Abstract
Background:
In human pancreatic adenocarcinoma, nuclear factor-kappa-B (NF-κB) transcription factor is constitutively activated that contributes to the resistance of the tumour cells to induced apoptosis. In our earlier studies, we have shown that brucein D (BD) mediated apoptosis through activation of the p38-mitogen-activated protein kinase (MAPK) signalling pathway in pancreatic cancer cells. This study investigated the function of reactive oxygen species (ROS) in BD-mediated p38-MAPK and NF-κB signalling pathways in PANC-1 cells.
Methods:
Glutathione and dihydroethidium assays were used to measure the antioxidant and superoxide levels, respectively. The protein expression of p22phox, p67phox and p38-MAPK were examined by western blot. The NF-κB activity was evaluated by electrophoretic mobility shift assay.
Results:
Treatment with BD depleted the intracellular glutathione levels in PANC-1 cells. Brucein D triggered the activation of NADPH oxidase isoforms, p22phox and p67phox while enhancing the generation of superoxide. Increases in both intracellular ROS and NADPH oxidase activity were inhibited by an antioxidant, N-acetylcysteine (NAC). Brucein D-mediated activation of p38-MAPK was also inhibited by NAC. However, inhibition of NF-κB activity in BD-treated cells was independent of ROS. In vivo studies showed that BD treatment effectively reduced the rate of xenograft human pancreatic tumour in nude mice with no significant toxicity.
Conclusion:
These data suggest that BD is an apoptogenic agent for pancreatic cancer cells through activation of the redox-sensitive p38-MAPK pathway and inhibition of NF-κB anti-apoptotic activity in pancreatic cancer cells.
Keywords: brucein D, PANC-1 cells, apoptosis, reactive oxygen species, pancreatic cancer
Human pancreatic adenocarcinoma is a highly aggressive malignancy intrinsically resistant to radiotherapy and chemotherapy (Wanebo and Vezeridis, 1996). It is well known that radio- and chemotherapy are to destroy cancer cells through an induction of cellular oxidative stress (Lau et al, 2008a). Oxidative stress is the result of enhanced production of intracellular reactive oxygen species (ROS) and/or impaired function of the cellular antioxidant defence system (Buttke and Sandstrom, 1994). Reactive oxygen species are highly reactive O2 metabolites, including superoxide radicals (O2•−), hydrogen peroxide (H2O2) and hydroxyl radicals (OH•) (Thannickal and Fanburg, 2000); they serve as key secondary messengers in numerous signalling pathways, eliciting a diverse array of biological responses such as transcriptional regulation, differentiation, proliferation and oncogenic transformation (Curtin et al, 2002; Martindale and Holbrook, 2002; Storz, 2006). Cellular ROS are thus essential to cell survival. On the other hand, ROS also have an important function in pro-apoptotic pathways (Curtin et al, 2002; Matsuzawa and Ichijo, 2005). As intracellular redox status is critical in determining the survival and death of cells (Hampton and Orrenius, 1998), many cancer therapeutics target to induce cellular apoptosis by disrupting the redox balance and depleting the intracellular thiol buffer system through extrusion or redistribution of glutathione (GSH) (Ghibelli et al, 1995).
One of the contributing factors to the aggressiveness, unresponsiveness and high mortality rate of pancreatic adenocarcinoma is the resistance of malignant pancreatic cells to apoptosis induced by radio- and chemo-therapeutic therapy (Wanebo and Vezeridis, 1996; Liptay et al, 2003). It has been well established that the transcription factor nuclear factor-kappa-B (NF-κB) mediates a central signalling pathway resulting in protection from apoptotic cell death (Karin and Lin, 2002; Kucharczak et al, 2003). There is mounting evidence to suggest that constitutive activation of anti-apoptotic proteins, such as NF-κB (Aggarwal, 2000), signal transducers and activators of transcription proteins (Battle and Frank, 2002), heat shock proteins (Beere and Green, 2001) and phosphatidylinositide-3-kinase (Nicholson and Anderson, 2002) all have a function in the resistance of pancreatic cancer cells to apoptosis. Nuclear factor-κB is a downstream effector of several growth receptors and is constitutively activated in human pancreatic tumour cells (Wang et al, 1999). Nuclear factor-κB is normally sequestered in the cytosol in its inactive form by inhibitory IκB proteins. Inducible phosphorylation of IκBs by IKKs targets for polyubiquitination and subsequent degradation, leading to activation of NF-κB (Yip-Schneider et al, 2005). The active NF-κB complex is a homo- or heterodimer composed of proteins from the NF-κB/Rel family, including NF-κB1 (p50/105), NF-κB2 (p52/100), RelA (p65), RelB and c-Rel (Verma et al, 1995; Baldwin, 1996). Complex assembly of c-Rel, RelA and RelB with their inhibitors, the IκBs, in the cytoplasm results in inactivity as the interaction masks the nuclear localisation signal of the NF-κB family members (Sen and Baltimore, 1986; Baeuerle and Baltimore, 1988). Several IκB proteins have been identified, such as IκB-α, IκB-β and IκB-ε (Verma et al, 1995; Whiteside and Israel, 1997). On stimulation, IκB is phosphorylated by IκB kinase, leading to its rapid degradation. Consequently, NF-κB proteins are released and translocate into the nucleus in which they transactivate target genes (Baldwin, 1996; Gilmore et al, 1996; Ghosh and Karin, 2002; Li and Verma, 2002).
With an objective to search for effective chemical agents for pancreatic cancer treatment, we have shown earlier that brucein D (BD) possesses potent antiproliferative and apoptogenic effects on human pancreatic cancer cells (Lau et al, 2008b). Moreover, we have recently reported that BD induces apoptosis in pancreatic cancer cells by activating the phosphorylation of p38-mitogen-activated protein kinase (MAPK) (Lau et al, 2009). Although the function of ROS in the activation of p38-MAPK (Yi et al, 2004; Lee et al, 2008; Chen et al, 2009), and the regulation of NF-κB activity (Hayakawa et al, 2003; Fujioka et al, 2004), has been characterised in substances-induced apoptosis in various cancer cells, the involvement of ROS in BD-induced pancreatic cancer cell apoptosis is yet to be elucidated. Given that ROS have an important function in an array of biological responses and diverse signalling pathways, we hypothesised that BD activates pro-apoptotic pathways through the enhancement of ROS production and the inhibition of NF-κB activity in pancreatic cancer cells. To test this hypothesis, we investigated (1) the function of oxidative stress and NF-κB activity in BD-induced apoptosis in a pancreatic cancer cell line, PANC-1; (2) the involvement of ROS in regulating the p38-MAPK and NF-κB signalling pathways in BD-treated PANC-1 cells; (3) the toxicity and anti-pancreatic cancer efficacy of BD in a pancreatic tumour xenograft animal model.
Materials and methods
Cell culture and BD treatment
Unless otherwise specified, the chemicals and reagents used in the project were obtained from Sigma-Aldrich (St Louis, MO, USA). PANC-1, a human pancreatic adenocarcinoma cell line obtained from American Type Culture Collection (Manassas, VA, USA), was grown in DMEM medium supplemented with 10% foetal bovine serum (Invitrogen, Grand Island, NY, USA), 4 mM L-glutamine, 4.5 g l–1 glucose, 1.5 g l–1 sodium bicarbonate, 100 U ml–1 penicillin and 10 U ml–1 streptomycin (Invitrogen) in a 5% CO2, 95% air humidified atmosphere. The procedure of the treatment of PANC-1 cells with BD was similar to that described in our earlier study (Lau et al, 2009).
