The ability of environmental factors to shape health and disease involves epigenetic mechanisms that mediate gene-environment interactions. Epigenetic gene regulation comprises the heritable changes in gene expression that occur in the absence of changes to the DNA sequence itself. Epigenetic mechanisms include chromatin folding and attachment to the nuclear matrix, packaging of DNA around nucleosomes, covalent modifications of histone tails (e.g. acetylation, methylation, phosphorylation), and DNA methylation. The influence of regulatory small RNAs and micro RNAs on gene transcription is also increasingly recognized as a key mechanism of epigenetic gene regulation.
Conventional gene-environment interaction studies strive to understand how individuals with different genotypes respond to various environmental factors and how these responses change over time. Such research efforts have highlighted the important contribution of both genetic and environmental variability in human diseases. However, it has been argued that a full understanding of gene-environment interactions requires that epigenetic mechanisms be taken into account. Therefore, the interdisciplinary field of environmental epigenomics emphasizes the potential for nutritional and environmental factors to influence fetal, adult, and transgenerational epigenetic gene regulation, resulting in numerous phenotypic consequences.1
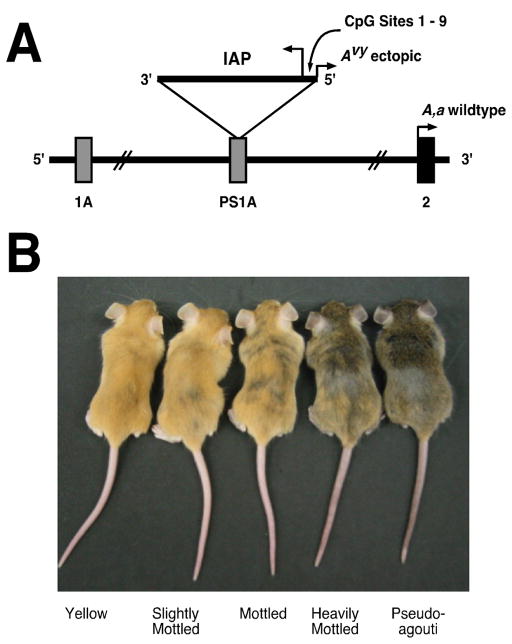
The viable yellow agouti (Avy) mouse model, in which coat color variation is correlated to epigenetic marks established early in development, has been used to investigate the impacts of nutritional and environmental influences on the fetal epigenome (Fig. 1A and B). The wild-type murine Agouti gene encodes a paracrine signaling molecule that produces either black eumelanin (a) or yellow phaeomelanin (A). Both A and a transcriptions are initiated from a developmentally regulated hair-cycle-specific promoter in exon 2 (Fig. 1A). Transient A expression in hair follicles during a specific stage of hair growth results in a sub-apical yellow band on each black hair shaft, causing the brown agouti coat color of wild-type mice.2 The Avy metastable epiallele resulted from the insertion of an intracisternal A particle (IAP) murine retrotransposon upstream of the transcription start site of the Agouti gene (Fig. 1A).2,3 A cryptic promoter in the proximal end of the Avy IAP promotes constitutive ectopic Agouti transcription not only in hair follicles, but throughout all cells, leading to yellow fur, as well as adult-onset obesity, diabetes, and tumorigenesis.4,5 Interestingly, CpG methylation in the Avy IAP correlates inversely with ectopic Agouti expression. The degree of methylation within the 5′ IAP long terminal repeat (LTR) varies dramatically among individual isogenic Avy/a mice, causing a wide variation in coat color ranging from yellow (unmethylated) to pseudoagouti (methylated) (Fig. 1B).
Figure 1. The viable yellow agouti (Avy) mouse model.
A) A contraoriented IAP insertion within pseudoexon 1A (PS1A) of the murine agouti gene contains a cryptic promoter (short arrowhead labeled Avy) that drives ectopic agouti expression. Transcription of A and a alleles initiates from a hair-cycle-specific promoter in exon 2 (short arrowhead labeled A, a). B) Genetically identical adult viable yellow agouti (Avy) mice representing the five coat color phenotypes. Yellow mice are hypomethylated at the transposable element upstream of the Agouti gene allowing maximal ectopic expression, whereas hypermethylation of this site silences ectopic agouti expression in the pseudoagouti animals. Mice that are predominately yellow are also clearly more obese than brown mice. Reprinted from Dolinoy et al. (2007)11 with permission.
