Abstract
PURPOSE
To safely assess new drugs, cancer patients in initial cohorts of phase I oncology studies receive low drug doses. Doses are successively increased until the maximum tolerated dose (MTD) is determined. Since traditional chemotherapy is often more effective near the MTD, ethical concerns have been raised regarding administration of low drug doses to phase I patients. However, a substantial portion of oncology trials now investigate targeted agents, which may have different dose-response relationships than cytotoxic chemotherapies.
EXPERIMENTAL DESIGN
Twenty-four consecutive trials treating 683 patients between 10-01-2004 and 6-30-2008 at MD Anderson Cancer Center were analyzed. Patients were assigned to a low-dose (≤25% MTD), medium-dose (25–75% MTD), or high-dose (≥75% MTD) group, and groups were compared for response rate, time-to-treatment-failure, progression-free survival, overall survival, and toxicity. To remove negatively biasing data from the high-dose group, in a second analysis patients treated above the MTD were excluded (high-dose group = 75–100% MTD). 97.7% of patients received targeted agents.
RESULTS
Even when excluding patients above the MTD, there was an early trend favoring the low- versus high-dose group in time-to-treatment-failure, with 32.9% versus 25.2% of patients on therapy at 3 months (p=0.08). Additionally, the low-dose group fared at least as well as the other groups in all other outcomes, including response rate, progression-free survival, overall survival, and toxicity.
CONCLUSIONS
These data may help alleviate concerns that patients who receive low drug doses on contemporary phase I oncology trials fare worse, and suggest targeted agents may have different dose-response relationships than cytotoxic chemotherapies.
INTRODUCTION
Critical to the development of novel anti-cancer treatments is the study of new drugs and drug combinations in clinical trials.(1) A new drug treatment’s optimal dose is usually determined in a phase I clinical trial, and for traditional chemotherapy the optimal dose has generally been considered to be near the drug’s maximum tolerated dose (MTD).(2) In a phase I trial, the MTD is determined by employing a dose-escalation scheme, in which consecutively enrolled patient cohorts receive increasing doses of study agent until unmanageable or unsafe side effects emerge.(3, 4) The highest tolerated dose defines the MTD. In contrast to phase I studies of other drugs, in which participants are usually healthy volunteers, phase I trials of anti-neoplastic agents are generally conducted in cancer patients due to the potentially toxic nature of some anti-cancer drugs. These patients have usually progressed through standard treatments, but they and their physicians often hope that there may be some clinical benefit from participating in a phase I study.(5–9) For these reasons, phase I trials carry ethical concerns. Some critics argue that patients in the low-dose cohorts of these dose-seeking studies receive suboptimal or even placebo doses of medication compared to those treated closer to the MTD.(3, 10–14) Additionally, physicians may be reluctant to refer patients to studies with potentially ineffective dose levels, and patients may be hesitant to participate in phase I trials for the same reason.(8, 15)
The paradigm of dosing cancer treatments at or near their MTD’s stems from early observations of positively-sloped dose-response relationships with classic cytotoxic chemotherapies, which showed increasing efficacy at higher doses.(2, 16–19) Many of these early observations were with agents that are indiscriminately cytotoxic, such as nucleoside analogues, and are not surprising given that since malignant cells generally divide faster than non-malignant cells, higher doses induce increased cell-kill through a kinetic mechanism.(20) However, many anti-cancer drugs currently undergoing clinical investigation are not classically cytotoxic, but rather are targeted agents that exploit some feature or pathway that is unique to or exaggerated in cancer cells, such as a growth factor receptor, kinase, or angiogenesis pathway. For this reason, and because of the ethical issues and patient and physician concerns noted above, we investigated whether patients receiving lower drug doses in contemporary phase I oncology trials are disadvantaged compared to patients receiving higher doses.
METHODS
Trial and Participant Inclusion and Data Collection
We evaluated 71 consecutive clinical trials enrolling 1420 participants between 10/01/2004 and 06/30/2008 in the Phase I Program at MD Anderson Cancer Center for eligibility in this analysis. To be included, a trial had to be a dose-escalation study of systemic therapy that had either reached a maximum tolerated dose (MTD), or in which at least one patient had received treatment at the maximum planned dose. For all studies, MTD is defined as the dose-level immediately below that dose which produced an unacceptable amount of dose-limiting toxicities as predefined in each study. The most common precise definition in our studies was the dose level below which at least one-third of patients had a dose-limiting toxicity. Twenty-four trials with 683 participants met criteria. Trials that had not yet reached an MTD or maximum planned dose (30 trials, 408 patients), were not dose-escalation studies (10 trials, 196 patients), were local-regional therapies (4 trials, 106 patients), and were not drug trials (3 trials, 27 patients) were excluded. Patients who started a study by 5/01/2008 were included, and response data through 6/30/2008 are used in the analysis. The majority of trials were multicenter (median = 2, range = 1–5), and MTD’s were determined based upon patient responses from all sites. All patients in the analysis were treated at MD Anderson, since efficacy data from other trial sites was limited.
