Abstract
The blood-brain barrier (BBB), which prevents the entry into the central nervous system (CNS) of most water-soluble molecules over 500 Da, is often disrupted after trauma. Post-traumatic BBB disruption may have important implications for prognosis and therapy. Assessment of BBB status is not routine in clinical practice because available techniques are invasive. The gold-standard measure, the cerebrospinal fluide (CSF)-serum albumin quotient (QA), requires the measurement of albumin in CSF and serum collected contemporaneously. Accurate, less invasive techniques are necessary. The objective of this study was to evaluate the relationship between QA and serum concentrations of monomeric transthyretin (TTR) or S100B. Nine subjects with severe traumatic brain injury (TBI; Glasgow Coma Scale [GCS] score ≤ 8) and 11 subjects with non-traumatic headache who had CSF collected by ventriculostomy or lumbar puncture (LP) were enrolled. Serum and CSF were collected at the time of LP for headache subjects and at 12, 24, and 48 h after ventriculostomy for TBI subjects. The QA was calculated for all time points at which paired CSF and serum samples were available. Serum S100B and TTR levels were also measured. Pearson's correlation coefficient and area under the receiver operating characteristic (ROC) curve were used to determine the relationship between the serum proteins and QA. Seven TBI subjects had abnormal QA's indicating BBB dysfunction. The remaining TBI and control subjects had normal BBB function. No significant relationship between TTR and QA was found. A statistically significant linear correlation between serum S100B and QA was present (r = 0.432, p = 0.02). ROC analysis demonstrated a significant relationship between QA and serum S100B concentrations at 12 h after TBI (AUC = 0.800; SE 0.147, 95% CI 0.511–1.089). Using an S100B concentration cutoff of 0.027 ng/ml, specificity for abnormal QA was 90% or higher at each time point. We conclude that serum S100B concentrations accurately indicate BBB dysfunction at 12 h after TBI.
Key words: biomarkers, blood-brain barrier dysfunction, traumatic brain injury
Introduction
Approximately 170,000 Americans suffer a moderate-severe traumatic brain injury (TBI) each year (Jager et al., 2000). Despite advances in early detection and surgical management, the mortality and resulting disability has improved little in the last 20 years (Masson et al., 2001). The observation of a time interval between injury, and irreversible brain swelling and death had raised hopes that pharmacologic interventions could prevent or limit this process and positively affect outcome. Multiple drug studies, however, have failed to show improvement (Narayan et al., 2002; Langham et al., 2003; Roberts et al., 2004; Alderson and Roberts, 2005). Careful analysis of these studies has suggested several ways in which future trials might have more success (Ikonomidou and Turski, 2002; Narayan et al., 2002).
The functional status of the blood-brain barrier (BBB) plays an important role in any pharmacologic treatment of TBI. The BBB consists of a single layer of brain capillary endothelium joined together by tight junctions (zonula occludens). Astrocytic foot processes make a substantial contribution to BBB integrity, covering more than 90% of the basement membrane of these capillaries (Bickel, 2001). This barrier prevents diffusion of most water-soluble molecules over 500 Da. A damaged, and thus open, BBB can facilitate both brain injury diagnosis and pharmacologic treatment. When the BBB is open, brain-related proteins such as S100B can enter the peripheral circulation where they can be measured, aiding in brain injury diagnosis (Raabe et al., 1998, 1999a, 2003; Dimopoulou et al., 2003). More importantly, if the BBB is opened after TBI, intravenously administered pharmacologic treatments would have the opportunity to reach the brain. Knowing in which patient and at what time the BBB is open will be key to the success of future pharmacologic studies.
The anatomical characteristics of the BBB and currently available technology limit the effectiveness of imaging for determination of BBB function. Brain endothelial capillaries average 7–12 μm in diameter and taken together have a surface area of approximately 20 m2 (Pardridge, 1997). Available imaging techniques for human research or clinical care lack the combination of very high-resolution and massive data processing capacity necessary to visualize this structure. Consequently, functional assessment of BBB status by calculation of the cerebrospinal fluid (CSF)-serum albumin quotient (QA) is widely accepted as the gold standard for BBB permeability (Andersson et al., 1994; Reiber and Peter, 2001). Albumin is an optimal candidate for measurement of BBB function, because it is synthesized peripherally, is not catabolized within the central nervous system (CNS), and does not readily diffuse across an intact BBB (Reiber and Peter, 2001). The effectiveness of QA for measurement of BBB function has been demonstrated by studies with radiolabeled albumin (Tourtellotte et al., 1980).
Calculation of QA requires the measurement of the albumin concentrations in serum and CSF collected simultaneously and is calculated as follows: QA = [albuminCSF]/[albuminserum] (Link and Tibbling, 1977; Tibbling et al., 1977). Repeated QA measurement from a single patient typically requires the placement of a ventriculostomy catheter for frequent sampling of CSF. Normal BBB permeability in adults is defined as a QA ≤ 0.007, and a damaged or open BBB is defined as mild (QA = 0.007–0.01), moderate (QA = 0.01–0.02), and severe (QA ≥ 0.02), respectively (Reiber and Felgenhauer, 1987; Stahel et al., 2001). These normal values were established by study of the QA in 396 patients with a normal BBB function or a pure BBB impairment without a CNS humoral immune response (Reiber and Felgenhauer, 1987; Stahel et al., 2001). Using QA as a measure of BBB permeability, several authors have detected BBB damage after moderate to severe TBI. With a QA value of 0.007 as upper limit of normal BBB permeability (Reiber and Felgenhauer, 1987), 61–90% of patients were found to have BBB damage (Pleines et al., 1998; Csuka E, 1999; Morganti-Kossmann et al., 1999; Maier et al., 2001; Stahel et al., 2001). The duration of BBB disruption varied from 30 min to 30 days, with the average duration being 7.2 days (Stahel et al., 2001).
The requirement for CSF to determine the QA limits its use both clinically and for research. A less invasive method for determination of BBB status such as a serum marker is necessary. Recently, the 14-kDa monomeric form of transthyretin (TTR) was identified as a non-invasive marker of blood-CSF barrier damage (Marchi et al., 2003a). This protein was identified via a proteomic analysis of serum from patients undergoing iatrogenic opening of their BBB and blood-CSF barrier by intra-arterial mannitol for treatment of CNS lymphoma. Monomeric TTR was identified in serum within minutes of the administration of mannitol, but was not present before barrier disruption or after barrier integrity was reestablished (Marchi et al., 2003a). Monomeric TTR is the major protein product of the choroid plexus constituting about 20% of the total amount of protein in the CSF and is normally absent in peripheral blood (Hamilton and Benson, 2001).
The measurement of serum concentrations of the astrocytic protein S100B has also been touted as a measure of BBB damage (Kapural et al., 2002; Marchi et al., 2003b). Elevated serum S100B concentrations were correlated with both mannitol-induced osmotic disruption (Kapural et al., 2002; Kanner et al., 2003; Marchi et al., 2003b) and physical disruption by fungal hyphae (Bertsch et al., 2001) of the BBB. Subsequently, several studies have used S100B as a non-invasive marker of BBB integrity. Elevations in serum S100B have been cited as evidence for BBB disruption in seizures (Marchi et al., 2007), exercise and hyperthermia (Watson et al., 2005, 2006), sepsis (Larsson et al., 2005), and brain ischemia during carotid artery cross clamping for carotid endarterctomy (Jaranyi et al., 2003).
