Abstract
Siderocalin is a secreted protein that binds to siderophores to prevent bacterial iron acquisition. While it has been shown to inhibit the growth of Mycobacterium tuberculosis (M.tb) in extracellular cultures, its effect on this pathogen within macrophages is not clear. Here, we show that siderocalin expression is up-regulated following M.tb infection of mouse macrophage cell lines and primary murine alveolar macrophages. Furthermore, siderocalin added exogenously as a recombinant protein or over-expressed in the RAW264.7 macrophage cell line inhibited the intracellular growth of the pathogen. A variant form of siderocalin, which is expressed only in the macrophage cytosol, inhibited intracellular M.tb growth as effectively as the normal, secreted form, an observation that provides mechanistic insight into how siderocalin might influence iron acquisition by the bacteria in the phagosome. Our findings are consistent with an important role for siderocalin in protection against M.tb infection and suggest that exogenously administered siderocalin may have therapeutic applications in tuberculosis.
Keywords: iron, infection, lipocalin 2, NGAL
INTRODUCTION
Mycobacterium tuberculosis (M.tb) is a bacterial pathogen that leads to the deaths of approximately 2 million people every year (Kaufmann and McMichael, 2005). Following inhalation, M.tb is taken up by macrophages and other cells in the lung, and can survive intracellularly for extended periods (Kaufmann, 2005). Iron is essential for M.tb to survive and grow intracellularly and to evade host immune defenses (Raghu et al., 1993; Kelley and Schorey, 2003; Serafin-Lopez et al., 2004). To ensure that its demand for iron is met, M.tb synthesizes and secretes the salicylate-type siderophore carboxymycobactin that tightly binds iron and delivers it to the bacteria via specific surface receptors and transport proteins (Ratledge and Dover, 2000). M.tb also expresses a more hydrophobic form of carboxymycobactin known as mycobactin that remains associated with the bacterial cell wall rather than being secreted (Rodriguez, 2006). Its role in iron acquisition is not clear, but it may facilitate the action of carboxymycobactin. Strains of M.tb that are unable to produce or utilize carboxymycobactin grow poorly in iron-restricted conditions, and exhibit attenuated growth inside macrophage cell lines and in vivo in mouse models of tuberculosis, confirming a role for siderophore-mediated iron acquisition in M.tb growth and virulence (De Voss et al., 2000; Rodriguez, 2006).
The host immune system has developed multiple strategies to limit the availability of iron to microbial pathogens (Doherty, 2007). One of these strategies involves the protein siderocalin (also known as lipocalin 2, 24p3 and neutrophil gelatinase-associated lipocalin or NGAL) (Goetz et al., 2002; Flo et al., 2004; Berger et al., 2006), a 21 kDa member of the lipocalin superfamily that was originally identified as a constituent of specific granules in human neutrophils (Kjeldsen et al., 1993), and later shown to be an acute-phase protein secreted by the liver in mice (Liu and Nilsen-Hamilton, 1995). Siderocalin does not bind iron directly (Yang et al., 2002), but rather, it binds specifically to iron-laden bacterial siderophores, including enterobactin secreted by E. coli (Goetz et al., 2002) and carboxymycobactin produced by mycobacteria (Holmes et al., 2005). The addition of recombinant siderocalin to cultures of E. coli or M.tb in vitro has a bacteriostatic effect that can be reversed with iron supplementation (Goetz et al., 2002; Flo et al., 2004; Martineau et al., 2007). Furthermore mice with a targeted disruption of the siderocalin gene exhibited a higher mortality rate than wild-type animals when challenged with intra-peritoneal injections of pathogenic E. coli (Flo et al., 2004; Berger et al., 2006). These observations point to an important role for siderocalin in anti-bacterial defense.
Although siderocalin has been shown to inhibit the growth of M.tb in extracellular culture, its effect on the growth of the pathogen in macrophages remains unclear. To clarify this issue, we have carried out experiments to examine the effect of siderocalin on the intra-macrophage multiplication of M.tb.
MATERIALS AND METHODS
M.tb infection of mice
Female C57BL/6J mice (Jackson Labs) were housed under specific pathogen-free conditions at the Harvard School of Public Health and were provided chow and water ad libitum. Following 3 weeks of acclimation in the BSL3 facility, 7-9 week old mice were infected with virulent M.tb (Erdmann strain; Trudeau Institute) via aerosol. Non-infected mice housed under the same conditions served as controls. Alveolar macrophages were prepared following a standard protocol (Zhang et al., 2008). Lungs were harvested from the mice at the time of sacrifice.
