Abstract
The RB-pathway, consisting of inhibitors and activators of cyclin-dependent kinases, the retinoblastoma tumor suppressor (RB), and the E2F-family of transcription factors, plays critical roles in the regulation of cell cycle progression and cell death. Components of this pathway, particularly p16Ink4a, cyclin D1, and RB, are frequently altered in sporadic human cancers to promote deregulated cellular proliferation. The consistent disruption of the RB-pathway in human cancers raises the possibility of exploiting tumor-specific RB-pathway defects to improve the efficacy of current therapies and to develop new therapeutic strategies. This chapter discusses how the RB-pathway status impacts the cellular responses to cytotoxic, cytostatic and hormone therapies, and how the components of the RB-pathway may be directly targeted to treat cancer.
A. BACKGROUND
A-1. Definition of the RB-Pathway
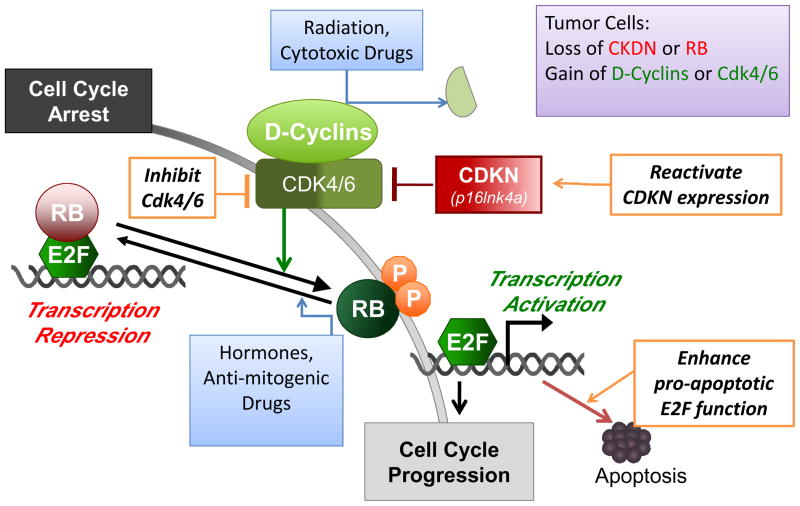
The RB-pathway that is discussed in this article consists of five families of proteins (Fig. 1) – CDKN (e.g., Ink4a), D-type cyclins, cyclin-dependent protein kinases (cdk4, cdk6), RB-family of pocket proteins (RB, p107, p130), and the E2F-family of transcription factors (heterodimers of E2F1–7, DP1, 2). This pathway plays a central role in the regulation of cell proliferation as its constituents are activated and/or inhibited by growth-promoting as well as growth-suppressing signals. Furthermore, several components of this pathway, i.e., p16Ink4a, cyclin D1 and RB, are frequently altered in cancer cells including, the deletion/silencing of the p16Ink4a locus, the amplification of the cyclin D1 focus, and the bialleleic mutation of the RB1 gene. Thus, components of this RB-pathway are rational targets in cancer therapy.
Figure 1. The RB-Pathway in Cancer Therapy.
The components of the RB-pathway, i.e., RB, E2F, D-type cyclins, Cdk4/6, p16Ink4a (CDKN2a) and their functional interactions, are depicted in the diagram. Genetic and epigenetic alternations in the RB-pathway are consistently detected in the majority of sporadic human cancers, and these defects are summarized in the purple box at the upper right-hand corner of the diagram. The status of the RB-pathway affects tumor cell responses to radiation and genotoxic drugs, which cause cell cycle arrest through the degradation of cyclin D1 and the consequent RB dephosphorylation. The status of the RB-pathway also affects tumor cell responses to hormone and other therapeutic strategies that block mitogenic signaling. Defects in the RB-pathway cause deregulated E2F activity, which stimulates gene expression to promote G1/S transition and apoptosis. Potential therapeutic strategies that directly target the RB-pathway defects are depicted in the diagram in orange boxes, and these include the reactivation of p16Ink4a expression in cases where the gene is silenced but not mutated, the inhibition of Cdk4/6 kinase activity, and the enhancement of E2F-dependent apoptosis.
