Abstract
Purpose
The goal of this study was to examine the effect of tobacco use on disease recurrence (local/regional recurrence, distant metastasis, or second primary) among HPV-positive patients with squamous cell carcinoma of the oropharynx (SCCOP) following a complete response to chemoradiation therapy.
Experimental Design
Between 1999 and 2007, 124 patients with advanced SCCOP (86% with stage IV) and adequate tumor tissue for HPV analysis who were enrolled in one of two consecutive University of Michigan treatment protocols were prospectively included in this study. Patients were categorized as never, former, or current tobacco users. The primary end-points were risk of disease recurrence and time to recurrence; secondary end-points were disease-specific survival and overall survival.
Results
One hundred and two patients (82.3%) had HPV-positive tumors. Over two-thirds (68%) of patients with HPV-positive tumors were tobacco users. Among HPV-positive patients, current tobacco users were at significantly higher risk of disease recurrence than never-tobacco users (hazard ratio = 5.2; confidence interval [1.1-24.4]; p=0.038). Thirty-five percent of HPV-positive ever tobacco users recurred compared to only 6% of HPV-positive never users and 50% of HPV-negative patients. All HPV-negative patients were tobacco users and had significantly shorter times to recurrence (p=0.002) and reduced disease-specific survival (p=0.004) and overall survival (p<0.001) compared to HPV-positive patients. Compared to HPV-positive never-tobacco users, those with a tobacco history showed a trend for reduced disease-specific survival (p=0.064) but not overall survival (p=0.221).
Conclusion
Current tobacco users with advanced, HPV-positive SCCOP are at higher risk of disease recurrence compared to never-tobacco users.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the eighth most common malignancy worldwide (1) and represents approximately 5% of new cancer diagnoses worldwide annually.(2) Over the past three decades, there has been a steady increase in the incidence of tonsil and tongue squamous cell carcinomas.(3, 4) Recent evidence has identified high-risk human papillomavirus (HPV), particularly HPV-16, as a causative agent for a subset of HNSCCs, accounting for over 50% of squamous cell carcinomas of the oropharynx (SCCOP) in the United States (5-9). HPV-positive SCCOP has a distinct risk factor profile (6) and oncogenic mechanism (10, 11) and likely portends a more favorable prognosis than HPV-negative SCCOP (5, 7, 12-17). Despite its impact on prognosis, tumor HPV status has not yet been used to alter therapeutic management. The most popular current treatment for advanced SCCOP, regardless of HPV status, involves concurrent chemoradiation therapy (CRT), (5, 18-22) which carries with it various morbidities. (19, 20)
Conflicting data exist on the combined effect of HPV and tobacco use on prognosis. Some investigators have found that non-smoking patients with HPV-positive tonsillar squamous cell carcinoma have a better disease-specific survival rate than their smoking counterparts.(13) Many studies, however, report no interaction between HPV and smoking on survival.(6, 12, 23, 24) To the best of our knowledge, no studies have focused on tobacco and HPV in relation to tumor recurrence or distant metastasis.
The purpose of this investigation was to test the hypothesis that tobacco use among HPV-positive advanced SCCOP patients increases the risk of disease recurrence, including local/regional recurrence (LR), distant metastasis (DM) and second primary cancer (SP).
Methods
Study Population
One hundred and twenty-four patients treated at the University of Michigan between 1999 and 2007 with stage III or IV SCCOP were prospectively included in this study. All patients were consented for and participated in the University of Michigan Head and Neck SPORE program, had a pretreatment biopsy with adequate tumor tissue for DNA extraction and HPV assessment, and were treated according to one of two consecutive prospective clinical trial protocols (described as ‘cohorts’ throughout the manuscript). Forty-one patients were treated on a Phase II trial (University of Michigan Cancer Center [UMCC]-9921) with induction chemotherapy (cisplatin and 5-fluorouracil [5-FU]) followed by concomitant cisplatin and full course radiation (70Gy) or surgical resection and full course radiation for non-responders to induction chemotherapy as previously reported (5); and 83 were treated with concomitant carboplatin, docetaxel, and IMRT (70Gy) on a more recent UMCC-0221 trial. A few patients who did not participate in the UMCC-0221 trial but received identical treatment were classified as UMCC-0221-like patients and therefore UMCC-0221 and UMCC-0221-like were considered one cohort.
Clinical and pathological characteristics
Clinical and pathological characteristics of interest included gender, age, primary tumor site, TNM staging as defined by the American Joint Committee on Cancer (AJCC), treatment cohort, and patient status at last follow-up. Primary tumor site was determined by direct laryngoscopy and biopsy performed at the University of Michigan. Charts were reviewed for disease recurrence, including LR, DM, or SP tumors. An LR was defined as a positive biopsy in the area of the primary tumor (local) or the cervical lymphatic region after a complete response to treatment as determined by a negative post-treatment biopsy and/or scan. A DM was identified via biopsy or PET scan and verified as such by the University of Michigan Multidisciplinary Tumor Board. A tumor was considered a related SP if it was squamous cell carcinoma, occurred over two years after the patient had been disease-free from the original SCCOP and was at least three centimeters distant from the original site.