This study was conducted in accordance with our Institutional Review Board’s guidelines. Data were obtained from the electronic patient record system and from standardized clinical trial overview tables used by the department. These tables are maintained on a secure institutional network as an abbreviated database for all trials performed in the department.
Treatment Dose
Doses administered on each trial were normalized as per the linear relationship [Dose Percentile] = [(Administered Dose − Minimum Dose)/(Maximum Dose − Minimum Dose)] * 100%. For trials that reached an MTD, Maximum Dose = MTD. For trials in which the maximum planned test dose was tolerated, Maximum Dose = the maximum planned dose. In this way, values ≤ 100% represent doses at or below the MTD, and values > 100% represent doses above the MTD. For trials with more than one agent, Dose Percentile is the arithmetic average of the Dose Percentile calculated for each agent.
Dose Percentiles were used to assign patients to arbitrarily defined low (25%), medium (>25% to <75%), and high (≥75%) dose groups. In this study, two analyses were performed: (i) all patients are included (i.e., doses from 0% to >100%), and (ii) only patients treated at or below the MTD are included (i.e., doses from 0–100%). In the latter, those receiving study drug above the MTD were excluded to reduce negative bias against the high-dose group’s outcomes due to increased toxicities.
Evaluation of Tumor Response to Treatment
Radiographic treatment responses were reported in each study. Using the World Health Organization (WHO)(21) criteria and Response Evaluation Criteria in Solid Tumors (RECIST),(22) responses were categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). CR is defined as tumor disappearance, PR as reduction of at least 50% (WHO) or 30% (RECIST), SD as any response between PR and PD, and PD as the appearance of new lesion(s) or increase by at least 25% (WHO) or 20% (RECIST). While imaging study was required for categorization of response as CR, PR, or SD, PD could additionally be assigned by a phase I physician for significantly worsening tumor-related symptoms (for example, hemoptysis in patient with tumor involving bronchus).
Statistical Analysis
All statistical analyses were performed with our statistician (JJL). To compare differences in baseline patient characteristics among dose groups, one-way analysis of variance was applied for age and number of prior treatments; Pearson’s chi-squared test for sex, race, performance status, and tumor type; and Kruskal-Wallis test for times from cancer diagnosis and end of last treatment to start of phase I treatment.
Percentages of participants with (CR/PR/SD) or PD were calculated by simple division, as was the percent of participants that came off study for toxicity (Figure 1). Estimates of time-to-treatment failure, progression-free survival (PFS), and overall survival (OS) were calculated by the Kaplan-Meier method(23) (Figures 2–4). Time-to-treatment failure and PFS differ as follows. In time-to-treatment failure, participants are assigned non-censored end dates when coming off study for any reason, and censored end dates only if on study on 6/01/2008. In PFS, participants are assigned non-censored end dates only at the time of PD or death. If a participant started a new treatment prior to PD or death, the first day of the new treatment is taken as a censored end date given any subsequent effect can not necessarily be attributed to the original treatment. As with time-to-treatment failure, when calculating PFS and OS patients are assigned censored end dates if still on study on 6/01/2008. Given these differences, PFS primarily captures the tumor response to therapy while time-to-treatment failure captures the tumor response in addition to other reasons a treatment may “fail,” for example due to toxicities.
Figure 1.
Response to Treatment 3–12 Months After Starting Study. A. Percentage of patients with a favorable response that stayed on study. B. Percentage off trial due to toxicity. C. Percentage off trial due to progressive disease or toxicity.
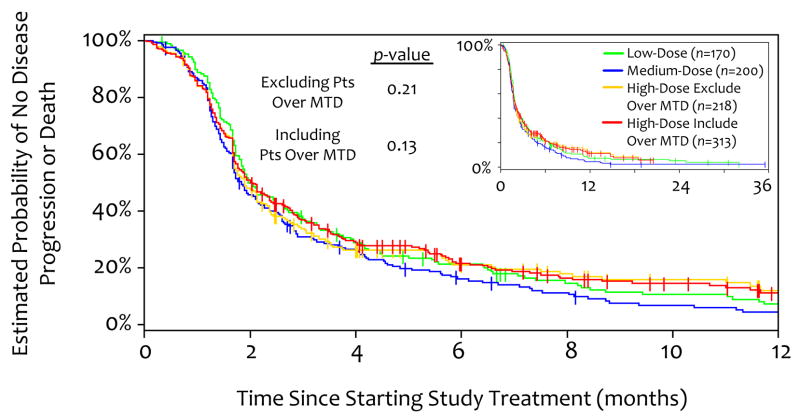
Figure 2.
