Abstract
Carboxyfluoroquinolones, such as ciprofloxacin, are employed for numerous infectious diseases. Renal secretion is a major determinant of their systemic and urinary concentration, but the specific transporters involved are virtually unknown. In vivo studies implicate the organic anion transporter (OAT) family as a pivotal component of carboxyfluoroquinolone renal secretion. Therefore, this study identified the specific renal basolateral OAT(s) involved, thereby highlighting potential sources of carboxyfluoroquinolone-drug interactions and variable efficacy. Two heterologous expression systems, Xenopus laevis oocytes and cell monolayers, were employed to determine the roles of murine and human renal basolateral mOat1/hOAT1 and mOat3/hOAT3. Ciprofloxacin was transported by mOat3 in both systems (Km, 70±6 μM), and demonstrated no interaction with mOat1 or hOAT1. Furthermore, ciprofloxacin, norfloxacin, ofloxacin, and gatifloxacin exhibited concentration-dependent inhibition of transport on mOat3 in cells, with inhibition constants of 198±39, 558±75, 745±165, and 941±232 μM, respectively. Ciprofloxacin and gatifloxacin also inhibited hOAT3. Subsequently, in vivo elimination of ciprofloxacin was assessed in wild-type and Oat3 null mice (Oat3(-/-)). Oat3(-/-) mice exhibited significantly elevated plasma levels of ciprofloxacin at clinically-relevant concentrations (P<0.05, males; P<0.01, females). Oat3(-/-) mice also demonstrated a reduced volume of distribution (27%, P<0.01, males; 14%, P<0.01, females) and increased area under the concentration-time curve (25%, P<0.05, males; 33%, P<0.01, females). Female Oat3(-/-) mice had a 35% (P<0.01) reduction in total clearance of ciprofloxacin relative to wild-type. Additionally, putative ciprofloxacin metabolites were significantly elevated in Oat3(-/-) mice. The present findings indicate that polymorphisms of, and drug interactions on, hOAT3 may influence carboxyfluoroquinolone efficacy, especially in urinary tract infections.
Introduction
Ciprofloxacin is a broad-spectrum antimicrobial that is employed in the treatment of numerous infectious diseases, including those afflicting the skin (Lipsky et al., 1999), bone (Gasem et al., 2003), gastrointestinal tract (Hsieh et al., 1998), genitourinary system (Wagenlehner et al., 2006), lungs (Jones, 2002), and meninges (Gogos et al., 1991). It is also a preferred agent for prevention and treatment of anthrax (Meyerhoff et al., 2004). It's mechanism of action is through effective inhibition of DNA gyrase, thus preventing DNA replication in susceptible bacteria (Gellert et al., 1977; Sugino et al., 1977). Ciprofloxacin is a carboxylic acid-containing fluoroquinolone (carboxyfluoroquinolone) that undergoes renal and hepatic elimination, with ∼50% (oral) or ∼80% (intravenous) appearing in the urine as parent compound and metabolites 24 hours after administration in humans (Hoffken et al., 1985). Approximately 20-40% of circulating ciprofloxacin is bound to serum protein (Bayer Product Information, 2002), and in humans the renal clearance of ciprofloxacin, and the related drug norfloxacin, is nearly triple the glomerular filtration rate, indicating that tubular secretion plays an important role in their elimination (Shimada et al., 1983; Jaehde et al., 1995). Moreover, in humans, concomitant probenecid administration has been demonstrated to diminish the renal clearance of ciprofloxacin and norfloxacin to values that approach the glomerular filtration rate, suggesting a major role for an anionic transport mechanism in carboxyfluoroquinolone renal elimination (Shimada et al., 1983; Jaehde et al., 1995). Probenecid also substantially reduced the total and renal clearance of another clinically used derivative, ofloxacin, in rats (Foote and Halstenson, 1998). However, the specific mechanism for renal basolateral uptake of these compounds has not been established.
This information is important for identifying potential renal etiologies of variable carboxyfluoroquinolone efficacy and concomitantly administered drug toxicity (e.g., methotrexate), such as xenobiotic competition for tubular transport (VanWert and Sweet, 2007) and transporter polymorphisms (Bleasby et al., 2005; Erdman et al., 2006), especially for the treatment of infectious diseases of the urinary tract. While a detailed mechanistic understanding of carboxyfluoroquinolone renal transport has not been achieved, some advances have been made in understanding the general flux of carboxyfluoroquinolones across tubular cells (Table 1). Basolateral uptake of carboxyfluoroquinolones appears to involve an organic anion transport mechanism, as levofloxacin inhibited basolateral p-aminohippurate uptake into opossum kidney cells (Matsuo et al., 2001), and basal uptake of levofloxacin was not inhibited by tetraethylammonium or cimetidine in LLC-PK1 cells derived from porcine kidney (Ohtomo et al., 1996). These observations are consistent with a role for the basolateral organic anion exchangers Oat1 and Oat3 in the renal uptake of carboxyfluoroquinolones (Sweet et al., 1997; Cihlar et al., 1999; Sweet et al., 1999; Sweet et al., 2002; Sweet et al., 2003). However, a recent study demonstrated that levofloxacin can inhibit creatinine transport by human renal basolateral organic cation transporter 2 (hOCT2) (Okuda et al., 2006). Investigations using LLC-PK1 cells and rat renal cortical apical membrane vesicles have demonstrated that ofloxacin and levofloxacin are capable of inhibiting tetraethylammonium transport across the apical membrane (Okano et al., 1990; Ohtomo et al., 1996). This finding suggests that zwitterionic fluoroquinolones interact with organic cation transporters at the brush border membrane as well. However, there are conflicting studies on the involvement of apical OATs in that one investigation reported no effect of ofloxacin on renal apical p-aminohippurate transport in rat (Okano et al., 1990), while another study reported that levofloxacin (an ofloxacin enantiomer) inhibits apical efflux of p-aminohippurate in opossum kidney cells (Matsuo et al., 2001). While this discrepancy may be the result of species differences in apical transport, clearly, the contribution of individual renal transporters in carboxyfluoroquinolone elimination remains ambiguous.
Table 1.
Carboxyfluoroquinolone renal transport literature.√, suggested role in transport; --, undetermined role in transport; no role, study specifically suggested no role for the transporter family and membrane; HEK293, human embryonic kidney cell line; hOCT2, human organic cation transporter 2; LLC-PK1, porcine renal proximal tubular cells; NMeN, N1-methyl-nicotinamide; OK, opossum renal proximal tubular cells; PAH, p-aminohippurate; TEA, tetraethylammonium.
| Carboxyfluoroquinolone | System | Major Finding | Suggested Possible Role for Transporter Family | Specific Transporter | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Anionic | Cationic | |||||||
| Apical | Basolateral | Apical | Basolateral | |||||
| ciprofloxacin | In vivo in humans (males and females) | Renal clearance was reduced to ∼1/3 of normal by probenecid | √ | √ | -- | -- | no | (Jaehde et al., 1995) |
| norfloxacin | In vivo in humans, rabbits, and dogs (all males) | Renal clearance was reduced to ∼1/2 of normal by probenecid, no effect was apparent in dogs | √ | √ | -- | -- | no | (Shimada et al., 1983) |
| ofloxacin | In vivo in humans | Reduced the plasma clearance of procainamide | -- | -- | √ | √ | no | (Martin et al., 1996) |
| ofloxacin | Rat renal cortical apical membrane vesicles | Inhibited uptake of TEA, cephalexin, and cephradine with no effect on PAH transport | √ | -- | √ | -- | no | (Okano et al., 1990) |
| ciprofloxacin, enoxacin, enrofloxacin, fleroxacin, norfloxacin, ofloxacin, and pefloxacin | In vivo: stop-flow renal peritubular capillary perfusion in rats | All inhibited renal basolateral uptake of PAH and NMeN (weakly to moderately) | -- | √ | -- | √ | no | (Ullrich et al., 1993) |
| ofloxacin | In vivo: i.v. administration in rats | Cimetidine and probenecid reduced total and renal clearance | √ | √ | √ | √ | no | (Foote and Halstenson, 1998) |
| levofloxacin | LLC-PK1 cells | Inhibited TEA apical transport; basolateral transport was not inhibited by TEA or cimetidine | -- | -- | √ | no role | no | (Ohtomo et al., 1996) |
| levofloxacin | OK cells | Inhibited PAH basolateral uptake and apical efflux; transport was unaffected by PAH | √ | √ | -- | -- | no | (Matsuo et al., 2001) |
| levofloxacin | hOCT2 expressing HEK293 cells | Inhibited creatinine uptake | -- | -- | -- | √ | hOCT2 | (Okuda et al., 2006) |
| levofloxacin | Isolated perfused rat kidney | Cimetidine and quinolones prolonged the transepithelial transit time | √ | -- | √ | -- | no | (Ito et al., 2000) |
Despite more than two decades of investigation into carboxyfluoroquinolone transport, the specific interaction of carboxyfluoroquinolones with organic anion transporters has not been examined using heterologous expression systems. Accordingly, we investigated the interaction of the two major renal basolateral organic anion transporters, Oat1 and Oat3, with ciprofloxacin and other carboxyfluoroquinolones, by conducting a series of experiments in which we employed Xenopus laevis oocytes and Chinese hamster ovary (CHO) cells expressing the murine and human transporters. Evidence was gathered indicating a significant involvement of murine Oat3 (mOat3) and human OAT3 (hOAT3). Further data corroborating this observation were obtained in vivo using intact wild-type and Oat3 knockout (Oat3(-/-)) mice.