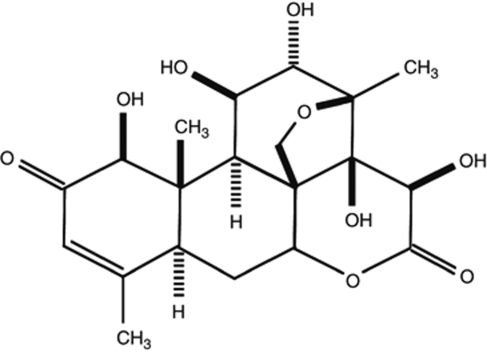
BD used in the present experiments was isolated from the fruit of Brucea javanica L. and its identity was confirmed by comparing its nuclear magnetic resonance and mass spectroscopy profiles with the published data (Yang et al, 1996), whereas its purity was calculated to be over 95% based on HPLC analysis (data not shown). Figure 1 shows the chemical structure of BD. For anti-oxidative experiments, an antioxidant, N-acetylcysteine (NAC; Sigma-Aldrich) was dissolved in phosphate-buffered saline (PBS) and incubated with the cells 1 h before BD treatment.
Figure 1.
Chemical structure of BD, a C-20 quassinoid.
Glutathione assay
Glutathione levels in PANC-1 cells were determined by an earlier described method (Ip et al, 2003; Chan and Leung, 2007). Briefly, PANC-1 cells were harvested and washed with PBS at the end of BD treatment. Trichloroacetic acid (25%) was mixed with PANC-1 cells at a ratio of 1 : 2 to precipitate protein. The mixture was subjected to centrifugation at 7000 g for 4 min at 4°C and the clear supernatant was obtained. The supernatant (200 μl) was diluted with 0.1 M phosphate buffer (1 ml) and GSH assay was started by adding 3 mM 5-5′-dithiobis[2-nitrobenzoic acid] (50 μl). The reaction was incubated at room temperature for 5 min and product measured by absorbance at 412 nm using a microplate spectrophotometer (Fluostar Optima, BMG Labtech, Durham, NC, USA). A standard curve was generated based on known concentration of GSH and was used to quantify the GSH content of each sample. Total GSH content was normalised by the protein concentration of PANC-1 cells.
In situ detection of superoxide
Dihydroethidium (DHE) staining for superoxide was carried out as described earlier (Cheng et al, 2008). In brief, after BD treatment, the cells were immediately fixed with 4% paraformaldehyde (ICN, Irvine, CA, USA) for 30 min. Dihydroethidium (5 μM) was dissolved in dimethyl sulphoxide and DAPI (1 : 1000, Jackson ImmunoResearch Laboratories, Burlingame, CA, USA) added to the slides and incubated at 37°C for 30 min. After washing with PBS, DHE staining was visualised using a confocal microscope (Leica DMLB, Wetzlar, Germany) equipped with a Leica DC 200 digital camera. Fluorescence intensity of DHE-stained PANC-1 cells was quantified using EZ-C1 Viewer 3.6 (Nikon, Tokyo, Japan).
Western blot analysis
The whole-cell lysate of PANC-1 cells was obtained by lysing with CytoBluster protein extraction reagent (Novagen, Darmstadt, Germany), and stored at −20°C. The cytoplasmic and nuclear proteins were extracted using nuclear extraction kit (Imgenex Corporation, San Diego, CA, USA). In brief, the cells were washed with 5 ml ice-cold PBS-PMSF after BD treatment. Buffer was aspirated and cells collected using cell scraper with 5 ml ice-cold PBS-PSMF (Tsang et al, 2004). The cell pellets were obtained by centrifugation for 5 min at 1000 rpm at 4°C. Then the pellets were incubated with 1 ml hypotonic buffer on ice for 5 min, followed by addition of 50 μl 10% detergent solution and vigorous vortexing for 10 s. After centrifugation at 14 000 rpm for 30 s at 4°C, the supernatant containing cytoplasmic protein and pellet containing the nuclear fraction were obtained. The nuclear pellet was resuspended in 100 μl nuclear lysis buffer and vortexed vigorously. The suspension was then incubated at 4°C for 30 min on a rocking platform, followed by centrifugation at 14 000 rpm for 10 min at 4°C and the supernatant containing nuclear protein was obtained. The cytoplasmic and nuclear proteins were stored at −20°C and −80°C, respectively. Protein lysates were then resolved by SDS–PAGE for 1.5 h at 120 V and transferred electrophoretically to PVDF membrane for 1 h at 17 V. Membranes were blocked with 5% (w/v) non-fat dry milk in PBS-T (0.1% v/v Tween-20 in PBS) for 1 h and subsequently incubated overnight at 4°C with primary antibodies at the following concentrations: rabbit anti-p22phox and p67phox (Santa Cruz, Santa Cruz, CA, USA) [1 : 500], rabbit anti-phospho-p38-MAPK (Cell Signaling, Beverly, MA, USA) [1 : 1000], rabbit anti-p38-MAPK (Cell Signaling) [1 : 1000], rabbit anti-IκB-α (Sigma-Aldrich) [1 : 500], rabbit anti-phospho-IκB-α (Cell Signaling) [1 : 1000], rabbit anti-phospho-NF-κB p65 (Cell Signaling) [1 : 1000] and mouse anti-β-actin (Sigma-Aldrich) [1 : 2500]. The membrane was incubated with horseradish peroxidase (HRP)-conjugated donkey anti-rabbit IgG or HRP-conjugated sheep anti-mouse IgG (Amersham Biosciences, Buckinghamshire, UK) for 1 h at room temperature. To verify equal loading of samples, the membranes were subsequently incubated with monoclonal antibody β-actin, followed by an HRP-conjugated sheep anti-mouse IgG. The protein bands were visualised by ECL western blotting detection reagents (Amersham Biosciences). For analysis of p38-MAPK, the cells were pre-incubated overnight in serum-free DMEM medium to remove any serum effects. The density of each band was determined by Leica IM computer software (Wetzlar, Germany).
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) was performed using a commercially available Gel Shift Assay System (Promega, Madison, WI, USA) based on our earlier study (Chan and Leung, 2007). Briefly, the nuclear extract (5 μg) or HeLa cell extract (positive control) was incubated with 32P-labelled oligonucleotide probe (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) in the presence of binding buffer [4% glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM dithiothreitol, 50 mM NaCl, 10 mM Tris–HCl, pH 7.5 and 50 μg ml–1 poly(dI-dC)·poly(dI-dC)] at room temperature for 20 min. After incubation, the DNA–protein complex was resolved by 4% non-denaturing polyacrylamide gel at 220 V. The gel was dried and exposed to X-ray film overnight at −80°C and the band densities were quantified by phosphorimager analysis using FlouroChem8000 (Alpha Innotech, San Leandro, CA, USA).