RNA isolation, cDNA synthesis and quantitative RT-PCR
Total RNA was prepared from whole lung tissue, alveolar macrophages, and RAW264.7 cells using TRIZOL (Invitrogen) according to the manufacturer's specifications. Gene expression was analyzed by qRT-PCR using the SYBR GreenER qPCR SuperMix for ABI PRISM (Invitrogen). Acidic ribosomal phosphoprotein P0 (Arbp, also known as 36B4) or GAPDH served as reference genes. The primer sequences for siderocalin were as follows: fwd, 5’- CCCCTGAACTGAAGGAACGTT-3’, rev, 5’-CGTCCTTGAGGCCCAGAGA-3’. Calculations for relative quantification were done using the comparative CT method (2−ΔΔCT).
Generation of a cytosolic mutant of human siderocalin
The primers, ngalF, 5’-GGATCCGCCACCATGCAGGACTCCACCTCAGACCTG-3’ and ngalR, 5’-GAATTCTCAGCCGTCGATACACTG-3’ were used to PCR amplify a 557 bp fragment using the plasmid pDNA3.1NGAL containing the full-length human siderocalin cDNA (Bundgaard et al., 1994) as the template. The amplification product, which retained the methionine start codon but lacked the secretion signal sequence, was cloned into the pCDNA3.1 vector to yield the construct pCDNA3.1cytoNGAL. Expression analysis was initially performed following transient trasfection into HeLa cells using Lipofectamine 2000 (Invitrogen). The cells were processed for Western blotting or immunofluorescence with an anti-human siderocalin monoclonal antibody (Kjeldsen et al., 1996) 48 h after transfection.
Construction and purification of recombinant GST-siderocalin
The insert from pCDNA3.1cytoNGAL was cloned into the vector pGEX2TK to generate the plasmid pGEXcytoNGAL, encoding an in-frame fusion with GST. pGEXcytoNGAL was transformed into E. coli strain BL21 to avoid binding of endogenous bacterial siderophores (Goetz et al., 2002). The GST-siderocalin fusion protein and GST were affinity purified from the relevant transformants using glutathione-agarose as described previously (Chaudhuri et al., 1997). The purity and identity of the fusion protein was confirmed by Western blotting with an anti-human siderocalin monoclonal antibody (Kjeldsen et al., 1996).
E. coli and M.tb growth assays
The ability of GST-siderocalin to inhibit bacterial growth was confirmed using the siderocalin susceptible E. coli strain H9049 (kindly provided by Dr. Michael Fischbach, Harvard Medical School) and M. tb in previously described in vitro growth inhibition assays (Fischbach et al., 2006) (Martineau et al., 2007).
Mycobacterial infection of murine macrophages
M.tb H37Rv (Wolf et al., 2007) and Mycobacterium bovis-BCG (BCG) (Teitelbaum et al., 1999) expressing green fluorescent protein (GFP) were cultured in 7H9 medium containing 50 μg mL−1 kanamycin (Sigma). M.tb and BCG in mid-growth phase were pelleted, resuspended in complete DMEM, sonicated, and passed through a 5 μM filter. For qRT-PCR analysis, RAW264.7 macrophages were infected at an MOI of 5:1 for the indicated time points and harvested in TRIZOL. For intracellular CFU, RAW264.7 macrophages were infected at a MOI of 1:1 to avoid artifact associated with bacterial clumping. To analyze the effect of recombinant siderocalin on intracellular M.tb and BCG growth, GST or GST-siderocalin was added to complete DMEM at a concentration of 10 μg mL−1. At the indicated time points, infected cells were washed with sterile PBS, lysed using 1% Trition X-100 in PBS, serially diluted and plated on 7H11 agar. Infected cells incubated with GST or GST-siderocalin grown on glass coverslips were fixed with 3.7% paraformaldehyde and mounted with DAKO fluorescent mounting medium for examination by fluorescence microscopy.
Generation of RAW264.7 cells stably expressing wild-type or cytosolic siderocalin
RAW264.7 cells were transfected with pCDNA3.1NGAL or pCDNA3.1cytoNGAL using Lipofectamine 2000. Stable transfectants were selected and grown in 400 μg mL−1 of G418.
Western blotting
Cells lysates were subjected to electrophoresis and Western blotting with the anti-siderocalin monoclonal antibodies specific for either the mouse (R&D Systems) or human (Kjeldsen et al., 1996) proteins and developed with an HRP-conjugated anti-mouse IgG antibody and enhanced chemiluminescence (Pierce).
Siderocalin immunofluorescence
Cells plated on cover-slips were fixed, permeabilized and stained with anti-siderocalin, followed by fluorochrome-conjugated secondary antibody. The cells were mounted in Vectashield before examination by fluorescence microscopy.
Statistical analysis
For statistical analysis, the means ± SEM are displayed. For intracellular M.tb growth, statistical analysis was performed using two-way ANOVA. The Student's t test was utilized for all other statistical comparisons. All statistical functions were executed using Graph Pad Prism version 4.0 for Windows (GraphPad Software).