Materials and Methods
Chemicals and Materials
[3H]p-aminohippurate and [3H]estrone-3-sulfate were purchased from PerkinElmer (Boston, MA) and American Radiolabeled Chemicals (St. Louis, MO), respectively. Ciprofloxacin and gatifloxacin were purchased from Fisher Scientific (Suwanee, GA) and LKT Laboratories, Inc. (St. Paul, MN), respectively. Norfloxacin and ofloxacin were purchased from MP Biomedicals (Solon, OH). The HPLC column and pre-column filter were acquired from Phenomenex (Torrance, CA).
Animals
Female Xenopus laevis were housed in 15-gallon aquariums and maintained on a 12 hr light/dark cycle. Frogs were fed and moved to clean, aged water every other day. Male and female Oat3(-/-) (backcrossed onto C57BL/6 8 times) and wild-type age-matched C57BL/6 mice (10-12 weeks old, ∼24 grams) were also used in the present study (VanWert et al., 2007; VanWert and Sweet, 2007). Mice were allowed food and water ad libitum and were housed in animal facilities maintained by the Medical University of South Carolina Division of Laboratory Animal Resources. The MUSC program for laboratory animal care has an assurance statement on file with the NIH Office for the Protection from Research Risks/Department of Health and Human Services and has maintained full accreditation with the Association for Assessment and Accreditation of Laboratory Animal Care since 1987. All animal procedures were approved by the MUSC Institutional Animal Care and Use Committee (AR#2080 [X. laevis] and AR#2082 [M. musculus]) and carried out in accordance with the Guide for the Care and Use of Laboratory Animals.
Isolation of Stably Transfected Cell Lines
Mouse organic anion transporter 1-expressing CHO cells (CHO-mOat1) were generated using a method adapted from that previously described for generation of mouse organic anion transporter 3-expressing (CHO-mOat3) and empty vector-transfected (CHO-FRT) CHO cell lines (VanWert and Sweet, 2007). Briefly, a full-length mOat1 restriction fragment was ligated into the pcDNA5/FRT vector and this construct was used to transfect CHO-FlpIn cells (Invitrogen, Carlsbad, CA) plated into a 12-well tissue culture plate (3×105 cells/well). Two days post plating, cells were transfected with 0.3 μg of pcDNA5/FRT-mOat1 plasmid DNA and 2.7 μg of pOG44 plasmid DNA for 24 hr using 2 μl of Transfectin Lipid Reagent (BioRad, Hercules, CA) according to the manufacturer's directions. After 24 h of transfection at 37°C/5% CO2, the medium was changed to fresh Ham's F12 with serum and the cells were grown for an additional 24 h. The cells were then thoroughly detached with trypsin and all cells were plated into a 25 cm2 tissue culture flask containing 700 μg/ml hygromycin B (Invitrogen) in Ham's F12 with serum. Untransfected cells were treated in parallel in order to detect positive resistance of transfected cells to hygromycin B. Transfected and untransfected cells were treated with hygromycin B for approximately 4 weeks. After testing positive for transport of [3H]p-aminohippurate, cells were maintained in 125 μg/ml hygromycin B.
The stable cell line expressing hOAT1 was generated previously in MDCKII cells (Aslamkhan et al., 2003). The HEK-293 cell line stably expressing hOAT3 was also established using the Flp-In expression system. Briefly, full-length hOAT3 cDNA was amplified from pcDNA3.1V5/His TOPO-hOAT3 and ligated into the pEF5/FRT/V5-DEST vector, resulting in the pEF5/FRT/V5-DEST-hOAT3 vector. Sequencing confirmed this construct to be 100% identical to GenBank accession no. AB042505 (Aslamkhan et al., 2006). HEK-293-FlpIn cells were plated into a 60 mm tissue culture dish (1×106 cells) using DMEM (High Glucose) media (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum. One day after plating, cells were transfected with 1 μg of pEF5/FRT/V5-DEST-hOAT3 plasmid DNA and 9 μg of pOG44 plasmid DNA using 20 μl of Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Twenty-four hours after transfection, the media was changed and the cells were grown for an additional 24 h, followed by selection via plating into a 25 cm2 tissue culture flask containing DMEM media with 10% fetal bovine serum and 50 μg/ml of hygromycin B (Invitrogen, Carlsbad, CA) for 4 weeks. An empty vector (pEF5/FRT/V5-DEST) HEK-293 stable cell line was also generated. Stable cell lines were maintained in 10 μg/ml of hygromycin B.
Xenopus laevis Oocyte Transport Assays
X. laevis oocytes were obtained from dissected ovaries by collagenase A treatment and substrate uptake assays were performed 3 days after injection with 20 ng of capped cRNA as previously described (Sweet et al., 2000; Youngblood and Sweet, 2004). Oocytes were randomly divided into experimental groups of 5 for estrone sulfate transport and 25 for ciprofloxacin transport and incubated for 60 min at room temperature in oocyte Ringer 2 (OR-2) medium (in mM: 82.5 NaCl, 2.5 KCl, 1 Na2HPO4, 3 NaOH, 1 CaCl2, 1 MgCl2, 1 pyruvic acid, and 5 HEPES, pH 7.6) containing 5 μM [3H]estrone-3-sulfate (0.5 μCi/ml) or 40 μM unlabeled ciprofloxacin. Unlabeled ciprofloxacin (500 μM) was present or absent in the [3H]estrone-3-sulfate uptake solution as indicated. Individual oocyte radioactivity was measured by liquid scintillation spectroscopy with external quench correction for measuring [3H]estrone-3-sulfate uptake. To determine unlabeled ciprofloxacin accumulation, high-performance liquid chromatography (HPLC) was employed on 5 pooled oocytes per sample (see below).
Cell Line Transport Assays
Procedures for cell uptake assays were adapted from previously reported protocols (Schnabolk et al., 2006; VanWert and Sweet, 2007). Cells were seeded in 24-well (2×105 cells per well for [3H]estrone-3-sulfate and [3H]p-aminohippurate uptake) or 12-well (4×105 cells per well for ciprofloxacin uptake) tissue culture plates and grown for 2 days (37°C/5% CO2) in the absence of antibiotics. Before initiation of transport experiments, the culture medium was removed and the cells were equilibrated for 10 min with transport buffer (Hank's balanced salt solution containing 10 mM HEPES, pH 7.4; Sigma-Aldrich, Saint Louis, MO). The equilibration medium was removed before a final application of 500 μl (24-well plate) or 1000 μl (12-well plate) of transport buffer containing 0.1-100 μM [3H]estrone-3-sulfate (0.5 μCi/ml), 0.144-10 μM [3H]p-aminohippurate (0.5 μCi/ml), or 20-1000 μM unlabeled ciprofloxacin in the absence or presence of inhibitors at the indicated concentrations. After incubation at room temperature or 37°C for the specified times, the uptake solutions were removed and the cells were rapidly rinsed three times with 1 ml of ice-cold transport buffer. In [3H]estrone-3-sulfate and [3H]p-aminohippurate transport experiments, the cells were dissolved in 500 μl of 1 M NaOH, neutralized with 50 μl of 10 M HCl, and assayed for radioactivity by liquid scintillation counting. In ciprofloxacin transport experiments, the cells were processed for HPLC (see below). For all cell transport experiments, aliquots were removed and analyzed for protein content using (mouse cell lines) a BCA protein assay kit (Pierce, Rockford, IL) or (human cell lines) Bradford assay kit (BioRad, Richmond, CA). Uptake was then normalized to protein content in lysates. Transformations for kinetic calculations were performed using GraphPad Prism® software, and the Km and Vmax values were calculated from the x and y intercepts of the Lineweaver-Burk plot, respectively. Inhibition constant (Ki) values were calculated assuming competitive inhibition. All experiments were performed at least three times in triplicate.
Ciprofloxacin Elimination
Ciprofloxacin (5 mg/kg body weight) was administered in normal saline (5 μl/g body weight) by bolus tail vein injection to unanesthetized animals. Serial blood samples (∼35 μL) were obtained at 1, 5, 10, 15, 20, and 30 min in heparinized capillary tubes and centrifuged to isolate plasma as previously described (VanWert et al., 2007; VanWert and Sweet, 2007). Ten microliters of plasma were then processed for HPLC as described in the following section.
