Abstract
Retinal pigment epithelial (RPE) cells, derived from the neuroectoderm, biosynthesize the novel lipid mediator neuroprotectin D1 (NPD1) from docosahexaenoic acid (DHA) in response to oxidative stress or to neurotrophins, and in turn, elicits cytoprotection. Here, we report the identification of a 16,17-epoxide-containing intermediate in the biosynthesis of NPD1 in ARPE-19 cells from 17S-hydro-(peroxy)-docosahexaenoic acid. We prepared and isolated tritium-labeled NPD1 ([3H]-NPD1) and demonstrate specific and high-affinity stereoselective binding to ARPE-19 cells (Kd = 31.3 ± 13.1 pmol/mg of cell protein). The stereospecific NPD1 interactions with these cells in turn gave potent protection against oxidative stress-induced apoptosis, and other structurally related compounds were weak competitors of NPD1 specific binding. This [3H]-NPD1/PD1 also displayed specific and selective high affinity binding with isolated human neutrophils (Kd = ~25 nM). Neither resolvin E1 nor lipoxin A4 competed for [3H]-NPD1/PD1 specific binding with human neutrophils. Together, these results provide evidence for stereoselective specific binding of NPD1/PD1 with retinal pigment epithelial cells as well as human neutrophils. Moreover, they suggest specific receptors for this novel mediator in both the immune and visual systems.
Keywords: Mediators, Neuroprotectin, Inflammation, Resolution, Photoreceptors, Binding
Introduction
Photoreceptor cells (cones and rods) are differentiated neurons that contain the phototransduction apparatus in the tightly organized outer segment membrane discs. These are the richest membranes containing the omega-3 fatty acid family member, docosahexaenoic acid (DHA) [1], as an acyl chain of phospholipids. Photoreceptor outer segment membranes are continuously assembled at the base of the outer segments throughout life, while at their tips, the oldest discs are shed and phagocytized by retinal pigment epithelial (RPE) cells. In turn, RPE cells recycle DHA and all-trans retinol (vitamin A, the precursor of the visual pigment chromophore) back to the photoreceptors. Recently, DHA was shown to be the precursor of neuroprotectin D1 (NPD1). NPD1 formation is upregulated in RPE cells during oxidative stress [2] or upon exposure to neurotrophins [3] and, in turn, promotes homeostasis and cell survival [1].
The RPE and photoreceptors comprise a region of the retina immersed in an environment prone to oxidative stress due to high oxygen consumption, a high metabolic rate, significant exposure to light, and an active flux of polyunsaturated fatty acids. Enhanced oxidative stress is involved in age-related macular degeneration and other retinal degenerations [1]. The ability of the retinal pigment epithelium to increase NPD1 production when confronted with excessive oxidative stress is a newly defined protective mechanism [4]. Moreover, the photoreceptor-phagocytosis enhancement of RPE cell survival (when confronted with oxidative stress) underlies the generation of NPD1 [3]. NPD1 biosynthesis is observed in response to injury in several instances, such as in RPE cells challenged by oxidative stress [1–3], in A2E oxirane accumulation [3], during ischemia-reperfusion in the brain [5] and kidneys [6], and in human brain cells in culture undergoing apoptosis upon exposure to beta amyloid peptide [7].
Resolving inflammatory exudates biosynthesize the D-series resolvins and protectins (i.e., 10,17-diHDHA) from DHA [8, 9]. NPD1/PD1 is a stereochemically defined 10,17-diHDHA molecule [10] that matches the endogenous product and possesses both specific and potent anti-inflammatory as well as modulatory actions in the immune [9–12] and inflammatory [13, 14] systems. For example, NPD1/PD1 is anti-inflammatory, stopping neutrophil infiltration and T cell migration in vivo [11]. Thus, NPD1/PD1 is active in both the neural and immune systems. We coined the term protectin D1 for when NPD1/PD1 is biosynthesized by immune cells; when NPD1/PD1 is specifically biosynthesized by neural and neural ectoderm cells, the prefix neuro is added (i.e., NPD1).
Here, we identify a 16,17-epoxytriene as a key intermediate in NPD1 biosynthesis and demonstrate the potent and stereospecific bioactivity of NPD1 with ARPE-19 cells. Using tritium-labeled NPD1 ([3H]-NPD1) and stereoisomers of NPD1, methyl esters, and structurally related compounds, we demonstrate high-affinity stereoselective binding of NPD1 with both human ARPE-19 cells and isolated human neutrophils.
Materials and Methods
The studies reported here have been reviewed and approved by the Institutional Animal Care and Use Committee at the Louisiana State University Health Sciences Center, New Orleans and the Harvard Medical Area Standing Committee on Animals, Boston (Protocol #02570). Synthetic NPD1, NPD1-Me, NPD1 stereoisomers, and RvD1-Me were prepared in stereochemically pure form via total organic synthesis [10].
ARPE-19 Cells, Induction of Oxidative Stress and Hoechst Staining
ARPE-19 cells at the 27th cell passage were grown in six well plates semi-confluent for 72 h in DMEM/F12, 10% fetal bovine serum (FBS) media. Cells were serum starved for 8 h before triggering oxidative stress by further incubation with 10 ng/ml of TNF-α plus 600 µM H2O2 during 15 h. Bioactivity was assayed by adding 50 nM of NPD1 or structurally-related compounds at the outset of oxidative stress and evaluated 15 h after by Hoescht 33342 staining. Cells were fixed with methanol for 15 min and washed once with phosphate-buffered saline (PBS) at room temperature. Then cells were stained with Hoechst solution (2 µM final concentration) for 15 min and apoptotic cell death was scored using a Nikon DIAPHOT 200 microscope under ultraviolet fluorescence. Oxidative stress-induced apoptosis followed by Hoechst staining in RPE cells correlated with [3H]-thymidine prelabeling and with Elisa determination of mono- and oligonucleosomes in these conditions [2].
