Abstract
Thyroid cancer is the most common type of endocrine malignancy and encompasses tumors with various levels of invasive growth and aggressiveness. Rap1GAP, a Rap1 GTPase activating protein, inhibits a RAS family member Rap1 by facilitating hydrolysis of GTP to GDP. In this study, we analyzed 197 thyroid tumor samples and showed that Rap1GAP was frequently lost or downregulated in various types of tumors, particularly in the most invasive and aggressive forms of thyroid cancer. The downregulation was due to promoter hypermethylation and/or loss of heterozygorosity, found in the majority of thyroid tumors. Treatment with demethylating agent 5-Aza and/or histone deacetylation inhibitor TSA induced gene re-expression in thyroid cells. A genetic polymorphism, Y609C, was seen in 7% of thyroid tumors, but was not related to gene downregulation. Loss of Rap1GAP expression correlated with tumor invasiveness, but not with specific mutations activating the MAPK pathway. In vitro, Rap1GAP downregulation was required for cell migration and matrigel invasion. Recovery of Rap1GAP expression inhibited thyroid cell proliferation and colony formation. Overall, our findings indicate that epigenetic and genetic loss of Rap1GAP is very common in thyroid cancer and promotes cell proliferation and invasion.
Keywords: thyroid cancer, Rap1GAP, epigenetic silencing, LOH
INTRODUCTION
Thyroid cancer is the most common type of endocrine malignancy and its incidence is rapidly growing in the United States and many other countries (1). Most of thyroid tumors originate from follicular epithelial cells and include benign follicular adenoma (FA), well differentiated follicular carcinoma (FC) and papillary carcinoma (PC), and undifferentiated anaplastic carcinoma (AC) (2). Patients with well differentiated carcinomas usually have a good prognosis, but a subset of these cancers recur and eventually cause patient death due to widely invasive local growth and metastasis to distant sites. Well differentiated cancers may undergo dedifferentiation and progression to AC, a formidable disease characterized by widespread invasion, early distant metastasis, and patient death within few months. The molecular mechanisms underlying proliferation, invasion and progression of thyroid tumors are not fully understood (3).
PC, the most common type of thyroid cancer, accounts for approximately 80% of all thyroid malignancies. BRAF, RAS, and RET/PTC mutations, usually mutually exclusive in the same tumor, a re found in approximately 70% of these tumors, indicating that RET/PTC-RAS-BRAF-MEK-ERK or MAPK signal pathway plays a pivotal role in the initiation and progression of PC (4). However, other genetic events are very likely to exist and contribute to significant variation in gene expression profiling, phenotypical features, and biological characteristics seen among PCs (5, 6).
Rap1, a member of the Ras family of small GTPases, has been implicated in the regulation of mitogenic and oncogenic pathways in thyroid (7–9) and biochemical studies demonstrated its role in regulating the ERK cascade and specifically its requirement for the RET/PTC-induced activation of BRAF-MEK-ERK (9–11). Like other small GTPases, Rap1 functions as a molecular switch which cycles between an inactive GDP-bound and active GTP-bound form. Rap1GAP, a GTPase activating protein, functions as a negative regulator of Rap1 activity by facilitating hydrolysis of GTP to GDP. Recent findings suggest that Rap1GAP is frequently inactivated in several tumor types and may function as a tumor suppressor (10–13). However its effects may vary in different cell types. In pancreatic cancer, Rap1GAP loss of heterozygorosity (LOH) is frequently seen and loss of Rap1GAP function promotes growth, survival, and invasion of pancreatic cancer cells in vitro and in vivo (10). In squamous cell carcinoma (SCC) of the head and neck, Rap1GAP has been shown to inhibit cell proliferation (11), but promote invasion (12). In thyroid tumors, expression of Rap1GAP protein has been found to be decreased in PCs when studied by immunohistochemistry (14), and in vitro Rap1GAP inhibited proliferation and invasion in thyroid cancer cell lines (13). Most recently, it has been shown that Rap1GAP is frequently downregulated in malignant melanoma via promoter hypermethylation, which promotes melanoma cell proliferation, survival and migration (15).
Rap1GAP alterations and expression have not been studied in various types of thyroid tumors and molecular mechanisms responsible for downregulation of Rap1GAP in thyroid tumors remain largely unknown. In this study, we demonstrate that Rap1GAP expression is lost with progressively higher frequency in more aggressive types of thyroid cancer via promoter hypermethylation and loss of heterozygorosity, and this plays an important role in promoting invasion of thyroid cancer cells.
