Abstract
Background
Right ventricular (RV) long-axis function is known to be depressed after cardiac surgery, but the mechanism is not known. We hypothesized that intraoperative transesophageal echocardiography could pinpoint the time at which this happens to help narrow the range of plausible mechanisms.
Method
Transthoracic echocardiography was conducted in 33 patients before and after elective coronary artery bypass graft. In an intensively monitored cohort of 9 patients, we also monitored RV function intraoperatively using serial pulsed wave tissue Doppler (PW TD) transesophageal echocardiography.
Results
There was no significant difference in myocardial velocities from the onset of the operation up to the beginning of pericardial incision, change in RV PW TD S′ velocities 3% ± 2% (P = not significant).
Within the first 3 minutes of opening the pericardium, RV PW TD S′ velocities had reduced by 43% ± 17% (P < .001). At 5 minutes postpericardial incision, 2 minutes later, the velocities had more than halved, by 54% ± 11% (P < .0001). Velocities thereafter remained depressed throughout the operation, with final intraoperative S′ reduction being 61% ± 11% (P < .0001).
One month after surgery, in the full 33-patient cohort, transthoracic echocardiogram data showed a 55% ± 12% (P < .0001) reduction in RV S′ velocities compared with preoperative values.
Conclusions
Minute-by-minute monitoring during cardiac surgery reveals that, virtually, all the losses in RV systolic velocity occurs within the first 3 minutes after pericardial incision. Right ventricular long-axis reduction during coronary bypass surgery results not from cardiopulmonary bypass but rather from pericardial incision.
Reduction in right ventricular (RV) long-axis velocities after coronary artery bypass graft (CABG) surgery is a widely recognized phenomenon1-3 and evident at 12 months or longer,1 in contrast to the transient mild reduction in left ventricular (LV) function that frequently recovers within 48 hours of surgery.4,5
There are many proposed mechanisms for the reduction in RV long-axis function, but they have been difficult to prove or disprove. One important step in reducing the number of hypotheses would be to identify the precise time at which RV annular velocities fall, because this would automatically exclude proposed injuries that occur afterward and make injuries that occur long before also look less plausible.
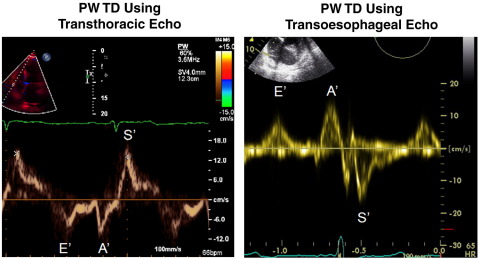
In this study, we use pulsed wave tissue Doppler (PW TD) as a means of assessing RV function.6,7 Right ventricular long-axis function can be easily assessed using TD, which provides a simple, reproducible quantification of systolic and diastolic myocardial velocities, which can be done by transthoracic echocardiography (TTE)8-10 at any time before or after surgery and by transesophageal echocardiography (TEE) during surgery (Figure 1).11,12
Figure 1.
Image on the left shows an example of a PW TD waveform obtained from transthoracic echocardiography. Images on the right show an example of PW TD waveform obtained from TEE.
In this study, we used TEE to assess RV long-axis velocities throughout CABG surgery procedures. In an additional larger group of patients, transthoracic echocardiography was used to assess RV long-axis function pre- and postoperatively, to identify the time point at which RV long-axis velocities decline.
Method
Subjects
Between July 2008 and January 2009, the number of patients who underwent isolated elective CABG surgery at St Mary's Hospital, Imperial College NHS Trust, Paddington, London, was 108. We prospectively recruited 33 patients (23 men; mean, 69 ± 8 years) referred for routine isolated elective first CABG surgery between July 2008 and January 2009. During that time, the number of patients who underwent isolated elective first CABG surgery was 83. Exclusion criteria were the need for concomitant valve surgery, prior history of cardiac surgery, and lone atrial fibrillation. We carried out transthoracic echocardiograms 1 week before and 1 month after surgery. Echocardiographic and TD measurements are shown in Table I.
Table I.
