravation of acute ischemic stroke by hyperthermia is related to an excitotoxic mechanism. Cerebrovasc. Dis. 1999;9:22–27. doi: 10.1159/000015891. [DOI] [PubMed] [Google Scholar]
- Colenbrander B. de Jong F. Wensing C. Changes in serum testosterone concentrations in the male pig during development. J. Reprod. Fertil. 1978;53:377–380. doi: 10.1530/jrf.0.0530377. [DOI] [PubMed] [Google Scholar]
- Dietrich D. Alonso O. Halley M. Busto P. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: A light and electron microscopic study in rats. Neurosurgery. 1996;38:533–541. doi: 10.1097/00006123-199603000-00023. [DOI] [PubMed] [Google Scholar]
- Dobbing J. Sands J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979;311:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Duhaime A.C. Hunter J. Grate L. Kim A. Demidenko E. Golden J. Harris C. Magnetic resonance imaging studies of age-dependent responses to scaled focal brain injury in the piglet. J. Neurosurg. 2003;99:542–548. doi: 10.3171/jns.2003.99.3.0542. [DOI] [PubMed] [Google Scholar]
- Duhaime A.C. Margulies S. Durham S. O'Rourke M. Golden J. Marwaha S. Raghupathi R. Maturation-dependent response of the piglet brain to scaled cortical impact. J. Neurosurg. 2000;93:455–462. doi: 10.3171/jns.2000.93.3.0455. [DOI] [PubMed] [Google Scholar]
- Emerson C. Headrick J. Vink R. Estrogen improves biochemical and neurologic outcome following traumatic brain injury in male rats, but not in females. Brain Res. 1993;608:95–100. doi: 10.1016/0006-8993(93)90778-l. [DOI] [PubMed] [Google Scholar]
- English M. Papernberg R. Farias E. Scott W. Hinchey J. Heat loss in an animal experimental model. J. Trauma. 1991;31:36–38. doi: 10.1097/00005373-199101000-00008. [DOI] [PubMed] [Google Scholar]
- Esbenshade K. Paterson A. Cantley T. Day B. Changes in plasma hormone concentrations associated with the onset of puberty in the gilt. J. Anim. Sci. 1982;54:320–324. doi: 10.2527/jas1982.542320x. [DOI] [PubMed] [Google Scholar]
- Flynn T. Developmental changes of myelin-related lipids in brain of miniature swine. Neurochem. Res. 1984;9:935–945. doi: 10.1007/BF00964525. [DOI] [PubMed] [Google Scholar]
- Ford J. Christenson R. Maurer R. Serum testosterone concentrations in embryonic and fetal pigs during sexual differentiation. Biol. Reprod. 1980;23:583–587. doi: 10.1095/biolreprod23.3.583. [DOI] [PubMed] [Google Scholar]
- Ford J. Differentiation of sexual behaviour in pigs. J. Reprod. Fert. Suppl. 1990;40:311–321. [PubMed] [Google Scholar]
- Ford J. Serum estrogen concentrations during postnatal development in male pigs. Proc. Soc. Exp. Biol. Med. 1983;174:160–164. doi: 10.3181/00379727-174-41719. [DOI] [PubMed] [Google Scholar]
- Grate L. Golden J. Hoopes P. Hunter J. Duhaime A.C. Traumatic brain injury in piglets of different ages: Techniques for lesion analysis using histology and magnetic resonance imaging. J. Neurosci. Methods. 2003;123:201–206. doi: 10.1016/s0165-0270(02)00361-8. [DOI] [PubMed] [Google Scholar]
- Hurn P. Macrae I. Estrogen as a neuroprotectant in stroke. J. Cereb. Blood Flow Metab. 2000;20:631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Hurn P. Vannucci S. Hagberg H. Adult or perinatal brain injury. Does sex matter? Stroke. 2005;36:193–195. doi: 10.1161/01.STR.0000153064.41332.f6. [DOI] [PubMed] [Google Scholar]
- Ingram D. Legge K. Variations in deep body temperature in the young unrestrained pig over the 24 hour period. J. Physiol. 1970;210:989–998. doi: 10.1113/jphysiol.1970.sp009253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. Busto R. Dietrich D. Kraydieh S. Ginsberg M. Delayed postischemic hyperthermia in awake rats worsens the histopathological outcome of transient focal cerebral ischemia. Stroke. 1996;27:2275–2281. doi: 10.1161/01.str.27.12.2274. [DOI] [PubMed] [Google Scholar]
