Abstract
Purpose
Low-molecular-weight cyclin E (LMW-E) in breast cancer cells induces genomic instability, and resistance to inhibition by p21, p27, and fulvestrant therapy. Here, we sought to determine if LMW-E renders breast cancer cells unresponsive to aromatase inhibitors (AI), elucidate the mechanism of such resistance and ascertain if inhibitors of LMW-E associated kinase activity could overcome this resistance.
Experimental Design
Antiproliferative effects of the AIs, were examined in aromatase-overexpressing MCF-7/Ac1 cells in the presence or absence of full length and LMW-E. Inhibition of LMW cyclin E kinase activity by roscovitine (a CDK inhibitor) was examined in letrozole-unresponsive MCF-7/Ac1 cells. The role of LMW-E and CDK2 in mediating recurrence following AI treatment were also assessed in breast cancer patients.
Results
Overexpression of LMW-E in postmenopausal patients was associated with a poor prognosis. Letrozole, but not exemestane or anastrozole, mediated a pronounced G1 arrest in MCF-7/Ac1 cells. Androstenedione (AD)-induced G1 exit correlated with increased cyclin E-associated kinase activity and increased CDK2 levels. Letrozole treatment inhibited cyclin E-CDK2 kinase activity by preventing the AD-induced increase in CDK2. LMW-E bypassed this effect and rendered the cells resistant to letrozole inhibition. Roscovitine blocked the AD-induced increase in CDK2 and LMW-E overexpression could not bypass this effect. Lastly, breast cancer patients whose tumor overexpress LMW-E were not responsive to AI treatment.
Conclusions
Roscovitine treatment can reverse intrinsic or acquired resistance to letrozole due to LMW-E expression in breast cancer cells. These data support clinical investigation of CDK2 inhibitor therapy for postmenopausal women with ER-positive, LMW-E-expressing breast cancer.
Keywords: cyclin E, low molecular weight forms, Roscovitine, letrozole, cell cycle
Introduction
Endocrine therapy is an important part of the management of patients with hormone receptor positive breast cancer. Approximately 75 percent of postmenopausal women with breast cancer have tumors that express the estrogen receptor (ER) and/or progesterone receptor (PR) suggesting that they may benefit from such targeted therapy. These patients will routinely be offered a third generation aromatase inhibitor (AI) such as anastrozole, exemestane or letrozole. These agents have been demonstrated to be well tolerated and their use results in improved disease-free survival (DFS) compared to the selective estrogen receptor modulator, tamoxifen, when used in the adjuvant setting (1–3). Letrozole has also been shown to result in greater reduction in tumor size and increased utilization of breast conserving surgery when compared with tamoxifen in the neoadjuvant setting (4).
Despite the effectiveness of AIs, not all patients respond to this treatment and in those who do, resistance develops after prolonged exposure. In a recent study, the value of proliferation as measured by Ki67, in predicting response to AIs was evaluated. This randomized, double blind, phase III study showed that letrozole improved disease-free survival compared to tamoxifen for postmenopausal women with hormone receptor-positive disease (1, 5). The investigators found a greater benefit from letrozole compared to tamoxifen in tumors with a higher Ki67 labeling index, suggesting that high Ki67 labeling index levels may identify a patient group that could benefit from letrozole as their initial adjuvant therapy (6). With respect to resistance to AI therapy, in the majority of cases, ERα expression is not lost (7), however there are alterations in downstream signaling genes and proteins. Increased growth factor signaling is also associated with resistance to endocrine therapy and suggests that inhibitors of signal transduction pathways could provide additional treatment options. The neoadjuvant setting provides the opportunity to identify genes that differ in expression with response (or lack thereof) to treatment. For example, in a recent neoadjuvant treatment study, increased expression of p44/p42 MAPK and HIF1a were independent predictors of resistance to letrozole (8). Taken together, these data suggest that identification and understanding of proteins that regulate response to AI treatment may provide critical information for the design of more effective treatment strategies.
Interest in cyclin E as a potential predictor of response to endocrine therapy originates from the associated cell cycle alterations of cyclin E, including: decreased length of the G1 phase, more rapid transition of G1 to S phase, increased cyclin E kinase activity and increased genomic instability (9–12). The LMW forms of cyclin E and their associated kinase activity are constitutively expressed and activated throughout the tumor cell cycle. These cyclin E-associated cell cycle disruptions affect not only the G1 checkpoint but also those at S, G2, and M (13, 14). The overlap between points in the cell cycle where cyclin E is deregulated and points in the cell cycle targeted by endocrine therapy raises the possibility that cyclin E modulation may be a predictor of response to endocrine therapy in breast cancer patients. Experimental models from a number of laboratories, including our own, have implicated the cyclin E-CDK2 complex and their associated inhibitors, p21 and p27 as important mediators of anti-estrogen therapy in cancer cells (10, 15). We have reported on resistance to fulvestrant mediated by the LMW forms of cyclin E, which are resistant to inhibition by p21 and p27 (10). Taken together, the data regarding estrogens and their effect on the G1/S transition of the cell cycle suggest that aberrations at this checkpoint, (i.e., cyclin E overexpression), may have a significant impact on the clinical benefit achieved from adjuvant endocrine therapy.
In this study, we set out to determine if overexpression of LMW cyclin E leads to lack of response to AI treatment in aromatase overexpressing cells. We further examined the mechanism of this resistance and have proposed targeted therapy to overcome it. Lastly, we provide evidence that breast cancer patients whose tumors overexpress LMW cyclin E are not responsive to AI treatment.
Materials and Methods
Chemicals
The aromatase inhibitors letrozole, exemestane and anastrozole were provided by Astra Zeneca. These drugs were dissolved in methanol and diluted in tissue culture medium. Androstenedione was obtained from Sigma Chemical Co. (St. Louis, MO) and the drug was dliluted in ethanol. Vehicles (methanol or ethanol) alone were used as controls.
Cell culture
MCF-7/Ac1 was cultured in improved modified Eagle’s medium with 5% fetal bovine serum, 1% penicillin/streptomycin solution and 600 µg/ml G418.
Cell growth
The effects of the aromatase inhibitors on MCF7/Ac1 cells growth were examined by counting the cells at the indicated time. The cells were detached from their flask using trypsin and counted using a Coulter counter machine.
Cell cycle analysis
MCF-7/Ac1 cells were cultured in estrogen-deprived media with or without 25 nM 4-androstenedione, and treated with different concentrations of aromatase inhibitors. Untreated and drug treated cells were collected 72 hours later for flow cytometry and lysates were prepared for western blot and kinase assays.
Flow cytometry analysis
Cells were pelleted and resuspended in 1.5 ml of PBS, then fixed in 3.5 ml of 95% ethanol overnight at −20°C. After being washed, the pellets were resuspended in a solution of PBS containing 10 µg/ml propidium iodide, 20 µg/ml RNase A, 0.5% Tween 20, and 0.5% BSA and incubated at 37°C for 30 min. The profiles of cells in the G0-G1, S, and G2-M phases of the cell cycle were analyzed at the M. D. Anderson Cancer Center Cytometry Core Facility on a FACSCaliber machine equipped with Cellquest or ModFit software.
Western blot analysis
Cell lysates were prepared and subjected to western blot analysis as described previously (16). Briefly, 50 µg of protein were subjected to electrophoresis on SDS-PAGE and transferred to Immobilon P overnight at 4°C at 35 mV constant voltage. The blots were blocked overnight at 4°C in BLOTTO [5% nonfat dried milk in 20 mM Tris, 137 mM NaCl, and 0.05% Tween (pH 7.6)]. After being washed, the blots were incubated in primary antibodies for 3 h. Primary antibodies used were cyclin E (HE-12; Santa Cruz Biotechnology), p21 (OP64; Oncogene Research Products, Boston, MA), p27 (K25020; BD Biosciences-Transduction Laboratories, Lexington, KY), cyclin-dependent kinase 2 (CDK2; Transduction Laboratories), and actin (Chemicon International, Inc., Temecula, CA). Blots were then incubated with goat anti-mouse or anti-rabbit immunoglobulin-horseradish peroxidase conjugate at a dilution of 1:5000 in BLOTTO for 1 h and finally washed and developed by using the Renaissance chemiluminescence system as directed by the manufacturer (Perkin-Elmer Life Sciences, Inc., Boston, MA). Western blots were quantitated by densitometric analysis using IPLab Gel software (Scientific Image Processing, Vienna, VA). Densitometric values of actin were used to standardize for equal protein loading. These values were introduced into the software Graph-Pad Prism version 4.0 (GraphPad Software, Inc., San Diego, CA) for statistical analysis.