NPD1 Biosynthesis and Trapping of Intermediates
The formation of epoxide-containing intermediates was studied with cultured ARPE-19 cells (5 × 106 cells/incubation) that were incubated at 37°C for 15 min with 1.8 µM 17S-hydro-(peroxy)-docosahexaenoic acid and 100 µg/ml zymosan A in 1.5 ml Dulbecco’s PBS (phosphate-buffered saline with Ca2+ and Mg2+, pH 7.4). The incubations were stopped with 10 volumes of cold acidified methanol, kept for 30 min at 4°C, and then rapidly extracted and taken for liquid chromatography (LC)-photodiode array-electrospray ionization-tandem mass spectrometry (MS/MS)-based lipidomic analysis (LC-MS/MS), using a hybrid quadrupole time-of-flight mass spectrometer (QSTAR XL, Applied Biosystems, Foster City, CA). The liquid chromatography instrument was mounted with a LUNA C-18 column (100 mm × 2 mm × 5 µm) (Phenomenex, Torrance, CA), and mobile phase was isocratic for 8 min with solvent C (water:methanol:acetic acid) (65%:35% :0.01%). Then a gradient was added for 22 min from 100% solvent C to 100% methanol, followed by isocratic for 5 min; flow rate was 200 µl/min. The mass spectrometry was operated in negative ion mode set at the following parameters: turbospray at 4.5 kV, nitrogen gas heated at 500°C, decluster potential −50 V, collision energy −20 V, mass scan range m/z 50 to 400, and data accumulation time per spectrum was 1.00 sec.
Radioligand Synthesis and Validation
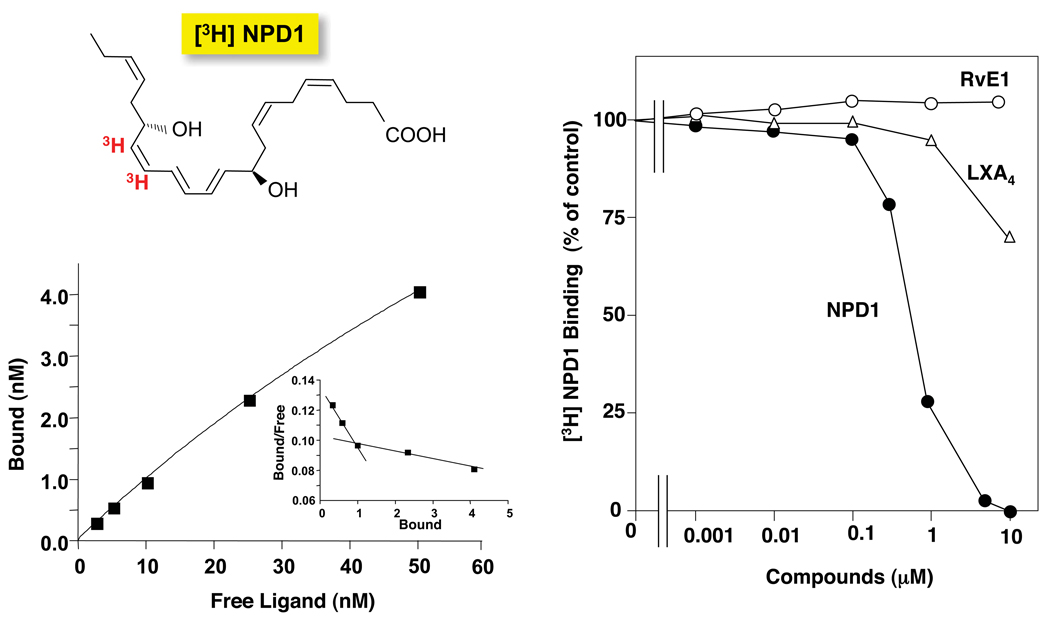
Tritium-labeled NPD1 ([3H]-NPD1: ~50 Ci/mmol) was obtained by custom catalytic tritiation of synthetic 15-acetylenic NPD1 methyl ester that was prepared as in Serhan et al. [10] (see Fig. 1), purified and supplied to American Radiolabeled Chemicals (St. Louis, MO) for labeling. The freshly isolated [3H]-NPD1 in its acid form was obtained by saponification followed by high performance liquid chromatography isolation, using LUNA C18-2 (100 mm × 2 mm × 5 µm) column and ultraviolet diode array detector, mobile phase (methanol:water:acetic acid) (78:22:0.01) with 0.2 ml/min flow rate. To minimize isomerization and degradation of the label, the isolated label was used immediately after isolation for specific binding experiments with ARPE-19 cells and human polymorphonuclear neutrophils (PMN).