MATERIALS AND METHODS
Human tissue samples and cell lines
We analyzed 204 snap frozen thyroid samples collected using the University of Pittsburgh IRB approved protocols. They included 7 normal thyroid samples, 40 hyperplastic nodules (HN), 49 follicular adenomas (FA), 27 follicular carcinomas (FC), 78 papillary carcinomas (PC) and 3 anaplastic carcinomas (AC). In addition, 92 neonatal cord blood samples from live births at Magee-Women's Hospital in Pittsburgh were used as a source of genomic DNA from unscreened, population based controls. In accordance with University of Pittsburgh IRB regulations, no information apart from ethnicity was available for these samples. All the samples were obtained from samples with Caucasian ancestry (maternal report). All thyroid cell lines were obtained from Dr. James Fagin (Memorial Sloan-Kettering Cancer Center) in 2003–2007. The cell lines were tested and authenticated in October 2008 in the Molecular Anatomic Pathology Laboratory at the University of Pittsburgh Medical Center using the ampFLSTR Identifiler PCR Amplification Kit (Applied Biosystems), which tests for 16 different polymorphic loci. All cell lines tested had unique genetic profiles and TPC1, Hth74, and TTA1 cell lines matched the size if alleles reported by Schweppe et al. (16).
qRT-PCR
To measure Rap1GAP mRNA expression, quantitative reverse transcription-PCR was performed on ABI PRISM 7500 (Applied Biosystems). Rap1GAP expression was assessed using SYBR Green (Applied Biosystems) and primers: forward 5'-ACGAGCATGTCATCAGCAAT-3'; reverse 5'-CCTTCTGGCCAAGAAATTCA-3'. Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization. The analysis was performed in duplicate. The Rap1GAP relative expression levels in tumor samples were calculated using the comparative CT method with the expression level averaged from 7 normal thyroids set as 1.0.
Detection of Rap1GAP genetic variations
Alterations in Rap1GAP gene were detected by direct sequencing and by LightCycler PCR followed with fluorescence melting curve analysis (FMCA). Direct sequencing of the coding sequence of Rap1GAP was performed from RNA following PCR amplification with four pairs of primers. For genomic DNA and LightCycler PCR, two pairs of primers flanking possible mutation sites 769T>C (C257R) and 1826A>G (Y609C) were used for amplification. For position 1826, a pair of primers flanking the mutation site was used (5'-AAGGTAGCCCTGCTTTG-3' and 5'-AGGAGAGCGTGTCATCC-3'), together with two fluorescent probes (sensor 5'-GGGACTCCTTCATCTATAGCACG-Fluorescein-3' and anchor 5'-LC Red-GGCTGGAGGACAGTGTCAGCAC-3').
Detection of BRAF and RAS mutations and RET/PTC rearrangements
V600E BRAF mutation was detected by real-time PCR and fluorescence melting curve analysis (FMCA) from DNA as previously reported (17). Point mutations of the RAS gene family most commonly found in thyroid cancer, NRAS codon 61, HRAS codon 61, and KRAS codon 12/13, were detected from DNA using PCR and FMCA on LightCycler as previously reported (18). Two main types of RET/PTC rearrangement, RET/PTC1 and RET/PTC3, were detected from RNA by RT-PCR with primers flanking the respective fusion point, followed by agarose gel electrophoresis of the PCR products as previously reported (19).
Immunohistochemistry
Immunohistochemistry for Rap1GAP was performed on formalin-fixed, paraffin-embedded tissues using avidin-biotin-complex method. Primary Rap1GAP antibody (Santa Cruz, sc-28189) were used at 1:200 dilution with overnight incubation at 4°C. The staining was scored as decreased when in 50% or more of tumor cells the intensity of staining was at least 50% weaker as compared to the adjacent non-neoplastic thyroid cells.
LOH analysis
Loss of heterozygosity (LOH) in the Rap1GAP region was studied using PCR amplification and capillary gel electrophoresis for two microsatellite loci (RAPGA1 and D1S2828) located on 1p36.1–p35 within the RAP1GAP gene.
Methylation-specific PCR
Two CpG islands within Rap1GAP promoter region (NSCI bioinformatics UCSC, http://genome.ucsc.edu), CpG24 and CpG74, contain 24 and 74 CpG individual sites, respectively (Supplemental Fig. 1). For methylation-specific PCR analysis, CpG24 island and two CpG units within CpG74 island close to transcription start site were selected. Three sets of methylation-specific and not methylation-specific primers were designed for CpG24, CpG74A and CpG74B using MethPrimer (20). Prior to amplification, DNA was subjected to bisulfite treatment using EpiTect® Bisulfite kit (Qiagen, Valencia, CA) following the manufacturer's instructions. Universal Methylated Human DNA Standard (Zymo Research Corp., Orange, CA), enzymatically methylated human genomic DNA by SssI methylase, was used as a positive control.
5-Aza and TSA treatment
DNA demethylating agent 5-Aza-deoxyline (5-Aza) and histone deacetylases inhibitor trichostatin A (TSA) were purchased from Sigma Chemical Co. (St. Louis, MO). TPC1 and Hth83 cells were plated in 24-well plate and grown for 24 h before treatment. 5-Aza was added in concentrations of 5 μM, 15 μM and 25 μM for 72 h treatment. TSA was added in concentration of 1 μM for only 24 h treatment, either alone or in combination with 5 μM of 5-Aza after cells having been treated with 5-Aza alone for 48 h. As control, cells were maintained in the regular medium without drug addition. Cells were harvested after treatment; protein was used for analysis of Rap1GAP expression and DNA was isolated for methylation-specific PCR.
