Abstract
The leukotrienes belong to a family of biologically active lipids derived from arachidonate that are often involved in inflammatory responses. In the central nervous system, a group of leukotrienes, known as the cysteinyl leukotrienes, is generated in brain tissue in response to a variety of acute brain injuries. Although the exact clinical significance of this excess production remains unclear, the cysteinyl leukotrienes may contribute to injury-related disruption of the brain-blood barrier and exacerbate secondary injury processes. In the present study, the formation and role of cysteinyl leukotrienes was explored in the fluid percussion injury model of traumatic brain injury in rats. The results showed that levels of the cysteinyl leukotrienes were elevated after fluid percussion injury with a maximal formation 1 hour after the injury. Neutrophils contributed to cysteinyl leukotriene formation in the injured brain hemisphere, potentially through a transcellular biosynthetic mechanism. Furthermore, pharmacological reduction of cysteinyl leukotriene formation after the injury, using MK-886, resulted in reduction of brain lesion volumes, suggesting that the cysteinyl leukotrienes play an important role in traumatic brain injury.
Key words: cysteinyl leukotrienes, inflammation, mass spectrometry, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of death and neurological morbidity. According to the Centers for Disease Control and Prevention (CDC), an estimated 1.4 million people sustain a TBI each year in the United States alone (Langlois et al., 2006). The CDC also estimates that over 5 million Americans are dependent on others to help perform simple activities of daily living as a result of TBI (Thurman et al., 1999), making the total direct and indirect costs of civilian TBI in the United States as high as $60 billion a year (Finklestein et al., 2006). The recent increased incidence of combat-related traumatic brain injury (TBI), the “signature injury” of the current conflicts in Iraq and Afghanistan, is also expected to have a substantial impact on budgeting and patient care resources within the military and Veterans' Affairs health care systems (Warden et al., 2005). Estimates of TBI incidence in wounded personnel in the two ongoing Middle East conflicts are as high as 25% (Fisher, 2008).
Brain damage from TBI results from two distinct processes: the primary injury, or the mechanical damage from the impact itself; and secondary injury resulting from a complex cascade of physiological reactions to the primary injury, ultimately leading to additional neuronal death. During the secondary injury phase, phospholipase A2 (PLA2) is activated, resulting in arachidonic acid (AA) release from neuronal membrane glycerophospholipids, ultimately leading to the generation of certain classes of lipid molecules, such as prostaglandins, leukotrienes (LTs), and thromboxanes (Phillis, 2003; Leslie, 2004). The biosynthesis of LTs begins with the conversion of AA to 5-hydroperoxyeicosatetraenoic acid (5-HpETE) by 5-lipoxygenase (5-LO) and 5-lipoxygenase activating protein (FLAP) and then into leukotriene A4 (LTA4) by 5-LO (Rouzer et al., 1986). The site for most of these biochemical events is the perinuclear region of the cell (Luo et al., 2003).
LTA4 is a very unstable epoxide that can be enzymatically converted either to leukotriene B4 (LTB4) by LTA4-hydrolase (LTA4-H) (Haeggstrom, 2004) or to leukotriene C4 (LTC4) through the action of LTC4-synthase (LTC4-S) (Lam, 2003). LTC4 is then converted to LTD4 and LTE4. LTC4, LTD4, and LTE4 are collectively known as the cysteinyl leukotrienes (cys-LTs). LTA4 can also be non-enzymatically hydrolyzed into Δ6-trans-LTB4s or 5,6-diHETEs (Borgeat and Samuelsson, 1979). Cys-LTs exert their biological activities through G protein-coupled receptors, cys-LT1, cys-LT2, and cys-LT3. The rank of potency of agonism for cys-LT1 is LTD4 > LTC4 > LTE4 (Lynch et al., 1999; Sarau et al., 1999) and LTD4 = LTC4 > LTE4 for cys-LT2 (Nothacker et al., 2000). Recently, a new cys-LT3 receptor has been reported with high affinity to LTE4 (Maekawa et al., 2008). The distribution of these receptors throughout the brain has not been fully characterized, but it is known that cys-LT2 receptor mRNA, but not cys-LT1 receptor mRNA, is highly expressed in multiple regions of the human brain parenchyma (Capra, 2004). Cys-LT1 and cys-LT2 are constitutively expressed in the human brain vasculature and their expression is induced after TBI in neuron and glial cells (Hu et al., 2005; Zhang et al., 2004), suggesting that both LTC4 and LTD4 could play a role in TBI.
In the periphery, such as the lungs, the biological activities of cysteinyl leukotrienes (cys-LTs) include altering vascular permeability, thus increasing plasma extravasation into peripheral tissue and contributing to the formation of inflammation and edema ( Boyce, 2005; Busse, 2005). In the central nervous system, cys-LTs are produced in response to a variety of acute brain injuries (Ciceri et al., 2001; Dhillon et al., 1996; Schuhmann et al., 2003). Much of the current published work involving the formation and role of cys-LTs in acute brain injuries has been done in experimental stroke models. For example, cys-LTs induce blood brain barrier disruption and brain edema after experimental stroke (Baba et al., 1991; Rao et al., 1999; Wang et al., 2006). In addition, intervention studies have demonstrated that 5-LO inhibitors and cys-LT receptor antagonists reduce the size of focal cerebral infarction from middle cerebral artery occlusion (Ciceri et al., 2001; Jatana et al., 2006; Yu et al., 2005; Zhao, 2005). Although the formation of cys-LTs has been documented in both brain tissue and CSF after TBI (Dhillon et al., 1996; Schuhmann et al., 2003), their cellular source and physiological role after experimental TBI have not been characterized.
In vitro studies demonstrate that as neurons and astrocytes (alone or in combination) are incapable of producing cys-LTs unless provided with an exogenous source of LTA4, the central nervous system production of these lipids likely relies on a transcellular biosynthetic process (Farias et al., 2007). Potential cellular sources of LTA4 for cys-LT synthesis after TBI could be either: (1) endogenous brain cells, such as microglia which, in vitro, are capable of producing cys-LTs upon stimulation (Ballerini et al., 2005); or (2) normally foreign cells that can infiltrate the brain parenchyma after disruption of the blood-barrier during TBI (Soares et al., 2005). These normally foreign cells, such as neutrophils and macrophages, can produce large amounts of LTA4 when activated. This LTA4 can then be further converted to cys-LTs by glial and neuronal cells.