The Avy allele is the most extensively studied murine metastable epiallele. Metastable epialleles are identical alleles that are variably expressed due to epigenetic modifications that are established very early in development.6 They are most often associated with retroelements and transgenesis. Three of the identified murine metastable epialleles (Avy, AxinFu, CabpIAP) are associated with contraoriented IAP insertions.2,7,8 The extent of DNA methylation at each allele is stochastic and dependent upon maternal nutrition and environmental exposures during early development.3,9–11 Approximately a thousand copies of IAP retrotransposons are present in the mouse genome,12 and about 40% of the human genome is comprised of transposable elements, of which approximately 9% are retrotransposons.13
The work summarized here utilizes the Avy mouse model as an epigenetic biosensor to characterize nutritional and environmental factors affecting epigenetic gene regulation and subsequent adult phenotype. First, the Avy model was recently employed to investigate the effects of a plant phytoestrogen on the fetal epigenome.9 Isoflavones represent a class of phytoestrogens present in soy and soy products that are active in multiple biological systems, including estrogen receptor- and non-estrogen receptor-mediated signaling pathways.14,15 Maternal dietary supplementation with genistein (250 mg/kg diet), the major isoflavone present in soy, shifted the coat color distribution of Avy/a offspring toward pseudoagouti (brown). This marked phenotypic change was mediated by increased DNA methylation of six CpG sites within the Avy IAP. The extent of DNA methylation in tissues from the three germ layers (brain, kidney, and liver) was correlated, indicating that genistein’s influence on DNA methylation occurs during early embryonic development. Moreover, the genistein-induced hypermethylation persisted into adulthood, decreasing ectopic Agouti expression and protecting adult offspring from obesity. The observed effects of genistein on the epigenome serve as a plausible explanation for the lower incidence of certain cancers in Asians compared to Westerners16 as well as the increased cancer incidence in Asians who immigrate to the United States.17 In the future, it will be important to determine if co-exposure to genistein and methyl donors, such as folic acid, or the presence of other isoflavones, can somehow inappropriately methylate the epigenome. This is especially critical for infants consuming soy formula and in areas where the local grain supply is supplemented with folic acid.
Many xenobiotics, ubiquitously present in the environment, have estrogenic and/or other hormonal properties and function as endocrine disruptors. Their potential to modify the epigenome remains largely unexplored. Recently, the Avy model was utilized to evaluate the effects of maternal exposure to the endocrine active chemical bisphenol A (BPA) on the fetal epigenome.11 BPA is a high-production volume chemical used in the manufacture of polycarbonate plastic and epoxy resins, and it is present in many commonly used items including food and beverage containers, baby bottles, and dental sealants. To evaluate the effects of maternal BPA exposure on the fetal epigenome, female a/a mice received phytoestrogen-free AIN-93G diet or phytoestrogen-free AIN-93G combined with 50 mg BPA/kg diet 2 weeks prior to mating with Avy/a males and throughout gestation and lactation. Maternal dietary BPA did not significantly (p>0.25) influence litter size, litter survival, wean weight, genotypic ratio, or sex ratio (data not shown). In contrast, maternal dietary BPA exposure shifted the coat color of Avy/a offspring toward yellow (Fig. 2A) and decreased methylation and nine CpG sites within the Avy IAP. CpG methylation was also decreased at the CabpIAP metastable locus, indicating that BPA-induced hypomethylation is not gene-locus specific, and may also impact as yet unidentified epigenetically labile genes in the mouse and, potentially, the human genome. Moreover, the BPA-induced hypomethylation of the fetal epigenome was abolished by maternal dietary nutritional supplementation with either methyl donors (folic acid, betaine, vitamin B12, and choline) (Fig. 2B) or the phytoestrogen genistein (Fig. 2C). These findings demonstrate that simple dietary changes can protect against the deleterious effects of environmental toxicants on the fetal epigenome.
Figure 2. Offspring coat color distributions following maternal BPA exposure or maternal BPA exposure combined with nutritional supplementation.
