Abstract
Surrogate markers have enormous potential for contributing to the diagnosis, prognosis, and therapeutic evaluation of acute brain damage, but extensive prior study of individual candidates has not yielded a biomarker in widespread clinical practice. We hypothesize that a panel of neuron-enriched proteins measurable in cerebrospinal fluid (CSF) and blood should vastly improve clinical evaluation and therapeutic management of acute brain injuries. Previously, we developed such a panel based initially on the study of protein release from degenerating cultured neurons, and subsequently on rodent models of traumatic brain injury (TBI) and ischemia, consisting of 14-3-3β, 14-3-3ζ, three distinct phosphoforms of neurofilament H, ubiquitin hydrolase L1, neuron-specific enolase, α-spectrin, and three calpain- and caspase-derived fragments of α-spectrin. In the present study, this panel of 11 proteins was evaluated as CSF and serum biomarkers for severe TBI in humans. By quantitative Western blotting and sandwich immunoassays, the CSF protein levels were near or below the limit of detection in pre-surgical and most normal pressure hydrocephalus (NPH) controls, but following TBI nine of the 11 were routinely elevated in CSF. Whereas different markers peaked coordinately, the time to peak varied across TBI cases from 24–96 h post-injury. In serum, TBI increased all four members of the marker panel for which sandwich immunoassays are currently available: a calpain-derived NH2-terminal α-spectrin fragment and the three neurofilament H phosphoforms. Our results identify neuron-enriched proteins that may serve as a panel of CSF and blood surrogate markers for the minimally invasive detection, management, mechanistic, and therapeutic evaluation of human TBI.
Key words: 14-3-3, biomarker panel, calpain, necrosis, neurofilament, traumatic brain injury, UCH-L1
Introduction
Acute brain injuries resulting from traumatic brain injury (TBI), cardiac arrest with resuscitation, or stroke result in lasting neurologic and cognitive problems to highly variable degrees. Currently, mild acute brain injuries are difficult to diagnose, and there are neither prognostic methods for identifying patients especially at risk for developing severe and sustained abnormalities, nor proven neuroprotective strategies for improving long-term functional outcome. To help circumvent these problems, considerable effort is being devoted to the establishment and validation of biochemical surrogate markers for acute brain damage. There are numerous reports that certain proteins enriched in the nervous system are detectable in human cerebrospinal fluid (CSF) and serum following acute brain injuries. Among the most widely studied candidate markers are the astroglial proteins S100β and glial fibrillary acidic protein, and the neuronal proteins neuron-specific enolase, neurofilament polypeptides, and tau. In many cases, CSF and serum alterations in these proteins are related to prognosis (Fassbender et al., 1997; McKeating et al., 1998; Wunderlich et al., 1999; Mussack et al., 2002; Zemlan et al., 2002; Rosen et al., 2004; Vos et al., 2004; Pineda et al., 2007; Grubb et al., 2007), and have shown promise in facilitating assessment of experimental neuroprotectant treatment strategies (Tanaka et al., 2007). Furthermore, both a hypophosphorylated form of neurofilament H (pNFH) and a proteolytic fragment of tau are expressed in neurons predominantly within axons, and CSF alterations in these proteins have been proposed as indices of axonal damage (Zemlan et al., 1999; Petzold et al., 2006).
Unfortunately, owing to limitations in sensitivity, specificity, and standardized quantification across multiple laboratories and studies, none of these proteins has emerged as a widely used diagnostic or prognostic clinical tool or a validated surrogate measure for irreversible brain damage. For example, although serum levels of S100β change in relation to short-term mortality and morbidity, as well as long-term neurologic outcome, the protein also markedly increases in serum during surgical procedures or in disorders unrelated to acute brain injuries (Anderson et al., 2001; Routsi et al., 2006; Teepker et al., 2008) as well as marathon runners (Hasselblatt et al., 2004), from whom it is derived from adipose and other extracranial sources (Kleine et al., 2003). Furthermore, acute alterations in serum S100β are not consistently predictive of brain dysfunction resulting from mild brain injury (Begaz et al., 2006). In an analysis of four candidate biomarkers in over 300 stroke patients, an efficacious tissue plasminogen activator treatment did not alter any of the stroke-induced serum marker increases (Jauch et al., 2006), suggesting none of these markers could be used as pharmacodynamic measures of brain damage. Finally, virtually all studies of the diagnostic and prognostic potential of surrogate markers have been conducted retrospectively, in that marker level cutoffs have been selected only after reviewing functional outcome data. Consequently, the need remains for new, highly sensitive biomarkers, which may be combined with other measures to improve in a clinically useful prospective fashion the diagnosis, prognosis and experimental therapeutic evaluation of acute brain injuries.
Rather than focusing on individual candidate biomarkers, and cognizant of advances in oncology with large-scale gene expression profiling (Konstantinopoulos et al., 2008; Ross et al., 2008) and cardiology with multimarker analyses (Morrow et al., 2007; Foussas et al., 2007), we hypothesize that a large panel of brain-enriched proteins may vastly improve the diagnosis and clinical evaluation of acute brain injuries. We have begun to develop such a panel based intially on the study of protein release from degenerating cultured neurons, and subsequently of rodent models of TBI and ischemia. Currently, the panel consists of 11 neuron-enriched proteins (i.e., expressed predominantly or exclusively in neurons): 14-3-3β, 14-3-3ζ, three distinct phosphoforms of neurofilament H, the ubiquitin hydrolase UCH-L1, neuron-specific enolase (NSE), α-spectrin, and three fragments of α-spectrin derived by either calpain or caspase proteolysis (Aitken et al., 2003; Goodman et al., 1987; Williams and Runge, 1983; Wilkinson et al., 1989; Day, 1992; Siman et al., 1988; 2004). Certain members of this biomarker panel have shown characteristics desirable for surrogate markers of acute brain damage, including large, rapid increases specifically following irreversible brain injury, and correlations with the severity of the initial injury and of the acute cerebral histopathology in experimental animals, as well as short-term neurological complications following surgically induced circulation arrest in humans (Siman et al., 2004, 2005, 2008; Lawrence et al., 2005). However, several of the proteins have not been studied before as individual markers for TBI in humans, and none have been examined in a combinatorial approach as an array of biomarkers. Consequently, in the present study this panel of proteins was evaluated as CSF and serum biomarkers for severe human TBI, compared with pre-surgical and normal pressure hydrocephalus (NPH) controls not suffering from acute brain damage.
Methods
Human subjects and samples
This study received Institutional Review Board (IRB) approval and written informed consent was obtained either from all study subjects or, in the cases of severe TBI, a legally authorized representative. Patients being treated for severe TBI were identified as having scores on the Glasgow Coma Scale (GCS) of 3–8 within 36 h of the time of injury. Nine TBI cases were enrolled in the present study. Patients were both males and non-pregnant females 18 years or older, five of whom received a ventriculostomy for management of intracerebral hypertension as part of their care. For all cases, blood was drawn from an in-place arterial catheter or venipuncture and allowed to clot. Some of the patients were enrolled in 2006 and early 2007, at a time when periventricular shunting was performed only for patients exhibiting hydrocephalus, and prior to subsequent guidelines identifying it as an acceptable method of controlling intracranial pressure in patients without hydrocephalus. Thus, four of the TBI patients did not receive a ventriculostomy, and so provided only blood samples. Sera and CSF freed of debris were obtained by centrifugation, then stored in aliquots at −80°C. In these and all subsequent cases, patient confidentiality was maintained by associating a subject identification number with each sample for accession by the investigators.