Kaplan-Meier Estimate of Time-to-Treament Failure. Tic marks represent censored patients (those still on study on June 30, 2008, the last date included in this analysis). p-values are for low, medium, and indicated high-dose group. Inset shows 3-year data.
Figure 4.
Kaplan-Meier analysis of OS. Overall survival for up to 3 years after starting phase I study.
p -values are calculated from Cox proportional hazards model, and p-values ≤ 0.05 are taken to be significant. Two-sided p-values are calculated as the distribution function of the z-test for the indicated comparisons (Figure 1). All statistical analyses were performed using R version 2.8.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Trial and Participant Characteristics
We analyzed 24 phase I studies including 683 participants, with a median of 22 participants per trial (range 11–68; Table 1). Most of the trials were of single agents (18 trials), while 5 combined 2 agents and one combined 3 agents. The median number of dose levels evaluated was 5 (range 3–13). The trials tested agents that span a broad range of anti-tumor mechanisms (Table 1), and at least one targeted or biological agent was part of the study regimen for 97.7% of participants.
Table 1.
Characteristics of Trials Included for Analysis
| Trial Target/Mechanism | Class | Number of Patients per Trial (n = 683) | Number of Patients Treated above MTD (n = 95) | Number of Dose Levels Tested |
|---|---|---|---|---|
| SINGLE AGENT | ||||
| Angiopoetin inhibitor | Biologic | 17 | 0 | 4 |
| Apoptosis | Biologic | 35 | 0 | 4 |
| Aurora kinase inhibitor | Small molecule | 16 | 0 | 9 |
| Cyclin dependent kinase inhibitor | Small molecule | 18 | 4 (22%) | 5 |
| Death receptor | Biologic | 23 | 0 | 5 |
| DNA synthesis inhibitor | Small molecule | 16 | 0 | 4 |
| EGFR/VEGFR inhibitor | Small molecule | 11 | 2 (18%) | 6 |
| Hypomethylating agent | Small molecule | 31 | 0 | 5 |
| Insulin growth factor receptor | Biologic | 21 | 0 | 4 |
| Mitotic inhibitor | Small molecule | 18 | 5 (28%) | 8 |
| Multikinase inhibitor | Small molecule | 11 | 2 (18%) | 3 |
| NF-kappa-B inhibitor | Small molecule | 21 | 3 (14%) | 10 |
| Ras inhibitor | Small molecule | 24 | 0 | 5 |
| STAT-3 inhibitor | Small molecule | 24 | 0 | 5 |
| Tyrosine kinase inhibitor | Small molecule | 49 | 15(31%) | 13 |
| Topoisomerase inhibitor | Small molecule | 14 | 0 | 5 |
| Multiple mechanisms, apoptosis inducer/antiangiogenic | Small molecule | 40 | 8 (20%) | 8 |
| Multiple mechanisms, apoptosis inhibitor/tubulin inhibitor | Small molecule | 11 | 0 | 4 |
| TWO AGENTS | ||||
| Anti-angiogenic + proteosome inhibitor | Biologic + small molecule | 54 | 0 | 9 |
| Hypomethylting agent + HDAC inhibitor | Small molecule | 68 | 6 (10%) | 7 |
| Immune + multiple mechanisms including Src inhibitor | Immune modulator + small molecule | 20 | 7 (35%) | 5 |
| Microtubule inhibitor + pro-apoptotic | Small molecule | 33 | 8 (24%) | 7 |
| Ras inhibitor + multikinase inhibitor | Small molecule | 58 | 14 (24%) | 5 |
| THREE AGENTS | ||||
| Proteosome + DNA synthesis inhibitors | Small molecule | 50 | 21 (42%) | 9 |
EGFR = epidermal growth factor receptor, HDAC = histone deacetylase, MTD = maximum tolerated dose, STAT-3 = signal transducer and activator of transcription 3, VEGFR = vascular epithelial growth factor receptor
Baseline participant characteristics were equally distributed among the low- (n=170), medium- (n=200), and high- (n=218 or 313 for excluding and not excluding patients treated over the MTD, respectively) dose groups, with no statistically significant difference in any parameter (Table 2). The mean age for all participants was 55.7 years, 55.2% were male, and 77.9% were white. Approximately 94% had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Gastrointestinal malignancies were the most common, reflecting referral patterns and disease prevalence. On average, participants had close to 6 prior treatments, including 3.7 systemic therapies, 1.3 surgeries, and 0.6 radiation treatments. There was an average of 2.0 months between finishing prior treatment and starting a phase I study agent, and 3.4 years between cancer diagnosis and starting a phase I study.
TABLE 2.