Western blot
Protein extracts were prepared from frozen tissue using T-PER Tissue Protein Extraction Reagent (Pierce, Rockford, IL, USA), and from cultures cells using Lysis-M Reagent (Roche Applied Science, Mannheim, Germany) with protease inhibitors (Complete, Mini, EDTA-free Protease Inhibitor Cocktail tablets, Roche). Samples were subjected to SDS-PAGE and immunoblotted with anti-Rap1GAP (Santa Cruz, sc-28189) antibodies.
Site-directed mutagenesis
HA-Rap1GAP609mut mutagenesis was done using the Quick Change Site-Directed Mutagenesis Kit (Stratagene), using standard-purified mutagenesis primers (designed with the QuikChange® Primer Design Program), and wtRap1GAP plasmid (kindly provided by Dr. Stork, Oregon Health Sciences University, Portland, OR) as DNA template, following the manufacturer's recommendations. Mutant sequence was then swapped into pmt2SM-HA-Rap1GAP vector (provided by Dr. J. Bos, Utrecht University Medical Center, Utrecht, The Netherlands).
Rap1 activation assay (RalGDS-RBD pull down)
HEK293 cells (~3×105 cells/well in 6-well plates) were transfected with 3μg total DNA and 6μg PEI (Polysciences). HA-Rap1b (250ng) was co-transfected with myc-Epac (500ng) and HA-RapGAP WT or Y609C (1–2.25μg). After 24h, cells were serum-starved for 2h followed by stimulation with forskolin (20μM). Cells were rinsed with cold PBS and lysed in buffer containing 200mM NaCl, 50mM Tris-HCl-pH7.5, 1% Nonidet P-40, 10% glycerol, 25mM MgCl2, 1mM PMSF, 2μM leupeptin, and 2μM aprotinin. Lysates were clarified by centrifugation at 13000 rpm for 15min at 4°C, and 10μg of bacterially expressed GST-RalGDS-RBD coupled to glutathione-Sepharose beads (Amersham Biosciences) were added to the supernatants. Upon 60min incubation at 4°C the beads were washed four times in the same lysis buffer. After the final wash, Laemmli sample buffer was added to the samples. Proteins were fractionated in a 12% SDS-PAGE and transferred to a PVDF membrane. Western blots were performed with anti-HA antibody (Covance).
Cell proliferation, tumorigenicity, and invasiveness assays
Transient transfection of Rap1GAP and control plasmids was carried out using Lipofectamine2000 (Invitrogen, Carlsbad, CA) according to manufacturer's protocol. For proliferation assay, cells were harvested 24 h post-transfection and seeded at 1×104 cell/well in 96-well plates. Cell proliferation was measured using the Rapid Cell Proliferation Kit (Calbiochem, San Diego, CA) for three days after plating. All samples were assayed in triplicate. For colony formation assay, cells were harvested after transfection and seeded at 1×103 cells per dish in10cm dishes in triplicate. The cells were cultured in growth medium containing 400 μg/ml of G418. After 14 days, colonies were stained with 0.25% crystal violet and counted. For in vitro cell invasion assay, cells were harvested after transfection and plated in RPMI 1640 medium with 1% FBS at density of 105 cell/well in the upper chamber of BioCoat Matrigel Invasion 24-well plate chambers (BD Biosciences, Bedford, MA), containing 8-μm-pore-size filter, and exposed to RPMI1640 with 10% FBS in the lower chamber. The invasion rate was evaluated after 22 h according to the manufacturer's instructions. The assay was performed in triplicate. For wound healing assay, cells transfected with Rap1GAP or vector plasmid were scraped with a pipette tip 48h later and washed with PBS to remove the floating cells. Photographs were taken at 0 h and 23 h time points.
Statistical analysis
One-way ANOVA (Post Hoc Bonferroni's test) was used to analyze the expression data for Rap1GAP in clinical cases of thyroid tumors, and Mann-Whitney U test were used to analyze data from LOH screening. Student's t test (unpaired, two-tailed) was used to analyze data from colony formation assay, invasion assay, wound healing assay and proliferation assay. Fisher's Exact Test (2-sided) was used to analyzed data from immunohistochemistry. All values were represented as mean±standard deviation. Significance was determined at P less than 0.05.
RESULTS
Rap1GAP is significantly downregulated in thyroid tumors
The expression of Rap1GAP mRNA was studied in 15 normal thyroid tissues and 197 samples that represent the entire spectrum of follicular-cell derived thyroid tumors and non-neoplastic nodular lesions including benign hyperplastic nodule (HN) and follicular adenoma (FA), well differentiated papillary carcinoma (PC) and follicular carcinoma (FC), and undifferentiated anaplastic carcinoma (AC). qRT-PCR results revealed that, as compared to normal thyroid tissue, Rap1GAP mRNA levels were maintained in HNs (relative expression 0.94), but were progressively lower in benign and particularly malignant tumors (Fig. 1A). In benign FAs, the decrease was not statistically significant (0.71) (P=0.07), whereas Rap1GAP levels were significantly downregulated in all malignant tumors including FCs (0.46), PCs (0.25) and ACs (0.04) (P<0.01).
