Abstract
Background
Electrophilic xenobiotics and endogenous products from oxidative stresses induce the glutathione S-transferases (GSTs), which form a large family within the phase II enzymes over both animal and plant kingdoms. The GSTs thus induced in turn detoxify these external as well as internal stresses. Because these stresses are often linked to ageing and damage to health, the induction of phase II enzymes without causing adverse effects would be beneficial in slowing down ageing and keeping healthy conditions.
Methodology/Principal Findings
We have tested this hypothesis by choosing allyl isothiocyanate (AITC), a functional ingredient in wasabi, as a candidate food ingredient that induces GSTs without causing adverse effects on animals' lives. To monitor the GST induction, we constructed a gst::gfp fusion gene and used it to transform Caenorhabditis elegans for use as a nematode biosensor. With the nematode biosensor, we found that AITC induced GST expression and conferred tolerance on the nematode against various oxidative stresses. We also present evidence that the transcription factor SKN-1 is involved in regulating the GST expression induced by AITC.
Conclusions/Significance
We show the applicability of the nematode biosensor for discovering and evaluating functional food substances and chemicals that would provide anti-ageing or healthful benefits.
Introduction
The free-living soil nematode Caenorhabditis elegans has become one of the most powerful biological research tools available today because of its ease of handling, short lifespan, large brood size, and amenability to molecular as well as classical genetics [1]. Recent studies have revealed that this nematode possesses not only innate defense mechanisms against infection by bacteria and fungi [2]–[4] but also detoxifying systems against xenobiotics [5]. Large numbers of genes in the C. elegans genome encode detoxification enzymes such as cytochrome P450 (CYP), short-chain type dehydrogenase (SDR), UDP-glucuronosyl/glucosyl transferase (UGT), and glutathione S-transferase (GST) (WormBase, http://www.wormbase.org/), and they are probably regulated by conserved signal transduction pathways.
Oxidative stresses derived from exogenous and endogenous substances are considered to cause cancer, atherosclerosis, inflammation, hypertension, diabetes, and several age-related diseases [6]. The phase II detoxification enzymes such as GST, glutathione reductase, glutathione peroxidase, UGT, and NAD(P)H:quinone oxidoreductase are recognized as playing major protective roles against oxidants [7]–[9]. Therefore, the induction of phase II enzymes without adverse effects may be one good strategy to reduce the risks of contracting cancer and age-related diseases. Diet-derived inducers of phase II enzymes, such as the isothiocyanates sulforaphane and allyl-isothiocyanate, have been reported as candidates for effective chemopreventives of diseases [10]–[13].
To take advantage of the nematode's defense systems for sensing and responding to xenobiotics, we constructed nematode biosensors, each transgenic animal containing a fusion gene of a phase II enzyme gene with the green fluorescence protein (GFP) gene. We previously reported that with such biosensors we could not only identify toxicants in foods and the environment, but also screen for foods or their ingredients, known or unknown, that would reduce their harmfulness or action [14], [15]. We here report the applicability of our nematode biosensors for the discovery and evaluation of functional food ingredients that serve as anti-ageing agents or protectives against health afflictions, by using allyl isothiocyanate (AITC) as a model functional food ingredient.
Results
Nematode Biosensor Detects gst-Inducing Activity in Chemicals
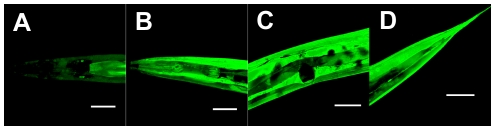
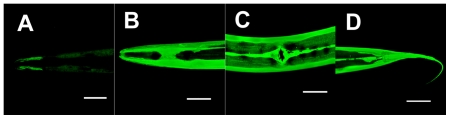
Among all of the known gst genes (WormBase, http://www.wormbase.org/), gst-4, which encodes a sigma class GST, was one of the most up-regulated genes and most prominently expressed when the nematode was treated with the harmful food substance acrylamide [15]. A transcriptional reporter gene (promoter-driven gfp transcription) constructed for the gst-4 promoter (gst-4p) was introduced into C. elegans to obtain the transgenic nematode MJCU032 {kEx32 [gst-4p::gfp, pDP#MM016B]}. Because an extrachromosomal (Ex) line sometimes shows unstable mosaic expression, we constructed the chromosomally-integrated line MJCU049 {kIs48 [gst-4p::gfp, pDP#MM016B] V} as a nematode biosensor. Its expression pattern did not differ from that of the Ex line and was very stable. We confirmed that it showed a typical response against a GST inducer; that is, the nematode biosensor MJCU049 constitutively emitted a residual dim amount of the GFP signal when it was grown on NGM plates and increased intensities when grown on NGM plates containing the GST inducer acrylamide (Figure 1).
Figure 1. Expression patterns in MJCU049 {kIs48 [gst-4p::gfp, pDP#MM016B] V}.
Photographs were taken by laser-scanned fluorescence microscopy. (A) Control without acrylamide. Weak GFP signal was detected from hypodermis, body wall muscle, and intestine. (B, C, D) With 500 mg/L acrylamide. Strong GFP signals were detected from the whole body. (B) Head region. (C) Vulval region. (D) Tail region. Scale bars, 50 µm.