BASELINE PATIENT CHARACTERISTICS
| Low-Dose# | Medium-Dose# | High-Dose# | p-value | |||
|---|---|---|---|---|---|---|
| ≤25% of MTD n = 170 | 25–75% of MTD n = 200 | 75–100% MTD (Exclude Patients Treated Over MTD) n = 218 | ≥75% of MTD (Include Patients Treated Over MTD) n = 313 | Exclude Patients Over MTD† | Include Patients Over MTD‡ | |
| Age (Mean ± SD*) | 55.8 ± 12.9 yrs | 55.1 ± 14.0 yrs | 56.1 ± 12.9 yrs | 56.0 ± 13.8 yrs | p = 0.79 | p = 0.74 |
| Sex | ||||||
| Male | 58.2% | 52.5% | 56.0% | 55.3% | p = 0.54 | p = 0.53 |
| Female | 41.8% | 47.5% | 44.0% | 44.7% | ||
| Race | ||||||
| White | 77.1% | 74.5% | 80.3% | 80.5% | p = 0.69 | p = 0.60 |
| Black | 9.4% | 11.0% | 6.9% | 6.4% | ||
| Hispanic | 9.4$ | 9.0% | 6.9% | 8.0% | ||
| Asian | 4.1% | 4.5% | 4.6% | 3.8% | ||
| Other | 0.0% | 1.0% | 1.4% | 1.3% | ||
| Performance Status (PS) | ||||||
| Patients with PS = 0 | 32.9% | 30.0% | 31.2% | 31.6% | p = 0.55 | p = 0.78 |
| Patients with PS = 1 | 60.6% | 66.0% | 63.8% | 61.0% | ||
| Patients with PS ≥ 2 | 6.5% | 4.0% | 5.0% | 7.3% | ||
| Tumor Typeϕ | ||||||
| Breast (n=61) | 7.1% | 11.5% | 8.7% | 8.3% | p = 0.32 | p = 0.73 |
| Gastrointestinal (n =206) | 37.6% | 31.0% | 29.4% | 25.6% | ||
| Genitourinary (n =63) | 7.1% | 8.0% | 9.6% | 11.2% | ||
| Head and Neck (n =50) | 7.6% | 6.0% | 8.3% | 8.0% | ||
| Melanoma (n =60) | 7.6% | 9.0% | 7.8% | 9.3% | ||
| Thoracic (n =54) | 9.4% | 6.5% | 6.9% | 8.0% | ||
| All Other (n =189) | 23.5% | 28.0% | 29.4% | 29.7% | ||
| Number of Prior Treatments (Mean ± SD*) | ||||||
| Systemic Treatments | 3.8 ± 2.2 | 3.9 ± 2.3 | 3.5 ± 2.4 | 3.5 ± 2.4 | p = 0.11^ | p = 0.13^ |
| Radiation Treatments | 0.6 ± 0.8 | 0.6 ± 0.8 | 0.7 ± 0.8 | 0.7 ± 0.9 | ||
| Surgical Treatments | 1.2 ± 1.2 | 1.4 ± 1.4 | 1.3 ± 1.0 | 1.3 ± 1.1 | ||
| Other Treatments∫ | 0.2 ± 0.6 | 0.3 ± 1.0 | 0.2 ± 0.5 | 0.2 ± 0.5 | ||
| 5.8 ± 2.8 | 6.2 ± 2.7 | 5.7 ± 2.8 | 5.7 ± 2.8 | |||
| Time From Cancer Diagnosis to C1D1 of Study Treatment% (Median with Range) | 3.2 years (0.4–26.6) | 3.5 years (0.3–33.1) | 3.1 years (0.1–36.7) | 3.4 years (0.1–36.7) | p = 0.79 | p = 0.63 |
| Time From End of Last Treatment to C1D1 of Trial§ (Median with Range) | 1.7 months (0.3–34.9) | 2.0 months (0.3–67.4) | 2.1 months (0.0–269.9) | 2.1 months (0–269.9) | p = 0.10 | p = 0.10 |
As per Methods, dose percentage is percent of MTD or percent of maximum tested dose if the maximum tested dose was not a MTD
p-values for analyses of Low, Medium,and High-Dose Exclude Patients Over MTD (n = 588)
p-values for analyses of Low, Medium, and High-Dose Include Patients Over MTD (n = 683)
SD = standard deviation
n-values include all patients. All Other includes endocrine, gynecological, lymphoma, myeloma, sarcoma, and other malignancies.
Concurrent chemotherapy and radiation therapy is categorized as “other treatment”
Given p-values are for the cumulative number of treatments by one-way ANOVA
C1D1 = cycle 1, day 1 of phase I treatment
Two patients (both in the high dose group) had no therapy prior to phase I treatment
Treatment Outcomes
As described in Methods, two analyses were conducted: one including all participants (n = 683), and another excluding those at doses greater than the MTD (n = 588). Radiographic responses and treatment induced toxicities are reported from 3–12 months after starting treatment (Figure 1), given the first planned imaging on most studies occurred after two cycles of treatment (cycles lasted on average 3–4 weeks).