RESULTS
M.tb infection induces a significant increase in siderocalin expression in murine macrophages and lung tissue
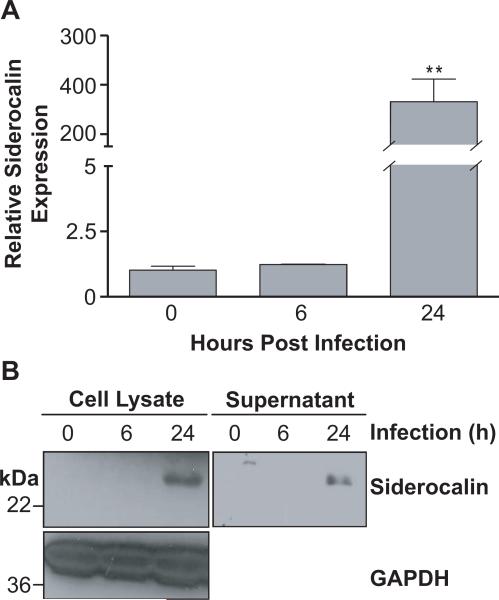
Since it is well known that M.tb targets host macrophages in order to establish infection (Kaufmann, 2004), we investigated the expression of siderocalin in RAW264.7 murine macrophages. Siderocalin was significantly up-regulated at 24 h post-infection, exhibiting approximately 300-fold induction when compared to non-infected controls (Figure 1A). This increase in siderocalin mRNA was accompanied by a corresponding increase in siderocalin protein expression and secretion (Figure 1B). Similar results were obtained with the J774 murine macrophage cell line (data not shown).
FIGURE 1. M.tb infection stimulates siderocalin expression in murine macrophages.
RAW264.7 murine macrophages were infected with M.tb at a MOI of 5:1. (A) Cells were harvested in TRIZOL reagent at the indicated time points. Relative siderocalin expression was determined using qRT-PCR. Gene expression corresponds to the 2−ΔΔCT between siderocalin and a reference gene (GAPDH). (B) Cell lysate and culture media (supernatant) were harvested and subjected to Western blotting with anti-siderocalin monoclonal antibody. GAPDH served as a loading control. For statistical analysis, the means ± SEM from an individual experiment (n=3) are shown. (**) denotes P ≤ 0.01 using the Student's t test. Similar results were obtained in separate experiments.
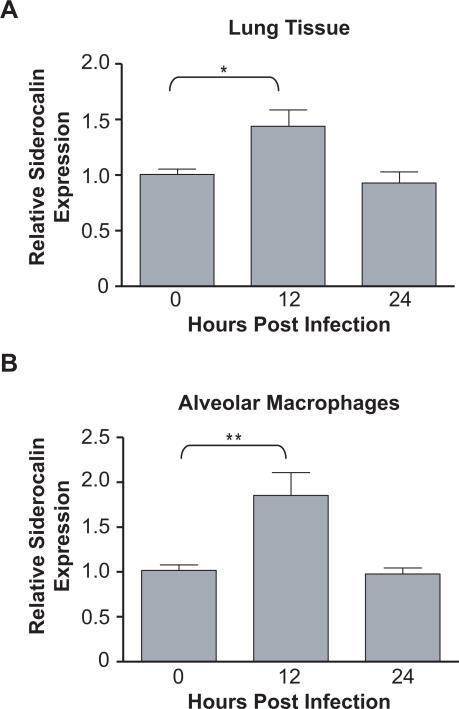
To examine expression of siderocalin in vivo, the lungs of mice infected with virulent M.tb were isolated and subjected to qRT-PCR analysis. Although the level of siderocalin expression in total lung tissue was much less than in the macrophage cell lines, there was a reproducible and statistically significant increase at 12 h post-infection which returned to baseline by 24 h (Figure 2A). We also examined siderocalin mRNA levels in alveolar macrophages from control and infected mice and found a more robust increase than in whole lung, although the time course of expression was similar (Figure 2B).
FIGURE 2. Siderocalin is induced in lung tissue and alveolar macrophages following M.tb infection.
Seven to nine week old female C57BL/6J mice were infected with the Erdmann strain of M.tb via aerosol. (A) Lungs were surgically removed 12 and 24 h post-infection and homogenized in TRIZOL to prepare total RNA. (B) Bronchoalveolar lavage fluid was collected at 12 and 24 h after infection and alveolar macrophages prepared. The cells were homogenized in TRIZOL. Siderocalin expression was determined in total lung and alveolar macrophages using qRT-PCR. Gene expression corresponds to the 2−ΔΔCT between siderocalin and a reference gene (36B4). For statistical analysis, the means ± SEM from a single experiment (n=5) for lung and alveolar macrophages are shown. (*) denotes P ≤ 0.05, (**) denotes P ≤ 0.01 using the Student's t test.