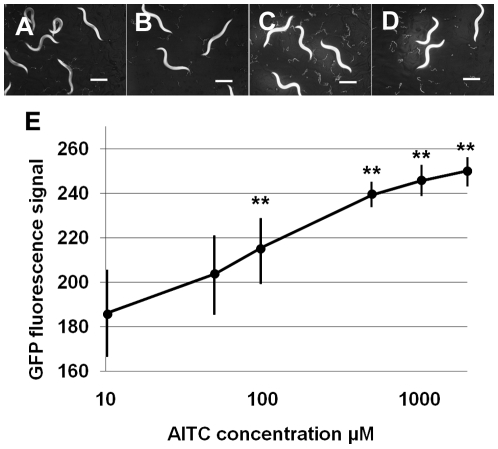
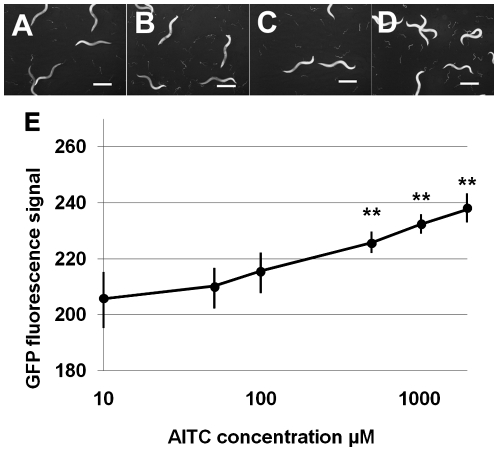
Next we used the software FL 1.0.0.1 to analyze the GFP signal kinetics of the nematode biosensor in response to AITC. Figure 2 shows the GFP fluorescence kinetics of MJCU049 in response to AITC exposure at concentrations from 10 µM to 2 mM. Graphs indicate the mean values ± S.E. (N = 23 to 31) for GFP fluorescence signals at the various AITC concentrations from two independent experiments. The GFP fluorescence signal reached the statistically significant strength at or over 100 µM AITC (Figure 2). All nematodes in every AITC concentration were alive 24 hours after exposure to AITC.
Figure 2. Fluorescence images of MJCU049 exposed to each AITC concentration.
(A) Control 0 µM AITC. (B) 100 µM AITC. (C) 1 mM AITC. (D) 2 mM AITC. (E) GFP fluorescence signals were measured and plotted. GFP signals increased at concentrations of more than 100 µM (Kruskal-Wallis test, ** p<0.005) in a dose-dependent manner. Scale bars, 500 µm.
AITC Effects on C. elegans Lifespan
Because AITC induced GST-4 expression at concentrations over 100 µM, we next analyzed the effects of AITC on the C. elegans lifespan. We treated the nematodes with various AITC concentrations (10 µM to 2 mM) from just-hatched L1 stage (L1 hatchee) or from L4 stage and measured lifespan at 25°C. The mean lifespan of nematodes grown on control plates (without AITC) was 11.4 days, and no adverse effects on the mean lifespan were observed at AITC concentrations of up to 1 mM. But the mean lifespan was significantly reduced when the nematodes were treated with 2 mM AITC from L1 (10.8 days, P<0.05) and L4 (10.3 days, P<0.005) stage (Table 1).
Table 1. Effect of AITC on C. elegans lifespan at 25°C.
| Concentration | Average ± SEM | Repeat | Med | Max | % differencea | P valueb | Total nematode |
| 0 µM | 11.4±0.13 | 4 | 11 | 19 | 394 | ||
| 10 µM L4- | 11.1±0.20 | 2 | 11 | 17 | −2.13 | 0.269 | 116 |
| 10 µM L1- | 11.0±0.21 | 2 | 11 | 18 | −3.42 | 0.139 | 117 |
| 50 µM L4- | 11.3±0.27 | 2 | 11 | 19 | −0.50 | 0.980 | 98 |
| 50 µM L1- | 11.6±0.23 | 2 | 12 | 17 | +1.89 | 0.713 | 109 |
| 100 µM L4- | 11.4±0.21 | 3 | 11 | 21 | +0.05 | 0.693 | 187 |
| 100 µM L1- | 11.3±0.20 | 3 | 11 | 19 | −0.34 | 0.965 | 179 |
| 500 µM L4- | 11.0±0.20 | 3 | 11 | 20 | −3.09 | 0.298 | 174 |
| 500 µM L1- | 10.8±0.20 | 3 | 11 | 20 | −5.36 | 0.052 | 175 |
| 1 mM L4- | 11.1±0.20 | 3 | 11 | 18 | −2.72 | 0.484 | 186 |
| 1 mM L1- | 10.8±0.20 | 3 | 10 | 19 | −4.92 | 0.067 | 168 |
| 2 mM L4- | 10.3±0.15 | 4 | 10 | 17 | −9.51 | 0.000 | 255 |
| 2 mM L1- | 10.8±0.16 | 4 | 10 | 19 | −5.21 | 0.018 | 209 |
L1- or L4- after the concentration unit µM or mM indicates that nematodes were treated with AITC from either L1 or L4 stage on, respectively.
a, Percent differences of the mean lifespan compared with that of the control.
b, P values were calculated by log-rank test (vs 0 µM).
AITC Effect on C. elegans Fecundity
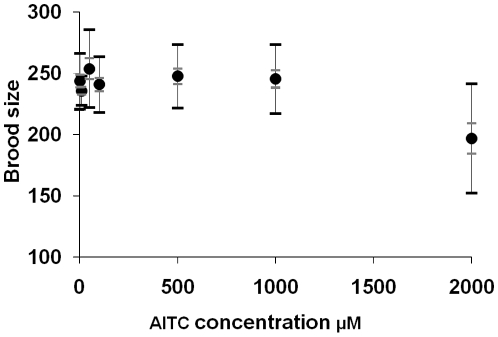
The effects of AITC on the nematodes' reproduction were analyzed by measuring the brood size of the wild-type N2. We treated nematodes from L1 hatchees with various AITC concentrations (0 µM and 10 µM to 2 mM). We did not detect the toxic effects of AITC until 2 mM at which concentration the brood size started to decrease (P<0.05, Figure 3).
Figure 3. AITC effects on the brood size of C. elegans grown at 20°C.
All viable progeny of each nematode parent were counted. The averages ± SEM (inner bars) and SD (outer bars) are shown. The significant differences in brood size were determined by Kruskal-Wallis test (** p<0.005).