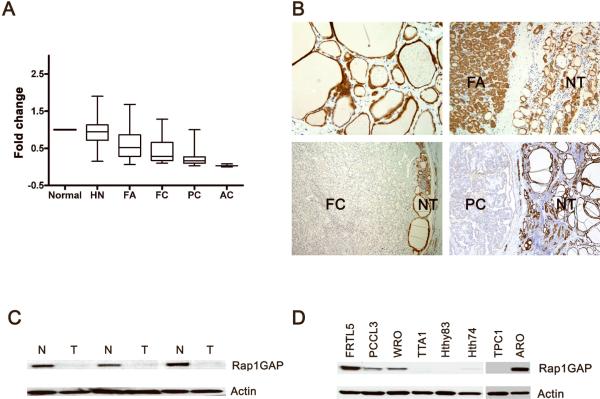
Figure 1. Rap1GAP is downregulated in thyroid tumors.
A: Rap1GAP mRNA expression in thyroid tumor as detected by qRT-PCR. Expression level averaged from 7 normal thyroid samples was set as 1.00. HN –hyperplastic nodule, FA – follicular adenoma; FC – follicular carcinoma, PC – papillary carcinoma, AC – anaplastic carcinoma. B: Rap1GAP immunostaining demonstrates strong cytoplasmic staining in normal thyrocytes (top left) and a FA (top right), and almost complete loss of staining in a FC (bottom left) and a PC (bottom right) as compared to the preserved staining in the adjacent normal thyroid (NT). Hematoxylin & eosin staining. C and D: Western blot analysis demonstrates loss of Rap1GAP protein in PCs (T) as compared to the paired normal thyroid tissues (N) (C) and in four out of five thyroid cancer cell lines (D).
To determine whether there was a downregulation of Rap1GAP at the protein level, 15 normal thyroid tissues and 105 tumor samples were studied by immunohistochemistry. Using semiquantitative assessment of the immunostain, a decrease or complete loss of Rap1GAP expression was observed in 0/28 HNs, 2/32 (6%) FAs, 6/16 (38%) FCs and 21/29 (72%) PCs (Fig 1B) (P<0.001). To further verify the loss of Rap1GAP protein in thyroid tumors, 3 PCs, which showed downregulation of mRNA and loss of protein expression by immunohistochemistry, were studied by western blotting. It confirmed the markedly decreased levels of RAP1GAP protein in all of these PCs as compared to the paired normal thyroid tissues (Fig 1C).
Similar findings were obtained in thyroid cell lines (Fig 1D). Rap1GAP expression was preserved in FRTL5 and PCCL3 cells, both of which are non-transformed rat thyroid cell lines, and was lost or markedly decreased in 4 out of 6 cancer cell lines including TTA1, Hth83, Hth74 and TPC1 cells, which are derived from human anaplastic (TTA1, Hth83, Hth74) and papillary carcinomas (TPC1). However, WRO and ARO cells showed preserved Rap1GAP expression. Whereas WRO cells are derived from thyroid follicular carcinoma, ARO cells, previously believed to be derived from thyroid anaplastic carcinoma, have been recently shown to be of non-thyroid origin (16).
The qRT-PCR results for Rap1GAP expression were correlated with the mutational status in 65 PCs. No difference between Rap1GAP expression levels and BRAF, RAS, or RET/PTC mutations was found (data not shown). This suggests that Rap1GAP downregulation, which is very common in PCs and other types of thyroid cancer, is not directly related to the activation of specific effectors of the MAPK signaling cascade.
Mechanisms of Rap1GAP down-regulation in thyroid tumors
To identify the mechanisms responsible for Rap1GAP down-regulation in thyroid tumors, the tumor samples were tested for mutations and other genetic variations in the Rap1GAP gene, loss of heterozygosity (LOH) in this region, and promoter hypermethylation.
Genetic variations in the Rap1GAP gene
First, the entire ORF of the Rap1GAP gene was analyzed in 12 PCs by direct sequencing. A heterozygous alternation (1826A>G) leading to Y609C was found in one tumor. No other alterations were identified. The Y609C variant, as well as Rap1GAP C257R, were two mutations previously reported in breast cancer (21). Then, we screened DNA from additional 45 cases of thyroid tumors for these two putative mutations by direct sequencing. Three additional Y609C alternations were found. We further studied the prevalence of Y609C alteration in 136 samples using LightCycler real-time PCR and fluorescence melting curve analysis. Totally, 151 thyroid tumors were screened and 10 (6.8%) were found to harbor a heterozygous Y609C allele (Table 1). Analysis of normal tissue in 3 cases with tumors positive for Y609C also identified Y609C in normal tissue, indicating that this was a germline genetic event.
Table 1.
Prevalence of Rap1GAP Y609C variant in patients with various thyroid tumors and benign nodules and in the general population
| Number of cases examined | Rap1GAP Y609C (%) | Prevalence of Y609C allele | ||
|---|---|---|---|---|
| HN | 42 | 0 (0%) | 0 | |
| Thyroid Tumors | FA | 49 | 3 (6.1%) | 10/302 (3.4%) |
| FC | 34 | 3 (8.8%) | ||
| PC | 65 | 3 (4.6%) | ||
| AC | 3 | 1 (33.3%) | ||
| General population | 92 | 2 (2.1%) | 2/184 (1.1%) | |
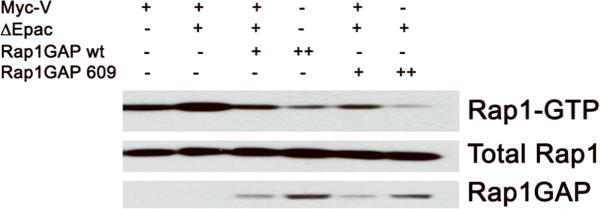
To assess whether Y609C may affect Rap1GAP catalytic activity we monitored Rap1-GTP by RalGDS-RBD pull-down assay. cAMP-Epac was utilized to increase Rap1-GTP levels, and hydrolytic activities of WT and Y609C Rap1GAP compared. As shown on Figure 2, both GAP proteins downregulated Rap GTP in a dose-dependent manner, indicating no effect of Y609C on Rap1GAP activity.