Aortic surgical patients eligible for the study were admitted to the Hospital of the University of Pennsylvania for thoracoabdominal aortic aneurysm repairs and had lumbar CSF drains placed as part of the standard care. Nineteen of these cases have been described in detail before and had normal neurological examinations at baseline (Siman et al., 2008). Three samples from this cohort were chosen for comparative evaluations in the present study and are representative of the entire group of surgical cases. Aliquots of CSF and serum from these patients were collected prior to the vessel clamping and circulation arrest performed as part of the aneurysm repair, and were stored at −80°C. Eight NPH cases also were part of the study. These patients exhibited ventricular enlargement on magnetic resonance imaging without evidence of obstruction or cortical atrophy beyond that expected for age, and had at least two of the following three clinical features of NPH: (i) development after age 45 of a progressive gait impairment; (ii) progressive urinary incontinence; and (iii) progressive cognitive impairment with onset after age 45. Patients were excluded if they exhibited either presence of Parkinsonism responsive to carbidopa, history of stroke, history of clinically meaningful depression or other major psychiatric disorder, history of substance abuse, or medical unsuitability for surgery. Eligible patients were scheduled for placement of a programmable ventriculoperitoneal shunt as part of their care. CSF and sera were obtained at the time of shunt placement. The samples were freed of debris as above and stored in aliquots at −80°C.
Western blot analysis of biomarker levels
Quantitative Western blotting was conducted using a near-infrared polypeptide labeling and image analysis method. Equivalent volumes of the CSF samples were fractionated under reducing and denaturing conditions by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and the polypeptides transferred to PVDF membranes using well-established methods (Siman et al., 2001, 2004, 2008). Six different intact biomarker polypeptides and two proteolytic fragments of α-spectrin were analyzed by immunoblotting: 14-3-3β (antibody dilution 1/1000; Santa Cruz), 14-3-3ζ (1/1000; Santa Cruz), neuron-specific enolase (Mab314, 1/1000; Millipore), UCH-L1 (1/2000; Millipore), α-spectrin (MAb1622, 1/1000; Millipore), and total neurofilament H (1/2000; Sigma). In addition, fragments of α-spectrin derived by calpain proteolysis were examined using cleavage site-specific antibodies reactive with the NH2- and COOH-terminal α-spectrin derivatives generated specifically by calpain (Abs 38 and 41, respectively) (Roberts-Lewis et al., 1994; Saatman et al., 1996), while a caspase-derived ∼120-kDa α-spectrin fragment was detected along with intact α-spectrin by MAb1622 (Wang, 2000). Immunostained polypeptides were labelled with the appropriate fluorescently tagged secondary antibodies emitting light at either 700 or 800 nm (1/15,000; Li-Cor), and band densities quantified using a Li-Cor Odyssey image capture and data analysis system. Serial dilution of individual samples confirmed that band density varied in direct linear proportion with antigen content over a 1,000-fold concentration range. Protein levels are represented as arbitrary units relative to the controls evaluated on a side-by-side basis, and a subset of samples were examined on every blot to permit comparative analyses of the entire sample pool.
Enzyme fluorescence immunoassay for three distinct neurofilament H phosphoforms
Three distinct variants of NFH were quantified by highly sensitive and specific sandwich immunoassays, using as capture reagents monoclonal antibodies highly specific for distinct NFH phosphoforms (Sternberger and Sternberger, 1983). The SMI-35 antibody recognizes lightly phosphorylated NFH, referred to as hypophospho-NFH. The SMI-32 antibody reacts only with dephosphorylated NFH, while the SMI-31 antibody recognizes only heavily phosphorylated NFH (referred to as phospho-NFH). The assays are modifications of those described originally by Petzold et al. (2003), converted to enzyme-linked immunofluorescence assays to enhance sensitivity and facilitate future development of a multiplex method of simultaneous analysis. Briefly, the mouse monoclonal antibodies to either hypophospho-NFH, dephospho-NFH or phospho-NFH (each at 1/10,000) were bound to 96-well microtiter plates in 50 mM sodium carbonate buffer 24–48 h at 4°C. Wells were treated for 45 min with 0.5% bovine serum albumin (BSA) in Tris-buffered saline with Tween-20 (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween-20; TTBS), washed four times with TTBS, and the samples were added at 10 μl/well along with BSA/TTBS to a final volume of 100 μl/well for 90 min at 22°C. Each antigen-containing sample was evaluated in triplicate. Wells were washed four times with TTBS, then incubated with the detecting antibody reactive with all forms of NFH (rabbit anti-NFH at 1/4000; Sigma) in BSA/TTBS for 60 min at 22°C. After washing, wells were incubated with mouse monoclonal anti-rabbit IgG-alkaline phosphatase (at 1/20,000; Sigma) in BSA/TTBS for 60 min at 22°C. After washing, the wells were treated with an alkaline phosphatase fluorogenic substrate consisting of 4-methyl-umbelliferylphosphate (250 μM) in 0.1 M Tris-HCl (pH 9.5)/1 mM MgCl2 at 37°C. The fluorescent product was measured for at least five time points in a microplate fluorimeter (Fluorocount; Perkin-Elmer). Negative controls included samples in which either the capture antibody or the CSF samples were omitted. Baseline fluorescence was determined from wells treated with the alkaline phosphatase substrate solution alone. Mean alkaline phosphatase activity was determined for each set of triplicate wells using linear regression analysis to determine the slope of the time-dependent increase in fluorescent product.
The NFH immunoassays were standardized using mouse spinal cord extract, which is enriched in all NFH phosphoforms. Cords from four adult CD-1 mice were excised rapidly and homogenized in 50 volumes of 20 mM Tris-HCl (pH 7.4) containing protease inhibitor cocktail (Sigma). Following centrifugation for 30 min at 40,000 × g, the neurofilament-rich supernatant was frozen in small batches at −80°C. Standard curves were made on each microtiter plate using serial dilutions of the spinal cord extract at 0.1–3 nl/well, over which the rate of alkaline phosphatase activity increased in proportion with the content of each NFH phosphoform. Fluorescence signal from all CSF samples was normalized to the standards, and is represented as the increase in relative fluorescence per unit time compared with negative control lacking any added standard. Each unit of antigen corresponds to the volume of standard in nanoliters that yields equivalent alkaline phosphatase activity. The dependence of each immunoassay on NFH phosphorylation status was confirmed by incubating standards overnight either with or without purified alkaline phosphatase (Sigma). As predicted, the dephosphorylation of spinal cord extract led to a decrease in signal for hypophospho-NFH but increased signal for dephospho-NFH.
Enzyme fluorescence immunoassay for calpain-derived α-spectrin NH2-terminal fragment
The ∼150-kDa NH2-terminal fragment (NTF) of α-spectrin generated by calpain proteolysis (Siman et al., 1984, 1988, 1989; Roberts-Lewis and Siman, 1993) was measured by a sandwich immunoassay similar in format to those used to quantify NFH phosphoforms. In this case, a monoclonal antibody to α-spectrin reactive with an epitope in the SH3 domain on the NH2-terminal half of the polypeptide was used as capture antibody (clone D8B7, 1/5,000; Abcam) (Xu et al., 2001) in 50 mM sodium bicarbonate/5 mM sodium carbonate buffer. The detection step employed a well characterized cleavage site-specific rabbit anti-α-spectrin NH2-terminal fragment antibody (Ab37, 1/3,000) (Roberts-Lewis et al., 1994; Siman et al., 2000).
The immunoassay for calpain-derived α-spectrin NTF was standardized using an extract from mouse brain treated with calcium to activate calpain and induce α-spectrin degradation. Brains from four adult CD-1 mice were excised rapidly and homogenized in 20 volumes of 20 mM Tris-HCl (pH 7.4)/5 mM β-mercaptoethanol/2 mM EDTA. Following centrifugation for 30 min at 40,000 × g, the supernatant was divided into two portions. One was maintained under calcium-free conditions while the second was brought to 10 mM calcium chloride, and both were incubated 20 min at 37°C. Standard curves were made on each microtiter plate using serial dilutions of the brain extracts ranging at 0.05–3 μl/well. The specificity of the immunoassay for a calpain-derived α-spectrin NTF was established by three findings. First, signal was more than 10-fold higher from the brain extract treated with calcium to activate endogenous calpain compared with the control extract. Second, signal was attenuated when the calcium treatment was performed in the presence of MDL-28170, a protease inhibitor that blocks calpain. Finally, the relative amounts of calpain-derived α-spectrin NTF measured by the immunoassay across a set of CSF samples from TBI cases matched the relative amounts determined by quantitative Western blot. For serum samples, fluorescent signal was normalized to the calcium-treated standards, and is represented as the increase in relative fluorescence units per unit time over a negative control lacking any added standard.