Immunoprecipitation and Immunoblotting
Two hundred fifty µg of cell extracts were used per immunoprecipitation with polyclonal antibody to cyclin E or polyclonal antibody to CDK2, coupled to protein A beads. After being washed, the immunoprecipitates were subjected to electrophoresis in 13% gels, transferred to Immobolin P, blocked, and incubated with the indicated antibodies as already described.
Protein Kinase Assays
For histone H1 kinase assays, the immunoprecipitates were incubated with kinase assay buffer containing 60 µM cold ATP, 5 µCi of [32P]ATP, and 5 µg of histone H1 (Roche Diagnostics Corporation, Indianapolis, IN) in a final volume of 30 µl at 37°C for 30 min. The products of the reaction were analyzed on 13% SDS PAGE gels, and the gels were stained, destained, dried, and exposed to X-ray film. For quantitation, the protein bands corresponding to histone H1 were excised, and the radioactivity of each band was measured by Cerenkov counting.
Study Patients
The clinical and pathologic data from 395 breast cancer patients, 390 of whom had data available regarding ER status, were previously reported by Keyomarsi et al. (17). Another group of patients included 128 women treated for breast cancer at MDACC with aromatase inhibitors (121 with anastrozole, 4 with letrozole, 2 with exemestane and 1 with letrozole followed by exemestane) between 2001 and 2009. This group of AI-treated women were selected from patients cohort enrolled in an IRB approved protocol to study the cyclin E deregulation in breast cancer. From all the patients enrolled in this study at the time of surgery, freshly resected breast cancer tissue samples were collected and subjected to protein extraction and western blot analysis of cyclin E expression. Demographic, clinical and pathologic data including the steroid-receptor status as well as the low molecular cyclin E levels are described in supplemental Table S1.
Statistical Analysis
Overall survival (OS) was calculated from the date of surgical excision of the primary tumor to the date of death or last follow-up. OS survival curves were computed by the Kaplan-Meier method (18). Univariate analyses of OS survival according to levels of ER and LMW cyclin E were performed with the use of a two-sided log-rank test (19). Results are shown as mean ± SD. Differences were considered significant when the two tailed Student’s t test showed differences at P < 0.05.
Results
Overexpression of LMW cyclin E in postmenopausal breast cancer patients is indicative of a poor prognosis irrespective of estrogen receptor status
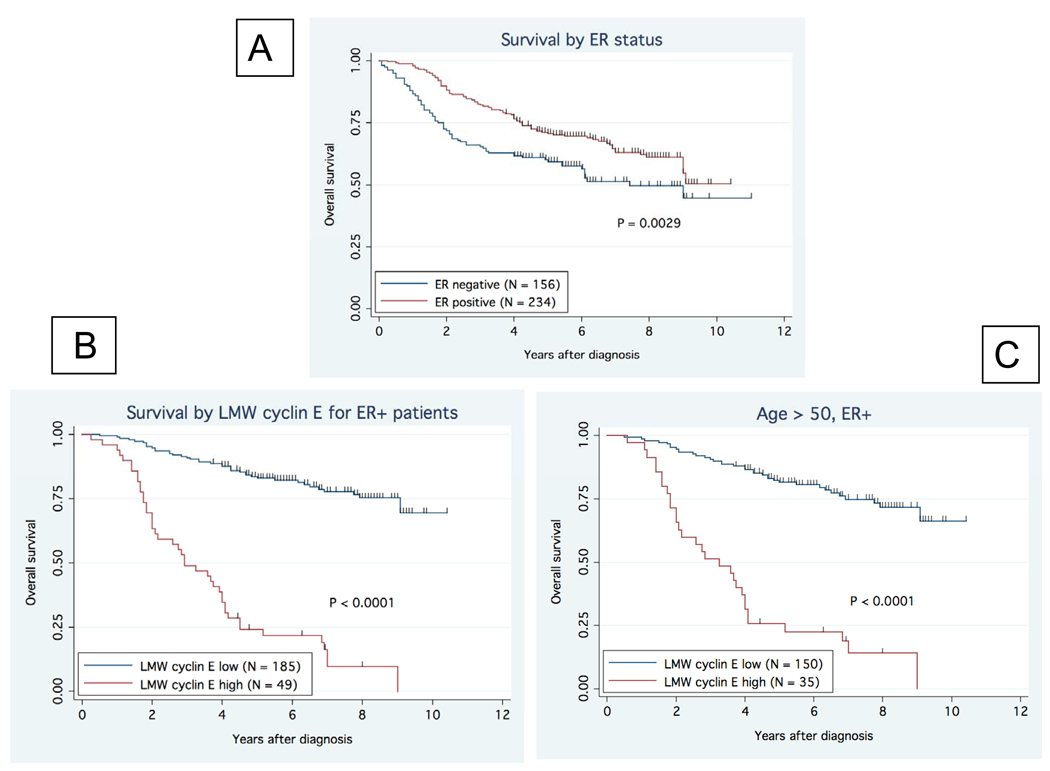
In a retrospective study of 395 patients, we have previously reported on the strong prognostic value of cyclin E in breast cancer (17). We have recently re-analyzed the data to determine the relevance of LMW cyclin E as a prognostic factor based on estrogen receptor status of the tumor (Fig 1). The 5-year overall survival (OS) rates were significantly higher in ER positive patients compared to ER negative patients (p=0.0029) (Fig 1A). We next stratified the 234 ER positive patients as a function of LMW cyclin E expression and found that those patients who had ER positive tumors who also had high levels of LMW cyclin E had worse outcome compared to those patients whose tumors were ER positive and had low levels of LMW cyclin E (p<0.0001) (Fig 1B). This relationship held when only the postmenopausal patients were included in the analysis (p<0.0001)(Fig 1C). Given this relationship between LMW cyclin E and ER status in this cohort of breast cancer patients, we sought to investigate whether LMW cyclin E may effect responsiveness to hormonal therapy. We specifically chose to investigate the effect on responsiveness to AIs, which currently are the standard of care for postmenopausal patients with HR positive breast cancer.
Figure 1. Relationship between ER status and cyclin E in breast cancer.
Breast cancer specimens from 395 patients were assessed for ER and cyclin E expression. (A) Overall survival (OS) was higher in patients with ER-positive (n-234) tumors (p=0.0029). (B) Patients with ER-positive tumors were stratified by LMW cyclin E expression. Patients with high LMW cyclin E had markedly decreased OS (median OS = 3.25 yrs) compared to those with low levels of LMW cyclin E (median OS not reached) (p<0.0001). (C) The relationship between OS, ER and LMW cyclin E was maintained in postmenopausal patients (p<0.001).
Effect of aromatase inhibitor treatment on proliferative response and cell cycle distribution of MCF-7/Ac1 cells
We used MCF-7/Ac1 cells, which are MCF7 breast cancer cells that have been transfected with the gene for aromatase, the enzyme responsible for the conversion of androgens to estrogens. These cells can be stimulated to grow using the aromatizable androgen, androstenedione (AD) that is transformed into estrogen by the aromatase activity of the cells as previously reported (20). This model system simulates the postmenopausal breast cancer patient.