Data obtained using transthoracic echocardiography on 33 patients before and after cardiac bypass surgery
| Characteristics | Preoperative TTE | Postoperative TTE | P |
|---|---|---|---|
| LV EF (%) | 60 ± 12.6 | 53.9 ± 15.2 | .090 |
| LV FS (%) | 27 ± 7.5 | 23.6 ± 8.2 | .08 |
| Transmitral E/A ratio | 0.94 ± 0.6 | 1.02 ± 0.4 | .530 |
| TDI E/E′ ratio | 11.9 ± 4.5 | 14.9 ± 7.5 | .03 |
| Septal PW TDI E′ (cm/s) | 6.2 ± 1.9 | 5.3 ± 1.4 | .02 |
| Septal PW TDI S′ (cm/s) | 6.0 ± 1.4 | 5.23 ± 1.4 | .02 |
| Lateral S′ (cm/s) | 5.49 ± 1.4 | 6.1 ± 2.4 | .10 |
| SAPSE (cm) | 1.2 ± 0.3 | 0.9 ± 0.3 | .0002 |
| Left atrial area (cm2) | 21.2 ± 4.2 | 22.2 ± 5.3 | .31 |
| RV EF (%) | 51 ± 14.7 | 53 ± 17.4 | .63 |
| RV FS (%) | 25.8 ± 6.1 | 23 ± 7.76 | .25 |
| RV end-diastolic volume (mL) | 55 ± 14.9 | 63.3 ± 26.3 | .06 |
| RV area (cm2) | 17.9 ± 3.5 | 18.8 ± 4.2 | .25 |
| TAPSE (cm) | 2.63 ± 0.1 | 1.1 ± 0.2 | .0001 |
| RV Tei index PW TDI | 0.47 ± 0.08 | 0.59 ± 0.09 | .0001 |
| RV PW TDI S′ (cm/s) | 13.7 ± 2.6 | 6.0 ± 1.4 | .0001 |
| RV PW TDI E′ (cm/s) | 9.5 ± 2.4 | 5.2 ± 1.9 | .0001 |
| Est PAP (mm Hg) | 22.4 ± 7.1 | 20.5 ± 6.1 | .33 |
| TR dP/dt (mm Hg/s) | 412 ± 113 | 377 ± 135 | .09 |
| Right atrial area (cm2) | 15.9 ± 3.7 | 18.8 ± 5.1 | .01 |
| RV circumference:length ratio | 3.1 ± 0.2 | 3.2 ± 0.2 | .13 |
| CO (L/min) | 4.8 ± 1.5 | 5.1 ± 1 | .39 |
Characteristics of 33 patients who underwent routine cardiac bypass surgery. These measurements were made by transthoracic echocardiography 1 week before and 1 month after surgery.
SAPSE, Septal annular plane systolic excursion; TAPSE, tricuspid annular plane systolic excursion; Est PAP, estimated pulmonary artery pressure.
Nine of these patients agreed to undergo continuous intraoperative monitoring by TEE. These patients (4 men and 5 women; mean, 66 ± 12 years) underwent recordings of RV PW TD myocardial velocities frequently from the onset of general anesthesia to the time of skin suturing. The timing of each stage of surgery was documented for each patient.
Measurements
Pre- and postoperative transthoracic echocardiography
Each patient was scanned using conventional 2-dimensional (2-D) PW and TD imaging (TDI) techniques 1 week before and 1 month after surgery. Scans were performed in the left lateral decubitus position using an IE33 Philips Medical System (Andover, MA). Parasternal and apical imaging windows were imaged using a S5-1, 3.5-MHz transducer at a mean depth of 16 cm. Ventricular fractional shortening (FS) and left atrial dimensions were calculated from the parasternal imaging windows, whereas ventricular volume estimates, atrial area estimates, annular M-mode excursion, TDI, and PW Doppler velocities were calculated from the apical window.
Left ventricular ejection fraction (EF) was assessed from the major axis using the Teichholz method. Right ventricular dimensions were taken in both minor and major axis positions. Dimension estimates were made in both parasternal long-axis and short axis views. These measures were repeated in the lateral-medial plane, perpendicular to the major axis from a point one third the length of this axis from the tricuspid valve annulus. Right ventricular major axis cavity length, area, and volume measurements were taken and ejection fraction estimates calculated.
Pulsed wave TDI (PW TDI) myocardial velocities and M-mode annular systolic excursion plane estimates were also measured by placing the PW and M-mode sample volume at the level of the basal RV free wall (tricuspid annular systolic plane excursion [TAPSE]) and basal septum (septal annular plane systolic excursion [SAPSE]), where the mean values of 4 consecutive beats of were recorded. The PW TDI sample volume length for each patient was set between 2 and 5 mm to minimize spectral broadening. Recordings were acquired during normal respiration and were used to estimate both RV and LV excursional distances (centimeter) and myocardial velocities (centimeter per second).
Ventricular filling was assessed using both transmitral and tricuspid PW Doppler sampled from the apical window. The PW Doppler sample volume was placed at the level of the mitral and tricuspid leaflet tips using the guidance of spectral color Doppler. All Doppler recordings were acquired during normal respiration and were used to calculate the following variables: (a) E, A, E′, A′, and S′ velocities; (b) E:A and E:E′ ratios; (c) stroke volume; (d) cardiac output; (e) estimated pulmonary artery pressures; and (f) tricuspid regurgitation dP/dt values.