Results
Clinical data on TBI and control patients
Nine severe TBI patients, eight NPH patients, and three presurgical cases were evaluated in the present study. Basic information on the TBI and NPH cases is summarized in Table 1. All nine TBI cases provided plasma samples, and the five who received periventricular shunts as part of their standard care were sources of CSF. As described previously (Siman et al., 2008), CSF and sera were obtained from surgical cases of aneurysm repair prior to vessel cross-clamping and circulation arrest.
Table 1.
Traumatic Brain Injury, Normal Pressure Hydrocephalus, and Control Presurgical Cases under Study
| Case | Gender | Age | (years) | Initial GCS type of injury | Fluids |
|---|---|---|---|---|---|
| TBI-2 | M | 19 | 4 | Auto accident | Serum |
| TBI-3 | M | 23 | 3 | Auto accident | CSF, Serum |
| TBI-7 | F | 17 | 3 | Auto accident | Serum |
| TBI-8 | M | 20 | 2 | Auto accident | CSF, Serum |
| TBI-9 | M | 19 | 3 | Fall | Serum |
| TBI-10 | M | 27 | 4 | Auto accident | CSF, Serum |
| TBI-12 | M | 75 | 6 | Fall | Serum |
| TBI-13 | F | 44 | 8 | Auto accident | CSF, Serum |
| TBI-17 | M | 18 | 9 | Gunshot wound | CSF, Serum |
| NPH-01 | M | 79 | CSF, Serum | ||
| NPH-31 | M | 77 | CSF. Serum | ||
| NPH-33 | F | 85 | CSF, Serum | ||
| NPH-34 | F | 69 | CSF, Serum | ||
| NPH-35 | F | 64 | CSF, Serum | ||
| NPH-37 | F | 78 | CSF, Serum | ||
| NPH-38 | M | 71 | CSF, Serum | ||
| NPH-39 | M | 83 | CSF, Serum | ||
| CONTROL-1 | M | 74 | CSF, Serum | ||
| CONTROL-3 | F | 80 | CSF, Serum | ||
| CONTROL-6 | F | 68 | CSF, Serum |
Western blot analyses of CSF biomarker alterations following severe TBI in humans
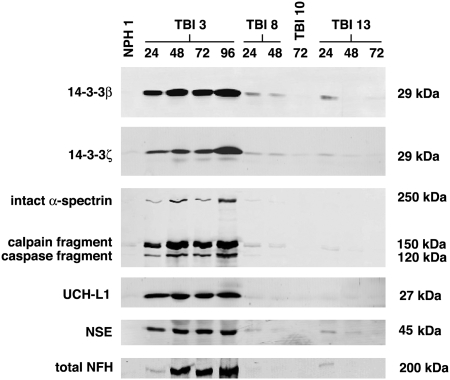
We recently identified a panel of neuron-enriched proteins released from degenerating neurons (Siman et al., 2004) that rise markedly in CSF in rodent experimental models for TBI and global cerebral ischemia (Siman et al., 2004, 2005), as well as in humans subjected to surgically induced transient circulation arrest (Siman et al., 2008). To begin evaluating whether these proteins might serve as a panel of biomarkers for human TBI, we first measured by quantitative Western blot analysis the levels of eight neuron-enriched proteins in ventricular CSF samples from cases of severe TBI (GCS 3–8), and compared them with cases of NPH and pre-surgical controls. Levels of 14-3-3β (∼29 kDa), 14-3-3ζ (∼29 kDa), the intact α-subunit of spectrin (∼250 kDa), a calpain-cleaved α-spectrin COOH-terminal fragment (∼150 kDa), a caspase-cleaved α-spectrin COOH-terminal fragment (∼120 kDa), UCH-L1 (∼27 kDa), NSE (∼45 kDa), and NFH (∼200 kDa) were near or below the limit of detection in ventricular CSF from NPH or lumbar CSF from aortic surgical cases prior to the surgical procedures. As exemplified in Figure 1, severe TBI consistently increased CSF levels of six of the eight neuron-enriched proteins at 24 h post-injury. Only for TBI-10 could biomarker elevations not be detected, and it is noteworthy that CSF was available for evaluation in this case only from the 72-h time point. Figure 1 also illustrates that the absolute magnitude of biomarker increases and their time to reach peak levels differed across the TBI cases. Among the four cases shown, TBI-3 exhibited the largest CSF elevations in 14-3-3β, 14-3-3ζ, intact α-spectrin, the α-spectrin calpain and caspase derivatives, UCH-L1, NSE, and NFH. In this case, all eight proteins were elevated by 24 h, but did not reach peak levels until 48–96 h. In contrast, cases TBI-8 and TBI-13 showed elevated levels of six of the proteins at 24 h, and all of the markers were highest at this time point, the earliest examined.
FIG. 1.
A panel of neuron-enriched proteins in human cerebrospinal fluid (CSF) following traumatic brain injury (TBI). By Western blot analysis, CSF levels of eight neuron-enriched proteins increased following severe human TBI. In comparison with CSF from cases of normal pressure hydrocephalus (NPH) or pre-surgical controls (not shown; see also Siman et al., 2008), TBI led to a marked rise in three of the four cases shown in CSF levels of the following members of our biomarker panel: the β and ζ isoforms of 14-3-3 protein, α-spectrin (∼250 kDa), a calpain-derived ∼150-kDa α-spectrin COOH-terminal fragment, a caspase-derived ∼120-kDa α-spectrin COOH-terminal fragment, ubiquitin C-terminal hydrolase L1 (UCH-L1), neuron-specific enolase (NSE), and neurofilament H (NFH). Immunodetection of the target polypeptides was confirmed by their appropriate apparent molecular weights (shown on the right). Note that levels of the calpain-derived fragment of α-spectrin always exceeded those of the caspase derivative, and the latter was not noticeably increased in a subset of TBI cases.
The profile of CSF biomarker alterations may be reflective of the underlying neurodegenerative mechanisms triggered by severe TBI. Although increases were noted for both calpain and caspase COOH-terminal derivatives of α-spectrin in human CSF following TBI, increased levels of the calpain fragment not only were more consistent, but predominated over the caspase derivative in every case. To confirm that human TBI leads to calpain activation and calpain-mediated α-spectrin degradation in the central nervous system (CNS), we probed blots of CSF proteins with cleavage site-specific antibodies reactive with the major calpain-derived NH2- or COOH-terminal fragments (Roberts-Lewis et al., 1994). Both calpain derivatives were below the detection limit in NPH and control CSF but increased markedly following severe TBI (data not shown). Collectively, our data provide further biomarker evidence that neurodegeneration following severe human TBI occurs through both calpain-driven necrosis and caspase-mediated apoptosis, with the necrotic component being the more prevalent pathophysiological mechanism (Pineda et al., 2007).
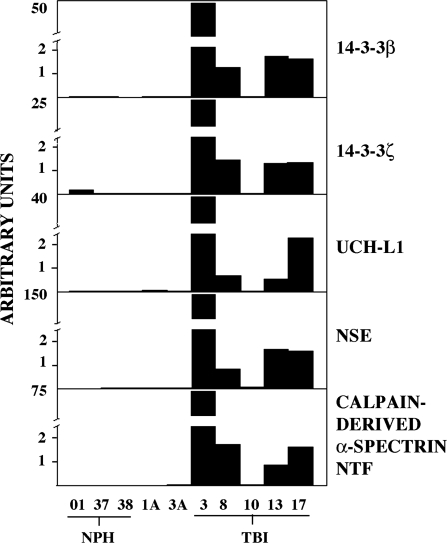
Quantitative analysis of CSF content of six of the neuron-enriched proteins across five control and five TBI cases is shown in Figure 2. All six biomarkers were near or below the limit of detection in the three cases of NPH and the two pre-surgical control cases (1A and 3A), but increased markedly in four of the five cases of TBI. Whereas case TBI-3 consistently showed the largest biomarker levels across the cases, the rank order among other cases varied slightly depending on the biomarker.