AITC Confers Resistance on C. elegans against Oxidative Stresses
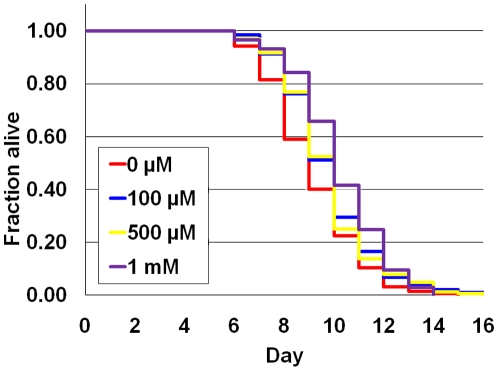
AITC induced GST-4 expression and did not have toxic effects on the nematodes at concentrations from 100 µM to 1 mM. Next, we analyzed the effects of AITC on C. elegans lifespan under oxidative stress conditions. Nematodes from L1 hatchees were treated for five days with AITC (0 µM, 100 µM, 500 µM, and 1 mM) before being transferred onto the NGM plates containing 10 mM paraquat, and then their lifespan was measured. When the nematodes were grown on 10 mM paraquat, the mean lifespan was 9.1 days, but the mean lifespan was extended significantly by treatment with AITC; that is, it increased by 6.98% (p<0.05) at 100 µM, 6.38% (p<0.05) at 500 µM, and 11.6% (p<0.001) at 1 mM (Figure 4, Table 2).
Figure 4. AITC increases resistance of nematodes against 10 mM paraquat.
Compared with nematodes without AITC treatment, lifespan was extended significantly when treated with AITC by 6.98% at 100 µM, by 6.38% at 500 µM, and 11.6% at 1 mM. Survival curves are presented based on three individual experiments.
Table 2. Protective effect of AITC on C. elegans against paraquat.
| Concentration | Average ± SEM | Repeat | Med | Max | % differencea | p valueb | Total nematode |
| 0 µM AITC | 9.13±0.12 | 3 | 9 | 15 | 222 | ||
| 100 µM AITC | 9.76±0.13 | 3 | 10 | 16 | +6.98% | 0.011 | 194 |
| 500 µM AITC | 9.71±0.14 | 3 | 10 | 16 | +6.38% | 0.027 | 168 |
| 1 mM AITC | 10.19±0.20 | 3 | 10 | 14 | +11.6% | 0.000 | 178 |
a, Percent differences of the mean lifespan compared with that of the 0 µM AITC.
b, P values were calculated by log-rank test (compared with 0 µM AITC).
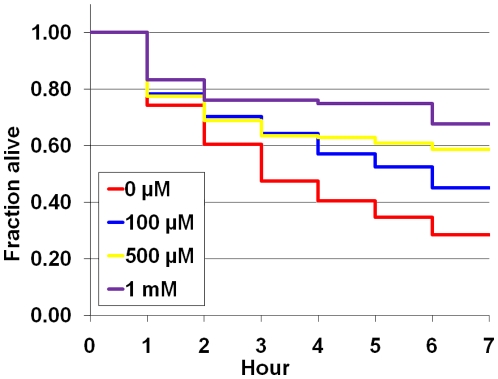
Next, the nematodes were treated for five days with AITC (0 µM, 100 µM, 500 µM, and 1 mM) from L1 hatchees, transferred into S medium containing 200 µM juglone, and checked every hour for viability. When the nematodes were grown on 200 µM juglone alone, the survival rate decreased in a time-dependent manner, i.e., 60.5% (2-hour), 40.5% (4-hour), and 28.4% (6-hour), but the AITC treatment extended the survival rate significantly (Figure 5, Table 3).
Figure 5. AITC increases resistance of C. elegans against 200 µM juglone.
Compared with nematodes without AITC treatment, survival rates were extended significantly when treated with AITC. Survival curves are presented based on three individual experiments.
Table 3. Protective effect of AITC on C. elegans against juglone.
| Survival % | ||||||
| Concentrationa | 2 hour | 4 hour | 6 hour | Repeat | p valueb | Total nematode |
| 0 µM AITC | 60.5% | 40.5% | 28.4% | 3 | 190 | |
| 100 µM AITC | 70.2% | 57.1% | 44.9% | 3 | 0.002 | 198 |
| 500 µM AITC | 68.8% | 62.9% | 58.6% | 3 | 0.000 | 186 |
| 1 mM AITC | 76.0% | 67.7% | 67.7% | 3 | 0.000 | 167 |
a, P values were calculated by log-rank test (compared with 0 µM AITC).
AITC Also Induces UGT-13
As we obtained good evidence that AITC induced GST-4, a member of the phase II enzymes that play central roles for protection against endogenous and exogenous oxidative stresses, we next wanted to examine whether AITC could induce other phase II enzymes. For that task, we chose UGT-13, a member of another large phase II enzyme family of UDP glucuronosyl transferases. We constructed the new transgenic nematode MJCU050 {kIs22 [ugt-13p::gfp, pDP#MM016B]} possessing the ugt-13 gene promoter (ugt-13p) fused with the gfp gene integrated in chromosome III. We confirmed that this nematode biosensor MJCU050, which emitted a residual constitutive level of the GFP signal similarly as did the GST biosensor, responded well against the harmful food substance acrylamide (Figure 6). When treated with AITC, MJCU050 started to express the GFP fluorescence signal over the control level from the 500 µM AITC level on (Figure 7).
Figure 6. Expression patterns in MJCU050 {kIs22 [ugt-13p::gfp, pDP#MM016B] III}.
Photographs were taken with laser-scanned fluorescence microscopy. (A) Control without acrylamide. Weak GFP signal was detected from head. (B, C, D) With 500 mg/L acrylamide. Strong GFP signals were detected from hypodermis. (B) Head region. (C) Vulval region. (D) Tail region. Scale bars, 50 µm.
Figure 7. AITC induces UGT-13 expression.
Fluorescence images of MJCU050 exposed to each AITC concentration from late L4 stage for 24 hours at 25°C. (A) Control 0 µM AITC. (B) 100 µM AITC. (C) 500 µM AITC. (D) 2 mM AITC. (E) GFP fluorescence signals were measured and plotted. GFP signals were increased at concentrations of 500 µM (Kruskal-Wallis test, ** p<0.005) in a dose-dependent manner. Scale bars, 500 µm.
AITC Induces GST-4 and UGT-13 Expression via the Transcription Factor SKN-1
Previously we reported that acrylamide-induced GST-4 expression was partially regulated by the transcription factor SKN-1 [15]. To examine whether the GST-4 and UGT-13 expressions induced by AITC were also regulated under SKN-1 control, we performed knockdown experiments by feeding RNAi.