In this paper, we examine the time course of production of the cys-LTs after lateral fluid percussion injury (FPI) in the rat using reverse phase liquid chromatography tandem mass spectrometry (RP-LC/MS/MS) in multiple reaction monitoring mode (MRM). We also determined the extent of involvement of circulating inflammatory cells in cys-LT synthesis after FPI by depleting peripheral neutrophils (the majority of circulating white blood cells) and comparing post-injury LTC4 formation of vinblastine-treated and vehicle-treated animals. Finally, the effect of pharmacological reduction of cys-LT production using a FLAP inhibitor (MK-886) was tested in multiple outcome measures after FPI.
The present study revealed that cys-LTs were formed after FPI and that circulating neutrophils were a contributing source of LTA4 for transcellular cys-LT formation in the injured brain. Furthermore, FLAP inhibitor (MK-886) pretreatment effectively reduced cys-LT formation after FPI and led to significant reduction of brain lesion volumes after FPI.
Materials and Methods
Materials
Reagents and solvents were purchased from Fisher Scientific (Pittsburgh, PA). Standard eicosanoids [d5]LTC4 (≥97 atom %D), [d4]LTB4 (≥97 atom %D), LTB4, LTC4, LTD4, and MK-886 (sodium salt) were purchased from Cayman Chemical Co. (Ann Arbor, MI). Vinblastine sulfate salt was purchased from Sigma-Aldrich (St. Louis, MO). Isoflurane was purchased from VEDCO Inc. (St. Joseph, MO). Dentral acrylic was purchased from Parkell Inc. (Edgewood, NJ).
Establishment of dural access and fluid percussion injury
Adult male Sprague-Dawley rats (250–300 g) were anesthetized with 3–3.5% isoflurane via nose cone and placed in a stereotaxic head frame. After scalp incision and reflection, a 3-mm diameter craniotomy was created and centered at −3 mm from bregma and 3.5 mm left of the sagittal suture. For support, two steel screws were placed 1 mm caudal to lambda and in the right parietal bone opposite the craniotomy site. A female Luer-Loc hub was centered over the craniotomy site and bonded to the skull with cyanoacrylate adhesive. Dental acrylic was poured around the Luer hub and support screws. After the acrylic hardened, antibiotic ointment was placed around the injury cap and the animal was removed from the stereotaxic frame and returned to his cage to recover.
Fifteen to 20 h after craniotomy and Luer hub implantation, the animals were anesthetized with isoflurane in an induction chamber. The animal was then removed from the chamber, immediately connected to the FPI apparatus, and received a 20 ms pulse of pressurized fluid (2.5–3.0 atm, moderate severity impact) on the intact dural surface before awakening from anesthesia (Frey et al., 2008). Sham-injured animals underwent establishment of dural access and were anesthetized and connected to the FPI apparatus, but the injury pulse was not triggered. All procedures as described were approved by the University of Colorado Institutional Animal Care and Use Committee.
Extraction of brain lipids and RP-LC/MS/MS analysis
Seven groups of four rats each were subjected to moderate FPI injury as detailed above. One group was euthanized at each of seven different time points after injury: 5 min, 30 min, 1 h, 3 h, 6 h, 12 h, and 24 h. An eighth group of four animals acted as sham-injury controls and were euthanized 1 h after sham injury. A ninth group of four animals acted as naïve controls. These animals were euthanized, but did not undergo any other study procedures. At time of euthanasia, all animals were deeply anesthetized with isoflurane, decapitated, and their brains were rapidly removed. Following dissection of the brain into left and right hemispheres, lipids were extracted for determination of eicosanoid levels by liquid chromatography/mass spectrometry techniques (LC/MS/MS) as previously described (Farias et al., 2008).
At each time point, the separated brain hemispheres were collected in 4 mL of 80% methanol and homogenized with a Dounce homogenizer. Internal standards (d4-LTB4, d5-LTC4) were added to the homogenate. Protein content was measured using BCA protein assay to normalize lipid levels to the amount of tissue. Samples were centrifuged and the supernatant was collected. Samples were diluted to a final methanol concentration of lower than 15% and then the lipids were extracted using a solid phase extraction cartridge (Strata C18-E, 100 mg/1 mL, Phenomenex, Torrence CA). The eluate (1 mL of methanol) was dried down and reconstituted in 70 μL of HPLC solvent A (8.3 mM acetic acid buffered to pH 5.7 with NH4OH) + 20 mL of solvent B (acetonitrile/methanol, 65/35, v/v). An aliquot of each sample (35 μL) was injected into an HPLC system and subjected to reversed phase chromatography using a C18 (Columbus 150 × 1 mm, 5 mm, Phenomenex) column eluted at a flow rate of 50 μL/min with a linear gradient from 25 to 100% of mobile phase B. Solvent B was increased from 25 to 85% by 24 min, to 100% by 26 min, and held at 100% for a further 12 min. The HPLC effluent was directly connected to the electrospray source of a triple quadrupole mass spectrometer (Sciex API 2000, PE-Sciex, Thornhill, Canada) and mass spectrometric analyses was performed in the negative ion mode using multiple reaction monitoring (MRM) of the specific transitions, m/z 624 → 272 for LTC4, m/z 495 → 177 for LTD4, m/z 438 → 333 for LTE4, m/z 335 → 195 for LTB4, m/z 339 → 197 for d4-LTB4, and m/z 629 → 277 for d5-LTC4. Quantitation was performed using a standard isotope dilution curve as previously described (Farias et al., 2007). The recovery of the deuterated standards was between 80 and 90%. The recovery of these internal standards reflects the recovery of endogenously produced lipid mediators as they have identical chemical structures and behavior. Comparisons of mean levels of the cys-LTs were made between homologous hemispheres in sham-injured and injured animals, as well as between hemispheres at each time point using one-way analysis of variance and the Student-Newman-Keuls test for multiple comparisons.
Vinblastine administration
Two groups of four animals each were subjected to moderate FPI injury as described above. Four days prior to injury, each animal in the first group was briefly anesthetized (less than 5 min) with 3 − 3.5% isoflurane and administered NaCl 0.9%, 2 mL/kg i.v. (vehicle) and the second group (n = 4) vinblastine sulfate (0.5 mg/kg i.v. in an identical volume) (Sigma-Aldrich). Neutrophil depletion was verified by complete cell blood counts (CBC) in vinblastine-treated animals 4 days after administration. Both groups were euthanized by decapitation and the brain lipids extracted 1 h after FPI. The amounts of LTC4 formed (measured by LC/MS/MS) were compared between groups using one-way analysis of variance and the Student-Newman-Keuls test for multiple comparisons.