In regard to favorable treatment outcomes, in each of the two analyses the percentage of patients whose disease was stable or better (indicating controlled disease) and who remained on treatment (indicating absent or tolerable side effects) was greater in the low-dose group than in the medium- and high-dose groups at 90 days, albeit not statistically significant, and the percentage remained statistically equivalent up to 1 year after starting therapy (Figure 1A). For example, the percentage of patients with (CR/PR/SD) and who were still on therapy at 90 days was 32.9% versus 25.2% for low-versus high-dose groups even when patients treated above the MTD are excluded (p=0.08).
Regarding unfavorable outcomes, the percentage of patients who came off trial for toxicity was lowest in the low-dose group and highest in the high-dose group at all time points, even if patients treated above the MTD were excluded. For example, these percentages are 1.8% versus 9.3% (p<0.01) at 3 months, and 2.4% versus 11.4% (p<0.01) at 12 months (Figure 1B). When patients treated above the MTD were included, the particularly large percentage of patients in the high-dose group who came off for toxicity (16.2% by 365 days) can be attributed to the fact that this group contained 95 patients treated at doses above the MTD. Other than toxicities, some patients came off study due to disease progression. Patients did not fail treatment more often in the low-dose group than in the medium or high-dose groups (for example, PD or toxicity at 3 months in 58.2% versus 65.9% of patients for low- versus high-dose group when those treated over the MTD are excluded, p=0.12; Figure 1C). A small percentage of patients (~10% in each dose group; data not shown) came off study for non-disease, non-treatment related reasons, such as co-morbidities and personal issues. In time-to-treatment failure analysis, these patients are still considered failures at the time drug was discontinued.
Time-to-treatment Failure, Progression-free Survival (PFS), Overall Survival (OS)
Time-to-treatment failure, PFS, and OS were estimated by the Kaplan-Meier method and were found to be similar for low-, medium-, and high-dose groups in analysis of all participants and in the analysis which excludes participants treated above the MTD. In fact, in no case does the low-dose group have a less desirable outcome than the medium- or high-dose groups (Figures 2–4). For all participants, median time-to-treatment failure was 1.7 months (Figure 2) and median PFS was 1.9 months (Figure 3). Median OS was 8.2 months for all participants (Figure 4), consistent with published data.(24) Of note is that upon restaging, a best response of SD, PR, or CR was observed in 46.8% of patients (first restaging on most studies occurs after 2 cycles of treatment, with cycles lasting 3–4 weeks). SD for at least 6 months, PR, or CR was observed in 11.9% of patients.
Figure 3.
Kaplan-Meier Estimate of PFS. Tic marks represent patients who started another treatment prior to disease progression or death, or who were on study on June 30, 2008. p-values are for low, medium, and indicated high-dose group. Inset shows 3-year data.
DISCUSSION
The practice of administering maximum tolerated drug doses to oncology patients originates from studies of childhood leukemia. Preclinical studies demonstrated that drug dose was proportional to the percentage of leukemic cells killed,(16) and the first clinical trial demonstrating increased benefit with higher dose therapy was published more than four decades ago.(17) While the practice of treating at or near the MTD has generally been maintained, the practical mechanics of how that dose is determined, specifically in phase I trials, is an issue of debate. Ethicists raise concerns over a large percentage of patients on phase I oncology trials receiving study drugs at potentially sub-therapeutic doses until the vicinity of the MTD is achieved.(10, 12–14)
To address this concern, we evaluated dose-response outcomes of 683 participants in 24 contemporary phase I oncology studies (conducted between 2004–2008; Methods). We included all consecutive studies in the specified time frame (Methods) to best capture the outcomes of all-comers to contemporary phase I studies, as opposed for example to including only single agent studies. Greater than 97% of study participants received at least one targeted agent (Table 1). As such, our results may primarily be helpful in the context of evaluating trials studying similar agents, given this study can not draw conclusions regarding relative outcomes for patients receiving lower versus higher doses of classically cytotoxic chemotherapy. We found that participants treated in low-dose cohorts of contemporary phase I studies are not clinically disadvantaged compared to their higher-dose counterparts (Figures 1–4). This is consistent with data from another analysis in a smaller group of patients.(25) Furthermore, advantageous effects were observed for the low-dose group in regard to the percentage of participants that came off trial for toxicity, even when patients treated above the MTD were excluded (2.4% versus 11.4% for low versus high-dose at one year, p < 0.01; Figure 1B). Consistent with this, one analysis of 149 phase I studies showed that trials with more aggressive dose escalation schemes had greater toxicities while response rates remained similar.(26)
Regarding dose groups, they were arbitrarily set to reasonable ranges (low ≤ 25% of MTD, medium = 25–75% of MTD, and high ≥ 75% of MTD; Methods). However, to further examine the relationship, another analysis was performed. Comparison of participants treated (i) below the MTD, n = 393, versus (ii) at the MTD (or maximum tested dose if no MTD was found), n = 195, demonstrated no discernable downside to being treated at the lower doses in regard to the described outcomes. For example, comparison of patients treated below the MTD versus those treated at the MTD (or maximum tested dose if no MTD was found) showed 29.0% versus 23.1% of patients had (CR/PR/SD) and were on therapy at 3 months (p=0.12), and 13.0% versus 9.2% had (CR/PR/SD) and were on therapy at 6 months (p=0.16). Further studies will be required to elucidate the basis for this observation, but it is possible that targeted agents exert their effects in a relatively dose-independent manner, or that even the lowest doses in contemporary phase I oncology studies are above a minimum threshold for impacting the target.