FIG. 2.
Quantitative analysis of biomarker elevations in human cerebrospinal fluid (CSF) following traumatic brain injury (TBI). Five biomarkers were quantified in human CSF by a near-infrared immunofluorescence labeling and image analysis approach, as described in Methods. The biomarkers were near or below the limit of detection in the three normal pressure hydrocephalus (NPH) cases and the two pre-surgical control cases (1A, 3A), but increased markedly at 24–96 h following severe TBI. For the five cases of TBI, peak biomarker levels are depicted. Note that only a single time point (72 h post-injury) was available for case TBI-10, which did not exhibit increased levels of any of the five biomarkers.
Sandwich immunoassays for quantifying three neurofilament H phosphoforms and a calpain-derived α-spectrin fragment
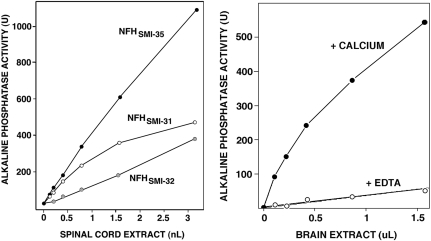
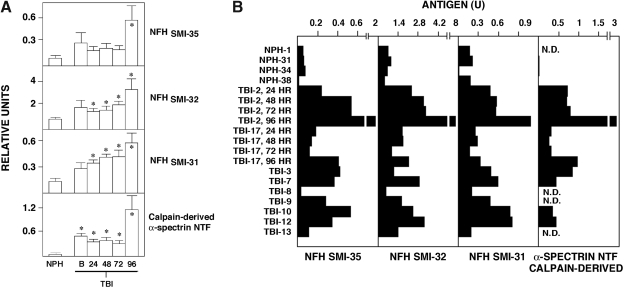
Several reports have raised the possibility that neurofilament polypeptides might be clinically useful CSF and serum biomarkers for degenerative brain disorders (Rosen et al., 2004; Petzold et al., 2003; Shaw et al., 2005; Anderson et al., 2008), and elevated levels of phosphorylated NFH have been linked to axonal damage accompanying both chronic disease (Petzold et al., 2005) and acute injuries (Anderson et al., 2008). NFH has more than 60 sites for serine-threonine phosphorylation, so we evaluated whether immunochemically distinct NFH phosphoforms may be additional biomarkers for severe human TBI. Using well-characterized discriminative monoclonal antibodies (Sternberger and Sternberger, 1983) for dephosphorylated NFH (SMI-32), hypophosphorylated NFH (SMI-35), or extensively phosphorylated NFH (SMI-31), we developed and validated enzyme-linked immunofluorescence assays for quantifying each phosphoform. As shown in Figure 3, spinal cord extract rich in neurofilaments contained levels of all three NFH phosphoforms readily measurable by immunoassay. Dephosphorylation of the extract by treatment with alkaline phosphatase led to a marked decrease in signal for hypophosphorylated NFHSMI-35 and increased signal for dephosphorylated NFHSMI-32, thereby confirming that the immunoassays measure distinct NFH phosphoforms (data not shown). The three immunoassays are extraordinarily sensitive, being capable of measuring NFH in as little as 1 pg of mouse spinal cord, and respond to a broad range of concentrations of spinal cord extract with proportionate changes in signal.
FIG. 3.
Enzyme-linked immunofluorescence sandwich assays for three distinct neurofilament H phosphoforms and a calpain-derived α-spectrin NH2-terminal fragment (NTF). Four sandwich immunoassays were validated and standardized using increasing amounts of spinal cord extract enriched in neurofilaments (left), or brain extract either treated with calcium to activate endogenous calpains, or maintained in EDTA (right). For distinguishing neurofilament H (NFH) phosphoforms, three distinct well-characterized capture antibodies were used: SMI-35, which preferentially reacts with hypophosphorylated NFH, SMI-32, which recognizes only nonphosphorylated NFH, and SMI-31, which binds specifically to highly phosphorylated NFH. The calpain-derived α-spectrin ∼150-kDa NTF was detected using Ab37, a cleavage site-specific antibody that only recognizes this calpain fragment (Roberts-Lewis et al., 1994; Siman et al., 2000).
A similar approach was used to develop a highly sensitive and specific fluorescence enzyme immunoassay for the ∼150-kDa calpain-derived NH2-terminal fragment (NTF) of α-spectrin (Siman et al., 1984, 1988, 1989; Roberts-Lewis et al., 1994). The assay captured intact α-spectrin as well as any NH2-terminal proteolytic fragments using a solid support coated with an antibody reactive with the SH3 domain (Xu et al., 2001), which is located in the NH2-terminal half of the α-subunit approximately 200 residues upstream of the preferred calpain cleavage site. The NH2-terminal calpain derivative was then detected using Ab37, a well-characterized neoepitope-specific antibody that reacts only with the NH2-terminal calpain derived fragment (Roberts-Lewis et al., 1994; Saatman et al., 1996; Siman et al., 2000). As shown in Figure 3B, treatment of mouse brain extract with calcium to activate calpain led to an approximately 15-fold increase in signal compared with the extract maintained in the calcium chelator EDTA, consistent with selective recognition of calpain-derived α-spectrin NTF. This was confirmed by attenuation of the calcium-induced increase in signal with MDL28170, a protease inhibitor that blocks calpain (data not shown).
CSF elevations in the 3 NFH phosphoforms following severe human TBI
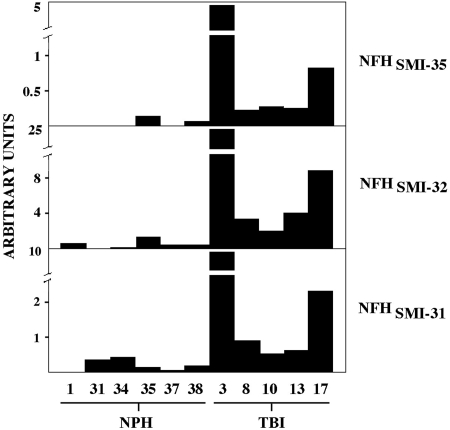
The discriminative immunoassays described above were applied initially to CSF analysis of the three NFH phosphoforms following TBI. As summarized in Figure 4, levels of each NFH variant were either relatively low or undetectable in CSF samples from six cases of NPH, but severe TBI increased all three NFH phosphoforms. The magnitude and time course of NFH phosphoform elevations were consistent with the eight markers measured by Western blotting. Thus, CSF levels of dephosphorylated NFHSMI-32, hypophosphorylated NFHSMI-35, and extensively phosphorylated NFHSMI-31 were highest in case TBI-3, in which they reached peak levels 96 h post-injury, next highest in TBI-17, and lowest in TBI-10.
FIG. 4.
Increased cerebrospinal fluid (CSF) levels of three neurofilament H (NFH) phosphoforms following severe human traumatic brain injury (TBI). CSF levels of hypophosphorylated NFH (SMI-35 reactive), nonphosphorylated neurofilament H (SMI-32 reactive), and highly phosphorylated neurofilament H (SMI-31 reactive) were quantified by fluorescence immunoassay from six cases of normal pressure hydrocephalus (NPH) and five cases of TBI. The graph depicts peak CSF levels at 24–72 h post-injury, except in the case of TBI-10, from whom only a 72-h sample was available.