RNA extraction, reverse transcription and real-time PCR
Gene expression was detected by RT–PCR. The QIAGEN RNeasy Mini Kit (QIAGEN, Valencia, CA, USA) was used to extract total RNA from PANC-1 cells from different treatment groups as described earlier (Leung et al, 1999; Lam and Leung, 2002). First-strand cDNA was generated from 2 μg of total RNA using oligo(dT) primer. β-actin cDNA was amplified as an endogenous control. Real time–PCR was performed using iQ5 Multicolor RT–PCR Detection System (Bio-Rad, Hercules, CA, USA) and the following steps: 95°C for 2 min; 95°C for 30 s, 63°C for 45 s, 95°C for 1 min (40 cycles), and 72°C for 10 min. The results are normalised to β-actin and expressed as relative mRNA expression level according to the 2-CT method (Livak and Schmittgen, 2001). Melting curve analysis was performed to confirm amplification specificity of the PCR products. Primer sequences for amplification of the human genes were as follows: bcl-2 forward, 5′-CGA GTT CAG TGG AGG AGA CC -3′ bcl-2 reverse, 5′- GCC TAA GGA AGG CAG CTA GG -3′ XIAP forward, 5′- CCT TGG GAA CAA CAT GCT AAA -3′ XIAP reverse, 5′- ACC AGA CAC TCC TCA AGT GAA -3′ β-actin forward, 5′-TGT CCA CCT TCC AGC AGA TGT-3′ and β-actin reverse, 5′-CGG ACT CGT CAT ACT CCT GCT T-3′ (Invitrogen, Hong Kong, China).
In vivo evaluation of tumour inhibition
Six-week-old male BALB/c nude mice were supplied by the Laboratory Animal Services Centre of The Chinese University of Hong Kong. The animals were housed under pathogen-free conditions in specifically designed air-controlled rooms with a 12-h light/dark cycle and fed with food and sterile water ad libitum. The care and use of the animals were in compliance with the institutional guidelines, and the experimental procedures were approved by the Animal Experimentation Ethics Committee of the CUHK (Ref. 08/032/MIS).
Xenografts were formed by subcutaneous inoculation of 5 × 106 CAPAN-2 cells (kindly provided by Dr Chaoyang Chen of the Faculty of Medicine, CUHK) in PBS. Treatment was initiated once the tumours reached a mean diameter of 8–10 mm. Mice were then randomised into different treatment groups of four mice each. Brucein D dissolved in 1.68% γ-cylodextrin was administered at various concentrations (0.375, 0.75 and 1.5 mg kg–1) once daily by tail vein injection for 10 consecutive days. The selection of the drug concentrations was based on the results of an earlier pilot study using a range of concentrations, that is 12, 6, 3, 1.5, 0.75 and 0.375 mg kg–1 per day, which showed that the concentrations of 1.5, 0.75 and 0.375 mg kg–1 per day elicited significant beneficial effects on the animal without causing any toxicity. Tumour volume (TV) and body weight were monitored daily and the TV was calculated by using the formula TV=4/3πr3, where r is half of the mean tumour diameter, measured in at least two directions (Sloss et al, 2008).
Plasma-specific enzyme level measurement
At the end of the treatment, the blood of the mice was obtained by cardiac puncture. After centrifugation, plasma was collected and stored at −20°C. Plasma aspartate transaminase (AST) and plasma alanin transminase (ALT) for liver damage, and plasma lactate dehydrogenase (LDH) and plasma creatine kinase (CK) for heart damage were analysed according to the manufacturer's instructions (Biosystems S.A., Barcelona, Spain).
Histological examination
At the end of the treatment, the mice were euthanised by cervical dislocation. Heart, liver and kidney were withdrawn and fixed in ice-cold 4% PFA in PBS at 4°C overnight. The fixed tissues were processed by Shando Pathcentre Tissue Processor (Thermo Scientific, Waltham, MA, USA). After dehydration, the tissues were embedded in molten paraffin (Merck, Germany) at 60°C and cast at room temperature. The paraffin-embedded tissue blocks were sectioned at 5 μm using a biocut rotary microtome machine (Reichert-Jung, Germany) onto gelatin-coated slides. The sections were then de-waxed by xylene and subsequently rehydrated by ethanol gradient (absolute to 70% ethanol, each for 30 s). After several washings, the sections were stained with haematoxylin for 5–8 min to locate the nucleus. Colour of haematoxylin was adjusted by rinsing with 1% acid alcohol and Scott's tap water. Then the sections were stained with 1% eosin for 5 min to visualise the cytoplasm. The slides were subjected to dehydration by ethanol gradient and xylene (three times, each for 30 s), and mounted using microscopic rapid-mounting media (Merck, Germany). Histological changes were examined under a light microscope (DFC 490, Olympus, Japan).
Statistical analysis
Multiple comparisons between data sets were performed using one-way analysis of variance followed by Dunnett's post hoc test. Statistical analyses were conducted using a GraphPad Prism 3.02 software package (GraphPad Software Inc., San Diego, CA, USA).
Results
Effects of BD on cellular glutathione concentration and superoxide production
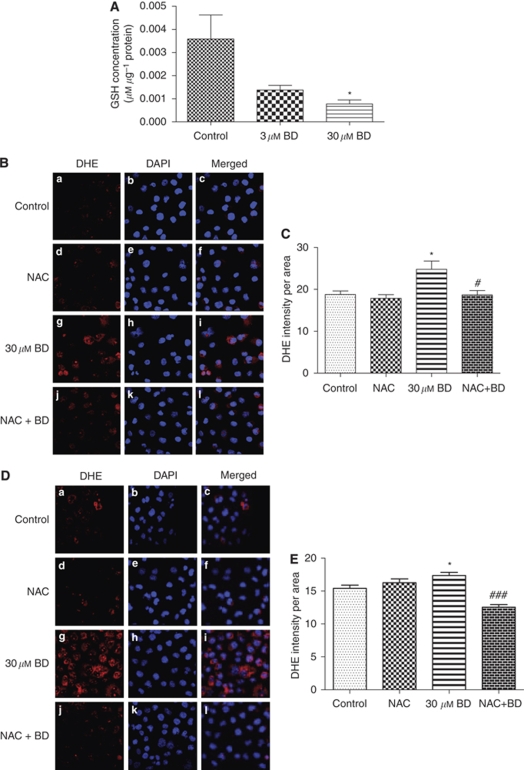
After treatment with 3 and 30 μM BD for 2 h, the GSH levels in PANC-1 cells decreased significantly (Figure 2A). The depletion of GSH levels in BD-treated PANC-1 cells showed a dose-dependent trend, and the GSH concentrations in both 3 and 30 μM BD-treated cells were significantly lower than that of the control cells. Next, the production of superoxide in BD-treated PANC-1 cells was measured by in situ DHE staining. PANC-1 cells treated with 30 μM BD for 1 and 2 h showed enhanced DHE staining in the nucleus of the cells (Figure 2B and D), indicating increased production of superoxide anions. Quantification of the fluorescence intensity revealed that 30 μM BD significantly accentuated the production of superoxide in both treatment time periods in PANC-1 cells (Figure 2C and E). Elevation of superoxide-free radical generation induced by BD was significantly inhibited by pre-incubation of the cells with an antioxidant, NAC (250 μM). Our results indicate that BD is able to induce ROS by depleting GSH and enhancing superoxide generation in PANC-1 cells.
Figure 2.
