Abstract
Purpose
This study assessed the safety/tolerability, pharmacokinetics, and clinical activity of three-times weekly intravenous 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in combination with once weekly intravenous cisplatin and daily pelvic radiation in patients with gynecologic malignancies. 3-AP is a novel small molecule inhibitor of ribonucleotide reductase (RNR) and is being tested as a potential radiosensitizer and chemosensitizer.
Experimental Design
Patients with stage IB2-IVB cervical cancer (n=10) or recurrent uterine sarcoma (n=1) were assigned to dose-finding cohorts of 2-hour 3-AP infusions during five weeks of cisplatin chemoradiation. Pharmacokinetic and methemoglobin samples and tumor biopsy for RNR activity were obtained on days 1 and 10. Clinical response was assessed.
Results
The maximum tolerated 3-AP dose is 25mg/m2 given three-times weekly during cisplatin and pelvic radiation. Two patients experienced manageable 3-AP-related grade 3 or 4 electrolyte abnormalities. 3-AP pharmacokinetics showed a 2-hour half-life, with median peak plasma concentrations of 277ng/mL (25mg/m2) and 467ng/mL (50mg/m2). Median methemoglobin levels peaked at 1% (25mg/m2) and 6% (50mg/m2) at 4 hours after initiating 3-AP infusions. No change in RNR activity was found on day 1 versus 10 in six early complete responders, while elevated RNR activity was seen on day 10 as compared to day 1 in four late complete responders (P =0.02). Ten (100%) patients with stage IB2-IVB cervical cancer achieved complete clinical response and remain without disease relapse with a median 18 months of follow-up (6-32 months).
Conclusions
3-AP was well tolerated at a three-times weekly intravenous 25mg/m2 dose during cisplatin and pelvic radiation.
Keywords: Triapine, cervical cancer, ribonucleotide reductase, radiosensitization
Introduction
Single- and double-strand DNA breaks resulting from therapeutic ionizing radiation (IR) and replication fork blocks resulting from cisplatin-induced DNA adduct formation must be effectively repaired for cell survival and replication. The rate-limiting step in de novo deoxyribonucleotide triphosphates (dNTPs) synthesis critical for DNA damage repair is catalyzed by ribonucleotide reductase (RNR). RNR has two constitutively expressed homodimeric active-site subunits (RNR-M1), and two tightly-regulated homodimeric small subunits (RNR-R2 or p53R2) which carry diferric irons stabilizing a tyrosyl free radical critical for catalytic function.(1, 2) RNR activity correlates with tumor proliferation rate and repair of IR-induced DNA damage.(3-6) Inhibiting RNR activity is not a new approach, as one of the earliest cervical cancer clinical trials targeted RNR with the chemotherapeutic hydroxyurea. The Gynecologic Oncology Group showed significant improvement in response (68% v. 49%), disease-free (13.6mo v. 7.6mo) and median survival (19.5mo v. 10.7mo) with hydroxyurea-radiation versus radiation treatment.(7) Leukopenia became a dose-limiting toxicity of oral daily hydroxyurea.(8-11)
The investigational chemotherapeutic drug 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine®, NSC#663249) is a 1000-fold more potent irreversible inhibitor of the RNR-R2 and p53R2 subunits of RNR as compared to hydroxyurea.(11-13) Preclinical studies have shown that 3-AP suppresses dNTP generation required for IR-related DNA damage repair, and thereby, enhances IR cytotoxicity.(14-16) Moreover, cervical cancer cells demonstrate a 17-fold increase in RNR-R2 protein and 4-fold rise in RNR activity 18 to 24 hours after IR,(5) suggesting that therapeutic RNR targeting may impact cervical cancer treatment. Our in vitro data shows cervical cancer cells exposed to IR plus 3-AP experience a G1 cell cycle block and increased IR cytotoxicty.(16) Given these findings, RNR inhibition following radiation is an appealing therapeutic strategy.
In phase 1 solid-cancer studies, single agent 3-AP was well tolerated at doses of 96 to 100mg/m2.(17-19) Pharmacokinetic data indicate that 3-AP concentrations peak at 1-10μM at 1-2 hours after a 2-hour intravenous infusion. In another phase 1 solid-cancer study, 2-hour 96mg/m2 intravenous 3-AP (day 1-5) plus 25mg/m2 or 37.5mg/m2 intravenous cisplatin (day 2 or day 3) given every other or every three weeks showed a pooled 33% clinical response rate.(20) Thus, 3-AP also appears to modify cisplatin-mediated cytotoxicity.
This study was designed to (1) assess safety/tolerability of three-times weekly 2-hour intravenous 3-AP infusion during daily pelvic radiation and weekly intravenous cisplatin chemotherapy; (2) assess 2-hour 3-AP infusion pharmacokinetics during radiation and cisplatin treatment; (3) evaluate methemoglobin levels and cancer tissue RNR activity as markers of 3-AP pharmacological inhibition; and (4) assess the clinical activity of radiation and cisplatin plus 3-AP chemotherapy.