MK-886 administration
Two groups of four animals each were subjected to moderate FPI injury. Thirty minutes prior to injury, each animal in the first group was briefly anesthetized (<5 min) with 3–3.5% isoflurane and administered a FLAP inhibitor, MK-886. The MK-886 was given at a dose of 6 mg/kg i.v. by tail vein, dissolved in sterile 0.9% saline with 10% DMSO to a total volume of 0.6 mL. The animals in the second group received anesthesia of similar duration and a pre-injury infusion of vehicle only (0.6 mL saline with 10% DMSO i.v. via tail vein). Both groups of animals were allowed to wake before receiving additional anesthesia for FPI. Both groups were euthanized by deep anesthesia with isoflurane, followed by decapitation, and the brain lipids extracted 1 h after FPI. The quantity of LTC4 formed was compared between groups using analysis of variance (ANOVA) test followed by Student-Newman-Keuls multiple comparisons test. Values of p < 0.05 were considered significant.
Estimation of brain lesion volume after TBI
Three days after FPI, six MK-886-treated and four vehicle-treated animals were anesthetized with isoflurane and decapitated. The brains were rapidly dissected out, mounted in the microtome cutting stage, and submerged in ice-cold HBSS buffer. The brains were then cut into 1 mm coronal sections. These slices were incubated at room temperature in a 4% solution of 2,3,5-triphenyltetrazolium chloride (TTC). Within 10–15 min, the viable cerebral tissue developed a red-pink color due to the reduction of TTC by functioning mitochondria to yield a deep red formazan (Goldlust et al., 1996). However, brain tissue damaged by the FPI was unable to convert TTC and remained unstained (white color). The TTC staining technique is a useful, rapid, and reproducible method for quantitation of lesion volume in multiple models of focal cerebral damage, including FPI (Perri et al., 1997). After the color development, sections were washed in HBSS buffer, fixed in 10% formalin, and kept at 4°C until pictures were recorded by a camera mounted to a dissecting microscope. Images were scanned in using a UMAX PowerLook 2100XL scanner at a resolution of 8000 dpi. Images were aligned and volume measurements were taken with Reconstruct version 1.1.0.0 (Fiala, 2005). The imaging and lesion volume calculations were performed by an independent investigator blinded to the rat treatment. For each section, the lesioned (unstained) areas of the brain hemisphere ipsilateral to the injury were determined by enlarging the image and, using signal intensities, identifying the demarcation between red tissue (viable) and white tissue (injured). Then, for each of the sections, the lesion area was multiplied by the thickness of the section (1 mm) to obtain a section-specific estimate of lesion volume. All single section lesion volumes were then summed over the extent of the lesion, resulting in a total (rostral-caudal) lesion volume, expressed in mm3. Mean lesion and brain volumes for the drug- and vehicle-treated groups were compared using a t test for two independent samples, assuming unequal variances.
Test of forelimb use for vertical-lateral exploration (“cylinder test”)
Using a previously published and validated protocol (Schallert, 2006; Frey et al., 2008), 10 MK-886-treated and 10 vehicle-treated animals were individually placed in a specially designed Plexiglass cylinder 30 cm high and 20 cm in diameter. When the animals reared to explore the wall of the cylinder, the number of times the right, left, or both forelimbs were used in the initial vertical exploratory placement was noted and video-recorded for later review and confirmation. After an animal made 20 vertical exploratory movements (or 20 min had passed), the test was concluded and the animal returned to its cage. Trials in which an animal made less than 15 vertical exploratory movements were excluded from further analysis. Each animal underwent testing at four different time points: pre-injury and 72 h, 1 week, and 10 days after injury. Limb use percent for each side was calculated using the formula: right limb use percent = ((right placements + (bilateral placements/2)/total number of placements) * 100. The percent change of right forelimb use from an individual animal's pretest performance was calculated, and the mean percent change in right forelimb use among drug-treated rats was compared to that of vehicle-treated animals at each time point using a two-tailed t test for independent samples assuming unequal variance.
Results
Time course of cys-LT production after moderate FPI
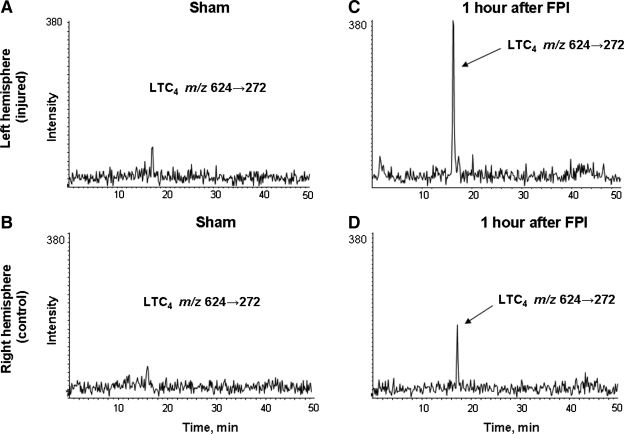
LTC4 was readily detected in the cerebral cortex of animals that underwent FPI, using mass spectrometric techniques (Fig. 1). The absolute quantity of LTC4 production in left and right brain hemispheres was measured in naïve, sham-injured, and injured animals using stable isotope dilution techniques (Farias et al., 2007). LTC4 was non-detectable (nd) in either brain hemisphere in naïve animals. As shown in Figure 1A and B, LTC4 was detected in very low amounts in the sham-injured animals (left, 1.0 ± 0.7 pg/mg protein; right: 0.97 ± 0.97 pg/mg protein, difference not significant). At 1 h after injury, a reproducible production of LTC4 in the left (Fig. 1C) and right (Fig. 1D) brain hemispheres was observed. This production was greater in the left hemisphere (14.35 ± 2.31 pg/mg of protein) than in the right hemisphere (3.62 ± 2.73 pg/mg protein). All comparisons of the mean levels of LTC4 production in naïve animals to the mean levels of LTC4 production in ipsilateral and contralateral sham-injured hemispheres were not statistically significant.
FIG. 1.
Comparison of LTC4 levels measured by MRM transition 624 → 272 in a single sham-injured (A, left hemisphere; B, right hemisphere) and a single FPI-injured animal 1 h after injury (C, left hemisphere; D, right hemisphere) using LC/MS/MS. The sham animal showed low amounts of LTC4 in both left and right hemispheres. In the FPI-injured animal, LTC4 formation was more abundant in the left (ipsilateral) than in the right (contralateral) brain hemisphere.