Figure 2. Y609C does not affect Rap1GAP activity.
HEK293 cells were transiently transfected with a total of 3.00μg of the combination of plasmids indicated: myc-V(+: 1.25μg; ++: 2.25μg; +++: 2.75μg), HA-Rap1(+: 0.25μg), myc-ΔEpac (+: 0.5μg), and Rap1GAP wt/609 (+: 1μg; ++: 2.25μg). Upon starvation, transfected cells were stimulated with forskolin. Lysates were prepared and Rap1 activation monitored by RalGDS-RBD pull-down assay (top); aliquots of each lysate (20 μg) were analyzed by HA blot (middle and bottom). Data are from a representative experiment that was reproduced twice.
To find if Rap1GAP Y609C is a rare mutation or if it represents a common single nucleotide polymorphism (SNP), the frequency of this variant was evaluated in 92 genomic DNA samples from population based controls (184 chromosomes). Two individuals were heterozygous for Y609C. Thus, the frequency of the G allele (Y609C) was 1.1%, indicating that Rap1GAP Y609C is probably a SNP. The frequency of this allele was somewhat greater among patients with thyroid tumors (3.4%), but the difference was not statistically significant (p=0.145).
Loss of heterozygosity (LOH)
The LOH status of this region was tested in 135 thyroid tumors using two microsatellite markers (Rap1GA1 and D1S2828) located within the Rap1GAP. The analysis was informative in 128 tumors, and 37 (29%) of them showed LOH in at least one of those two loci (Supplemental Table 1). The frequency of LOH was 16% in FA, 21% in FC, 41% in PC, and 67% in AC. A significant correlation between LOH and decreased Rap1GAP mRNA expression level was found (p=0.03, Mann-Whitney U test), indicating that LOH is likely to be responsible for the decreased expression of Rap1GAP in a subset of thyroid tumors.
Promoter methylation
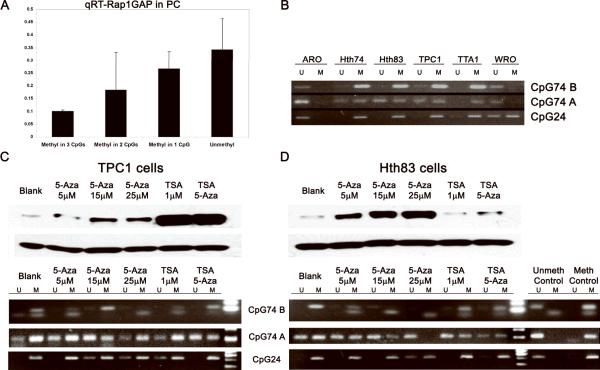
Two known CpG islands within Rap1GAP promoter region, CpG24 and CpG74, contain 24 and 74 individual CpG units, respectively (Supplemental Fig. 1). For methylation analysis of Rap1GAP promoter region, CpG24 island and two individual CpG units within CpG74 island closest to the transcription start site (CpG74A and CpG74B) were used. Methylation status of these three CpG areas was analyzed in 58 PCs. Whereas 17 (29%) tumors were found unmethylated at these sites, 26 (45%) revealed methylation at one site, 10 (17 %) at two sites, and 5 (9%) at all three sites. The presence and extent of methylation of CpG islands in the promoter region showed a strong inverse correlation with Rap1GAP expression at the mRNA level (R2=0.9993), with tumors hypermethylated in all three sites showing the lowest levels of Rap1GAP expression (Fig 3A). As a control, methylation status was also examined in 5 normal thyroid tissues obtained after thyroidectomy and showed no methylation at most of these sites (Supplemental Fig 2).
Figure 3. Promoter hypermethylation is responsible for down-regulation of Rap1GAP in human PCs and thyroid tumor cell lines.
A: An inverse correlation between the extent of methylation at 3 CpG sites in Rap1GAP promoter region and mRNA expression in 58 PCs. B: An inverse correlation between the extents of methylation at CpG74B, CpG74A and CpG24 sites in Rap1GAP promoter in thyroid cancer cell lines. M, methylated; U, unmethylated. C: Reactivation of Rap1GAP expression in TPC1 and Hth83 cells treated with 5-Aza, TSA and TSA plus Aza as detected Western blot (upper panels). Methylation status of the Rap1GAP was analyzed by MSP at 3 CpG sites (lower panels).