Effects of BD on redox balance in PANC-1 cells. (A) BD attenuates antioxidant levels in PANC-1 cells. Cells were treated with BD for 2 h and GSH levels were determined. (B, D) Representative diagram showing dihydroethidium (DHE) staining of PANC-1 cells after treatment with vehicle (a–c), 250 μM NAC (d–f), 30 μM BD (g–i) and 250 μM NAC + 30 μM BD (j–l) for 1 or 2 h. Cells were fixed in 4% PFA and then incubated with DHE to detect the superoxide production (red). Nuclei were counterstained with DAPI (blue). Merged images are shown for each treatment (c, f, i and l). (C, E) Effects of BD on superoxide production in PANC-1 cells. Cells were pre-incubated with 250 μM NAC for 1 h and then treated with 30 μM BD for 1 or 2 h. The intensity of DHE staining was measured. *P<0.05 vs vehicle-control group; #P<0.05 and ###P<0.001 vs 30 μM BD-treatment group. The results were obtained from at least three independent experiments. (The colour reproduction of this figure is available on the html full text version of the manuscript.)
Role of NADPH oxidase in BD-treated PANC-1 cells
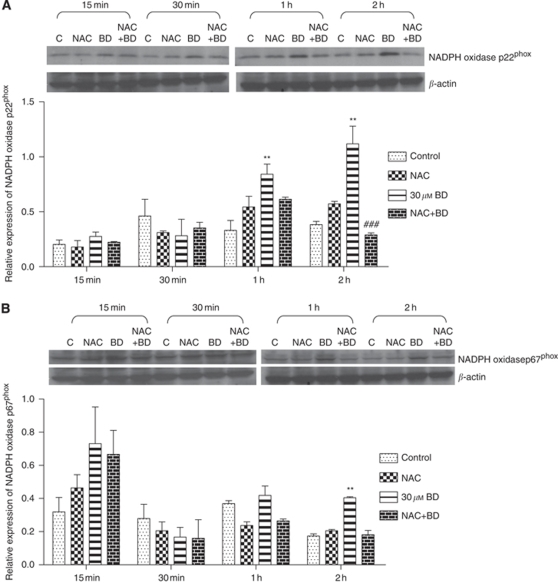
NADPH oxidase activity was measured in BD-treated PANC-1 cells for different incubation periods (15 min to 2 h). Western blot results indicated that treatment with 30 μM BD increased protein expression of both p22 and p67 subunits of NADPH oxidase in PANC-1 cells (Figure 3A and B, respectively). When compared with the control, the expression of p22phox NADPH oxidase increased significantly after treatment with 30 μM BD for 1 and 2 h. However, a significant increase of p67phox NADPH oxidase seemed only after 2 h incubation with BD. Pre-treatment of PANC-1 cells with 250 μM NAC effectively prevented BD-mediated augmentation in the expression of p22phox and p67phox NADPH oxidase.
Figure 3.
Brucein D-treated results in an increase in the expression of NADPH oxidase. Effects of BD on the protein expression of NADPH oxidase p22phox (A) and p67phox (B) in PANC-1 cells in the absence and presence of 250 μM NAC. Representative images and statistical analysis of band intensity are shown in upper and lower panels, respectively. The results were obtained from at least three independent experiments. **P<0.01 vs vehicle-control group; ###P<0.001 vs 30 μM BD-treatment group. The results were obtained from at least three independent experiments.
Effects of BD-induced ROS on p38-MAPK activation
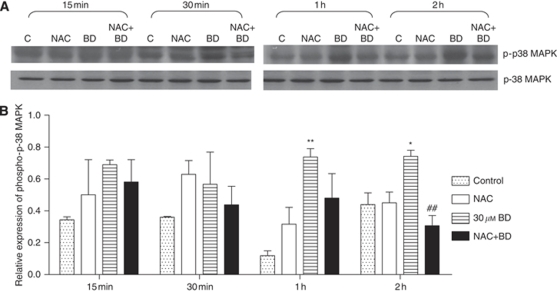
To examine the function of BD-induced ROS in mediating the activation of p38-MAPK in PANC-1 cells, the expression of p38-MAPK protein in each of the treatment groups was analysed. A distinct phosphorylation of p38-MAPK in PANC-1 cells was observed after 30 μM BD treatment for 1 and 2 h (Figure 4). Pre-treatment with 250 μM NAC effectively abolished the phosphorylation of p38-MAPK in BD-treated cells, indicating the requirement of ROS for activation of p38-MAPK.
Figure 4.
Effects of pre-treatment with NAC of the phosphorylation of p38-MAPK after treatment with BD. Cells were pre-treated with 250 μM NAC for 1 h, then incubated with 30 μM BD for 15 min to 2 h. (A) Western bolt analysis of samples using total p38 (p38-MAPK) and phosphorylation-specific p38 (p-p38-MAPK) antibodies. The results were obtained from three independent experiments. (B) Quantitative analysis of western blot band intensities. *P<0.05 vs vehicle control, **P<0.01 vs control and ##P<0.01 vs 30 μM BD treatment.
Involvement of the NF-κB signalling pathway in BD-treated PANC-1 cells
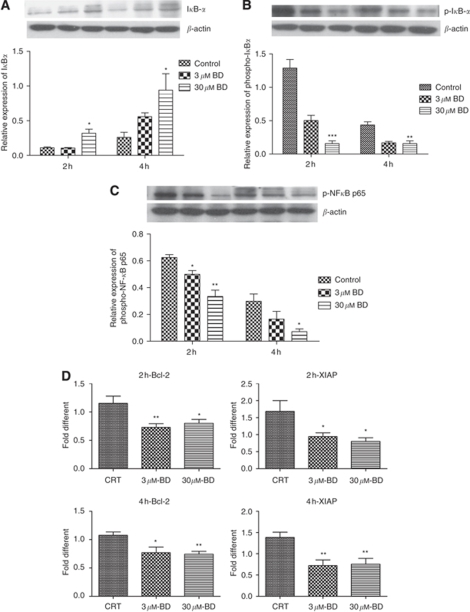
To determine the effects of BD on the NF-κB pathway, PANC-1 cells were treated with BD for 2 or 4 h. The levels of phosphorylated IκB-α in PANC-1 cells significantly decreased with increasing concentrations of BD (Figure 5A). Treatment with 30 μM BD significantly accentuated IκB-α protein levels (Figure 5B), suggesting that BD suppressed the growth of PANC-1 cells by inhibiting NF-κB activation through augmenting IκB protein levels. In addition, phospho-NF-κB p65 protein expression was examined in PANC-1 cell nuclear fractions. The result showed that phospho-NF-κB p65 expression was significantly attenuated in the nucleus after treatment with 3 and 30 μM BD for 2 or 4 h (Figure 5C).
Figure 5.
Effects of BD on the activation of NF-κB. PANC-1 cells were treated with 3 and 30 μM BD for 2–4 h. (A, B) Western blots of cytoplasmic protein extracts (20 μg per lane) using antibodies specific for IκB-α and phospho-IκB-α. (C) Western blots of nuclear protein extracts (20 μg per lane) using a phospho-NF-κB p65 antibody. (D) RT–PCR analysis for the mRNA expression of NF-κB-regulated genes, bcl-2 and XIAP. *P<0.05 and **P<0.01 vs vehicle control. The results were obtained from three independent experiments.