The presence of dephospho-NFH in axonal swellings is a reliable histological marker for axonal injury in response to brain trauma (Chen et al., 1999), but owing to the low amount of dephosphorylated NFH in normal (uninjured) spinal cord relative to phosphorylated NFH variants (Petzold et al., 2003), this dephosphoform has not received attention before as a CSF marker for brain injury. Instead, prior studies of NFH phosphoforms as surrogate markers for brain damage have focused on the hypophosphorylated or extensively phosphorylated variants. We found that following standardization of the three immunoassays against spinal cord extract, for a given CSF sample dephosphorylated NFHSMI-32 showed the largest increase following TBI, followed by heavily phosphosphorylated NFHSMI-31, with the relative level of hypophosphorylated NFHSMI-35 increasing the least among the three.
Application of the fluorescence enzyme immunoassay for a calpain-derived NH2-terminal fragment of α-spectrin demonstrated a pronounced increase in CSF levels of this calpain cleavage product following severe TBI, thus confirming our findings from independent Western blot analysis, and paralleling results for the three NFH phosphoforms (data not shown).
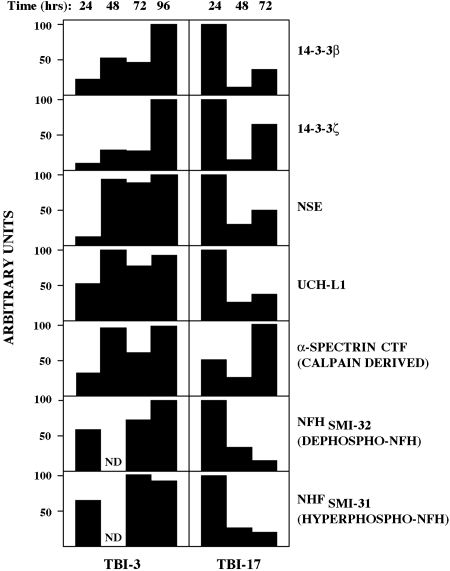
Different cases of TBI have distinct time courses for CSF increases in the biomarker panel
We have begun evaluating the kinetics of CSF alterations in the biomarker panel following human TBI. Figure 5 depicts that, for two representative TBI cases, seven different biomarkers change generally in a coordinated fashion. For example, in case TBI-3 elevations in all seven markers were apparent by 24 h post-injury but continued to rise thereafter, in many cases only reaching peak levels at the 96-h time point. Longer time points were not examined, and so it is unclear whether CSF marker levels exhibit still further elevations at even later time points. Similarly for case TBI-17, six of the seven biomarkers rose over time in a coordinated manner. However, the time course for marker elevations differed from TBI-3, in which highest measurable levels of six of the markers were reached at 24 h and then declined thereafter. Only the time-dependent rise in a calpain-derived COOH-terminal α-spectrin fragment differed for TBI-17, taking 72 h to reach highest levels. Thus, the time courses for post-injury CSF elevations in members of our biomarker panel can vary markedly across TBI cases. Furthermore, although most of the markers rise coordinately within a given case, at least one marker may differ in time to reach peak levels.
FIG. 5.
Biomarker elevations are coordinated over time and vary across traumatic brain injury (TBI) cases. The graph depicts time-dependent increases in seven members of the biomarker panel for two cases of TBI. For case TBI-3, marker elevations were detectable at 24 h and increased thereafter, reaching peak levels at 72–96 h post-injury. In sharp contrast, for case TBI-17, elevations in six of the seven biomarkers were highest at 24 h and declined thereafter, with only the calpain-cleaved α-spectrin NH2-terminal fragment (NTF) reaching maximal levels at the later time point.
Serum elevations in 3 NFH phosphoforms and calpain-derived α-spectrin NTF following TBI
Whereas there are a number of potential clinical applications for CSF markers for acute brain damage in the diagnosis, prognosis, mechanistic, and therapeutic evaluation of severe human TBI, a panel of serum biomarkers could be even more useful, since for the vast majority of TBI cases CSF is not obtained during routine care. Consequently, we began evaluating serum alterations in our biomarker panel following severe TBI, starting with four panel members for which we have established thus far highly sensitive and specific fluorescence enzyme immunoassays: dephosphorylated NFHSMI-32, hypophosphorylated NFHSMI-35, extensively phosphorylated NFHSMI-31, and a calpain-derived NH2-terminal α-spectrin fragment (NTF). As shown from data of Figure 6A pooled for all nine cases, severe TBI led to statistically significant and time-dependent elevations in all four markers for acute brain damage. Compared with NPH cases, TBI led on average to three- to fourfold increases in each of the NFH phosphoforms, and more than a 20-fold increase in calpain-derived α-spectrin NTF. Serum elevations for each marker occurred rapidly, being detectable at baseline (sampled the same day as the TBI), but not reaching peak levels on average until 96 h post-injury, the longest time point examined. On average, the temporal profile of biomarker elevations appeared biphasic, with relatively little change from the day of injury to 72 h post-injury, followed by a later secondary increase.
FIG. 6.
Four members of the protein panel are blood biomarkers for human traumatic brain injury (TBI). (A) Fluorescence enzyme immunoassays for the three distinct neurofilament H (NFH) phosphoforms and a calpain-derived α-spectrin NH2-terminal fragment (NTF) were applied to sera taken from normal pressure hydrocephalus (NPH) and pre-surgical cases, four of which are depicted here, as well as the nine cases of severe TBI. The graph shows the mean biomarker levels (±SEM) at various times post-injury. Not all time points were available for all TBI cases, and the means are derived from three to nine determinations. Serum levels of the four neuron-enriched proteins were elevated by 24 h post-injury (*p < 0.05), but did not reach peak levels until 96 h post-injury, the longest time point examined. (B) Case-by-case serum biomarker elevations. In comparison with NPH cases, marker levels were consistently higher for eight of the nine cases of TBI. As illustrated for cases TBI-2 and TBI-17, peak serum levels of each of the four biomarkers was not reached until 96 h post-injury.
It is noteworthy that there was considerable variation in serum marker levels among the NPH cases. Most of the NPH cases had consistently low levels of the four markers comparable to sera obtained from presurgical cases exhibiting no CNS pathology, and were uniformly below serum levels for eight of the nine TBI cases (Fig. 6). On the other hand, cases NPH-33 and NPH-37 had distinctly measurable serum marker levels that overlapped with some of the severe TBI cases (data not shown). Elevated levels of putative brain injury markers have been reported before in a subset of NPH cases (Agren-Wilsson et al., 2007; Gloeckner et al., 2008), possibly reflecting either an evolving white matter damage or the presence of Alzheimer's disease.
Within-case serum levels of the three NFH phosphoforms and the calpain-derived α-spectrin NTF were related, so that those cases with largest peak increases in one marker also exhibited the largest peak elevations in the other three markers (Fig. 6B). Nevertheless, the overall rank-order of serum marker levels across all the TBI cases varied depending on the marker. Among the nine TBI cases examined in this study, only TBI-8 showed consistently low serum biomarker levels that matched those of most of the cases of NPH.
Discussion
Here we demonstrate that among a panel of 11 neuron-enriched proteins, severe human TBI leads to rapid, marked, and consistent increases in nine of the proteins in CSF and at least four of them in serum. Although extensive prior studies over the past 20 years have evaluated candidate CSF and serum biomarkers for acute brain damage in humans, our findings describe the first panel of multiple biomarkers with potential applications in the diagnosis, prognosis, mechanistic and therapeutic evaluation of TBI in human adults. Five of the proteins described herein have not been evaluated before, either individually or in combination, as surrogate markers for TBI in humans: the beta and zeta isoforms of 14-3-3 protein, the deubiquitinating enzyme UCH-L1, the dephosphorylated form of NFH (NFHSMI-32), and a calpain-derived NH2-terminal fragment of α-spectrin. These five novel biomarkers are highly abundant neuronal proteins (Aitken et al., 2003; Wilkinson et al., 1989; Goodman et al., 1987; Williams and Runge, 1983) and so their presence in CSF and serum offers not only high sensitivity but also increased specificity for acute neuronal injury in comparison with the widely studied glial-enriched biomarkers S100β and glial fibrillary acidic protein. Whereas spectrin is an actin-binding protein with widespread expression in a large number of cell types both within and outside the nervous system, calpain-mediated cleavage of the α-subunit occurs in the brain predominantly under pathological but not physiological conditions, and is triggered by stimuli that elicit acute neurodegeneration (Siman et al., 1988, 1989; Roberts-Lewis and Siman, 1993; Roberts-Lewis et al., 1994; Saatman et al., 1996). The presence of these neuron-enriched proteins in the CSF and serum compartments following severe human TBI likely results from the spillage of these proteins from dying neurons, along with at least a transient breakdown of the barriers separating the CSF and blood from the brain parenchyma. This concept is supported by prior studies in cell culture and experimental models for acute traumatic and ischemic brain damage, which identified members of the current panel as proteins released by dying neurons that also rise markedly in accessible body fluids, some in direct correlation with the magnitude of acute cerebral histopathology (Siman et al., 2004, 2005).