Recombinant siderocalin significantly inhibits M.tb growth within murine macrophages
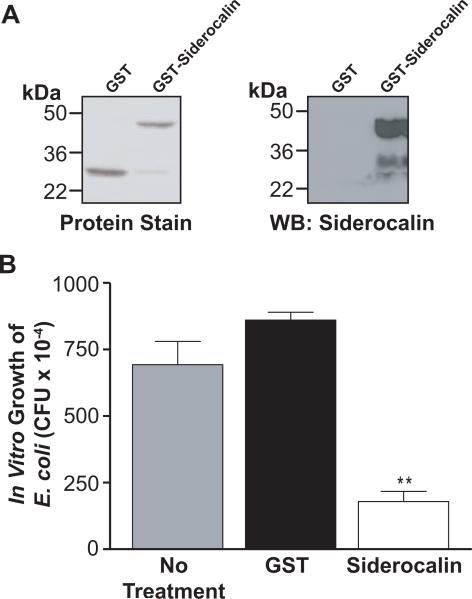
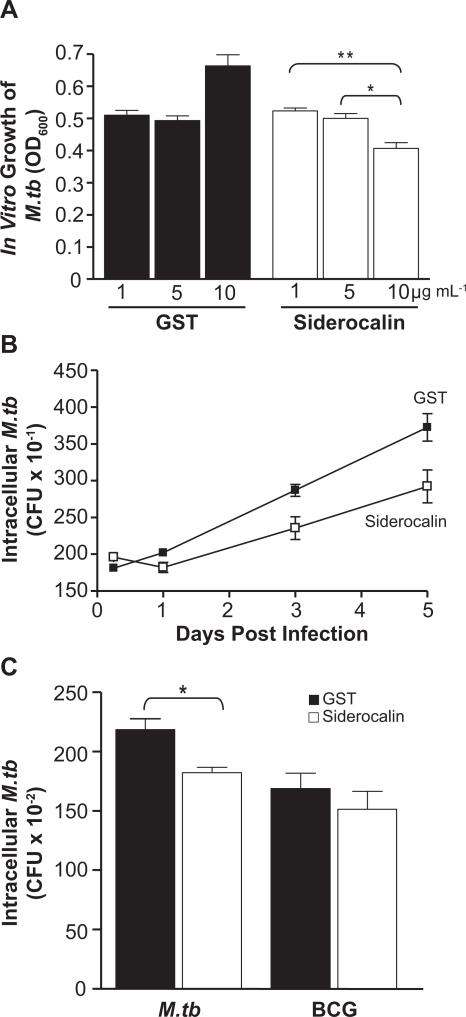
To address the impact of siderocalin on the growth of M.tb within macrophages, we prepared a recombinant GST-siderocalin fusion protein (Figure 3A) and confirmed its ability to inhibit the growth of a siderocalin susceptible strain of E. coli (Figure 3B). When added directly to the bacterial culture, recombinant siderocalin also significantly inhibited growth of virulent H37Rv M.tb in a dose dependent manner (Figure 4A) and at levels consistent with previous studies (Martineau et al., 2007). Since 10 μg mL−1 of siderocalin had the greatest effect in these experiments, this concentration of protein was used to treat RAW264.7 cells infected with M.tb. Figure 4B demonstrates that addition of recombinant siderocalin into the macrophage culture medium resulted in a significant reduction of intracellular M.tb CFU over a 5 day time course, but had no effect on the intracellular growth of BCG (Figure 4C).
FIGURE 3. Purification and efficacy of recombinant siderocalin.
(A) Coomassie staining was used to assess the purity and molecular weight of recombinant protein preparations. The identity of the recombinant siderocalin was determined using Western blotting with a monoclonal anti-siderocalin antibody. (B) E. coli was treated for 6 h with 10 μg mL−1 recombinant GST or GST-siderocalin. Treated cultures were then serially diluted in order to quantify CFU. For statistical analysis, the means ± SEM from a single experiment (n=3) are shown. (**) denotes P ≤ 0.01, using the Student's t test.
FIGURE 4. Recombinant siderocalin inhibits M.tb growth.
(A) 3 × 104 CFU mL−1 of M.tb was grown in 7H9 broth containing the indicated concentrations of GST or GST-siderocalin. Growth was assayed by determining the OD600. For statistical analysis, the means ± SEM from a single experiment (n=3) are shown. (**) denotes P ≤ 0.01, (*) denotes P ≤ 0.05 using the Student's t test. (B) RAW264.7 cells were infected with M.tb at an MOI of 1:1. 10 μg mL−1 GST or GST-siderocalin was added to the culture medium daily over the time course. The replication of intracellular bacteria was assessed by determining CFU. The means ± SEM from one experiment (n = 3) are shown. Statistical analysis using two-way ANOVA determined that the curves were significantly different P < 0.0001. (C) RAW264.7 cells were infected with M.tb or BCG at an MOI of 1:1. 10 μg mL−1 GST or GST-siderocalin was added to the culture medium daily for 3 days. The replication of intracellular bacteria was determined by counting CFU. The means ± SEM from one experiment (n = 3) are shown. (*) denotes P ≤ 0.05 using the Student's t test. For all experiments, similar results were obtained on separate occasions.