The functional interactions among the five families of proteins in this pathway are well established. The Ink4-family of proteins, p16Ink4a, p15Ink4b, p18Ink4c and p19Ink4d are small heat-stable proteins containing the AKN (ankyrin repeat) domain. Each of the Ink4 proteins can bind to and inhibit the activity of cdk4 and cdk6. The cdk4/6 are D-cyclin-dependent protein kinases. Each of the D-cyclin proteins can associate with cdk4 or cdk6 to form the active kinase complex. The Ink4 proteins compete with the D-cyclins for cdk4/6 to prevent the formation of the active kinase complex. During regulated cell proliferation, the complex of D-cyclin/cdk4/6 is activated as cells respond to mitogenic signals and commit to cell cycle entry. The major cellular targets of the D-cyclin/cdk4/6 complexes are the RB-family of pocket proteins, which contain multiple peptide-binding pockets and assemble nuclear protein-complexes to regulate chromatin structures and transcription factor activities. The RB-family proteins are recruited to specific promoters through their interactions with sequence-specific DNA binding proteins. In the pathway discussed here (Fig. 1), the critical interactions are between the RB-pocket proteins and the E2F-family of transcription factors. When recruited to E2F-regulated promoters, RB-pocket proteins inhibit transcription by directly suppressing the transactivation function of E2F and by recruiting factors that mediate transcriptional repression. Phosphorylation of the RB-pocket proteins by D-cyclin/cdk4 and 6 invariably disrupts the RB•E2F interaction, leading to the activation of E2F-regulated gene expression. E2F binds to and regulates the promoters of multiple genes involved in cell cycle progression (e.g. cyclin E and cyclin A), nucleotide biosynthesis (e.g. thymidylate synthase and ribononucleotide reductase), DNA replication (e.g. MCM7 and cdc6), and mitotic progression (e.g. cyclin B1 and cdk1). As will be discussed below, E2F also stimulates the expression of pro-apoptotic genes (e.g., caspases and Apaf-1) (Fig. 1), and thus alterations in the RB-pathway can affect tumor cell response to cytotoxic agents.
A-2. Alterations in the RB-Pathway in Cancer Cells
Cancer researchers have been interested in the RB-pathway because it is consistently altered in cancer cells to promote deregulated cell proliferation. In this pathway, the Ink4-family and the RB-family proteins function as tumor suppressors, whereas the D-cyclins, cdk4/6 and E2F promote tumor cell proliferation. Recently, a comprehensive analyses of the genome and transcriptome of 206 primary glioblastoma tumors together with the selected sequencing of 601 genes in 91 of the 206 tumor samples have shown that the RB-pathway is altered in 78% of the primary glioblastoma tumor samples. These alterations in the RB-pathway include homozygous deletion and mutation of CDKN2A (p16Ink4a) and RB1 (RB) in 52% and 11% of the samples, respectively, and homozygous deletion of CDKN2B (p15Ink4b) and CDKN2C (p18Ink4c) in 47% and 2% of the tumor samples, respectively. On the other hand, the CDK4, CDK6 and CCND2 (cyclin D2) genes are amplified in 18%, 1% and 2% of the glioblastoma tumors examined (1). Taken together, the frequent yet distinct alterations of components of the RB-pathway in cancer raise the possibility for rationally designed therapeutic strategies that exploit defects in this pathway.
A-3. Role of RB-Pathway in Cellular Responses to Genotoxins
Cytotoxic chemotherapeutic agents and ionizing radiation remain the mainstay therapeutic approaches in the treatment of cancer. These agents almost always cause DNA damage, and the molecular mechanisms underlying the cellular response to genotoxic stresses have been the subject of intense research (2, 3). In this context, it is well appreciated that the RB-pathway is regulated at multiple points to instill the appropriate cell cycle inhibition that is induced by DNA damage. For example, cyclin D1 is rapidly degraded following DNA damage (4, 5) (Fig. 1). Correspondingly, blockade of cyclin D1 degradation in damaged cells leads to aberrant cell cycle progression that is associated with a breakdown in genome integrity (4, 5). The degradation of cyclin D1, together with p53-mediated induction of p21Cip1 and the activity of protein phosphatases cause the dephosphorylation and activation of RB to block cell cycle progression (Fig. 1). The bialleleic loss of RB1 results in a proclivity of deregulated DNA replication in the presence of DNA damage, and thus resulting in additional secondary DNA lesions and enhanced cellular death (6, 7). In addition to cyclin D1 and RB, p16ink4a is implicated in enforcing senescence-like growth arrest in response to the DNA damage (8). Together, several interesting features of the RB-pathway have emerged from these analyses: First, the amplification of cyclin D1 does NOT equate with RB loss in the context of DNA damage response, because the overproduced cyclin D1 can still be efficiently attenuated through proteolytic degradation (5). Second, RB loss may affect the DNA damage response in ways that are not found with either the loss of p16ink4a loss or the gain of cyclin D1. In other words, RB function can be controlled by factors beyond those comprising the canonical RB-pathway (9, 10). Thus, in the context of genotoxic response, the individual components of the RB-pathway are important, yet their defects are likely to have common as well as distinct biological consequences.