High-Performance Liquid Chromatography
Ciprofloxacin was extracted from Xenopus oocytes as follows: five oocytes were placed in a microcentrifuge tube, dried with a gentle stream of nitrogen gas, and homogenized in 40 μl of mobile phase A (50 mM formic acid, pH 3.5 via ammonia) with an ultrasonic probe. The homogenate was centrifuged at 21,000 ×g for 5 min and 25 μl of supernatant was directly injected into the HPLC system via an autosampler. The HPLC system consisted of a Waters 2695 separations module (Waters, Milford, MA), a Phenomenex Luna C18(2) 250 mm × 4.6 mm, 5 μm reversed-phase column preceded by a KrudKatcher guard/filter (Phenomenex, Torrance, CA), and a Waters 474 scanning fluorescence detector. To resolve ciprofloxacin an isocratic elution was employed (60% A / 40 % B [HPLC-grade methanol], 800 μl/min, retention time ∼8 min). Quantification was performed via a published procedure (Joos et al., 1985) using peak areas with fluorescence detection (respective excitation and emission λ of 278 and 456 nm). The external standard curve was consistently linear (R2 > 0.998) and was repeated for each day of analysis.
Ciprofloxacin was extracted from cell monolayers in the 12-well plate by applying 250 μl of 70°C distilled and deionized water and pipetting aggressively to lift the cells. Cellular detachment was verified microscopically and cells were placed in a microcentrifuge tube before disrupting with an ultrasonic probe. Membranous debris was pelleted by centrifugation at 21,000 ×g for 20 min and 230 μl of supernatant were then placed in a fresh tube. Twenty μl of the supernatant were taken and diluted to 60 μl with water for duplicate 25 μl samples in the BCA protein assay. In order to precipitate protein that could potentially block the column, 10 μl of 4-hydroxyphenylacetic acid were added to the remaining 210 μl of supernatant to achieve a final concentration of 500 ng/μl. This turbid solution was vortexed vigorously and protein was pelleted at 21,000 ×g for 10 min. Thirty microliters of this supernatant were injected for HPLC under identical conditions to those for the oocyte assay.
Ciprofloxacin was quantified in mouse plasma using a reported method with minor adaptations (Joos et al., 1985). Standards made in plasma and 10 μl plasma samples from injected animals were vortexed after adding 4 volumes of 6% (w/v) trichloroacetic acid in HPLC-grade water. They were then centrifuged at 21,000 ×g for 5 min to pellet the protein. Thirty microliters of supernatant were used directly for HPLC separation and detection as described above for oocytes.
Kinetic and Statistical Analyses
Transport and inhibition curves and kinetics were determined using Prism® software (GraphPad, San Diego, CA). Ki values were determined assuming competitive inhibition. Pharmacokinetic determinations were performed using WinNonlin® software (Pharsight Corporation, Mountain View, CA). Non-compartmental i.v. bolus modeling was used and yielded lower coefficients of variation as compared to other models. Statistical significance of elimination curves was determined using two-way ANOVA. One-way ANOVA with Tukey's multiple comparison test was used for Figures 1 and 2. A two-tailed student's t test was used for all other comparisons. The α for significance was set at 0.05.
Figure 1.
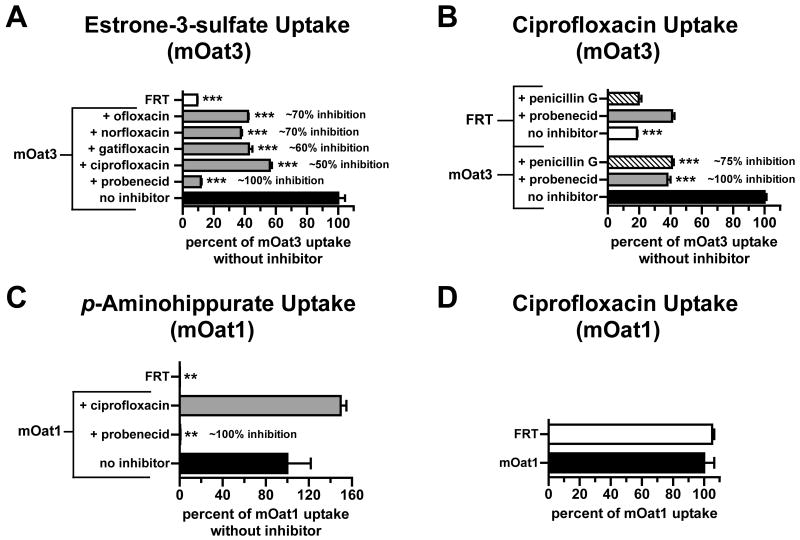
Carboxyfluoroquinolone inhibition and transport in mouse organic anion transporter-expressing Chinese hamster ovary cells. [3H]Estrone-3-sulfate, [3H]p-aminohippurate, or unlabeled ciprofloxacin uptake was measured at room temperature for 15 min in cells expressing mOat1 or mOat3. Empty-vector transfected Chinese hamster ovary cells (FRT) serve as a reference for nonspecific accumulation. (A) [3H]Estrone-3-sulfate (5 μM) uptake in CHO-mOat3 cells in the absence (no inhibitor) or presence of carboxyfluoroquinolones or probenecid (1 mM). (B) Ciprofloxacin (100 μM) uptake in CHO-mOat3 cells in the absence (no inhibitor) or presence of Oat3 inhibitors (1 mM). (C) [3H]p-aminohippurate (145 nM) uptake in CHO-mOat1 cells in the absence (no inhibitor) or presence of ciprofloxacin or probenecid (1 mM). (D) Ciprofloxacin (100 μM) uptake in CHO-mOat1 cells. Values shown are means ± S.E. and significant differences were assessed between mOat3 or mOat1 without inhibition and the indicated bars (**P<0.01, ***P<0.001). Percent inhibition was calculated after correcting for nonspecific accumulation (i.e., in FRT cells).
Figure 2.
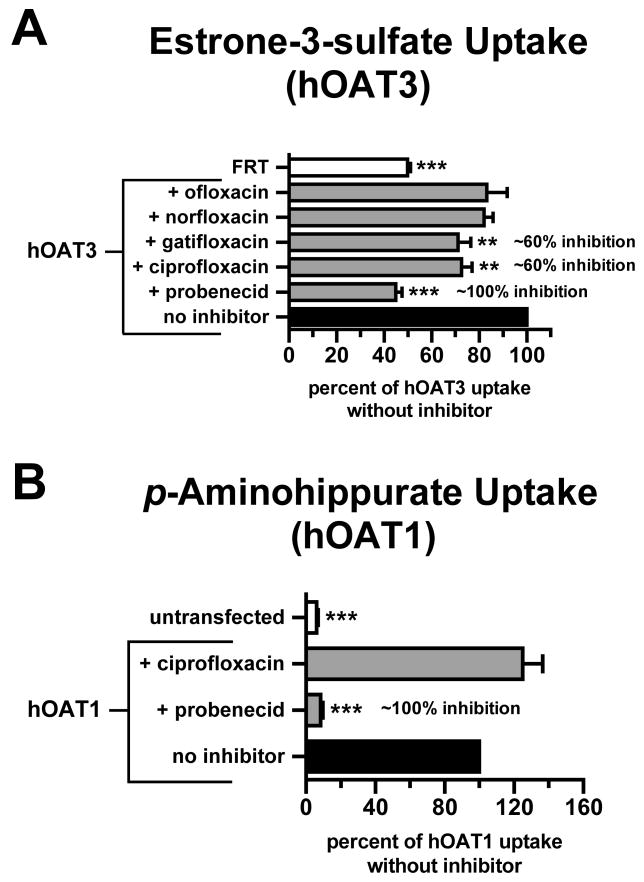
Carboxyfluoroquinolone inhibition in human organic anion transporter-expressing cells. [3H]Estrone-3-sulfate or [3H]p-aminohippurate uptake was measured for 15 min in cells expressing hOAT3 (HEK293 cells) or hOAT1 (MDCKII cells), respectively. Empty-vector transfected HEK293 cells (FRT) or untransfected MDCKII cells served as a reference for nonspecific accumulation. (A) [3H]Estrone-3-sulfate (100 nM) uptake in hOAT3 cells in the absence (no inhibitor) or presence of carboxyfluoroquinolones or probenecid (1 mM). (B) [3H]p-aminohippurate (10 μM) uptake in hOAT1 cells in the absence (no inhibitor) or presence of ciprofloxacin or probenecid (1 mM). Values shown are means ± S.E. and significant differences were assessed between hOAT3 or hOAT1 without inhibition and the indicated bars (**P<0.01, ***P<0.001). Percent inhibition was calculated after correcting for nonspecific accumulation (i.e., in FRT or untransfected cells).
Results
Interaction of Ciprofloxacin with mOat3 in Xenopus laevis Oocytes
In mOat3-expressing Xenopus laevis oocytes, ciprofloxacin (500 μM) significantly inhibited mOat3-mediated estrone-3-sulfate (5 μM) uptake (∼40% in 60 min at room temperature, P < 0.05, data not shown). When examined directly as a substrate, ciprofloxacin (40 μM) accumulated in mOat3-expressing oocytes to a level significantly higher than in water-injected/control oocytes (∼2-fold greater in 60 min at room temperature, P < 0.01, data not shown).