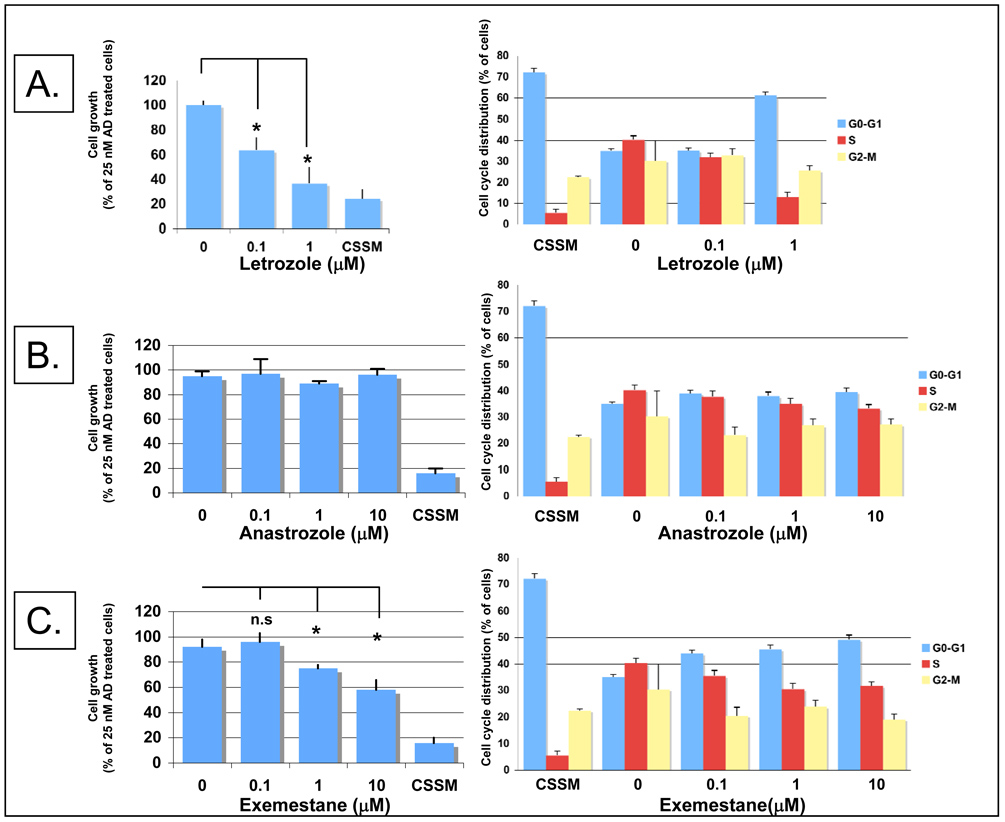
To examine the effects of the three different aromatase inhibitors on the proliferation of MCF-7/Ac1 cells, cells were cultured in estrogen deprived, charcoal stripped serum media (CSSM) for 4 days before treatment. The response of the cells to AD alone or AD plus one of the 3 aromatase inhibitors letrozole (Fig. 2A), anastrozole (Fig. 2B), and exemestane (Fig. 2C) was measured after 3 days of treatment. Cells maintained in CSSM were used as controls. Compared to control cells (CSSM), 25 nM AD treatment resulted in a 3.9 +/− 1.3--fold increase in cell number. The AD-induced growth of MCF-7/Ac1 cells was inhibited by letrozole by 37.2+/−10.4% at 0.1 µM and 63.5+/−13.8% at 1 µM (Fig. 2A). The antiproliferative effect of letrozole is comparable to that in cells cultured in estrogen deprived media (CSSM). Anastrozole did not inhibit the AD-induced growth of MCF-7/Ac1 cells at any of the concentrations tested while exemestane partially inhibited their growth by 25+/−3% at 1 µM and 42+/−8% at 10 µM (P<0.05 vs untreated cells). These results show that MCF-7/Ac1 cells are more responsive to letrozole than to exemestane or anastrozole.
Figure 2. Effect of aromatase inhibitor treatment on proliferative response and cell cycle distribution of MCF-7/Ac1 cells.
Cells were cultured in IMEM with 10% charcoal-stripped serum medium (CSSM) without phenol red and with 600 µg/ml of G418 for 4 days before plating. Cells (100, 000) were seeded in 100 mm dishes and, 24 hrs later, were exposed for 3 days to the specific treatment. (A), antiproliferative effect of increasing concentrations of letrozole in the presence of 25 nM of androstenedione (AD) on MCF-7/Ac1 cell growth (left) and cell cycle distribution (right). Cell growth is expressed as the percentage of the cells compared with the control cells (25 nM AD treated cells, 575,000 cells at day 3). Columns, mean of two to three experiments, each in triplicates; bars, S.D. *, P<0.05, when compared to cells only treated with 25 nM AD; n.s, not significant. CSSM, untreated cells cultured in charcoal-stripped serum medium without phenol red and with 600 µg/ml of G418. (B), antiproliferative effect of increasing concentrations of anastrozole in the presence of 25 nM of androstenedione (AD) on MCF-7/Ac1 cell growth (left) and cell cycle distribution (right). (C), antiproliferative effect of increasing concentrations of exemestane in the presence of 25 nM of androstenedione (AD) on MCF-7/Ac1 cell growth (left) and cell cycle distribution (right).
To investigate the causes of the antiproliferative effects of aromatase inhibitors, cells were stained with PI and cell cycle analysis was performed by flow cytometry (Figure 2, right panels). AD treatment increased the fraction of cells in S phase by 7.3-fold compared to vehicle treated cells (40.3+/−1.9% versus 5.5+/−1.7%) with a concomitant decrease in G0/G1. Among all the treatments, letrozole caused the greatest accumulation of cells (61.2+/−1.5%) in G0/G1 compared to control cells (72.1+/−1.9%) and a significant decrease in the number of cells in S phase (13.1+/−2.3%) compared to control cells (5.5+/−1.7%). Exemestane at 10 µM caused an increase in G0-G1 cells from 35.1+/−0.8% to 49.2+/−0.7% with a decrease in the number of cells in S phase from 40.3+/−1.9% to 31.7+/−2.5% while anastrozole at 10 µM had a more subtle effect. The flow cytometry data correlate with the effect observed on cell number with letrozole having the strongest antiproliferative effects on MCF-7/Ac1 cells due to disruption of cell cycle progression by causing growth arrest at the G1 phase of the cell cycle.
Mechanism of letrozole induced G1 arrest
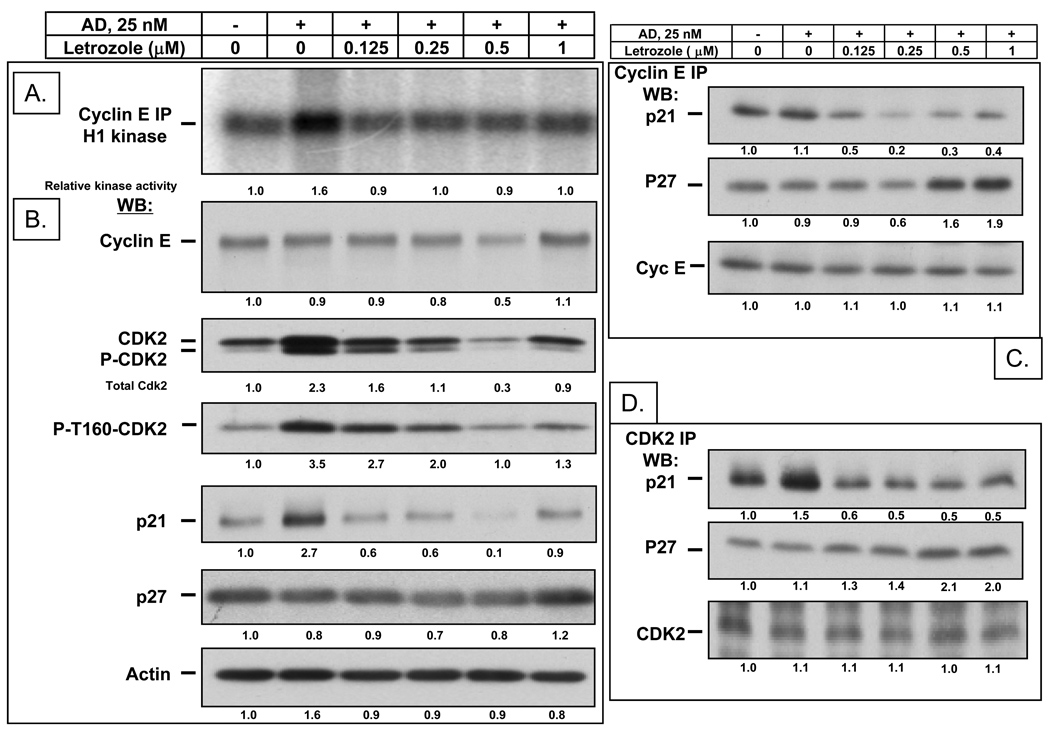
After observing induction of G1 arrest by aromatase inhibitors, we set out to examine the mechanism involved. Because letrozole was the most effective of the 3 inhibitors, we tested this drug in subsequent experiments. We investigated the effect of increasing concentrations of letrozole on the cyclin E-associated kinase activity (Fig. 3A) and on the CDK2-associated kinase activity (Fig. S1). AD treatment increased the cyclin E-associated kinase activity by 1.6-fold and the CDK2-associated kinase activity by 2.6 to 3.5-fold compared to vehicle treated cells. Letrozole blocked this increase in cyclin E-and CDK2- associated kinase activity at a concentration as low as 0.125 µM (Fig. 3A and Fig. S1). Western blot analysis showed that AD treatment increased the CDK2 protein levels by 2.3-fold when compared to vehicle treated cells while letrozole treatment blocked the increase in CDK2 protein levels in a dose dependent manner (Fig. 3B). Active CDK2 is depicted by an increase in phospho-CDK2 band shown both in western blot analysis using pan CDK2 antibody or using a phospho-specific CDK2 antibody which increased by 3.5 fold in AD treated cells. We also show that increasing concentration of letrozole leads to a block of AD-induced increase in CDK2 kinase activity that parallel decreased phospho-T160-CDK2 (Fig S1). Additionally, letrozole treatment also results in the decreased phosphorylation of the endogenous CDK2 substrate, pRb (Fig. S1). Cyclin E protein levels were not affected by AD treatment and slightly decreased at 0.5 µM letrozole. P27 protein levels remained stable and were independent of drug treatments. In order to define the molecular basis of the cyclin E and CDK2 kinase inhibition, we performed immunoprecipitation with cyclin E (Fig. 3C) and CDK2 (Fig. 3D) antibodies followed by western blot for p21 and p27. While AD treatment did not affect p21 binding to cyclin E, it slightly increased the binding to CDK2 by 1.5-fold while letrozole treatment slightly decreased p21 binding to both cyclin E and CDK2. In contrast, even though p27 protein levels remained unchanged after drug treatments, p27 binding to both cyclin E and CDK2 increased in a dose-dependent manner following letrozole treatment by up to 2-fold greater than the levels in AD treated cells. These results suggest that AD induced cell proliferation and G1 exit are mediated by an increase in phospho-CDK2 activity and that letrozole inhibits these effects by preventing the AD induced increase in CDK2 activity and by inducing increased binding of p27 to cyclin E and CDK2 complexes.