In evaluating the data, it is somewhat surprising that the low-dose group’s outcomes were as favorable as the high-dose group’s given that the high-dose group is enriched with patients more likely to respond to therapy. This enrichment exists because if a treatment shows promising results in a patient(s) during a study’s dose-escalation phase, then the study may allow for additional patients who have the same tumor type(s) as the one(s) who responded to be enrolled at the MTD (or maximum tested dose). Fourteen of the 24 studies in this analysis include patients treated in such MTD dose-expansion cohorts. The most likely explanation for this surprising result may be that patients treated at the MTD - which is defined based upon toxicities observed within the first three to four weeks of treatment - had more cumulative toxicities with prolonged administration than those treated at lower doses, thus necessitating their withdrawal. Additionally, while a higher dose can cause greater toxicity, it does not necessarily result in greater efficacy; targeted agents may fully modulate their target or have distinct activities at doses lower than the MTD. Such a precedent has been shown for decitabine, an agent used to treat myelodysplastic syndrome, which is both better tolerated and more effective at lower doses.(27–29) Collectively, these observations suggest that the issue of optimum dosing of targeted therapies is complex, and that exploring doses lower than the MTD may be worthwhile for some agents. Consistent with this notion, a discussion of the prudence of the classic MTD approach in the era of targeted therapies is taking place. (30–32) Some argue that too little is known to abandon historical approaches,(33) while others argue that efforts should be made to guide treatment based on the minimal effective dose(34) or optimum biological dose.(35, 36)
Finally, while the aim of this study was to evaluate potential downsides to low-dose cohort participants on phase I oncology studies, and not to evaluate the efficacy of phase I studies, the latter point deserves some mention. Disease response rates seen in this analysis are consistent with previously published results.(2, 37–40) For example, Hortsmann, et al evaluated 460 phase I trials enrolling 11,935 participants from 1991–2002, of whom 10,402 (87.2%) were evaluated for response.(39, 41) A best response of CR or PR was observed in 10.6% of participants, while an additional 34.1% had SD, for an overall “benefit rate” of 44.7%. In our study, 622 of 683 participants (91.1%) were evaluated for response, while 8.9% came off study for toxicities or for other reasons before a first assessment of disease response. Of participants evaluated, 46.8% achieved a best response of CR, PR, or SD, which is similar to the Hortsmann study. Additionally, in our study 11.9% of patients achieved CR, PR, or prolonged SD (prolonged SD = stable disease for 6 months or greater).
The ethical issues surrounding phase I trials are complex, and may benefit from data gleaned through investigational analyses. In this study, we evaluated a large cohort of patients participating in contemporary phase I oncology trials of predominantly targeted agents. We found no discernable downside to being treated in the low- versus the medium- or high-dose groups. These data may help to alleviate concerns about a relative lack of benefit for patients in low-dose cohorts of phase I trials, and support the notion that further investigation of targeted agents may be required to fully understand their optimal dosing and potentially beneficial effects.
Acknowledgments
We thank Drs. Steve Lockless and Richard Theriault for critical manuscript review and Dr. Fadi Braiteh and Karin Hahn for discussions regarding conceptual design of the study. The study was supported in part by Grant RR024148 to R.K. from the National Center for Research Resources, a component of the NIH Roadmap for Medical Research (http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp), and by Grant CA16672 to J.J.L. from the National Cancer Institute.
Research Support:
The study was supported in part by Grant RR024148 to R.K. from the National Center for Research Resources, a component of the NIH Roadmap for Medical Research (http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp), and by Grant CA16672 to J.J.L. from the National Cancer Institute.
Footnotes
Disclosures:
None of the authors have any conflicts of interest relevant to the subject of this manuscript.
Statement of Translational Relevance
The data presented in this manuscript are immediately and directly translatable to the clinic. These data should alleviate ethical concerns that patients who receive lower drug doses on contemporary phase I oncology trials fare worse than those at higher doses, and thereby may impact the decision to use aggressive dose-escalation schemes in phase I studies. Additionally, the data suggest targeted agents may have different dose-response relationships than cytotoxic chemotherapies, which may not only affect dose escalation schemes, but guide translational studies of targeted agents.