Interaction of Carboxyfluoroquinolones with mOat3/hOAT3 and mOat1/hOAT1 in Cell Monolayers
The stably-transfected mOat3-expressing (CHO-mOat3) and mOat1-expressing (CHO-mOat1) cell lines demonstrated marked accumulation of standard substrates relative to empty vector-transfected (CHO-FRT) cells: more than 10-fold greater for estrone-3-sulfate on mOat3 (Fig. 1A) and more than 150-fold greater for p-aminohippurate on mOat1 (Fig. 1C). In CHO-mOat3 cells, mOat3-mediated estrone-3-sulfate uptake was significantly inhibited (50-70%) by carboxyfluoroquinolones, while probenecid inhibited virtually all transport (Fig. 1A). Ciprofloxacin accumulated in CHO-mOat3 cells to more than 5 times its concentration in CHO-FRT cells (Fig. 1B). Penicillin G and probenecid inhibited mOat3-mediated ciprofloxacin uptake by 75% and 100%, respectively, after correction for nonspecific accumulation (i.e., in CHO-FRT cells, Fig. 1B). In CHO-mOat1 cells, mOat1-mediated p-aminohippurate uptake was not inhibited by a 7,000-fold molar excess of ciprofloxacin, but was abolished by the same molar excess of probenecid (Fig. 1C). Ciprofloxacin accumulation in CHO-mOat1 and CHO-FRT cells was virtually identical (Fig. 1D).
The stably-transfected hOAT3- and hOAT1-expressing cell lines also demonstrated significantly greater accumulation of standard substrates relative to control cells (Fig. 2). Similar to mOat3, hOAT3-mediated estrone-3-sulfate uptake was significantly inhibited by ciprofloxacin and gatifloxacin (∼60% inhibition after correction for nonspecific accumulation, Fig. 2A). Additionally, there was a nonsignificant trend for inhibition (∼20%) by both norfloxacin and ofloxacin. Probenecid again inhibited all mediated transport. As with mOat1, hOAT1-mediated p-aminohippurate uptake was unaffected by ciprofloxacin, but was abolished by probenecid (Fig. 2B).
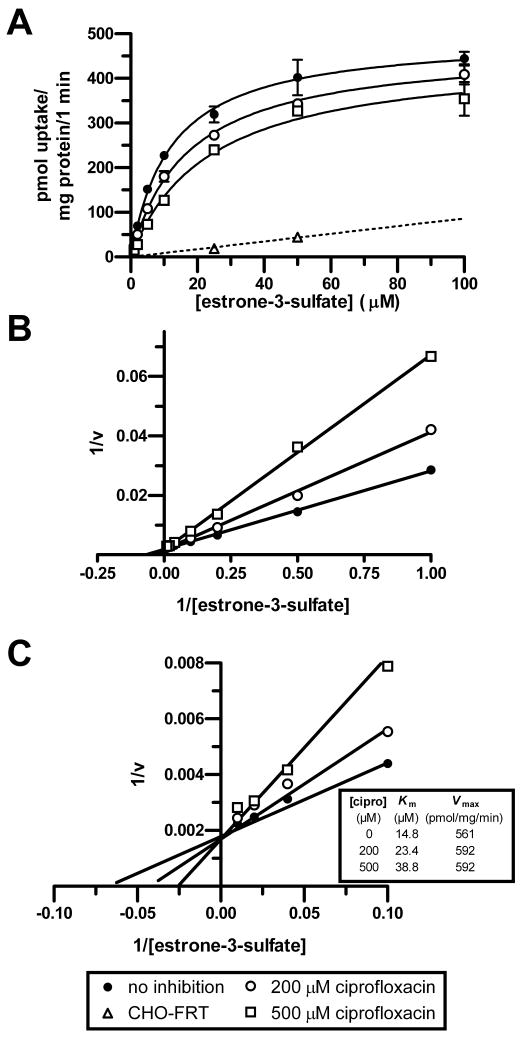
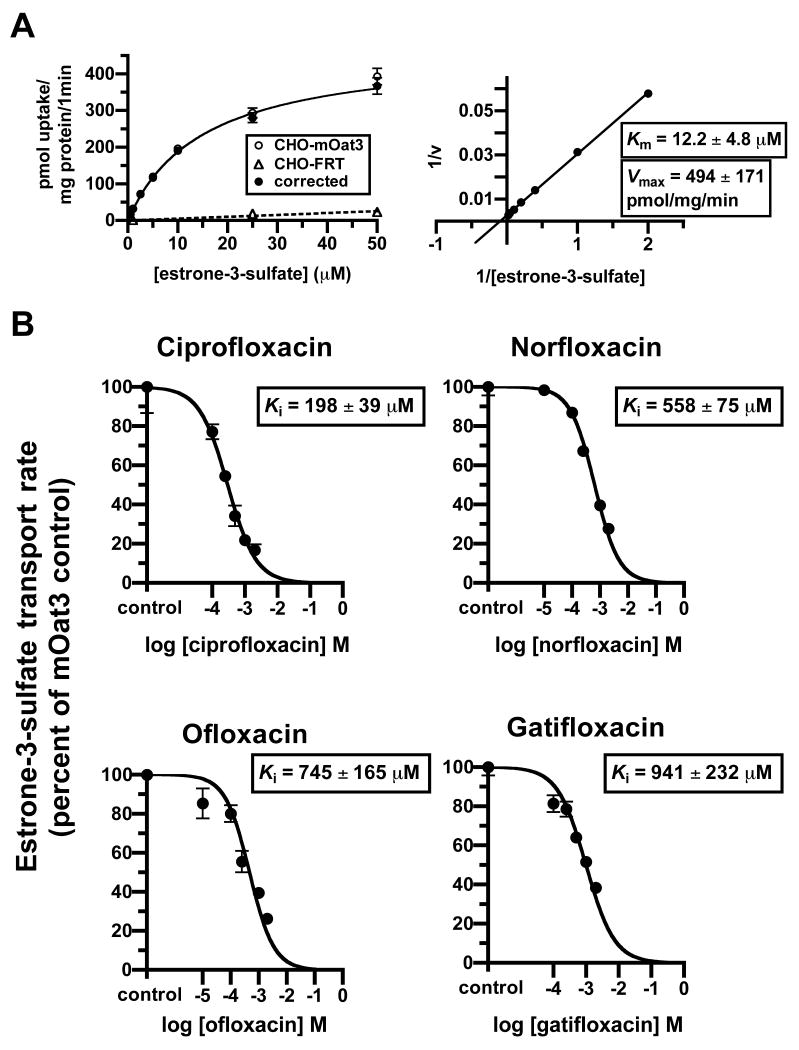
After demonstrating that carboxyfluoroquinolones interact with mOat3 and hOAT3, experiments were conducted in order to characterize their inhibition type and potency. It was determined that ciprofloxacin is a competitive-type inhibitor of estrone-3-sulfate transport on mOat3 (Fig. 3). This was reflected in the positive correlation between the ciprofloxacin concentration and the observed Km, together with an unaltered Vmax (Fig. 3C, embedded table). Subsequently, inhibition constants (Ki) were determined for four carboxyfluoroquinolones assuming competitive inhibition; ciprofloxacin, norfloxacin, ofloxacin, and gatifloxacin (Fig. 4B). In order to calculate the Ki values, estrone-3-sulfate's Km for transport was first determined in CHO-mOat3 cells (12.2 ± 4.8 μM, Fig. 4A). All four carboxyfluoroquinolones demonstrated a concentration-dependent inhibition of estrone-3-sulfate transport on mOat3, and ciprofloxacin exhibited the greatest potency.
Figure 3.
Competitive-type inhibition of mOat3 by ciprofloxacin. The Vmax and observed Km for [3H]estrone-3-sulfate uptake were determined in CHO-mOat3 cells at room temperature in the absence or presence of ciprofloxacin at 200 or 500 μM. (A) The [3H]estrone-3-sulfate transport rate was assessed at 1, 2, 5, 10, 25, 50, and 100 μM for 1 min. The saturation curves shown were generated after correction for nonspecific accumulation in empty-vector transfected cells (CHO-FRT). (B) Lineweaver-Burk transformation of data shown in A. (C) The graph from panel B was magnified to illustrate the x and y intercepts. Data points are shown as means ± S.E.
Figure 4.
mOat3 inhibition constants (Ki) of carboxyfluoroquinolones. The Km for [3H]estrone-3-sulfate uptake was determined in CHO-mOat3 cells and was subsequently used to calculate Ki values for carboxyfluoroquinolones. mOat3-mediated [3H]estrone-3-sulfate uptake was determined by subtracting accumulation in empty-vector transfected cells (CHO-FRT). (A) Left panel: [3H]estrone-3-sulfate uptake was measured for 1 min at 0.5, 1, 2.5, 5, 10, 25, and 50 μM to determine saturable transport (corrected). Right panel: Lineweaver-Burk transformation using corrected data to calculate the Km for [3H]estrone-3-sulfate. (B) [3H]estrone-3-sulfate (1 μM) uptake was measured for 1 min in the absence or presence of carboxyfluoroquinolones (ciprofloxacin and gatifloxacin at 100, 250, 500, 1000, and 2000 μM; norfloxacin and ofloxacin at 10, 100, 250, 1000, and 2000 μM) to determine Ki values. Inhibition curves, and thus Ki values, were determined using the “One site competition” model in GraphPad Prism® (i.e., assuming competitive-type inhibition). Kinetic constants shown are means ± S.E of 3 to 5 experiments performed in triplicate and graphs are single representative experiments.