Tobacco history
History of tobacco use was determined via two methods: chart review and self-reporting at the time of study enrollment. Patients were categorized according to their use of cigarettes, cigars, pipes, or chewing tobacco as current (including those who quit < 1 year prior to diagnosis), former (quit ≥ 1 year prior to diagnosis), or never-tobacco users. Never-tobacco users were those who had never used chewing tobacco, cigars, or pipes in their lifetime and those who smoked less than the equivalent of one pack per day per year – one pack-year – in their lifetime. Former tobacco users were then separated into an early-cessation group (quit ≥ 20 years prior to diagnosis) and a late-cessation group (quit < 20 years prior to diagnosis). To evaluate the cigarette dose-effect, a subgroup analysis was conducted on cigarette users.
HPV Detection
Using DNA extracted from tumor within the pretreatment biopsy, HPV analysis was accomplished with a sensitive, specific, quantitative method developed in Dr. David Kurnit’s laboratory at the University of Michigan as described previously (5, 25) or by SensiGen LLC of Ann Arbor, MI, (now owned by Sequenom, San Diego, CA) using Dr. Kurnit’s licensed technology. In brief, HPV analysis involved real-time competitive polymerase chain reaction and matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectroscopy separation of products on a matrix-loaded silicon chip array. This method uses primers unique to the HPV-E6 region of each of 13 high-risk HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) as well as HPV-6 and HPV-11. HPV type positivity was scored as positive by the presence of a discrete mass spectroscopy peak for the high risk HPV type-specific E6 amplicon corresponding to the internal HPV type-specific competitor (see references 5,26).
Immunohistochemistry
As described previously (26) TMA slides were deparaffinized, rehydrated, and peroxidase quenched (Dako Cytomation, Glostrup, Denmark). For p16 staining, antigen retrieval using citrate buffer was employed. Slides were blocked with horse serum, washed, incubated with primary antibody, (16P04 - Lab-Vision, Fremont CA (UMCC-9921), or the CINtec p16ink4a antibody-MTM Laboratories, Inc,Westborough, MA, UMCC-0221) washed and probed with avidin/biotin peroxidase (ABC Kit; Vector Laboratories, Burlingame, CA). Proportion and intensity of staining were scored by a pathologist (K.G.C., who was blinded to the clinical outcome) using a scale of 1 to 4: 1, < 5%; 2, 5% to 20%; 3, 21% to 50%; and 4, 51% to 100% tumor staining. Intensity was scored as 1 = no staining; 2, low intensity; 3, moderate; and 4, high intensity. Scores for multiple cores from each patient were averaged. A re-test confirmed that both antibodies give the same result on the same tumors.
Study End Points
The primary end point was time to recurrence. Secondary end points were disease-specific survival (DSS) and overall survival (OS). DSS was measured from the date of study enrollment to the date of death caused by an LR, DM, or SP. Overall survival was measured from the date of study enrollment to the date of death from any cause.
Statistical Analysis
The analysis assessed the tobacco effect among HPV-positive patients on time to recurrence, DSS and OS. Patients with persistent disease following treatment were excluded from the analysis of time to recurrence but included in the analysis of DSS and OS. The Kaplan-Meier method was used to depict the survival estimates between categories of a single discrete variable, whereas the Cox proportional hazard models were used to assess single-variable and multi-variable effects while adjusting for the cohort effect.
Patients from the two treatment cohorts were analyzed together. However, each reported statistical analysis accounted for the cohort effect. To investigate the association between discrete variables, such as gender and T class, Cochran-Mantel-Haenszel statistics were used. The association between HPV and continuous variables, such as age, was examined by logistic regression.
To analyze the prognostic effects of T class and tobacco status on outcome, three models were constructed: 1) a model with T class and cohort effect, 2) a model with tobacco status and cohort effect, and 3) a model with both T class and tobacco status in addition to cohort effect. Models 1 and 3, as well as 2 and 3, were used to assess one variable effect beyond the effects of the other variable while controlling for cohort effect. Likelihood ratio statistics were used to compare the models. Including HPV-negative subjects a similar analysis was used to assess the effect of tobacco beyond HPV status and T class. All statistical analyses were done using SAS v9.1 (Carey, North Carolina). A two-tailed p-value of 0.05 or less was considered to be statistically significant.
Results
Study population
The 124 patients from both cohorts were similar in age, race, primary tumor site, clinical stage, tumor classification (T class), and nodal classification (N class). The UMCC-9921 trial was completed prior to the start of the UMCC-0221 trial and therefore had longer follow-up times. The median follow-up time was 76 months (95% confidence interval [71, 85] months) and 36 months (95% confidence interval [31, 39] months) for UMCC-9921 and UMCC-0221, respectively. The incidence of HPV-positive tumors was significantly higher (p=0.001) in the more recent UMCC-0221 trial (90% HPV-positive) compared to the UMCC-9921 trial (66% HPV-positive). In the more recent UMCC-0221 trial there was a higher proportion of never-tobacco users and former tobacco users in the early cessation group (quit ≥ 20 years prior to diagnosis) compared to the UMCC-9921 trial (p=0.03). In addition, the proportion of women (12%) in the UMCC-0221 trial was lower than in the UMCC-9921 trial (27%) (p=0.04). The patients in UMCC-9921 who did not respond to an initial cycle of neoadjuvant chemotherapy underwent definitive surgery with post-operative radiation, while all patients in UMCC-0221 completed definitive chemoradiation and were followed for recurrence and possible salvage surgery as necessary.