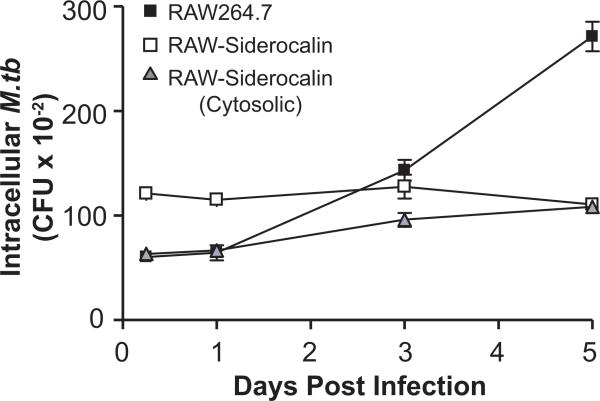
Over-expression of wild-type and a cytosolic form of siderocalin significantly reduces intracellular M.tb proliferation
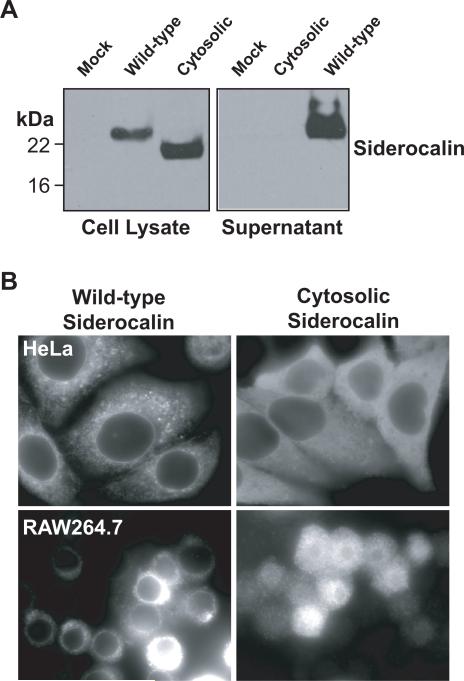
Although both siderocalin and M.tb carboxymycobactin are secreted (Liu and Nilsen-Hamilton, 1995; Holmes et al., 2005), the location in which these molecules interact in the context of intra-macrophage infection is not well understood. In order to investigate this issue, we constructed a variant of siderocalin that lacked the signal sequence required for secretion. When transiently over-expressed in HeLa cells, this variant was present in whole cell lysates, but unlike wild-type siderocalin, it could not be detected in the cell culture supernatant (Figure 5A). Immunofluorescence analysis of transiently transfected HeLa cells and stably transfected RAW264.7 macrophages demonstrated a reticular and punctate distribution of the wild-type siderocalin, consistent with localization to the secretory pathway. On the other hand, the cytosolic variant demonstrated a diffuse staining pattern characteristic of soluble cytoplasmic proteins (Figure 5B). Infection of RAW264.7 cells stably expressing wild-type or cytoplasmic siderocalin demonstrated that both proteins had similar bacteriostatic effects on the intracellular growth of M.tb (Figure 6).
FIGURE 5. Generation of cells over-expressing siderocalin.
(A) HeLa cells were transfected with the indicated siderocalin expression plasmid using Lipofectamine 2000. Expression levels were evaluated by Western blotting with a monoclonal anti-siderocalin antibody. (B) HeLa cells and RAW246.7 cells transfected with the indicated expression plasmid were analyzed by immunofluorescence to determine siderocalin expression.
FIGURE 6. Over-expression of wild-type or cytosolic siderocalin significantly reduces intracellular M.tb growth.
RAW264.7 cells stably expressing wild-type or cytosolic siderocalin were infected with M.tb at an MOI of 1:1. The replication of intracellular bacteria was analyzed by determining CFU at the indicated time points. The means ± SEM from one experiment (n = 3) are shown. Statistical analysis using two-way ANOVA determined that the curves for the over-expressing cell lines were significantly different from the control P < 0.0001. Similar results were obtained on separate occasions.