Patients and Methods
Eligibility Criteria
Enrolled patients were females age ≥18 years with histologically-confirmed primary or recurrent gynecologic malignancies not amenable to curative surgery. Patients had a Karnofsky performance status of ≥50%; life expectancy of ≥12 weeks; hemoglobin concentration ≥10g/dL, absolute granulocyte count ≥1,500/μL, platelet count ≥100,000/μL, total bilirubin ≤2.0mg/dL, AST(SGOT)/ALT(SGPT) ≤2.5X and PT/aPTT ≤1.5X institutional normal limits, and plasma creatinine ≤2.0mg/dL. Previously treated patients were off therapy for four weeks. Patients with symptomatic cardiac and/or pulmonary disease were excluded.
The study protocol was approved by the National Cancer Institute (NCI) Cancer Therapeutics Evaluation Treatment (CTEP) committee and the institutional review board of University Hospitals of Cleveland Case Medical Center and monitored by the Case Comprehensive Cancer Center Data Safety and Monitoring Board. All patients provided a signed informed consent.
Safety Assessments
Patients underwent examination and hematologic, hepatic, and renal blood testing, and baseline computed tomography (CT) or 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography scans (18F-FDG PET/CT) within 28 days before the first 3-AP infusion. Physical examinations, adverse event assessments (NCI Common Toxicity Criteria version 3.0), and bloodwork were repeated weekly. Post-study examinations and adverse event assessments were required at 1- and 3-months after completing all radiation. Patients were followed every 3-months thereafter. Optional 3-month post-study 18F-FDG PET/CT studies were recommended.
Protocol Treatments
This was a dose-finding phase 1 study of three-times weekly intravenous 3-AP (Triapine®) in combination with once weekly intravenous cisplatin chemotherapy and daily pelvic radiation therapy administered for five weekly cycles. 3-AP was supplied by Vion Pharmaceuticals (New Haven, CT) to NCI-CTEP (Bethesda, MD) in 50 mg viscous liquid vials, and for 2-hour intravenous infusion was diluted in 0.9% sodium chloride to a final concentration of 0.01 to 2mg/mL. A Fibonacci 3+3 cohort trial design was implemented for 2-hour intravenous 3-AP dose escalation levels of 25, 50, 75, and 100mg/m2. A single observed dose limiting toxicity (DLT) event led to an additional three patients treated at the dose level where the DLT event occurred. Dose-finding escalation continued if no additional DLTs were observed. Two observed DLTs stopped dose escalation, with the prior dose level declared the maximum tolerated dose (MTD) as long as 6 patients had been treated with ≤1 instance of DLT.
Cisplatin (40mg/m2) was given intravenously prior to radiation therapy on a once every week for five weeks with an optional week six dosing. Cisplatin and 3-AP were not given on the same day.
Pelvic radiation consisted of parallel-opposed anteroposterior-posterioranterior (AP/PA) and lateral pelvic external-beam treatment fields, delivering 25 fractions of 1.8Gy daily fractions for a total dose of 45.0Gy using 6-MV to 18-MV photons (Table 1). An optional parametrial boost (n=10) of 5 fractions of 1.8Gy daily fractions for a total dose of 9.0Gy was administered using parallel-opposed AP/PA fields. Brachytherapy in patients with cervical cancer (n=10) followed pelvic radiation such that total radiation treatment time was less than 56 days. Intracavitary (n=9) or interstitial (n=1) low-dose rate brachytherapy was allowed. Brachytherapy treatment increased total point A dose to a median 80.0Gy (median 43 hours; median 62cGy/hour). No cisplatin nor 3-AP infusions were given during brachytherapy.
Table 1.
Patient Characteristics by 3-AP Dose Cohort
| Number of Patients |
||||
|---|---|---|---|---|
| Characteristic | 25mg/m2 | 50mg/m2 | ||
| Age, years | ||||
| Median | 60 | 62 | ||
| Range | 34-68 | 54-69 | ||
| Race | ||||
| White | 5 | 4 | ||
| African American | 1 | 1 | ||
| Disease Site | ||||
| Cervix | ||||
| Stage IB2 | 1 | 0 | ||
| Stage IIA | 1 | 2 | ||
| Stage IIB | 0 | 1 | ||
| Stage IIIB | 2 | 1 | ||
| Stage IVA | 1 | 0 | ||
| Stage IVB | 1 | 0 | ||
| Uterus | ||||
| Stage IV | 0 | 1 | ||
3-AP Plasma Pharmacokinetic and Serum Methemoglobin Measurements
Heparinized intravenous blood samples determined 3-AP concentrations on day 1 and day 10 before and at 2, 4, 6, and 24 hours after start of 2-hour infusion. Plasma was centrifuged at 3,000rpm (15min) in a refrigerated centrifuge, and then stored (−80°C). 3-AP concentrations were measured by liquid chromatography tandem mass spectrometry, as previously described.(21) The lower limit of quantification was 20ng/mL. Median peak plasma concentrations (Cmax) and terminal elimination constants for drug half-life were calculated by noncompartmental methods (SPSS 12.0, Chicago, IL).