A-4. Role of RB-Pathway in Cellular Responses to Anti-Mitogens
There is a growing class of therapeutic agents that target intrinsic oncogenic or growth stimulatory pathways that are required for tumor maintenance or growth. As such, the plethora of studies that evaluated the importance of the RB-pathway in anti-mitogenic signaling (10, 11) may have applicability to therapeutic agents that perturb these pathways. In general, attenuation of mitogenic signaling results in reduced cyclin D1 levels, limited CDK4/6 activity, and the resultant dephosphorylation/activation of RB (Fig. 1). In this context, cyclin D1 is typically down regulated via a combination of transcriptional and post-transcriptional mechanisms (12). Some anti-proliferative stresses also cause the proteolytic turnover and/or the nuclear exclusion of cyclin D1 (12). Importantly, formation of the cyclin D1-CDK4/6 complex is also dependent on mitogenic signaling. Because of these multiple mechanisms of regulation, the functional impact of deregulated cyclin D1 expression can be highly context dependent.
Unlike cyclin D1 and Cdk4/6, p16ink4a is not generally responsive to mitogenic factors and it is not strongly implicated in the response to antimitogenic perturbations. The loss of RB alone is not sufficient to render cells mitogen-independent, in part due to the activity of the RB-related pocket proteins p107 and p130 that can inhibit cell cycle progression via compensatory mechanisms (13, 14). However, the deletion of RB can limit the effectiveness of specific anti-proliferative signals. For example, TGF-beta mediated cell cycle arrest and the anti-proliferative effect of ERK-inhibitors are largely dependent on RB in simple genetic models (15, 16). Thus, there are clear distinctions through which the RB-pathway functions in relation to anti-mitogenic signaling that would be expected to modulate therapeutic response and have implications for clinical response.
B. CLINICAL-TRANSLATIONAL ADVANCES
B-1. RB-Pathway Alterations as Determinants of Therapeutic Responses in Preclinical Models and Clinical Correlates
The findings discussed above from cell culture models have been interrogated in a number of preclinical and clinical settings that have focused largely on two central therapeutic approaches.
Cytotoxic Agents and Radiation
As discussed above radiation and the majority of cytotoxic chemotherapeutic agents function at least in part by inducing DNA damage. In keeping with results from model systems, cyclin D1 over-expression is associated with diverse impacts on therapeutic response that seem to be dependent on the preclinical model and the form of therapeutic challenge. However, the number of published studies that interrogated the effects of cyclin D1 over-expression on tumor responses to genotoxins is surprising limited. These studies have found a role for cyclin D1 in either sensitizing to therapy, or compromising therapeutic response (17–20); however, there has not been sufficient validation of these results across patient populations.
Analyses of p16ink4a loss have demonstrated diverse impact of this tumor suppressor on therapeutic response. It may be anticipated that lack of p16ink4a and failure to establish senescence would be associated with poor response to chemotherapy (21); however, this concept has yet to be clearly supported in the analyses of clinical specimens. Interestingly, some studies have found that elevated levels of p16ink4a in pretreatment biopsies are associated with improved response to cytotoxic therapeutic agents (22–24). Increased expression of p16ink4a is indicative of functional inactivation of RB, as was first appreciated over fifteen years ago in the analyses of cells transformed by viral oncoproteins or harboring RB deletion. In particular, HPV-positive tumors are characterized by elevated p16ink4a staining (24–26), and HPV-positive head and neck tumors exhibit a better response to radiation therapy (24, 27). These findings with the p16ink4a-high tumors indirectly suggest that RB inactivation may also contribute to their sensitivity to chemo or radiation therapy.
In breast cancer, lung cancer, and several other preclinical models, loss of RB is associated with increased sensitivity to cisplatin, adriamycin, ionizing radiation, and other genotoxic drugs (28, 29). Recent studies suggest that cell cycle deregulation, metabolic stress, and other genetic factors associated with the loss of RB may be particularly relevant co-determinants of tumor responses to cytotoxic drugs (7)(Kay MacLeod personal communication). Because the role of RB in genotoxic response may be context dependent (30), identifying those RB-deficient tumor types that will be particularly sensitive or resistant to cytotoxic chemotherapy or radiation remains a challenge. Nevertheless, in ER-negative breast cancer, RB loss is associated with an improved response to cytotoxic therapy (31). This finding has been further corroborated by gene expression profiling that revealed a statistically significant impact of an RB loss-of-heterozygosity signature on improved therapeutic response (32). In bladder cancer, tumors lacking RB have been shown to display an improved response to radiation (33). The aforementioned radiosensitivity of HPV-positive head and neck cancers, which are defective in RB function, would represent another tumor type supporting the concept (24, 27). While these clinical findings are consistent with preclinical studies, it is important to appreciate that RB loss per se may not be the only determining factor, but rather the status of RB may be a marker for a tumor sub-type that is intrinsically more sensitive to genotoxins (32). Thus, additional studies will be required to clearly elucidate the utility of RB-pathway markers in directing chemotherapy, as this will likely be critically dependent on the tumor type and the therapeutic modality.