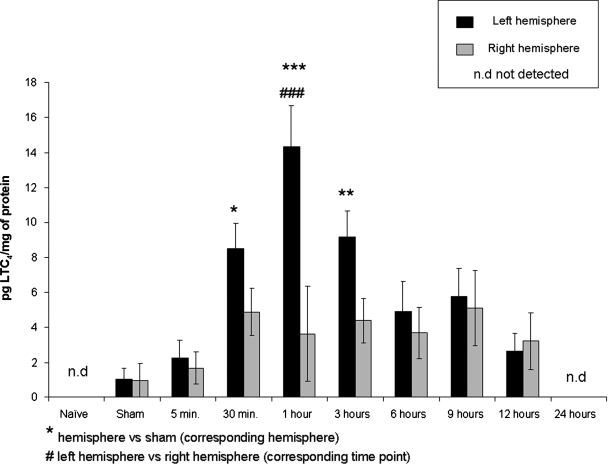
LTC4 production in the injured brains was assessed at six different time points (5 min, 30 min, 1 h, 3 h, 6 h, 12 h and 24 h) after FPI (Fig. 2). In these animals, LTC4 generation increased with time in the left hemisphere, reaching a maximum of 14.4 ± 2.3 pg/mg protein at 1 h after FPI. These measured levels were statistically significantly different from the sham-injured left hemisphere levels of LTC4 production at 30 min (p < 0.05), 1 h (p < 0.001), and 3 h (p < 0.01) after injury. After 3 h, LTC4 levels gradually decreased with time and were no longer detectable 24 h after injury.
FIG. 2.
LTC4 formation time course after FPI. Measurements of LTC4 in left and right brain hemispheres of naïve, sham-injured, and FPI animals by LC/MS/MS. In injured animals, LTC4 levels were assessed at different time points after FPI (5 min, 30 min, 1 h, 3 h, 6 h, 12 h, and 24 h). Four animals were used in each time point group, except n = 5 for both hemispheres at 5 min and 3 h; n = 3 for the right hemisphere at 1 h and both hemispheres at 12 h after injury. Results are expressed as the average ± SEM. nd, not detected. Significant difference from homologous hemisphere of sham-injured animals (***p < 0.001; **p < 0.01; *p < 0.05). Significant difference from contralateral hemisphere (###p < 0.001).
In the right hemisphere of animals that received FPI, a subtle increase in LTC4 levels was observed at 30 min following injury. This increased production was maintained up to 12 h after injury, but never reached statistical significance in comparison with LTC4 levels in sham-injured right hemispheres. LTC4 was no longer detectable in this hemisphere by 24 h after FPI. At each time point, levels of LTC4 production were higher in the left hemisphere (ipsilateral to the injury) than in the right hemisphere (contralateral to the injury). These differences reached significance at 1 h after injury.
LTD4, measured by MRM transition m/z 495 → 177, was non-detectable in either brain hemisphere of naïve or sham-injured animals and was increased after FPI with a similar temporal profile formation to LTC4 (data not shown), consistent with the rapid metabolism of LTC4 by neuronal tissue (Farias et al., 2007). LTE4, the metabolic product of LTD4, was not detected in the rat brain after FPI. No LTB4 was detected in any of the samples.
Effect of pretreatment with vinblastine on LTC4 production at 1 h after FPI
Vinblastine is a chemotherapeutic agent and its administration results in an almost complete reduction of circulating neutrophils (Beray-Berthat et al., 2003). Four days after administration of vinblastine (n = 4) or vehicle (n = 4), the number of neutrophils circulating in the blood was assessed by complete cell blood counts. In vehicle-treated animals, the neutrophil count in the blood was approximately 2.5 × 109/L while in vinblastine-treated animals neutrophils could not be detected.
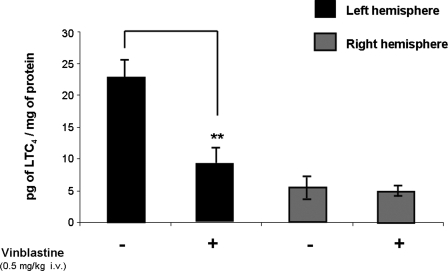
LTC4 production was detected in the left and right brain hemispheres of vinblastine- or vehicle-treated animals by LC/MS/MS at 1 h after injury (Fig. 3). Mean LTC4 levels were significantly lower in the left (ipsilateral to the injury) brain hemisphere of vinblastine-treated animals (9.03 ± 2.25 pg LTC4/mg of protein) compared to that of the vehicle-treated animals (22.75 ± 2.85 pg LTC4/mg of protein). The right brain hemispheres showed no difference in LTC4 production between groups.
FIG. 3.
Neutropenic agent vinblastine (n = 4) reduced LTC4 formation significantly in ipsilateral brain hemisphere 1 h after FPI compared with that of vehicle-treated animals (n = 4) by approximately 50% while the contralateral hemisphere showed no significant difference between groups. Results are expressed as the average ± SEM. Significant difference from homologous hemisphere of vehicle-treated animals (**p < 0.01).
Effect of pretreatment with MK-886 on LTC4 production at 1 h after FPI
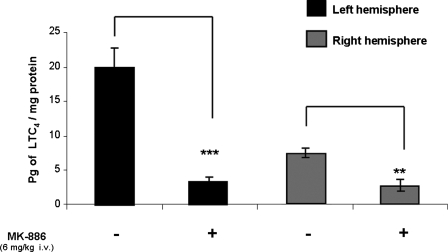
In order to inhibit the leukotriene formation in this model, animals were given the FLAP inhibitor MK-886 prior to FPI. Levels of LTC4 were measured in left (ipsilateral to injury) and right brain hemispheres separately 1 h after injury in MK-886- and vehicle-treated animals (Fig. 4). Mean LTC4 level in the MK-886-treated left hemispheres was 3.39 ± 0.42 pg/mg protein. Mean LTC4 level in the vehicle-treated left hemispheres was 20.00 ± 2.71 pg/mg protein. Mean LTC4 level in the MK-886-treated right hemispheres was 2.6 ± 0.80 pg/mg protein. Mean LTC4 level in the vehicle-treated right hemispheres was 7.19 ± 0.63 pg/mg protein. On each side, the difference between the MK-886-treated and homologous vehicle-treated hemispheres was statistically significant (p = 0.009 on the left hemisphere; p = 0.003 on the right hemisphere).