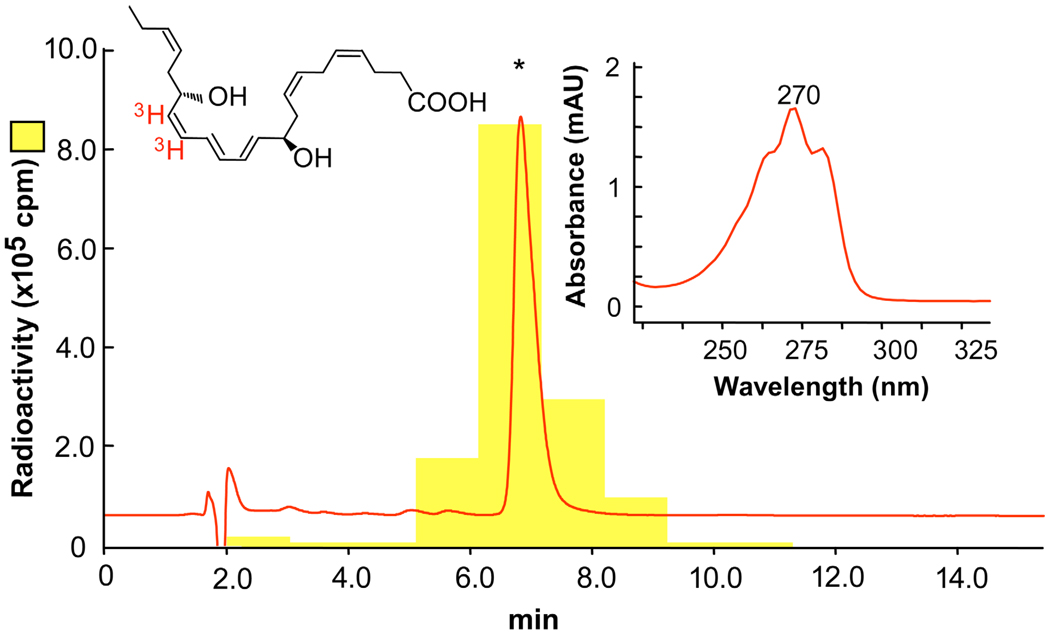
Figure 1.
[3H]-NPD1: elution profile and UV spectrum. Reverse phase HPLC of [3H]-NPD1 was performed and recorded at 270 nm absorbance, showing identical retention time (asterisk) as standard NPD1. The locations of the [3H] in red are indicated on the chemical structure of NPD1. Insert shows the characteristic online UV spectrum.
Specific Binding with ARPE-19 Cells
Stereoselective binding for NPD1 with ARPE-19 cells was carried out using a radioactive ligand [3H]2-NPD1 (50 Ci/mmol). The unlabeled cold-ligand was synthetic NPD1 [see ref. 10]. ARPE-19 cells, cultured for 7 days at the 27th passage, were detached from culture flasks and used as whole cells (100,000 cells/assay tube) for binding experiments. Saturation binding studies were performed within a range of 0.1 to 50,000 pM [3H]2-NPD1, using 0.1 µM of unlabeled ligand for non-specific binding detection. Analysis of saturation was performed by non-linear least-square curve fitting of total and non-specific raw data using a Gauss-Newton method. Competition studies were performed at 5 pM radio-ligand, and the competitive ligand ranged from 10−11 to 10−6 M. Fitting of sigmoid curves was performed on Prism 4 software (GraphPad Software, La Jolla, CA) and an SAS program.
PD1 Specific Binding to Human PMN
Binding studies were carried out with tritiated PD1 synthesized using custom tritiation (American Radiolabeled Chemicals) followed by HPLC isolation. Aliquots of isolated human PMNs (2×106 cells / 100µl) and indicated concentrations of [3H]-PD1 with or without unlabeled competitors were incubated in Dulbecco’s PBS with CaCl2 and MgCl2 for 1 h at 4°C. For determination of non-specific binding, at least 1,000x concentration of unlabeled NPD1/PD1 was used. The bound and unbound radioligands were separated by filtration through Whatman GF/C glass microfiber filters and radioactivity was determined. Data analyses were carried out using Prism (GraphPad).
Results
NPD1 Biosynthesis in ARPE-19 Cells Involves a 16,17-Epoxide Intermediate
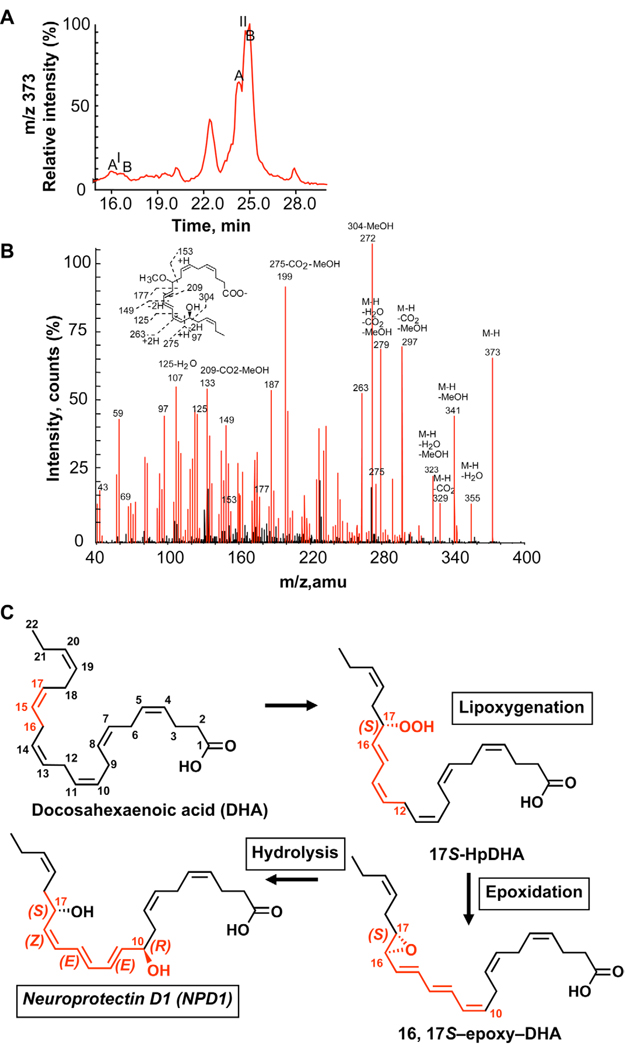
ARPE-19 cells were incubated with 17S-hydro(peroxy)docosa-4Z,7Z,10Z,13Z,15E,19Z-hexaenoic acid, and the incubations were stopped with the addition of 10-vol/vol excess acidic methanol. Following extraction, LC-MS/MS analyses were used to identify whether methoxy-trapping products were generated from the proposed epoxide-containing intermediate in these cells [2, 7] as observed in exudates, glial cells and T cells [8, 9, 11]. The identified methoxy-trapping products from ARPE-19 cells are shown in Figure 2. The compounds were two stereoisomers, namely the S and R diastereoisomers of the 16-methoxy,17S-hydroxy containing