Heparinized intravenous blood samples drawn into arterial blood gas syringes determined serum methemoglobin concentrations on days 1 and 10 before and at 2, 4, 6, and 24 hours after start of 2-hour infusion. Methemoglobin levels were reported as a percentage of total hemoglobin observed by direct spectrophotometry.(22, 23)
Sequential Tumor Biopsies, Immunohistochemistry, and Ribonucleotide Reductase Assay
Tumor biopsies were obtained before radiation plus 3-AP (day 1) and again on day 10 by transvaginal punch biopsy (~500mg, 0.5cm3), then snap-frozen (<30min) and stored (−80°C). For immunohistochemistry (IHC), the distal 0.5mm biopsy ends were sectioned and stained with hematoxylin and eosin to confirm presence of tumor.(24) Modified IHC assays were performed using RNR-R2 mouse monoclonal (0.5mg/mL, 1:100, Abcam, Inc. [Cambridge, MA]) and RNR-p53R2 rabbit polyclonal (0.2mg/mL, 1:250; Novus Biologicals [Littleton, CO]) antibodies.(25) Adapting previous methods and blinded to treatment and response,(26) two pathologists scored IHC specimens for RNR-R2 and p53R2 protein positivity: negative 0 (<5%), positive 1+ (5% to <25%), positive 2+ (25% to <75%), and positive 3+ (≥75%).
Tumor and stromal intracellular deoxycytidine (dCTP) pools were quantified for RNR activity using a DNA-polymerase extension assay.(16) Tumor biopsies were thawed, homogenized by glass micro-bead pulverization, and intracellular dNTPs extracted by ice-cold 60% methanol. The DNA-polymerase extension assay template was 5′-AAAGAAAGAAAGAAAGAAAGGGCGGTGGAGGCGG-3′ and the primer was 5′-CCGCCTCCACCGCC-3′ (Integrated DNA Technologies, Coralville, IA). A liquid scintillation counter quantified radioactivity, with incorporated H3-dTTP radioactivity linearly proportional to dCTP (nM/mg).
Evaluation of Clinical Activity and Statistical Methods
The study design reflected the desire to detect differential tumor response for translational biology endpoints (IHC and RNR activity, day 1 and 10) before any planned brachytherapy (i.e., after 5 weeks of pelvic radiation and cisplatin plus 3-AP chemotherapy), even though complete study treatment concluded after brachytherapy. Thus, differences in tumor response could be compared among treated patients whom did or did not receive brachytherapy. Early complete responders were defined as having disappearance of all active cancer after 5 weeks of protocol therapy and before any brachytherapy. Late complete responders were defined as having disappearance of all active cancer after all protocol therapy and at the 1-month follow-up assessment. Tumor response was reassessed following all protocol therapy at 1-month by physical examination and at 3-months by physical examination and repeat CT or 18F-FDG PET/CT imaging. Patients were followed every three months. T-tests, analysis of variance (ANOVA), and Wilcoxon rank sum statistics (α = 0.05) were computed (SPSS 12.0, Chicago, IL).
Results
Patient Characteristics
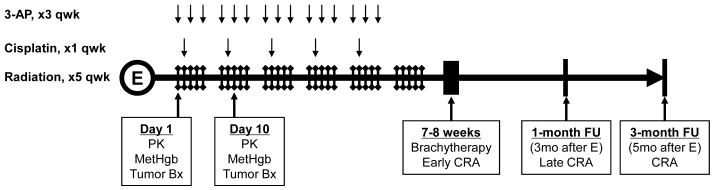
Eleven patients were enrolled between May, 2006 and August, 2008 (Table 1); 10 had cervical cancer. Patients were assigned to dose-finding cohorts of 25mg/m2 and 50mg/m2 3-AP during radiation and cisplatin therapy (Fig. 1). Further dose escalation was not performed because of dose-limiting toxicities. Patients included primarily women with new diagnoses, as only a single patient diagnosed with uterine stromal sarcoma had received previous 3 cycles of gemcitabine-docetaxel chemotherapy. No patients had received prior pelvic radiation.
Figure 1.
Phase 1 clinical trial schema. Abbreviations: E = enrollment, qwk = per week, PK = pharmacokinetic sampling, MetHgb = methemoglobin sampling, Tumor Bx = transvaginal tumor biopsy, FU = after all protocol therapy completion follow-up, CRA = clinical response assessment.
Safety and Tolerability
Eleven patients received 145 intravenous doses of 3-AP (median 15 doses). The 2-hour intravenous 3-AP infusion was well tolerated at the 25mg/m2 and 50mg/m2 doses, with no immediate infusion-related sequelae reported. One patient received nine of 15 50mg/m2 3-AP infusions; 3-AP was stopped for non-DLT leukocytopenia resulting in two delays of cisplatin administration. One patient, who had 9cm abdominopelvic relapse of her uterine stromal sarcoma, received a single 50mg/m2 3-AP infusion and four pelvic radiation doses for abdominopelvic disease prior to symptomatic metastatic pulmonary disease progression.
Eighteen 3-AP drug-related adverse events occurred in four (36%) of 11 patients (Table 2). Most 3-AP drug-related adverse events (14 of 18) were mild to moderate in intensity (i.e., ≤ grade 3, resolving to grade 0-2 within two days). The four dose-limiting toxicities occurred in two patients. One patient at the 50mg/m2 3-AP dose level had grade 3 anorexia requiring hospitalization; her anorexia resolved within four days. The other patient enrolled at the 50mg/m2 3-AP dose level had grade 3 nausea and dehydration requiring hospitalization for intravenous hydration where a grade 3 rise in blood urea nitrogen, a grade 4 lowering of serum bicarbonate, and a grade 4 rise in serum creatinine were observed and attributed to cisplatin administration. Grade 4 serum bicarbonate corrected to grade 2 after two days; grade 3 blood urea nitrogen and grade 4 creatinine corrected to grade 2 after eight days of intravenous hydration in this one diabetic patient.