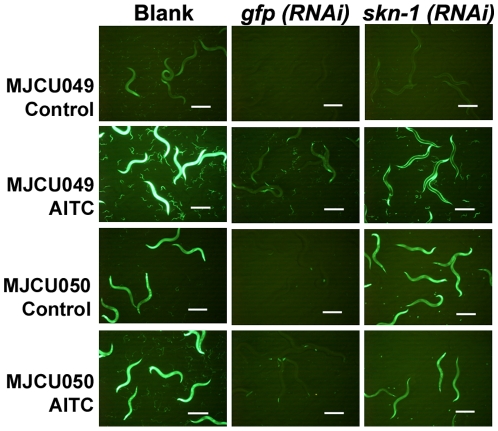
Whereas without AITC both MJCU049 and MJCU050 constitutively emitted a dim amount of the GFP signals, upon exposure to 2 mM AITC they each emitted much stronger GFP signals from the whole body (Figures 8, S1, S2). The knockdown of gfp by feeding RNAi inhibited the residual constitutive expression for both GST-4 and UGT-13, as expected. Although the RNAi feeding knockdown of skn-1 prevented the constitutive GST-4 expression, it failed to prevent the constitutive UGT-13 expression, thus suggesting that such expression is not under SKN-1 control (Figure 8). SKN-1, however, did regulate the AITC-induced UGT-13 expression. We also found that SKN-1 regulated AITC-induced GST-4 expression except for that in the body-wall muscle.
Figure 8. AITC induces GST-4 and UGT-13 expression via the transcription factor SKN-1.
Although the residual amounts of both GST-4 and UGT-13 were observed to be expressed constitutively without AITC (Control), their expressions were induced well over the constitutive level after AITC treatment. Both constitutive and inducible expressions of GST-4 were suppressed by gfp and skn-1 (RNAi). Inducible expression of UGT-13 was also suppressed by gfp and skn-1 (RNAi), but constitutive expression of UGT-13 was suppressed by only gfp (RNAi), but not by skn-1 (RNAi). Inducible GST-4 expression in neither pharynx nor body-wall muscle was suppressed by skn-1 (RNAi) (Hasegawa et al., 2008). Inducible GST-4 expression in the pharynx was not suppressed by gfp (RNAi) indicating that RNAi, itself, does not work in the pharynx. Scale bars, 500 µm.
Discussion
Primarily because of improvements in public health and lifestyle, our life expectancy today is much longer than before. It means at the same time that we have a higher chance of suffering from age-related diseases as we live longer. Thus, we need to seek ways to improve the quality of our life, especially later in our life. One way to do so is to find foods or food ingredients that reduce the risks of suffering from cancer or age-related diseases. Inducers of phase II enzymes without adverse effects would be candidates for reducing such risks.
GSTs and UGTs comprise a large family of enzymes, which generally exist in every organism from bacteria to humans, and they are considered major players in the phase II detoxification of both endogenous oxidative stress products and exogenous electrophilic chemical compounds [9], [16], [17]. The C. elegans genome contains 52 gst- and 72 ugt-coding genes. The 52 gst genes belong to the Alpha, Sigma, Omega, Zeta, and Pi classes (WormBase, http://www.wormbase.org/), and their expression patterns were found to vary uniquely [15]. GST-4, which encodes a sigma class GST, was one of the most prominently up-regulated genes when the nematode was treated with the harmful food substance acrylamide [15], the food contaminant methylmercury (Hasegawa and Miwa, unpublished result), and the oxidative stressors paraquat and juglone [18], [19]. The GST-4 expression correlated with oxidative stress tolerance in a dose-dependent manner [19].
AITC, formed from glucosinolate, is an allelochemical compound having an isothiocyanate (ITC, –N = C = S) group, which is widely found among various cruciferous vegetables, such as broccoli, cabbage, and cauliflower [10], [20]. Isothiocyanates have been reported to induce several phase II enzymes in vitro and in vivo [11]–[13] and protect mammalian cultured cells against oxidative damage [7], [21], [22]. The nematode biosensor successfully evaluated AITC as being a very good GST-4 and UGT-13 inducer, suggesting that AITC might confer on the nematode resistance to oxidative stress. We could determine the optimal AITC concentration for the nematode biosensor; that is, AITC concentrations from 100 µM to 1 mM strongly induced both GST-4 and UGT-13 in vivo without causing adverse effects on either nematode fecundity or lifespan. The high concentration of 2 mM AITC, however, exerted a toxic effect on the nematodes, as it decreased both their fecundity and lifespan. Not only beneficial effects, but also toxic effects such as carcinogenicity and mutagenicity have been reported [23], [24]. High concentrations of isothiocyanates were reported to kill the potato cyst nematode Globodera rostochiensis and the eggs of the black vine weevil Otiorhynchus sulcatus, and their potential use as a pesticide for controlling nematodes or weevils in the soil was discussed [25], [26]. The mechanism of toxicity such as killing, however, remains to be investigated. AITC was also reported to elicit acute pain and neurogenic inflammation by activating TRPA1, a member of the mammalian transient receptor potential ion channel family [27], [28]. C. elegans TRPA-1, the ortholog of the mammalian TRPA1, was suggested to function in mechanosensation [29]. It is interesting to ask if TRPA-1 exerts a protective role against juglone or paraquat in response to AITC and/or if TRPA-1 is also under the SKN-1 regulation (Figures S3, S4).
With C. elegans as a monitoring organism, many synthetic and natural compounds that increased C. elegans lifespan have been discovered, including the anticonvulsants ethosuximide, trimethadione, and valproic acid, the antidepressant 3,3-diethyl-2-pyrrolidones [30]–[32], blueberry polyphenol [33], extracts of Eleutherococcus senticosus and Rhodioa rosea [34], and an extract of Ginkgo biloba [35], the last of which also increased survival rate against several stresses. Epigallocatechin gallate (EGCG) did not extend C. elegans lifespan but attenuated the age-related behavioral decline [36] and extended survival rate under heat stress and oxidative stress by inducing SOD-3 and HSP-16.2 [37]. Noticeably, EGCG induced skn-1 mRNA [37], possibly suggesting that EGCG, like AITC in our present report, might have induced phase II enzymes, which consequently would have conferred oxidative stress tolerance on the nematode. Our biosensor would easily test this hypothesis.