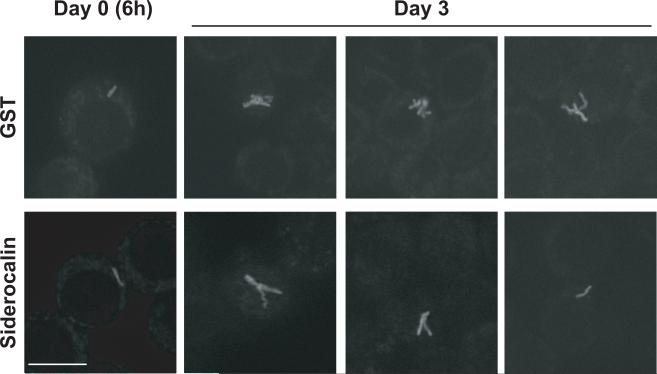
Intracellular M.tb undergoes growth arrest following addition of recombinant siderocalin
In order to determine whether the addition of siderocalin affected intracellular growth or cell-to-cell spread of M.tb, RAW264.7 cells infected with GFP-M.tb and incubated with recombinant siderocalin were subjected to immunofluorescence analysis. Although no difference could be detected in the number of infected cells, there were distinct morphological changes observed in the bacteria present in the cells treated with recombinant siderocalin. Three days post-infection, M.tb from control cells increased from one to numerous small bacilli (Figure 7, panel A). However, M.tb from siderocalin treated cells did not exhibit an obvious increase in the number of bacilli. Instead, only one or two elongated, filamentous bacilli were detected (Figure 7 panel B) a morphologic appearance consistent with bacteria that are growth arrested (Liu and Nilsen-Hamilton, 1995; Hett et al., 2008; Hett and Rubin, 2008).
FIGURE 7. Siderocalin treatment results in intracellular growth arrest of M.tb.
RAW264.7 cells grown on glass coverslips were infected with virulent H37Rv GFP-M.tb and incubated with recombinant GST-siderocalin or GST for 3 days. Cells were fixed using 3.7% paraformaldehyde and M.tb morphology was visualized using immunofluorescence.
DISCUSSION
The observations presented here indicate that siderocalin expression is up-regulated by M.tb in mouse macrophage cell lines after infection in vitro and in alveolar macrophages following aerosol infection of mice in vivo. Moreover, our results demonstrate clearly that siderocalin inhibits M.tb multiplication both extracellularly, as has been shown previously (Martineau et al., 2007), and also when the bacteria are growing in macrophages. Similar findings, based on both in vitro and in vivo experiments, have been reported recently by others (Saiga et al., 2008). However, in that study, the siderocalin-dependent inhibition of intracellular growth of M.tb and BCG was apparent only in alveolar epithelial cells and not alveolar macrophages. The reason for the difference between our findings and those of Saiga et al. with respect to the effects of siderocalin on growth of M.tb in macrophages is not clear. It could relate to the fact that we used a macrophage cell line while they used primary alveolar macrophages. The difference would then suggest that the effects of siderocalin on intracellular bacterial growth are influenced by the characteristics of specific host cell types.
Our data indicate that siderocalin does not simply act on extracellular M.tb during the course of cell-to-cell spread, but actually limits the replication of bacteria inside the macrophages. This result implies that siderocalin must somehow influence iron acquisition by M.tb residing within the macrophage. One possibility is that siderocalin is internalized by receptor-mediated endocytosis into the intracellular compartment in which the bacteria reside. We have looked for evidence in support of this idea, but unlike results obtained by others with alveolar epithelial cells (Saiga et al., 2008), we have not been successful in co-localizing over-expressed or exogenously administered siderocalin with M.tb residing within RAW264.7 macrophages (C. Srikanth and E. Johnson, unpublished data). Another potential explanation is that siderocalin may reduce the concentration of intracellular free iron via interactions with its putative receptor, as has been suggested previously (Devireddy et al., 2005). The mechanism of this effect is not well understood, but it is believed to involve a hypothetical mammalian siderophore that shuttles iron into and out of the cell. Since intracellular iron is utilized by M.tb (Olakanmi et al., 2002; Olakanmi et al., 2006), siderocalin-mediated depletion of this nutrient source offers a plausible explanation for the ability of siderocalin to inhibit the intra-macrophage growth of the pathogen. However, we have not observed any consistent effects of exogenously added siderocalin on intracellular free iron concentrations in RAW264.7 cells based on a calcein fluorescence assay (Wang et al., 2008)(L. Wang, unpublished data). A third possibility is that siderophores produced by intracellular M.tb may traverse the phagosomal or plasma membranes to scavenge iron from both cytoplasmic and extracellular sites, and that extracellular siderocalin may interfere with this process by sequestering the siderophores without necessarily accessing the compartment containing the bacteria or altering intracellular iron stores. This idea is consistent with the relatively hydrophobic character of carboxymycobactin (Holmes et al., 2005; Luo et al., 2005), and is also supported by our finding that a cytosolic form of siderocalin is as effective at inhibiting the intracellular growth of M.tb as the normal, secreted form (Figure 6).