Table 2.
Number of patients with Drug-related Adverse Events that occurred in ≥ 5% of patients receiving 3-AP*
| n = 11 |
|||||||
|---|---|---|---|---|---|---|---|
| Adverse Event | 25mg/m2 (n = 6) | 50mg/m2 (n = 5) | Total | Grade 3 | Grade 3† | Grade 4† | |
| Cardiovascular | |||||||
| Transient flushing | 0 | 1 | 1 | 1 | 0 | 0 | |
| Electrocardiogram QTc ≥ 0.48 seconds | 0 | 1 | 1 | 1 | 0 | 0 | |
| Pulmonary | |||||||
| Hypoxia | 0 | 1 | 1 | 1 | 0 | 0 | |
| Gastrointestinal | |||||||
| Anorexia | 0 | 1 | 1 | 1 | 1 | 0 | |
| Nausea | 0 | 2 | 2 | 2 | 0 | 0 | |
| Dehydration | 0 | 1 | 1 | 1 | 0 | 0 | |
| Constipation | 1 | 0 | 1 | 1 | 0 | 0 | |
| Abdominal Cramping | 1 | 1 | 2 | 1 | 0 | 0 | |
| Metabolic/Laboratory | |||||||
| Bicarbonate (<11 - 8 mmol/L) | 0 | 1 | 1 | 0 | 0 | 1 | |
| Blood urea nitrogen (severe elevation) | 0 | 1 | 1 | 1 | 1 | 0 | |
| Creatinine (>3.0 - 6.0 x upper limit of normal) | 0 | 2 | 2 | 0 | 0 | 1 | |
| Neurological | |||||||
| Confusion | 0 | 2 | 2 | 1 | 0 | 0 | |
| Sensory neuropathy | 0 | 1 | 1 | 1 | 0 | 0 | |
| Dermatological | |||||||
| Skin decubitus ulcer | 0 | 1 | 1 | 0 | 0 | 0 | |
Adverse events were summarized by the dose to which the patient was initially assigned. Grade 3 adverse events were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (NCI CTCAE).
Grade 3 adverse event not resolving to grade 0-2 over 2 days, considered dose-limiting toxicity. Grade 4 toxicity considered dose-limiting toxicity.
No significant 3-AP drug-related symptomatic dyspnea or methemoglobinemia were reported in the 10 cervical cancer patients. The one uterine sarcoma patient who had pulmonary metastases and prior chemotherapy experienced grade 3 hypoxia with peak methemoglobin level of 11% requiring continuous oxygen supplementation four hours after her first 50mg/m2 3-AP 2-hour infusion. After 24-hour continuous oxygen supplementation, her room-air oxygen saturation normalized and methemoglobin levels lowered to 1%.
Clinical Activity
Ten (91%) of 11 enrolled patients were assessed for tumor response, each with squamous cervical cancer. All 10 (100%) patients achieved a complete clinical response at post-treatment one-month follow-up. Of the 10 complete responders, six (60%) had an early complete clinical response (i.e., no disease detected after 5 weeks of radiation and cisplatin plus 3-AP chemotherapy). These six patients had a median tumor size of 7.5cm (range 4-8cm). The four late complete responders had a 7cm (range 6-8cm) median tumor size, which decreased to a 1cm (range 1-1.5cm) median after 5 weeks of radiation and cisplatin plus 3-AP chemotherapy. One late complete responder had a solitary pulmonary lesion at clinical presentation, achieved complete response of her pelvic cervical cancer following all protocol therapy, and had biopsy-confirmed non-viable pulmonary metastatic disease two months after completing cisplatin plus 3-AP chemotherapy. With a median follow-up of 18 months (range 6-32 months), the 10 evaluable patients have no disease progression. Five of these 10 patients had 18F-FDG PET/CT metabolically-avid pelvic or lower (L4-L5) para-aortic lymphadenopathy before treatment; none have had disease relapse as assessed by repeat CT or 18F-FDG PET/CT imaging.
Tumor tissue ribonucleotide reductase protein levels and activity
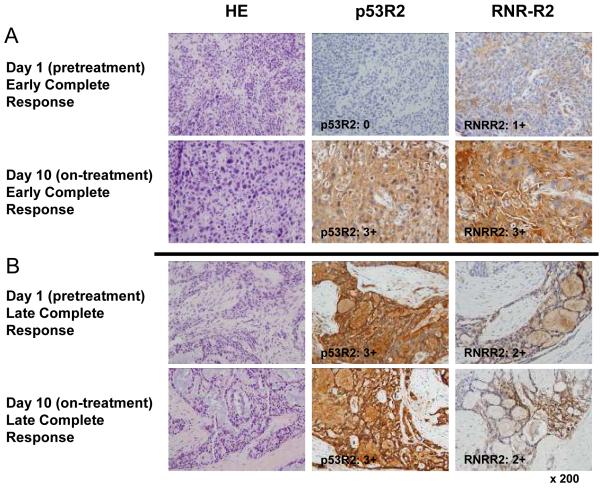
One study objective was to identify biomarkers of the targeted enzyme RNR from day 1 and day 10 tumor biopsies using IHC and biochemical assays that might distinguish responders from nonresponders. Figure 2 shows representative IHC tumor biopsies from day 1 (pretreatment) and day 10 in early and late complete response cervical cancer patients. p53R2 and RMR-R2 protein expression levels by IHC on day 1 and day 10 increased in six early complete responders (Fig. 2A). Four late complete responders exhibited unchanged, elevated day 1 and day 10 p53R2 and RNR-R2 protein levels (Fig. 2B).