HPV-positive versus HPV-negative patients
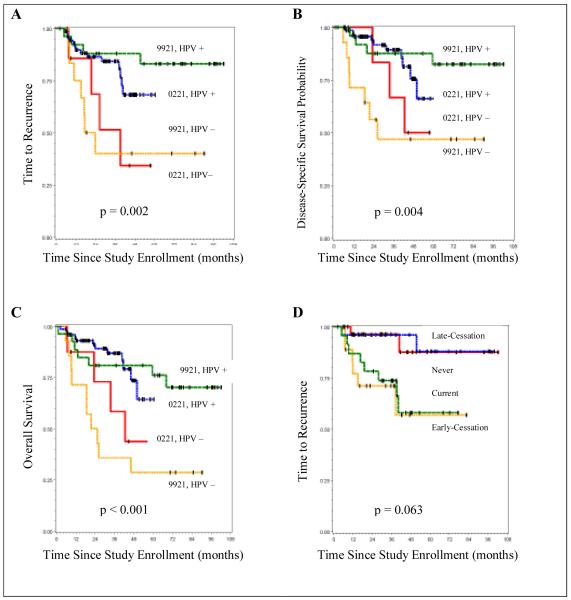
Controlling for the cohort effect, HPV-positive and HPV-negative patients were similar with respect to race, clinical stage and N class. HPV-positive patients were significantly younger (p=0.04), more likely to be male (p=0.04), had less advanced T class (p=0.002), and were more likely to be never-tobacco users (p<0.0001) (Table 1A). Compared to HPV-negative patients, those with HPV-positive tumors had significantly longer times to recurrence (p=0.002; Figure 1A) and more favorable DSS (p=0.004; Figure 1B) and OS (p<0.001; Figure 1C) among both UMCC-9921 and UMCC-0221 cohorts, regardless of tobacco history. HPV-negative patients were 3.5 times more likely (p=0.002) to develop disease recurrence (Hazard Ratio = 3.5, 95% CI = [1.6, 7.9]) than HPV-positive patients. These findings are consistent with our previous reports (5, 26) and those of other groups (4, 12-16, 24, 27, 28).
Table 1A. Patient Demographics, Tumor Pathology, Tobacco Use, and Disease Recurrence for Oropharyngeal Cancer Patients.
| Demographics | Total | HPV+ (N=102) | HPV− (N=22) | |
|---|---|---|---|---|
| number (percent) | ||||
| Gender | ||||
| Female | 21 | 13 (12.8) | 8 (36.4) | |
| Male | 103 | 89 (87.3) | 14 (63.6) | |
| Age | 57.2 | 56.5 | 60.3 | |
| Race or Ethnicity | ||||
| White, non-Hispanic | 116 | 98 (96) | 18 (81.8) | |
| Black, non-Hispanic | 5 | 2 (2.0) | 3 (13.6) | |
| Unknown | 3 | 2 (2.0) | 1 (4.6) | |
| Primary Tumor Site | ||||
| Base of Tongue (BOT) | 62 | 51 (50) | 11 (50) | |
| Tonsil | 57 | 49 (48) | 8 (36.4) | |
| Oropharynx Unspecified | 5 | 2 (2.0) | 3 (13.6) | |
| Tumor Stage | ||||
| III | 17 | 13 (12.7) | 4 (18.2) | |
| IVA | 92 | 76 (74.5) | 16 (72.7) | |
| IVB | 15 | 13 (12.8) | 2 (9.1) | |
| Primary Tumor (T class) | ||||
| 1 | 13 | 13 (12.8) | 0 (0) | |
| 2 | 38 | 35 (34.3) | 3 (13.6) | |
| 3 | 35 | 27 (26.5) | 8 (36.4) | |
| 4 | 38 | 27 (26.5) | 11 (50) | |
| Lymph Nodes (N class) | ||||
| 0 | 15 | 11 (10.8) | 4 (18.2) | |
| 1 | 16 | 11 (10.8) | 5 (22.7) | |
| 2a | 17 | 13 (12.7) | 4 (18.2) | |
| 2b | 37 | 32 (31.4) | 5 (22.7) | |
| 2c | 24 | 22 (21.6) | 2 (9.1) | |
| 3 | 15 | 13 (12.7) | 2 (9.1) | |
| Tobacco Use | ||||
| Never | 33 | 33 (32.4) | 0 (0) | |
| Former early-cessation | 23 | 20 (19.6) | 3 (13.6) | |
| Former late-cessation | 29 | 26 (25.5) | 3 (13.6) | |
| Current | 39 | 23 (22.6) | 16 (72.7) | |
| Disease Progression Events | ||||
| Local/regional Recurrence | 10 | 6 (5.9) | 4 (18.2) | |
| Distant Metastasis | 17 | 12 (11.8) | 5 (22.7) | |
| Second Primary | 6 | 5 (4.9) | 1 (4.6) | |
SCCOP, squamous cell carcinoma of the oropharynx
Only includes second primaries related to original SCCOP (this excludes a prostate cancer, B-cell lymphoma, and lung adenocarcinoma – see Table 2B)
Figure 1.