What is the reason for the differential susceptibility of M. tb and BCG to siderocalin observed in our experiments (Figures 4B and 4C)? An analysis of siderophores secreted by various mycobacterial species grown under iron limiting conditions may shed some light on this question. This study demonstrated that BCG produced significantly lower amounts of carboxymycobactin than M.tb and also expressed certain carboxymycobactins with side chain structures not found in M. tb (Gobin et al., 1999). These differences raise the possibility that BCG carboxymycobactin may be relatively resistant to inhibition by siderocalin, particularly since the structure of the carboxymycobactin side chain is known to influence binding to siderocalin (Holmes et al., 2005). It is also possible that M. tb and BCG may reside in intracellular locations that are differentially accessible to siderocalin. It was recently reported that M.tb and Mycobacterium leprae are able to escape endomembranous compartments and enter the cytosol, while BCG remains restricted to the phagosome (van der et al., 2007). Differences in sub-cellular localization between M.tb and BCG may contribute to the differences in their susceptibility to siderocalin. Cytosolic residence of M. tb may also account for our observation that a cytosol-restricted variant of siderocalin was able to inhibit growth of this pathogen (Figure 6).
Competition for iron between host and pathogen is crucial to the outcome of infection (Schaible and Kaufmann, 2004; Doherty, 2007). One of the mechanisms used by the host to deprive pathogens of iron is production of hepcidin, an acute phase response protein that decreases expression of the macrophage and enterocyte iron exporter ferroportin, thereby blocking the release of iron into the circulation (Nicolas et al., 2002; Nemeth et al., 2003; Nemeth et al., 2004). While this mechanism may be effective against extracellular microorganisms, it may actually favor the growth of pathogens such as M.tb that reside within macrophages, since hepcidin-mediated down-regulation of ferroportin would increase intracellular iron levels in these cells. An increase in siderocalin expression, which occurs locally and systemically during the response to infection (Liu and Nilsen-Hamilton, 1995; Cowland et al., 2003; Saiga et al., 2008), may help to counteract the effects of hepcidin by disrupting iron acquisition by intracellular pathogens.
Siderocalin is emerging as a significant factor in implementing the strategy of iron deprivation that is an important component of anti-microbial defense. Our findings, together with observations reported recently by others (Martineau et al., 2007; Saiga et al., 2008), highlight the role of this molecule in protection against M.tb, and raise the possibility that exogenously administered siderocalin may find use as an adjunct to standard chemotherapy in the treatment of tuberculosis.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Michael Fischbach for providing the H9049 bacterial strain, and to Dr. Marianne Wessling-Resnick for encouragement and advice. This work was supported by grants to BJC from the NIH (R21 AI065619) and the Milton Fund of Harvard University. EEJ was supported by an NIH Roadmap Grant to Dr. Marianne Wessling-Resnick and LW by an unrestricted grant from Wyeth Nutrition.
REFERENCES
- 1.Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, Fong HE, Cheung CC, Mak TW. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1834–1839. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bundgaard JR, Sengelov H, Borregaard N, Kjeldsen L. Molecular cloning and expression of a cDNA encoding NGAL: a lipocalin expressed in human neutrophils. Biochem. Biophys. Res. Commun. 1994;202:1468–1475. doi: 10.1006/bbrc.1994.2096. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri A, Orme S, Eilam S, Cherayil BJ. CD40-mediated signals inhibit the binding of TNF receptor-associated factor 2 to the CD40 cytoplasmic domain. J. Immunol. 1997;159:4244–4251. [PubMed] [Google Scholar]
- 4.Cowland JB, Sorensen OE, Sehested M, Borregaard N. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1 beta, but not by TNF-alpha. J. Immunol. 2003;171:6630–6639. doi: 10.4049/jimmunol.171.12.6630. [DOI] [PubMed] [Google Scholar]
- 5.De Voss JJ, Rutter K, Schroeder BG, Su H, Zhu Y, Barry CE., III The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1252–1257. doi: 10.1073/pnas.97.3.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–1305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Doherty CP. Host-pathogen interactions: the role of iron. J. Nutr. 2007;137:1341–1344. doi: 10.1093/jn/137.5.1341. [DOI] [PubMed] [Google Scholar]