Figure 2.
Representative cervical cancer histopathology and ribonucleotide reductase (RNR) R2 and p53R2 protein levels day 1 and day 10 are depicted in (A) a early complete response and (B) an late complete response cervical cancer patient after radiation and cisplatin (40mg/m2) plus 3-AP (25mg/m2). Histopathology (hematoxalin eosin: HE) demonstrates high grade cervical cancer with evident radiation-drug treatment effect comparing biopsy specimens pretreatment (day 1) to on-treatment (day 10) sections. Immunohistochemistry (IHC) staining shows rise in pretreatment day 1 to day 10 RNR-R2 and p53R2 protein levels in an early complete response patient (A), but unchanged elevated RNR-R2 and p53R2 protein levels in a late complete response patient (B).
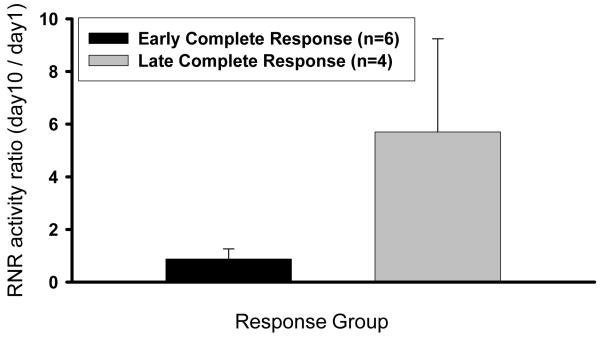
Figure 3 shows RNR activity using a biochemical assay from cervical cancer biopsies expressed as a ratio of day 10 to day 1 and correlated with treatment response. Our recent in vitro data suggests that radiation treatment significantly increases RNR activity, while radiation plus 3-AP treatment significantly reduces RNR activity.(16) Among six early complete responders, radiation and cisplatin plus 3-AP chemotherapy resulted in no substantial RNR activity ratio change day 1 to day 10, ranging between 0.37 and 1.30 (Fig. 3). Among four late complete responders, day 10 RNR activity levels were substantially higher than day 1, with ratios ranging between 2.15 and 9.55 (P =0.02; Fig. 3). On average, the four late complete responders demonstrated a 2.5-fold elevation in dCTP pool levels on day 10. The patient that had the highest RNR activity day10:day1 ratio (i.e., 9.55) had a clinical 8cm tumor shrink to 2cm after five weeks of radiation and cisplatin plus 3-AP chemotherapy; the patient then achieved a late complete response after all protocol therapy including brachytherapy.
Figure 3.
Ribonucleotide reductase (RNR) activity in early and late complete clinical responders after radiation and cisplatin (40mg/m2) plus 3-AP (25 or 50mg/m2) is illustrated. Mean RNR activity (dCTP [nM/mg]), expressed as a ratio of day 10 to day 1 levels, is higher in late complete response as compared to early complete response patients (P =0.02).
Pharmacokinetics and Methemoglobin Levels
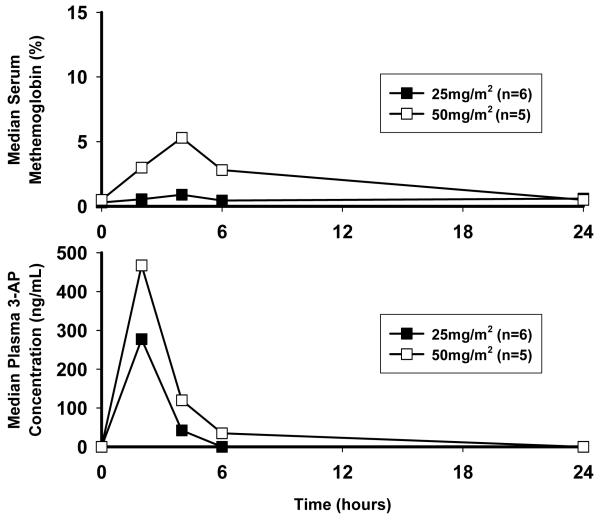
Median (i.e., both day 1 and day 10) 3-AP plasma concentration profiles are shown in Figure 4 (lower panel). Plasma concentrations reached Cmax at the completion of a 2-hour 3-AP infusion, with median peak plasma concentrations of 277ng/mL (25mg/m2) and 467ng/mL (50mg/m2) and a half-life of 2 hours with no change in day 1 and 10 levels (25mg/m2, P =0.18; 50mg/m2, P =0.35). 3-AP plasma concentrations on average decayed to 2% (25mg/m2) and 13% (50mg/m2) of observed Cmax at 6 hours after start of 2-hour infusion.
Figure 4.
Median steady-state plasma concentration profiles for each 3-AP dose group (lower panel) and corresponding median serum methemoglobin proportion (upper panel).