While non-invasive, it is unclear whether serum TTR or S100B can replace the QA. The evidence for these proteins as serum biomarkers of BBB integrity is correlative, and characterization of their sensitivity and specificity relative to a gold standard test of BBB integrity has not been reported to date. This limits the usefulness of these markers in several ways. The ability of TTR to detect BBB damage in moderate to severe TBI patients has not been tested. In addition, it is unclear if TTR can detect subtle disruptions in BBB integrity. While serum S100B has been correlated with BBB disruption, it may also rise due to parenchymal brain injury or extra-cranial injuries. Indeed, serum S100B concentrations have been reported to rise in the setting of boney fractures, lacerations, melanoma, mania associated with bipolar disorder, schizophrenia, multiple sclerosis, human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS), and strenuous physical activity (Anderson et al., 2001a; Wiesmann et al., 1999; Adami et al., 2001; Stalnacke et al., 2003).
In the current study, we measured serum S100B and monomeric TTR levels in a cohort of moderate to severe TBI patients who had a ventriculostomy catheter placed as part of their clinical care and in adult emergency department (ED) patients with benign headache who had a lumbar puncture. Albumin concentrations in paired samples of CSF and serum from these patients were also measured and the CSF-serum QA calculated. We report here the relationship between serum levels of these two putative biomarkers of BBB dysfunction and the gold standard CSF-serum QA.
Methods
Subjects
A prospective convenience sample of adult patients presenting to the ED of an academic medical and level I trauma center (University of Rochester Medical Center [URMC]) were enrolled. Patients were enrolled into two groups, one presenting with TBI requiring ventriculostomy catheter placement (n = 10) and the other presenting with headache (n = 10). A third group of normal, healthy control patients (n = 10) was also enrolled. TBI subjects were eligible for inclusion if they were ≥18 years old, had a history or physical exam evidence of blunt, non-penetrating head trauma, and had a ventriculostomy catheter placed as part of clinical care within 12 h of injury. Ventriculostomy catheters are typically placed to monitor intracerebral pressure in patients with severe TBI (Glasgow Coma Scale [GCS] score ≤8) and an intracranial injury on computed tomography (CT) scan. However, these catheters are also placed in those with severe TBI and a normal CT if two or more of the following factors are present: age >40, unilateral/bilateral motor posturing, and systolic blood pressure (SBP) < 90 mm Hg (Bratton et al., 2007). In some cases, subjects with initial GCS scores above 8 received ventriculostomy catheters because of subsequent clinical deterioration and were included in the study. Study subjects were identified in the ED or intensive care unit (ICU), and informed consent was obtained from a proxy prior to enrollment in the study. Subjects with TBI were excluded from the study for the following criteria: age < 18 years, penetrating head trauma, diagnosis of TBI not clear, patient expected to die within 12 h, ventriculostomy catheter placed >12 h after injury, prisoners, and wards of the state.
Subjects were eligible for inclusion as a headache subject if they were ≥18 years old, presented to the ED with a chief complaint of headache, had spare blood drawn as part of their clinical care, had a normal head CT, and had a lumbar puncture (LP) with normal results performed as part of their clinical care. A normal LP was defined as CSF pressure (if measured) of 50–180 mm H2O, glucose of 40–85 mg/dL, total protein of 15–50 mg/dL, total nucleated cells less than 5 per mL, and red blood cells less than 50/mm2 in tube 4. Headache subjects were excluded from the study for the following criteria: age less than 18 years, head trauma within last month requiring ED visit, blood not drawn as part of clinical care, or abnormal LP. Informed consent was obtained from all headache patients prior to inclusion in the study.
Subjects were eligible to serve as normal, healthy controls if they were ≥18 and ≤65 years old. Patients were excluded if they had a condition known to affect the BBB or serum levels of S100B. These include the following: history of migraine headaches, stroke, age over 65 years, brain tumor, melanoma, bipolar disorder, schizophrenia, multiple sclerosis, HIV/AIDS, TBI requiring ED visit; strenuous physical activity within the last 24 h; surgery or laceration repair within the last month; boney fracture or laceration within the last month. Informed consent was obtained from all control patients before inclusion in the study. This study was approved by the URMC Research Subjects Review Board.
Sample collection and processing
Headache and normal control subjects had one blood draw each: just prior to LP and at the time of enrollment into the study, respectively. TBI subjects had blood draws at four time points: on ED presentation before the ventriculostomy was placed, just after insertion of the ventriculostomy catheter within 12 h of injury, at 24 h after injury, and at 48 h after injury. For all blood draws, 4 ml of venous or arterial blood were drawn into serum separator tubes and centrifuged at 3000 rpm for 10 min at room temperature. The cellular components were discarded and the serum stored at −80°C until used for assays.
CSF was collected at the time of LP for headache subjects. In TBI subjects, CSF was obtained at the same time as serum: at 12, 24, and 48 h after injury. For each CSF sample, 5–10 ml of CSF was collected into a 15-ml polyproylene tube, immediately placed on ice and centrifuged at 3000 rpm for 10 min at room temperature. The cellular components were discarded and the remaining sample stored at −80°C until used for assays.
Albumin measurements and calculation of the CSF/serum albumin quotient
Serum albumin concentrations were measured in the URMC Clinical Laboratory with the bromocresol green dye-binding method. CSF albumin concentrations were measured by nephelometry at a national reference laboratory (ARUP Laboratories, Salt Lake City, UT). The albumin quotient (QA) was calculated using the following formula: [albuminCSF]/[albuminserum].
Transthyretin measurement
Measurements of monomeric serum TTR were made following the methods of Marchi et al. (2003a). Serum samples were loaded into Centricon YM50 filter units (Millipore, Billerica, MA) and centrifuged to concentrate the low molecular weight fraction. Protein concentrations for the low molecular weight (<50 kDa) fractions were determined with the Biorad DC Protein Assay kit (Hercules, CA). Proteins in the concentrated samples were loaded onto a large-format 12% polyacrylamide gel and separated by electrophoresis under non-denaturing conditions. A total of 12.5 μg of protein from each sample was loaded. Each gel included a lane loaded with 5 ng of purified TTR (Sigma-Aldrich, St. Louis, MO) as a positive control. After electorphoresis, proteins were transferred onto polyvinyl difluoride (PVDF) membranes by electroblotting overnight at 4°C. Blots were blocked with 5% nonfat dry milk in TBST (20 mM Tris, 500 NaCl, 0.1%, Tween-20) for 1 h, and then washed three times in TBST. Following washes, the membranes were incubated with primary antibody to TTR (Dakocytomation, Carpinteria, CA) at a 1:1000 dilution for 1 h at room temperature. After exposure to primary antibody, blots were rinsed as above and incubated for 1 h with secondary goat anti-rabbit antibody conjugated to horseradish peroxidase (Jackson Immuno Research, West Grove, PA) at a 1:2000 dilution. Chemiluminescence was detected with the Western Lightning Chemiluminescence Reagent Plus detection system (Perkin Elmer, Waltham, MA). Optical densities of the TTR bands were measured with Biorad Microplate Reader 3 (Hercules, CA). For each gel, quantification of band optical densities were determined by comparison with the optical density measured from the purified TTR protein band of known concentration.