In mammals, several phase II enzymes, like GST, are regulated by the bZIP transcriptional factor Nrf2 in response to oxidative or electrophilic stresses [8]. Without such stresses, Nrf2 is normally repressed by the oxidative stress sensor Keap1 [38], which binds and degrades Nrf2 through the ubiquitin-proteasome system. Under oxidative stress conditions, Nrf2 is released from Keap1 into the nucleus where it induces the expression of phase II enzymes [38]. In C. elegans, the transcription factor SKN-1 is found to function similarly to Nrf2 by inducing GCS-1 expression in response to oxidative stresses [39] and GST-4 expression in response to acrylamide [15]. And also in C. elegans, WDR-23, a C. elegans homolog of mammalian WDR23 and a WD40 repeat protein, seems to function similarly to the mammalian Keap1 [40] (Hasegawa and Miwa, unpublished results). Although without stresses MJCU049 and MJCU050 expressed a very low amount of a constitutive level for both GST-4 and UGT-13, treating them with AITC induced these enzymes well over the constitutive level. SKN-1 regulated both the constitutive and inductive expressions of GST-4; however, it did not regulate the constitutive expression of UGT-13. This result suggests that some factor other than SKN-1 should regulate the constitutive expression of UGT-13. The present nematode biosensor is not only a tool to evaluate and discover functional food ingredients but also would be a powerful genetic tool to dissect the GST and UGT expression pathways and to analyze their regulatory mechanisms (Hasegawa, Nonomura, Kondo, and Miwa, unpublished results).
Because the nematode biosensor we have established here takes direct advantage of its own sensing and responding systems against xenobiotic chemicals, we could apply it to (a) detect and evaluate harmful chemicals (15), (b) discover chemicals that ameliorate, reduce, or neutralize harmful chemicals [14], and (c) discover chemicals that improve our health or extend our longevity. As such is the case, the nematode biosensor serves as a handy, rapid, and inexpensive method not only to evaluate but also to discover chemicals, both harmful and useful, whether they are known or unknown, albeit an obvious caution is required when results so obtained are applied to humans.
Materials and Methods
Nematode Strains and Culturing
C. elegans var. Bristol, strain N2 [41] and unc-119 (ed3) [42] were obtained from the Caenorhabditis elegans Genetics Center (University of Minnesota, Minnesota, Minneapolis, MN, USA). The nematodes were cultured and handled according to the procedures described by Brenner [41]. Allyl-isothiocyanate (AITC)-containing NGM plates were prepared as follows: 100 µL of AITC (Tokyo Chemical Industry, Tokyo, Japan) was diluted in 5 ml of 100% ethanol (Wako, Tokyo, Japan) to prepare the 200 mM stock solution. This was then serially diluted in 100% ethanol as described [43]. Fifty micro liters of each AITC concentration or ethanol as a control was added directly into a 6 cm Petri dish, and 5 mL of NGM agar was pipetted into the dish and immediately mixed by gentle swirling. All AITC plates were prepared two days before use and seeded with Escherichia coli OP50 one day before use.
Nematode Biosensors
Because of their potent responses against several xenobiotics (acrylamide, methylmercury, and chloropropanols, Hasegawa and Miwa, unpublished results), we selected two phase II enzymes, UGT-13 and GST-4, and made their GFP reporter constructs for use as nematode biosensors. PCR was performed with KOD Plus DNA polymerase (TOYOBO, Osaka, Japan) on N2 genomic DNA. The PCR primers for ugt-13 and gst-4 were designed to amplify each predicted promoter upstream from each predicted start site; UGT-13For_SphI, 5′ – AAG GGG CAT GCA TTA TTA TGT TCC TAT TTC TTT TA – 3′, UGT-13pRev_BamHI, 5′ – CGG GAT CCC ATT GGG AAT TTT TCT TGA AAC AA – 3′, GST-4ForVer3_SalI, 5′ – GGG TCG ACT TTT GCA GAC TAA AAA TAA CTA CTC TG – 3′, GST-4pRev_BamHI, 5′ – CGG GAT CCC ATA ATT AGA ATT CAG TAA TTG AAT CG – 3′. PCR-amplified DNA fragments were digested with the appropriate restriction enzymes and then ligated into the gfp vector pPD95.77 (kindly provided by A. Fire, Stanford University) to obtain the reporter constructs. Each reporter construct (100 µg/mL) so obtained was co-injected with an equal concentration of pDP#MM016B into the gonadal arms of unc-119 (ed3) adult hermaphrodites as described [44] to obtain MJCU020 {kEx20 [ugt-13p::gfp, pDP#MM016B]} and MJCU032 {kEx32 [gst-4p::gfp, pDP#MM016B]}. Each transgene extrachromosomal array was chromosomally integrated by the method of Mitani [45] and outcrossed two times with N2 to obtain MJCU049 {kIs48 [gst-4p::gfp, pDP#MM016B]} and MJCU050 {kIs22 [ugt-13p::gfp, pDP#MM016B]}. SNP (Single Nucleotide Polymorphism) -based mapping [46] located the fusion gene gst-4p::gfp of MJCU049 integrated in the linkage group V and, similarly, the fusion gene ugt-13p::gfp of MJCU050 in the linkage group III (data not shown). GFP expression patterns were observed with both a Nikon SMZ800 dissection microscope equipped with a fluorescence filter (GFP LP filter) and a ZEISS Axiovert 200 microscope equipped with a confocal laser-scanning module.