Hormonal Therapy-Breast/Prostate Cancer
The hormonal therapy utilized in the treatment of breast and prostate cancer represents one the most commonly prescribed targeted therapies for cancer. In the case of prostate cancer these therapies affect the activity of the androgen receptor (AR), while in breast cancer the estrogen receptor (ER) is the target. Hormonal therapies potently activate the RB function to elicit a cytostatic response. A key factor in the general response to such endocrine therapy is the reduction in cyclin D1 (34, 35). In the case of breast cancer this is through complex transcriptional mechanisms (36), while in prostate cancer an mTOR dependent pathway regulates cyclin D1 translation (37). Enforced expression of cyclin D1 has varying impact on the response to endocrine therapies in preclinical models of breast cancer (38–40). Correspondingly, higher cyclin D1 levels are not generally associated with poor prognosis in ER-positive breast cancer (41–44). In prostate cancer, cyclin D1 can function as an antagonist of AR signaling but it is not frequently over-expressed in this tumor type (45).
In contrast to cyclin D1, RB appears to have a potent function in the response to hormonal therapies. Preclinical prostate and breast cancer models demonstrate therapeutic bypass with compromised RB function (34, 46–48). In breast cancer, disruption of RB function is associated with a subtype that exhibits poor response to Tamoxifen and related agents, and a benefit of adjuvant chemotherapy (31, 32, 46). A number of response-predictive gene expression profiles, such as OncotypeDx (49), actually measure RB activity because they interrogate the expression levels of proliferation associated E2F-regulated genes. While the analyses of prostate cancer specimens has been more limited, RB loss was observed progressively in castrate resistant prostate cancer (50). These combined findings suggest that knowledge of RB-pathway perturbations and the regulation of RB function in breast and prostate cancer may contribute to more effectively deployed hormonal therapies to ameliorate the rate of recurrence.
B-2. Novel Therapeutic Approaches—Targeting the RB-Pathway Tationally
There are currently two distinct approaches that are gaining traction in directly targeting the RB pathway to therapeutic effect.
Taking Advantage of RB-pathway Deregulation
The concept of exploiting the loss of RB and the consequential deregulation of E2F-activity to kill tumor cells (Fig. 1) was first demonstrated by the result that agents with the capability of aberrantly stimulating E2F activity can elicit increased cytotoxic response in tumor cells with perturbations in the RB-pathway (51, 52). Subsequently, a number of groups have explored pathways or agents that have specific activity against the RB-pathway. From these analyses a few different concepts have begun to emerge. First, there are cellular signaling pathways that can protect cells with deregulated E2F-activity from death (53, 54). Inhibitors of these pathways, by definition, could be “synthetically lethal” with disruption of the RB-pathway in cancer cells. Second, the pro-apoptotic activity of p53 is restrained by the RB-pathway (55). As such, RB-deficient tumor cells could be sensitized to p53-mediated cell death. In keeping with this concept, it has been shown that RB-deficient tumor lines or those exhibiting deregulated E2F activity are more sensitive to compounds that have the capacity to activate p53 (56). Third, because the RB-pathway impacts E2F transcriptional activity, oncolytic viruses have been produced that would specifically replicate and kill tumor cells that harbor deregulated E2F activity (57).
Activating the Tumor Suppression Function of the RB-Pathway
When RB1 was first cloned over 20 years ago, it was hoped that the discovery of the first tumor suppressor would lend itself to therapeutic approaches for retinoblastoma and additional tumor types. In keeping with that general concept, the enforced expression of p16Ink4a or of constitutively active alleles of RB1 potently inhibits cellular proliferation and can induce persistent cell cycle arrest that is characterized by certain molecular elements of senescence (58, 59). Correspondingly, gene-transfer approaches that specifically target the RB-pathway have been deployed in preclinical models, but not yet in the clinical setting (60, 61). It is well appreciated that re-activating compromised tumor suppressors is a significant pharmacological challenge. In terms of the RB-pathway the closest approach to achieving this goal is the use of inhibitors of DNA methylation or histone deacetylases which although cytotoxic and leading to numerous other endpoints, can also lead to the activation of epigenetically silenced p16Ink4a (Fig. 1) (62).