Figure 3. In vitro antiproliferative effect of increasing concentrations of letrozole in the presence of 25 nM AD on MCF-7/Ac1 human breast cancer cells.
(A), Letrozole blocks the AD-induced increase in cyclin E-associated kinase activity in MCF-7/Ac1 cells. MCF-7/Ac1 cells were treated with the indicated concentrations of letrozole and AD for 3 days. Cyclin E kinase assays were performed by immunoprecipitating equal amounts of cell lysate (250 µg) with monoclonal antibodies to cyclin E coupled to protein A beads, using histone H1 as substrate. (B) Letrozole blocks the AD-induced increase in CDK2 protein levels. The same cell lysates were subjected to western blot analysis (50 µg of protein) with the indicated antibodies. (C) Letrozole induced increased binding of p27 to cyclin E complexes. The same cell lysates were first immunoprecipitated with cyclin E followed by western blot for p21 and p27. (D) Letrozole induced increased binding of p27 to CDK2 complexes. The same cell lysates were first immunoprecipitated with CDK2 followed by western blot for p21 and p27. The levels of proteins were measured by densitometric scanning of the corresponding bands and normalized using actin values. The values indicated at the bottom were compared to the values obtained with untreated cells.
LMW cyclin E, but not full-length cyclin E, overexpressing MCF-7/Ac1 cells partially override the letrozole inhibition of AD-induced S-phase entry and AD-induced CDK2 protein levels
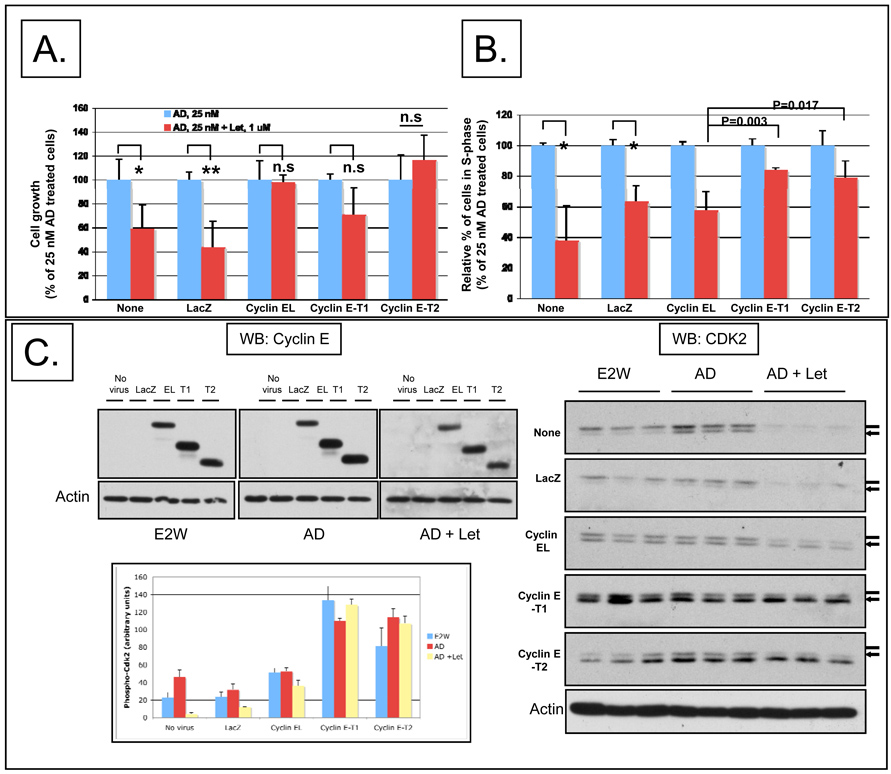
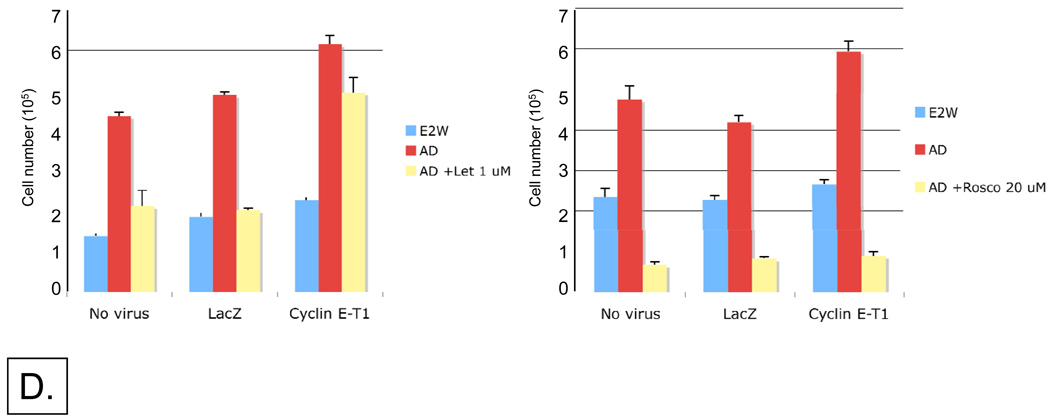
Since overexpression of LMW cyclin E deregulates the G1 to S transition, we interrogated the role of full-length and LMW cyclin E in letrozole response. To this end, we examined the sensitivity of cyclin E overexpressing MCF-7/Ac1 cells to the growth inhibitory effect of letrozole using adenoviruses to overexpress full-length and LMW cyclin E (Figure 4). MCF-7/Ac1 cells were cultured in charcoal stripped serum media (CSSM) for 4 days before infecting them with 4000 m.o.i. of either LacZ, full-length cyclin E (cyclin EL) or LMW cyclin E (cyclin E-T1 and cyclin E-T2). Twenty-four hours later, cells were left either untreated, treated with 25 nM AD alone, or treated with 25 nM AD plus 1 µM letrozole for an additional 3 days (Fig. 4A,B). Following the treatment, cells were enumerated or subjected to flow cytometry analysis. The results revealed that AD-induced growth of MCF-7/Ac1 cells was inhibited by letrozole by 40.8% (P=0.029) in uninfected cells and by 56.1% (P<0.01) in LacZ infected cells, while no significant growth inhibition was observed in cyclin EL, T1, and T2 infected cells (P>0.05) (Fig 4A). However, flow cytometric analysis revealed that letrozole treatment caused a significant decrease in the number of cells in S phase in uninfected (62%), LacZ-infected cells (37%), and cyclin EL-infected cells (42%) while cyclin E-T1 and T2-infected cells were partially resistant to letrozole induced decrease in S phase fraction (16% and 21%, P<0.01 versus cyclin EL) (Fig 4B). These results demonstrate that while cyclin E overexpressing cells could override the letrozole inhibition of AD-induced increase in cell number (Fig. 4A), only the LMW cyclin E overexpressing cells could override the letrozole inhibition of AD-induced S-phase entry (Fig. 4B).