- 8.Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, Liu DR, Raymond KN, Wanner BL, Strong RK, Walsh CT, Aderem A, Smith KD. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16502–16507. doi: 10.1073/pnas.0604636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 10.Gobin J, Wong DK, Gibson BW, Horwitz MA. Characterization of exochelins of the Mycobacterium bovis type strain and BCG substrains. Infect. Immun. 1999;67:2035–2039. doi: 10.1128/iai.67.4.2035-2039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 12.Hett EC, Chao MC, Deng LL, Rubin EJ. A mycobacterial enzyme essential for cell division synergizes with resuscitation-promoting factor. PLoS. Pathog. 2008;4:e1000001. doi: 10.1371/journal.ppat.1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hett EC, Rubin EJ. Bacterial growth and cell division: a mycobacterial perspective. Microbiol. Mol. Biol. Rev. 2008;72:126–56. doi: 10.1128/MMBR.00028-07. table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes MA, Paulsene W, Jide X, Ratledge C, Strong RK. Siderocalin (Lcn 2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Structure. 2005;13:29–41. doi: 10.1016/j.str.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann SH. New issues in tuberculosis. Ann. Rheum. Dis. 2004;63(Suppl 2):ii50–ii56. doi: 10.1136/ard.2004.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann SH. Recent findings in immunology give tuberculosis vaccines a new boost. Trends Immunol. 2005;26:660–667. doi: 10.1016/j.it.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann SH, McMichael AJ. Annulling a dangerous liaison: vaccination strategies against AIDS and tuberculosis. Nat. Med. 2005;11:S33–S44. doi: 10.1038/nm1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelley VA, Schorey JS. Mycobacterium's arrest of phagosome maturation in macrophages requires Rab5 activity and accessibility to iron. Mol. Biol. Cell. 2003;14:3366–3377. doi: 10.1091/mbc.E02-12-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 20.Kjeldsen L, Koch C, Arnljots K, Borregaard N. Characterization of two ELISAs for NGAL, a newly described lipocalin in human neutrophils. J. Immunol. Methods. 1996;198:155–164. doi: 10.1016/s0022-1759(96)00153-6. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Nilsen-Hamilton M. Identification of a new acute phase protein. J. Biol. Chem. 1995;270:22565–22570. doi: 10.1074/jbc.270.38.22565. [DOI] [PubMed] [Google Scholar]
- 22.Luo M, Fadeev EA, Groves JT. Mycobactin-mediated iron acquisition within macrophages. Nat. Chem. Biol. 2005;1:149–153. doi: 10.1038/nchembio717. [DOI] [PubMed] [Google Scholar]
- 23.Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. Neutrophil-mediated innate immune resistance to mycobacteria. J. Clin. Invest. 2007;117:1988–1994. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 25.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 26.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J. Clin. Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olakanmi O, Schlesinger LS, Ahmed A, Britigan BE. Intraphagosomal Mycobacterium tuberculosis acquires iron from both extracellular transferrin and intracellular iron pools. Impact of interferon-gamma and hemochromatosis. J. Biol. Chem. 2002;277:49727–49734. doi: 10.1074/jbc.M209768200. [DOI] [PubMed] [Google Scholar]
- 28.Olakanmi O, Schlesinger LS, Britigan BE. Hereditary hemochromatosis results in decreased iron acquisition and growth by Mycobacterium tuberculosis within human macrophages. J. Leukoc. Biol. 2006 doi: 10.1189/jlb.0606405. [DOI] [PubMed] [Google Scholar]
- 29.Raghu B, Sarma GR, Venkatesan P. Effect of iron on the growth and siderophore production of mycobacteria. Biochem. Mol. Biol. Int. 1993;31:341–348. [PubMed] [Google Scholar]
- 30.Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez GM. Control of iron metabolism in Mycobacterium tuberculosis. Trends Microbiol. 2006;14:320–327. doi: 10.1016/j.tim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Saiga H, Nishimura J, Kuwata H, Okuyama M, Matsumoto S, Sato S, Matsumoto M, Akira S, Yoshikai Y, Honda K, Yamamoto M, Takeda K. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J. Immunol. 2008;181:8521–8527. doi: 10.4049/jimmunol.181.12.8521. [DOI] [PubMed] [Google Scholar]
- 33.Schaible UE, Kaufmann SH. Iron and microbial infection. Nat. Rev. Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 34.Serafin-Lopez J, Chacon-Salinas R, Munoz-Cruz S, Enciso-Moreno JA, Estrada-Parra SA, Estrada-Garcia I. The effect of iron on the expression of cytokines in macrophages infected with Mycobacterium tuberculosis. Scand. J. Immunol. 2004;60:329–337. doi: 10.1111/j.0300-9475.2004.01482.x. [DOI] [PubMed] [Google Scholar]
- 35.Teitelbaum R, Cammer M, Maitland ML, Freitag NE, Condeelis J, Bloom BR. Mycobacterial infection of macrophages results in membrane-permeable phagosomes. Proc. Natl. Acad. Sci. U. S. A. 1999;96:15190–15195. doi: 10.1073/pnas.96.26.15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der WN, Hava D, Houben D, Fluitsma D, van ZM, Pierson J, Brenner M, Peters PJ. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Johnson EE, Shi HN, Walker WA, Wessling-Resnick M, Cherayil BJ. Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J. Immunol. 2008;181:2723–2731. doi: 10.4049/jimmunol.181.4.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, Barasch J. An iron delivery pathway mediated by a lipocalin. Mol. Cell. 2002;10:1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008;83:14.1.1–14.1.14. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]