Intraoperative TEE
Intraoperative TEE examinations were carried out in 9 of the 33 patients using a multiplane 5-MHz transesophageal transducer and Vivid I system (GE Healthcare, Waukesha, WI). The probe was placed at a mean depth of 30 cm at a mean angle of 22°, where a mid-esophageal 4-chamber view was obtained.
Pulsed wave TD myocardial velocities estimates were measured with the sample volume 1 cm from the level of the tricuspid annulus during ventricular systole. The mean of 4 consecutive beats was recorded. The sample volume length was set between 2 and 5 mm to minimize spectral broadening. Recording commenced once a working view was located, which permitted the operator to replicate the tricuspid annular velocities obtained during the preoperative transthoracic echocardiogram.
Right ventricular annular velocities were frequently recorded, initially from the onset of general anesthesia up until the end of the operation when the skin was sutured; the mean values of 4 consecutive beats were calculated. Recordings were acquired during normal ventilated respiration.
In patients undergoing off-pump “beating heart” surgery, velocity recordings were made continuously until the point of skin suturing. In patients receiving on-pump coronary bypass, where the heart needed to be stopped using anterograde crystalloid cardioplegia and the patient placed on a heart and lung bypass machine, recordings were taken until bypass cannulae insertion. Recording resumed after full weaning from cardiopulmonary bypass and after protamine administration. All perioperative TD data were recorded by a senior, highly experienced TEE operator.
Statistical analysis
Statistical analysis was performed using Statview 5.0 (SAS Institute Inc, Cary, NC, USA). Continuous data are expressed as mean ± SD. Comparisons between patients before and after surgery were made using paired Student t test. Comparisons between subgroups were made using unpaired t tests. A P value of <.05 was considered significant.
Extramural funding was provided by the British Heart Foundation to support this work.
Results
The transthoracic echocardiographic measurements of all 33 subjects scanned before and after surgery are shown in Table I. All subjects have good ventricular function with no regional wall motion abnormalities and no valve dysfunction other than mild regurgitation.
Intraoperative findings
Overall, 90% of the reduction in RV long-axis velocities occurred within the first few minutes immediately after pericardial incision. There was no significant difference in velocities from the onset of the operation up to the beginning of pericardial incision (change in RV S′ 3% ± 2%, P = not significant [ns]).
Within the first 3 minutes of opening the pericardium, the RV S′ velocities had reduced by 44% ± 17% (P < .0001). Five minutes after pericardial incision, the RV S′ velocities had more than halved by 54% ± 11% (P < .0001). This reduction persisted throughout the operation in all 9 subjects and, at the time where the skin on the chest was sutured, remained 61% ± 11% (P < .0001) below the preoperative value.
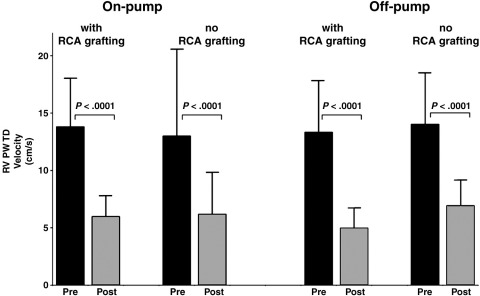
Of the 9 patients, 2 were placed on cardiopulmonary bypass and 5 required right coronary vein grafting. After pericardial opening, no differences were seen in the onset or rate at which RV long-axis velocities reclined regardless of whether surgery was conducted on or off pump and regardless of whether right coronary vein grafting was carried out (Table II).
Table II.
Transthoracic echocardiographic data on the 9 patients in the intensive subgroup versus the other 24 patients in the study
| Characteristics | Subgroup studied intraoperatively (n = 9) | Subgroup not studied intraoperatively (n = 24) | P |
|---|---|---|---|
| Age | 66 ± 12 | 68 ± 8 | |
| Sex | 4 men/5 women | 19 men/5 women | |
| Surgery off pump | 6 | 15 | |
| Surgery on pump | 3 | 9 | |
| LV FS (%) | 26 ± 7 | 28 ± 7 | .52 |
| LV EF (%) | 57 ± 12 | 63 ± 11 | .02 |
| RV FS (%) | 27 ± 6.3 | 30 ± 6.1 | .27 |
| RV EF (%) | 48 ± 17 | 51 ± 14 | .22 |
| TAPSE (cm) | 2.5 ± 0.4 | 2.6 ± 0.5 | .51 |
| RV PW TD S′ (cm/s) | 12.5 ± 2.7 | 14 ± 3.3 | .21 |
| RV PW TD E′ (cm/s) | 9.4 ± 2.2 | 9.6 ± 2.5 | .71 |
| Est PAP (mm Hg) | 21 ± 2 | 22 ± 7 | .66 |
Characteristics of 9 the intensively monitored patients compared with 24 other patients.