Figure 4. LMW but not full-length cyclin E overexpressing MCF-7/Ac1 cells could partially override the letrozole inhibition of AD-induced G1 exit and AD-induced CDK2 protein levels.
(A) Cyclin E overexpression overrides the letrozole inhibition of AD-induced proliferation. MCF-7/Ac1 cells were cultured in IMEM with 10% charcoal-stripped serum medium (CSSM) without phenol red and with 600 µg/ml of G418 for 4 days before plating. Triplicate wells of 6-well plates were then infected with the indicated adenoviruses (at 4000 m.o.i.) 24 hours before drug treatment. Cells were then left untreated (E2W, estrogen withdrawal) or treated with 25 nM AD (AD) or treated with 25 nM AD and 1 µM letrozole (AD + Let) and collected 3 days later for cell number. Cell growth is expressed as the percentage of the cells compared with the control cells (25 nM AD treated cells). (B) LMW cyclin E overexpressing MCF-7/Ac1 cells could partially override the letrozole inhibition of AD-induced G1 exit. MCF-7/Ac1 cells were treated as described in A, and collected for flow cytometry analysis. Histograms represent the S-phase fraction expressed as the percentage of the cells in S-phase compared with the control cells (25 nM AD treated cells). (C) Cyclin E overexpression prevented the block by letrozole of AD-induced CDK2 protein levels. The same cell lysates as in A and B were subjected to western blot analysis (50 µg of protein) with cyclin E and CDK2 antibodies. The bar graph represents the densitometric values of the phosphorylated CDK2 bands. (D) LMW cyclin E overexpressing MCF-7/Ac1 cells cannot bypass the block by roscovitine of AD-induced increase in cell number. Left, MCF-7/Ac1 cells were cultured in IMEM with 10% charcoal-stripped serum medium (CSSM) without phenol red and with 600 µg/ml of G418 for 4 days before plating. Cells were then infected with the indicated adenoviruses (at 4000 m.o.i.) 24 hours before drug treatment. Cells were then treated with 25 nM AD and 1 µM letrozole and collected 3 days later for cell number. Right, cells were treated as in A except that letrozole was replaced by 20 µM of roscovitine. Columns, mean of two to three experiments, each in triplicates; bars, S.D.
To determine if cyclin E overexpression could rescue the block by letrozole of AD-induced CDK2 protein levels, the same samples were used to determine the cyclin E and CDK2 protein levels by western blot analysis. The exogenous forms of cyclin E were expressed at 2-fold to 4-fold higher levels than endogenous cyclin E and the western blot in Fig.4C demonstrates that the cyclin E protein levels were not affected by the drug treatments. We also show that the LMW cyclin E protein levels achieved by adenoviral expression is comparable to the levels seen in human breast tumor samples (Fig. S2). AD treatment induced a 1.8-fold increase in total CDK2 protein levels in uninfected and LacZ infected cells while letrozole treatment downregulated the total CDK2 protein levels to 10% of the level in uninfected cells. Letrozole treatment of cyclin EL overexpressing cells downregulated the total CDK2 protein levels to only 50% of the level found in untreated, uninfected cells. In sharp contrast, in untreated, LMW cyclin E overexpressing cells, the CDK2 protein levels were already 3.6-fold (for cyclin E-T1) and 1.6-fold (for cyclin E-T2) higher than in untreated uninfected cells and did not drop following letrozole treatment. Densitometric scanning of the western blots revealed a 1.5 to 2-fold increase in the amount of phosphorylated, active CDK2 bands (lower band) in LMW cyclin E overexpressing cells compared to cyclin EL overexpressing cells consistent with higher CDK2 kinase activity that is resistant to letrozole inhibition (Fig 4C, bar graph). Furthermore, increasing concentrations of the cyclin E-T1 virus increase the CDK2 kinase activity in a dose-dependent manner, 1.8- to 5-fold at 500 m.o.i, 5- to 9.7-fold at 1000 m.o.i and 5.8- to 14.4-fold at 4000 m.o.i when compared to the CDK2 kinase activity in AD-treated LacZ cells (Fig. S3). Lastly, we show that 1 uM Letrozole treatment of LMW cyclin E (T1) expressing cells cannot block the CDK2 kinase activity at any of the cyclin E-T1 adenovirus m.o.i while in LacZ expressing cells letrozole completely blocks the AD-induced increase in CDK2 kinase activity (Fig. S3). These results demonstrate that cyclin E overexpression can prevent the block by letrozole of AD-induced CDK2 partially for cyclin EL and completely for cyclin E-T1 and cyclin E-T2 overexpressing cells. Cyclin E-T1 overexpressing cells maintain a high CDK2 kinase activity that is insensitive to letrozole inhibition. (Fig. S3).
LMW cyclin E overexpressing MCF-7/Ac1 cells cannot bypass the block by roscovitine of AD-induced increase in cell number
Since our results thus far demonstrated that AD and LMW cyclin E induced cell proliferation and that G1 exit is mediated by increased CDK2 protein levels and activity, we questioned if a cyclin-dependent kinase inhibitor such as roscovitine could block this effect. To directly address this question, MCF-7/Ac1 cells were cultured in charcoal stripped serum media (CSSM) for 4 days before adding medium with no virus or with 4000 m.o.i. of either LacZ, or LMW cyclin E (cyclin E-T1) adenoviruses. 24 hours later, cells were left untreated, treated with 25 nM AD alone, or treated with 25 nM AD plus 1 µM letrozole for 3 days (Fig. 4D). The AD-induced growth of MCF-7/Ac1 cells was inhibited by letrozole by 51% in uninfected cells and by 58% in LacZ infected cells while the growth of cyclin E-T1 infected cells was inhibited by only 20% (Fig. 4D, left). In sharp contrast, 20 uM of Roscovitine completely inhibited the AD-induced increase in cell number in uninfected, LacZ or cyclin E-T1 infected cells (Fig. 4D, right). These results demonstrate that LMW cyclin E overexpressing MCF-7/Ac1 cells cannot bypass the block by roscovitine of AD-induced increase in cell number.