Median (i.e., both day 1 and day 10) methemoglobin percentages are shown in Figure 4 (upper panel). 3-AP is an iron chelator, able to inhibit iron-dependent cytochrome b5 and methemoglobin reductases in red blood cells.(12) Pharmacologic inhibition of red blood cell reductases leads to an accumulation of ferric (III) methemoglobin through naturally-occurring, spontaneous oxidation of red blood cell ferrous (II) hemoglobin.(27) Methemoglobin is incapable of binding oxygen and manifests biologically as tissue hypoxia and clinically as dyspnea. In a prior phase 1 clinical trial, every 4-week infusion of 3-AP (105mg/m2, day 1) plus gemcitabine (600-1000mg/m2, day 1, 8, 15) resulted in dose-limiting, symptomatic methemoglobinemia (>10% MHgb) in 3 (10%) of 29 patients.(28) In this study, median peak methemoglobin was 1% (range 0-2%) for the 25mg/m2 and 6% (range 1-11%) for the 50mg/m2 dosing four hours after start of 2-hour 3-AP infusion (P <0.001) and no difference in day 1 and day 10 (P =1.00).
Discussion
3-AP was well tolerated at a three-times weekly intravenous dosing of 25mg/m2 during daily pelvic radiation and weekly cisplatin treatment. Complete clinical responses were observed in all six patients with advanced stage cervical cancer receiving 25mg/m2 3-AP infusions. With 25mg/m2 3-AP doses, toxicities were minor. Dose-limiting grade 3 gastrointestinal and grade 4 electrolyte changes were restricted to 50mg/m2 3-AP infusions.
Ten patients had advanced stage IB2-IVB cervical cancer, with a median 7.5cm tumor size often leading to parametrial tissue or pelvic wall muscle invasion and nephropathy. Early complete clinical responses were achieved in 6 of 10 patients (60%) after 5 weeks of daily pelvic radiation and cisplatin plus 3-AP chemotherapy and prior to intracavitary brachytherapy. At 1- and 3-months after completing all protocol therapy including brachytherapy, all 10 (100%) cervical cancer patients achieved complete tumor response. With a median 18 months of follow-up (range 6-32 months), there has been no documented local or distant disease relapse. Historically, pelvic radiation plus cisplatin chemotherapy followed by brachytherapy achieve a 70% complete clinical response and 73% 18-month progression-free survival in advanced stage cervical cancer patients.(29-31)
Here, 3-AP pharmacokinetics did not display a time-dependent increase in the 24-hour pharmacokinetic evaluations performed on both day 1 and day 10 and no corresponding symptomatic rise in serum methemoglobin. As such, 3-AP treatment was scheduled three-times weekly to provide repeated drug-induced RNR inhibition, and thereby, prolonged inhibition of on-demand deoxyribonucleotide synthesis during radiation. We observed 3-AP drug concentrations sufficient to achieve tumor responses up to four hours postdose (Fig. 4), and as such, frequent 3-AP dosing appears reasonable for 3-AP mediated radiosensitization. Using experimental cervical cancer cell models, we found that 3-AP treatment significantly enhanced IR-related cytotoxicity through a significant 3-AP induced reduction in RNR activity, sustained IR-induced DNA damage, and a long G1-phase cell cycle arrest perhaps indicating a p53-independent radiosensitizing mechanism.(16)
In this clinical trial, we measured the target enzyme RNR in day 1 and day 10 tumor biopsies using two different assays, an IHC assay of RNR-R2 and RNR p53R2 protein expression levels and a biochemical assay of total RNR activity. Based on this small patient series, an early complete response (six patients) was associated with moderate change in the IHC assay (Fig. 2) and no change in the biochemical assay (Fig. 3) comparing day 1 to day 10 cancer biopsies. In early complete responders in whom low pretreatment RNR-R2 and RNR-p53R2 protein levels were observed, it can be argued that 3-AP treatment effectively reduced a predictably higher RNR activity level in these cervical cancers, based on our prior in vitro data, resulting in no change in the RNR biochemical assay.(5, 6, 16) In late complete responders, where we found no change in the elevated RNR-R2 and RNR-p53R2 protein levels by immunohistochemistry, comparing pretherapy (day 1) to on-therapy (day 10) tumor biopsy sections (Fig. 2) and actually higher RNR activity levels (Fig. 3), one could argue that the overall effect of 3-AP treatment was less than optimal. However, these late complete responders have experienced durable responses with a median follow-up of 18 months similar to early complete responders, suggesting that additional 3-AP mechanisms of radiosensitization and chemosensitization are operative. As discussed in detail in our recent publication, multiple mechanisms of radiosensitization by 3-AP may contribute to the overall tumor cytotoxicity including an enhanced G1/S cell cycle delay and reduced radiation-mediated DNA damage reapir. Enahnced tumor cytotoxicity in these patients could also result from ionizing radiation-cisplatin interactions, independent of 3-AP treatment.
Durable clinical activity was observed with a median 18-month follow-up after administering intravenous 25mg/m2 3-AP doses given three-times weekly during pelvic radiation and cisplatin chemotherapy in advanced stage cervical cancer patients. The favorable adverse event profile of this combination makes this regimen an exciting new cervical cancer treatment for women. A confirmatory phase 2 study of daily pelvic radiation and once weekly cisplatin (40mg/m2) plus three-times weekly 3-AP (25mg/m2) is underway.