GFP Signal Kinetics
Synchronized L1-stage transgenic nematodes were obtained by treating egg-containing adults with sodium hypochlorite [47] and allowed to grow on NGM plates seeded with E. coli OP50 at 20°C for 48 hours until the late L4 stage. About one hundred of these nematodes were collected and put onto each OP50-seeded 6 cm NGM plate, with each concentration of AITC or without it (control), and incubated at 25°C. After 24 hours of incubation, stereo photomicrographs of individual nematodes were taken with a Nikon SMZ800 equipped with a GFP fluorescence module. GFP fluorescence signals from the individual nematodes were measured from the photomicrographs with a nematode image analyzer “FL ver. 1.0.0.1” (http://www.vision.cs.chubu.ac.jp/fl1001/). Nonparametric one-way ANOVA (Excel Tokei 2006, SSRI, Tokyo, Japan) was used to determine the significance of differences in the mean GFP signal values.
Brood Size Counting
All experiments were carried out at 20°C. Synchronized first larval stage (L1) nematodes (N2 strain) were transferred onto the bacterial-lawned NGM plates with AITC at various concentrations or without it (control) and incubated for two days. Single late L4 stage nematodes were then transferred onto the bacterial-lawned NGM spot plates with AITC at various concentrations or without it (control), and total numbers of fertilized eggs laid were counted by using a Nikon SMZ800. Nonparametric one-way ANOVA (Excel Tokei 2006, SSRI, Tokyo, Japan) was used to determine the significance of differences in the mean brood sizes.
Lifespan Measurement
Synchronized L1 stage nematodes (N2 strain) were transferred onto the bacterial-lawned NGM plate with AITC at various concentrations or without it (control) and incubated for two days at 20°C. Two-day old (Late L4 stage) nematodes were then transferred onto 2.5 mg/L 5-fluoro-2-deoxyuridine (FdUrd, Sigma) -containing NGM plates seeded with E. coli OP50 with each AITC concentration or without it, and incubated at 25°C. This time point represented the first day of life-span analysis. Three-day old nematodes (five-days after synchronization) were again transferred onto the fresh AITC or control FdUrd plates and checked daily for viability by using a Nikon SMZ800. A nematode was considered dead when it no longer responded to light prodding with a platinum wire. Survival curves were analyzed by the Kaplan-Meier procedure, and significant differences between survival curves were calculated by the log-rank test with statistical software Excel Tokei 2006 (SSRI, Tokyo, Japan). All fresh AITC plates were prepared on the day before use.
Stress Resistance Test
Synchronized three-day old (five days after synchronization) adults treated with each AITC concentration were prepared as described above. For the oxidative stress resistance assay, AITC-treated three-day old nematodes (five days after hatching) were transferred onto NGM plates containing 10 mM paraquat (Wako, Osaka, Japan), or into wells of a 48-well plate containing S medium [47] and 200 µM juglone (Calbiochem, Darmstadt, Germany) in a total volume of 200 µL per well. Both assays were incubated at 20°C and were checked for viability daily (for the paraquat test) or every hour (for the juglone test) by using a Nikon SMZ800.
RNAi
Gene fragments of skn-1 or gfp were prepared by PCR amplification of C. elegans N2 cDNA or plasmid vector pPD95.77 with the following primers: Ceskn1EcoRI_For, 5′ – GGA ATT CGG CCA ATC CAA ATA TGA TTA TCC A – 3′; Ceskn1EcoRI_Rev, 5′ – GGA ATT CGG GCA GCA ACC TTG TTC TTT CCG – 3′ or GFPFor_EcoRI, 5′ – GGG AAT TCA CTG GAG TTG TCC CAA TTC TT – 3′; GFPRev_EcoRI, 5′ – GGG AAT TCA TCC ATG CCA TGT GTA ATC CC – 3′. Both of the skn-1- or gfp-derived PCR fragments were digested with EcoRI and cloned into the EcoRI restriction site of the RNAi vector pPD129.36 (Kindly provided by Fire, A., Stanford University). The PCR fragment-ligated plasmids or the blank vector pPD129.36 were used to transform E. coli HT115 [48].
For RNAi experiments, synchronized L1-stage transgenic nematodes were first grown for 48 hours at 20°C with NGM (containing 50 µg/ml ampicillin and 12.5 µg/ml tetracycline) plates seeded with E. coli HT115 transformed with each different RNAi plasmid. Thereafter, the nematodes were collected and transferred onto NGM plates, with or without 2 mM AITC, seeded with each different E. coli HT115 RNAi bacteria, and grown for 24 hours at 20°C. The nematodes were observed for GFP expression by using a Nikon SMZ800 dissection microscope equipped with a GFP fluorescence filter.
Supporting Information
GFP fluorescence signals were measured. GST-4 expression was induced with 2 mM AITC (p<0.005) over its constitutive expression. Both constitutive and inducible GST-4 expressions were suppressed by skn-1 (RNAi) (p<0.005) except for in the body-wall muscle and pharynx. For a more detailed explanation, refer to the Figure 8 legend. Significance of differences in the mean GFP signal values was calculated by nonparametric one-way ANOVA.
(0.10 MB TIF)
GFP fluorescence signals were measured. UGT-13 expression was induced with 2 mM AITC (p<0.005) over its constitutive expression. Inducible UGT-13 expression was suppressed by skn-1 (RNAi) (p<0.005), but constitutive UGT-13 expression was not. For a more detailed explanation, refer to the Figure 8 legend. Significance of differences in the mean GFP signal values was calculated by nonparametric one-way ANOVA.
(0.10 MB TIF)
AITC conferred resistance against 200 µM juglone, but this resistance essentially disappeared by skn-1 (RNAi), suggesting that the AITC-induced protection observed arose only through the AITC-activated skn-1 activity. Animals were treated with 1 mM AITC with or without skn-1 (RNAi) from L1 stage; and juglone resistance assay was performed as described in Materials and Methods. Survival curves (based on three individual experiments) were analyzed by the Kaplan-Meier procedure, and significant differences between survival curves were calculated by the log-rank test.
(0.10 MB TIF)
AITC conferred resistance against 10 mM paraquat. Not only this resistance disappeared by skn-1 (RNAi) but resistance reduced below even that for controls, suggesting that SKN-1 has a protective role against paraquat without AITC. Animals were treated with 1 mM AITC with or without skn-1 (RNAi) from L1 stage, and the paraquat resistance assay was performed as described in Materials and Methods. Survival curves (based on two individual experiments) were analyzed by the Kaplan-Meier procedure, and significant differences between survival curves were calculated by the log-rank test.
