Abstract
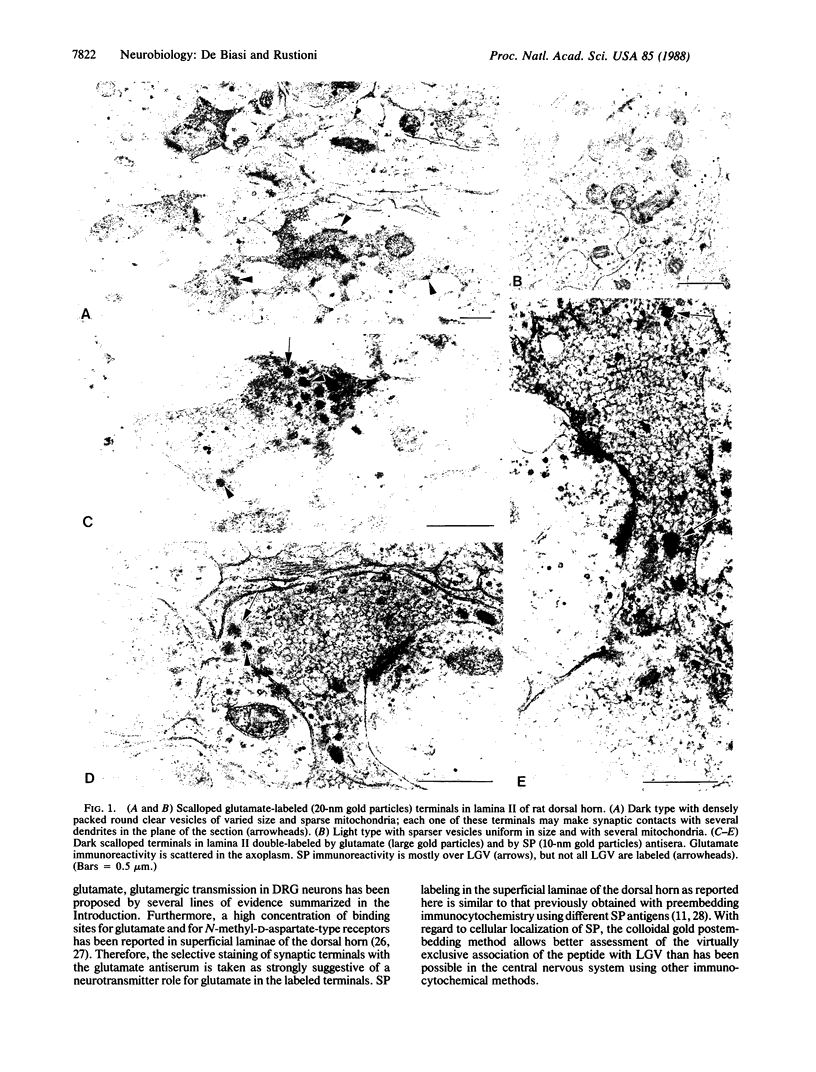
By light microscopic immunocytochemistry it has been previously shown that approximately equal to 70% of the neurons in rat dorsal root ganglia are labeled with an antiserum for glutamate conjugated to hemocyanin; the smaller among these neurons are also positive for substance P. By using a postembedding ImmunoGold method and electron microscopy, it is shown here that synaptic terminals in the superficial laminae of the spinal cord of rats selectively stain for the same glutamate antiserum. Immunolabeling is in small dome-shaped and in large scalloped synaptic terminals. Scalloped terminals are of two types. One type consists of dark terminals with many agranular vesicles of different size and a few large granular vesicles; these are probably endings of unmyelinated and small myelinated primary afferent fibers. The other type consists of light terminals with small agranular vesicles homogeneous in size with neurofilaments and many mitochondria; these are probably endings of larger myelinated primary afferent fibers. By means of double-labeling electron microscopic immunocytochemistry with colloidal gold particles of two different sizes, it is also shown here that substance P is present in only the dark type of glutamate-labeled scalloped terminals. The primary afferent origin of the terminals labeled by the antisera for glutamate and for substance P is demonstrated by a triple-labeling strategy: immunocytochemistry for both antisera on sections from rats in which dorsal rhizotomy or dorsal root ganglion injection of horseradish peroxidase conjugated to wheat germ agglutinin was performed. It is proposed that glutamate is the neurotransmitter in primary afferents mediating input from different peripheral receptor classes, including nociceptors. Effects of glutamate and substance P on spinal dorsal horn neurons may result from co-release of these two mediators from the same dorsal root afferent terminal.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbaresi P., Rustioni A., Cuénod M. Retrograde labeling of dorsal root ganglion neurons after injection of tritiated amino acids in the spinal cord of rats and cats. Somatosens Res. 1985;3(1):57–74. doi: 10.3109/07367228509144577. [DOI] [PubMed] [Google Scholar]
- Barber R. P., Vaughn J. E., Slemmon J. R., Salvaterra P. M., Roberts E., Leeman S. E. The origin, distribution and synaptic relationships of substance P axons in rat spinal cord. J Comp Neurol. 1979 Mar 15;184(2):331–351. doi: 10.1002/cne.901840208. [DOI] [PubMed] [Google Scholar]
- Bendayan M., Zollinger M. Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J Histochem Cytochem. 1983 Jan;31(1):101–109. doi: 10.1177/31.1.6187796. [DOI] [PubMed] [Google Scholar]