(A), Time to recurrence by HPV status for each cohort; (B), Disease-specific survival by HPV status for each cohort; (C), Overall survival by HPV status for each cohort; (D), Time to recurrence by tobacco use among HPV-positive patients for each cohort
HPV status and disease recurrence
Of the 124 patients, 102 (82%) had HPV-positive tumors (Table 1A). HPV-16 was detected in 95% of HPV-positive tumors, followed by HPV-35 in 3% and HPV-18 in 2% of tumors. Of the 102 HPV-positive patients, 33 (32.3%) were never-tobacco users and 69 (67.6%) had used tobacco in their lifetime. Thus, the majority of patients with HPV-positive tumors were tobacco users. In fact, 23/102 (22.6%) were current tobacco users and 46/102 (45%) were former users. The patient demographics, tumor pathology, tobacco status, and disease recurrence events (LR, DM, and histologically related SP) are depicted in Tables 1A and 1B. Note that the study population consisted of patients with very advanced disease, with the majority, 107/124 (86.3%), having stage IV disease at diagnosis. Thirty patients (30/124) (24.2%) suffered disease recurrence events, including SP tumors of squamous cell histology (Table 2A). Of these 30 patients, 23 (76.7%) have died of their SCCOP. Three other patients with histologically unrelated SP tumors were censored at last follow-up for the recurrent disease analysis (Table 2B). Fourteen patients used cigars (n=7), oral tobacco (n=4), or pipes (n=3) instead of or in addition to cigarettes. Three of the 14 (21.4%) suffered disease recurrence – one LR and two DMs – and all three were cigar users.
Table 1B. Patients with Recurrent Disease (LR, DM, and related SP) by HPV Status and Tobacco History (Never, Former, or Current).
| HPV+ (N=102) | HPV− (N=22) | |
|---|---|---|
| Never | 2/33 (6.1%) | 0/0 |
| LR | 0 | 0 |
| DM | 1 | 0 |
| SP | 1 | 0 |
| Former | 9/46 (19.6%) | 3/6 (50%) |
| LR | 3 | 1 |
| DM | 5 | 1 |
| SP | 1 | 1 |
| Current | 8/23 (34.8%)* | 8/16 (50%) |
| LR | 3 | 4 |
| DM | 6 | 4 |
| SP | 2 | 2 |
| Total | 19/102 (18.6%) | 11/22 (50%) |
LR, local/regional disease; DM, distant metastasis; SP, second primary tumor
Three HPV-positive current tobacco users suffered two events each (LR/DM; LR/DM; LR/SP), thus 11 events occurred in eight patients
Table 2A. Clinical and Pathological Characteristics of all Patients who Developed Disease Recurrence (LR, DM, & histologically related SP).
| Patient No. |
HPV status |
Tobacco History | Pack- Years (Cigarette use only) |
Primary Tumor Site |
T Class |
N Class |
Disease Recurrence Event (LR, DM or SP) |
Site of Disease Recurrence Event |
Patient status at last follow- up (in reference to SCCOP) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Positive | Never† | 0 | Tonsil | 4 | 2b | DM | Lung | Dead with disease |
| 2 | Positive | Never | 0 | Tonsil | 3 | 3 | SP | Mandibular gingiva | Alive with disease |
| 3 | Positive | Former cigarette; quit 37 yr prior | 10 | BOT | 4 | 3 | DM | Lung, bone | Dead with disease |
| 4 | Positive | Former cigarette; quit 37 yr prior | 10 | BOT | 4 | 2b | LR | Suprasternal lymph node | No evidence of disease |
| 5 | Positive | Former cigarette; quit 35 yr prior | 6 | Tonsil | 4 | 3 | DM | Lung | Dead with disease |
| 6 | Positive | Former cigarette; quit 28 yr prior | 12 | BOT | 2 | 2b | DM | Bone | Dead with disease |
| 7 | Positive | Former 1 cigar per day x 8 years; quit 25 yr prior | 0 | Tonsil | 1 | 3 | LR | Level II lymph node | Dead with disease |
| 8 | Positive | Former cigarette; quit 21 yr prior | 120 | Tonsil | 2 | 1 | LR | Tonsil, level III lymph node | No evidence of disease |
| 9 | Positive | Former cigarette; quit 20 yr prior | 12 | Tonsil | 2 | 2b | SP | Tongue | Alive with disease |
| 10 | Positive | Former cigarette; quit 4 yr prior | 100 | BOT | 4 | 2c | DM | Lung | Dead with disease |
| 11 | Positive | Former cigarette; quit 2 yr prior | 60 | BOT | 2 | 3 | DM | Dermis, lung | Dead with disease |
| 12 | Positive | Current cigarette | 35 | BOT | 4 | 0 | DM | Liver | Dead with disease |
| 13 | Positive | Current cigarette | 35 | Tonsil | 4 | 0 | DM | Lung | Dead with disease |
| 14 | Positive | Current cigarette | 30 | BOT | 4 | 2c | LR; DM | LR – retropharyngeal space DM – lung, bone |
Dead with disease |