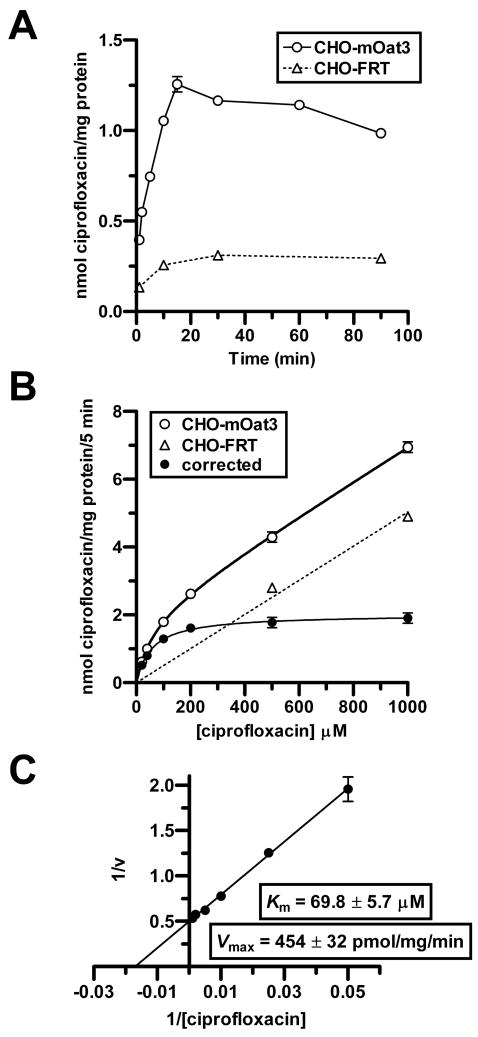
Kinetics of ciprofloxacin transport in CHO-mOat3 cells were then determined in order to demonstrate saturability and provide an additional means of assessing its affinity for mOat3. A time course study indicated that ciprofloxacin accumulation in CHO-mOat3 cells was very rapid over the first 1-2 min, and then approximately constant for the next 14 min (Fig. 5A). Saturation kinetics were then determined for ciprofloxacin transport in CHO-mOat3 cells using a 5 min uptake duration to approximate the initial rate (Fig. 5B). Background accumulation in control CHO-FRT cells was also determined. mOat3-mediated ciprofloxacin accumulation was clearly saturable with a Km of 69.8 ± 5.7 μM and a Vmax of 454 ± 32 pmol/mg of protein/min (Fig. 5, B and C).
Figure 5.
Ciprofloxacin transport kinetics in CHO-mOat3 cells. Ciprofloxacin uptake was measured in CHO-mOat3 or empty vector-transfected (CHO-FRT) cells at room temperature. (A) Ciprofloxacin (100 μM) accumulation was determined by HPLC at 1, 2, 5, 10, 15, 30, 60, and 90 min to determine the linear accumulation phase. (B) Ciprofloxacin uptake at 5 min was determined over a range of concentrations (20, 40, 100, 200, 500, and 1000 μM). “Corrected” curve represents accumulation mediated by mOat3 (i.e., the difference between uptake in CHO-mOat3 and CHO-FRT cells). (C) Lineweaver-Burk transformation using corrected data from B to calculate Km and Vmax values for ciprofloxacin from the x and y intercepts. Values shown are means ± S.E. from 3 determinations performed in triplicate.
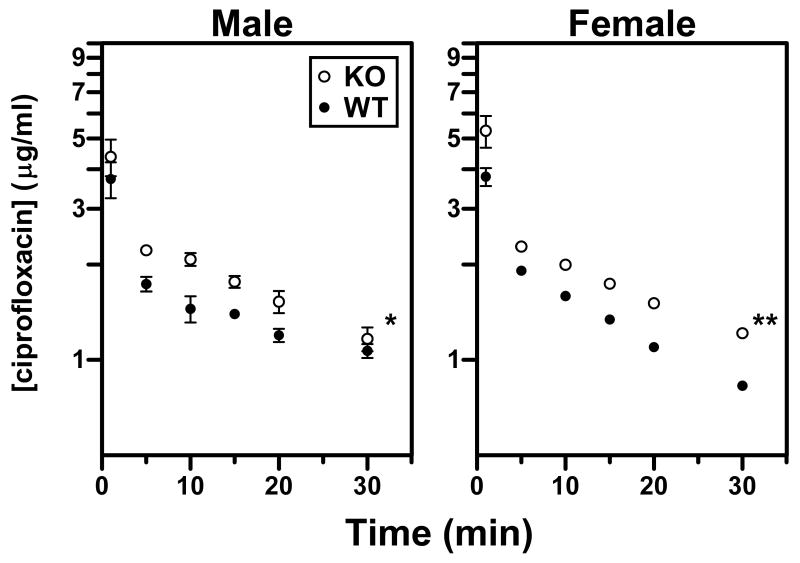
Ciprofloxacin Plasma Elimination in Wild-Type and Oat3(-/-) Mice
The administered ciprofloxacin dose (5 mg/kg) was chosen to yield plasma concentrations that are clinically observed in humans (i.e., 3.6-5.0 μg/ml; Fig. 6). Notably, ciprofloxacin plasma levels were significantly higher in male and female Oat3(-/-) mice as compared to wild-type (Fig. 6). Female knockout mice demonstrated an apparent reduction in the terminal elimination rate (i.e., slope) as compared to wild-type, while this trend was not obvious in males. Non-compartmental pharmacokinetic analysis also revealed abnormalities in Oat3(-/-) mice (Table 2). Both genders of Oat3(-/-) mice exhibited a significantly lower apparent volume of distribution (Vdss) and higher area under the concentration-time curve (AUC) compared to wild-type (Vdss reduction of 27% for males, 14% for females; AUC heightened by 25% for males, 33% for females). For female Oat3(-/-) mice exclusively, these abnormalities resulted in a significant (35%) reduction in total plasma clearance of ciprofloxacin (Table 2). Furthermore, gender differences in pharmacokinetics of ciprofloxacin were observed exclusively in wild-type mice, and indicated that females have a significantly lower volume of distribution (∼18%), shorter half-life (∼40%), and greater plasma clearance (∼39%). Oat3 deletion abolished these gender differences.
Figure 6.
Ciprofloxacin plasma elimination after i.v. bolus administration. Ciprofloxacin was administered to male and female wild-type (WT) and Oat3(-/-) (KO) mice at a dose of 5 mg/kg in order to achieve clinically observed plasma levels. Serial plasma samples were acquired at 1, 5, 10, 15, 20, and 30 min and assayed by HPLC to determine total (free and bound) ciprofloxacin levels. Values shown are means ± S.E. (n = 4 WT and 6 KO males; 3 WT and 5 KO females) and significant differences are between entire curves using two-way ANOVA (*P<0.05, **P<0.01).
Table 2.
Pharmacokinetic analysis of i.v. bolus ciprofloxacin in wild-type and Oat3(-/-) mice. Ciprofloxacin was administered by i.v. bolus injection at a 5 mg/kg dose and serial plasma samples were analyzed (see Fig. 6). Non-compartmental pharmacokinetic analysis was performed. Data are shown as mean ± S.E. and significant differences are for either Oat3(-/-) compared to wild-type for the same gender (*P<0.05, **P<0.01), or female compared to male for the same genotype (†P<0.05, ††P<0.01). AUC, area under the plasma concentration-time curve from 0 to 30 min; Vdss, estimated volume of distribution at steady state. Plasma clearance was calculated using the AUC from zero to infinity.