The combined results of methylation and LOH analyses of 53 PCs demonstrated that in this group of tumors, 7 (13%) had LOH, 26 (45%) methylation, 15 (28%) both LOH and methylation, and 7 (13%) had neither of these events LOH or methylation, demonstrating the presence of one or both of these alterations in the majority of human PCs.
Next, we examined the methylation status of these CpG sites in thyroid cancer cell lines. Hypermethylation of all three CpG sites in the Rap1GAP promoter region was detected in Hth74, Hth83, TPC1 and TTA1 cells (Fig 3B). Importantly, all of them had no Rap1GAP expression as demonstrated earlier by Western blot (Fig 1D). In ARO cells that preserved Rap1GAP expression, none of those three CpG sites were methylated, and in WRO cells with slightly decreased Rap1GAP expression, only CpG24 was methylated.
To confirm that epigenetic mechanisms cause decreased Rap1GAP expression in thyroid cancer cells, we treated TPC1 and Hth83 cells with 5-Aza and TSA to find whether demethylation of Rap1GAP promoter and/or acetylating histones could induce re-expression of Rap1GAP. In both cell lines, treatment with 5-Aza (5–25μM) resulted in various levels of restoration of Rap1GAP expression (Fig 3C,D). In Hth83 cells, 5-Aza treatment induced Rap1GAP re-expression in a dose-dependent manner and in correlation with alteration of methylation status. Complete demethylation achieved by 25μM of 5-Aza resulted in the highest levels of Rap1GAP expression. In TPC1 cells, 5-Aza treatment resulted in modest increase in Rap1GAP expression. However, TSA induced pronounced restoration of Rap1GAP expression in TPC1 cells. In Hth83 cells, treatment with TSA had not effect on Rap1GAP expression. These results provide direct evidence that epigenetic mechanisms underlie downregulation of Rap1GAP expression in many thyroid tumors.
Correlation between Rap1GAP loss and thyroid tumor invasiveness
In the mRNA and immunohistochemistry studies described above, loss or reduction in Rap1GAP expression generally correlated with the degree of invasiveness of specific tumor types, being lowest in PCs and ACs, which have the most widespread infiltrative growth. To assess the in vivo effects of loss of Rap1GAP on invasiveness in thyroid tumors more accurately, we evaluated the expression levels of Rap1GAP by immunohistochemistry in 49 PCs and 20 FCs, in which tumors were grouped by invasiveness. Among PCs, the decreased or loss of Rap1GAP immunostaining was observed in 28/29 (97%) invasive PCs but only in 4/20 (20%) encapsulated PCs (p<0.001). Among FCs, the decrease or loss of immunostaining was found in 5/5 (100%) widely invasive FCs and 2/15 (13%) minimally invasive FCs (P=0.001). The role of loss of Rap1GAP in invasiveness of thyroid cells was further confirmed in vitro. Re-expression of Rap1GAP in TPC1 and Hth83 thyroid cells led to inhibition of matrigel invasion and decreased migration ability in the wound healing assay (Fig 4).
Figure 4. Re-expression of Rap1GAP inhibits invasion and migration of human thyroid carcinoma cells.
A: Rap1GAP significantly impaired ability of TPC1 and Hth83 cells to invade through Matrigel. The percentage of cells that penetrated through Matrigel-coated Transwell chambers is shown based on three experimental replicates. B: Wound healing assay. Confluent Hth83 and TPC1 cells transfected with either Rap1GAP or vector plasmid for 48h were wounded, and images were acquired immediately and 23h later.
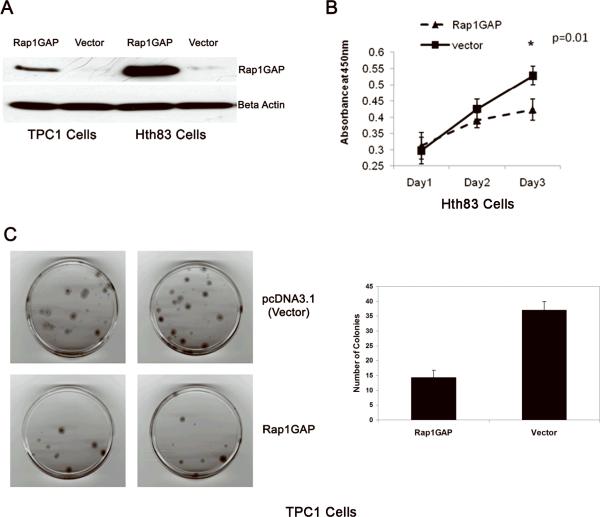
Recovery of Rap1GAP expression inhibits thyroid cancer cell growth in vitro
To examine whether Rap1GAP may serve as a potential therapeutic target, we studied the effect of restoration of Rap1GAP expression on TPC1 and Hth83 thyroid cells using proliferation assay and colony formation assay. Restoration of Rap1GAP expression by transfection with Rap1GAP, as confirmed by western blotting (Fig 5A), resulted in the inhibition of cell growth (Fig 5B) and decrease in their colony formation ability (Fig 5C).
Figure 5. Rap1GAP inhibits thyroid cancer cell growth in vitro.