- Coimbra A., Ribeiro-da-Silva A., Pignatelli D. Effects of dorsal rhizotomy on the several types of primary afferent terminals in laminae I-III of the rat spinal cord. An electron microscope study. Anat Embryol (Berl) 1984;170(3):279–287. doi: 10.1007/BF00318731. [DOI] [PubMed] [Google Scholar]
- Conti F., Rustioni A., Petrusz P., Towle A. C. Glutamate-positive neurons in the somatic sensory cortex of rats and monkeys. J Neurosci. 1987 Jun;7(6):1887–1901. doi: 10.1523/JNEUROSCI.07-06-01887.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A. W., Johnston G. A. Glutamate and related amino acids in cat spinal roots, dorsal root ganglia and peripheral nerves. J Neurochem. 1970 Aug;17(8):1205–1208. doi: 10.1111/j.1471-4159.1970.tb03369.x. [DOI] [PubMed] [Google Scholar]
- Engberg I., Marshall K. C. Reversal potential for Ia excitatory post synaptic potentials in spinal motoneurones of cats. Neuroscience. 1979;4(11):1583–1591. doi: 10.1016/0306-4522(79)90021-6. [DOI] [PubMed] [Google Scholar]
- Greenamyre J. T., Young A. B., Penney J. B. Quantitative autoradiographic distribution of L-[3H]glutamate-binding sites in rat central nervous system. J Neurosci. 1984 Aug;4(8):2133–2144. doi: 10.1523/JNEUROSCI.04-08-02133.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Henry J. L. Effects of substance P on functionally identified units in cat spinal cord. Brain Res. 1976 Sep 24;114(3):439–451. doi: 10.1016/0006-8993(76)90965-3. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Toomim C. S., McCarthy K. D., Conti F., Battaglia G., Rustioni A., Petrusz P. Characterization of antisera to glutamate and aspartate. J Histochem Cytochem. 1988 Jan;36(1):13–22. doi: 10.1177/36.1.2891743. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Substance p: localization in the central nervous system and in some primary sensory neurons. Science. 1975 Nov 28;190(4217):889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L., Cuello A. C. Capsaicin-induced depletion of substance P from primary sensory neurones. Brain Res. 1978 Aug 18;152(1):183–188. doi: 10.1016/0006-8993(78)90146-4. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Yoshioka K., Jahr C. E. Amino acid receptor-mediated transmission at primary afferent synapses in rat spinal cord. J Exp Biol. 1986 Sep;124:239–258. doi: 10.1242/jeb.124.1.239. [DOI] [PubMed] [Google Scholar]
- Knyihar-Csillik E., Csillik B., Rakic P. Ultrastructure of normal and degenerating glomerular terminals of dorsal root axons in the substantia gelatinosa of the rhesus monkey. J Comp Neurol. 1982 Oct 1;210(4):357–375. doi: 10.1002/cne.902100404. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O., Dahlström A., Geffard M., Ahlman H., Ericson L. E. An improved immunocytochemical method for subcellular localization of serotonin in rat enterochromaffin cells. J Histochem Cytochem. 1987 Mar;35(3):319–326. doi: 10.1177/35.3.3819375. [DOI] [PubMed] [Google Scholar]