trapping products, corresponding to peak IA and IB in HPLC chromatogram (Fig. 2 A) that were identified by MS/MS (Fig. 2 B). Two stereoisomers (S, R) of 10-methoxy, 17S-hydroxy trapping product, were isolated in peak IIA and IIB on HPLC chromatogram (Fig. 2 A) and were identified by MS/MS (Fig. 2 C). The 16-methoxy-containing trapping products were identified based on MS/MS spectral analysis that demonstrated diagnostic ions at m/z 373 (Fig. 2 B) of the corresponding LC peaks in Figure 2 A. The presence in both IA and IB of the same diagnostic MS/MS ions of m/z 373 (M-H), 355 (M-H-H2O), 341 (M-H-MeOH), 329 (M-H-CO2), 311 (M-H-H2O-CO2), 297 (M-H-CO2-MeOH), 127, 139, 143, 153, 167, 205, 207, 231, 247, 275, 83 (127-CO2), 95 (139-CO2), 109 (153-CO2), 135 (167-MeOH), 161 (205-CO2), 175 (207-MeOH), and 215 (247-MeOH) was consistent with racemic methoxy-trapping products at position 16, denoted A and B stereoisomers – namely, 16R/S-methoxy-17S-hydroxydocosahexenoic acid (Fig. 2). The 10-methoxy-containing trapping products were also identified based on the diagnostic MS/MS (373) ions at m/z 373 (M-H), 355 (M-H-H2O), 341(M-H-MeOH), 329 (M-H-CO2), 323 (M-H-H2OMeOH), 297 (M-H-CO2-MeOH), 279 (M-H-CO2-MeOH), 153, 177, 149, 125, 263, 275, 97, 107 (125-H2O), 133 (209-CO2-MeOH), 199 (275-CO2-MeOH), 272 (304-MeOH). Together, the formation and identification of these alcohol trapping products indicate that a 16,17-epoxide is the intermediate in NPD1 biosynthesis from the precursor lipoxygenase-generated product, namely 17S-hydro-(peroxy)-docosahexaenoic acid in ARPE-19 cells. This epoxide is likely enzymatically hydrolyzed to NPD1 to produce the correct stereochemical assignment in NPD1. These results indicate that the hydrolysis is very likely enzymatic because it stereoselectively inserts oxygen derived from water and sets the double bond configuration in the conjugated triene to generate the NPD1 carrying cis,trans,trans-conjugated triene geometry responsible for its potent bioactions. Moreover, these results support the proposed pathway depicted in Figure 2 C for NPD1 biosynthesis by ARPE-19 cells.
Figure 2.
Epoxide-containing intermediate in NPD1 biosynthesis: Identification of alcohol-trapping products. ARPE-19 cells (5 × 106 cells/incubation) were incubated with 1.85 µg 17S-hydro-(peroxy)-docosahexaenoic acid and alcohol-trapping products were analyzed. (A) LC-MS/MS selective ion chromatogram for methoxy-trapping products at m/z 373 shows two 16-OCH3 (1A and 1B) and two 10-OCH3 (IIA and IIB) isomers. (B) LC-MS/MS spectrum of 16-OCH3 product I; C) LC-MS/MS of 10-OCH3 product II. (C) Proposed pathway for NPD1 biosynthesis from 17S-hydroperoxy-DHA through a 16,17-epoxytriene intermediate.