Another means to re-activate RB function in tumor cells is through the use of CDK-inhibitors that would prevent RB phosphorylation and maintain efficient transcriptional repression (Fig. 1). First generation compounds such a flavopiridol, did result in the blockade of RB phosphorylation, and the RB status did play a role in the response to flavopridol (63). However, such compounds are relatively toxic due to effects on CDKs that are involved in the regulation of transcription (64). As such, whether these compounds exert their primary effect through the RB-pathway remains an open question. Second-generation CDK4/6 inhibitors have been developed that are highly selective and seemingly have no off-target effects based on biochemical and cell based assays (64–66). Consistent with expectation, such agents are dependent on the presence of RB for therapeutic effect in preclinical models (66). Of these agents the Pfizer compound, PD-0332991 is the most broadly deployed in the clinic. The Phase I trial of PD-0332991 represented the first targeted use of an agent that specifically activates RB in the clinic. In keeping with the preclinical data, the Phase I trial utilized RB-deficiency as an exclusion criterion, and several Phase I/II single-agent or combination trials are currently in progress (cancer.gov/clinicaltrials). A key question for such highly specific cytostatic agents is whether they will have therapeutic efficacy, because tumor evolution may readily develop bypass mechanisms to overcome such a single agent. While more trials will be required to answer this question, the preliminary indication from the Phase I trial is that there exist certain tumor types wherein PD-0332991 or related means to chronically activate RB will be therapeutically effective. Specifically, in malignant teratoma continual treatment with PD-0332991 has prevented progression of the disease in patients for approximately two years (67). Together, these analyses suggest that RB-pathway activation could be utilized therapeutically.
Key questions moving forward
As discussed above there is promise in considering the RB-pathway as a node upon which to make treatment decisions or deploy specific therapeutic approaches. However, in spite of substantial preclinical work and analyses of clinical specimens, a number of impediments preclude the implementation of these ideas broadly in the clinic. First, there remains considerable uncertainty about the functional importance of the RB-pathway in reference to therapeutic response in multiple tumor types. Second, the means for monitoring RB-pathway function in biopsy specimens is not standardized. Third, there have yet to be any study that has demonstrated the ideal means to therapeutically target distinct RB-pathway lesions. Fourth, the overall efficacy of prolonged RB-pathway mediated tumor-stasis as a therapeutic option remains uncertain. The address of these issues will require substantial additional studies, and critical re-evaluation of the extensive literature to develop idealized strategies for exploiting the wealth of knowledge in this pathway for the benefit of the cancer patient.
Acknowledgments
The authors thank the members of their laboratories and Beth Gosnell for assistance with manuscript preparation, editorial contributions, and thought-provoking discussion. There are many outstanding studies that contribute to this field, and the author’s regret the inability to fully acknowledge many studies in the context of this relatively short report. ESK and JYJW are supported by grants from the NCI.
References
- 1.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Connor MJ, Martin NM, Smith GC. Targeted cancer therapies based on the inhibition of DNA strand break repair. Oncogene. 2007;26:7816–24. doi: 10.1038/sj.onc.1210879. [DOI] [PubMed] [Google Scholar]
- 3.Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26:7773–9. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- 4.Pontano LL, Aggarwal P, Barbash O, Brown EJ, Bassing CH, Diehl JA. Genotoxic stress-induced cyclin D1 phosphorylation and proteolysis are required for genomic stability. Mol Cell Biol. 2008;28:7245–58. doi: 10.1128/MCB.01085-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agami R, Bernards R. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell. 2000;102:55–66. doi: 10.1016/s0092-8674(00)00010-6. [DOI] [PubMed] [Google Scholar]