- Luerssen T. Klauber M. Outcome from pediatric head injury: On the nature of prospective and retrospective studies. Pediatr. Neurosurg. 1995;23:34–41. doi: 10.1159/000120933. [DOI] [PubMed] [Google Scholar]
- Margulies S. Thibault L. Gennarelli T. Physical model simulations of brain injury in the primate. J. Biomech. 1990;23:823–836. doi: 10.1016/0021-9290(90)90029-3. [DOI] [PubMed] [Google Scholar]
- Miller A. Blith B. Lack of insulating effect of body fat during exposure to internal and external heat loads. J. Appl. Physiol. 1958;12:17–19. doi: 10.1152/jappl.1958.12.1.17. [DOI] [PubMed] [Google Scholar]
- Mount L. Rowell R. Body size, body temperature and age in relation to the metabolic rate of the pig in the first five weeks after birth. J. Physiol. 1960;154:408–416. doi: 10.1113/jphysiol.1960.sp006587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison B., III Saatman K. Meaney D. McIntosh T. In vitro central nervous system models of mechanically induced trauma: A review. J. Neurotrauma. 1998;15:911–928. doi: 10.1089/neu.1998.15.911. [DOI] [PubMed] [Google Scholar]
- Natale J. Joseph J. Helfaer M. Shaffner D. Early hyperthermia after traumatic brain injury in children: Risk factors, influence on length of stay, and effect on short-term neurologic status. Crit. Care Med. 2000;28:2608–2615. doi: 10.1097/00003246-200007000-00071. [DOI] [PubMed] [Google Scholar]
- Pampiglione G. Some aspects of development of cerebral function in mammals. Proc. Roy. Soc. Med. 1971;64:429–435. [PMC free article] [PubMed] [Google Scholar]
- Pond W. Boleman S. Fiorotto M. Ho H. Knabe D. Mersmann H. Savell J. Su D. Perinatal ontogeny of brain growth in the domestic pig. Proc. Soc. Exp. Biol. Med. 2000;223:102–108. doi: 10.1177/153537020022300114. [DOI] [PubMed] [Google Scholar]
- Povlishock J. Hayes R. Michel M. McIntosh T. Workshop on animal models of traumatic brain injury. J. Neurotrauma. 1994;11:723–732. doi: 10.1089/neu.1994.11.723. [DOI] [PubMed] [Google Scholar]
- Raghupathi R. Mehr M. Helfaer M. Margulies S. Traumatic axonal injury is exacerbated following repetitive closed head injury in the neonatal pig. J. Neurotrauma. 2004;21:307–316. doi: 10.1089/089771504322972095. [DOI] [PubMed] [Google Scholar]
- Roof R. Hall E. Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J. Neurotrauma. 2000a;17:1155–1169. doi: 10.1089/neu.2000.17.1155. [DOI] [PubMed] [Google Scholar]
- Roof R. Hall E. Gender differences in acute CNS trauma and stroke: Neuroprotective effects of estrogen and progesterone. J. Neurotrauma. 2000b;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- Shaver E. Duhaime A.C. Curtis M. Gennarelli L.M. Barrett R. Experimental acute subdural hematoma in infant piglets. Pediatr. Neurosurg. 1996;25:123–129. doi: 10.1159/000121109. [DOI] [PubMed] [Google Scholar]
- Shear D. Galani R. Hoffman S. Stein D. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp. Neurol. 2002;178:59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- Shreiber D. Bain A. Ross D. Smith D. Gennarelli T. McIntosh T. Meaney D. Experimental investigation of cerebral contusion: Histopathological and immunohistochemical evaluation of dynamic cortical deformation. J. Neuropathol. Exp. Neurol. 1999;58:153–164. doi: 10.1097/00005072-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Stein D. Brain damage, sex hormones and recovery: A new role for progesterone and estrogen? Trends Neurosci. 2001;24:386–391. doi: 10.1016/s0166-2236(00)01821-x. [DOI] [PubMed] [Google Scholar]