| 15 | Positive | Current cigarette | 44 | BOT | 4 | 0 | DM | bone, dermis | Dead with disease |
| 16 | Positive | Current cigarette | 35 | Tonsil | 3 | 0 | LR; SP | LR – digastric lymph node SP – soft palate |
Dead with disease |
| 17 | Positive | Current cigarette | 45 | BOT | 4 | 2b | SP | Lung | No evidence of disease |
| 18 | Positive | Current 3 cigars per week x 15 years | 0 | BOT | 4 | 2c | DM | Lung, bone | Dead with disease |
| 19 | Positive | Current 2-4 cigars per week x 20 years | 0 | Tonsil | 4 | 2c | LR; DM | LR – tonsil DM – lung |
Dead with disease |
| 20 | Negative | Former cigarette; quit 35 yr prior | 80 | BOT | 4 | 1 | SP | Esophagus | No evidence of disease |
| 21 | Negative | Former cigarette; quit 25 yr prior | 50 | Oropharynx^ | 3 | 2a | LR | Tongue | Dead with disease |
| 22 | Negative | Former cigarette; quit 20 yr prior | 40 | BOT | 4 | 1 | DM | Lung | Dead with disease |
| 23 | Negative | Current cigarette | 25 | Tonsil | 3 | 2a | DM | Lung, liver | Dead with disease |
| 24 | Negative | Current cigarette | 30 | BOT | 4 | 3 | DM | Bone, liver | Dead with disease |
| 25 | Negative | Current cigarette | 50 | BOT | 4 | 0 | DM | Bone | Dead with disease |
| 26 | Negative | Current cigarette | 75 | Tonsil | 4 | 0 | LR | Pretracheal tissue | Dead with disease |
| 27 | Negative | Current cigarette | 35 | Oropharynx^ | 4 | 2b | LR | Tonsil | No evidence of disease |
| 28 | Negative | Current cigarette | 60 | BOT | 4 | 2c | LR | Tongue | Dead with disease |
| 29 | Negative | Current cigarette | 40 | BOT | 4 | 2a | DM | Dermis, lung, abdomen, bone | Dead with disease |
| 30 | Negative | Current cigarette | 59 | BOT | 2 | 2a | SP | Lung | Dead with disease |
BOT, base of tongue; LR, local/regional recurrence; DM, distant metastasis; SP, second primary tumor; yr, year; SCCOP, squamous cell carcinoma of the oropharynx
This patient had daily exposure to second hand smoke and welding fumes
Tumor site within oropharynx unspecified
Table 2B. Clinical and Pathological Characteristics of Patients with Histologically Unrelated Second Primary Tumors.
| Patient No. |
HPV status |
Tobacco History | Pack- Years (Cigarette use only) |
Primary Tumor Site |
T Class |
N Class |
Disease Recurrence Event (LR, DM or SP) |
Site of Disease Recurrence Event |
Patient status at last follow- up (in reference to SCCOP) |
|---|---|---|---|---|---|---|---|---|---|
| 31 | Positive | Never | 0 | Oropharynx^ | 2 | 3 | SP | Prostate* | No evidence of disease |
| 32 | Positive | Former cigarette; quit 17 yr prior | 20 | Tonsil | 1 | 2b | SP | Lung (B-cell lymphoma)* | No evidence of disease |
| 33 | Negative | Former cigarette; quit 12 yr prior | 70 | Oropharynx^ | 3 | 1 | SP | Lung (adenocarcinoma)* | Dead without disease |
LR, local/regional recurrence; DM, distant metastasis; SP, second primary tumor; yr, year; SCCOP, squamous cell carcinoma of the oropharynx
SP tumor unrelated to original SCCOP based on location (patient 31) or tumor cell histology (patients 32 and 33). Patients 31 and 32 are alive with no evidence of SCCOP recurrence after eight years and 1.5 years of follow-up, respectively. Patient 33 died from the lung adenocarcinoma after four years without evidence of SCCOP recurrence. These three patients were censored at the last follow-up time for statistical analysis of time-to-disease recurrence.
Tumor site within oropharynx unspecified
HPV status, smoking, and disease recurrence
HPV-positive current tobacco users were over five times more likely to develop a recurrence compared to never users (p=0.038, hazard ratio=5.2, 95% confidence interval [1.1, 24.4]; Table 3). As depicted in Table 1B, the rate of disease recurrence among HPV-positive patients was lowest among never-tobacco users (6.1%), followed by former users (19.6%), and highest among current users (34.8%). Recurrence events occurred in 17/69 (24.6%) HPV-positive former (n=9) or current (n=8) tobacco users, a 3.7 times higher rate of recurrence compared to never-tobacco users. Three HPV-positive tobacco users suffered two distinct recurrence events each (LR/DM, LR/SP, and LR/DM; Table 2A, patients 14, 16 and 19). Among HPV-negative patients (all of whom were tobaccos users), 50% (11/22) had disease recurrence, among both former (3/6) and current (8/16) tobacco users.