Nuclear factor-κB-regulated genes were also examined to determine whether BD affected their expression. The mRNA expression of anti-apoptotic and NF-κB target genes, bcl-2 and XIAP, was significantly decreased after BD treatment (Figure 5D). These results indicate that inhibition of NF-κB activity by BD-treatment affects the transcription of anti-apoptotic genes, resulting in decreased resistance to apoptosis in PANC-1 cells.
Effects of BD-induced ROS in NF-κB signalling activity
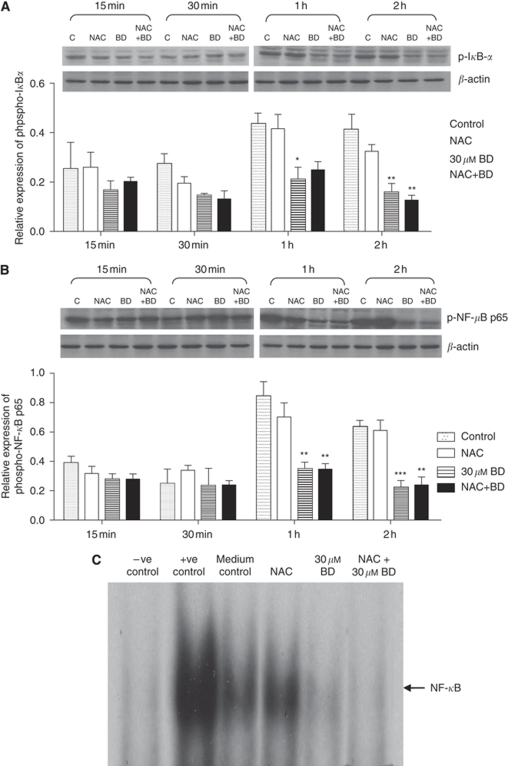
The phosphorylation of IκB-α significantly decreased after BD treatment for 1 or 2 h (Figure 6A). Similarly, NF-κB p65 activity was significantly reduced in the nuclear portion of PANC-1 cells when treated with BD for 1 or 2 h. To evaluate the involvement of BD-induced ROS in NF-κB activation, PANC-1 cells were pre-treated with NAC. Interestingly, no difference was observed in the levels of phospho-IκB-α and phospho-NF-κB p65 between the BD alone treated cells and that pre-treated with NAC (Figure 6A and B). Cells pre-treated with NAC alone (without BD treatment) exhibited no change in the activity of both phosphor-IκB-α and phosphor-NF-κB p65 compared with the vehicle-control group.
Figure 6.
Brucein D-induced inhibition of NF-κB activation is independent of ROS in PANC-1 cells. Cells were pre-treated with 250 μM NAC or vehicle for 1 h, then incubated with 30 μM BD for 15 min to 2 h. Western blot analysis was performed on cytoplasmic lysate using anti-phospho-IκBα antibodies (A) and nuclear lysate using anti-phospho-NF-κB p65 antibodies (B). *P<0.05, **P<0.01 and ***P<0.001 vs vehicle control. The results were obtained from three independent experiments. Quantitative analysis of band intensities is shown in lower panels, respectively. (C) Representative image showing the results from EMSA of nuclear extracts of PANC-1 cells after BD treatment for 2 h. Lane 1: distilled water (negative control); Lane 2: HeLa cell extract (positive control); Lane 3: vehicle control; Lane 4: 250 μM NAC treatment; Lane 5: 30 μM BD treatment; Lane 6: 250 μM NAC + 30 μM BD treatment. Similar results were obtained in three independent experiments.
The ability of BD to inhibit the DNA-binding activity of NF-κB in PANC-1 cells was also examined by EMSA. As shown in Figure 6C, constitutive NF-κB binding was evident in exponentially growing PANC-1 cells when cultured in medium only. Treatment with 30 μM BD for 2 h markedly inhibited NF-κB DNA-binding activity in PANC-1 cells. Electrophoretic mobility shift assay results confirmed that pre-treatment of cells with NAC did not affect the DNA-binding activity in cells treated with either vehicle or BD.
BD inhibits the growth of CAPAN-2 human pancreatic tumour xenografts in vivo
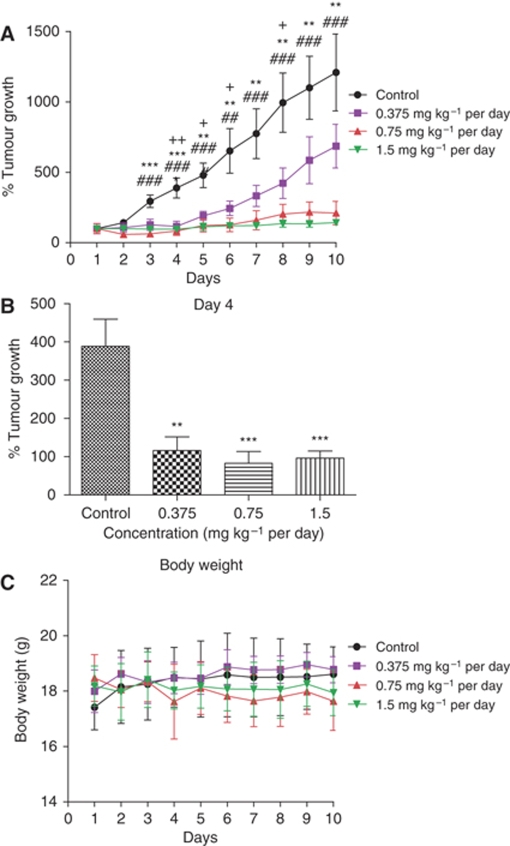
Pancreatic tumours were successfully established in nude mice after transplanting human pancreatic adenocarcinoma CAPAN-2 cells. In Figure 7A, the size of the xenograft tumours formed in the control-treatment mice were markedly larger than that of the 1.5 mg kg–1 per day BD-treated mice. Daily TV measurements revealed that the tumour sizes in BD-treated mice were, in general, smaller than those of the vehicle-control group. These data indicate that BD is able to significantly suppress the tumour growth in a xenograft model in a dose-dependent manner. It should be noted that the tumours grew exponentially in the vehicle-control mice. When exposed to BD at the concentrations of 0.75 and 1.5 mg kg–1 body weight, the rate of tumour growth in the xenograft mice remained at a very low level throughout the entire treatment period. It is worth noting that mice treated with BD started to show significant inhibition of tumour growth on day 4 (Figure 7B).
Figure 7.
In vivo analysis of the anti-tumour effects of BD. CAPAN-2 cells were xenografted by subcutaneous inoculation into nude mice. (A) Mice were treated with BD daily at the indicated concentrations through i.v. administration for 10 consecutive days. The tumour volume (TV) of each mouse was determined using the formula: TV=4/3πr3; n=4, where +P<0.05 and ++P<0.01 comparing 0.375 mg kg–1 per day vs vehicle control; **P<0.01 and ***P<0.001 comparing 0.75 mg kg–1 per day vs vehicle control; ##P<0.01 and ###P<0.001 comparing 1.5 mg kg–1 per day vs vehicle control. (B) Effects of BD on tumour xenograft growth in nude mice after 4 days of treatment at the indicated concentrations. The percentage of tumour growth was calculated after 4 days BD treatment; n=4, **P<0.01 and ***P<0.001 vs vehicle control. (C) The body weight of each mouse was measured for 10 days.