(0.10 MB TIF)
Acknowledgments
We are grateful to the Caenorhabditis elegans Genetics Center for providing nematode strains. We thank Dr. Hironobu Fujiyoshi and Dr. Shoichi Shimizu, Chubu University, for providing the fluorescence analyzing software FL ver. 1.0.0.1.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a Japan Science and Technology (JST) research fund to J.M., a Japan Society for the Promotion of Science (JSPS) research fund to K.H. of the Japanese Ministry of Education, Culture, Sports, Science and Technology, and a Chubu University Special Fund to J.M.. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Riddle DL, Blumenthal T, Meyer BJ, Priess JR. Introduction to C. elegans. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. New York: Cold Spring Harbor Laboratory; 1997. pp. 1–22. [Google Scholar]
- 2.Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science. 2002;297:623–626. doi: 10.1126/science.1073759. [DOI] [PubMed] [Google Scholar]
- 3.Kurz LC, Tan MW. Regulation of aging and innate immunity in C. elegans. Aging Cell. 2004;3:185–193. doi: 10.1111/j.1474-9728.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim DH, Ausubel FM. Evolutionary perspectives on innate immunity from the study of Caenorhabditis elegans. Curr Opin Immunol. 2005;17:4–10. doi: 10.1016/j.coi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Lindblom TH, Dodd AK. Xenobiotic detoxification in the nematode Caenorhabditis elegans. J Exp Zool A Comp Exp Biol. 2006;305:720–730. doi: 10.1002/jez.a.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benz CC, Yau C. Ageing, oxidative stress and cancer: paradigms in parallax. Nature Reviews Cancer. 2008;8:875–879. doi: 10.1038/nrc2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao X, Dinkova-Kostova AT, Talalay P. Powerful and prolonged protection of human retinal pigment epithelial cells, keratinocytes, and mouse leukemia cells against oxidative damage: the indirect antioxidant effects of sulforaphane. Proc Natl Acad Sci USA. 2001;98:15221–15226. doi: 10.1073/pnas.261572998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Ann Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 9.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferase. Ann Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 10.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochem. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 11.Munday R, Munday CM. Induction of phase II detoxification enzymes in rat by plant-derived isothiocyanates: comparison of allyl isothiocyanate with sulforaphane and related compounds. J Agric Food Chem. 2004;52:1867–1871. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Munday R, Jobson HE, Munday CM, Lister C, et al. Induction of GST and NQO1 in cultured bladder cells and in the urinary bladders of rats by an extract of broccoli (Brassica oleracea italica) sprouts. J Agric Food Chem. 2006;54:9370–9376. doi: 10.1021/jf062109h. [DOI] [PubMed] [Google Scholar]
- 13.Angeloni C, Leoncini E, Malaguti M, Angelini S, Hrelia P, et al. Modulation of phase II enzymes by sulforaphane: implications for its cardioprotective potential. J Agric Food Chem. 2009;57:5615–5622. doi: 10.1021/jf900549c. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa K, Miwa S, Tajima T, Tsutsumiuchi K, Taniguchi H, et al. A rapid and inexpensive method to screen for common foods that reduce the action of acrylamide, a harmful substance in food. Toxicol Letters. 2007;175:82–88. doi: 10.1016/j.toxlet.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa K, Miwa S, Isomura K, Tsutsumiuchi K, Taniguchi H, et al. Acrylamide-responsive genes in the nematode Caenorhabditis elegans. Toxicol Sci. 2008;101:215–225. doi: 10.1093/toxsci/kfm276. [DOI] [PubMed] [Google Scholar]
- 16.Hundle BS, O'Brien DA, Alberti M, Beyer P, Hearst JE. Functional expression of eaxanthin glucosyltransferase from Erwinia herbicola and a proposed uridine diphosphate binding site. Proc Natl Acad Sci USA. 1992;89:9321–9325. doi: 10.1073/pnas.89.19.9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bock KW. Vertebrate UDP-glucuronosyltransferases: functional and evolutionary aspect. Biochem Pharmacol. 2003;66:691–696. doi: 10.1016/s0006-2952(03)00296-x. [DOI] [PubMed] [Google Scholar]
- 18.Tawe WN, Eschbach ML, Walter RD, Henkle-Dührsen K. Identification of stress-responsive genes in Caenorhabditis elegans using RT-PCR differential display. Nuc Acid Research. 1998;26:1621–1627. doi: 10.1093/nar/26.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leiers B, Kampkötter A, Grevelding CG, Link CD, Johnson TE, et al. A stress-responsive glutathione S-transferase confers resistance to oxidative stress in Caenorhabditis elegans. Free Rad Biol Med. 2003;34:1405–1415. doi: 10.1016/s0891-5849(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 20.Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, et al. Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem. 1999;47:1541–1548. doi: 10.1021/jf980985s. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Jia Z, Strobl JS, Ehrich M, Misra HP, et al. Potent induction of total cellular and mitochondrial antioxidants and phase 2 enzymes by cruciferous sulforaphane in rat aortic smooth muscle cells: cytoprotection against oxidative and electrophilic stress. Cardiovasc Toxicol. 2008;8:115–125. doi: 10.1007/s12012-008-9020-4. [DOI] [PubMed] [Google Scholar]
- 22.Higgins LG, Kelleher MO, Eggleston IM, Itoh K, Yamamoto M, et al. Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox-cycling agents. Toxicol Appl Pharmacol. 2009;237:267–280. doi: 10.1016/j.taap.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 23.IARC (International Agency for Research on Cancer) monographs on the evaluation of carcinogenic risks to humans. Allyl isothiocyanate. 1999. (IARC, Lyon) vol. 73 pp37-48. http://monographs.iarc.fr/ENG/Monographs/vol73/mono73-6.pdf.