TAPSE, Tricuspid annular plane systolic excursion; Est PAP, estimated pulmonary artery pressure.
Pre- and postoperative transthoracic findings: impact of surgery on the RV
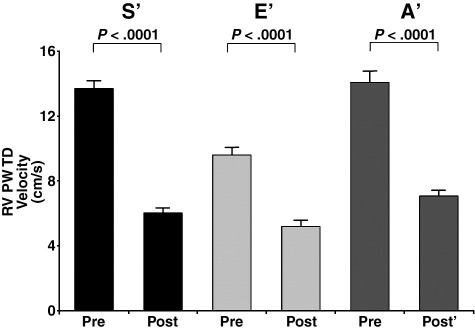
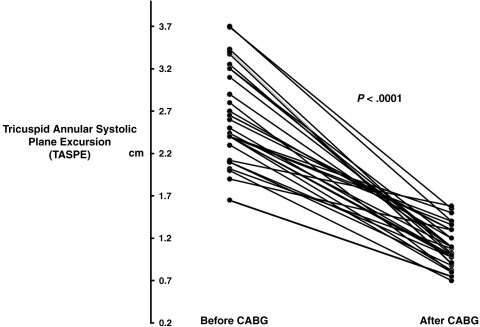
The overall group of 33 patients showed the same pattern of decline in RV velocities, which was seen in the intensively monitored intraoperative cohort, with long-axis S′ velocities falling from 13.7 ± 2.6 to 6.0 ± 1.4 cm/s (by 55% ± 12%, P < .0001). E′ velocities fell from 9.5 ± 2.4 to 5.2 ± 1.9 cm/s (by 45%, P < .0001), and TAPSE estimates fell from 2.6 ± 0.1 to 1.1 ± 0.2 cm/s (by 58%, P < .0001) (Figures 2 and 3).
Figure 2.
Reduction in RV PW TD S′ (black), E′ (light gray), and A′ (dark gray) in 33 patients after routine CABG surgery.
Figure 3.
Changes in RV TAPSE distance in 33 patients before and after routine CABG surgery.
Right atrial area increased by from 15.9 ± 3.7 to 18.8 ± 5.1 cm2 (by 15%, P < .01). However, there was no significant change in RV ejection fraction, FS, end-diastolic area, or pulmonary pressures and tricuspid regurgitation (TR) dP/dt estimates (P = ns).
Impact of right coronary artery grafting
Of the 33 operations, 19 involved saphenous vein grafting of either the main, posterior marginal, or acute marginal segments of the right coronary artery. Of these, the RV S′ velocities fell by 60% from 13.6 ± 2.8 cm/s 1 week before surgery to 5.5 ± 1.2 cm/s 1 month after. Of the patients who did not require right coronary grafts, the velocities also reduced significantly by 48% from 13.1 ± 2.6 cm/s 1 week preoperatively to 6.77 ± 1.4 cm/s 1 month after. In the 19 patients who received grafting to the right coronary, the additional 11% reduction in postoperative RV S′ velocities was significantly greater (P < .05) than seen in the other 14 patients.
On pump versus off pump
There was no difference between the 15 patients who were placed on cardiopulmonary bypass and the 18 who were not. Of those patients placed onto bypass, the RV PW TD S′ velocities fell by 56% from 14.0 ± 2.3 cm/s 1 week before surgery to 6.0 ± 1.6 cm/s 1 month after. Similarly, of the patients who underwent “beating heart” off-pump surgery, the RV PW TD S′ velocities fell by 56% from 13.7 ± 3 cm/s 1 week before surgery to 6.0 ± 1.6 cm/s 1 month after. There were no significant differences between those who had cardiopulmonary bypass and those who had not (P = ns).
Discussion
Intraoperative TEE pinpoints the moment that RV velocities begin to fall
The role of transesophageal PW TD in an intraoperative setting has been well validated and identified 6-9. The unique contribution of this study is the large number of measurements made sequentially while carefully documenting the context of the progress of the operation. Measurements were frequent and encompassed the time at which the RV velocities declined. In all 9 intraoperative patients, the moment at which RV velocities began to decline was almost immediately after the opening of the pericardium. It remains evident weeks after surgery.
Cardioplegia delivery is too late to prevent RV velocity decline
Many mechanisms have been proposed for the precise cause of postoperative RV long-axis dysfunction. Cardioplegia is an obvious suspect and has been the focus of many previous studies trying to identify the cardioplegia fluid,13 temperature, and delivery method14-18 that minimized a decline in RV function.
In our present study, we examined both off-pump procedures (ie, no cardioplegia) and on-pump procedures (anterograde crystalloid cardioplegia). We found that the off-pump surgery affected RV systolic velocities just as much as on-pump surgery (Figure 4). Most tellingly, our study shows that RV velocity decline occurs long before the bypass cannulae are inserted.