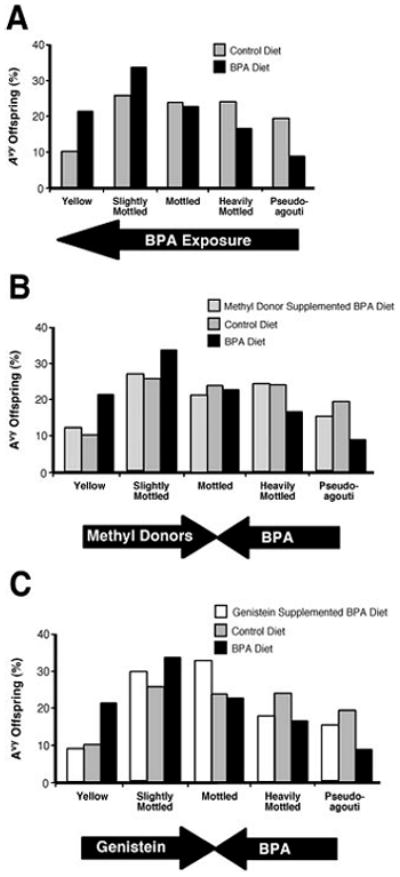
A) Coat color distribution of Avy/a offspring born to 16 control (n=60) and 17 BPA-exposed (n=73) litters (50 mg BPA/kg diet). Maternal BPA exposure shifts offspring coat color distribution toward yellow (p=0.007). B) Coat color distribution yellow. C) Coat color distribution of Avy/a offspring born to 13 BPA-exposed/genistein-supplemented (n=39), 16 control (n=60), and 17 BPA-exposed (n=73) mothers. Maternal nutritional supplementation of the of Avy/a offspring born to 14 BPA-exposed/methyl donor-supplemented (n=54), 16 control (n=60), and 17 BPA-exposed (n=73) mothers. Maternal nutritional supplementation with methyl donors counteracts BPA-induced DNA hypomethylation, and the shift in coat-color distribution toward BPA diet with the phytoestrogen genistein also counteracts BPA-induced DNA hypomethylation and the shift in coat color distribution toward yellow. Redrawn from Dolinoy et al. (2007)11 with permission.
The BPA study represents the first time the Avy model was employed to examine the effects of an environmental contaminant rather than a nutritional agent on the fetal epigenome. Unfortunately, current risk assessment policies have yet to incorporate environmental influences on the fetal epigenome in standard practice. While our studies with the Avy mouse have determined that maternal nutrition3,9 and environmental exposures11 influence the fetal epigenome, the characterization of other key chemical and physical environmental factors affecting the fetal epigenome is imperative to protect individuals from environmentally induced changes in the fetal epigenome. Although the Avy mouse serves as an excellent in vivo epigenetic biosensor for epigenetic alterations, the eventual development of high-throughput assays for rapid toxicological evaluation of environmental agents on the epigenome will significantly advance the field of environmental epigenomics.
In our maternal exposure studies, Avy methylation was similar in tissues from the three germ layers, indicating that methyl donors, genistein, and BPA act early in development.3,9,11 Clearly, embryogenesis is a critical window of vulnerability for environmentally induced epigenetic alterations. In fact, epigenetic marks, including CpG methylation, are generally stable in somatic cells; however, during at least two developmental time periods, the epigenome undergoes extensive reprogramming. These critical windows of development include gametogenesis as well as early preimplantation embryos.18 Therefore, in order to fully characterize environmental epigenomics, a fuller analysis of timing of exposure will be essential. In fact, a recent study of maternal nutrition using the Axinfu metastable epiallele observed tissue-specific DNA methylation changes, indicating that environmentally induced epigenetic changes can be dependent on the developmental time point of exposure.10 Epigenetic risk assessment strategies should consider the developing embryo as well as other critical time points (e.g. puberty and old age) when assessing environmental risk of a particular compound.
Epigenetic modifications affecting metastable epiallele regulation appear to be not only mitotically heritable, but also transgenerationally heritable through inefficient reprogramming of epigenetic marks during gametogenesis.4,19,20 Additionally, it has been shown that the influence of environmental factors on epigenetic gene regulation may also persist transgenerationally despite lack of continued exposure in subsequent generations.21,22 Breeding studies conducted with both Avy and AxinFu mice have revealed transgenerational inheritance of coat color4 or tail kink phenotype.19 However, when Whitelaw et al.23 investigated DNA methylation as the inherited epigenetic mark within developing Avy embryos, DNA methylation was entirely absent from the blastocyst, indicating that CpG methylation is not the inherited mark. Thus, though it is clear that environmental effects on the epigenome can be inherited in the mammalian germ-line, the mechanisms supporting this inheritance are unknown. Histone modifications and non-coding RNAs are two likely mechanisms mediating transgenerational epigenetic inheritance, although their exact roles remain to be elucidated.