| Gender | Genotype | Pharmacokinetic Parameter | |||
|---|---|---|---|---|---|
| Vdss (ml/kg) |
Half-Life (min) |
AUC (min*ng/μl) |
Plasma Clearance (ml/min/kg) |
||
| Male | wild-type (n = 4) | 2,391 ±141 | 35.3 ±2.6 | 47.9 ±2.5 | 49.5 ±3.3 |
| Oat3(-/-) (n = 6) | 1,755 ±107 ** | 30.8 ±6.1 | 60.0 ±2.7 * | 47.9 ±7.0 | |
| Female | wild-type (n = 3) | 1,949 ±66 † | 21.2 ±1.0 †† | 47.3 ±1.4 | 69.0 ±2.8 †† |
| Oat3(-/-) (n = 5) | 1,673 ±13 ** | 28.7 ±2.9 | 62.8 ±2.2 ** | 44.9 ±3.0 ** | |
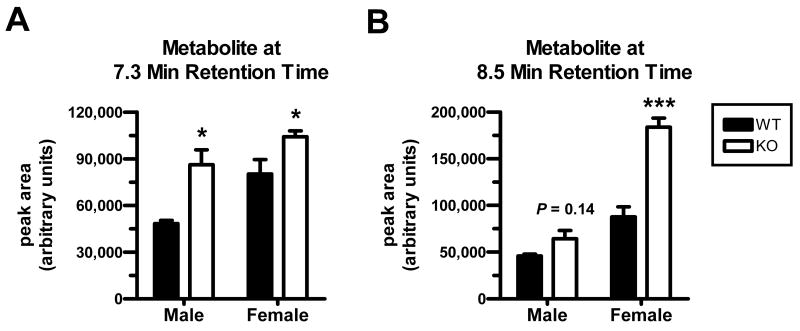
Two putative ciprofloxacin metabolite peaks were observed in serum samples from all mice (Figs. 7 and 8). The peaks were absent at one minute after administration, and became overt at later time points. An attempt was made to identify the putative metabolites by administering 50 mg/kg of ciprofloxacin (i.v. bolus) to a mouse, acquiring total blood at 30 min, and isolating the “metabolite” fractions during subsequent HPLC for analysis by mass spectrometry. The early-eluting putative metabolite (Figs. 7 and 8; retention time of ∼7.3 min) yielded an abundant peak at a mass-to-charge ratio of 346.3 when assessed with electrospray ionization in the positive ion mode. The late-eluting “metabolite” (Figs. 7 and 8; retention time of ∼8.5 min) showed an abundant peak at a mass-to-charge ratio of 316.1. Male Oat3(-/-) mice exhibited significantly heightened accumulation of the early-eluting “metabolite” compared to wild-type (Fig. 8A), while female Oat3(-/-) mice exhibited significantly heightened accumulation of both “metabolites” (Fig. 8, A and B). Furthermore, while the female-to-male ciprofloxacin plasma ratio was not far from unity within each genotype at 30 min (i.e., ratio of 1.04 for Oat3(-/-) and 0.78 for wild-type), female mice showed far greater levels of the “metabolites”, particularly the late-eluting one, compared to males (Fig. 8).
Figure 7.
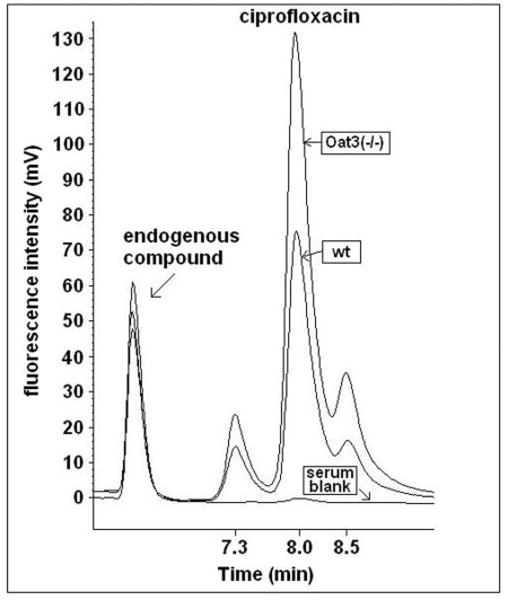
Representative HPLC chromatograms of ciprofloxacin extracted from plasma acquired 30 min post-administration. Isocratic elution and fluorescence detection was employed for quantifying ciprofloxacin in wild-type and Oat3(-/-) mice. Overlaid ciprofloxacin peaks are shown from wild-type (wt) and Oat3(-/-) mouse plasma, in addition to a serum blank. Peaks to the immediate left and right of ciprofloxacin, i.e., putative metabolites, were not found in spiked plasma or samples acquired one minute after administration. The unidentified endogenous compound was observed in blank serum (highest endogenous peak) and both genotypes (two lower endogenous peaks), providing an indication of HPLC sample injection consistency.
Figure 8.
Comparison of putative ciprofloxacin metabolites between wild-type and Oat3(-/-) mice 30 min after i.v. bolus administration. Metabolites were apparent in chromatograms at later time points after ciprofloxacin administration as shown in Figure 7. (A) Metabolite eluting at 7.3 min. (B) Metabolite eluting at 8.5 min. Values shown are means ± S.E. (n = 4 WT and 6 KO males; 3 WT and 5 KO females) and significance was assessed between genotypes within the same gender (*P<0.05, ***P<0.001).
Discussion
Fluoroquinolones exhibit an impressive spectrum of antimicrobial activity and remain one of the most commonly prescribed classes of pharmacotherapeutics. Some carboxylic acid-containing fluoroquinolones, or carboxyfluoroquinolones, such as ciprofloxacin and levofloxacin, are among the most useful in treating afflicted patients. In general, carboxyfluoroquinolones can undergo both renal and hepatic elimination, with the renal component demonstrating a marked bias for secretory transport (Shimada et al., 1983; Jaehde et al., 1995). Therefore, perturbations in tubular secretory transport function can significantly increase systemic, and decrease urinary, levels of carboxyfluoroquinolones (Jaehde et al., 1995), potentially resulting in enhanced systemic efficacy, but failure to eradicate renal and post-renal pathogens. This is of pivotal importance to patients with ascending or complicated urinary tract infections, as carboxyfluoroquinolones are employed as first-line treatment (Warren et al., 1999).
While renal secretion of carboxyfluoroquinolones is well accepted as a major route of elimination, the specific transport mechanism for this pathway remains ambiguous. Thus far, investigators have suggested possible roles for organic anion, organic cation, and undetermined transporter families, expressed on both basolateral and apical plasma membranes (Table 1). A recent in vitro study suggested a role for basolateral human organic cation transporter 2 (hOCT2) specifically, based on the ability of levofloxacin and a pre-market fluoroquinolone to inhibit creatinine transport (Okuda et al., 2006). The promiscuous interaction of carboxyfluoroquinolones with various transport systems stems from their zwitterionic nature, bearing both amine and carboxylate moieties on opposite ends.
Although a role for an individual organic anion transporter in carboxyfluoroquinolone renal secretion has not been reported, three in vivo studies in particular provide substantial evidence that the OAT family plays a pivotal role (Shimada et al., 1983; Jaehde et al., 1995; Foote and Halstenson, 1998). Two of the investigations demonstrated that probenecid can reduce the renal clearance of ciprofloxacin and norfloxacin to approximately one-third or one-half, respectively, of that in the uninhibited state in humans (Shimada et al., 1983; Jaehde et al., 1995). In a subsequent investigation it was shown that probenecid can produce a similar effect on ofloxacin in rats, reducing renal clearance to approximately one-half of normal (Foote and Halstenson, 1998). In the latter investigation, cimetidine also markedly reduced renal clearance of ofloxacin in rats, maintaining the putative role for organic cation transporters. However, it is now established that cimetidine also interacts substantially with organic anion transporters 1 and 3 (Burckhardt et al., 2003; Tahara et al., 2005; Erdman et al., 2006). Since it is primarily uncharged at physiological pH (Burckhardt et al., 2003), its effects on ofloxacin secretion may be the result of inhibition of either organic cation or anion transporter families. Given the convincing in vivo evidence substantiating the role of the OAT family, we probed further into the mechanism of this interaction by assessing the ability of carboxyfluoroquinolones to interact with murine and human renal basolateral Oat1/OAT1 and Oat3/OAT3 in vitro, and subsequently, Oat3 in vivo using wild-type and Oat3(-/-) mice.
The present results indicate that carboxyfluoroquinolones, especially ciprofloxacin, exhibit a significant interaction with murine and human Oat3/OAT3 (Figs. 1 and 2). Furthermore, ciprofloxacin interacts with mOat3/hOAT3 selectively, demonstrating no inhibition of mOat1/hOAT1, and no transport by mOat1, the other major renal basolateral transporter (Figs. 1 and 2). Ciprofloxacin's concentrative accumulation by, and affinity for, mOat3 suggested that this transporter may play a significant role in vivo in the elimination of ciprofloxacin (Figs. 4 and 5). mOat3's in vivo role was thus confirmed by comparing elimination in wild-type and Oat3(-/-) mice (Fig. 6 and Table 2). Pharmacokinetic differences between genotypes in this investigation are expected to be caused by differences in transport, and not differences in hemodynamics or glomerular function, as these potential confounding factors have been ruled out in two previous studies by measuring inulin plasma elimination in the same mouse colony (VanWert et al., 2007; VanWert and Sweet, 2007). The consequence of Oat3 deletion in the present study, at clinically relevant ciprofloxacin levels, is similar to the effect of probenecid in previous studies in humans and rats (Shimada et al., 1983; Jaehde et al., 1995; Foote and Halstenson, 1998). Therefore, it is likely that OAT3 plays a significant role in humans in the renal secretion of carboxyfluoroquinolones. Thus, the clear role of mOat3 in carboxyfluoroquinolone elimination, along with the lack of interaction with mOat1/hOAT1, and previous reports of hOAT3 polymorphisms yielding non-functional hOAT3 protein, suggest there is a human population that may respond poorly to carboxyfluoroquinolone therapy when prescribed for eradication of renal and post-renal pathogens.