References
- 1.Critical role of phase I clinical trials in cancer treatment. American Society of Clinical Oncology. J Clin Oncol. 1997;15:853–9. doi: 10.1200/JCO.1997.15.2.853. [DOI] [PubMed] [Google Scholar]
- 2.Von Hoff DD, Turner J. Response rates, duration of response, and dose response effects in phase I studies of antineoplastics. Invest New Drugs. 1991;9:115–22. doi: 10.1007/BF00194562. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhauer EA, O’Dwyer PJ, Christian M, Humphrey JS. Phase I clinical trial design in cancer drug development. J Clin Oncol. 2000;18:684–92. doi: 10.1200/JCO.2000.18.3.684. [DOI] [PubMed] [Google Scholar]
- 4.Rogatko A, Babb JS, Tighiouart M, Khuri FR, Hudes G. New paradigm in dose-finding trials: patient-specific dosing and beyond phase I. Clin Cancer Res. 2005;11:5342–6. doi: 10.1158/1078-0432.CCR-05-0458. [DOI] [PubMed] [Google Scholar]
- 5.Daugherty CK. Ethical issues in the development of new agents. Invest New Drugs. 1999;17:145–53. doi: 10.1023/a:1006371200296. [DOI] [PubMed] [Google Scholar]
- 6.Markman M. The needs of science vs the needs of patients: ethical concerns in cancer clinical trials. Cleve Clin J Med. 2003;70:1008–9. 13–4, 16. doi: 10.3949/ccjm.70.12.1008. [DOI] [PubMed] [Google Scholar]
- 7.Meropol NJ, Weinfurt KP, Burnett CB, et al. Perceptions of patients and physicians regarding phase I cancer clinical trials: implications for physician-patient communication. J Clin Oncol. 2003;21:2589–96. doi: 10.1200/JCO.2003.10.072. [DOI] [PubMed] [Google Scholar]
- 8.Daugherty C, Ratain MJ, Grochowski E, et al. Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol. 1995;13:1062–72. doi: 10.1200/JCO.1995.13.5.1062. [DOI] [PubMed] [Google Scholar]
- 9.Markman M. Assessing cancer clinical trials: will your patient benefit from a ‘breakthrough’? Cleve Clin J Med. 2002;69:368–9. 75–6. doi: 10.3949/ccjm.69.5.368. [DOI] [PubMed] [Google Scholar]
- 10.Angelos P. Ethical Issues in Cancer Patient Care. 2. Springer; 1999. [Google Scholar]
- 11.Chen EX, Tannock IF. Risks and benefits of phase 1 clinical trials evaluating new anticancer agents: a case for more innovation. Jama. 2004;292:2150–1. doi: 10.1001/jama.292.17.2150. [DOI] [PubMed] [Google Scholar]
- 12.Ratain MJ, Mick R, Schilsky RL, Siegler M. Statistical and ethical issues in the design and conduct of phase I and II clinical trials of new anticancer agents. J Natl Cancer Inst. 1993;85:1637–43. doi: 10.1093/jnci/85.20.1637. [DOI] [PubMed] [Google Scholar]
- 13.Lipsett MB. On the nature and ethics of phase I clinical trials of cancer chemotherapies. Jama. 1982;248:941–2. [PubMed] [Google Scholar]
- 14.Joffe S, Miller FG. Rethinking risk-benefit assessment for phase I cancer trials. J Clin Oncol. 2006;24:2987–90. doi: 10.1200/JCO.2005.04.9296. [DOI] [PubMed] [Google Scholar]
- 15.Dunn S. Steve’s Strategic Guide to Phase I Cancer Clinical Trials. 2004 [cited; Available from: http://www.cancerguide.org/trials_phase1.html.
