Abstract
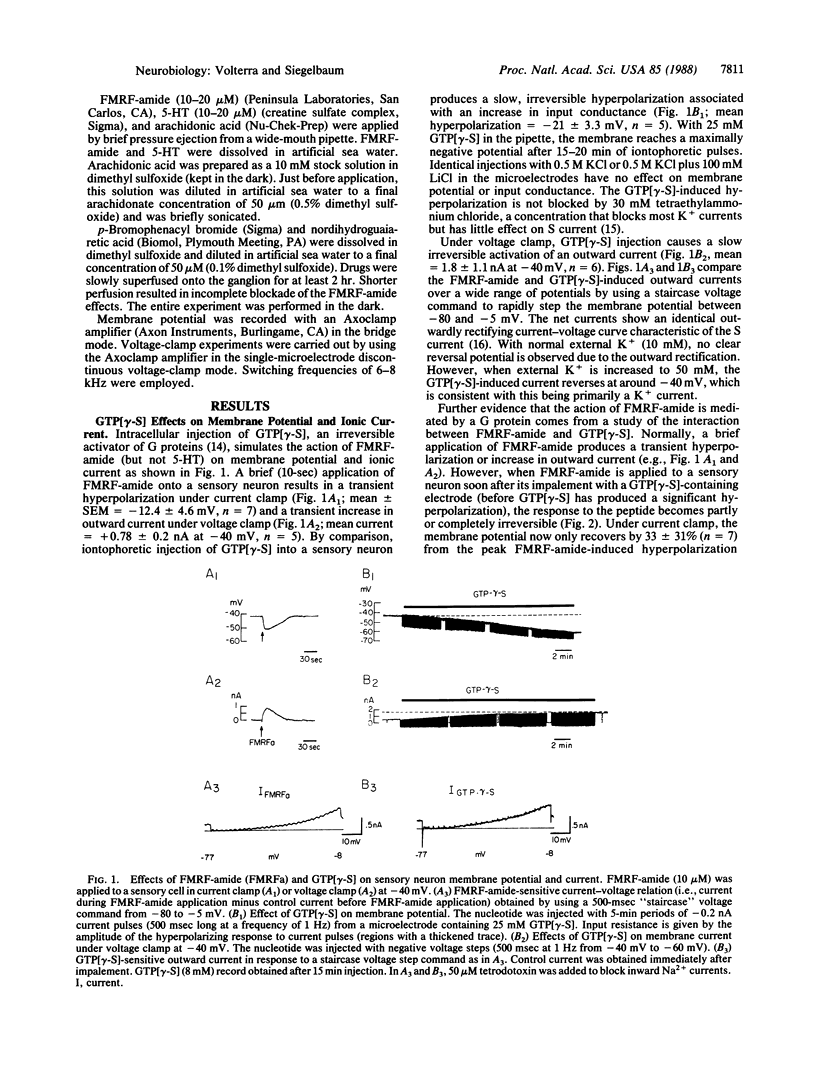
The role of guanine nucleotide-binding proteins (G proteins) in the cAMP-dependent action of serotonin (5-HT) and the antagonistic action of the neuropeptide Phe-Met-Arg-Phe-NH2 (FMRF-amide), mediated by the lipoxygenase metabolites of arachidonic acid, was investigated in Aplysia sensory neurons. Intracellular injection of guanosine 5'-[gamma-thio]triphosphate (GTP[gamma-S]) mimics the hyperpolarizing action of FMRF-amide due to activation of the S K+ current and alters the transient response to FMRF-amide into an irreversible (or only partially reversible) response. At higher concentrations, GTP[gamma-S] occludes the response to FMRF-amide. Injection of activated pertussis toxin inhibits the response to FMRF-amide but not to 5-HT. Injection of guanosine 5'-[beta-thio]diphosphate inhibits the response to FMRF-amide by approximately equal to 50% and completely blocks the response to 5-HT. Three lines of evidence suggest that the FMRF-amide-activated G protein is involved at an early stage of the arachidonic acid cascade, prior to the release of arachidonate. (i) Pertussis toxin injection blocks the hyperpolarizing response to FMRF-amide but not to exogenously applied arachidonic acid. (ii) Two blockers of the arachidonic acid cascade inhibit the hyperpolarizing responses to both FMRF-amide and GTP[gamma-S] (and unmask a 5-HT-like depolarizing response to the nucleotide). (iii) Concentrations of GTP[gamma-S] that alter the kinetics of the FMRF-amide response have no effect on the hyperpolarizing response to arachidonic acid. We conclude that a pertussis toxin-sensitive G protein most likely acts to couple the FMRF-amide receptor to phospholipase activation and arachidonic acid release, whereas a pertussis toxin-insensitive G protein couples the 5-HT receptor to adenylate cyclase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Burch R. M., Jelsema C. L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988 Mar;11(3):117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- Belardetti F., Siegelbaum S. A. Up- and down-modulation of single K+ channel function by distinct second messengers. Trends Neurosci. 1988 May;11(5):232–238. doi: 10.1016/0166-2236(88)90132-4. [DOI] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Brezina V., Eckert R., Erxleben C. Suppression of calcium current by an endogenous neuropeptide in neurones of Aplysia californica. J Physiol. 1987 Jul;388:565–595. doi: 10.1113/jphysiol.1987.sp016632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critz S. D., Harper J. F., Byrne J. H. Evidence for the inhibitory subunit of adenylate cyclase (Ni) in nervous and heart tissue of Aplysia. Neurosci Lett. 1986 Feb 28;64(2):145–150. doi: 10.1016/0304-3940(86)90090-x. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Jelsema C. L. Light activation of phospholipase A2 in rod outer segments of bovine retina and its modulation by GTP-binding proteins. J Biol Chem. 1987 Jan 5;262(1):163–168. [PubMed] [Google Scholar]