The present findings also suggest that mOat3 contributes significantly to the distribution of ciprofloxacin in both genders, but its role in total clearance is more overt in females (Fig. 6 and Table 2). Furthermore, deletion of Oat3 abolished all pharmacokinetic differences between males and females, indicating that differential Oat3 function exists between genders, and females may depend more on this transporter than males. Similar observations have been reported for two other pharmacotherapeutic Oat3 substrates, namely, penicillin G and methotrexate, in that Oat3 deletion had a greater impact on their total clearance in females as compared to males (VanWert et al., 2007; VanWert and Sweet, 2007). Moreover, the observation that females may depend more on Oat3 function than males is also consistent with known gender differences in expression of Oat3 mRNA in the currently used C57BL/6 mouse colony (i.e., female kidney expressed twice the level of Oat3 mRNA relative to male (VanWert et al., 2007). Given our current knowledge, it is unclear if this gender difference in mOat3 dependence can be extrapolated to other species. For example, female rats express lower levels of Oat3 protein in the proximal tubule than males (Ljubojevic et al., 2004) and rabbits failed to exhibit any gender differences in Oat3 mRNA or protein levels (Groves et al., 2006). Gender differences in humans have not been reported, therefore, clinical predictions regarding gender differences in dependence on hOAT3 are not warranted at this time.
Finally, two putative ciprofloxacin metabolites were observed during HPLC analysis, both of which were completely absent in spiked plasma standards and early time points, but highly evident in later time points (Fig. 7). Each of the suspected metabolites exhibited heightened accumulation in Oat3(-/-) mice (Figs. 7 and 8), suggesting that ciprofloxacin levels are not elevated as a result of impaired metabolism in Oat3(-/-) mice, but rather as a consequence of perturbed secretory transport. For both genotypes, the metabolites accumulated to a greater extent in females. Additionally, the late-eluting metabolite was markedly heightened in female Oat3(-/-) mice compared to wild-type or males of either genotype (Fig. 8B).
Four ciprofloxacin metabolites have been identified in humans, desethyleneciprofloxacin (M1, or the 2-aminoethylamino metabolite), sulfociprofloxacin (M2), oxociprofloxacin (M3), and formylciprofloxacin (M4) (Zeiler et al., 1987). The M1 and M3 metabolites have also been observed in animals (rats and monkeys) (Siefert et al., 1986). All of these metabolites retain the carboxylate moiety, suggesting that they may also be substrates for Oat3. In support of this, probenecid has been shown to prolong the half-life of the 2-aminoethylamino metabolite (M1) and impact its plasma level to a greater extent than the parent compound in humans (Jaehde et al., 1995). The early-eluting (7.3 minute) HPLC peak in the present study yielded an abundant mass spectrometry peak with a mass-to-charge ratio of 346.3, and is consistent with the metabolite oxociprofloxacin (M3), which has a molecular weight of 345.3 prior to protonation and has previously been identified in animals (Siefert et al., 1986). The late-eluting (8.5 minute) HPLC peak does not appear to correspond to any of the previously reported metabolites (M1-M4) of ciprofloxacin. Further studies are required to positively identify the compound represented by this peak.
In conclusion, the results of this investigation show that renal basolateral mOat3/hOAT3 interacts with carboxyfluoroquinolones in vitro, and the murine ortholog plays a significant role in ciprofloxacin elimination at clinically observed concentrations in vivo. In contrast, mOat1/hOAT1 does not interact with ciprofloxacin, indicating that this other major renal basolateral OAT is not involved in elimination of ciprofloxacin. Therefore, hOAT3 polymorphisms, some of which have already been demonstrated as highly dysfunctional (Erdman et al., 2006), should be considered a potential source of variable carboxyfluoroquinolone efficacy in tissues and especially throughout the urinary tract. Furthermore, numerous drug interactions involving carboxyfluoroquinolones, whether impacting the carboxyfluoroquinolone level or concomitant drug level, likely occur via competition for transport on hOAT3. Such carboxyfluoroquinolone-drug interactions have been documented in humans and are known to involve substrates/inhibitors which have been demonstrated to interact with mOat3 in vivo (e.g., methotrexate (VanWert and Sweet, 2007) and probenecid). Pharmacotherapeutic OAT3 substrates, e.g., penicillin G and non-steroidal anti-inflammatory drugs, should therefore be administered with caution in patients who are receiving a carboxyfluoroquinolone for a urinary tract infection, as blood and tissue levels of the quinolone may be therapeutic or supertherapeutic, while urinary levels may fail to reach the minimum effective concentration.
Acknowledgments
Financial Support: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-067216 (D.H.S.), by fellowship provisions from the American Foundation for Pharmaceutical Education (A.L.V.), and by the National Institutes of Health Grant Number C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources.
Nonstandard Abbreviations
- ANOVA
analysis of variance
- AUC
area under the concentration-time curve
- CHO
Chinese hamster ovary cells
- CHO-FRT
empty vector-transfected CHO
- CHO-mOat1
mOat1-transfected CHO
- CHO-mOat3
mOat3-transfected CHO
- hOCT2
human organic cation transporter 2
- HPLC
high-performance liquid chromatography
- Ki
inhibitory constant
- Km
Michaelis-Menten constant
- LLC-PK1
porcine kidney cells
- mOat1
murine organic anion transporter 1
- mOat3
murine organic anion transporter 3
- OAT/Oat
organic anion transporter
- OAT1/hOAT1
human organic anion transporter 1
- OAT3/hOAT3
human organic anion transporter 3
- Oat3(-/-)
Oat3 null
- Vdss
apparent volume of distribution at steady state
- Vmax
maximum transport rate
References
- Aslamkhan A, Han YH, Walden R, Sweet DH, Pritchard JB. Stoichiometry of organic anion/dicarboxylate exchange in membrane vesicles from rat renal cortex and hOAT1-expressing cells. Am J Physiol Renal Physiol. 2003;285(4):F775–783. doi: 10.1152/ajprenal.00140.2003. [DOI] [PubMed] [Google Scholar]
- Aslamkhan AG, Thompson DM, Perry JL, Bleasby K, Wolff NA, Barros S, Miller DS, Pritchard JB. The flounder organic anion transporter fOat has sequence, function, and substrate specificity similarity to both mammalian Oat1 and Oat3. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1773–1780. doi: 10.1152/ajpregu.00326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer. Product Information: Cipro(R), ciprofloxacin oral tablets and suspension. Bayer Pharmaceutical Division; West Haven, CT: 2002. 2002f. [Google Scholar]
- Bleasby K, Hall LA, Perry JL, Mohrenweiser HW, Pritchard JB. Functional consequences of single nucleotide polymorphisms in the human organic anion transporter hOAT1 (SLC22A6) J Pharmacol Exp Ther. 2005;314(2):923–931. doi: 10.1124/jpet.105.084301. [DOI] [PubMed] [Google Scholar]
- Burckhardt BC, Brai S, Wallis S, Krick W, Wolff NA, Burckhardt G. Transport of cimetidine by flounder and human renal organic anion transporter 1. Am J Physiol Renal Physiol. 2003;284(3):F503–509. doi: 10.1152/ajprenal.00290.2002. [DOI] [PubMed] [Google Scholar]
- Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, Sweet DH. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol. 1999;56(3):570–580. doi: 10.1124/mol.56.3.570. [DOI] [PubMed] [Google Scholar]
- Erdman AR, Mangravite LM, Urban TJ, Lagpacan LL, Castro RA, de la Cruz M, Chan W, Huang CC, Johns SJ, Kawamoto M, Stryke D, Taylor TR, Carlson EJ, Ferrin TE, Brett CM, Burchard EG, Giacomini KM. The human organic anion transporter 3 (OAT3; SLC22A8): genetic variation and functional genomics. Am J Physiol Renal Physiol. 2006;290(4):F905–912. doi: 10.1152/ajprenal.00272.2005. [DOI] [PubMed] [Google Scholar]
- Foote EF, Halstenson CE. Effects of probenecid and cimetidine on renal disposition of ofloxacin in rats. Antimicrob Agents Chemother. 1998;42(2):456–458. doi: 10.1128/aac.42.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasem MH, Keuter M, Dolmans WM, Van Der Ven-Jongekrijg J, Djokomoeljanto R, Van Der Meer JW. Persistence of Salmonellae in blood and bone marrow: randomized controlled trial comparing ciprofloxacin and chloramphenicol treatments against enteric fever. Antimicrob Agents Chemother. 2003;47(5):1727–1731. doi: 10.1128/AAC.47.5.1727-1731.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M, Mizuuchi K, O'Dea MH, Itoh T, Tomizawa JI. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos CA, Maraziotis TG, Papadakis N, Beermann D, Siamplis DK, Bassaris HP. Penetration of ciprofloxacin into human cerebrospinal fluid in patients with inflamed and non-inflamed meninges. Eur J Clin Microbiol Infect Dis. 1991;10(6):511–514. doi: 10.1007/BF01963940. [DOI] [PubMed] [Google Scholar]