Table 3. Risk of Recurrence and Disease-Specific Survival (DSS) by Tobacco Use among HPV-Positive Patients.
| Tobacco group | Hazard Ratio | 95% Confidence Interval |
p value | |
|---|---|---|---|---|
|
Risk of Recurrence |
Current vs. Never | 5.2 | (1.1-24.4) | 0.038* |
| Former vs. Never | 2.9 | (0.6-13.6) | 0.18 | |
| Current vs. Former | 1.8 | (0.7-4.8) | 0.24 | |
| DSS | Current vs. Never | 7.2 | (0.88-58.4) | 0.07 |
| Former vs. Never | 3.6 | (0.43-30.1) | 0.24 | |
| Current vs. Former | 2 | (0.66-6.03) | 0.22 |
Statistical significance
Twenty-nine of 33 (87.9%) HPV-positive never-tobacco users are currently alive and disease-free. Of the two HPV-positive never-tobacco users (Table 2A, patients 1 and 2) with disease recurrence, one died and one is alive with disease. Patient no. 1 developed lung metastases and died two years following primary diagnosis; of note, he was a welder by occupation with a history of second-hand smoke exposure. Patient no. 2 developed a second primary squamous cell carcinoma of the gingiva and is alive with disease. Another two HPV-positive never-tobacco users died disease-free from other causes: suicide and an idiopathic lung/kidney disorder. The fourth HPV-positive never-tobacco user who died had an undiagnosed dihydropyrimidine dehydrogenase deficiency and died 2 months after primary diagnosis from 5-fluorouracil toxicity; he was never disease-free and therefore excluded from the time-to-disease recurrence analysis. One other HPV-positive, former tobacco user was never disease free and was excluded from the time-to-disease recurrence analysis.
The overall effect of ever using tobacco exhibited a strong trend for increased risk of disease recurrence among HPV-positive patients, but after adjusting for cohort effect, did not reach statistical significance (p=0.063; Figure 1D). Similarly, the necessary adjustment for the cohort effect also influenced the significance level of tobacco use, which showed a strong trend for an adverse effect on DSS (p=0.064), but not OS (p=0.221), among HPV-positive tobacco users. While HPV-positive current tobacco users had a 7.2 times greater risk of dying from their disease (DSS) than never-tobacco users, after adjusting for the cohort effect, this also fell slightly short of statistical significance (p=0.07; Table 3).
Curiously, when HPV-positive former tobacco users were subdivided into an early-cessation group (quit ≥ 20 years prior to diagnosis; n=20) and a late cessation group (quit < 20 years prior to diagnosis; n=26), the tobacco effect on time-to-disease recurrence was significant (p=0.043) using the Cox model and adjusting for cohort effect. Among HPV-positive former tobacco users, early-cessation users were five times more likely to develop a recurrence than the never users (p=0.03, hazard ratio = 5, 95% confidence interval [1.07, 23.8]). Conversely, former late-cessation users had nearly the same low risk of recurrence as never users (p=0.97; hazard ratio=1.05, 95% confidence interval [0.14, 7.7]).
HPV-positive status, T class, and pack-years
Among HPV-positive patients, more advanced T class was associated with current tobacco usage (p=0.02). As a single variable, T class was a significant prognostic indicator for recurrence among HPV-positive subjects (p=0.01, hazard ratio=2, 95% CI [1.2, 3.5]). Among both HPV-positive and HPV-negative patients, T class was significantly associated with a higher likelihood of recurrence (p=0.0004). We explored the possible tobacco pack-year dose effect among combined former and current HPV-positive tobacco users on disease recurrence and did not find a dose-response relationship.
HPV status with p16 expression
Of the 124 patients tested for HPV, p16 staining data was available for 113. Of these, 11 of 113, (9.7%) had discrepancies between p16 expression and HPV status. Seven tumors were HPV-positive/p16-negative and four were HPV-negative/p16-positive. Of the HPV-positive/p16-negative cases, the majority (4/7) had low viral copy number. HPV-positive/p16-negative tumors are thought to contain transcriptionally inactive E7 (29), which our series suggests may be more common in tumors with low HPV copy number. Interestingly, more than one-third of the discordant HPV/p16 results were HPV-negative/p16-positive. Whether these represent tumors with high risk HPV types not represented in our panel or another mechanism, such as mutation or inactivation of the retinoblastoma protein, remains to be determined. Curiously, only 2/11 patients with discordant HPV/p16 died of their disease; both were in the UMCC 9921 cohort, developed DM, were current or former smokers, had less than one HPV copy/cell, and were p16-negative.