Specific Cytoprotective Action of NPD1 in Oxidative Stress-Challenged ARPE-19 Cells
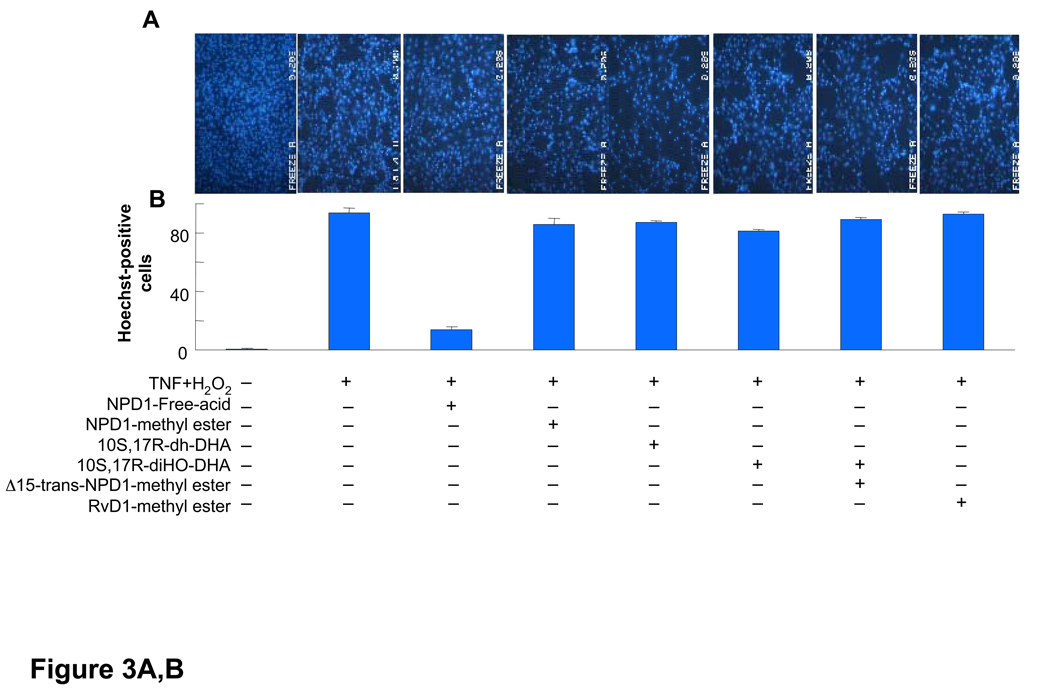
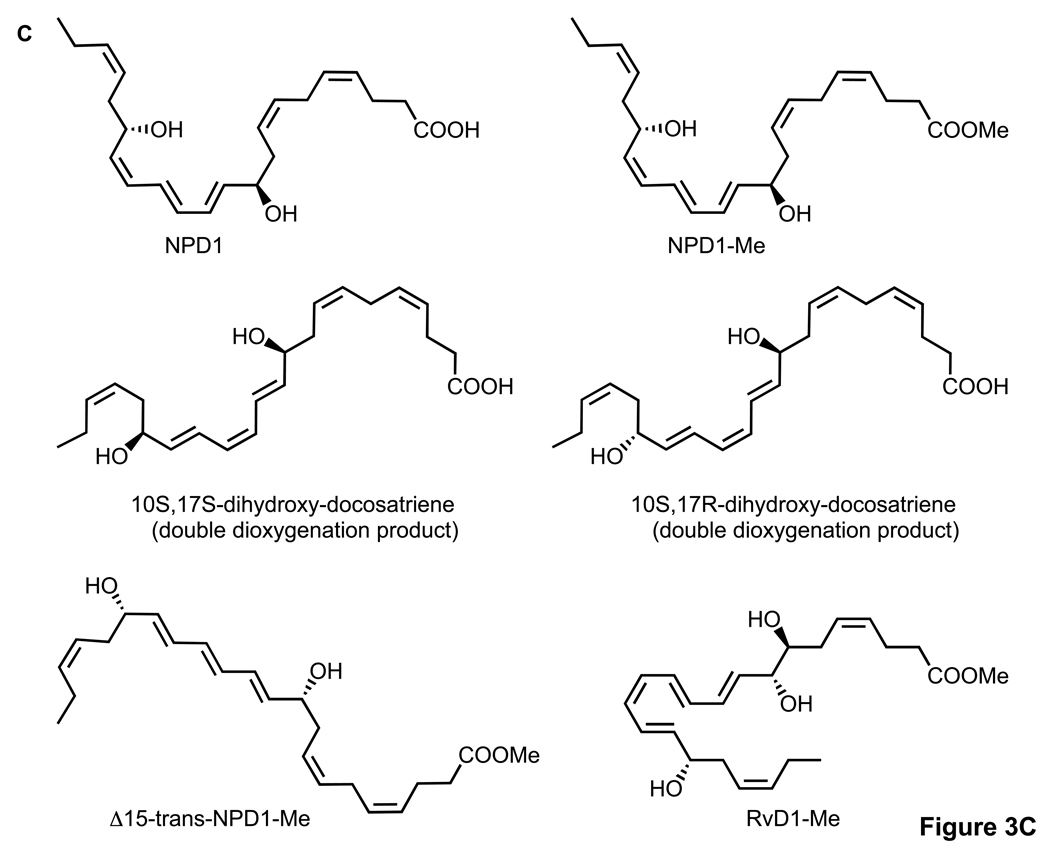
Since NPD1 reduces oxidative stress-mediated apoptotic cell death in ARPE-19 cells [1], in the present experiments we directly compared the cytoprotective actions of synthetic NPD1 with NPD1 stereoisomers and related compounds, each prepared by total organic synthesis. These included NPD1 methyl ester and other docosanoids, i.e., 10S,17R-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid (10S,17R-dihydroxy-docosatriene), 10S,17S-dihydroxy-docosa-4Z,7Z,11E,13Z,15E,19Z-hexaenoic acid (10S,17S-dihydroxy-docosatriene), 10R,17S-dihydroxy-docosa 4Z,7Z,11E,13E,15E,19Z-hexaenoic methyl ester (Δ15-trans-NPD1-Me), and resolvin D1, 7S,8R,17S-trihydroxy-docosa-4Z,9E,11E,13Z,15E,19Z-hexaenoic acid methyl ester (RvD1-Me) [10, 15]. Figure 3 shows the actions of synthetic NPD1, its stereoisomers, methyl esters, and related compounds on apoptotic cell death as assessed by Hoechst 33342 nuclear staining in ARPE-19 cells induced by serum starvation/H2O2/TNF-α. This assay, under these conditions, correlates with other markers of apoptotic cell death, including oligo- and mono-nucleosomes formation, [3H]-thymidine DNA degradation, and caspase-3 activation [2]. It is evident that NPD1 at 50 nM prevented oxidative stress-induced apoptotic cell death by 80–85% (Fig. 3). The NPD1-carboxy-methyl ester (NPD1-Me, Fig. 3) was less potent at the same concentration, with only 10–15% inhibition of Hoechst 33342 nuclear staining. The specific NPD1 stereoisomers and related docosanoids, i.e., 10S,17R-dihydroxy-docosatriene, 10S,17S-dihydroxydocosatriene, Δ15-trans-NPD1-Me, and RvD1-Me (see structures in Fig. 3), were only weak inhibitors, giving ~5–10% reduction of cell apoptosis at the equimolar concentration 50 nM. Note that, while both RvD1 and NPD1/PD1 are potent anti-inflammatory mediators [15], only NPD1/PD1 gave results suggesting stereoselective and specific interactions of NPD1 and actions with ARPE-19 cells.