A: Re-expression of Rap1GAP in TPC1 and Hth83 cells by transient transfection confirmed by Western blots at 48h post-transfection. B: Colony formation assay in TPC1 cells. The cells transfected with Rap1GAP plasmid or empty vector, both containing neomycin resistance gene, were cultured in the presence of G418. After 14 days, colonies were fixed and stained (upper panels), and counted in two separate experiments (lower panel). C: Proliferation assay in Hth83 cells. Cells were plated at 24 h post-transfection and proliferation was assessed over 3 days by WST-1 method. Results are from three separate experiments and show that Rap1GAP inhibited cells growth on day 3 in Hth83 cells.
DISCUSSION
In this study, we demonstrate that Rap1GAP has important tumor suppressor functions in thyroid cells and is frequently lost through epigenetic and genetic mechanisms in most aggressive forms of thyroid cancer.
Common mechanisms of gene silencing and inactivation of tumor suppressor genes include inactivating mutations, LOH and epigenetic alterations. Our results showed that the major mechanisms of Rap1GAP inactivation in thyroid cells are LOH and hypermethylation of the promoter region. Rap1GAP is located at human chromosome 1 p36.1–p35, a region in which high frequency of LOH has been reported in several types of human cancer, including pancreatic carcinoma and oral squamous cell carcinoma. A recent study of pancreatic cancers showed a 33% frequency of LOH in the Rap1GAP gene region in these tumors (10). It appears that this mechanism is also common in thyroid cancer, as we observed high frequency of LOH in this region, particularly in most invasive tumor types.
Another mechanism of Rap1GAP identified in this study was through hypermethylation of CpG islands in the promoter region of the gene. The extent of hypermethylation correlated with expression levels of Rap1GAP. Moreover, treatment with 5-Aza and/or TSA in thyroid cancer cell lines with depleted Rap1GAP expression and hypermethylation in promoter restored the expressions of Rap1GAP. During the preparation of the manuscript, down-regulation of Rap1GAP via promoter hypermethylation was identified in melanoma (15). Since epigenetic gene silencing through promoter methylation and changes in chromatin structure is reversible, our data suggest that Rap1GAP may serve as a potential target for thyroid cancers therapy. This would be of particular importance for anaplastic thyroid carcinoma, which is one of the most aggressive human tumors with short survival and lack of effective therapeutic strategies.
Our data indicate that in thyroid cells, Rap1GAP loss in important for tumor cell invasion. Strong correlation with tumor invasiveness was observed in the most common types of thyroid cancer, i.e. papillary and follicular carcinomas, as in both tumor types the loss of Rap1GAP was largely limited to the tumor with extensive invasion. Similarly, expression of Rap1GAP in thyroid cells in vitro significantly inhibited cell migration and invasion. Similar results have been recently shown in human follicular thyroid carcinoma FTC-133 cells (13) and in pancreatic cancer cells (10). However, in oropharyngeal squamous cell carcinomas, Rap1GAP appears to have an opposite effect on invasion, as it has been shown to promote invasion via induction of matrix metalloproteinase (MMP) 9 secretion, which is associated with poor survival in low N-stage SCC (12). The reasons for different effect of Rap1GAP on tumor invasiveness in oropharyngeal squamous cell carcinoma as compared to pancreatic and thyroid cancer are not clear. It is conceivable that this may be due to tissue-specific effects of Rap1GAP expression on MMP production. In thyroid cancer cells, a link between enhanced productions of MMPs and BRAF mutation has been found, which may help to explain the more invasive behavior of thyroid cancers carrying BRAF mutation (22). However, gene expression array data revealed no significant association between Rap1GAP expression and expression of MMPs 2, 3, 9, and 13 in thyroid PCs (5).
We also observed that restoration of Rap1GAP inhibits thyroid tumor cell proliferation and growth. These findings corroborate the results recently reported in pancreatic cancer, where Rap1GAP expression suppresses tumor formation and progression in vitro and in vivo and also increases apoptotic rates of cancer cells in response to chemotherapeutic drugs 5-FU and etoposide (10). In oropharyngeal squamous cell carcinoma, Rap1GAP expression inhibits tumor growth in vivo (11). These findings provide additional evidence for the role of Rap1GAP downregulation in tumor growth and invasion in thyroid and other cell types, and suggest that restoration of Rap1GAP expression may be exploited as a potential therapeutic approach for these tumors.
Two genetic alterations in the Rap1GAP gene (C257R and Y609C) were previously identified in breast cancer, and those believed to be mutations (21); these genetic variants have not been listed in most SNPs databases. However, the results of our study indicate that the Y609C allele is present with 1.1% incidence in the general population and therefore likely represents a SNP. The incidence of this allelic variant is 3.4% in patients with thyroid tumors, as detected in a series of 151 patients. The difference is not statistically significant; however, whether or not this genetic variation is associated with predisposition to thyroid tumors will require the analysis of a much larger cohort of patients. It is unlikely that Y609C directly affect the function of the gene, as the Y609C Rap1GAP revealed no effect on Rap1 activity in the pull-down assay as compared to wide type Rap1GAP. This finding is not unexpected since Y609C is located outside of the core of Rap1GAP protein, which is considered to be essential for its function. Indeed, it has been demonstrated that out of 663 amino acid residues, only amino acids 75 to 416 are required for full GAP activity (23).