Discussion
It is now well established that HPV-positive SCCOP patients have a more favorable outcome and are more likely to be non-smokers (4, 5, 9, 13, 23, 27, 28) than HPV-negative SCCOP patients. The patients in our study with HPV-positive tumors had a lower risk of disease recurrence and more favorable DSS and OS, when compared to patients with HPV-negative tumors. However, never-tobacco users comprised only a minority of the HPV-positive patients. Over two-thirds (68%) of our HPV-positive patients were former or current tobacco users. Strikingly, HPV-positive current tobacco users were five times more likely to develop disease recurrence, including local/regional recurrence, distant metastasis, or second primary tumor, compared to never-tobacco users. Former tobacco users were nearly three times more likely to have recurrence than never users in HPV-positive patients. Over the duration of this study, the recurrence rate was only 6% among HPV-positive never users, compared to 20% of former users and 35% of current users, all of which were lower than the recurrence rate of 50% among HPV-negative patients.
Tobacco use also exhibited a strong statistical trend for an adverse effect on DSS among HPV-positive patients. Consistent with our observations, Hafkamp et al. [13] found that non-smoking HPV-positive tonsillar cancer patients had better DSS than their smoking counterparts. Although the sample size in that study was small (10 of 33 HPV-positive patients were never-smokers), and only three of those 33 patients received some form of chemotherapy (most underwent surgery and/or radiation), nevertheless, similar effects of tobacco were observed. Moreover, Gillison et al. (30) reported at ASCO this year (2009) that smoking > 20 pk/yrs was associated with an increased hazard risk of death =1.79 in HPV-positive oropharynx cancer patients treated on RTOG 0129 relative to HPV-positive OPC with <20 pk/yrs. However, they did not assess risk of recurrence or DM as Worden et al. reported at the same meeting (31). Further studies investigating the prognostic effect of tobacco use in HPV-positive patients are warranted.
It is not surprising that current tobacco use increases the risk of recurrence among HPV-positive patients. However, the lack of a significant difference in risk of recurrence between current and former smokers in our two cohorts was puzzling. We noted a striking difference in risk of recurrence among the former early-cessation tobacco users and the former late-cessation users. This was unexpected and counterintuitive. Those who quit over 20 years prior to diagnosis had a much higher risk of recurrence than both the never-tobacco users and the late-cessation group. This suggests that early tobacco cessation in HPV-positive individuals does not lower the risk of recurrence to that of a never-tobacco user. Possibly, the mutagenic effects of tobacco exposure at the time of HPV infection, which likely occurs soon after becoming sexually active, [5] may create an environment more suitable for HPV DNA integration into the host genome as well as increase the risk of associated errors in the somatic DNA. Contrarily, these differences in recurrence rates among the early- and late-cessation groups may reflect other non-tobacco related biologic factors or could be the result of a comparatively small sample size. It is also possible that because there were significantly more early-cessation users in the UMCC-0221 cohort, this treatment type may be less effective. Furthermore, the role of HPV integration in SCCOP remains unknown. Integration of HPV has been reported to occur in 40-50% of SCCOP, (13) however integration alone has not been linked to poorer outcome. (13, 15)
There were several important differences in the two treatment cohorts that should be noted. For one, we observed a surprising increase in the proportion of HPV-positive oropharyngeal cancers among our two cohorts; from 66% HPV-positive in the UMCC-9921 cohort to 90% in the more recent UMCC-0221 cohort. Similarly, the U.S. incidence of tonsil and tongue cancer has been increasing linearly at rates of nearly 4% and 2% per year, respectively, over the past thirty years. [3] Our results indicate that HPV-related SCCOP may be rising in incidence more rapidly in our patient population than has been reported in the literature. This is consistent with a recent report of changing incidence trends. (32) Gender was also significantly different among the two cohorts, possibly reflecting changes in the epidemiology of SCCOP or changes in the patient population presenting at our institution. The UMCC-0221 trial had shorter follow-up times and slightly different treatments, therefore it was important to take cohort effects into account during all statistical analyses.
In conclusion, a current tobacco history among HPV-positive SCCOP patients increases the risk of LR, DM, or SP tumors, compared to never-tobacco users. Our results also emphasize that the majority of HPV-positive SCCOP patients are current or former tobacco users and that an HPV-positive tumor does not necessarily confer as good a prognosis in smokers. Clinical trials are warranted to investigate whether targeting treatments with respect to tobacco history will improve survival and quality of life. Furthermore, it is yet to be determined whether reducing the intensity of treatment for some HPV-positive, non-smoking, SCCOP patients will compromise prognosis.
Acknowledgments
Funding: This study was supported in part by Grants No. R01 DE 13346 and P30 DC 05188 from the National Institutes of Health (NIH) NIDCR; Head and Neck Cancer SPORE Grant No. P50 CA97248; Cancer Center Support Grant No. P30 CA46592; Michigan Institute for Clinical & Health Research (MICHR) UL1RR024986, and Grants from the state of Michigan.