Figure 3.
Activity of NPD1, its stereoisomers and related compounds on oxidative stress (OS)-induced apoptosis in ARPE-19 cells. Evaluation of neuroprotective bioactivity in ARPE-19 cells under OS induced by 600 µM H2O2 and 10 ng/ml TNF-α. Cells grown for 72 h after plating were serum starved for 8 h before OS induction. Compounds were added (50 nM) at the time of OS induction. After 14 h of treatment, cells were 33342 Hoechst stained, and apoptotic cells scored as described in Materials and Methods. Hoechst 33342 nuclear staining in these conditions correlates with oligo- and mono nucleosomes formation, [3H]-thymidine DNA degradation, and caspase-3 activation, additional assays of monitoring cell death [2]. (A) Representative pictures of ARPE-19 cells stained with 33342 Hoechst. Apoptosis (condensed and shiny nuclei) upon exposure to OS, and OS + NPD1 or other lipids. (B) Quantitative analysis of apoptotic cell populations. Results are expressed as percent of total population and are average of three independent experiments, ± standard error (SE). (C) Chemical structures of NPD1, NPD1 methyl ester (NPD1-Me), 10S,17S-dihydroxy-docosatriene, 10S,17R-dihydroxy-docosatriene, 10S,17S-dihydroxy-docosatriene, Δ15-trans-NPD1-Me and resolvin D1-Me.
NPD1 Stereoselective and Specific Binding
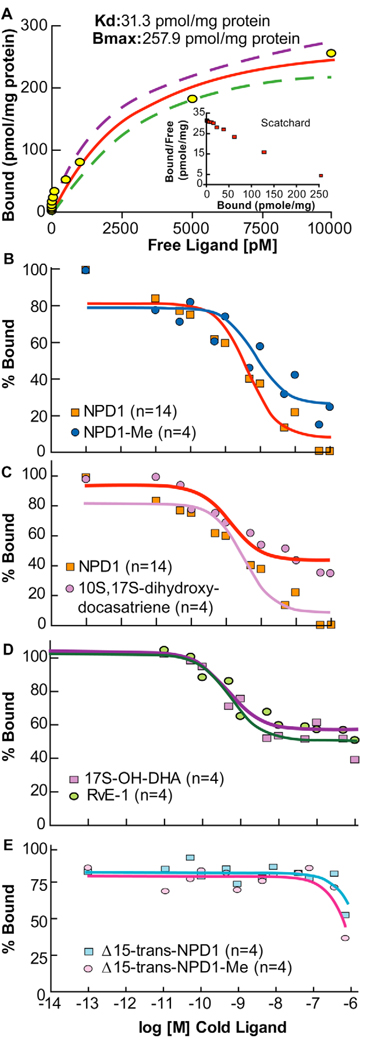
[3H]-NPD1 was bond with a Kd of 31.3 ± 13.1 pmol/mg of cell protein, and the maximal binding capacity of NPD1 for ARPE-19 cells was 257.9 ± 25.8 pmol/mg of cell protein (Fig. 4 A). Saturation binding showed proper performance in the lower range. However, at concentrations of radio-ligand above 10 nM, non-specific binding was apparent and in line with the hydrophobic nature of the ligand; hence results above this range were excluded. Scatchard plot analysis in the inset of Figure 4 A indicates a linear relationship consistent with a single specific binding site on these cells. Competitive curves of unlabeled ligand or its methyl ester derivative display a step sigmoidal curve (Fig. 4 A) for NPD1 (free acid form), with 90–100% binding displacement contained within two log units of cold ligand concentrations and a calculated EC50 of 3.6 nmol/mg protein. The methyl ester NPD1 derivative otherwise shows a shallow curve that drifts to the right, indicating less affinity for binding sites (Fig. 4 B). The maximum NPD1 binding displacement of 74% was at 1 µM. The NPD1 stereoisomer (10S,17S-dihydroxy-docosatriene) displacement curve appeared very shallow, reaching only 60% of total binding at 1 µM (Fig. 4 C). Other structurally related omega-3 fatty acid-derived compounds were 17S-hydroxy-DHA, resolvin E1 (RvE1), Δ15-trans-NPD1 (free acid), and Δ15-trans-NPD1 (methyl ester). Figures 4 D and 4 E report only ~40% maximal displacement activity for 17S-hydroxy-DHA, and with RvE1 indicates that these compounds display only minimal interactions with NPD1 binding sites at these concentrations. Also, the Δ15-trans NPD1 isomer, tested in the acid form or the NPD1 methyl ester derivative, did not give significant competitive displacement, indicating that they do not interact with the high-affinity NPD1 binding site in this concentration range. Taken together, these results indicate that NPD1 binding is specific, high-affinity, and is present at significant levels on ARPE-19 cells. These results are consistent with the potent and stereoselective actions of NPD1 on RPE cells. Moreover, in direct comparison with the actions of NPD1, both RvE1 and RvD1 were less active than NPD1 in regulating apoptosis of RPE cells (Table 1).
Figure 4.