In vivo toxicity test of BD
Mice treated with BD intravenously at dose as high as 1.5 mg kg–1 for 10 days did not show any drug-related side effects. The body weight of mice with BD injection for 10 consecutive days did not show significant differences to that of the γ-cylodextrin-control group (Figure 7C). Plasma was collected at the end of the experiment and the presence of heart and liver-specific enzymes in plasma because of organ damage was assessed. No significant difference was found between γ-cylodextrin and BD-treatment groups regarding the activities of plasma enzymes CK, LDH, ALT and AST (Supplementary Figure 1). Furthermore, histological examination revealed no apparent alterations in the structural organisation of the heart, liver and kidney tissues between the BD-treated and control groups (Supplementary Figure 2), suggesting that BD treatment at 1.5 mg kg–1 for 10 days caused no toxicity to the heart, liver and kidney tissues in the tumour-bearing mice.
Discussion
Our earlier studies identified BD, a quassinoid found abundantly in Chinese medicine Fructus Bruceae javanica, to exert potent antiproliferative activity in cultured human pancreatic adenocarcinoma cells through the induction of apoptosis involving activation of p38-MAPK signalling (Lau et al, 2008b, 2009). In this study, we attempted to further elucidate the underlying mechanism by which BD mediates anti-proliferative action on pancreatic cancer cells. Our data have shown that BD mediates activation ROS-regulated p38-MAPK phosphorylation, whereas inhibiting NF-κB anti-apoptotic activity in pancreatic cancer cells.
The intracellular redox status reflects a precise balance between oxidative stress and the endogenous thiol-buffering system. It has been shown that an imbalance of the intracellular redox state towards oxidative stress triggers downstream cellular events, such as alteration of mitochondrial function and cell signalling pathways leading to apoptotic cell death (Zhang et al, 2004). Our results showed that in pancreatic cancer cells, treatment with BD caused reduction of intracellular GSH in a does-dependent manner. Glutathione is the most abundant bulk antioxidant, which prevents apoptosis and maintains viability of the cells (Slater et al, 1995). PANC-1 cells deprived of GSH on exposure to BD became more vulnerable to oxidative stress when their ability to scavenge and detoxify the various metabolically generated reactive oxygen intermediates was impaired. In addition, BD treatment of PANC-1 cells resulted in an increased generation of superoxide-free radicals. Pre-treatment of PANC-1 cells with NAC, an antioxidant capable of inhibiting apoptosis induced by ROS (Yang et al, 2007), diminished the production of superoxide induced by BD. We further showed that membrane NADPH oxidase activity was present in PANC-1 cells, and was elevated with BD treatment. Non-mitochrondrial NADPH oxidase is a multi-component complex consisting of two membrane proteins, gp91phox and p22phox, and four cytosolic factors, p47phox, p67phox, p40phox and Rac (Babior et al, 2002). The gp91phox and p22phox proteins comprise the catalytic centre of the enzyme flavocytochrome b558. Activation of the enzyme catalyses the conversion of molecular oxygen (O2) to superoxide-free radical (O2•−), which generates oxidative stress (Griendling et al, 2000). Our data showed that PANC-1 cells expressed the key components of NADPH oxidase p22phox and p67phox, and after treatment with BD, protein expression of both p22phox and p67phox were up-regulated. The up-regulation of p22phox and p67phox by BD was effectively abolished by pre-treatment with NAC. These data indicate that NADPH oxidase is a source of ROS on BD treatment.
Considerable evidence suggests that ROS have an important function in key intracellular signal transduction pathways for a variety of cellular process (Aslan and Ozben, 2003; Poli et al, 2004; Chiarugi, 2005). Recently, ROS has been proposed to be involved in tumour metastasis (Radisky et al, 2005; Storz, 2005). One of the important downstream signals involved in tumour invasion is activation of MAPKs, including ERK, p38 and JNK (Berra et al, 1995; Schonwasser et al, 1998). All MAPKs are activated by dual phosphorylation of threonine and tyrosine motifs within the activation loop. Once activated, MAPKs translocate to the nucleus in which they phosphorylate target transcription factors. Our earlier work unequivocally showed that treatment with BD increased phosphorylation of p38-MAPK, and subsequently activated the caspase cascade in PANC-1 cells leading to cellular apoptosis (Lau et al, 2009). Given that BD is able to induce oxidative stress and activate NADPH oxidase, the effect of NAC on p38-MAPK activation in PANC-1 cells was assessed. Immunoblotting analyses showed that pre-treatment of NAC markedly abrogated p38-MAPK activation in BD-treated cells, suggesting that ROS was upstream of p38-MAPK activation.
Nuclear factor-κB has been reported to be activated in various tumours, such as human leukaemia and lymphoma, the lung, breast, colon and pancreatic carcinomas (Rayet and Gelinas, 1999; Karin and Lin, 2002; Wu and Kral, 2005; Braeuer et al, 2006). The p65 subunit of NF-κB is constitutively activated in most human pancreatic cancer tissues and cell lines (Wang et al, 1999), and this activation has been shown to have important functions in tumourigenesis and liver metastasis of pancreatic adenocarcinoma (Fujioka et al, 2003). Therefore, inhibition of NF-κB has become a major strategy in anti-cancer therapy for pancreatic tumours.
According to our data, BD treatment inhibited phosphorylation and degradation of the inhibitory subunit, IκB-α, thereby sequestering NF-κB and preventing nuclear translocation and DNA binding. The important function of NF-κB in protecting cells against diverse apoptotic stimuli including chemo- and radiotherapy is well known. Anti-apoptotic NF-κB-regulated genes include genes that encode bcl-2-like proteins and IAP proteins (IAP-1, IAP-2, XIAP) (Barkett and Gilmore, 1999). Increased activation of p65 in PANC-1 cells was abolished by BD treatment, leading to a decrease in the transcription of bcl-2 and XIAP.
It has been proposed that ROS are involved in the activation of NF-κB. Certain types of compounds with antioxidant properties block NF-κB activation and most agents that activate NF-κB are known to trigger ROS formation. For instance, NF-κB activation can be induced by treatment with H2O2 in certain cell lines. However, the contribution of redox regulation in NF-κB activation is a topic for intense debate (Brennan and O'Neill, 1995; Korn et al, 2001). In addition, several research groups have reported that H2O2-induced NF-κB activation is highly cell type dependent (Anderson et al, 1994; Bowie et al, 1997; Li and Karin, 1999). Thus, we initially hypothesised that ROS induced by BD treatment serve as secondary messengers mediating NF-κB activity. Surprisingly, pre-treatment of the most widely used antioxidant, NAC, did not show any regulatory effect on NF-κB in PANC-1 cells with or without BD treatment. Hayakawa et al (2003) showed that endogenous ROS produced through Rac/NADPH oxidase do not mediate NF-κB signalling. It is thus plausible that the inhibition of NF-κB activity was independent of the ROS induced by BD treatment.
In drug development, it is essential to evaluate the toxicity and potency of drug candidates in vivo. In our current project, we evaluated the anti-tumour efficacy of BD in a nude mice model xenografted with CAPAN-2 human pancreatic cancer cells. We found that BD was able to inhibit the tumour growth without causing mortality, significant weight loss or other noticeable side effects on the heart, liver and kidney tissues (Supplementary Figures 1 and 2). These observations are in agreement with our in vitro studies showing that treatment of pancreatic cancer cells with BD resulted in a concentration-dependent induction of apoptosis. The potent anti-tumour activity of BD together with the absence of toxicity renders this plant-derived quassinoid a promising drug candidate in pancreatic cancer chemotherapy. It seems that further in-depth pre-clinical animal studies and, ultimately, clinical trials on BD is warranted for development of this chemical into anti-cancer pharmaceutical.