Figure 4.
Effect of surgery on RV PW TD velocities, separating patients according to whether the operation was on or off pump and whether they received right coronary artery graft. All 4 groups had similar declines in RV velocity.
Thus, cardioplegia cannot be the cause. Therefore, the choice of cardioplegia solution, its temperature, the method of delivery (antegrade vs retrograde), or even the decision to place the patient onto bypass (on pump vs off pump) is not likely to be able to help preserve RV velocities.
Right coronary artery disease is not a major factor
An alternative, long-held, hypothesis for RV impairment after CABG surgery has been right coronary artery stenosis not considered severe enough to receive a graft. It has been proposed that this could cause insufficient delivery of cardioplegia19 and, therefore, inadequate myocardial hypothermia to the RV, which—lying anteriorly—would also be heavily exposed to a localized increase in atmospheric temperatures.20,23
The fall in RV velocities occurs so early, at pericardial opening, and with such consistency, that many of the commonly proposed mechanisms can immediately be cleared of suspicion.
Similarly, additional interventions that precede pericardial incision such as sternal cutting and opening cannot be blamed for this decline in PW TD velocities. In our study, the sternum was often cut and left spread open, whereas leg veins were harvested. During this time, often 20 minutes or longer, the pericardium remained intact. In this time, RV velocities remained unchanged. It was only after pericardial incision that the rapid reduction in annular excursional velocities occurred (Figures 5-7).
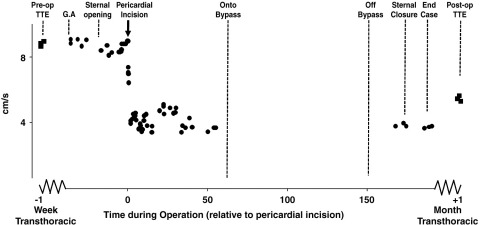
Figure 5.
Intraoperative TEE RV S′ during a routine on-pump CABG to left anterior descending and circumflex arteries. The preoperative TTE PW TD velocities are replicated at the time of general anesthesia and remain unchanged after skin incision and sternal opening. Immediately after pericardial incision, there is an immediate marked reduction in RV TD S′ velocities, which remains until the time of bypass cannulae insertion when measurements had to be suspended temporarily. After myocardial reperfusion, rewarming, and then weaning from cardiopulmonary bypass, data collection is resumed. Right ventricular TD S′ velocities remain depressed at the same level and do not improve during the rest of the operation. The last measurement at the time of skin closure show the same depressed velocities. One month later, the velocity remains equally depressed.
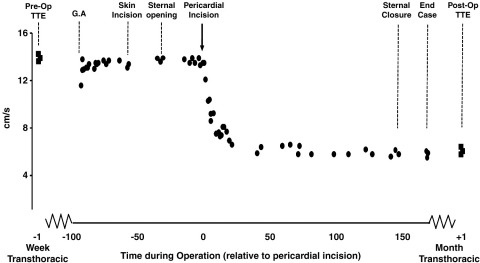
Figure 6.
Intraoperative S′ velocity of the RV free wall during a routine off-pump CABG to left anterior descending and circumflex arteries.
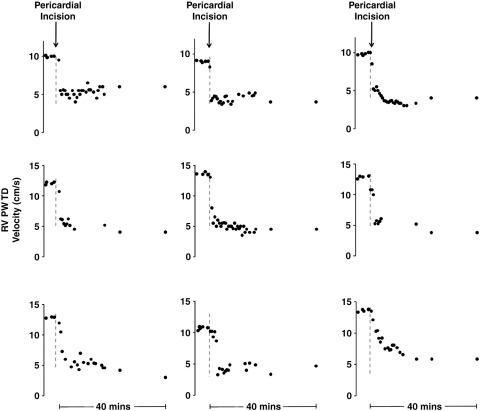
Figure 7.
The time course of RV velocities of all 9 patients in the intensively monitored cohort, zooming in to show the prelude to, and the first 40 minutes after, pericardial opening for each case. The gray dashed line indicates the time when the pericardium was incised. All 9 patients demonstrate an immediate decline in RV S′ myocardial velocities. This reduction occurs mostly within the first 3 minutes after pericardial incision and persists throughout the operation.