- 24.Masutomi N, Toyoda K, Shibutani M, Niho N, Uneyama C, et al. Toxic effects of benzyl and allyl isothiocyanates and benzyl-isoform specific metabolites in the urinary bladder after a single intravesical application to rats. Toxicol Pathol. 2001;29:617–622. doi: 10.1080/019262301753385942. [DOI] [PubMed] [Google Scholar]
- 25.Borek V, Elberson LR, McCaffrey JP, Morra MJ. Toxicity of isothiocyanates produced by glucosinolates in Brassicaceae species to black vine weevil eggs. J Agric Food Chem. 1998;46:5318–5323. [Google Scholar]
- 26.Buskov S, Serra B, Rosa E, Sørensen H, Sørensen JC. Effects of intact glucosinolates and products produced from glucosinolates in myrosinase-catalyzed hydrolysis on the potato cyst nematode (Globodera rostochiensis Cv. Woll) J Agric Food Chem. 2002;50:690–695. doi: 10.1021/jf010470s. [DOI] [PubMed] [Google Scholar]
- 27.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinman A, Chuang H-h, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, et al. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nature Neuroscience. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- 30.Evason K, Huang C, Yamben I, Covey DF, Kornfeld K. Anticonvulsant medications extend worm life-span. Science. 2005;307:258–261. doi: 10.1126/science.1105299. [DOI] [PubMed] [Google Scholar]
- 31.Petrascheck M, Ye X, Buck LB. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature. 2007;450:553–557. doi: 10.1038/nature05991. [DOI] [PubMed] [Google Scholar]
- 32.Evason K, Collins JJ, Huang C, Hughes S, Kornfeld K. Valproic acid extends Caenorhabditis elegans lifespan. Aging Cell. 2008;7:305–317. doi: 10.1111/j.1474-9726.2008.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, et al. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiegant FAC, Surinova S, Ytsma E, Langelaar-Makkinje M, Wikman G. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontol. 2008;10:27–42. doi: 10.1007/s10522-008-9151-9. [DOI] [PubMed] [Google Scholar]
- 35.Kampkötter A, Pielarski T, Rohring R, Timpel C, Chovolou Y, et al. The Ginkgo biloba extract EGb761 reduced stress sensitivity, ROS accumulation and expression of catalase and glutathione S-transferase 4 in Caenorhabditis elegans. Pharmacol Research. 2006;55:139–147. doi: 10.1016/j.phrs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Brown MK, Evans JL, Luo Y. Beneficial effects of natural antioxidants EGCG and α-lipolic acid on life span and age-dependent behavioral decline in Caenorhabditis elegans. Pharmacol Biochem Behavior. 2006;85:620–628. doi: 10.1016/j.pbb.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Jie G, Zhang J, Zhao B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Rad Biol Med. 2009;46:414–421. doi: 10.1016/j.freeradbiomed.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Bio. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–1893. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choe KP, Przybysz AJ, Strange K. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol. 2009;29:2704–2715. doi: 10.1128/MCB.01811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenner S. The genetics of the nematode Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maduro M, Pilgrim D. Conservation of function and expression of unc-119 from two Caenorhabditis species despite divergence of non-coding DNA. Gene. 1996;183:77–85. doi: 10.1016/s0378-1119(96)00491-x. [DOI] [PubMed] [Google Scholar]
- 43.Hasegawa K, Miwa S, Tsutsumiuchi K, Taniguchi H, Miwa J. Extremely low dose of acrylamide decreases lifespan in Caenorhabditis elegans. Toxicol Letters. 2004;152:183–189. doi: 10.1016/j.toxlet.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 44.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitani S. Genetic regulation of mec-3 gene expression implicated in the specification of the mechanosensory neuron cell types in Caenorhabditis elegans. Dev Growth Differ. 1995;37:551–557. doi: 10.1046/j.1440-169X.1995.t01-4-00010.x. [DOI] [PubMed] [Google Scholar]
- 46.Davis MW, Hammarlund M, Harrach T, Hullett P, Olsen S, et al. Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics. 2005;6:118. doi: 10.1186/1471-2164-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stiernagle T. Maintenance of C. elegans. . In: Hope I, editor. C. elegans, a practical approach. Oxford: Oxford University Press; 1999. pp. 51–67. [Google Scholar]
- 48.Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2001;2:research0002. doi: 10.1186/gb-2000-2-1-research0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GFP fluorescence signals were measured. GST-4 expression was induced with 2 mM AITC (p<0.005) over its constitutive expression. Both constitutive and inducible GST-4 expressions were suppressed by skn-1 (RNAi) (p<0.005) except for in the body-wall muscle and pharynx. For a more detailed explanation, refer to the Figure 8 legend. Significance of differences in the mean GFP signal values was calculated by nonparametric one-way ANOVA.
(0.10 MB TIF)
GFP fluorescence signals were measured. UGT-13 expression was induced with 2 mM AITC (p<0.005) over its constitutive expression. Inducible UGT-13 expression was suppressed by skn-1 (RNAi) (p<0.005), but constitutive UGT-13 expression was not. For a more detailed explanation, refer to the Figure 8 legend. Significance of differences in the mean GFP signal values was calculated by nonparametric one-way ANOVA.
(0.10 MB TIF)
AITC conferred resistance against 200 µM juglone, but this resistance essentially disappeared by skn-1 (RNAi), suggesting that the AITC-induced protection observed arose only through the AITC-activated skn-1 activity. Animals were treated with 1 mM AITC with or without skn-1 (RNAi) from L1 stage; and juglone resistance assay was performed as described in Materials and Methods. Survival curves (based on three individual experiments) were analyzed by the Kaplan-Meier procedure, and significant differences between survival curves were calculated by the log-rank test.
(0.10 MB TIF)
AITC conferred resistance against 10 mM paraquat. Not only this resistance disappeared by skn-1 (RNAi) but resistance reduced below even that for controls, suggesting that SKN-1 has a protective role against paraquat without AITC. Animals were treated with 1 mM AITC with or without skn-1 (RNAi) from L1 stage, and the paraquat resistance assay was performed as described in Materials and Methods. Survival curves (based on two individual experiments) were analyzed by the Kaplan-Meier procedure, and significant differences between survival curves were calculated by the log-rank test.
(0.10 MB TIF)