- Potashner S. J., Tran P. L. Decreased uptake and release of D-aspartate in the guinea pig spinal cord after dorsal root section. J Neurochem. 1984 Apr;42(4):1135–1144. doi: 10.1111/j.1471-4159.1984.tb12722.x. [DOI] [PubMed] [Google Scholar]
- Randić M., Miletić V. Effect of substance P in cat dorsal horn neurones activated by noxious stimuli. Brain Res. 1977 Jun 3;128(1):164–169. doi: 10.1016/0006-8993(77)90245-1. [DOI] [PubMed] [Google Scholar]
- Salt T. E., Hill R. G. Neurotransmitter candidates of somatosensory primary afferent fibres. Neuroscience. 1983 Dec;10(4):1083–1103. doi: 10.1016/0306-4522(83)90101-x. [DOI] [PubMed] [Google Scholar]
- Schneider S. P., Perl E. R. Comparison of primary afferent and glutamate excitation of neurons in the mammalian spinal dorsal horn. J Neurosci. 1988 Jun;8(6):2062–2073. doi: 10.1523/JNEUROSCI.08-06-02062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S. P., Perl E. R. Selective excitation of neurons in the mammalian spinal dorsal horn by aspartate and glutamate in vitro: correlation with location and excitatory input. Brain Res. 1985 Dec 23;360(1-2):339–343. doi: 10.1016/0006-8993(85)91251-x. [DOI] [PubMed] [Google Scholar]
- Schouenborg J., Sjölund B. H. First-order nociceptive synapses in rat dorsal horn are blocked by an amino acid antagonist. Brain Res. 1986 Aug 6;379(2):394–398. doi: 10.1016/0006-8993(86)90798-5. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J., Leknes A. K., Bore A. T., Vaaland J. L., Edminson P., Haug F. M., Ottersen O. P. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature. 1983 Feb 10;301(5900):517–520. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- Urbán L., Randić M. Slow excitatory transmission in rat dorsal horn: possible mediation by peptides. Brain Res. 1984 Jan 9;290(2):336–341. doi: 10.1016/0006-8993(84)90952-1. [DOI] [PubMed] [Google Scholar]
- Wang B. L., Larsson L. I. Simultaneous demonstration of multiple antigens by indirect immunofluorescence or immunogold staining. Novel light and electron microscopical double and triple staining method employing primary antibodies from the same species. Histochemistry. 1985;83(1):47–56. doi: 10.1007/BF00495299. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Puil E. A. Actions of glutamic acid on spinal neurones. Exp Brain Res. 1973 Mar 29;17(1):35–49. doi: 10.1007/BF00234562. [DOI] [PubMed] [Google Scholar]
- de Lanerolle N. C., LaMotte C. C. Ultrastructure of chemically defined neuron systems in the dorsal horn of the monkey. I. Substance P immunoreactivity. Brain Res. 1983 Sep 5;274(1):31–49. doi: 10.1016/0006-8993(83)90519-x. [DOI] [PubMed] [Google Scholar]