The advantage of PW TD in identifying precise time points
Historically, RV function has been difficult to quantify. Invasive measurements have been usually made using either Swan-Ganz thermodilution catheters or visualization of the right side of the heart contraction by catheterization and injection of contrast. These methods are not practical to carry out on a fine-grained temporal basis intraoperatively or in longer-term follow-up. Radionuclide ventriculography21,22 and magnetic resonance imaging23-25 are useful in the assessment of RV function, but they cannot realistically be used intraoperatively. Conventional 2-D echocardiography is of limited use in assessing RV function because of difficulty in defining endocardial surfaces and because of the shape of the right ventricle. Tissue Doppler echocardiography has improved the echocardiographic assessment of RV function using lower gain settings to eliminate the low amplitude signals and omits the high pass filter to detect the low velocity signal from tissue movements. It therefore allows accurate detection of systolic and diastolic motion of specific areas of both the left and RV walls.1,26-34 Decreased velocities are reported to correlate with impaired 2-D ventricular function in heart failure of ischemic and nonischemic etiology, and this agrees well with radionuclide measures26,27,34,35 and is a technique that has been validated to be an accurate and reproducible method of noninvasively measuring RV function.35 Because RV function is dominated by motion in the long-axis, TDI is a good mode of assessment.
The RV is vulnerable during cardiac surgery for many reasons. The RV wall is much thinner than the left. Because, for a given transmural pressure, wall stress is proportional to radius of curvature and inversely proportional to wall thickness, both effects conspire to increase wall stress. During cardiac surgery, loss of the pericardial support and the resultant increased transmural pressure and consequently increased wall stress, which could injure the myocardium or make it susceptible to a change in behaviors when the pericardial constraint is lost. This is especially important in high operative risk patients because the development of RV dysfunction after isolated CABG in patients with LV EF <40% is reported to be associated with higher morbidity and mortality.12,36
Myocardial velocities are not synonymous with myocardial function, and therefore, a reduction in these velocities may not represent a decline in RV function. In particular, it is quite possible that although the peak PW TD velocities are sensitive for assessing RV dysfunction as has been reported, they may not be specific for it, with many other factors perhaps affecting velocities also. Nevertheless, these velocities are frequently measured and their decline at the time of cardiac surgery is often remarked upon and the mechanism sought after. We believe that this study, by isolating the velocity reduction in time, helps prune the range of possible mechanisms, which, in turn, may help others working to understand its meaning for the patient.
Study limitations
This study focused on the PW TD assessment of RV long-axis annular velocities. Right ventricle geometry is complex, and so this is far from comprehensive. However, longitudinal excursion plays the dominant role in RV function7 and can be monitored intraoperatively with exquisite temporal resolution. It is therefore a good choice of variable to monitor both intraoperative and pre-/postlongitudinal function,34,37 providing good sensitivity and reliability.
We carried out postoperative transthoracic echocardiograms 1 month after surgery, unlike previous studies where transthoracic echocardiograms have been conducted 18 months after surgery.8 It is well known that there is a persistent and significant reduction in RV long-axis velocities after cardiac surgery.1,8,11,30 The aim of this study was to identify the precise time point at which RV long-axis velocities began to decline during surgery itself, a question that until now had been unanswered. The decision to select a 1-month follow-up transthoracic echocardiography does not weaken the findings of this study, because the trajectory of RV function from 1 month onward has been well documented. What has been missing until now is the novel information about exactly when during the surgery RV velocities decline: our study shows that in all 9 of 9 intraoperative monitored patients, the decline occurred at the same time, namely, the incision of the pericardium.
Conclusion
This study shows that the reduction in RV long-axis myocardial velocities occurs at the time of pericardial opening during CABG surgery. In our patient cohort, other factors such as cardiopulmonary bypass, inadequate myocardial protection, or even the act of sternal closure are not plausible mechanisms for the immediate fall in RV velocities at the time of pericardial incision, because these factors occur long after this velocity reduction is fully established. However, it remains possible that they and other factors may contribute additionally to the reduction in RV velocities, and in other cohorts of patients, the relative contribution of pericardial incision to the overall fall could be different.
Beat-by-beat intraoperative TEE assessment of RV function reveals that the instantaneous reduction in myocardial annular velocities coincides with the opening of the pericardial sac. The reason for this long-lasting selective reduction remains unclear, but the intense and consistent temporal association with pericardial opening (which removes external support to the RV from the pericardium) suggests that the pericardium contributes much more to the preservation of peak RV myocardial annular velocities than previously supposed. Virtually, all of the RV long-axis reduction seen during coronary surgery occurs at the time of pericardial incision.
Acknowledgements
We thank the cardiac anesthetic and cardiothoracic surgical staff at St Mary's Hospital, Imperial College NHS Trust, Paddington, London. The authors would like to acknowledge the National Institute for Health Research Biomedical Research Centre.