S100B measurement
Serum S100B concentrations were measured with the Nexus Dx S100B test kit (Nanogen, San Diego, CA). This detection kit employs the sandwich enzyme-linked immunosorbent assay (ELISA) technique and consists of a 96-well plate coated with an S100B capture antibody. Samples, controls, and standards were loaded into appropriate wells of the plate followed by the addition of an S100B detecting antibody. Finally, enzyme substrate was added, and the resulting absorbance of the samples at 450 nm was measured. S100B concentrations in the serum samples were determined using the standard curve generated from the absorbance of the standards. The lower detection limit of this assay is 0.1 ng/ml.
Statistical analysis
The statistical analysis was conducted using SPSS (SPSS Inc., Chicago, IL) and R (open source software). Student's t-test was used to compare mean QA's, TTR concentrations, or S100B concentrations between two groups. The analysis of variance (ANOVA) was used to compare mean QA's, TTR concentrations, or S100B concentrations amongst three or more groups. Significance was defined as p ≤ 0.05.
To assess the accuracy of serum S100B and TTR concentrations for detecting BBB disruption in headache and TBI subjects, receiver operating characteristic (ROC) curves were constructed for each TBI time point as well as for the combined data from all three TBI time points. In each of these ROC curves, headache data was also included. For the purpose of this analysis, the QA was dichotomized into normal (≤0.007) and abnormal (>0.007). The ROC curve plots the sensitivity of a measure on the y-axis and (1-sensitivity) on the x-axis, and measures the overall accuracy of a test. The most important summary index of the ROC curve is the area under the ROC curve (AUC). An ROC curve with AUC >0.5 means the test is informative in the sense that it is better than classifying subjects in completely random fashion. In general, the closer the curve to the upper-left corner (point (0, 1)), the bigger the AUC and the more accurate the test. For each possible threshold based on the sample, we computed the estimates of the corresponding sensitivity and specificity.
For each of the ROC curve estimates (based on the empirical estimates of the sensitivities and specificities at the observed test levels), the AUC and the associated standard error were estimated. For the combined analysis, correlation between values from the same subject at different time points was considered. Methods developed by Obuchowski (1997), which addresses such within-subject correlations, were used. These methods better estimate the accuracy of within-subject measurements and result in smaller confidence intervals without altering the AUC.
Results
Clinical characteristics of study subjects
Ten subjects were enrolled in each of the study groups. The TBI group consisted of eight males and two females, with a mean age of 50.7 years (range, 39–63 years). The headache group consisted of five males and five females, with a mean age of 40.6 years (range, 26–77 years). The healthy control group consisted of six males and four females, with a mean age of 34.0 years (range, 24–43 years).
Eight of the TBI subjects enrolled in the study were intubated at the time of presentation. In the study region, patients with GCS ≤8 are often intubated in the field for airway protection. Two subjects (TBI 4 and TBI 9) with high initial GCS scores rapidly decompensated after arrival. Contusion was evident on CT scan for six of 10 patients. Five of 10 had subarachnoid blood, while three of 10 had subdural or epidural bleeding. Table 1 summarizes the clinical characteristics of the TBI subjects enrolled in the study.
Table 1.
Clinical Characteristics of TBI Subjects
| Age | Sex | Mechanism | GCS | Head CT results | Extra cranial injuries | |
|---|---|---|---|---|---|---|
| TBI 1 | 53 | M | Fall | 3T | CONT, SAH | None |
| TBI 2 | 46 | F | MVA | 6T | SAH, IVH, IPH, CONT | Skull Fx, L1-L4 Fx, pelvic Fx |
| TBI 3 | 56 | F | Pedestrian struck | 4T | SAH, IPH, IV | Facial Fx, C3-C5 cord cont |
| TBI 4 | 44 | M | Assault | 15 | EDH, SDH, SAH, CONT | Forearm Fx, C5-C7 cord cont |
| TBI 5 | 63 | M | MVA | 3T | CONT, SAH | Skull Fx, rib Fx |
| TBI 6 | 50 | M | MVA | 5T | SAH, IPH | Rib Fx, PTX, liver lac |
| TBI 7 | 47 | M | MVA | 4T | CONT, SAH | Skull Fx, rib Fx, PTX, lung cont |
| TBI 8 | 57 | M | MVA | 9T/8T | IPH, SAH, SDH | L1-L2 Fx, ankle Fx |
| TBI 9 | 39 | M | Pedestrian struck | 14/8T | EDH, CONT, SDH | None |
| TBI 10 | 52 | M | MVA | not recorded | SDH, SAH | C7 Fx, humerus Fx, PTX, splenic lac |
CONT, contusion; EDH, epidural hematoma; IPH, intraparenchymal hemorrhage; MVA, motor vehicle accident; IVH, intraventricular hemorrhage; SAH, subarachnoid hemorrhage; SDH, subdural hematoma; lac, laceration; GCS, Glasgow Coma Scale; Fx, fracture; PTX, pneumothorax.
Albumin quotient
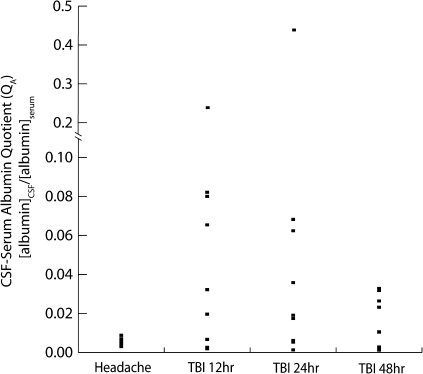
The individual QA values for TBI and headache subjects are shown in Figure 1. QA values for all headache subjects were normal, with a mean QA of 0.0055 (SD 0.0019). Seven of 10 TBI subjects had abnormal BBB function, with a mean QA for all time points of 0.049 (SD 0.0914). The QA was significantly greater in TBI than in headache subjects (p = 0.02; Student's t-test). Mean QA values at 12, 24, and 48 h after TBI were 0.0588 (SD 0.0747), 0.0727 (SD 0.1393), and 0.0156 (SD 0.013), respectively. The QA was not determined in one headache subject and in one TBI subject at 12 h (TBI 1) because a sufficient quantity of CSF was not available for analysis. For one subject (TBI 6), only a 12-h QA was measured because the patient died shortly after enrollment in the study.
FIG. 1.
Cerebrospinal fluid (CSF)/serum albumin quotients (QA) were calculated for headache and traumatic brain injury (TBI) subjects by measuring the concentrations of albumin in CSF and serum collected contemporaneously. The albumin quotient was then calculated by dividing the CSF albumin concentration by the serum albumin concentration. A QA value of 0.007 or less is considered normal. All headache subjects had normal QA values, with the exception of one whose QA was marginally elevated (0.0088 in HA 1). Seven TBI patients had abnormal QA values at all three time points (12, 24, and 48 h after TBI). The remaining three TBI subjects (TBI 4, TBI 7, and TBI 9) had normal QA values at all three time points. Mean QA values for headache subjects and for TBI subjects at 12, 24, and 48 h were 0.0055 (SD 0.0019), 0.0588 (SD 0.0747), 0.0727 (SD 0.1393), and 0.0156 (SD 0.013), respectively. The QA was significantly greater in TBI than in headache subjects (p = 0.02).