FIG. 4.
MK-886 pretreatment (n = 4) reduced LTC4 production significantly 1 h after FPI compared to that of vehicle-pretreated animals (n = 4) in both left and right brain hemispheres. Results are expressed as the average ± SEM. Significant difference from homologous hemisphere of vehicle-treated animals (***p < 0.001; **p < 0.01).
Effect of pretreatment with MK-886 on lesion volume 72 h after FPI
TTC staining is a frequently used technique to differentiate viable tissue from infarction macroscopically (Chiamulera et al., 1993). TTC-stained slices were used to calculate brain lesion volumes for drug-treated (n = 6) and vehicle-treated (n = 4) animals 72 h after FPI. The uninjured brain hemisphere stained uniformly red and the border between the red and the white tissue was easily detected in the injured cortex. Subcortical white matter did not stain in either injured or uninjured brain hemispheres. The mean brain lesion volume for vehicle-treated animals (6.99 ± 0.64 mm3) was significantly larger (p = 0.02) than the lesion volume for the drug-treated animals (3.70 ± 0.95 mm3). Mean total brain volumes between drug- and vehicle-treated animals were not significantly different (data not shown).
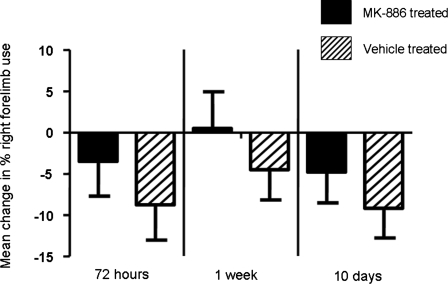
Effect of pretreatment with MK-886 on persistence of gross motor dysfunction after FPI
Ten injured and 10 sham-injured animals underwent neurological testing using the cylinder test at three different time points after injury (72 h, 1 week, and 10 days) and their performance compared to that obtained during pre-injury testing (Fig. 5). No animals had asymmetric limb use greater than 60:40 in naïve, preinjury testing. Pretreatment with MK-886 showed a trend toward mitigating the neurological deficit resulting from FPI at all three time points, although the difference in mean change in right forelimb use between MK-886- and vehicle-treated animals never reached statistical significance.
FIG. 5.
MK-886 pretreatment reduced gross motor dysfunction after TBI. Comparison of vehicle (n = 10) vs. MK-886 (n = 10) pretreated animals 72 h, 1 week, and 10 days after FPI. Results are expressed as the average ± SEM.
Discussion
Overall, moderate FPI caused a significantly increased production of LTC4, and LTD4 but no measurable increase in levels of LTB4 or LTE4 in brain parenchyma within the first 24 h after injury. The elevation in LTC4 levels was detectable within 30 min after injury, peaked at 1 h after injury, and remained significant until 3 h after injury. Schuhmann et al. (2003) reported cys-LT levels in CSF after controlled cortical impact injury in the rat measured by GC/MS/MS, with a similar time course. They found that CSF LTC4 levels were significantly elevated by 1 h after trauma, with a more than 5-fold maximum at 4 h after injury. Likewise, using immunological techniques, Dhillon et al. (1996) found significantly elevated LTC4 levels in injured cortex tissue at 10 min, 30 min, and 1 h following lateral fluid percussion model of TBI, although the absolute levels they detected are larger than ours. It is noteworthy that the detection limits of the mass spectrometric and immunological techniques used to measure cys-LT are different. The detection limit for the LC/MS/MS method employed in this study is 40 pg injected on column for LTC4, whereas the detection limit for enzyme immunoassay (EIA) used in the Dhillon et al. (1996) study is approximately 0.5 pg per assay. Although LC/MS/MS method is less sensitive, this technique is a more specific method of detecting cys-LTs because it is based on the physical-chemical property of elution retention time from a reverse phase HPLC column and the gas phase ion chemistry of the precursor (molecular ion of LTC4) decomposition to the correct product ion, as compared to antibody binding in an immunoassay. In addition, the sensitivity of the LC/MS/MS assay employed is adequate to detect levels of LTs physiologically active via the cys-LT receptors. Most importantly, however, all three studies are in agreement that excess LTC4 production is an early response of brain tissue to traumatic injury.
Interestingly, a small amount of cys-LT formation was detected in sham animals, but not in injured animals at 24 h post injury. A possible explanation for this difference is the time at which these animals were euthanized with respect to the implant surgery. Sham animals were killed 22 h after the implant surgery while the 24 h post-injury animals were killed 48 h after the implant, suggesting that the implant effect on cys-LT production can only be detected within 24 h after the implant. In naïve animals, no cys-LT formation was observed showing that decapitation has no effect on cys-LT production.
The change in LTD4 levels largely paralleled those of LTC4. LTE4 was not detected at any time point in response to FPI, most likely because its level was below the detection limit of the techniques employed in this study.
The increased post-injury production of both LTC4 and LTD4 occurred bilaterally, but to a much greater extent in the brain hemisphere ipsilateral to the injury, suggesting that their production overall was in response to both diffuse and focal (injury site-specific) post-injury stimuli. As detailed in the Introduction, neurons and astrocytes (alone or in combination) are most likely incapable of producing cys-LTs unless provided with an exogenous source of LTA4, so central nervous system production of these lipids likely relies on a transcellular biosynthetic process (Farias et al., 2007). The interhemisphere differential production of these compounds may result from the differential availability of tissue sources of LTA4. Because the depletion of circulating neutrophils via vinblastine had a significant effect on LTC4 production in the ipsilateral brain hemisphere, while no change was observed in the contralateral hemisphere, it is possible that injury-related extravasation of circulating cells (such as neutrophils) can contribute to cys-LT formation locally, but not in distant, uninjured tissue. The remaining LTC4 production in both the ipsilateral brain hemisphere and contralateral hemisphere would then likely derive from an endogenous source of LTA4, such as microglia (Ballerini et al., 2005). Our finding that treatment with the FLAP inhibitor MK-886 significantly reduced LTC4 formation in both ipsilateral and contralateral brain hemisphere after FPI suggests that MK-886 affects both circulating cells and endogenous brain cells.