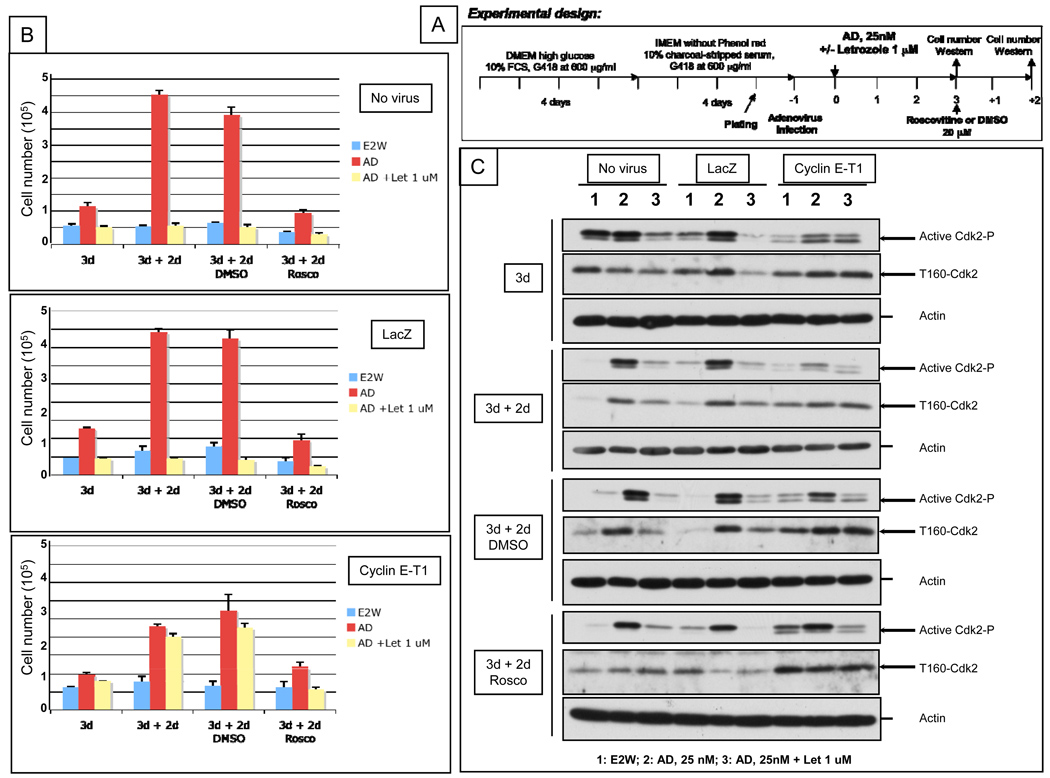
Roscovitine blocks the AD-induced increase in active (phosphorylated) CDK2 and LMW cyclin E overexpression cannot bypass this effect
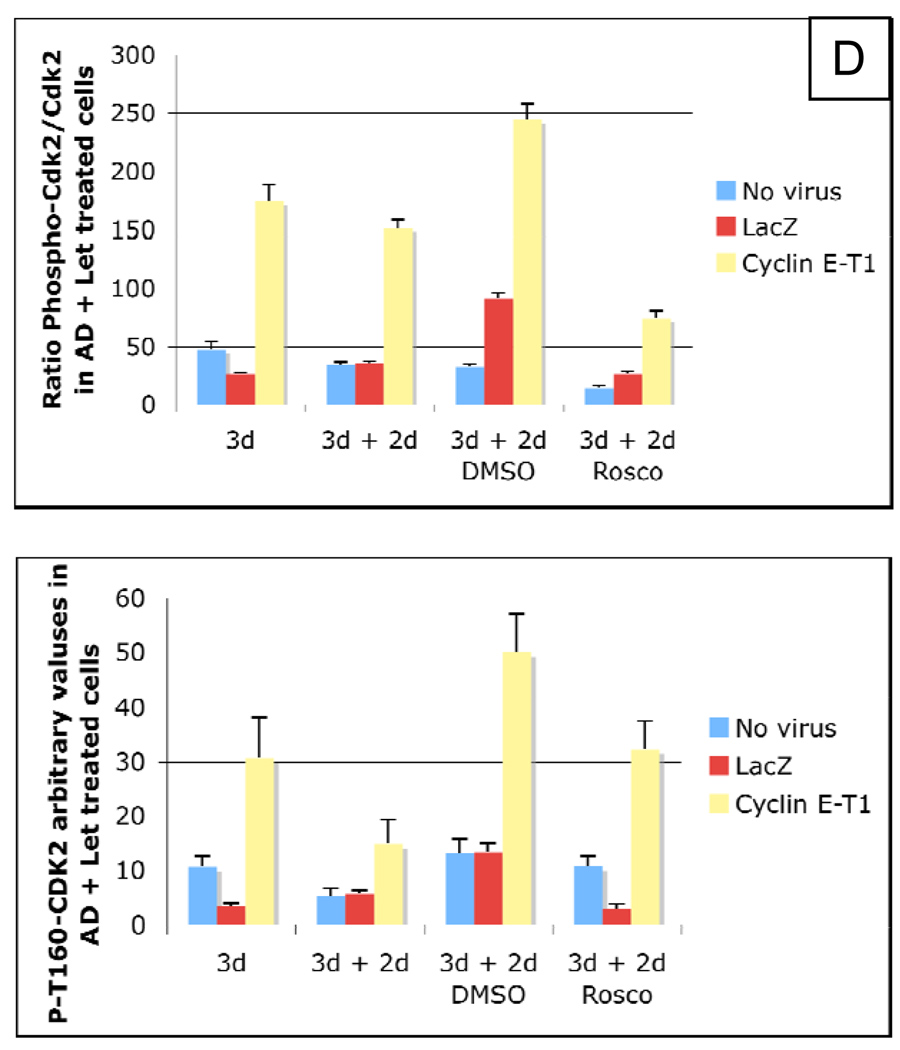
We next examined if roscovitine could also block the growth of letrozole-resistant LMW cyclin E overexpressing MCF-7/Ac1 cells. To this end, cyclin E-T1 (i.e. LMW) infected MCF-7/Ac1 cells were sequentially treated with AD in the presence or absence of letrozole for 3 days, followed by 20 µM of Roscovitine for an additional 2 days (Fig. 5). Medium alone or medium plus DMSO were used as controls. A schematic of the treatment strategy is depicted in figure 5A. At the conclusion of the treatment, cells were enumerated and subjected to western blot analysis with CDK2 (total) and phospho-CDK2 antibodies. The results revealed that three days of letrozole treatment blocked the AD-induced increase in cell number in uninfected and LacZ infected MCF-7/Ac1 cells while LMW cyclin E overexpressing cells were resistant to letrozole inhibition (Fig. 5B). Culturing of cells for an additional two days in charcoal stripped serum medium or medium plus DMSO led to a 4- and 3.5-fold increase in AD-induced proliferation for uninfected and LacZ infected cells, respectively and a 2.3 and 2.8-fold increase in AD-induced proliferation for LMW cyclin E overexpressing cells. This AD-induced proliferation was blocked by roscovitine concomitant with the disappearance or decrease in phosphorylated, active CDK2 protein as shown by western blot analysis (Fig. 5C, lower band). In LMW cyclin E overexpressing cells, letrozole treatment did not prevent a 2.5-fold and 2.8-fold increase in AD-induced proliferation nor did it decrease the phosphorylated/unphosphorylated CDK2 ratio (134–234%) (Fig 5D, upper). On the other hand, treatment of cells with 20 uM of roscovitine was sufficient to completely block the proliferation of letrozole resistant cells concomitant with a decrease in phosphorylated/unphosphorylated CDK2 ratio to 69% (Fig 5D, lower). These results show that roscovitine blocks the AD-induced increase in active (phosphorylated) CDK2 and LMW cyclin E overexpression cannot bypass this effect. These results also suggest that roscovitine treatment of breast cancer cells can reverse intrinsic or acquired resistance to letrozole as a result of LMW cyclin E expression.
Figure 5. Roscovitine blocks the AD-induced increase in active (phosphorylated) CDK2 and LMW cyclin E overexpression cannot bypass this effect.
(A) Schematic representation of the experimental design. (B) MCF-7/Ac1 cells were cultured in IMEM with 10% charcoal-stripped serum medium (CSSM) without phenol red and with 600 µg/ml of G418 for 4 days before plating at a density of 100,000 cells for a 100 mm dish. Cells were then infected with the indicated adenoviruses (at 4000 m.o.i.) 24 hours before drug treatment. Cells were then treated with 25 nM AD and 1 µM letrozole and collected 3 days later for cell number (3d) followed by 2 days in medium (CSSM) alone (3d + 2d) or medium (CSSM) plus DMSO (3d + 2d DMSO) or medium (CSSM) plus 20 µM of roscovitine (3d + 2d Rosco). (C) The same cells used for counting were collected and lysates were subjected to western blot analysis (25 µg of protein) with either the CDK2 (D-12) or phospho-T160-CDK2 antibody. (D) upper, Ratio of densitometric values of phosphorylated CDK2/total CDK2. Lower, Densitomertic values of the phospho-T160-CDK2 bands in AD + Let treated cells.