Transthyretin measurements
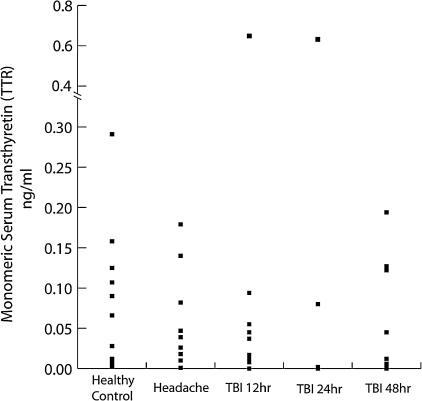
The individual TTR concentrations for all subjects are shown in Figure 2. Healthy control and headache subjects had mean TTR concentrations of 0.089 (SD 0.089) and 0.055 (SD 0.060) ng/ml, respectively. For two headache subjects, sufficient serum was not available for TTR measurement. The mean serum TTR concentration in TBI subjects for all time points was 0.083 (SD 0.154) ng/ml. Serum TTR concentrations at 12, 24, and 48 h after TBI were 0.028 (SD 0.030), 0.102 (SD 0.235), and 0.071 (SD 0.072) ng/ml, respectively. There were no significant differences between any groups (p ≤ 0.98, ANOVA). Given the exceedingly variable TTR concentrations and the lack of significant relationships between groups further analysis for correlation with QA are not shown.
FIG. 2.
The monomeric form of transthyretin (TTR) is abundantly present in cerebrospinal fluid (CSF) and is normally absent in serum. The presence of monomeric TTR in serum has been reported to be a marker for blood-brain barriet (BBB) dysfunction. Monomeric TTR was measured in the serum of healthy control, headache, and traumatic brain injury (TBI subjects) by densitometry of Western blots. TTR concentrations were quite variable, with no significant differences between groups. Normal control and headache subjects had mean TTR concentrations of 0.089 (SD 0.089) and 0.055 (SD 0.060) ng/ml, respectively. TTR concentrations at 12, 24, and 48 h after TBI were 0.028 (SD 0.030), 0.102 (SD 0.235), and 0.071 (SD 0.072) ng/ml, respectively. There were no significant differences between any groups (p ≤ 0.98, analysis of variance [ANOVA]).
Serum S100B concentrations
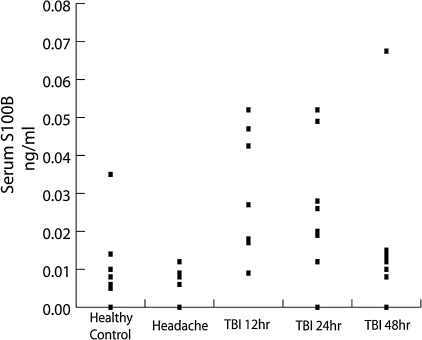
The individual S100B concentrations for all subjects are shown in Figure 3. Healthy control and headache subjects had mean serum concentrations of 0.0065 ng/ml (SD 0.0043) and 0.0089 ng/ml (SD 0.0101), respectively. There was no difference between these groups (p = 0.50). For two headache subjects, sufficient serum was not available for S100B measurement. The mean serum S100B concentration in TBI subjects for all time points was 0.0238 ng/ml (SD 0.0183). Serum S100B concentrations at 12, 24, and 48 h after TBI were 0.0304 ng/ml (SD 0.0168), 0.0258 ng/ml (SD 0.0176), and 0.0168 ng/ml (SD 0.0195), respectively. S100B values were significantly elevated relative to headache (p < 0.02) and healthy control (p < 0.04) patients at 12 and 24, but not at 48 h after TBI. To investigate the possibility that ventriculostomy placement independently results in elevated serum S100B concentrations, serum was drawn from TBI subjects at the time of their presentation to the ED prior to ventriculostomy placement. For each TBI subject, the pre-ventriculostomy S100B concentrations were greater than subsequent S100B concentrations (data not shown). S100B concentrations were not measured prior to ventriculostomy placement in one subject (TBI 4), at 12 h in one subject (TBI 1), or at 24 h for one subject (TBI 5) because insufficient serum was available for the analysis. One subject (TBI 6) died shortly after enrollment, and only pre-ventriculostomy S100B concentrations were measured.
FIG. 3.
S100B is an astrocyte protein that has been noted to be released into serum under conditions of blood-brain barriet (BBB) compromise. We measured serum S100B concentrations in the serum of health control, headache, and traumatic brain injury (TBI) subjects by enzyme-linked immunosorbent assay (ELISA). Serum S100B concentrations were elevated in TBI subjects at all time points relative to healthy control and headache patients with a general trend towards decreasing S100B concentrations as time after injury increased. Normal control and headache subjects had mean serum concentrations of 0.0065 ng/ml (SD 0.0043) and 0.0089 ng/ml (SD 0.0101), respectively. There was no difference between these groups (p = 0.50). S100B concentrations at 12, 24, and 48 h after TBI were 0.0304 ng/ml (SD 0.0168), 0.0258 ng/ml (SD 0.0176), and 0.0168 ng/ml (SD 0.0195), respectively. S100B values were significantly elevated relative to headache (p < 0.02) and healthy control (p < 0.04) patients at 12 and 24, but not 48 h after TBI.
S100B concentrations predict abnormal QA
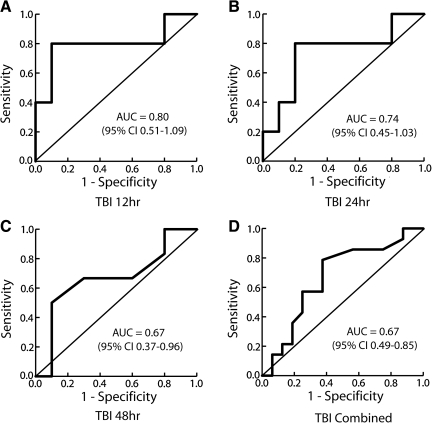
Using ROC analyses, a significant relationship was found between QA and serum S100B concentrations at 12 h after TBI (AUC = 0.800; SE 0.147, 95% confidence interval [CI] 0.511–1.089) (Fig. 5). Using a cutoff of 0.022 ng/ml, serum S100B concentration was 80% sensitive and 90% specific for abnormal QA. For the ROC curves at 24 and 48 h after TBI, AUC approached but did not reach significance (24 h: AUC = 0.740 [SE 0.150, 95% CI 0.446–1.034]; 48 h: AUC = 0.667 [SE 0.152, 95% CI 0.369–0.964]). When TBI and headache data from all time points were considered together, the relationship between S100B and QA approaches, but does not reach statistical significance (AUC 0.670; SE 0.0931, 95% CI 0.488–0.852, p = 0.0679). At a S100B concentration of 0.027 ng/ml, specificity for abnormal QA is 90% or higher at each time point (Table 2).
FIG. 5.