Other members of our biomarker panel have been studied before in acute brain injuries on an individual basis: NSE, a calpain-derived COOH-terminal α-spectrin fragment, a caspase-derived COOH-terminal α-spectrin fragment, hypophosphorylated NFHSMI-35, and an extensively phosphorylated NFH variant similar to NFHSMI-31 (Herrmann et al., 2000; Shaw et al., 2005; Begaz et al., 2006; Pineda et al., 2007; Anderson et al., 2008). However, owing to limitations in sensitivity, specificity, and consistent prospective analyses, neither these nor any other individual glial- or neuron-enriched protein has been developed yet as a biomarker with utility for the prospective diagnosis and management of human TBI. The simultaneous evaluation of a panel of biomarkers for acute brain damage might provide a number of advantages over the measure of individual markers. In addition to the aforementioned specificity for neuronal versus astroglial degeneration, a panel of neuron-enriched biomarkers is unlikely to be influenced by contributions from extracranial sources unrelated to acute brain damage. This is a significant limitation with serum S100β, which can be derived not only from damaged CNS tissue, but also from adipose and other non-CNS sources (Anderson et al., 2001; Kleine et al., 2003; Hasselblatt et al., 2004). Furthermore, the multi-variate measure of a protein array might detect subtle biomarker alterations with much greater fidelity than single markers, particularly when the inter- and intra-assay variability for each individual marker may be significant relative to the magnitude of any TBI-induced change. In this regard, the relative magnitude and time course for CSF and serum elevations in each member of the current biomarker panel generally are coordinated within each case (Figs. 2, 5, and 6). This raises the intruiging but still untested possibility that the simultaneous measure of a large number of neuron-enriched proteins may vastly increase the discriminative power of biomarkers to diagnose and quantify milder forms of TBI and other acute brain injuries than studied here. This concept is supported by evidence that several members of our biomarker panel rise in human CSF following surgically-induced circulation arrest, even for patients exhibiting no overt short-term neurological complications (Siman et al., 2008).
Radiological, neurological and neurophysiological studies of severe TBI have highlighted the diversity of pathophysiological processes contributing to brain dysfunction following TBI. It is unlikely that this complexity can be appreciated fully from the measure of any single biomarker. Indeed, the simultaneous evaluation of just two serum markers (NSE and S100β) reportedly revealed different release patterns over time for cases of primary cortical contusion, diffuse axonal injury, and cerebral edema without focal mass lesions (Herrmann et al., 2000). Potentially, the combined assessment of CSF and blood alterations in large biomarker panels may provide clinically useful information related to the diversity of pathophysiological mechanisms and responses to inverventive measures designed to reduce evolving brain damage. Particularly intruiging is the prospect for discerning on a per-patient basis the relative contributions of distinct neurodenegerative mechanisms. Calpains and caspases are cysteine protease families that differentially mediate necrotic and apoptotic modes of neuronal death, respectively (Wang et al., 2000; Harwood et al., 2005). Consequently, TBI-induced increases in the CSF and serum of α-spectrin fragments derived by either calpain or caspase activity likely reflect the relative contributions of necrotic and apoptotic mechanisms. Our findings that a calpain-mediated α-spectrin derivative predominates over a caspase derivative in CSF following severe TBI (Fig. 1) substantiates an earlier report (Pineda et al., 2007), as well as studies of rodent experimental models of TBI (Saatman et al., 1996; Raghupathi et al., 2000; Pike et al., 2001; Siman et al., 2004) and cerebral ischemia (Zhang et al., 2002). Furthermore, using a novel sandwich immunoassay selective for a calpain-derived NH2-terminal fragment of α-spectrin, we provide the first evidence that injury-induced brain calpain activity is measurable in the blood following TBI (Figs. 3 and 6). The noninvasive quantitation of this and other mechanism-based biomarkers could facilitate clinical studies aimed at stratifying TBI cases based on their underlying pathophysiologies. Pooled temporal analysis of nine TBI cases indicates that serum levels of neuron-enriched proteins rise rapidly following severe TBI and suggests that the elevations might be biphasic (Fig. 6). Given the considerable evidence that secondary injury mechanisms contribute to the outcome following TBI, it is interesting to consider that kinetic analyses using a biomarker panel might be informative for the onset and severity of these secondary pathophysiological processes.
Blood-based biomarkers for acute brain damage like the three NFH phosphoforms and calpain-cleaved α-spectrin NTF described in the present study might have clinical applications beyond those feasible with CSF, including the vast majority of cases of TBI, stroke, and cardiac arrest with resuscitation, from whom CSF is not obtained routinely in the course of patient care. There are also opportunities for applying blood markers to preclinical studies of the onset, evolution, mechanisms, and treatment of TBI-induced brain damage in living animals. Heretofore, measures of brain dysfunction have been restricted predominantly to behavioral studies, which reflect in complex and indirect ways the underlying pathophysiological processes, or to post-mortem histopathological and neurochemical analyses, which provide only limited information on brain mechanisms contributing to the onset and progression of damage and dysfunction.
Given that all four of the proteins evaluated in both CSF and serum in the present study increase markedly following severe TBI in humans, we expect that additional members of our panel also will serve as serum markers for acute brain damage. Furthermore, there are still more neuron-enriched proteins that have the potential to serve as biomarkers and could be added to the current panel. The 11 proteins examined herein are only a fraction of those proteins released preferentially from dying neurons (Siman et al., 2004), and this pool likely contains additional candidate biomarkers that are expressed abundantly and preferentially in neurons. Nevertheless, significant issues need to be addressed before clinical application of members of this biomarker panel may be considered. Most importantly, it has yet to be established whether relationships exist between alterations our biomarker panel and the early detection, the mechanistic evaluation, and the short- and long-term prognosis of human TBI. Additional future studies must address whether members of this protein panel respond to interventions that normalize brain physiology and elicit neuroprotection, and so might serve as pharmacodynamic markers for human TBI. Simultaneous measures of CSF and blood biomarker alterations might be informative on the time course and severity of blood-brain barrier dysfunction following injury, but will require study of biomarker kinetics and turnover in these distinct compartments. The clinical validation and implementation of a large biomarker panel will be challenging. Analyses of a multiplicity of markers over several time points offers a huge number of variables, and considerable further research will be required to identify constellations of biomarker measures that provide clinically useful information for directing patient management and care. Finally, the development and optimization of a multiplex immunoassay method for the simultaneous measure of this biomarker panel may facilitate clinical utility by fostering implementation of the panel across multiple laboratories and hospitals.
Acknowledgments
This research was supported by the National Institutes of Health (grant NS048234 to R.S.).
Author Disclosure Statement
No conflicting financial interests exist.