NPD1 saturation binding on ARPE-19 cells. (A) Increasing concentrations of radio-ligand (NPD1) gave saturation with a Kd, indicating very high affinity. Scatchard plot analysis (see inset) appears to be linear, suggesting a single binding site. Kd and Bmax values were obtained by non-linear least-square curve fitting analysis of raw data, 8 interactions, (5 separate experiments, n=20 per data point), p < 0.0001. (B – E) Competitive displacement for NPD1. (B) Comparison of NPD1 free acid with its methyl ester. NPD1 gave a sharper sigmoidal curve compared to its carboxy methyl ester. Competitive binding was obtained at a constant radio-ligand concentration of 5 pM, and cold competitive ligand ranging 10−11 to 10−6 M performed with 100,000 cells. Four individual displacement curves were performed for NPD1, and two for NPD1 methyl ester (NPD1-Me). (C) Comparison of NPD1 to its stereoisomer 10S,17S dihydroxy-docosatriene. Displacement curve for 10S,17S di-dihydroxy-docosatriene shows shallow. This is a strong indication of receptor stereospecificity for the specific ligand, with a maximal displacement of 60% of specific binding at highest concentration tested. (D) Competition by 17S-hydroxy-DHA and RvE1. Both compounds show very low competitive activity for NPD1, reaching only to about 40% maximum displacement activities at the highest concentrations. (E) Competition by Δ15-trans-NPD1 acid, or its methyl ester. Both compounds showed little to no competitive ability for [3H]-NPD1 bound to these cells. Data collection and assay conditions for C, D and E were as in B.
Table 1.
NPD1, RvD1 and RvE1 dose-dependent inhibition of apoptosis induced by oxidative stress in RPE cells
| Treatment | Dose (nM) | % Inhibition |
|---|---|---|
| NPD1 | 10 | 2.6 |
| 25 | 44.8 | |
| 50 | 92.4 | |
| RvD1 | 10 | 0.0 |
| 25 | 4.8 | |
| 50 | 38.4 | |
| RvE1 | 10 | 0.0 |
| 25 | 3.2 | |
| 50 | 29.2 |
The 72-h grown ARPE-19 cells in six-well plates were serum starved for 8 h. Oxidative stress was introduced by H2O2/TNF-α as before [2]. Stressed cells were challenged with NPD1, RvD1, and RvE1 at different concentrations as indicated for 15 h. Results are averages of five repeated points each from three independent experiments.
Specific NPD1/PD1 Binding with Human PMN
NPD1/PD1 displays potent stereoselective anti-inflammatory and pro-resolving actions [9–11]. Specific binding experiments with [3H]-NPD1 and isolated human neutrophils demonstrated high affinity specific binding to PMN at 4°C and showed a 2-site fit for sites of high and low affinity binding (Kd ~ 25 nM and ~ 200 nM) with isolated human PMN (see Fig. 5 and inset). Two other anti-inflammatory and pro-resolving mediators that bind specific receptors on human neutrophils and specifically human PMN, namely lipoxin A4 (LXA4) [16] and RvE1 [17, 18] did not compete with [3H]-NPD1 (Fig. 5, Panel B). Thus, these results suggest that NPD1/PD1 specifically binds to sites not shared by the ALX GPCR ALX-FPR, BLT1, or ChemR23 that bind RvE1 on human and murine neutrophils [18]. Moreover, they suggest that NPD1/PD1 specifically binds to saturable high affinity sites on human neutrophils (Fig. 5).
Figure 5.
[3H]-NPD1/PD1-specific binding to human PMN. (A) Isolated human PMNs were incubated with the indicated concentrations of [3H]-NPD1/PD1 in the presence or absence of 10 µM unlabeled NPD1/PD1. Saturation curve and Scatchard plot (inset) are the average of triplicates and representative of results from two separate donors. (B) Competition for [3H]-PD1 (3nM)-specific binding to isolated human PMN with increased concentrations of PD1, RvE1 or LXA4. Results are the average of triplicates and representative of n=3.
Discussion
Here we demonstrate high-affinity stereoselective binding of NPD1 in ARPE-19 cells and human neutrophils. For this purpose, we prepared [3H]-NPD1, NPD1 isomers, and structurally-related compounds to test their actions with ARPE-19 cells as in acute inflammation and resolution [10].
NPD1 is a potent bioactive product biosynthesized from the omega-3 fatty acid DHA that is endowed with anti-inflammatory and pro-survival bioactivity generated in response to H2O2/TNFα-triggered oxidative stress [2], ischemia reperfusion [5, 6], photoreceptor outer segment phagocytosis in RPE cells exposed to oxidative stress [4], Aβ-mediated cell damage [7], and neurotrophic growth factors [3] including APPα [7]. A convergence of immune-mediated inflammatory signaling is implicated in age-related macular degeneration (AMD) at the interface of photoreceptor cells and RPE cells. In fact, elements of the alternative pathway of complement system activation make immune-mediated dysfunction a salient feature of the pathophysiology of AMD, since the variants (single nucleotide polymorphisms) in the genes encoding factor H [19], factor B and complement component C [20] are major risk factors for AMD. Factor H is an inhibitor of the alternative pathway of complement system activation, resulting in modulation of cell injury and inflammatory signaling [20, 21]. Furthermore, inflammatory and cytotoxic proteins accumulate beneath the RPE cells, and then pathoangiogenesis develops in the neovascular form of AMD [22].
In the non-vascular form of AMD, apoptosis triggers RPE and photoreceptor loss. The stereoselective binding of NPD1 in RPE cells may have relevance in both forms of macular degenerations because this lipid mediator is anti-inflammatory and anti-angiogenic [23], as well as a downregulator of apoptosis in RPE cells [1]. Indeed, NPD1 potently inhibits interleukin-1-mediated upregulation of cyclooxygenase-2 in RPE cells [2]. NPD1 downregulates oxidative stress-induced apoptosis [2] as well as A2E-mediated RPE cell apoptosis [3]. A2E is a product of phototransduction that accumulates in the aging retinal pigment epithelium and that excessively accumulates in the RPE of patients with Stargardt disease, an inherited form of AMD [2]. Defining the operational context of the biosynthetic pathway leading to NPD1 formation was necessary in order to characterize key intermediates of this route.