In order to determine the relationship between the albumin quotient (QA) and serum S100B concentrations, receiver operating characteristic (ROC) curves were constructed for each traumatic brain injury (TBI) time point as well as for all TBI time points (A–C) taken together (D). Each of these analyses includes data from the headache subjects. The combined analysis accounts for intra-patient correlation between different time points. A significant relationship was found between QA and S100B at 12, but not at 24 or 48 h, after TBI. When data from all time points was considered together, the relationship between QA and S100B approached but did not reach significance.
Table 2.
S100B Is 90% Specific for the Detection of Abnormal QA
|
TBI 12 h |
TBI 24 h |
TBI 48 h |
||||||
|---|---|---|---|---|---|---|---|---|
| S100B (ng/μl) | Sensitivity | Specificity | S100B (ng/μl | Sensitivity | Specificity | S100B (ng/μl) | Sensitivity | Specificity |
| 0 | 1.000 | 0.000 | 0.0000 | 1.000 | 0.000 | 0.0000 | 1.000 | 0.000 |
| 0.0030 | 1.000 | 0.200 | 0.0030 | 1.000 | 0.200 | 0.0030 | 1.000 | 0.200 |
| 0.0070 | 0.800 | 0.200 | 0.0070 | 0.800 | 0.200 | 0.0070 | 0.833 | 0.200 |
| 0.0085 | 0.800 | 0.400 | 0.0085 | 0.800 | 0.400 | 0.0085 | 0.677 | 0.400 |
| 0.0105 | 0.800 | 0.600 | 0.0105 | 0.800 | 0.600 | 0.0095 | 0.667 | 0.600 |
| 0.0145 | 0.800 | 0.700 | 0.0155 | 0.800 | 0.800 | 0.0110 | 0.677 | 0.700 |
| 0.0175 | 0.800 | 0.800 | 0.0195 | 0.600 | 0.800 | 0.0125 | 0.500 | 0.900 |
| 0.0220 | 0.800 | 0.900 | 0.0230 | 0.400 | 0.800 | 0.0135 | 0.333 | 0.900 |
| 0.0265 | 0.600 | 0.900 | 0.0270 | 0.400 | 0.900 | 0.0145 | 0.167 | 0.900 |
| 0.0345 | 0.400 | 0.900 | 0.0385 | 0.200 | 0.900 | 0.0395 | 0.000 | 0.900 |
| 0.0445 | 0.400 | 1.000 | 0.0505 | 0.200 | 1.000 | 0.0640 | 0.000 | 1.000 |
| 0.0495 | 0.200 | 1.000 | 0.0520 | 0.000 | 1.000 | |||
| 1.0520 | 0.000 | 1.000 | ||||||
Discussion
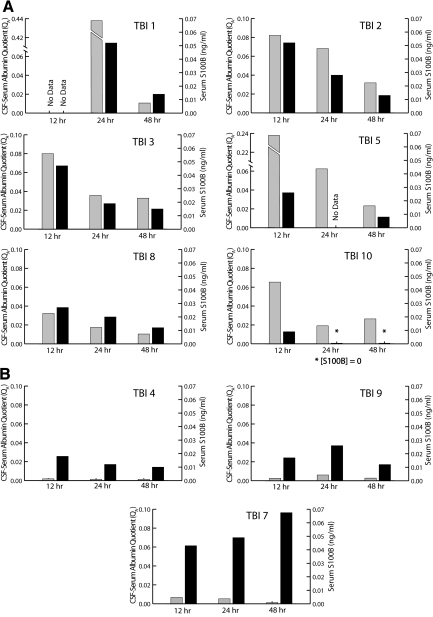
Our studies were designed to evaluate the relationship between QA, a gold standard measure of BBB function, and serum concentrations of monomeric TTR and S100B. S100B and monomeric TTR are found principally in astrocytes and CSF, respectively. Peripheral elevations of these proteins have been associated with disruption of BBB function in prior studies; however the evidence for this association is correlative (Marchi et al., 2003a,b; Bertsch et al., 2001; Kapural et al., 2002). In the current study, BBB barrier function was determined in 10 subjects with moderate to severe TBI and nine subjects with benign headache. Elevated QA, indicative of BBB dysfunction, was present in seven TBI subjects. In these subjects, QA decreased with time (Figs. 1 and 4). This temporal pattern is consistent with prior studies of QA after severe TBI (Morganti-Kossmann et al., 1999). The remaining three TBI subjects and all of the headache subjects had normal QA.
FIG. 4.
Seven traumatic brain injury (TBI) subjects had abnormal albumin quotients (QA) values. (A) For these subjects, peak S100B concentrations and (QA values occurred at the earliest time point, after which both values fell at each successive time point. Three TBI subjects had normal QA values. (B) Two of the subjects, TBI 4 and TBI 9, suffered from epidural hematoma and differed from the remaining TBI subjects in that they arrived at the emergency department lucid after which they had rapid deterioration. The third subject, TBI 7, had evidence of a stroke on CT and subsequently died before discharge from the hospital. Gray bars represent albumin quotients; black bars represent serum S100B concentrations.
Serum S100B concentrations correlated well with QA, typically declining with time in the TBI patients with abnormal BBB function (Fig. 4A). For our analysis of the relationship between S100B and QA, we constructed ROC curves at three post-TBI time points as well as a combined ROC curve using data from all time points (Fig. 5). The combined analysis also accounted for correlation within the data from each individual TBI patient. A significant area under the curve was found at 12 h after TBI. At this time point, a serum S100B cutoff of 0.022 ng/ml is 80% sensitive and 90% specific for predicting an abnormal QA. At 24 and 48 h, the area under the curve approached but did not reach statistical significance. However, at all three time points after TBI, a serum S100B concentration of greater than 0.027 ng/ml was 90% specific for elevated QA (Table 2). At the later two time points, the sensitivity for S100B was poor. We did not find a relationship between QA and monomeric TTR.
Although this study is limited by a small sample size, we believe the statistically significant relationship between 12-h QA and S100B to be a true positive finding. The probability that this result is a false positive (Type 1 error) is quite small (≤0.05) and is not related to our small sample size. We are less certain about our negative results, that is, the lack of a statistical relationship between S100B and QA at combined time points. The probability that this result is a false negative (Type 2 error) is indeed related to small sample size. With a larger sample size and more statistical power, we might have detected a statistically significant relationship.
Head CT findings from the TBI patients demonstrate typically heterogeneous injuries that include intraventricular, subarachnoid, epidural, subdural, and intraparenchymal hemorrhages as well as contusions. There was no injury type common in all patients (Table 1). Although diffuse axonal injury (DAI) is a common to all severities of TBI (Geddes et al., 2000), it was not detectable in any of the TBI subjects on head CT. Head CT is notably insensitive for the detection of DAI (Mittl et al., 1994), and it is likely that DAI was present but not detected in many of our TBI subjects. Our study design is limited in that it does not account for differences between specific sub-types of injury in the TBI subjects. Consequently, we cannot discern differences in S100B and QA that are related to specific injury patterns. Our detection of a statistically significant relationship between S100B and QA despite the heterogeneous makeup of our cohort suggests that this relationship is strong.