References
- Agren-Wilsson A. Lekman A. Sjöberg W. Rosengren L. Blennow K. Bergenheim A.T. Malm J. CSF biomarkers in the evaluation of idiopathic normal pressure hydrocephalus. Acta Neurol. Scand. 2007;116:333–339. doi: 10.1111/j.1600-0404.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- Aitken A. Jones D. Soneji Y. Howell S. 14-3-3 proteins: biological function and domain structure. Biochem. Soc. Trans. 2003;23:605–611. doi: 10.1042/bst0230605. [DOI] [PubMed] [Google Scholar]
- Anderson K.J. Scheff S.W. Miller K.M. Roberts K.N. Gilmer L.K. Yang C. Shaw G. The phosphorylated axonal form of the neurofilament subunit NF-H (pNF-H) as a blood biomarker of traumatic brain injury. J. Neurotrauma. 2008;25:1079–1085. doi: 10.1089/neu.2007.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.E. Hansson L.O. Nilsson O. Dijlai-Merzoug R. Settergren G. High serum S100β levels for trauma patients without head injuries. Neurosurgery. 2001;48:1255–1258. doi: 10.1097/00006123-200106000-00012. [DOI] [PubMed] [Google Scholar]
- Begaz T. Kyriacou D.N. Segal J. Bazarian J.J. Serum biochemical markers for post-concussion syndrome in patients with mild traumatic brain injury. J. Neurotrauma. 2006;23:1201–1210. doi: 10.1089/neu.2006.23.1201. [DOI] [PubMed] [Google Scholar]
- Chen X.H. Meaney D.F. Xu B.N. Nonaka M. McIntosh T.K. Wolf J.A. Saatman K.E. Smith D.H. Evolution of neurofilament subtype accumulation in axons following diffuse brain injury in the pig. J. Neuropathol. Exp. Neurol. 1999;58:588–596. doi: 10.1097/00005072-199906000-00003. [DOI] [PubMed] [Google Scholar]
- Day I.N. Enolases and PGP9.5 as tissue-specific markers. Biochem. Soc. Trans. 1992;20:637–642. doi: 10.1042/bst0200637. [DOI] [PubMed] [Google Scholar]
- Fassbender K. Schmidt R. Schreiner A. Fatar M. Muhlhauser F. Daffertshofer M. Hennerici M. Leakage of brain-originated proteins in peripheral blood: temporal profile and diagnostic value in early ischemic stroke. J. Neurol. Sci. 1997;148:101–105. doi: 10.1016/s0022-510x(96)05351-8. [DOI] [PubMed] [Google Scholar]
- Foussas S.G. Zairis M.N. Makrygiannis S.S. Manousakis S.J. Anastassiadis F.A. Apostolatos C.S. Patsourakos N.G. Glyptis M.P. Papadopoulos J.K. Xenos D.C. Adamopoulou E.N. Olympios C.D. Argyrakis S.K. The significance of circulating levels of both cardiac troponin I and high-sensitivity C reactive protein for the prediction of intravenous thrombolysis outcome in patients with ST-segment elevation myocardial infarction. Heart. 2007;93:952–956. doi: 10.1136/hrt.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloeckner S.F. Meyne F. Wagner F. Heinemann U. Krasnianski A. Meissner B. Zerr I. Quantitative analysis of transthyretin, tau and amyloid-beta in patients with dementia. J. Alzheimers Dis. 2008;14:17–25. doi: 10.3233/jad-2008-14102. [DOI] [PubMed] [Google Scholar]
- Goodman S.R. Zagon I.S. Riederer B.M. Spectrin isoforms in mammalian brain. Brain Res. Bull. 1987;18:787–792. doi: 10.1016/0361-9230(87)90217-6. [DOI] [PubMed] [Google Scholar]
- Grubb N.R. Simpson C. Sherwood R.A. Abraha H.D. Cobbe S.M. O'Carroll R.E. Deary I. Fox KA. Prediction of cognitive dysfunction after resuscitation from out-of-hospital cardiac arrest using serum neuron-specific enolase and protein S-100. Heart. 2007;93:1268–1273. doi: 10.1136/hrt.2006.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood S.M. Yaqoob M.M. Allen D.A. Caspase and calpain function in cell death: bridging the gap between apoptosis and necrosis. Ann. Clin. Biochem. 2005;42:415–431. doi: 10.1258/000456305774538238. [DOI] [PubMed] [Google Scholar]
- Hasselblatt M. Mooren F.C. von Ahsen N. Keyvani K. Fromme A. Schwarze-Eicker K. Senner V. Paulus W. Serum S100beta increases in marathon runners reflect extracranial release rather than glial damage. Neurology. 2004;62:1634–1636. doi: 10.1212/01.wnl.0000123092.97047.b1. [DOI] [PubMed] [Google Scholar]
- Herrmann M. Jost S. Kutz S. Ebert A.D. Kratz T. Wunderlich M.T. Synowitz H. Temporal profile of release of neurobiochemical markers of brain damage after traumatic brain injury is associated with intracranial pathology as demonstrated in cranial computerized tomography. J. Neurotrauma. 2000;17:113–122. doi: 10.1089/neu.2000.17.113. [DOI] [PubMed] [Google Scholar]
- Jauch E.C. Lindsell C. Broderick J. Fagan S.C. Tilley B.C. Levine S.R. NINDS rt-PA Stroke Study Group. Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke. 2006;37:2508–2513. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- Kleine T.O. Benes L. Zofel P. Studies of the brain specificity of S100β and neuron-specific enolase (NSE) in blood serum of acute care patients. Brain Res. Bull. 2003;61:265–279. doi: 10.1016/s0361-9230(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Konstantinopoulos P.A. Spentzos D. Cannistra S.A. Gene-expression profiling in epithelial ovarian cancer. Nat. Clin. Pract. Oncol. 2008;5:577–587. doi: 10.1038/ncponc1178. [DOI] [PubMed] [Google Scholar]
- Lawrence E.J. Dentcheva E. Curtis K.M. Roberts V.L. Siman R. Neumar R.W. Neuroprotection with delayed initiation of prolonged hypothermia after in vitro transient global brain ischemia. Resuscitation. 2005;64:383–388. doi: 10.1016/j.resuscitation.2004.07.016. [DOI] [PubMed] [Google Scholar]
- McKeating E. Andrews P. Mascia L. Relationship of neuron specific enolase and S100 concentrations in systemic and jugular venous system to injury severity and outcome after traumatic brain injury. Acta Neurochir. 1998;71:117–119. doi: 10.1007/978-3-7091-6475-4_35. [DOI] [PubMed] [Google Scholar]
- Morrow D.A. Cannon C.P. Jesse R.L. Newby L.K. Ravkilde J. Storrow A.B. Wu A.H. Christenson R.H. National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115:e356–e375. doi: 10.1161/CIRCULATIONAHA.107.182882. [DOI] [PubMed] [Google Scholar]
- Mussack T. Biberthaler P. Kanz K.-G. Wiedemann E. Gippner-Steppert C. Mutschler W. Jochum M. Serum S100β and interleukin-8 as predictive markers for comparative neurologic outcome analysis of patients after cardiac arrest and severe traumatic brain injury. Crit. Care Med. 2002;30:2669–2674. doi: 10.1097/00003246-200212000-00010. [DOI] [PubMed] [Google Scholar]
- Nylen K. Ost M. Csajbok L.Z. Nilsson I. Hall C. Blennow K. Nellgård B. Rosengren L. Serum levels of S100B, S100A1B and S100BB are all related to outcome after severe traumatic brain injury. Acta Neurochir. 2008;150:221–227. doi: 10.1007/s00701-007-1489-2. [DOI] [PubMed] [Google Scholar]
- Petzold A. Keir G. Green A.J. Giovannoni G. Thompson E.J. A specific ELISA for measuring neurofilament heavy chain phosphoforms. J. Immunol. Methods. 2003;278:179–190. doi: 10.1016/s0022-1759(03)00189-3. [DOI] [PubMed] [Google Scholar]