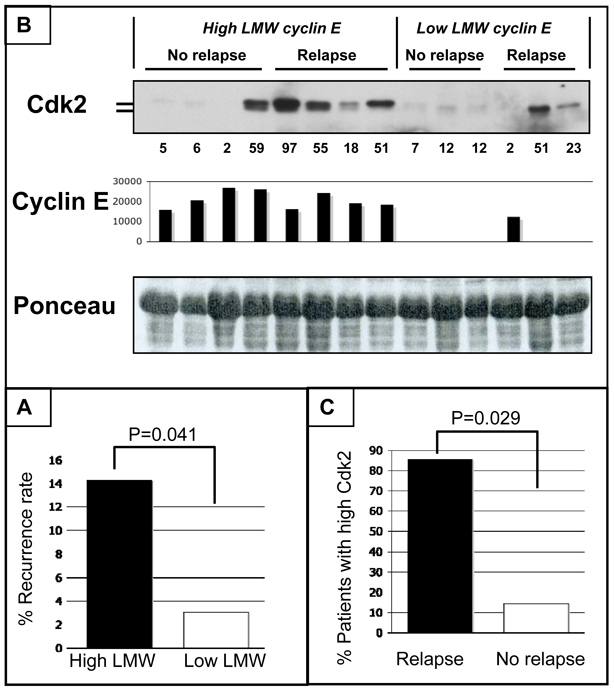
Increased risk of recurrence in AI-treated patients with high LMW levels in primary tumors
To determine the relationship between levels of LMW cyclin E in breast cancer tissues and resistance to AI treatment, we performed an analysis of recurrence rate in 128 AI-treated breast cancer patients with high (28/128) and low (100/128) LMW levels in primary tumors. (Table S1, Fig. 6A). Patient demographics are depicted in Table S1. We found that AI-treated patients with high LMW tumors have increased frequency of recurrence (4/28, 14.3%) when compared to patients with low LMW tumors (3/100, 3.0%; Fisher’s exact test P=0.041) The relative risk of disease relapse in AI-treated patients with high LMW tumors was 4.76 higher than in women with low LMW tumors. Furthermore, we measured the levels of CDK2 in primary tumor tissues from patients resistant to AI based on disease relapse (n=7) as well as in breast cancer samples from patients being disease-free after AI treatment at the time of the last contact (n=7) (Fig. 6B). These results revealed that 6 out of 7 patients with recurrent disease had increased CDK2 protein levels compared to 1 out of 7 patients with no relapse (P= 0.0291, Fisher’s exact test, Fig. 6C). Among the high LMW cyclin E group, 4 out of 4 AI-resistant patients (who had relapse) had increased CDK2 protein levels compared to 1 out of 4 patients with no relapse (P= 0.1429, Fisher’s exact test). These results suggest that overexpression of LMW cyclin E and increased CDK2 protein levels not only can predict potential AI treatment failure, but also provide a rational basis of treatment of these patients with CDK inhibitors.
Figure 6. AI-treated patients with high LMW primary breast tumors have increased frequency of recurrence and increase levels of CDK2.
(A) AI-treated patients with high LMW primary tumors have increased frequency of recurrence (4/28, 14.3%) when compared to patients with low LMW tumors (3/100, 3.0%; Fisher’s exact test P=0.041). (B) Western blot analysis to measure CDK2 protein levels in breast cancer tissues from patients with high LMW cyclin E levels but did not relapse (n=4), from patients with high LMW cyclin E levels who relapsed (n=4), from patients with low LMW cyclin E levels but did not relapse (n=3), and from patients with low LMW cyclin E levels who relapsed (n=3). Lysates were subjected to western blot analysis (50 µg of protein) with CDK2 (D-12, sc-6248). Total cyclin E levels were determined by western blot analysis and densitometry was used to quantitate full length and LMW forms for each sample. The densitometric values of LMW cyclin E are presented in the bar graph. Units used are arbituary. (C) AI-resistant tumors have increased levels of CDK2. 6 out of 7 patients with recurrent disease had increased CDK2 protein levels compared to 1 out of 7 patients with no relapse (P= 0.0291, Fisher’s exact test).
Discussion
In this report we show that overexpression of the LMW forms of cyclin E render letrozole therapy ineffective in breast cancer cells which express both aromatase and estrogen receptor. The mechanism of this effect is through LMW cyclin E-mediated induction of CDK2 activity. When LMW cyclin E is present, it results in higher CDK2 activity and resistance to p21 and p27 inhibition. Treatment of cells with letrozole leads to increased binding of p27 to CDK2 resulting in inactivation of CDK2. An event such as overexpression of LMW cyclin E, which can bypass this process will render letrozole ineffective in mediating a growth arrest in these cells. We also show that treatment of cells with roscovitine can overcome this LMW cyclin E-mediated letrozole resistance. As such, our data provides an alternative treatment option for those postmenopausal breast cancer patients whose tumors are ER positive, but express the LMW forms of cyclin E. We show that this subgroup of patients has a poor prognosis, with a median survival time of only 3.25 years. We provide in vitro evidence, that if these patients were to be treated with letrozole, it is likely that they will not respond effectively to this treatment.
A major issue in the treatment of hormone receptor positive breast cancer is resistance to endocrine therapy. This resistance is intrinsic in up to 50% of patients and acquired in all patients with metastatic disease. Mechanisms of resistance to letrozole include a genetic polymorphism in the aromatase gene CYP19 (21), high levels of ER expression driving transcription (22) or a constitutively active estrogen receptor ER that does not require estrogen for activation (23). Cancer cells can also acquire resistance to letrozole by activation of the HER-2/MAPK pathway and in these cases trastuzumab plus letrozole has been shown to be more effective than either drug alone in letrozole-refractory tumors (24). Other growth factor pathways, including IGF receptor and the PI3K/AKT/mTOR pathways, have been demonstrated to play a role in resistance to endocrine agents and combination treatments targeting multiple pathways are more effective (25, 26). Here we show that activation of CDK2 by overexpression of the LMW forms of cyclin E is a novel mode of letrozole resistance; one that can be circumvented with CDK inhibitors.
Cyclin E protein is overexpressed and post-translationally cleaved by elastase into LMW isoforms (27). LMW cyclin E accumulation is tumor-specific and these isoforms have been found in multiple tumor types including breast, ovarian and colorectal cancers, and melanomas (28–32). Furthermore, LMW cyclin E proteins have been shown to be strong correlative biomarkers in breast and ovarian cancers (17, 31). The LMW cyclin E isoforms have a more profound effect on cell cycle deregulation than the full-length cyclin E (EL) protein (16, 27, 29, 30, 33, 34) and transgenic mice expressing the LMW cyclin E isoforms have more mammary tumor development and metastasis than transgenic mice with the full-length cyclin E (EL) (35) Thus the LMW cyclin E isoforms appear more aggressive than EL in cell cycle abrogation and mammary tumor initiation and maintenance. Cyclin E has also been implicated in anti-estrogen resistance. A study found that the association between cyclin E and disease outcome was restricted to patients who were treated with tamoxifen in the adjuvant setting (15). Another study using MCF-7 cells reported that overexpression of cyclin E could counteract tamoxifen-mediated growth arrest in human breast cancer patients (36). Our laboratory has previously shown that overexpression of LMW cyclin E in breast cancer cells is associated with resistance to fulvestrant (16). Here, we describe a novel mechanism of letrozole resistance through overexpression of LMW cyclin E leading to sustained activation of CDK2. Patients with high LMW cyclin E levels and ER positive tumors would likely not respond to letrozole treatment but could benefit from a therapy targeting the cyclin E/CDK2 complexes such as Roscovitine (Seliciclib or CYC202).
Until now, the use of CDK inhibitors in human malignancies has been of limited success. This may be due to suboptimal selection of the group of patients that would benefit the most from the therapy. We show in our model system that the conversion of androstenedione into estrogen by the aromatase enzyme activity strongly stimulates the growth of breast cancer cells by increasing the CDK2 kinase activity leading to increase in the S-phase fraction. Our study shows that letrozole treatment blocks the AD-induced increase in S-phase fraction which would be translated to a low Ki67 labeling index in a responding tumor. The Ki67 labeling index before and after neoadjuvant endocrine therapy could identify the non-responding ER-positive, LMW cyclin E positive tumors that could benefit from a CDK2 targeted therapy. Additionally, this study suggests that there is a need to identify the population of patients that may benefit from CDK inhibitors (i.e. overcoming the weaknesses of prior studies that were limited by poor patient selection) and that our data suggest that tumors from patients with ER positive disease should be assessed for expression of LMW cyclin E in an effort to predict who may respond to letrozole and who could also benefit from CDK2 targeted therapy.
Translational Relevance
Although letrozole treatment of postmenopausal estrogen receptor-positive breast cancer reduces risk of early metastasis, resistance develops with time. Inhibition of cyclin E/CDK2 kinase activity through increased binding of the cell cycle inhibitor p27 to the complex is a key mediator of the antiproliferative effects of letrozole. Overexpression of LMW cyclin E can bypass this process and renders letrozole ineffective in mediating growth arrest. Treatment of the cells with roscovitine overcomes the LMW cyclin E-mediated letrozole resistance. Lastly, we show that breast cancer patients whose tumors overexpress LMW cyclin E are more likely to recur after AI treatment compared to those with low or no expression of LMW cyclin E. These data support clinical investigation of CDK2 inhibitor therapy for postmenopausal women with ER-positive, LMW cyclin E expressing breast cancer.