Interestingly, two of the three subjects with normal QA values (TBI 4 and TBI 9), presented with epidural hematoma (EDH) and a clinical course consisting of a lucid presentation followed by rapid neurological deterioration. The lucid interval commonly seen in patients with EDH may be due to a rapidly expanding extra-axial hematoma caused by arterial bleeding compressing brain that is relatively uninjured. These patients have a better prognosis than patients with other types of traumatic extra-axial bleeding (Stieg and Kase, 1998). While the two subjects with EDH in this study had other associated brain injuries identified on CT, it is possible that they did not have sufficient direct insult to the brain to cause BBB dysfunction. Although our study was not designed to differentiate between different sub-types of TBI, this finding may be an important consideration in the design of future studies that focus on specific TBI subtypes.
The third subject with normal BBB function (TBI 7) did not have EDH, and had a markedly unusual relationship between S100B and QA relative to that observed in the remaining TBI subjects (Fig. 4B). Despite normal QA values, the serum concentrations of S100B increased at each successive time point for this subject. Furthermore, S100B concentrations were very high at all time points. At 48 h, the serum concentration of S100B in this subject was sevenfold higher than in any other subject at the same time point. Review of brain imaging results showed that this subject had hypo-attenuation in the left middle cerebral artery (MCA), distribution suggesting an infarction. This finding was not evident on the initial scan but was present on a subsequent CT at 16 h later. The temporal pattern of serum S100B concentrations in patients with MCA stroke closely parallels the observed S100B concentrations in this TBI subject (Büttner et al., 1997).
Post-stroke BBB dysfunction occurs early after stroke and appears to evolve and worsen with time. BBB dysfunction is detectable as early as 3 h after stroke with the low molecular weight MRI contrast agent gadolinium (157 Da) (Hofmeijer et al., 2004), whereas albumin, which weighs 68 kDa, does not cross the BBB until 3 days after the event (Gotoh et al., 1985). S100B, which weighs 21 kDa, is intermediate in size between gadolinium and albumin, and is elevated in the systemic circulation at 6 h after stroke (Foerch et al., 2007; Wunderlich et al., 2004). It is possible that the S100B elevations seen in this subject are related to post-stroke BBB dysfunction that has not yet progressed sufficiently to allow albumin to cross into the CSF.
In the setting of trauma, late elevations in serum S100B are also associated with poor outcomes. Raabe et al. (1999b) studied 84 patients with severe TBI and report that patients with poor outcomes had higher initial S100B levels that rose substantially over time in contrast to patients with good outcome who had lower initial serum S100B levels that fell with time. Similarly, Pelinka et al. (2003b) report that non-survivors after severe TBI generally have elevated and sustained or rising plasma S100B levels lasting longer than 48 h, whereas survivors have S100B levels that fall to normal within 48 h. Within our study, two TBI subjects died as a result of their injuries. One, TBI 6, died early after enrollment and only data from the 12-h time are available. The other subject, TBI 7, did have elevated S100B concentrations that rose with time. It is possible that this late rise in S100B was due to brain trauma that was ultimately fatal.
Extra-cranial release of S100B in the setting of trauma has been identified in prior studies (Anderson et al., 2001a; Savola et al., 2004; Pelinka et al., 2003a) and may have occurred in our subjects. Significant amounts of S100B are present in bone marrow, blood, and fat (Anderson et al., 2001b). Fractures in particular are thought to be an important source of S100B after trauma. The timing of S100B release from extra-cranial tissue is variable and may depend on the specific injury. For isolated fracture in an animal model, plasma S100B was increased from 30 to 120 min following injury, after which levels were not significantly elevated above baseline (Pelinka et al., 2003a). Others note that trauma patients with fractures or other injuries in the absence of TBI have elevated S100B concentrations the day after the injury (Anderson et. al., 2001a). Eight of 10 of our TBI subjects had extra-cranial injuries (Table 1). All of the patients with extra-cranial injuries had some type of fracture. Again, the small sample size limits our ability to discern relationships between specific injuries and serum S100B concentrations. While there were no obvious differences in serum S100B profiles between the two isolated TBI and the eight multi-system trauma subjects, it remains possible that some serum S100B may have originated from extra-cranial injuries.
Other known causes of peripheral S100B elevation include Alzheimer's disease, Down's syndrome, and schizophrenia (Wiesmann et al., 1999; Adami et al., 2001). Elevated levels have also be found in healthy individuals after distance running (Otto et al., 2000; Hasselblatt et al., 2004), and after playing soccer (Stalnacke et al., 2004), basketball, or hockey (Stalnacke et al., 2003). While the control patients were screened and excluded if they had any of these conditions or recent strenuous physical activity, the headache and TBI subjects were not. In retrospect, none of these subjects had Down's syndrome, schizophrenia, or clinically diagnosed dementia, although it is possible that some may have had preclinical Alzheimer's disease. We did not collect data regarding recent strenuous physical activity in any of these subjects.
Given the concerns regarding the specificity of S100B, we also investigated the effectiveness of serum monomeric TTR for the prediction of abnormal QA. Monomeric TTR is found only in CSF and appears in serum after iatrogenic BBB disruption (Marchi et al., 2003a). We did not find a relationship between monomeric TTR and QA. Technical issues may have affected the TTR values obtained. Both serum and CSF specific forms of TTR share the same amino acid sequence and differ only in quaternary structure making the development of quantitative antibody-based tests (such as ELISA) difficult. Thus, we were limited to using the more error prone semi-quantitative technique of densitometry of Western blots. A further difficulty that may have affected the accuracy of our measurements is that the highly abundant serum tetramer may have broken down into monomer during sample processing. The development of an ELISA specific for monomeric TTR would address these technical issues. Other biomarkers with greater specificity for the CNS such as neurofilament proteins and UCHL-1 have recently been described (Anderson, 2008; Papa, .008) and could also be used in future studies of BBB dysfunction after trauma.
In summary, serum S100B is an accurate predictor of QA at 12 h after TBI. These results reinforce findings in prior, correlative studies that relate serum S100B elevations with osmotic (Kapural et al., 2002b; Kanner et al., 2003; Marchi et al., 2003b) and physical (Bertsch et al., 2001) BBB disruption. The observed relationship between S100B and QA became weaker with time as evidenced by smaller area under the curve values for each successive time point. The small sample size of our study and the very high S100B levels in subject TBI 7 at the 24- and 48-h time points may have obscured a significant relationship at these points. Future studies should include larger study populations to allow the identification of significant relationships between QA and S100B at these time points as well. Furthermore, patients who may be suffering acute stroke as well as patients that die in the immediate post-injury period should also be excluded. Finally, further studies of other biomarkers with greater CNS specificity such as UCHL-1 and neurofilament proteins should be performed. Our current findings support the use of serum S100B within 12 h of TBI to determine the functional status of the BBB. This non-invasive tool should allow the design of improved studies to examine the relationship of BBB status to the diagnosis, prognosis, and treatment of TBI in clinical populations.
Acknowledgments
This work was supported by the following: American Geriatrics Society Jahnigen Career Development Scholars Award (to B.J.B.) and the National Institutes of Health (grant 5R01HD051865-02 to J.J.B., B.J.B., and A.N.).
Author Disclosure Statement
No competing financial interests exist.