- 6.Bosco EE, Mayhew CN, Hennigan RF, Sage J, Jacks T, Knudsen ES. RB signaling prevents replication-dependent DNA double-strand breaks following genotoxic insult. Nucleic Acids Res. 2004;32:25–34. doi: 10.1093/nar/gkg919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Knabb JR, Spike BT, Macleod KF. Elevated Poly-(ADP-Ribose)-Polymerase Activity Sensitizes Retinoblastoma-Deficient Cells to DNA Damage-Induced Necrosis. Mol Cancer Res. 2009 doi: 10.1158/1541-7786.MCR-08-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartkova J, Rezaei N, Liontos M, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–7. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 9.Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–809. doi: 10.1038/sj.onc.1208619. [DOI] [PubMed] [Google Scholar]
- 10.Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8:671–82. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang JY, Knudsen ES, Welch PJ. The retinoblastoma tumor suppressor protein. Adv Cancer Res. 1994;64:25–85. doi: 10.1016/s0065-230x(08)60834-9. [DOI] [PubMed] [Google Scholar]
- 12.Gladden AB, Diehl JA. Location, location, location: the role of cyclin D1 nuclear localization in cancer. J Cell Biochem. 2005;96:906–13. doi: 10.1002/jcb.20613. [DOI] [PubMed] [Google Scholar]
- 13.Sage J, Mulligan GJ, Attardi LD, et al. Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev. 2000;14:3037–50. doi: 10.1101/gad.843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera RE, Sah VP, Williams BO, Makela TP, Weinberg RA, Jacks T. Altered cell cycle kinetics, gene expression, and G1 restriction point regulation in Rb-deficient fibroblasts. Mol Cell Biol. 1996;16:2402–7. doi: 10.1128/mcb.16.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrera RE, Makela TP, Weinberg RA. TGF beta-induced growth inhibition in primary fibroblasts requires the retinoblastoma protein. Mol Biol Cell. 1996;7:1335–42. doi: 10.1091/mbc.7.9.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Abaco GM, Hooper S, Paterson H, Marshall CJ. Loss of Rb overrides the requirement for ERK activity for cell proliferation. J Cell Sci. 2002;115:4607–16. doi: 10.1242/jcs.00161. [DOI] [PubMed] [Google Scholar]
- 17.Sarbia M, Stahl M, Fink U, et al. Prognostic significance of cyclin D1 in esophageal squamous cell carcinoma patients treated with surgery alone or combined therapy modalities. Int J Cancer. 1999;84:86–91. doi: 10.1002/(sici)1097-0215(19990219)84:1<86::aid-ijc16>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Pelosio P, Barbareschi M, Bonoldi E, et al. Clinical significance of cyclin D1 expression in patients with node-positive breast carcinoma treated with adjuvant therapy. Ann Oncol. 1996;7:695–703. doi: 10.1093/oxfordjournals.annonc.a010718. [DOI] [PubMed] [Google Scholar]
- 19.Akervall J, Brun E, Dictor M, Wennerberg J. Cyclin D1 overexpression versus response to induction chemotherapy in squamous cell carcinoma of the head and neck--preliminary report. Acta Oncol. 2001;40:505–11. doi: 10.1080/028418601750288244. [DOI] [PubMed] [Google Scholar]
- 20.Shintani S, Mihara M, Ueyama Y, Matsumura T, Wong DT. Cyclin D1 overexpression associates with radiosensitivity in oral squamous cell carcinoma. Int J Cancer. 2001;96:159–65. doi: 10.1002/ijc.1014. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt CA, Fridman JS, Yang M, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335–46. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 22.Bruland O, Fluge O, Immervoll H, et al. Gene expression reveals two distinct groups of anal carcinomas with clinical implications. Br J Cancer. 2008;98:1264–73. doi: 10.1038/sj.bjc.6604285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong CS, Narasimhan B, Cao H, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74:553–61. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendenhall WM, Logan HL. Human Papillomavirus and Head and Neck Cancer. Am J Clin Oncol. 2009 doi: 10.1097/COC.0b013e31818b8fee. [DOI] [PubMed] [Google Scholar]
- 25.Mellin Dahlstrand H, Lindquist D, Bjornestal L, et al. P16(INK4a) correlates to human papillomavirus presence, response to radiotherapy and clinical outcome in tonsillar carcinoma. Anticancer Res. 2005;25:4375–83. [PubMed] [Google Scholar]
- 26.Stanley MA. Prognostic factors and new therapeutic approaches to cervical cancer. Virus Res. 2002;89:241–8. doi: 10.1016/s0168-1702(02)00192-2. [DOI] [PubMed] [Google Scholar]