- Petzold A. Eikelenboom M.J. Keir G. Grant D. Lazeron R.H. Polman C.H. Uitdehaag B.M. Thompson E.J. Giovannoni G. Axonal damage accumulates in the progressive phase of multiple sclerosis: three year follow up study. J. Neurol. Neurosurg. Psychiatry. 2005;76:206–211. doi: 10.1136/jnnp.2004.043315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold A. Keir G. Kay A. Kerr M. Thompson E.J. Axonal damage and outcome in subarachnoid hemorrhage. J. Neurol. Neurosurg. Psychiatry. 2006;77:753–759. doi: 10.1136/jnnp.2005.085175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike B.R. Flint J. Dutta S. Johnson E. Wang K.K. Hayes R.L. Accumulation of non-erythroid alpha II-spectrin and calpain-cleaved alpha II-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J. Neurochem. 2001;78:1297–1306. doi: 10.1046/j.1471-4159.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- Pineda J.A. Lewis S.B. Valadka A.B. Papa L. Hannay H.J. Heaton S.C. Demery J.A. Liu M.C. Aikman J.M. Akle V. Brophy G.M. Tepas J.J. Wang K.K. Robertson C.S. Hayes R.L. Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J. Neurotrauma. 2007;24:354–366. doi: 10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- Raghupathi R. Graham D.I. McIntosh T.K. Apoptosis after traumatic brain injury. J. Neurotrauma. 2000;17:927–938. doi: 10.1089/neu.2000.17.927. [DOI] [PubMed] [Google Scholar]
- Roberts-Lewis J.M. Siman R. Spectrin proteolysis in the hippocampus: a biochemical marker for neuronal injury and neuroprotection. Ann. N. Y. Acad. Sci. 1993;679:78–86. doi: 10.1111/j.1749-6632.1993.tb18290.x. [DOI] [PubMed] [Google Scholar]
- Roberts-Lewis J.M. Savage M.J. Marcy V.R. Pinsker L.R. Siman R. Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J. Neurosci. 1994;14:3934–3944. doi: 10.1523/JNEUROSCI.14-06-03934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H. Karlsson J.E. Rosengren L. CSF levels of neurofilament is a valuable predictor of long-term outcome after cardiac arrest. J. Neurol. Sci. 2004;221:19–24. doi: 10.1016/j.jns.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Ross J.S. Hatzis C. Symmans W.F. Pusztai L. Hortobágyi G.N. Commercialized multigene predictors of clinical outcome for breast cancer. Oncologist. 2008;13:477–493. doi: 10.1634/theoncologist.2007-0248. [DOI] [PubMed] [Google Scholar]
- Routsi C. Stamataki E. Nanas S. Psachoulia C. Stathopoulos A. Koroneos A. Zervou M. Jullien G. Roussos C. Increased levels of serum S100B protein in critically ill patients without brain injury. Shock. 2006;26:20–24. doi: 10.1097/01.shk.0000209546.06801.d7. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Bozyczko-Coyne D. Marcy V. Siman R. McIntosh T.K. Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J. Neuropathol. Exp. Neurol. 1996;55:850–860. doi: 10.1097/00005072-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Siman R. Baudry M. Lynch G. Brain fodrin: substrate for calpain I, an endogenous calcium-activated protease. Proc. Natl. Acad. Sci. USA. 1984;81:3572–3576. doi: 10.1073/pnas.81.11.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G. Yang C. Ellis R. Anderson K. Parker Mickle J. Scheff S. Pike B. Anderson D.K. Howland D.R. Hyperphosphorylated neurofilament NF-H is a serum biomarker of axonal injury. Biochem. Biophys. Res. Commun. 2005;336:1268–1277. doi: 10.1016/j.bbrc.2005.08.252. [DOI] [PubMed] [Google Scholar]
- Siman R. Noszek J.C. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988;1:279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Siman R. Noszek J.C. Kegerise C. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J. Neurosci. 1989;9:1579–1590. doi: 10.1523/JNEUROSCI.09-05-01579.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R. Flood D.G. Thinakaran G. Neumar R.W. Endoplasmic reticulum stress-induced cysteine protease activation in cortical neurons: effect of an Alzheimer's disease-linked presenilin-1 knock-in mutation. J. Biol. Chem. 2001;276:44736–44743. doi: 10.1074/jbc.M104092200. [DOI] [PubMed] [Google Scholar]
- Siman R. McIntosh T.K. Soltesz K.M. Chen Z. Neumar R.W. Roberts V.L. Proteins released from degenerating neurons are surrogate markers for acute brain damage. Neurobiol. Dis. 2004;16:311–320. doi: 10.1016/j.nbd.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Siman R. Zhang C. Roberts V.L. Pitts-Kiefer A. Neumar R.W. Novel surrogate markers for acute brain damage: cerebrospinal fluid levels correlate with severity of ischemic neurodegeneration in the rat. J. Cereb. Blood Flow Metab. 2005;25:1433–1444. doi: 10.1038/sj.jcbfm.9600138. [DOI] [PubMed] [Google Scholar]
- Siman R. Roberts V.L. McNeil E. Dang A. Bavaria J.E. Ramchandren S. McGarvey M. Biomarker evidence for mild central nervous system injury after surgically-induced circulation arrest. Brain Res. 2008;1213:1–11. doi: 10.1016/j.brainres.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L.A. Sternberger N.H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc. Natl. Acad. Sci. USA. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y. Marumo T. Omura T. Yoshida S. Serum S100beta indicates successful combination treatment with recombinant tissue plasminogen activator and MK-801 in a rat model of embolic stroke. Brain Res. 2007;1154:194–199. doi: 10.1016/j.brainres.2007.03.085. [DOI] [PubMed] [Google Scholar]
- Teepker M. Munk K. Mylius V. Haag A. Moller J.C. Oertel W.H. Schepelmann K. Serum concentrations of S100beta and NSE in migraine. Headache. 2008. (in press). [DOI] [PubMed]
- Vos P.E. Lamers K.J. Hendriks J.C. van Haaren M. Beems T. Zimmerman C. van Geel W. de Reus H. Biert J. Verbeek M.M. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004;62:303–310. doi: 10.1212/01.wnl.0000120550.00643.dc. [DOI] [PubMed] [Google Scholar]
- Xu J. Ziemnicka D. Scalia J. Kotula L. Monoclonal antibodies to alphaI spectrin Src homology 3 domain associate with macropinocytic vesicles in nonerythroid cells. Brain Res. 2001;898:171–177. doi: 10.1016/s0006-8993(01)02156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.K. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson K.D. Lee K.M. Deshpande S. Duerksen-Hughes P. Boss J.M. Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- Williams R.C., Jr. Runge M.S. Biochemistry and structure of mammalian neurofilaments. Cell Muscle Motil. 1983;3:41–56. doi: 10.1007/978-1-4615-9296-9_2. [DOI] [PubMed] [Google Scholar]
- Wunderlich M.T. Ebert A.D. Kratz T. Goertler M. Jost S. Herrmann M. Early neurobehavioral outcome after stroke is related to release of neurobiochemical markers of brain damage. Stroke. 1999;30:1190–1195. doi: 10.1161/01.str.30.6.1190. [DOI] [PubMed] [Google Scholar]
- Zemlan F.P. Rosenberg W.S. Luebbe P.A. Campbell T.A. Dean G.E. Weiner N.E. Cohen J.A. Rudick R.A. Woo D. Quantification of axonal damage in traumatic brain injury: affinity purification and characterization of cerebrospinal fluid tau protein. J. Neurochem. 1999;72:741–750. doi: 10.1046/j.1471-4159.1999.0720741.x. [DOI] [PubMed] [Google Scholar]
- Zemlan F.P. Jauch E.C. Mulchahey J.J. Gabbita S.P. Rosenberg W.S. Speciale S.G. Zuccarello M. C-tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain Res. 2002;947:131–139. doi: 10.1016/s0006-8993(02)02920-7. [DOI] [PubMed] [Google Scholar]
- Zhang C. Siman R. Xu Y.A. Mills A.M. Frederick J.R. Neumar R.W. Comparison of calpain and caspase activities in the adult rat brain after transient forebrain ischemia. Neurobiol. Dis. 2002;10:289–305. doi: 10.1006/nbdi.2002.0526. [DOI] [PubMed] [Google Scholar]