References
- 1.Alam M., Hedman A., Norlander R. Right ventricular function before and after an uncomplicated coronary artery bypass graft as assessed by pulsed wave Doppler tissue imaging of the tricuspid annulus. Am Heart J. 2003;146:520–525. doi: 10.1016/S0002-8703(03)00313-2. [DOI] [PubMed] [Google Scholar]
- 2.Carr-White G.S., Kon M., Koh T.W. Right ventricular function after pulmonary autograft replacement of the aortic valve. Circulation. 1999;100:II–36. doi: 10.1161/01.cir.100.suppl_2.ii-36. [DOI] [PubMed] [Google Scholar]
- 3.Brookes C.I., White P.A., Bishop A.J. Validation of a new intraoperative technique to evaluate load-independent indices of right ventricular performance in patients undergoing cardiac operations. J Thorac Cardiovasc Surg. 1998;116:468–476. doi: 10.1016/S0022-5223(98)70013-3. [DOI] [PubMed] [Google Scholar]
- 4.Kloner R.A. Clinical evidence for stunned myocardium after CABG. J Card Surg. 1994;9(suppl):397–402. doi: 10.1111/jocs.1994.9.3s.397. [DOI] [PubMed] [Google Scholar]
- 5.Roberts A.J. Serial assessment of left ventricular performance following CABG. J Thorac Cardiovasc Surg. 1981;81:69–84. [PubMed] [Google Scholar]
- 6.Meluzin J., Spinarova L., Soucek M. Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion; a new, rapid, and non-invasive method of evaluating right ventricular systolic function. Eur Heart J. 2001;22:340–348. doi: 10.1053/euhj.2000.2296. [DOI] [PubMed] [Google Scholar]
- 7.Hammarstrom E., Wranne B., Popp R.L. Tricuspid annular motion. J Am Soc Echocardiogr. 1991;4:131–139. doi: 10.1016/s0894-7317(14)80524-5. [DOI] [PubMed] [Google Scholar]
- 8.Diller G.P., Wasan B.S., Mayet J. Effect of coronary artery bypass surgery on myocardial function as assessed by tissue Doppler echocardiography. Eur J Cardiothorac Surg. 2008;34:995–998. doi: 10.1016/j.ejcts.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Dokainish H., Sengupta R., Lakkis N. Usefulness of right ventricular tissue Doppler imaging to predict outcome in left ventricular heart failure independent of left ventricular diastolic function. Am J Cardiol. 2007;99:961–965. doi: 10.1016/j.amjcard.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Alam M., Wardell J., Andersson E. Characteristics of mitral and tricuspid annular velocities determined by pulsed was Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr. 1999;12:618–628. doi: 10.1053/je.1999.v12.a99246. [DOI] [PubMed] [Google Scholar]
- 11.JD C.l.a.u.d.e., Tousignant P., Bowry R. Tricuspid annular velocity in patients undergoing cardiac operation using transesophageal echocardiography. J Am Society Cardiol. 2006;19:329–334. doi: 10.1016/j.echo.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Derumeaux G., Ovize M., Loufoua J. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation. 1998;97:1970–1977. doi: 10.1161/01.cir.97.19.1970. [DOI] [PubMed] [Google Scholar]
- 13.Mullen J.C. Right ventricular function: a comparison between blood and crystalloid cardioplegia. Ann Thorac. Surg. 1987;43:17–24. doi: 10.1016/s0003-4975(10)60159-2. [DOI] [PubMed] [Google Scholar]
- 14.Christakis G.T. Randomised study of right ventricular function with intermittent warm or cold cardioplegia. Ann Thorac. Surg. 1996;61:128–134. doi: 10.1016/0003-4975(95)00933-7. [DOI] [PubMed] [Google Scholar]
- 15.Allen B.S., Winkelmann J.W., Hanafy H. Retrograde cardioplegia does not adequately perfuse the right ventricle. J Thorac Cardiovasc Surg. 1995;109:1116–1124. doi: 10.1016/S0022-5223(95)70195-8. [DOI] [PubMed] [Google Scholar]
- 16.Christakis G.T., Fremes S.E., Weisel R.D. Right ventricular dysfunction following cold potassium cardioplegia. J Thorac Cardiovasc Surg. 1985;90:243–250. [PubMed] [Google Scholar]
- 17.Kaukoranta P.K., Lepojarvi M.V., Kivilouma K.T. Myocardial protection during anterograde versus retrograde cardioplegia. Ann Thorac Surg. 1998;66:697–698. doi: 10.1016/s0003-4975(98)00459-7. [DOI] [PubMed] [Google Scholar]
- 18.Kamalot A., Bellows S., Kay G.L. Is warm retrograde blood cardioplegia better than cold for myocardial protection? Ann Thorac Surg. 1997;97:605–612. doi: 10.1016/s0003-4975(96)01074-0. [DOI] [PubMed] [Google Scholar]
- 19.Schirmer U., Calzia E., Georgieff M. Right ventricular function after coronary artery bypass grafting in patients with and without revascularization of the right coronary artery. J Cardiothorac Vasc Anesth. 1995;9:659–664. doi: 10.1016/s1053-0770(05)80226-5. [DOI] [PubMed] [Google Scholar]