- 27.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 28.Du W, Searle JS. The rb pathway and cancer therapeutics. Curr Drug Targets. 2009;10:581–9. doi: 10.2174/138945009788680392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knudsen ES, Knudsen KE. Tailoring to RB: tumour suppressor status and therapeutic response. Nat Rev Cancer. 2008 doi: 10.1038/nrc2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ianari A, Natale T, Calo E, et al. Proapoptotic function of the retinoblastoma tumor suppressor protein. Cancer Cell. 2009;15:184–94. doi: 10.1016/j.ccr.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derenzini M, Donati G, Mazzini G, et al. Loss of retinoblastoma tumor suppressor protein makes human breast cancer cells more sensitive to antimetabolite exposure. Clin Cancer Res. 2008;14:2199–209. doi: 10.1158/1078-0432.CCR-07-2065. [DOI] [PubMed] [Google Scholar]
- 32.Herschkowitz JI, He X, Fan C, Perou CM. The functional loss of the retinoblastoma tumour suppressor is a common event in basal-like and luminal B breast carcinomas. Breast Cancer Res. 2008;10:R75. doi: 10.1186/bcr2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agerbaek M, Alsner J, Marcussen N, Lundbeck F, von der Maase H. Retinoblastoma protein expression is an independent predictor of both radiation response and survival in muscle-invasive bladder cancer. Br J Cancer. 2003;89:298–304. doi: 10.1038/sj.bjc.6601063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knudsen KE, Arden KC, Cavenee WK. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J Biol Chem. 1998;273:20213–22. doi: 10.1074/jbc.273.32.20213. [DOI] [PubMed] [Google Scholar]
- 35.Watts CK, Sweeney KJ, Warlters A, Musgrove EA, Sutherland RL. Antiestrogen regulation of cell cycle progression and cyclin D1 gene expression in MCF-7 human breast cancer cells. Breast Cancer Res Treat. 1994;31:95–105. doi: 10.1007/BF00689680. [DOI] [PubMed] [Google Scholar]
- 36.Eeckhoute J, Carroll JS, Geistlinger TR, Torres-Arzayus MI, Brown M. A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev. 2006;20:2513–26. doi: 10.1101/gad.1446006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–92. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- 38.Pacilio C, Germano D, Addeo R, et al. Constitutive overexpression of cyclin D1 does not prevent inhibition of hormone-responsive human breast cancer cell growth by antiestrogens. Cancer Res. 1998;58:871–6. [PubMed] [Google Scholar]
- 39.Wang Y, Dean JL, Millar EK, et al. Cyclin D1b is aberrantly regulated in response to therapeutic challenge and promotes resistance to estrogen antagonists. Cancer Res. 2008;68:5628–38. doi: 10.1158/0008-5472.CAN-07-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hui R, Finney GL, Carroll JS, Lee CS, Musgrove EA, Sutherland RL. Constitutive overexpression of cyclin D1 but not cyclin E confers acute resistance to antiestrogens in T-47D breast cancer cells. Cancer Res. 2002;62:6916–23. [PubMed] [Google Scholar]
- 41.Han S, Park K, Bae BN, et al. Cyclin D1 expression and patient outcome after tamoxifen therapy in estrogen receptor positive metastatic breast cancer. Oncol Rep. 2003;10:141–4. [PubMed] [Google Scholar]
- 42.Rudas M, Lehnert M, Huynh A, et al. Cyclin D1 expression in breast cancer patients receiving adjuvant tamoxifen-based therapy. Clin Cancer Res. 2008;14:1767–74. doi: 10.1158/1078-0432.CCR-07-4122. [DOI] [PubMed] [Google Scholar]
- 43.Barnes DM, Gillett CE. Cyclin D1 in breast cancer. Breast Cancer Res Treat. 1998;52:1–15. doi: 10.1023/a:1006103831990. [DOI] [PubMed] [Google Scholar]
- 44.Gillett C, Smith P, Gregory W, et al. Cyclin D1 and prognosis in human breast cancer. Int J Cancer. 1996;69:92–9. doi: 10.1002/(SICI)1097-0215(19960422)69:2<92::AID-IJC4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 45.Comstock CE, Augello MA, Benito RP, et al. Cyclin D1 splice variants: polymorphism, risk, and isoform-specific regulation in prostate cancer. Clin Cancer Res. 2009;15:5338–49. doi: 10.1158/1078-0432.CCR-08-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bosco EE, Wang Y, Xu H, et al. The retinoblastoma tumor suppressor modifies the therapeutic response of breast cancer. J Clin Invest. 2007;117:218–28. doi: 10.1172/JCI28803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma A, Comstock CE, Knudsen ES, et al. Retinoblastoma tumor suppressor status is a critical determinant of therapeutic response in prostate cancer cells. Cancer Res. 2007;67:6192–203. doi: 10.1158/0008-5472.CAN-06-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varma H, Conrad SE. Reversal of an antiestrogen-mediated cell cycle arrest of MCF-7 cells by viral tumor antigens requires the retinoblastoma protein-binding domain. Oncogene. 2000;19:4746–53. doi: 10.1038/sj.onc.1203827. [DOI] [PubMed] [Google Scholar]