The absence of detectable production of LTB4 after FPI in the face of robust neutrophil activation and participation in the production of the cys-LTs was unexpected. The most likely explanation for this finding was that LTB4 was produced but metabolized too quickly to be detected. Various cells have been shown to metabolize LTB4 within minutes (Murphy and Gijon, 2007), lending support to this idea. Another possible contributing factor is improved efficiency of transcellular biosynthesis coupling of LTA4 metabolism in vivo, when compared to in vitro studies (Farias et al., 2007). Efficient shunting of LTA4 into a transcellular biosynthetic route could result in preferential production of the cys-LTs over LTB4.
A key step for the biosynthesis of LTs is the conversion of AA to form LTA4 by the action of 5-LO and FLAP (Evans et al., 2008). MK-886 is an inhibitor of leukotriene synthesis known to act by inhibiting the function of FLAP. As such, inhibition of FLAP activity would be expected to markedly reduce cys-LT production by blocking the biosynthetic pathway of these compounds. The ability of intravenously administered MK-886 to reduce cerebral pathology in the central nervous system had been previously demonstrated in a rat focal model of cerebral ischemia (Ciceri et al., 2001). There is no dose of MK-886 routinely used in the literature at present in rats. Across species the intravenous doses used range from 0.3 to 10 mg/kg (Guhlmann et al., 1989; Provost et al., 1998; Ciceri et al., 2001; Uz et al., 2008). Our 6 mg/kg dose regimen was the median dose represented and the first we tested. Because this dose resulted in a robust decrease in cys-LT levels and did not alter animal morbidity or mortality, we chose to stay with this dose for the remainder of our experiments. As shown in Figure 4, pretreatment with MK-886 resulted in the expected significant, bihemispheric decrease in excess LTC4 production 1 h after injury compared to the vehicle-treated homologous hemispheres. This decrease was 83% and 70% in the left and right brain hemispheres, respectively.
Importantly, pretreatment with MK-886 also resulted in a significant 52.9% reduction in brain lesion volume at 72 h after injury. This is the first report demonstrating a clear relationship between the inhibition of cys-LTs production and a reduction in the extent of brain damage after TBI. Although the pathophysiology of transient or permanent global ischemia may be different from that of TBI, our data are comparable to the lesion volume reductions seen after treatment with MK-886 in rat models of focal cerebral ischemia (Ciceri et al., 2001; Jatana et al., 2006). Our studies also showed that pretreatment with MK-886 (6 mg/kg) shows a trend towards reduction in injury-induced right forelimb use deficits at 72 h, 1 week, and 10 days after experimental TBI (Fig. 5). The test of forelimb use for vertical-lateral exploration, or the cylinder test, has been shown to be a reliable measure of primary motor forelimb function after unilateral cerebral ischemia or hemorrhage in rats and mice (Schallert, 2006) and after FPI (Frey et al., 2008). Measuring the asymmetry in forepaw placing during vertical exploratory movements provides an index of lateralized brain injury. The cylinder test has also been widely used in the experimental stroke and Parkinson's disease literature (Schallert, 2006). FPI-injured animals show a significant decrease in contralateral (right) forelimb use during vertical exploratory behavior for at least 1 week post-injury, a length of injury effect similar to many commonly used motor function measures after FPI (Fujimoto et al., 2004; Frey et al., 2008).
In conclusion, the present study demonstrates that cys-LTs are elevated in response to TBI in an animal model and that circulating neutrophils are a contributing source of LTA4 for cys-LT formation in injured brain tissue. Furthermore, the reduction of cys-LT formation by MK-886 pretreatment led to a significant decrease in brain lesion volumes and a trend towards improvement in injury-induced right forelimb use deficits compared to vehicle-treated animals. In the future, it would be of special interest to further explore the cellular source and mechanism of cys-LT synthesis after injury and the effect of MK-886 post-treatment in this TBI animal model to facilitate future studies in human TBI.
Acknowledgments
This project was supported by grants from the Colorado TBI Trust Fund Research Program, the Colorado Injury Control and Research Center, the Eastern Colorado Health Care System, the National Institute of Heart, Lung and Blood (HL25785), and the National Institutes of Health (HL025785). The authors wish to thank Erin Genova and Storey Wilson (Pathology Department, UCDenver) for their help with the histological studies, and Aaron Lepkin and Cheri Unkart for their help with the behavioral testing.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Baba T. Black K.L. Ikezaki K. Chen K.N. Becker D.P. Intracarotid infusion of leukotriene C4 selectively increases blood-brain barrier permeability after focal ischemia in rats. J. Cereb. Blood Flow Metab. 1991;11:638–643. doi: 10.1038/jcbfm.1991.115. [DOI] [PubMed] [Google Scholar]
- Ballerini P. Di Iori P. Cicarelli R. Caciagli F. Poli A. Beraudi A. Buccella S. D'Alimonte I. D'Auro M. Nargi E. Patricelli P. Visini D. Traversa U. P2Y1 and cysteinyl leukotriene receptor mediate purine and cysteinyl lukoriene co-release in primary cultures of rat microglia. Int. J. Immunopathol. Pharmacol. 2005;18:255–268. doi: 10.1177/039463200501800208. [DOI] [PubMed] [Google Scholar]
- Beray-Berthat V. Croci N. Plotkine M. Margaill I. Polymorphonuclear neutrophils contribute to infarction and oxidative stress in the cortex but not in the striatum after ischemia-reperfusion in rats. Brain Res. 2003;987:32–38. doi: 10.1016/s0006-8993(03)03224-4. [DOI] [PubMed] [Google Scholar]