- Groves CE, Suhre WB, Cherrington NJ, Wright SH. Sex differences in the mRNA, protein, and functional expression of organic anion transporter (Oat) 1, Oat3, and organic cation transporter (Oct) 2 in rabbit renal proximal tubules. J Pharmacol Exp Ther. 2006;316(2):743–752. doi: 10.1124/jpet.105.094979. [DOI] [PubMed] [Google Scholar]
- Hoffken G, Lode H, Prinzing C, Borner K, Koeppe P. Pharmacokinetics of ciprofloxacin after oral and parenteral administration. Antimicrob Agents Chemother. 1985;27(3):375–379. doi: 10.1128/aac.27.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh WJ, Lin HC, Hwang SJ, Hou MC, Lee FY, Chang FY, Lee SD. The effect of ciprofloxacin in the prevention of bacterial infection in patients with cirrhosis after upper gastrointestinal bleeding. Am J Gastroenterol. 1998;93(6):962–966. doi: 10.1111/j.1572-0241.1998.00288.x. [DOI] [PubMed] [Google Scholar]
- Ito T, Yano I, Hashimoto Y, Inui K. Transepithelial transport of levofloxacin in the isolated perfused rat kidney. Pharm Res. 2000;17(2):236–241. doi: 10.1023/a:1007533817835. [DOI] [PubMed] [Google Scholar]
- Jaehde U, Sorgel F, Reiter A, Sigl G, Naber KG, Schunack W. Effect of probenecid on the distribution and elimination of ciprofloxacin in humans. Clin Pharmacol Ther. 1995;58(5):532–541. doi: 10.1016/0009-9236(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Jones RN. Microbiology of newer fluoroquinolones: focus on respiratory pathogens. Diagn Microbiol Infect Dis. 2002;44(3):213–220. doi: 10.1016/s0732-8893(02)00436-4. [DOI] [PubMed] [Google Scholar]
- Joos B, Ledergerber B, Flepp M, Bettex JD, Luthy R, Siegenthaler W. Comparison of high-pressure liquid chromatography and bioassay for determination of ciprofloxacin in serum and urine. Antimicrob Agents Chemother. 1985;27(3):353–356. doi: 10.1128/aac.27.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky BA, Miller B, Schwartz R, Henry DC, Nolan T, McCabe A, Magner DJ, Talbot GH. Sparfloxacin versus ciprofloxacin for the treatment of community-acquired, complicated skin and skin-structure infections. Clin Ther. 1999;21(4):675–690. doi: 10.1016/S0149-2918(00)88319-8. [DOI] [PubMed] [Google Scholar]
- Ljubojevic M, Herak-Kramberger CM, Hagos Y, Bahn A, Endou H, Burckhardt G, Sabolic I. Rat renal cortical Oat1 and Oat3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition. Am J Physiol Renal Physiol. 2004;287(1):F124–138. doi: 10.1152/ajprenal.00029.2004. [DOI] [PubMed] [Google Scholar]
- Martin DE, Shen J, Griener J, Raasch R, Patterson JH, Cascio W. Effects of ofloxacin on the pharmacokinetics and pharmacodynamics of procainamide. J Clin Pharmacol. 1996;36(1):85–91. doi: 10.1002/j.1552-4604.1996.tb04156.x. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Yano I, Habu Y, Katsura T, Hashimoto Y, Inui K. Transport of levofloxacin in the OK kidney epithelial cell line: interaction with p-aminohippurate transport. Pharm Res. 2001;18(5):573–578. doi: 10.1023/a:1011012822437. [DOI] [PubMed] [Google Scholar]
- Meyerhoff A, Albrecht R, Meyer JM, Dionne P, Higgins K, Murphy D. US Food and Drug Administration approval of ciprofloxacin hydrochloride for management of postexposure inhalational anthrax. Clin Infect Dis. 2004;39(3):303–308. doi: 10.1086/421491. [DOI] [PubMed] [Google Scholar]
- Micromedex (R) Healthcare Series. Thomson Micromedex; Greenwood Village, CO: nd. [Oct. 8, 2007]. ( http://www.thomsonhc.com) [Google Scholar]
- Ohtomo T, Saito H, Inotsume N, Yasuhara M, Inui KI. Transport of levofloxacin in a kidney epithelial cell line, LLC-PK1: interaction with organic cation transporters in apical and basolateral membranes. J Pharmacol Exp Ther. 1996;276(3):1143–1148. [PubMed] [Google Scholar]
- Okano T, Maegawa H, Inui K, Hori R. Interaction of ofloxacin with organic cation transport system in rat renal brush-border membranes. J Pharmacol Exp Ther. 1990;255(3):1033–1037. [PubMed] [Google Scholar]
- Okuda M, Kimura N, Inui K. Interactions of fluoroquinolone antibacterials, DX-619 and levofloxacin, with creatinine transport by renal organic cation transporter hOCT2. Drug Metab Pharmacokinet. 2006;21(5):432–436. doi: 10.2133/dmpk.21.432. [DOI] [PubMed] [Google Scholar]
- Schnabolk GW, Youngblood GL, Sweet DH. Transport of estrone sulfate by the novel organic anion transporter Oat6 (Slc22a20) Am J Physiol Renal Physiol. 2006;291(2):F314–321. doi: 10.1152/ajprenal.00497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada J, Yamaji T, Ueda Y, Uchida H, Kusajima H, Irikura T. Mechanism of renal excretion of AM-715, a new quinolonecarboxylic acid derivative, in rabbits, dogs, and humans. Antimicrob Agents Chemother. 1983;23(1):1–7. doi: 10.1128/aac.23.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefert HM, Maruhn D, Maul W, Forster D, Ritter W. Pharmacokinetics of ciprofloxacin. 1st communication: absorption, concentrations in plasma, metabolism and excretion after a single administration of [14C]ciprofloxacin in albino rats and rhesus monkeys. Arzneimittelforschung. 1986;36(10):1496–1502. [PubMed] [Google Scholar]
- Sugino A, Peebles CL, Kreuzer KN, Cozzarelli NR. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DH, Chan LM, Walden R, Yang XP, Miller DS, Pritchard JB. Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am J Physiol Renal Physiol. 2003;284(4):F763–769. doi: 10.1152/ajprenal.00405.2002. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Miller DS, Pritchard JB. Localization of an organic anion transporter-GFP fusion construct (rROAT1-GFP) in intact proximal tubules. Am J Physiol. 1999;276(6 Pt 2):F864–873. doi: 10.1152/ajprenal.1999.276.6.F864. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Miller DS, Pritchard JB. Basolateral localization of organic cation transporter 2 in intact renal proximal tubules. Am J Physiol Renal Physiol. 2000;279(5):F826–834. doi: 10.1152/ajprenal.2000.279.5.F826. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem. 2002;277(30):26934–26943. doi: 10.1074/jbc.M203803200. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Wolff NA, Pritchard JB. Expression cloning and characterization of rOat1. The basolateral organic anion transporter in rat kidney. J Biol Chem. 1997;272(48):30088–30095. doi: 10.1074/jbc.272.48.30088. [DOI] [PubMed] [Google Scholar]
- Tahara H, Kusuhara H, Endou H, Koepsell H, Imaoka T, Fuse E, Sugiyama Y. A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J Pharmacol Exp Ther. 2005;315(1):337–345. doi: 10.1124/jpet.105.088104. [DOI] [PubMed] [Google Scholar]
- Ullrich KJ, Rumrich G, David C, Fritzsch G. Bisubstrates: substances that interact with both, renal contraluminal organic anion and organic cation transport systems. II. Zwitterionic substrates: dipeptides, cephalosporins, quinolone-carboxylate gyrase inhibitors and phosphamide thiazine carboxylates; nonionizable substrates: steroid hormones and cyclophosphamides. Pflugers Arch. 1993;425(34):300–312. doi: 10.1007/BF00374180. [DOI] [PubMed] [Google Scholar]
- VanWert AL, Bailey RM, Sweet DH. Organic anion transporter 3 (Oat3/Slc22a8) knockout mice exhibit altered clearance and distribution of penicillin G. Am J Physiol Renal Physiol. 2007;293(4):F1332–1341. doi: 10.1152/ajprenal.00319.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanWert AL, Sweet DH. Impaired clearance of methotrexate in organic anion transporter 3 (Slc22a8) knockout mice: A gender specific impact of reduced folates. Pharm Res. 2007 doi: 10.1007/s11095-007-9407-0. in press, available online July 28 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenlehner FM, Kinzig-Schippers M, Tischmeyer U, Wagenlehner C, Sorgel F, Naber KG. Urinary bactericidal activity of extended-release ciprofloxacin (1,000 milligrams) versus levofloxacin (500 milligrams) in healthy volunteers receiving a single oral dose. Antimicrob Agents Chemother. 2006;50(11):3947–3949. doi: 10.1128/AAC.00477-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA) Clin Infect Dis. 1999;29(4):745–758. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- Youngblood GL, Sweet DH. Identification and functional assessment of the novel murine organic anion transporter Oat5 (Slc22a19) expressed in kidney. Am J Physiol Renal Physiol. 2004;287(2):F236–244. doi: 10.1152/ajprenal.00012.2004. [DOI] [PubMed] [Google Scholar]
- Zeiler HJ, Petersen U, Gau W, Ploschke HJ. Antibacterial activity of the metabolites of ciprofloxacin and its significance in the bioassay. Arzneimittelforschung. 1987;37(2):131–134. [PubMed] [Google Scholar]