In summary, the results of this study demonstrate that Rap1GAP is likely to serve as an important tumor suppressor in thyroid cells and its loss during carcinogenesis contributes to tumor progression and invasion. Restoration of Rap1GAP expression by releasing the epigenetic block or by other mechanisms have an inhibitory effect on invasion and proliferation of thyroid cancer cells, suggesting that Rap1GAP may serve as a novel therapeutic target for most aggressive types of thyroid cancer.
Supplementary Material
Acknowledgements
The authors thank Dr. P. Stork (Oregon Health Sciences University, Portland, OR) for providing wtRapGAP plasmid and Dr. J. Bos (Utrecht University Medical Center, Utrecht, The Netherlands) for pCGN-HA-Rap1, pCMV-myc-Epac and pmt2SM-HA-Rap1GAP plasmids.
Supported by the NIH grant R01 CA88041 (YEN) and DK063069 (DLA)
References
- 1.Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–91. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikiforov YE. Thyroid tumors: Classification and general considerations. Diagnostic pathology and molecular genetcis of the thyroid. In: Nikiforov YE, Biddinger PW, Thompson LDR, editors. Lippincott Williams & Wilkins; Baltimore: 2009. pp. 94–102. [Google Scholar]
- 3.Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8:83–95. doi: 10.1586/14737159.8.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–7. [PubMed] [Google Scholar]
- 5.Giordano TJ, Kuick R, Thomas DG, et al. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene. 2005;24:6646–56. doi: 10.1038/sj.onc.1208822. [DOI] [PubMed] [Google Scholar]
- 6.Adeniran AJ, Zhu Z, Gandhi M, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–22. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 7.De Falco V, Castellone MD, De Vita G, et al. RET/papillary thyroid carcinoma oncogenic signaling through the Rap1 small GTPase. Cancer Res. 2007;67:381–90. doi: 10.1158/0008-5472.CAN-06-0981. [DOI] [PubMed] [Google Scholar]
- 8.Gao L, Feng Y, Bowers R, et al. Ras-associated protein-1 regulates extracellular signal-regulated kinase activation and migration in melanoma cells: two processes important to melanoma tumorigenesis and metastasis. Cancer Res. 2006;66:7880–8. doi: 10.1158/0008-5472.CAN-06-0254. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Dillon TJ, Pokala V, et al. Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation. Mol Cell Biol. 2006;26:2130–45. doi: 10.1128/MCB.26.6.2130-2145.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Chenwei L, Mahmood R, et al. Identification of a putative tumor suppressor gene Rap1GAP in pancreatic cancer. Cancer Res. 2006;66:898–906. doi: 10.1158/0008-5472.CAN-05-3025. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Mitra RS, Henson BS, et al. Rap1GAP inhibits tumor growth in oropharyngeal squamous cell carcinoma. Am J Pathol. 2006;168:585–96. doi: 10.2353/ajpath.2006.050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitra RS, Goto M, Lee JS, et al. Rap1GAP promotes invasion via induction of matrix metalloproteinase 9 secretion, which is associated with poor survival in low N-stage squamous cell carcinoma. Cancer Res. 2008;68:3959–69. doi: 10.1158/0008-5472.CAN-07-2755. [DOI] [PubMed] [Google Scholar]
- 13.Tsygankova OM, Prendergast GV, Puttaswamy K, et al. Downregulation of Rap1GAP contributes to Ras transformation. Mol Cell Biol. 2007;27:6647–58. doi: 10.1128/MCB.00155-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nellore A, Paziana K, Ma C, et al. Loss of Rap1GAP in Papillary Thyroid Cancer. J Clin Endocrinol Metab. 2009;94:1026–32. doi: 10.1210/jc.2008-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng H, Gao L, Feng Y, Yuan L, Zhao H, Cornelius LA. Down-regulation of Rap1GAP via promoter hypermethylation promotes melanoma cell proliferation, survival, and migration. Cancer Res. 2009;69:449–57. doi: 10.1158/0008-5472.CAN-08-2399. [DOI] [PubMed] [Google Scholar]
- 16.Schweppe RE, Klopper JP, Korch C, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–41. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003;120:71–7. doi: 10.1309/ND8D-9LAJ-TRCT-G6QD. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Z, Ciampi R, Nikiforova MN, Gandhi M, Nikiforov YE. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: effects of the detection methods and genetic heterogeneity. J Clin Endocrinol Metab. 2006;91:3603–10. doi: 10.1210/jc.2006-1006. [DOI] [PubMed] [Google Scholar]
- 20.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–31. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 21.Sjoblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 22.Mesa C, Jr., Mirza M, Mitsutake N, et al. Conditional activation of RET/PTC3 and BRAFV600E in thyroid cells is associated with gene expression profiles that predict a preferential role of BRAF in extracellular matrix remodeling. Cancer Res. 2006;66:6521–9. doi: 10.1158/0008-5472.CAN-06-0739. [DOI] [PubMed] [Google Scholar]
- 23.Rubinfeld B, Crosier WJ, Albert I, et al. Localization of the rap1GAP catalytic domain and sites of phosphorylation by mutational analysis. Mol Cell Biol. 1992;12:4634–42. doi: 10.1128/mcb.12.10.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.