Footnotes
Present Address for Dr. Teknos and Ms. Kumar: Department of Otolaryngology, Ohio State University, Columbus, OH
Present Address for Dr. Maxwell: Department of Otolaryngology, University of Pittsburgh, Pittsburgh, PA
Statement of Translational Relevance
This study demonstrates that patients with advanced, HPV-positive squamous cell carcinoma of the oropharynx (SCCOP) who are current smokers are at higher risk of disease recurrence and tend to have worse disease-specific survival compared to never-smokers with HPV-positive SCCOP. This supports the use of less aggressive treatment for never-smokers with HPV-positive cancer compared to their smoking counterparts. The development of clinical protocols to this effect is already under way at the University of Michigan based on the results of this study. This research is entirely original, applicable to clinical practice, and based on prior evidence that HPV-positive patients have better outcomes and are more likely to be non-smokers than HPV-negative patients. We also test a well-supported hypothesis with reliable and robust statistics. We feel that this study will be of utmost interest to the readership of Clinical Cancer Research and trigger further research into the management of patients with SCCOP.
References
- 1.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–41. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103:1843–9. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 4.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429–35. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 5.Worden FP, Kumar B, Lee JS, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26:3138–46. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–20. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 7.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Koo BS, Kang S, et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120:1418–25. doi: 10.1002/ijc.22464. [DOI] [PubMed] [Google Scholar]
- 9.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 10.Herrero R. Chapter 7: Human papillomavirus and cancer of the upper aerodigestive tract. J Natl Cancer Inst Monogr. 2003:47–51. doi: 10.1093/oxfordjournals.jncimonographs.a003482. [DOI] [PubMed] [Google Scholar]
- 11.Ragin CC, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res. 2007;86:104–14. doi: 10.1177/154405910708600202. [DOI] [PubMed] [Google Scholar]
- 12.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 13.Hafkamp HC, Manni JJ, Haesevoets A, et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer. 2008;122:2656–64. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Thompson CH, O’Brien CJ, et al. Human papillomavirus positivity predicts favourable outcome for squamous carcinoma of the tonsil. Int J Cancer. 2003;106:553–8. doi: 10.1002/ijc.11261. [DOI] [PubMed] [Google Scholar]
- 15.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–6. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 16.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–20. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 17.Sisk EA, Soltys SG, Zhu S, Fisher SG, Carey TE, Bradford CR. Human papillomavirus and p53 mutational status as prognostic factors in head and neck carcinoma. Head Neck. 2002;24:841–9. doi: 10.1002/hed.10146. [DOI] [PubMed] [Google Scholar]
- 18.Bensadoun RJ, Etienne MC, Dassonville O, et al. Concomitant b.i.d. radiotherapy and chemotherapy with cisplatin and 5-fluorouracil in unresectable squamous-cell carcinoma of the pharynx: clinical and pharmacological data of a French multicenter phase II study. Int J Radiat Oncol Biol Phys. 1998;42:237–45. doi: 10.1016/s0360-3016(98)00235-1. [DOI] [PubMed] [Google Scholar]
- 19.Bernier J, Thames HD, Smith CD, Horiot JC. Tumor response, mucosal reactions and late effects after conventional and hyperfractionated radiotherapy. Radiother Oncol. 1998;47:137–43. doi: 10.1016/s0167-8140(97)00221-1. [DOI] [PubMed] [Google Scholar]
- 20.Denis F, Garaud P, Bardet E, et al. Late toxicity results of the GORTEC 94-01 randomized trial comparing radiotherapy with concomitant radiochemotherapy for advanced-stage oropharynx carcinoma: comparison of LENT/SOMA, RTOG/EORTC, and NCI-CTC scoring systems. Int J Radiat Oncol Biol Phys. 2003;55:93–8. doi: 10.1016/s0360-3016(02)03819-1. [DOI] [PubMed] [Google Scholar]
- 21.Langer CJ, Harris J, Horwitz EM, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Protocol 9911. J Clin Oncol. 2007;25:4800–5. doi: 10.1200/JCO.2006.07.9194. [DOI] [PubMed] [Google Scholar]
- 22.Lee DJ, Cosmatos D, Marcial VA, et al. Results of an RTOG phase III trial (RTOG 85-27) comparing radiotherapy plus etanidazole with radiotherapy alone for locally advanced head and neck carcinomas. Int J Radiat Oncol Biol Phys. 1995;32:567–76. doi: 10.1016/0360-3016(95)00150-W. [DOI] [PubMed] [Google Scholar]
- 23.Applebaum KM, Furniss CS, Zeka A, et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst. 2007;99:1801–10. doi: 10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 24.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Yang K, Khafagi A, et al. Sensitive detection of human papillomavirus in cervical, head/neck, and schistosomiasis-associated bladder malignancies. Proc Natl Acad Sci U S A. 2005;102:7683–8. doi: 10.1073/pnas.0406904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–37. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24:2606–11. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–13. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–84. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 30.Gillison ML, J H, Westra W, et al. Survival Outcomes by tumor papillomavirus (HPV) status in stage III-IV oropharyngeal cancer (OPC) in RTOG 0129. J Clin Oncol. 2009:27. [Google Scholar]
- 31.Worden FP, Hooton JM, Lee J, et al. Association of tobacco (T) use with risk of distant metastases (DM), tumor recurrence, and death in patients (pts) with HPV-positive (+) squamous cell cancer of the oropharynx (SCCOP) J Clin Oncol. 2009:27. [Google Scholar]
- 32.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]