Supplementary Material
Acknowledgments
We thank Dr. Elizabeth Mittendorf for the critical reading of this manuscript. This work was supported by National Institutes of Health grant CA87458 and National Cancer Institute grant P50CA116199 and a grant from Clayton Foundation to Khandan Keyomarsi.
References
- 1.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 2.Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007;369:559–570. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 3.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 4.Ellis MJ, Ma C. Letrozole in the neoadjuvant setting: the P024 trial. Breast Cancer Res Treat. 2007;105 Suppl 1:33–43. doi: 10.1007/s10549-007-9701-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 6.Viale G, Giobbie-Hurder A, Regan MM, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1–98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26:5569–5575. doi: 10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Normanno N, Di Maio M, De Maio E, et al. Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr Relat Cancer. 2005;12:721–747. doi: 10.1677/erc.1.00857. [DOI] [PubMed] [Google Scholar]
- 8.Generali D, Buffa FM, Berruti A, et al. Phosphorylated ERalpha, HIF-1alpha, and MAPK signaling as predictors of primary endocrine treatment response and resistance in patients with breast cancer. J Clin Oncol. 2009;27:227–234. doi: 10.1200/JCO.2007.13.7083. [DOI] [PubMed] [Google Scholar]
- 9.Akli S, Keyomarsi K. Cyclin E and its low molecular weight forms in human cancer and as targets for cancer therapy. Cancer Biol Ther. 2003;2:S38–S47. [PubMed] [Google Scholar]
- 10.Akli S, Zheng PJ, Multani AS, et al. Tumor-Specific Low Molecular Weight Forms of Cyclin E Induce Genomic Instability and Resistance to p21, p27, and Antiestrogens in Breast Cancer. Cancer Res. 2004;64:3198–3208. doi: 10.1158/0008-5472.can-03-3672. [DOI] [PubMed] [Google Scholar]
- 11.Dulic V, Drullinger L, Lees E, Reed S, Stein G. Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-cdk2 and cyclin D1-cdk2 complexes. Proc Natl Acad Sci. 1993;90:11034–11038. doi: 10.1073/pnas.90.23.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spruck CH, Won K-A, Reed SI. Deregulated cyclin E induces chromosome instability. Nature. 1999;401:297–300. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]
- 13.Keyomarsi K, Conte D, Jr, Toyofuku W, Fox MP. Deregulation of cyclin E in breast cancer. Oncogene. 1995;11:941–950. [PubMed] [Google Scholar]
- 14.Sgambato A, Han EK, Zhou P, Schieren I, Weinstein IB. Overexpression of cyclin E in the HC11 mouse mammary epithelial cell line is associated with growth inhibition and increased expression of p27(Kip1) Cancer Res. 1996;56:1389–1399. [PubMed] [Google Scholar]
- 15.Span PN, Tjan-Heijnen VC, Manders P, Beex LV, Sweep CG. Cyclin-E is a strong predictor of endocrine therapy failure in human breast cancer. Oncogene. 2003;22:4898–4904. doi: 10.1038/sj.onc.1206818. [DOI] [PubMed] [Google Scholar]
- 16.Akli S, Zheng PJ, Multani AS, et al. Tumor-specific low molecular weight forms of cyclin E induce genomic instability and resistance to p21, p27, and antiestrogens in breast cancer. Cancer Res. 2004;64:3198–3208. doi: 10.1158/0008-5472.can-03-3672. [DOI] [PubMed] [Google Scholar]
- 17.Keyomarsi K, Tucker SL, Buchholz TA, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347:1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EP, Meier P. Nonparametric estimation of incomplete observatons. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 20.Zhou DJ, Pompon D, Chen SA. Stable expression of human aromatase complementary DNA in mammalian cells: a useful system for aromatase inhibitor screening. Cancer Res. 1990;50:6949–6954. [PubMed] [Google Scholar]
- 21.Colomer R, Monzo M, Tusquets I, et al. A single-nucleotide polymorphism in the aromatase gene is associated with the efficacy of the aromatase inhibitor letrozole in advanced breast carcinoma. Clin Cancer Res. 2008;14:811–816. doi: 10.1158/1078-0432.CCR-07-1923. [DOI] [PubMed] [Google Scholar]
- 22.Kuske B, Naughton C, Moore K, et al. Endocrine therapy resistance can be associated with high estrogen receptor alpha (ERalpha) expression and reduced ERalpha phosphorylation in breast cancer models. Endocr Relat Cancer. 2006;13:1121–1133. doi: 10.1677/erc.1.01257. [DOI] [PubMed] [Google Scholar]
- 23.Masri S, Phung S, Wang X, et al. Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer Res. 2008;68:4910–4918. doi: 10.1158/0008-5472.CAN-08-0303. [DOI] [PubMed] [Google Scholar]
- 24.Sabnis G, Schayowitz A, Goloubeva O, Macedo L, Brodie A. Trastuzumab Reverses Letrozole Resistance and Amplifies the Sensitivity of Breast Cancer Cells to Estrogen. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-08-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lisztwan J, Pornon A, Chen B, Chen S, Evans DB. The aromatase inhibitor letrozole and inhibitors of insulin-like growth factor I receptor synergistically induce apoptosis in in vitro models of estrogen-dependent breast cancer. Breast Cancer Res. 2008;10:R56. doi: 10.1186/bcr2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beeram M, Tan QT, Tekmal RR, Russell D, Middleton A, DeGraffenried LA. Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann Oncol. 2007;18:1323–1328. doi: 10.1093/annonc/mdm170. [DOI] [PubMed] [Google Scholar]
- 27.Porter DC, Zhang N, Danes C, et al. Tumor-specific proteolytic processing of cyclin E generates hyperactive lower-molecular-weight forms. Mol Cell Biol. 2001;21:6254–6269. doi: 10.1128/MCB.21.18.6254-6269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bales E, Mills L, Milam N, et al. The low molecular weight cyclin E isoforms augment angiogenesis and metastasis of human melanoma cells in vivo. Cancer Res. 2005;65:692–697. [PubMed] [Google Scholar]
- 29.Bedrosian I, Lu KH, Verschraegen C, Keyomarsi K. Cyclin E deregulation alters the biologic properties of ovarian cancer cells. Oncogene. 2004;23:2648–2657. doi: 10.1038/sj.onc.1207408. [DOI] [PubMed] [Google Scholar]
- 30.Corin I, Di Giacomo MC, Lastella P, Bagnulo R, Guanti G, Simone C. Tumor-specific hyperactive low-molecular-weight cyclin E isoforms detection and characterization in non-metastatic colorectal tumors. Cancer Biol Ther. 2006;5:198–203. doi: 10.4161/cbt.5.2.2356. [DOI] [PubMed] [Google Scholar]
- 31.Davidson B, Skrede M, Silins I, Shih Ie M, Trope CG, Florenes VA. Low-molecular weight forms of cyclin E differentiate ovarian carcinoma from cells of mesothelial origin and are associated with poor survival in ovarian carcinoma. Cancer. 2007;110:1264–1271. doi: 10.1002/cncr.22918. [DOI] [PubMed] [Google Scholar]
- 32.Keyomarsi K, Pardee AB. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci U S A. 1993;90:1112–1116. doi: 10.1073/pnas.90.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wingate H, Bedrosian I, Akli S, Keyomarsi K. The low molecular weight (LMW) isoforms of cyclin E deregulate the cell cycle of mammary epithelial cells. Cell Cycle. 2003;2:461–466. [PubMed] [Google Scholar]
- 34.Wingate H, Zhang N, McGarhen MJ, Bedrosian I, Harper JW, Keyomarsi K. The tumor-specific hyperactive forms of cyclin E are resistant to inhibition by p21 and p27. J Biol Chem. 2005;280:15148–15157. doi: 10.1074/jbc.M409789200. [DOI] [PubMed] [Google Scholar]
- 35.Akli S, Van Pelt CS, Bui T, et al. Overexpression of the low molecular weight cyclin E in transgenic mice induces metastatic mammary carcinomas through the disruption of the ARF-p53 pathway. Cancer Res. 2007;67:7212–7222. doi: 10.1158/0008-5472.CAN-07-0599. [DOI] [PubMed] [Google Scholar]
- 36.Dhillon NK, Mudryj M. Ectopic expression of cyclin E in estrogen responsive cells abrogates antiestrogen mediated growth arrest. Oncogene. 2002;21:4626–4634. doi: 10.1038/sj.onc.1205576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.