- 20.Boldt J., Kling D., Hempelmann G. Myocardial temperature during cardiac operations: influence on right ventricular function. J Thorac Cardiovasc Surg. 1990;100:562–568. [PubMed] [Google Scholar]
- 21.Wilson N.J., Neutze J.M., Ramage M.C. Transthoracic echocardiography for right ventricular function late after the Mustard operation. Am Heart J. 1996;131:360–367. doi: 10.1016/s0002-8703(96)90367-1. [DOI] [PubMed] [Google Scholar]
- 22.Kaul S., Tei C.h., Shah P.M. Assessment of right ventricular function using two dimensional echocardiography. Am Heart J. 1984;107:526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 23.Heling W.A., Bosch H.G., Maliepaard C.h. Comparison of echocardiographic methods with magnetic resonance imaging for assessment of right ventricular function in children. Am J Cardiol. 1995;76:589–594. doi: 10.1016/s0002-9149(99)80161-1. [DOI] [PubMed] [Google Scholar]
- 24.Haber I., Metaxas D.N., Geva T., Axel L. Three-dimensional systolic kinematics of the right ventricle. Am J Physiol Heart Circ Physiol. 2005;289:1826–1833. doi: 10.1152/ajpheart.00442.2005. [DOI] [PubMed] [Google Scholar]
- 25.Haber I., Metaxas D.N., Axel L. Three-dimensional motion reconstruction and analysis of the right ventricle using tagged MRI. Med Image Anal. 2000;4:335–355. doi: 10.1016/s1361-8415(00)00028-1. [DOI] [PubMed] [Google Scholar]
- 26.Bruch C., Schmermund A., Bartel T. Tissue Doppler imaging for on-line detection of regional early diastolic ventricular asynchrony in patients with coronary artery disease. Int J Card Imaging. 1999;15:379–390. doi: 10.1023/a:1006255329288. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Fernandez M.A., Azevedo J., Moreno M. Regional diastolic function in ischaemic heart disease using pulsed wave Doppler tissue imaging. Eur Heart J. 1999;20:496–505. doi: 10.1053/euhj.1998.1278. [DOI] [PubMed] [Google Scholar]
- 28.Nagueh S.F., Middleton K.J., Kopelen H.A. Doppler tissue imaging: a new non-invasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1535. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 29.Galiuto L., Ignone G., DeMaria A.N. Contraction and relaxation velocities of the normal left ventricle using pulsed-wave tissue Doppler echocardiography. Am J Cardiol. 1998;81:609–614. doi: 10.1016/s0002-9149(97)00990-9. [DOI] [PubMed] [Google Scholar]
- 30.Sohn D.W., Chai I.H., Lee D.J. Assessment of mitral annular velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 31.Alam M., Wardell J., Andersson E. Right ventricular function in patients with first inferior myocardial infarction: assessment by tricuspid annular motion and tricuspid annular velocity. Am Heart J. 2000;139:710–715. doi: 10.1016/s0002-8703(00)90053-x. [DOI] [PubMed] [Google Scholar]
- 32.Galderisi M., Severino S., Caso P. The usefulness of pulsed tissue Doppler for the clinical assessment of right ventricular function. Ital Heart J. 2002;3:241–247. [PubMed] [Google Scholar]
- 33.Galderisi M., Severino S., Caso P. Systolic right ventricular function assessment by pulsed wave tissue Doppler imaging of the tricuspid annulus. Swiss Med Wkly. 2005;6:461–468. doi: 10.4414/smw.2005.11043. [DOI] [PubMed] [Google Scholar]
- 34.Meluzin J., Spinarova L., Soucek M. Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion; a new, rapid and non-invasive method of evaluating right ventricular systolic function. Eur Heart J. 2001;22:340–348. doi: 10.1053/euhj.2000.2296. [DOI] [PubMed] [Google Scholar]
- 35.Bleeker G.B., Steendijk P., Bax J.J. Assessing right ventricular function: the role of echocardiography and complementary technologies. Heart. 2006;92(Suppl 1):i19–26. doi: 10.1136/hrt.2005.082503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boldt J., Zickmann B., Hempelmann G. Right ventricular function in patients with reduced left ventricular function undergoing myocardial revascularization. J Cardiothorac Vasc Anesth. 1992;6:24–28. doi: 10.1016/1053-0770(91)90040-Z. [DOI] [PubMed] [Google Scholar]
- 37.Alam M., Wardell J., Andersson E. Characteristics of mitral and tricuspid annular velocities determined by pulsed wave Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr. 1999;12:618–628. doi: 10.1053/je.1999.v12.a99246. [DOI] [PubMed] [Google Scholar]