When developmental biologist Conrad Waddington first envisioned the epigenetic landscape as a metaphor for cellular determination during development, it is doubtful he could have predicted the full complexity of the field 50 years later.24 Throughout the years, the definition of epigenetics has evolved, while the number of molecular phenomena involving epigenetic pathways has grown. Recently, yet another metaphor was put forward to describe the field of epigenetics as a “bridge between genotype and phenotype” that alters gene expression without changing the underlying DNA sequence.25 The notion that early environmental exposures interact with epigenetic gene regulation to influence phenotype and adult disease suggests that epigenetic gene regulation serves as a link between nature and nurture.
Acknowledgments
The author thanks Randy L. Jirtle for critical reading of this manuscript.
Funding. This work was supported by NIH grants ES13053, ES08823, ES015165, and T32-ES07031.
Footnotes
Declaration of interest. The author declares no competing financial interests.
References
- 1.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duhl D, Vrieling H, Miller KA, Wolff GL, Barsh GS. Neomorphic agouti mutations in obese yellow mice. Nat Genet. 1994;8:59–65. doi: 10.1038/ng0994-59. [DOI] [PubMed] [Google Scholar]
- 3.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan H, Sutherland H, Martin D, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23:314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 5.Miltenberger R, Mynatt R, Wilkinson J, Woychik R. The role of the agouti gene in the yellow obese syndrome. J Nutr. 1997;127(Suppl):S1902–S1907. doi: 10.1093/jn/127.9.1902S. [DOI] [PubMed] [Google Scholar]
- 6.Rakyan VK, Blewitt ME, Druker R, Preis JI, Whitelaw E. Metastable epialleles in mammals. Trends Genet. 2002;18:348–351. doi: 10.1016/s0168-9525(02)02709-9. [DOI] [PubMed] [Google Scholar]
- 7.Vasicek T, Zeng L, Guan X, Zhang T, Costantini F, Tilghman S. Two dominant mutations in the mouse fused gene are the result of transposon insertions. Genetics. 1997;147:777–786. doi: 10.1093/genetics/147.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Druker R, Bruxner TJ, Lehrbach NJ, Whitelaw E. Complex patterns of transcription at the insertion site of a retrotransposon in the mouse. Nucl Acids Res. 2004;32:5800–5808. doi: 10.1093/nar/gkh914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolinoy DC, Wiedman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waterland RA, Dolinoy DC, Lin JR, Smith CA, Shi X, Tahiliani K. Maternal methyl supplements increase offspring DNA methylation at Axin (fused) Genesis. 2006;44:401–406. doi: 10.1002/dvg.20230. [DOI] [PubMed] [Google Scholar]
- 11.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuff E, Lueders K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- 13.International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature. 2001;209:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 14.Lamartiniere C, Cotroneo M, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132(Suppl):S552–S558. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 15.Valachovicova T, Slivova V, Sliva D. Cellular and physiological effects of soy flavonoids. Mini Rev Med Chem. 2004;4:881–887. doi: 10.2174/1389557043403387. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Gourley L, Duffy S, Esteve J, Lee J, Day N. Dietary effects on breast-cancer risk in Singapore. Lancet. 1991;337:1197–1200. doi: 10.1016/0140-6736(91)92867-2. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler R, Hoover R, Pike M, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 18.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 19.Rakyan VK, Chong S, Champ ME, et al. Transgenerational inheritance of epigenetic states at the murine AxinFu allele occurs after maternal and paternal transmission. Proc Natl Acad Sci. 2003;100:2538–2543. doi: 10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakyan VK, Preis J, Morgan HD, Whitelaw E. The marks, mechanisms and memory of epigenetic states in mammals. Biochem J. 2001;356:1–10. doi: 10.1042/0264-6021:3560001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang HS, Anway MD, Rekow SS, Skinner MK. Transgenerational epigenetic imprinting of the male germline by endocrine disruptor exposure during gonadal sex determination. Endocrinology. 2006;147:5524–5541. doi: 10.1210/en.2006-0987. [DOI] [PubMed] [Google Scholar]
- 23.Blewitt ME, Vickaryous NK, Paldi A, Koseki H, Whitelaw E. Dynamic reprogramming of DNA methylation at an epigenetically sensitive allele in mice. PLoS Genet. 2006;2:399–405. doi: 10.1371/journal.pgen.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waddington C. A Discussion of Some Aspects of Theoretical Biology. Allen and Unwin; London: 1957. The Strategy of the Genes. [Google Scholar]
- 25.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]