- Borgeat P. Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J. Biol. Chem. 1979;254:7865–7869. [PubMed] [Google Scholar]
- Boyce J.A. Eicosanoid mediators of mast cells: receptors, regulation of synthesis, and pathobiologic implications. Chem. Immunol. Allergy. 2005;87:59–79. doi: 10.1159/000087571. [DOI] [PubMed] [Google Scholar]
- Busse W. Kraft M. Cysteinyl leukotrienes in allergic inflammation: strategic target for therapy. Chest. 2005;127:1312–1326. doi: 10.1378/chest.127.4.1312. [DOI] [PubMed] [Google Scholar]
- Capra V. Molecular and functional aspects of human cysteinyl leukotriene receptors. Pharmacol. Res. 2004;50:1–11. doi: 10.1016/j.phrs.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Chiamulera C. Terron A. Reggiani A. Cristofori P. Qualitative and quantitative analysis of the progressive cerebral damage after middle cerebral artery occlusion in mice. Brain Res. 1993;606:251–258. doi: 10.1016/0006-8993(93)90992-v. [DOI] [PubMed] [Google Scholar]
- Ciceri P. Rabuffetti M. Monopoli A. Nicosia S. Production of leukotrienes in a model of focal cerebral ischaemia in the rat. Br. J. Pharmacol. 2001;133:1323–1329. doi: 10.1038/sj.bjp.0704189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch J. Rapoport S.I. Purdon A.D. Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochem. Res. 1997;22:759–765. doi: 10.1023/a:1022030306359. [DOI] [PubMed] [Google Scholar]
- Dhillon H.S. Dose J.M. Prasad M.R. Regional generation of leukotriene C4 after experimental brain injury in anesthetized rats. J. Neurotrauma. 1996;13:781–789. doi: 10.1089/neu.1996.13.781. [DOI] [PubMed] [Google Scholar]
- Evans J.F. Ferguson A.D. Mosley R.T. Hutchinson J.H. What's all the FLAP about? 5-lipoxygenase-activating protein inhibitors for inflammatory diseases. Trends Pharmacol. Sci. 2008;29:72–78. doi: 10.1016/j.tips.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Farias S.E. Basselin M. Chang L. Heidenreich K.A. Rapoport S.I. Murphy R.C. Formation of eicosanoids, E2/D2 isoprostanes, and docosanoids following decapitation-induced ischemia, measured in high-energy-microwaved rat brain. J. Lipid Res. 2008;49:1990–2000. doi: 10.1194/jlr.M800200-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias S.E. Zarini S. Precht T. Murphy R.C. Heidenreich K.A. Transcellular biosynthesis of cysteinyl leukotrienes in rat neuronal and glial cells. J. Neurochem. 2007;103:1310–1318. doi: 10.1111/j.1471-4159.2007.04830.x. [DOI] [PubMed] [Google Scholar]
- Feldmann H. Klages G. Gärtner F. Scharfenberg J. The prognostic value of intracranial pressure monitoring after severe head injuries. Acta Neurochir. Suppl (Wien) 1979;28:74–77. doi: 10.1007/978-3-7091-4088-8_15. [DOI] [PubMed] [Google Scholar]
- Fiala J.C. Reconstruct: a free editor for serial section microscopy. http://fiala-fantoccini.com/resources/jmi_1466.pdf. J. Microscopy. 2005;218:52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein E. Corso P. Miller T., et al. The Incidence and Economic Burden of Injuries. Oxford University; New York: 2006. [Google Scholar]
- Fisher H. CRS Report for Congress. Washington, D.C: 2008. U.S. Military Casualty Statistics: Operation Iraqi Freedom and Operation Enduring Freedom. [Google Scholar]
- Frey L.C. Hellier J. Unkart C. Lepkin A. Howard A. Hasebroock K. Serkova N. Liang L. Patel M. Soltesz I. Staley K. A novel apparatus for lateral fluid percussion injury in the rat. J. Neurosci. Methods. 2009;177:267–272. doi: 10.1016/j.jneumeth.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S.T. Longhi L. Saatman K. Conte V. Stocchetti N. McIntosh T.K. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 2004;28:365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Goldlust E.J. Paczynski R.P. He Y.Y. Hsu C.Y. Goldberg M.P. Automated measurement of infarct size with scanned images of triphelnyl chloride-stained rat brain. Stroke. 1996;27:1657–1662. doi: 10.1161/01.str.27.9.1657. [DOI] [PubMed] [Google Scholar]
- Guhlmann A. Keppler A. Kästner S. Krieter H. Brückner U.B. Messmer K. Keppler D. Prevention of endogenous leukotriene production during anaphylaxis in the guinea pig by an inhibitor of leukotriene biosynthesis (MK-886) but not by dexamethasone. J. Exp. Med. 1989;170:1905–1918. doi: 10.1084/jem.170.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeggstrom J.Z. Leukotriene A4 hydrolase/aminopeptidase, the gatekeeper of chemotactic leukotriene B4 biosynthesis. J. Biol. Chem. 2004;279:50639–50642. doi: 10.1074/jbc.R400027200. [DOI] [PubMed] [Google Scholar]
- Hall L.M. Murphy R.C. Electrospray mass spectrometric analysis of 5-hydroperoxy and 5-hydroxyeicosatetraenoic acids generated by lipid peroxidation of red blood cell ghost phospholipids. J. Am. Soc. Mass Spectrom. 1998;9:527–532. doi: 10.1016/S1044-0305(98)00013-0. [DOI] [PubMed] [Google Scholar]
- Hu H. Chen G. Zhang J.M. Zhang W.P. Zhang L. Ge Q.F. Yao H.T. Ding W. Chen Z. Wei E.Q. Distribution of cysteinyl leukotriene receptor 2 in human traumatic brain injury and brain tumors. Acta Pharmacol. Sin. 2005;26:685–690. doi: 10.1111/j.1745-7254.2005.00092.x. [DOI] [PubMed] [Google Scholar]
- Jatana M. Giri S. Ansari M.A. Elango C. Singh A.K. Singh I. Khan M. Inhibition of NF-kappaB activation by 5-lipoxygenase inhibitors protects brain against injury in a rat model of focal cerebral ischemia. J. Neuroinflammation. 2006;11:3–12. doi: 10.1186/1742-2094-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K. Matsumoto M. Hori M. Cerebral ischemia in 5-lipoxygenase knockout mice. Brain Res. 2004;1004:198–202. doi: 10.1016/j.brainres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Lam B.K. Leukotriene C4 synthase. Prostaglandins Leukot. Essent. Fatty Acids. 2003;69:111–116. doi: 10.1016/s0952-3278(03)00071-1. [DOI] [PubMed] [Google Scholar]
- Langois J.A. Rutland-Brown W. Thomas K.E. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Atlanta. 2006.