- Katada T., Tamura M., Ui M. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch Biochem Biophys. 1983 Jul 1;224(1):290–298. doi: 10.1016/0003-9861(83)90212-6. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Levitan I. B. Intracellular injection of guanyl nucleotides alters the serotonin-induced increase in potassium conductance in Aplysia neuron R15. J Gen Physiol. 1984 Feb;83(2):269–285. doi: 10.1085/jgp.83.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Nakashima S., Tohmatsu T., Hattori H., Suganuma A., Nozawa Y. Guanine nucleotides stimulate arachidonic acid release by phospholipase A2 in saponin-permeabilized human platelets. J Biochem. 1987 Apr;101(4):1055–1058. doi: 10.1093/oxfordjournals.jbchem.a121948. [DOI] [PubMed] [Google Scholar]
- Ocorr K. A., Byrne J. H. Membrane responses and changes in cAMP levels in Aplysia sensory neurons produced by serotonin, tryptamine, FMRFamide and small cardioactive peptideB (SCPB). Neurosci Lett. 1985 Apr 9;55(2):113–118. doi: 10.1016/0304-3940(85)90004-7. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- Piomelli D., Shapiro E., Feinmark S. J., Schwartz J. H. Metabolites of arachidonic acid in the nervous system of Aplysia: possible mediators of synaptic modulation. J Neurosci. 1987 Nov;7(11):3675–3686. doi: 10.1523/JNEUROSCI.07-11-03675.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D., Volterra A., Dale N., Siegelbaum S. A., Kandel E. R., Schwartz J. H., Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature. 1987 Jul 2;328(6125):38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- Salari H., Braquet P., Borgeat P. Comparative effects of indomethacin, acetylenic acids, 15-HETE, nordihydroguaiaretic acid and BW755C on the metabolism of arachidonic acid in human leukocytes and platelets. Prostaglandins Leukot Med. 1984 Jan;13(1):53–60. doi: 10.1016/0262-1746(84)90102-1. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Sato M. A single GTP-binding protein regulates K+-channels coupled with dopamine, histamine and acetylcholine receptors. Nature. 1987 Jan 15;325(6101):259–262. doi: 10.1038/325259a0. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Takahashi J., Matsumoto M., Takashima K., Hakozaki S., Sato M. Islet activating protein-sensitive guanosine triphosphate-binding protein regulates K+-channels coupled with FMRFamide receptors. Jpn J Physiol. 1987;37(3):551–557. doi: 10.2170/jjphysiol.37.551. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Bernier L., Castellucci V. F., Palazzolo M., Saitoh T., Stapleton A., Kandel E. R. What molecular steps determine the time course of the memory for short-term sensitization in Aplysia? Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):811–819. doi: 10.1101/sqb.1983.048.01.084. [DOI] [PubMed] [Google Scholar]
- Shuster M. J., Siegelbaum S. A. Pharmacological characterization of the serotonin-sensitive potassium channel of Aplysia sensory neurons. J Gen Physiol. 1987 Oct;90(4):587–608. doi: 10.1085/jgp.90.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]