Along these lines, we identified a 16,17-epoxide-containing intermediate (using alcohol trapping) in the biosynthesis of NPD1 from the precursor 17S-hydro-(peroxy)- docosahexaenoic acid in ARPE-19 cells. The identification of these markers points to the epoxide as a key intermediate in the pathway for NPD1 biosynthesis by ARPE-19 cells and served as the basis for the design of experiments directed to assess both stereospecific NPD1 binding and stereoselective actions in ARPE-19 cells. The biosynthesis of NPD1 in ARPE-19 cells proceeds in a pathway identical to both glial cells [9] and human T cells [11]. The resolvins, in particular RvE1 and RvD1, have potent anti-inflammatory and pro-resolving actions in several experimental animal models of inflammatory diseases [23–25]. These local mediators, together with NPD1, are generated during the resolution phase of an acute inflammatory response [8]. When directly compared to the actions of NPD1, both RvE1 and RvD1 were less active than NPD1 in regulating apoptosis of RPE cells (Table 1), while they show potent anti-inflammatory and pro-resolving actions in vivo [14, 26]. Together, the results of the present report demonstrate: a) key components of the proposed biosynthetic pathway of NPD1 in RPE cells; b) direct evidence for the stereoselective actions of NPD1 and its complete stereochemical assignment proved identical to that of PD1 biosynthesized in the immune system [10]. Importantly, related bioactive mediators such as resolvins or NPD1 stereoisomers prepared by total organic synthesis were either less active or not active in regulating the protective actions originally uncovered for NPD1 [2]; c) for the first time (using a new radiolabel prepared from the acetylenic NPD1 precursor) the specific binding of NPD1 to RPE cells; and d) human neutrophils. Although both ARPE-19 cells and human PMN specifically bind [3H]-labeled NPD1/PD1 with stereoselectivity, these sites appear to have different binding characteristics for the two human cell types. In this regard, the ARPE-19 cells gave results that fit a one-site binding model, and results obtained with PMN fit a two-site model of high and low affinity binding sites. Importantly, these findings establish that the biosynthesis of NPD1 in ARPE-19 cells and immune cells of PD1 [11] is essentially identical and that the NPD1/PD1 stereochemical assignment in these two systems is the same, yet the site of action in these cells appears to have different characteristics.
In summation, the results of the present study demonstrate that upon activation, RPE cells convert DHA via specific enzymatic steps (initially catalyzed by a lipoxygenase) into a novel 16,17-epoxytriene-containing intermediate that, in turn, is enzymatically converted to NPD1. This signal, namely NPD1, has stereoselective sites of action and specific binding in RPE cells. NPD1/PD1 specific binding sites were also present on human neutrophils, where PD1 is both anti-inflammatory and pro-resolving [14, 27]. Thus, the present findings provide the first evidence for specific high affinity receptors for this novel mediator in retinal epithelial pigment cells and human neutrophils.
Acknowledgements
We thank M. H. Small for expert assistance in manuscript preparation and Dr. N. Chiang (BWH-HMS) for calculating the Kd values for human PMN.
This work was supported by National Institutes of Health grants R01 EY005121 and P20 RR016816 (N.G.B.), and grants GM38765 and P50 DE016191 (C.N.S.).
Abbreviations
- PD1
protectin D1, 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid
- NPD1
neuroprotectin D1
- PMN
polymorphonuclear neutrophils
- RPE
retinal pigment epithelial
- RvE1
resolvin E1, 5S,12R,18R,trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid
- RvD1
resolvin D1, 7S,8R,17S-trihydroxy-docosa-4Z,9E,11E,13Z,15E,19Z-hexaenoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bazan NG. Homeostatic regulation of photoreceptor cell integrity: significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid: the Proctor Lecture. Invest Ophthalmol Vis Sci. 2007;48:4866–4881. doi: 10.1167/iovs.07-0918. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci USA. 2007;104:13152–13157. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukherjee PK, Marcheselli VL, de Rivero Vaccari JC, Gordon WC, Jackson F, Bazan NG. Photoreceptor outer segment phagocytosis attenuates oxidative stress-induced apoptosis with concomitant neuroprotectin D1 synthesis. Proc Natl Acad Sci USA. 2007;104:13158–13163. doi: 10.1073/pnas.0705963104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 6.Duffield JS, Hong S, Vaidya V, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 7.Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong S, Gronert K, Devchand P, Moussignac R-L, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN, Gotlinger K, Hong S, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 11.Ariel A, Li P-L, Wang W, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 12.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:249–261. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and pro-resolving lipid mediators. Annu Rev Pathol Mech Dis. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y-P, Oh SF, Uddin J, et al. Resolvin D1 and its aspirin-triggered 17R epimer: stereochemical assignments, anti-inflammatory properties and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 16.Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 19.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bok D. Evidence for an inflammatory process in age-related macular degeneration gains new support. Proc Natl Acad Sci USA. 2005;102:7053–7054. doi: 10.1073/pnas.0502819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penfold PL, Madigan MC, Gillies MC, Provis JM. Immunological and aetiological aspects of macular degeneration. Prog Retin Eye Res. 2001;20:385–414. doi: 10.1016/s1350-9462(00)00025-2. [DOI] [PubMed] [Google Scholar]
- 23.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dona M, Fredman G, Schwab JM, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153 Suppl. 1:S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]