- 49.Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26:721–8. doi: 10.1200/JCO.2007.15.1068. [DOI] [PubMed] [Google Scholar]
- 50.Mack PC, Chi SG, Meyers FJ, Stewart SL, deVere White RW, Gumerlock PH. Increased RB1 abnormalities in human primary prostate cancer following combined androgen blockade. Prostate. 1998;34:145–51. doi: 10.1002/(sici)1097-0045(19980201)34:2<145::aid-pros10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 51.Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–98. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 52.Chen YN, Sharma SK, Ramsey TM, et al. Selective killing of transformed cells by cyclin/cyclin-dependent kinase 2 antagonists. Proc Natl Acad Sci U S A. 1999;96:4325–9. doi: 10.1073/pnas.96.8.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris EJ, Michaud WA, Ji JY, Moon NS, Rocco JW, Dyson NJ. Functional identification of Api5 as a suppressor of E2F-dependent apoptosis in vivo. PLoS Genet. 2006;2:e196. doi: 10.1371/journal.pgen.0020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moon NS, Di Stefano L, Dyson N. A gradient of epidermal growth factor receptor signaling determines the sensitivity of rbf1 mutant cells to E2F-dependent apoptosis. Mol Cell Biol. 2006;26:7601–15. doi: 10.1128/MCB.00836-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer. 2009;9:738–48. doi: 10.1038/nrc2718. [DOI] [PubMed] [Google Scholar]
- 56.Kitagawa M, Aonuma M, Lee SH, Fukutake S, McCormick F. E2F-1 transcriptional activity is a critical determinant of Mdm2 antagonist-induced apoptosis in human tumor cell lines. Oncogene. 2008;27:5303–14. doi: 10.1038/onc.2008.164. [DOI] [PubMed] [Google Scholar]
- 57.Lim MJ, Min SH, Lee JJ, et al. Targeted therapy of DNA tumor virus-associated cancers using virus-activated transcription factors. Mol Ther. 2006;13:899–909. doi: 10.1016/j.ymthe.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 58.Knudsen KE, Weber E, Arden KC, Cavenee WK, Feramisco JR, Knudsen ES. The retinoblastoma tumor suppressor inhibits cellular proliferation through two distinct mechanisms: inhibition of cell cycle progression and induction of cell death. Oncogene. 1999;18:5239–45. doi: 10.1038/sj.onc.1202910. [DOI] [PubMed] [Google Scholar]
- 59.Craig C, Kim M, Ohri E, et al. Effects of adenovirus-mediated p16INK4A expression on cell cycle arrest are determined by endogenous p16 and Rb status in human cancer cells. Oncogene. 1998;16:265–72. doi: 10.1038/sj.onc.1201493. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X, Multani AS, Zhou JH, et al. Adenoviral-mediated retinoblastoma 94 produces rapid telomere erosion, chromosomal crisis, and caspase-dependent apoptosis in bladder cancer and immortalized human urothelial cells but not in normal urothelial cells. Cancer Res. 2003;63:760–5. [PubMed] [Google Scholar]
- 61.Roig JM, Molina MA, Cascante A, et al. Adenovirus-mediated retinoblastoma 94 gene transfer induces human pancreatic tumor regression in a mouse xenograft model. Clin Cancer Res. 2004;10:1454–62. doi: 10.1158/1078-0432.ccr-0442-03. [DOI] [PubMed] [Google Scholar]
- 62.Egger G, Aparicio AM, Escobar SG, Jones PA. Inhibition of histone deacetylation does not block resilencing of p16 after 5-aza-2′-deoxycytidine treatment. Cancer Res. 2007;67:346–53. doi: 10.1158/0008-5472.CAN-06-2845. [DOI] [PubMed] [Google Scholar]
- 63.Budak-Alpdogan T, Chen B, Warrier A, Medina DJ, Moore D, Bertino JR. Retinoblastoma tumor suppressor gene expression determines the response to sequential flavopiridol and doxorubicin treatment in small-cell lung carcinoma. Clin Cancer Res. 2009;15:1232–40. doi: 10.1158/1078-0432.CCR-08-0810. [DOI] [PubMed] [Google Scholar]
- 64.Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–66. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 65.Toogood PL, Harvey PJ, Repine JT, et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem. 2005;48:2388–406. doi: 10.1021/jm049354h. [DOI] [PubMed] [Google Scholar]
- 66.Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–38. [PubMed] [Google Scholar]
- 67.Vaughn DJ, Flaherty K, Lal P, et al. Treatment of growing teratoma syndrome. N Engl J Med. 2009;360:423–4. doi: 10.1056/NEJMc0808558. [DOI] [PubMed] [Google Scholar]