References
- Adami C. Sorci G. Blasi E., et al. S100B expression in and effects on microglia. Glia. 2001;33:131–142. [PubMed] [Google Scholar]
- Alderson P. Roberts I. Corticosteroids for acute traumatic brain injury. Cochrane Database Syst. Rev. 2005;1 doi: 10.1002/14651858.CD000196.pub2. CD000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K.J. Scheff S.W. Miller K.M., et al. The phosphorylated axonal form of the neurofilament subunit NF-H (pNF-H) as a blood biomarker of traumatic brain injury. J. Neurotrauma. 2008;25:1079–1085. doi: 10.1089/neu.2007.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.E. Hansson L.O. Nilsson O., et al. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001a;48:1255–1258. doi: 10.1097/00006123-200106000-00012. [DOI] [PubMed] [Google Scholar]
- Anderson R.E. Hansson L.O. Nilsson O., et al. Increase in serum S100A1-B and S100BB Turing cardiac surgery arises from extracerebral sources. Ann. Thorac. Surg. 2001b;71:1512–1517. doi: 10.1016/s0003-4975(01)02399-2. [DOI] [PubMed] [Google Scholar]
- Andersson M. Alvarez-Cermeno J. Bernardi G., et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J. Neurol. Neurosurg. Psychiatry. 1994;57:897–902. doi: 10.1136/jnnp.57.8.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch T. Casarin W. Kretschmar M., et al. Protein S-100B: a serum marker for ischemic and infectious injury of cerebral tissue. Clin. Chem. Lab. Med. 2001;39:319–323. doi: 10.1515/CCLM.2001.050. [DOI] [PubMed] [Google Scholar]
- Bickel U. Yoshikawa T. Pardridge W.M. Delivery of peptides and proteins through the blood-brain barrier. Adv. Drug Deliv. Rev. 2001;46:247–279. doi: 10.1016/s0169-409x(00)00139-3. [DOI] [PubMed] [Google Scholar]
- Bratton S.L. Chestnut R.M. Ghajar J., et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J. Neurotrauma. 2007;24:S37–S44. doi: 10.1089/neu.2007.9990. [DOI] [PubMed] [Google Scholar]
- Büttner T. Weyers S. Postert T., et al. S-100 protein: serum marker of focal brain damage after ischemic territorial MCA infarction. Stroke. 1997;28:1961–1965. doi: 10.1161/01.str.28.10.1961. [DOI] [PubMed] [Google Scholar]
- Csuka E. Morganti-Kossmann M.C. Lenzlinger P.M., et al. IL-10 levels in cerebrospinal fluid and serum of patients with severe tramatic brain injury: relationship to IL-6, TNF-&, TGF-B1 and blood-brain barrier function. J. Neuroimmunol. 1999;101:211–221. doi: 10.1016/s0165-5728(99)00148-4. [DOI] [PubMed] [Google Scholar]
- Dimopoulou I. Korfias S. Dafni U., et al. Protein S-100b serum levels in trauma-induced brain death. Neurology. 2003;60:947–951. doi: 10.1212/01.wnl.0000049931.77887.7f. [DOI] [PubMed] [Google Scholar]
- Foerch C. Wunderlich M.T. Dvorak F., et al. Elevated serum S100B levels indicate a higher risk of hemorrhagic transformation after thrombolytic therapy in acute stroke. Stroke. 2007;38:2491–2495. doi: 10.1161/STROKEAHA.106.480111. [DOI] [PubMed] [Google Scholar]
- Geddes J.F. Whitwell H.L. Graham D.I. Traumatic axonal injury: practical issues for diagnosis in medicolegal cases. Neuropathol. Appl. Neurobiol. 2000;26:105–116. doi: 10.1046/j.1365-2990.2000.026002105.x. [DOI] [PubMed] [Google Scholar]
- Gotoh O. Asano T. Koide T., et al. Ischemic brain edema following occlusion of the middle cerebral artery in the rat. I: The time courses of the brain water, sodium and potassium contents and blood-brain barrier permeability to 125I-albumin. Stroke. 1985;16:101–109. doi: 10.1161/01.str.16.1.101. [DOI] [PubMed] [Google Scholar]
- Hamilton J.A. Benson M.D. Transthyretin: a review from a structural perspective. Cell. Mol. Life Sci. 2001;58:1491–1521. doi: 10.1007/PL00000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselblatt M. Mooren F.C. von Ahsen N., et al. Serum S100beta increases in marathon runners reflect extracranial release rather than glial damage. Neurology. 2004;62:1634–1636. doi: 10.1212/01.wnl.0000123092.97047.b1. [DOI] [PubMed] [Google Scholar]
- Hofmeijer J. Veldhuis W.B. Schepers J., et al. The time course of ischemic damage and cerebral perfusion in a rat model of space-occupying cerebral infarction. Brain Res. 2004;1013:74–82. doi: 10.1016/j.brainres.2004.03.057. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C. Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- Jager T.E. Weiss H.B. Coben J.H., et al. Traumatic brain injuries evaluated in U.S. emergency departments, 1992–1994. Acad. Emerg. Med. 2000;7:134–140. doi: 10.1111/j.1553-2712.2000.tb00515.x. [DOI] [PubMed] [Google Scholar]
- Jaranyi Z. Szekely M. Bobek I., et al. Impairment of blood-brain barrier integrity during carotid surgery as assessed by serum S-100B protein concentrations. Clin. Chem. Lab. Med. 2003;41:1320–1322. doi: 10.1515/CCLM.2003.201. [DOI] [PubMed] [Google Scholar]
- Kanner A.A. Marchi N. Fazio V., et al. Serum S100beta: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer. 2003;97:2806–2813. doi: 10.1002/cncr.11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapural M. Bengez-Krizanac L.J. Barnett G., et al. Serum S-100B as a possible marker of blood-brain barrier disruption. Brain Res. 2002;940:102–104. doi: 10.1016/s0006-8993(02)02586-6. [DOI] [PubMed] [Google Scholar]
- Langham J. Goldfrad C. Teasdale G., et al. Calcium channel blockers for acute traumatic brain injury. Cochrane Database Syst. Rev. 2003;4 doi: 10.1002/14651858.CD000565. CD000565. [DOI] [PubMed] [Google Scholar]
- Larsson A. Lipcsey M. Sjolin J., et al. Slight increase of serum S-100B during porcine endotoxemic shock may indicate blood-brain barrier damage. Anesth. Analg. 2005;101:1465–1469. doi: 10.1213/01.ANE.0000180193.29655.6A. [DOI] [PubMed] [Google Scholar]
- Link H. Tibbling G. Principles of albumin and IgG analyses in neurological disorders. II. Relation of the concentration of the proteins in serum and cerebrospinal fluid. Scand. J. Clin. Lab. Invest. 1977;37:391–396. doi: 10.1080/00365517709091497. [DOI] [PubMed] [Google Scholar]
- Maier B. Schwerdtfeger K. Mautes A., et al. Differential release of interleukines 6, 8, and 10 in cerebrospinal fluid and plasma after traumatic brain injury. Shock. 2001;15:421–426. doi: 10.1097/00024382-200115060-00002. [DOI] [PubMed] [Google Scholar]
- Marchi N. Fazio V. Cucullo L., et al. Serum Transthyretin monomer as a possible marker of blood-to-CSF barrier disruption. J. Neurosci. 2003a;23:1949–1955. doi: 10.1523/JNEUROSCI.23-05-01949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N. Rasmussen P. Kapural M., et al. Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor. Neurol. Neurosci. 2003b;21:109–121. [PMC free article] [PubMed] [Google Scholar]