- Leslie C.C. Regulation of the specific release of arachidonic acid by phospholipase A2. Prostaglandins Leukot. Essent. Fatty Acids. 2004;70:373–376. doi: 10.1016/j.plefa.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Levin H.S. Prediction of recovery from traumatic brain injury. J. Neurotrauma. 1995;12:913–922. doi: 10.1089/neu.1995.12.913. [DOI] [PubMed] [Google Scholar]
- Lewis R.A. Austen K.F. Soberman R.J. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N. Engl. J. Med. 1990;10:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- Luo M. Jones S.M. Peters-Golden M. Brock T.G. Nuclear localization of 5-lipoxygenase as a determinant of leukotriene B4 synthetic capacity. Proc. Natl. Acad. Sci. USA. 2003;100:12165–12170. doi: 10.1073/pnas.2133253100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch K.R. O'Neill G.P. Liu Q. Im D.S. Sawyer N. Metters K.M. Coulombe N. Abramovitz M. Figueroa D.J. Zeng Z. Connolly B.M. Bai C. Austin C.P. Chateauneuf A. Stocco R. Greig G.M. Kargman S. Hooks S.B. Hosfield E. Williams D.L., Jr. Ford-Hutchinson A.W. Caskey C.T. Evans J.F. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–789. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- Maekawa A. Kanaoka Y. Xing W. Austen K.F. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc. Natl. Acad. Sci. USA. 2008;105:16695–16700. doi: 10.1073/pnas.0808993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg. Focus. 2007;22:E1. doi: 10.3171/foc.2007.22.5.2. [DOI] [PubMed] [Google Scholar]
- Murphy R.C. Gijon M.A. Biosynthesis and metabolism of leukotrienes. J. Biochemistry. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- Nothacker H.P. Wang Z. Zhu Y. Reinscheid R.K. Lin S.H. Civelli O. Molecular cloning and characterization of a second human cysteinyl leukotriene receptor: discovery of a subtype selective agonist. Mol. Pharmacol. 2000;58:1601–1608. doi: 10.1124/mol.58.6.1601. [DOI] [PubMed] [Google Scholar]
- Palmblad J. Gyllenhammar H. Lindgren J.A. Malmsten C.L. Effects of leukotrienes and f-Met-Leu-Phe on oxidative metabolism of neutrophils and eosinophils. J. Immunol. 1984;6:3041–3045. [PubMed] [Google Scholar]
- Perri B.R. Smith D.H. Murai H. Sinson G. Saatman K.E. Raghupathi R. Bartus R.T. McIntosh T.K. Metabolic quantification of lesion volume following experimental traumatic brain injury in the rat. J. Neurotrauma. 1997;14:15–22. doi: 10.1089/neu.1997.14.15. [DOI] [PubMed] [Google Scholar]
- Phillis J.W. O'Regan M.H. The role of phospholipases, cyclooxygenases, and lipoxygenases in cerebral ischemic/traumatic injuries. Crit. Rev. Neurobiol. 2003;15:61–90. doi: 10.1615/critrevneurobiol.v15.i1.30. [DOI] [PubMed] [Google Scholar]
- Provost P. Borgeat P. Merhi Y. Platelets, neutrophils, and vasoconstriction after arterial injury by angioplasty in pigs: effects of MK-886, a leukotriene biosynthesis inhibitor. Br. J. Pharmacol. 1998;123:251–258. doi: 10.1038/sj.bjp.0701611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A.M. Hatcher J.F. Kindy M.S. Dempsey R.J. Arachidonic acid and leukotriene C4: role in transient cerebral ischemia of gerbils. Neurochem. Res. 1999;24:1225–1232. doi: 10.1023/a:1020916905312. [DOI] [PubMed] [Google Scholar]
- Rouzer C.A. Matsumoto T. Shimizu T. Samuelsson B. The human leukocyte 5-lipoxygenase: enzyme purification, multi-component regulatory system, and LTA4 synthase activity. Adv. Prostaglandin Thromboxane Leukot. Res. 1986;16:3–16. [PubMed] [Google Scholar]
- Sarau H.M. Ames R.S. Chambers J. Ellis C. Elshourbagy N. Foley J.J. Schmidt D.B. Muccitelli R.M. Jenkins O. Murdock P.R. Herrity N.C. Halsey W. Sathe G. Muir A.I. Nuthulaganti P. Dytko G.M. Buckley P.T. Wilson S. Bergsma D.J. Hay DW. Identification, molecular cloning, expression, and characterization of a cysteinyl leukotriene receptor. Mol. Pharmacol. 1999;56:657–663. doi: 10.1124/mol.56.3.657. [DOI] [PubMed] [Google Scholar]
- Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann M.U. Mokhtarzadeh M. Stichtenoth D.O. Skardelly M. Klinge P.M. Gutzki F.M. Samii M. Brinker T. Temporal profiles of cerebrospinal fluid leukotrienes, brain edema and inflammatory response following experimental brain injury. Neurol. Res. 2003;25:481–491. doi: 10.1179/016164103101201896. [DOI] [PubMed] [Google Scholar]
- Soares H.D. Hicks R.R. Smith D. McIntosh T.K. Inflammatory leukocyte recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J. Neuroscience. 1995;15:8223–8233. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman D.J. Alverson C. Dunn K.A. Guerrero J. Sniezek J.E. Traumatic brain injury in the United States: a public health perspective. J. Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Uz T. Dimitrijevic N. Imbesi M. Manev H. Manev R. Effects of MK-886, a 5-lipoxygenase activating protein (FLAP) inhibitor, and 5-lipoxygenase deficiency on the forced swimming behavior of mice. Neurosci Lett. 2008;436:269–272. doi: 10.1016/j.neulet.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.L. Huang X.J. Fang S.H. Yuan Y.M. Zhang W.P. Lu Y.B. Ding Q. Wei E.Q. Leukotriene D4 induces brain edema and enhances CysLT2 receptor-mediated aquaporin 4 expression. Biochem. Biophys. Res. Commun. 2006;350:399–404. doi: 10.1016/j.bbrc.2006.09.057. [DOI] [PubMed] [Google Scholar]
- Warden D.L. Ryan L.M. Helmick K.M., et al. War neurotrauma: the Defense and Veterans Brain Injury Center (DVBIC) experience at Walter Reed Army Medical Center (WRAMC) J. Neurotrauma 22, 2005:1178. [Google Scholar]
- Yu G.-L. Wei E.-Q. Zhang S.-H. Xu H.-M. Chu L.-S. Zhang W.-P. Chen Z. Mei R.-H. Zhao M.-H. Montelukast, a cysteinyl leukotriene receptor-1 antagonist, dose- and time-dependently protects against focal cerebral ischemia in mice. Pharmacology. 2005;73:31–40. doi: 10.1159/000081072. [DOI] [PubMed] [Google Scholar]
- Zhang W.P. Hu H. Zhang L. Ding W. Yao H.T. Chen K.D. Sheng W.W. Chen Z. Wei E.Q. Expression of cysteinyl leukotriene receptor 1 in human traumatic brain injury and brain tumors. Neurosci. Lett. 2004;17:247–251. doi: 10.1016/j.neulet.2004.03.088. [DOI] [PubMed] [Google Scholar]