Acknowledgments
Grant support: NIH grant K12 CA76917 (C.A. Kunos). Supported in part by NIH grant P30 CA43703 for use of the Radiation Core Facility, Translational Research Core Facility, and Cytometry Core Facility, Case Western Reserve University and the CASE Comprehensive Cancer Center, University Hospitals Case Medical Center. The authors would like to thank Ramon Adams for efforts in data collection and monitoring; Anita Merriam and Song-mao Chiu, PhD for RNR activity assay processing; Adam Kresak, Christina Bagby, DO and Fadi Abdul-Karim, MD for p53R2/RNR-R2 immunohistochemistry staining and analysis; and Charles Hoppel, MD and Steve Ingalls for pharmacokinetic analysis.
SOURCES OF SUPPORT: NIH grant K12 CA76917 (C.A. Kunos). Supported in part by NIH grants UO1 CA62502 and P30 CA43703-17 for Clinical Trials Unit, Pharmacology, and Translational Research Core at CASE Comprehensive Cancer Center, University Hospitals Case Medical Center.
Footnotes
CONFLICT OF INTEREST: There are no potential conflicts of interest among the authors and this manuscript. This manuscript has been seen, read, and agreed upon in its content by all designated authors. This manuscript has not been submitted or published elsewhere.
RELEVANCE: Worldwide, one-half million women are diagnosed annually with cervical cancer, with 90 percent of new cervical cancer cases related to human papillomavirus-silenced p53. Cancer cell replication depends on ribonucleotide reductase, the rate-limiting enzyme catalyzing de novo deoxyribonucleotide production needed for DNA synthesis. After ionizing radiation, ribonucleotide reductase activity increases, facilitating DNA repair and decreasing cancer cell sensitivity to this important cancer treatment. A new intravenous and oral anti-tumor drug, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP), potently inhibits ribonucleotide reductase. In this study, we report the NCI—CTEP sponsored Phase 1 safety and translational science clinical trial of radiation and cisplatin plus 3-AP in patients with locally advanced stage IB2-IVa cervical cancer. In this 10 patient study population at high-risk for relapse and cancer-related death, a 100% complete response rate was observed and no disease progression was documented through 18 months of median follow-up.
References
- 1.Eklund H, Uhlin U, Farnegardh M, Logan DT, Nordlund P. Structure and function of the radical enzyme ribonucleotide reductase. Progress in Biophysics & Molecular Biology. 2001;77:177–268. doi: 10.1016/s0079-6107(01)00014-1. [DOI] [PubMed] [Google Scholar]
- 2.Kolberg M, Strand KR, Graff P, Andersson KK. Structure, function, and mechanism of ribonucleotide reductases. Biochimica et Biophysica Acta. 2004;1699:1–34. doi: 10.1016/j.bbapap.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Elford HL, Freese M, Passamani E, Morris HP. Ribonucleotide reductase and cell proliferation. I. Variations of ribonucleotide reductase activity with tumor growth rate in a series of rat hepatomas. Journal of Biological Chemistry. 1970;245:5228–33. [PubMed] [Google Scholar]
- 4.Takeda E, Weber G. Role of ribonucleotide reductase in expression in the neoplastic program. Life Sci. 1981;28:1007–14. doi: 10.1016/0024-3205(81)90746-3. [DOI] [PubMed] [Google Scholar]
- 5.Kuo M-L, Kinsella T. Expression of ribonucleotide reductase after ionizing radiation in human cervical carcinoma cells. Cancer Res. 1998;58:2245–52. [PubMed] [Google Scholar]
- 6.Kuo M-L, Hwang H-S, Sosnay PR, Kunugi KA, Kinsella TJ. Overexpression of the R2 subunit of ribonucleotide reductase in human nasopharyngeal cancer cells reduces radiosensitivity. Cancer J. 2003;9:277–85. doi: 10.1097/00130404-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Hreshchyshyn MM, Aron BS, Boronow RC, Franklin EW, 3rd, Shingleton HM, Blessing JA. Hydroxyurea or placebo combined with radiation to treat stages IIIB and IV cervical cancer confined to the pelvis. International Journal of Radiation Oncology, Biology, Physics. 1979;5:317–22. doi: 10.1016/0360-3016(79)91209-4. [DOI] [PubMed] [Google Scholar]
- 8.Stehman FB, Bundy BN, Thomas G, et al. Hydroxyurea versus misonidazole with radiation in cervical carcinoma: long-term follow-up of a Gynecologic Oncology Group trial. Journal of Clinical Oncology. 1993;11:1523–8. doi: 10.1200/JCO.1993.11.8.1523. [DOI] [PubMed] [Google Scholar]
- 9.Beitler J, Anderson P, Haynes H, et al. Phase I clinical trial of parenteral hydroxyurea in combination with pelvic and para-aortic external radiation and brachytherapy for patients with advanced squamous cell cancer of the uterine cervix. Int J Radiation Oncology Biol Phys. 2002;52:637–42. doi: 10.1016/s0360-3016(01)02662-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhou B, Hsu N, Pan B, Doroshow J, Yen Y. Overexpression of ribonucleotide reductase in transfected human KB cells increases their resistance to hydroxyurea: M2 but not M1 is sufficient to increase resistance to hydroxyurea in transfected cells. Cancer Res. 1995;55:1328–33. [PubMed] [Google Scholar]
- 11.Finch RA, Liu M, Grill SP, et al. Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone): A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochemical Pharmacology. 2000;59:983–91. doi: 10.1016/s0006-2952(99)00419-0. [DOI] [PubMed] [Google Scholar]