In conclusion, this study is the first report to delineate the mechanistic pathways associated with BD-mediated apoptosis in pancreatic cancer cells. Brucein D depleted the intracellular GSH levels, favouring the onset of apoptosis by passively allowing oxidative stress to build up. Oxidative stress generated by NADPH oxidase activation leads to p38-MAPK activation, causing apoptosis in pancreatic cancer cells. In addition, BD treatment inhibits anti-apoptotic gene expression by blocking NF-κB activation, which may increase cell susceptibility to cytotoxic agents. Taking together the in vitro and the preliminary, but promising, in vivo data, we believe that BD has good potential for further development into a clinical treatment for pancreatic cancer.
Acknowledgments
This work was supported by a Direct Grant from The Chinese University of Hong Kong (Project 2030326, awarded to ZXL and PSL), by a General Research Fund from the Research Grants Council of Hong Kong (Project CUHK470709) and by the Focused Investment Scheme (The Chinese University of Hong Kong, awarded to PSL).
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Conflict of interest
The authors declare no conflict of interest.
Supplementary Material
References
- Aggarwal BB (2000) Apoptosis and nuclear factor-kappa B: a tale of association and dissociation. Biochem Pharmacol 60: 1033–1039 [DOI] [PubMed] [Google Scholar]
- Anderson MT, Staal FJ, Gitler C, Herzenberg LA, Herzenberg LA (1994) Separation of oxidant-initiated and redox-regulated steps in the NF-kappa B signal transduction pathway. Proc Natl Acad Sci USA 91: 11527–11531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan M, Ozben T (2003) Oxidants in receptor tyrosine kinase signal transduction pathways. Antioxid Redox Signal 5: 781–788 [DOI] [PubMed] [Google Scholar]
- Babior BM, Lambeth JD, Nauseef W (2002) The neutrophil NADPH oxidase. Arch Biochem Biophys 397: 342–344 [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D (1988) I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science 242: 540–546 [DOI] [PubMed] [Google Scholar]
- Baldwin Jr AS (1996) The NF-kappa B and I kappa B proteins: new discoveries and insights. Ann Rev Immunol 14: 649–683 [DOI] [PubMed] [Google Scholar]
- Barkett M, Gilmore TD (1999) Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene 18: 6910–6924 [DOI] [PubMed] [Google Scholar]
- Battle TE, Frank DA (2002) The role of STATs in apoptosis. Curr Mol Med 2: 381–392 [DOI] [PubMed] [Google Scholar]
- Beere HM, Green DR (2001) Stress management – heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol 11: 6–10 [DOI] [PubMed] [Google Scholar]
- Berra E, Az-Meco MT, Lozano J, Frutos S, Municio MM, Sanchez P, Sanz L, Moscat J (1995) Evidence for a role of MEK and MAPK during signal transduction by protein kinase C zeta. EMBO J 14: 6157–6163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie AG, Moynagh PN, O'Neill LA (1997) Lipid peroxidation is involved in the activation of NF-kappaB by tumor necrosis factor but not interleukin-1 in the human endothelial cell line ECV304. Lack of involvement of H2O2 in NF-kappaB activation by either cytokine in both primary and transformed endothelial cells. J Biol Chem 272: 25941–25950 [DOI] [PubMed] [Google Scholar]
- Braeuer SJ, Buneker C, Mohr A, Zwacka RM (2006) Constitutively activated nuclear factor-kappaB, but not induced NF-kappaB, leads to TRAIL resistance by up-regulation of X-linked inhibitor of apoptosis protein in human cancer cells. Mol Cancer Res 4: 715–728 [DOI] [PubMed] [Google Scholar]
- Brennan P, O'Neill LA (1995) Effects of oxidants and antioxidants on nuclear factor kappa B activation in three different cell lines: evidence against a universal hypothesis involving oxygen radicals. Biochim Biophys Acta 1260: 167–175 [DOI] [PubMed] [Google Scholar]
- Buttke TM, Sandstrom PA (1994) Oxidative stress as a mediator of apoptosis. Immunol Today 15: 7–10 [DOI] [PubMed] [Google Scholar]
- Chan YC, Leung PS (2007) Angiotensin II type 1 receptor-dependent nuclear factor-kappaB activation-mediated proinflammatory actions in a rat model of obstructive acute pancreatitis. J Pharmacol Exp Ther 323: 10–18 [DOI] [PubMed] [Google Scholar]
- Chen KC, Kao PH, Lin SR, Chang LS (2009) Upregulation of Fas and FasL in Taiwan cobra phospholipase A2-treated human neuroblastoma SK-N-SH cells through ROS- and Ca2+-mediated p38 MAPK activation. J Cell Biochem 106: 93–102 [DOI] [PubMed] [Google Scholar]
- Cheng Q, Law PK, de GM, Leung PS (2008) Combination of the dipeptidyl peptidase IV inhibitor LAF237 [(S)-1-[(3-hydroxy-1-adamantyl)ammo]acetyl-2-cyanopyrrolidine] with the angiotensin II type 1 receptor antagonist valsartan [N-(1-oxopentyl)-N-[[2′-(1H-tetrazol-5-yl)-[1,1′-biphenyl]-4-yl]methyl]-L-valine] enhances pancreatic islet morphology and function in a mouse model of type 2 diabetes. J Pharmacol Exp Ther 327: 683–691 [DOI] [PubMed] [Google Scholar]
- Chiarugi P (2005) PTPs versus PTKs: the redox side of the coin. Free Radic Res 39: 353–364 [DOI] [PubMed] [Google Scholar]
- Curtin JF, Donovan M, Cotter TG (2002) Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods 265: 49–72 [DOI] [PubMed] [Google Scholar]
- Fujioka S, Schmidt C, Sclabas GM, Li Z, Pelicano H, Peng B, Yao A, Niu J, Zhang W, Evans DB, Abbruzzese JL, Huang P, Chiao PJ (2004) Stabilization of p53 is a novel mechanism for proapoptotic function of NF-kappaB. J Biol Chem 279: 27549–27559 [DOI] [PubMed] [Google Scholar]
- Fujioka S, Sclabas GM, Schmidt C, Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C, Chiao PJ (2003) Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin Cancer Res 9: 346–354 [PubMed] [Google Scholar]
- Ghibelli L, Coppola S, Rotilio G, Lafavia E, Maresca V, Ciriolo MR (1995) Non-oxidative loss of glutathione in apoptosis via GSH extrusion. Biochem Biophys Res Commun 216: 313–320 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M (2002) Missing pieces in the NF-kappaB puzzle. Cell 109(Suppl): S81–S96 [DOI] [PubMed] [Google Scholar]
- Gilmore TD, Koedood M, Piffat KA, White DW (1996) Rel/NF-kappaB/IkappaB proteins and cancer. Oncogene 13: 1367–1378 [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M (2000) NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501 [DOI] [PubMed] [Google Scholar]
- Hampton MB, Orrenius S (1998) Redox regulation of apoptotic cell death. Biofactors 8: 1–5 [DOI] [PubMed] [Google Scholar]