- Marchi N. Angelov L. Masaryk T., et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson F. Thicoipe M. Aye P., et al. Epidemiology of severe brain injuries: a prospective population-based study. J. Trauma. 2001;51:481–489. doi: 10.1097/00005373-200109000-00010. [DOI] [PubMed] [Google Scholar]
- Mittl R.L. Grossman R.I. Hiehle J.F., et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am. J. Neuroradiol. 1994;15:1583–1589. [PMC free article] [PubMed] [Google Scholar]
- Morganti-Kossmann M.C. Hans V.H. Lenzlinger P.M., et al. TGF-beta is elevated in the CSF of patients with severe traumatic brain injuries and parallels blood-brain barrier function. J. Neurotrauma. 1999;16:617–628. doi: 10.1089/neu.1999.16.617. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. Ansell B., et al. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuchowski N.A. Nonparametric analysis of clustered ROC curve data. Biometrics. 1997;53:567–578. [PubMed] [Google Scholar]
- Otto M. Holthusen S. Bahn E., et al. Boxing and running lead to a rise in serum levels of S-100B protein. Int. J. Sports Med. 2000;21:551–555. doi: 10.1055/s-2000-8480. [DOI] [PubMed] [Google Scholar]
- Papa L. Akinyi L. Pineda J., et al. Levels of UCH-L1 in human CSF and outcome following severe traumatic brain injury. Acad. Emerg. Med. 2008;15:S73. [Google Scholar]
- Pardridge W.M. Drug delivery to the brain. J. Cereb. Blood Flow Metab. 1997;17:713–731. doi: 10.1097/00004647-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Pelinka L.E. Szalay L. Jafarmadar M., et al. Circulating S100B is increased alter bilateral femur fracture without brain injury in the rat. Br. J. Anaesth. 2003a;91:595–597. doi: 10.1093/bja/aeg225. [DOI] [PubMed] [Google Scholar]
- Pelinka L.E. Toegel E. Mauritz W., et al. Serum S100B: a marker of brain damage in traumatic brain injury with and without multiple trauma. Shock. 2003b;19:195–200. doi: 10.1097/00024382-200303000-00001. [DOI] [PubMed] [Google Scholar]
- Pleines U.E. Stover J.F. Kossmann T., et al. Soluble ICAM-1 in CSF coincides with the extent of cerebral damage in patients with severe traumatic brain injury. J. Neurotrauma. 1998;15:399–409. doi: 10.1089/neu.1998.15.399. [DOI] [PubMed] [Google Scholar]
- Raabe A. Grolms C. Seifert V. Serum markers of brain damage and outcome prediction in patients after severe head injury. Br. J. Neurosurg. 1999a;13:56–59. doi: 10.1080/02688699944195. [DOI] [PubMed] [Google Scholar]
- Raabe A. Grolms C. Sorge O., et al. Serum S100B protein in severe head injury. Neurosurgery. 1999b;45:477–483. doi: 10.1097/00006123-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Raabe A. Grolms C. Keller M., et al. Correlation of computed tomography findings and serum brain damage markers following severe head injury. Acta Neurochir. (Wien) 1998;140:787–791. doi: 10.1007/s007010050180. [DOI] [PubMed] [Google Scholar]
- Raabe A. Kopetsch L. Woszczyk A., et al. Serum S-100B protein as a molecular marker in severe traumatic brain injury. Restor. Neurol. Neurosci. 2003;21:159–169. [PubMed] [Google Scholar]
- Reiber H. Felgenhauer K. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin. Chim. Acta. 1987;163:319–328. doi: 10.1016/0009-8981(87)90250-6. [DOI] [PubMed] [Google Scholar]
- Reiber H. Peter J.B. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J. Neurol. Sci. 2001;184:101–122. doi: 10.1016/s0022-510x(00)00501-3. [DOI] [PubMed] [Google Scholar]
- Roberts I. Yates D. Sandercock P., et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomized placebo-controlled trial. Lancet. 2004;364:1321–1328. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
- Savola O. Pyhtinen J. Leino T.K., et al. Effects of head and extracranial injuries on serum protein S100B levels in trauma patients. J. Trauma. 2004;56:1229–1234. doi: 10.1097/01.ta.0000096644.08735.72. [DOI] [PubMed] [Google Scholar]
- Stahel P.F. Morganti-Kossmann M.C. Perez D., et al. Intrathecal levels of complement-derived soluable membrane attack complex (sC5b-9) correlate with blood-brain barrier dysfunction in patients with traumatic brain injury. J. Neurotrauma. 2001;18:773–781. doi: 10.1089/089771501316919139. [DOI] [PubMed] [Google Scholar]
- Stalnacke B.M. Tegner Y. Sojka P. Playing ice hockey and basketball increases serum levels of S-100B in elite players; a pilot study. Clin. J. Sport Med. 2003;13:292–302. doi: 10.1097/00042752-200309000-00004. [DOI] [PubMed] [Google Scholar]
- Stalnacke B.M. Tegner Y. Sojka P. Playing soccer increases serum concentrations of the biochemical markers of brain damage S-100B and neuron-specific enolase in elite players: a pilot study. Brain Inj. 2004;18:899–909. doi: 10.1080/02699050410001671865. [DOI] [PubMed] [Google Scholar]
- Stieg P.E. Kase C.S. Intracranial hemorrhage: diagnosis and emergency management. Neurol. Clin. 1998;16:373–390. doi: 10.1016/s0733-8619(05)70069-4. [DOI] [PubMed] [Google Scholar]
- Tibbling G. Link H. Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand. J. Clin. Lab. Invest. 1977;37:385–390. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- Tourtellotte W.W. Potvin A.R. Fleming J.O., et al. Multiple sclerosis: measurement and validation of central nervous system IgG synthesis rate. Neurology. 1980;30:240–244. doi: 10.1212/wnl.30.3.240. [DOI] [PubMed] [Google Scholar]
- Watson P. Shirreffs S.M. Maughan R.J. Blood-brain barrier integrity may be threatened by exercise in a warm environment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R1689–R1694. doi: 10.1152/ajpregu.00676.2004. [DOI] [PubMed] [Google Scholar]
- Watson P. Black K.E. Clark S.C., et al. Exercise in the heat: effect of fluid ingestion on blood-brain barrier permeability. Med. Sci. Sports Exerc. 2006;38:2118–2124. doi: 10.1249/01.mss.0000235356.31932.0a. [DOI] [PubMed] [Google Scholar]
- Wiesmann M. Wandinger K.P. Missler U., et al. Elevated plasma levels of S-100b protein in schizophrenic patients. Biol. Psychiatry. 1999;45:1508–1511. doi: 10.1016/s0006-3223(98)00217-0. [DOI] [PubMed] [Google Scholar]
- Wunderlich M.T. Wallesch C.W. Goertler M. Release of neurobiochemical markers of brain damage is related to the neurovascular status on admission and the site of arterial occlusion in acute ischemic stroke. J. Neurol. Sci. 2004;227:49–53. doi: 10.1016/j.jns.2004.08.005. [DOI] [PubMed] [Google Scholar]