- 12.Chaston TB, Lovejoy DB, Watts RN, Richardson DR. Examination of the antiproliferative activity of iron chelators: multiple cellular targets and the different mechanism of action of triapine compared with desferrioxamine and the potent pyridoxal isonicotinoyl hydrazone analogue 311. Clin Cancer Res. 2003;9:402–14. [PubMed] [Google Scholar]
- 13.Shao J, Zhou B, Zhu L, et al. In vitro characterization of enzymatic properties and inhibition of p53R2 subunit of human ribonucleotide reductase. Cancer Res. 2004;64:1–6. doi: 10.1158/0008-5472.can-03-3048. [DOI] [PubMed] [Google Scholar]
- 14.Guittet O, Hakansson P, Voevodskaya N, et al. Mammalian p53R2 protein forms an active ribonculeotide reductase in vitro with the R1 protein, which is expressed both in resting cells in response to DNA damage and in proliferating cells. J Biol Chem. 2001;276:40647–51. doi: 10.1074/jbc.M106088200. [DOI] [PubMed] [Google Scholar]
- 15.Barker C, Burgan W, Carter D, et al. In vitro and in vivo radiosensitization induced by the ribonucleotide reductase inhibitor Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone) Clin Cancer Res. 2006;12:2912–8. doi: 10.1158/1078-0432.CCR-05-2860. [DOI] [PubMed] [Google Scholar]
- 16.Kunos C, Chiu S, Pink J, Kinsella T. Modulating radiation resistance by inhibiting ribonucleotide reductase in cancers with virally or mutationally silenced p53 protein. Radiation Res. 2009;172:666–76. doi: 10.1667/RR1858.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feun L, Modiano M, Lee K, et al. Phase I and pharmacokinetic study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) using a single intravenous dose schedule. Cancer Chemotherapy & Pharmacology. 2002;50:223–9. doi: 10.1007/s00280-002-0480-0. [DOI] [PubMed] [Google Scholar]
- 18.Murren J, Modiano M, Clairmont C, et al. Phase I and pharmacokinetic study of triapine, a potent ribonucleotide reductase inhibitor, administered daily for five days in patients with advanced solid tumors. Clin Cancer Res. 2003;9:4092–100. [PubMed] [Google Scholar]
- 19.Wadler S, Makower D, Clairmont C, Lambert P, Fehn K, Sznol M. Phase I and pharmacokinetic study of the ribonucleotide reductase inhibitor, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone, administered by 96-hour intravenous continuous infusion. Journal of Clinical Oncology. 2004;22:1553–63. doi: 10.1200/JCO.2004.07.158. [DOI] [PubMed] [Google Scholar]
- 20.Murren J, Modiano M, Plezia P, et al. A phase 1 study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (Triapine) in combination with cisplatin (CDDP) Proc Am Soc Clin Oncol. 2003;22:160a. abstract 643. [Google Scholar]
- 21.Hsieh S, Tobien T, Koch K. Increasing throughput of parallel on-line extraction liquid chromatography/electrospray ionization tandem mass spectrometry system for GLP quantitative bioanalysis in drug development. Rapid Commun Mass Spectrom. 2004;18:285–92. doi: 10.1002/rcm.1327. al. e. [DOI] [PubMed] [Google Scholar]
- 22.Dennis R, Valeri C. Measuring percent oxygen saturation of hemoglobin, percent carboxyhemoglobin and methemoglobin, and concentrations of toal hemoglobin and oxygen in blood of man, dog, and baboon. Clin Chem. 1980;26:1304–8. [PubMed] [Google Scholar]
- 23.Copeland B, Dyer P, Pesce A. Hemoglobin by first derivative spectrophotometry: extent of hemolysis in plasma and serum collected in vacuum container devices. Ann Clin Lab Sci. 1989;19:383–8. [PubMed] [Google Scholar]
- 24.Dowlati A, Haaga J, Remick S, et al. Sequential tumor biopsies in early phase clinical trials of anticancer agents for pharmacodynamic evaluation. Clin Cancer Res. 2001;7:2971–6. [PubMed] [Google Scholar]
- 25.Liu L, Zhou B, Xue L, et al. Metastasis-supressing potential of ribonucleotide reductase small subunit p53R2 in human cancer cells. Clin Cancer Res. 2006;12:6337–44. doi: 10.1158/1078-0432.CCR-06-0799. [DOI] [PubMed] [Google Scholar]
- 26.Xue L, Zhou B, Liu X, et al. Ribonucleotide reductase small subunit p53R2 facillitates p21 induction of G1 arrest under UV irradiation. Cancer Res. 2007;67:16–21. doi: 10.1158/0008-5472.CAN-06-3200. [DOI] [PubMed] [Google Scholar]
- 27.Mansouri A, Lurie AA. Concise review: methemoglobinemia. American Journal of Hematology. 1993;42:7–12. doi: 10.1002/ajh.2830420104. [DOI] [PubMed] [Google Scholar]
- 28.Yen Y, Margolin K, Doroshow J, et al. A phase I trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone in combination with gemcitabine for patients with advanced cancer. Cancer Chemother Pharmacol. 2004;54:331–42. doi: 10.1007/s00280-004-0821-2. [DOI] [PubMed] [Google Scholar]
- 29.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. New England Journal of Medicine. 1999;340:1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 30.Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. Journal of Clinical Oncology. 1999;17:1339–48. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 31.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. New England Journal of Medicine. 1999;340:1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]