- Hayakawa M, Miyashita H, Sakamoto I, Kitagawa M, Tanaka H, Yasuda H, Karin M, Kikugawa K (2003) Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J 22: 3356–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip SP, Wong TP, Tsai SJ, Leung PS (2003) The recovery of some components of the renin angiotensin system in the rat pancreas after chronic exposure to hypoxic condition. J Mol Endocrinol 31: 563–571 [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A (2002) NF-kappaB at the crossroads of life and death. Nat Immunol 3: 221–227 [DOI] [PubMed] [Google Scholar]
- Korn SH, Wouters EF, Vos N, Janssen-Heininger YM (2001) Cytokine-induced activation of nuclear factor-kappa B is inhibited by hydrogen peroxide through oxidative inactivation of IkappaB kinase. J Biol Chem 276: 35693–35700 [DOI] [PubMed] [Google Scholar]
- Kucharczak J, Simmons MJ, Fan Y, Gelinas C (2003) To be, or not to be: NF-kappaB is the answer – role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene 22: 8961–8982 [DOI] [PubMed] [Google Scholar]
- Lam SY, Leung PS (2002) A locally generated angiotensin system in rat carotid body. Regul Pept 107: 97–103 [DOI] [PubMed] [Google Scholar]
- Lau AT, Wang Y, Chiu JF (2008a) Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. J Cell Biochem 104: 657–667 [DOI] [PubMed] [Google Scholar]
- Lau ST, Lin ZX, Liao Y, Zhao M, Cheng CH, Leung PS (2009) Brucein D induces apoptosis in pancreatic adenocarcinoma cell line PANC-1 through the activation of p38-mitogen activated protein kinase. Cancer Lett 281: 42–52 [DOI] [PubMed] [Google Scholar]
- Lau ST, Lin ZX, Zhao M, Leung PS (2008b) Brucea javanica fruit induces cytotoxicity and apoptosis in pancreatic adenocarcinoma cell lines. Phytotherapy Res 22: 477–486 [DOI] [PubMed] [Google Scholar]
- Lee KB, Kim KR, Huh TL, Lee YM (2008) Proton induces apoptosis of hypoxic tumor cells by the p53-dependent and p38/JNK MAPK signaling pathways. Int J Oncol 33: 1247–1256 [PubMed] [Google Scholar]
- Leung PS, Wong RP, Sernia C (1999) Angiotensinogen expression by rat epididymis: evidence for an intrinsic, angiotensin-generating enzyme. Mol Cell Endocrinol 155: 115–122 [DOI] [PubMed] [Google Scholar]
- Li N, Karin M (1999) Is NF-kappaB the sensor of oxidative stress? FASEB J 13: 1137–1143 [PubMed] [Google Scholar]
- Li Q, Verma IM (2002) NF-kappaB regulation in the immune system. Nat Rev Immunol 2: 725–734 [DOI] [PubMed] [Google Scholar]
- Liptay S, Weber CK, Ludwig L, Wagner M, Adler G, Schmid RM (2003) Mitogenic and antiapoptotic role of constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J Cancer 105: 735–746 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192: 1–15 [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Ichijo H (2005) Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid Redox Signal 7: 472–481 [DOI] [PubMed] [Google Scholar]
- Nicholson KM, Anderson NG (2002) The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal 14: 381–395 [DOI] [PubMed] [Google Scholar]
- Poli G, Leonarduzzi G, Biasi F, Chiarpotto E (2004) Oxidative stress and cell signalling. Curr Med Chem 11: 1163–1182 [DOI] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ (2005) Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436: 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayet B, Gelinas C (1999) Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18: 6938–6947 [DOI] [PubMed] [Google Scholar]
- Schonwasser DC, Marais RM, Marshall CJ, Parker PJ (1998) Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol 18: 790–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R, Baltimore D (1986) Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 47: 921–928 [DOI] [PubMed] [Google Scholar]
- Slater AF, Nobel CS, Maellaro E, Bustamante J, Kimland M, Orrenius S (1995) Nitrone spin traps and a nitroxide antioxidant inhibit a common pathway of thymocyte apoptosis. Biochem J 306(Pt 3): 771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloss CM, Wang F, Liu R, Xia L, Houston M, Ljungman D, Palladino MA, Cusack Jr JC (2008) Proteasome inhibition activates epidermal growth factor receptor (EGFR) and EGFR-independent mitogenic kinase signaling pathways in pancreatic cancer cells. Clin Cancer Res 14: 5116–5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz P (2005) Reactive oxygen species in tumor progression. Front Biosci 10: 1881–1896 [DOI] [PubMed] [Google Scholar]
- Storz P (2006) Reactive oxygen species-mediated mitochondria-to-nucleus signaling: a key to aging and radical-caused diseases. Sci STKE 2006: re3. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028 [DOI] [PubMed] [Google Scholar]
- Tsang SW, Cheng CHK, Leung PS (2004) The role of the pancreatic renin-angiotensin system in acinar cell digestive secretion and in acute pancreatitis. Regul Pept 119: 213–219 [DOI] [PubMed] [Google Scholar]
- Verma IM, Stevenson JK, Schwarz EM, Van AD, Miyamoto S (1995) Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev 9: 2723–2735 [DOI] [PubMed] [Google Scholar]
- Wanebo HJ, Vezeridis MP (1996) Pancreatic carcinoma in perspective. A continuing challenge. Cancer 78: 580–591 [DOI] [PubMed] [Google Scholar]
- Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ (1999) The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res 5: 119–127 [PubMed] [Google Scholar]
- Whiteside ST, Israel A (1997) I kappa B proteins: structure, function and regulation. Semin Cancer Biol 8: 75–82 [DOI] [PubMed] [Google Scholar]
- Wu JT, Kral JG (2005) The NF-kappaB/IkappaB signaling system: a molecular target in breast cancer therapy. J Surg Res 123: 158–169 [DOI] [PubMed] [Google Scholar]
- Yang J, Su Y, Richmond A (2007) Antioxidants tiron and N-acetyl-L-cysteine differentially mediate apoptosis in melanoma cells via a reactive oxygen species-independent NF-kappaB pathway. Free Radic Biol Med 42: 1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZQ, Xie HY, Wang JY, Sun TM, Li X (1996) Chemical studies of the active antitumor components from the fruits of Brucea javanica (L. Merr.). Nat Prod Res Dev 8: 35–39 [Google Scholar]
- Yi J, Yang J, He R, Gao F, Sang H, Tang X, Ye RD (2004) Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res 64: 108–116 [DOI] [PubMed] [Google Scholar]
- Yip-Schneider MT, Nakshatri H, Sweeney CJ, Marshall MS, Wiebke EA, Schmidt CM (2005) Parthenolide and sulindac cooperate to mediate growth suppression and inhibit the nuclear factor-kappa B pathway in pancreatic carcinoma cells. Mol Cancer Ther 4: 587–594 [DOI] [PubMed] [Google Scholar]
- Zhang S, Lin ZN, Yang CF, Shi X, Ong CN, Shen HM (2004) Suppressed NF-kappaB and sustained JNK activation contribute to the sensitization effect of parthenolide to TNF-alpha-induced apoptosis in human cancer cells. Carcinogenesis 25: 2191–2199 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.