- 16.Skipper HE, Schabel FR, Wilcox WS. Experimental evaluation of potential anticancer agents. XII. On the criteria and kinetics associated with “curability” of experimental leukemia. Cancer Chemotherapy Reports. 1964;35:1–111. [PubMed] [Google Scholar]
- 17.Pinkel D, Hernandez K, Borella L, et al. Drug dosage and remission duration in childhood lymphocytic leukemia. Cancer. 1971;27:247–56. doi: 10.1002/1097-0142(197102)27:2<247::aid-cncr2820270202>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Henderson IC, Hayes DF, Gelman R. Dose-response in the treatment of breast cancer: a critical review. J Clin Oncol. 1988;6:1501–15. doi: 10.1200/JCO.1988.6.9.1501. [DOI] [PubMed] [Google Scholar]
- 19.Bonadonna G, Valagussa P. Dose-response effect of adjuvant chemotherapy in breast cancer. N Engl J Med. 1981;304:10–5. doi: 10.1056/NEJM198101013040103. [DOI] [PubMed] [Google Scholar]
- 20.Pratt WB, Ruddon RW, Ensminger WD, Maybaum J. The Anticancer Drugs. 2. Oxford University Press; 1994. [Google Scholar]
- 21.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Jiang H, Fine JP. Survival analysis. Methods Mol Biol. 2007;404:303–18. doi: 10.1007/978-1-59745-530-5_15. [DOI] [PubMed] [Google Scholar]
- 24.Wheler J, Tsimberidou AM, Hong D, et al. Survival of patients in a Phase 1 clinic: the M. D. Anderson Cancer Center Experience. Cancer. 2009;115:1091–9. doi: 10.1002/cncr.24018. [DOI] [PubMed] [Google Scholar]
- 25.Postel-Vinay S, Arkenau HT, Olmos D, et al. Clinical benefit in Phase-I trials of novel molecularly targeted agents: does dose matter? Br J Cancer. 2009;100:1373–8. doi: 10.1038/sj.bjc.6605030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koyfman SA, Agrawal M, Garrett-Mayer E, et al. Risks and benefits associated with novel phase 1 oncology trial designs. Cancer. 2007;110:1115–24. doi: 10.1002/cncr.22878. [DOI] [PubMed] [Google Scholar]
- 27.Jabbour E, Issa JP, Garcia-Manero G, Kantarjian H. Evolution of decitabine development: accomplishments, ongoing investigations, and future strategies. Cancer. 2008;112:2341–51. doi: 10.1002/cncr.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Groeningen CJ, Leyva A, O’Brien AM, Gall HE, Pinedo HM. Phase I and pharmacokinetic study of 5-aza-2′-deoxycytidine (NSC 127716) in cancer patients. Cancer Res. 1986;46:4831–6. [PubMed] [Google Scholar]
- 29.Wijermans P, Lubbert M, Verhoef G, et al. Low-dose 5-aza-2′-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: a multicenter phase II study in elderly patients. J Clin Oncol. 2000;18:956–62. doi: 10.1200/JCO.2000.18.5.956. [DOI] [PubMed] [Google Scholar]
- 30.Parulekar WR, Eisenhauer EA. Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. J Natl Cancer Inst. 2004;96:990–7. doi: 10.1093/jnci/djh182. [DOI] [PubMed] [Google Scholar]
- 31.Seymour L. The design of clinical trials for new molecularly targeted compounds: progress and new initiatives. Curr Pharm Des. 2002;8:2279–84. doi: 10.2174/1381612023393099. [DOI] [PubMed] [Google Scholar]
- 32.Hoekstra R, Verweij J, Eskens FA. Clinical trial design for target specific anticancer agents. Invest New Drugs. 2003;21:243–50. doi: 10.1023/a:1023581731443. [DOI] [PubMed] [Google Scholar]
- 33.Sleijfer S, Wiemer E. Dose selection in phase I studies: why we should always go for the top. J Clin Oncol. 2008;26:1576–8. doi: 10.1200/JCO.2007.15.5192. [DOI] [PubMed] [Google Scholar]
- 34.Haines IE. Dose selection in phase I studies: why we should always go for the most effective. J Clin Oncol. 2008;26:3650–2. doi: 10.1200/JCO.2008.17.6719. author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 35.Fox E, Curt GA, Balis FM. Clinical trial design for target-based therapy. Oncologist. 2002;7:401–9. doi: 10.1634/theoncologist.7-5-401. [DOI] [PubMed] [Google Scholar]
- 36.Wolf M, Swaisland H, Averbuch S. Development of the novel biologically targeted anticancer agent gefitinib: determining the optimum dose for clinical efficacy. Clin Cancer Res. 2004;10:4607–13. doi: 10.1158/1078-0432.CCR-04-0058. [DOI] [PubMed] [Google Scholar]
- 37.Decoster G, Stein G, Holdener EE. Responses and toxic deaths in phase I clinical trials. Ann Oncol. 1990;1:175–81. doi: 10.1093/oxfordjournals.annonc.a057716. [DOI] [PubMed] [Google Scholar]
- 38.Estey E, Hoth D, Simon R, Marsoni S, Leyland-Jones B, Wittes R. Therapeutic response in phase I trials of antineoplastic agents. Cancer Treat Rep. 1986;70:1105–15. [PubMed] [Google Scholar]
- 39.Horstmann E, McCabe MS, Grochow L, et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med. 2005;352:895–904. doi: 10.1056/NEJMsa042220. [DOI] [PubMed] [Google Scholar]
- 40.Roberts TG, Jr, Goulart BH, Squitieri L, et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA. 2004;292:2130–40. doi: 10.1001/jama.292.17.2130. [DOI] [PubMed] [Google Scholar]
- 41.Kurzrock R, Benjamin RS. Risks and benefits of phase 1 oncology trials, revisited. N Engl J Med. 2005;352:930–2. doi: 10.1056/NEJMe058007. [DOI] [PubMed] [Google Scholar]