Abstract
Rationale: The critical innate immune mechanisms that regulate granulomatous inflammation in sarcoidosis are unknown. Because the granuloma-inducing component of sarcoidosis tissues has physicochemical properties similar to those of amyloid fibrils, we hypothesized that host proteins capable of forming poorly soluble aggregates or amyloid regulate inflammation in sarcoidosis.
Objectives: To determine the role of the amyloid precursor protein, serum amyloid A, as an innate regulator of granulomatous inflammation in sarcoidosis.
Methods: Serum amyloid A expression was determined by immunohistochemistry in sarcoidosis and control tissues and by ELISA. The effect of serum amyloid A on nuclear factor (NF)-κB induction, cytokine expression, and Toll-like receptor-2 stimulation was determined with transformed human cell lines and bronchoalveolar lavage cells from patients with sarcoidosis. The effects of serum amyloid A on regulating helper T cell type 1 (Th1) granulomatous inflammation were determined in experimental models of sarcoidosis, using Mycobacterium tuberculosis catalase–peroxidase.
Measurements and Main Results: We found that the intensity of expression and distribution of serum amyloid A within sarcoidosis granulomas was unlike that in many other granulomatous diseases. Serum amyloid A localized to macrophages and giant cells within sarcoidosis granulomas but correlated with CD3+ lymphocytes, linking expression to local Th1 responses. Serum amyloid A activated NF-κB in Toll-like receptor-2–expressing human cell lines; regulated experimental Th1-mediated granulomatous inflammation through IFN-γ, tumor necrosis factor, IL-10, and Toll-like receptor-2; and stimulated production of tumor necrosis factor, IL-10, and IL-18 in lung cells from patients with sarcoidosis, effects inhibited by blocking Toll-like receptor-2.
Conclusions: Serum amyloid A is a constituent and innate regulator of granulomatous inflammation in sarcoidosis through Toll-like receptor-2, providing a mechanism for chronic disease and new therapeutic targets.
Keywords: sarcoidosis, serum amyloid A, innate immunity, granuloma, cytokines
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Studies support a mycobacterial etiology for sarcoidosis. The critical innate immune mechanisms that regulate chronic granulomatous inflammation in sarcoidosis in the absence of active infection remain unknown.
What This Study Adds to the Field
Serum amyloid A is an innate receptor ligand that aggregates and regulates granulomatous inflammation in sarcoidosis, providing a novel mechanism for chronic disease.
Sarcoidosis is a multisystem granulomatous disease that can lead to chronic debilitating lung, heart, skin, and neurological disease and has no safe, effective treatment (1). Sarcoidosis occurs worldwide, with the highest annual incidence in northern European countries (estimated cumulative lifetime risk, >1%); in North America sarcoidosis compromises preferentially African-Americans with more severe disease (2). Immunopathologic hallmarks include epithelioid noncaseating granulomas with activated CD4+ T cells and macrophages, oligoclonal expansions of αβ+ T cells consistent with antigen stimulation, highly polarized helper T cell type 1 (Th1) cytokine expression, and local production of immunoregulatory cytokines such as tumor necrosis factor (TNF), IL-12, IL-18, and IL-10 (1, 3–7).
Our studies provide evidence of a mycobacterial etiology for sarcoidosis (8, 9). Using a novel proteomic approach, we identified Mycobacterium tuberculosis catalase–peroxidase (mKatG) as a candidate pathogenic tissue antigen that stimulates B- and T-cell responses in sarcoidosis (8, 9). Our approach, which required no a priori hypothesis regarding specific microbial or autoantigens, capitalized on the assumption that pathogenic “sarcoidosis” antigens would be a poorly soluble component of sarcoidosis granulomas. Although these and other studies (10, 11) support a mycobacterial etiology of sarcoidosis, the mechanisms that result in chronic granulomatous inflammation (in the absence of reactivation of mycobacterial or other infection) remain unclear.
The Kveim reaction provides a biologically relevant in vivo model of granuloma formation in sarcoidosis (12). In this reaction, insoluble homogenates of sarcoidosis tissues injected intradermally into patients with sarcoidosis induce epithelioid granulomas indistinguishable from spontaneously arising granulomas. Our previous studies demonstrated clonal T-cell populations at Kveim reaction sites consistent with antigen-driven responses (13). Because the granuloma-inducing properties of sarcoidosis tissue extracts (Kveim reagent) have physicochemical properties similar to those of amyloid or prion fibrils (neutral detergent insolubility; relative heat, acid, nuclease, and protease resistance; and sensitivity to alkali and potent denaturants) (14), we hypothesized that host proteins with the potential to form poorly soluble aggregates or amyloid fibrils play a role in the development of epithelioid granulomas in sarcoidosis.
We report herein that serum amyloid A (SAA), an ancient, highly inducible acute-phase reactant and amyloid precursor protein (15), may act as an immunological switch, amplifying ongoing Th1 granulomatous responses to mycobacterial antigens. We demonstrate that SAA is expressed with an intensity and distribution pattern characteristic of sarcoidosis compared with many other granulomatous processes. Further, our data indicate that SAA regulates granuloma formation and cytokine production in experimental models of mKatG-induced granulomatous lung inflammation as well as in lung macrophages from patients with sarcoidosis, effects mediated through Toll-like receptor-2 (TLR2).
Some of the results of these studies have been previously reported in the form of an abstract (16).
METHODS
Study Population
Patients recruited for bronchoalveolar lavage (BAL) studies underwent a clinically indicated diagnostic bronchoscopy, participated voluntarily, and provided informed consent under protocols approved by The Johns Hopkins Medical Institutions (Baltimore, MD) Institutional Review Board. A diagnosis of sarcoidosis was established either by tissue biopsy or by initial manifestations consistent with Löfgren syndrome according to worldwide consensus criteria (1). Control patients were either those who underwent a clinically indicated bronchoscopy and who later were determined not to have sarcoidosis or infection, or healthy nonatopic or atopic individuals recruited for research study under a separate protocol approved by The Johns Hopkins Medical Institutions Institutional Review Board. Patients with sarcoidosis and control patients were not receiving systemic corticosteroids or other immunosuppressive drugs at the time of their bronchoscopy. Demographic and clinical characteristics of these subject groups are listed in Table 1.
TABLE 1.
CHARACTERISTICS OF SUBJECTS RECRUITED FOR BRONCHOALVEOLAR LAVAGE STUDIES
| Subjects with Sarcoidosis | Control Subjects | |
|---|---|---|
| Total subjects, n | 57 | 29 |
| Female | 38 | 17 |
| Male | 19 | 12 |
| Mean age, years | 44.5 | 48.7 |
| CXR stage, 0/I/II/III/IV* | 0/15/34/8/0 | N/A |
| Smoking status, P/Q/N† | 5/15/37 | 3/4/22 |
| BAL cell differential, %‡ | ||
| Macrophages | 66.4 ± 2.5 | 88.1 ± 1.9 |
| Lymphocytes | 30.6 ± 2.5 | 9.9 ± 1.5 |
| Neutrophils | 2.1 ± 0.3 | 1.5 ± 0.3 |
| Eosinophils | 0.6 ± 0.1 | 0.5 ± 0.2 |
Definition of abbreviations: BAL = bronchoalveolar lavage; CXR = chest X-ray; N/A = not applicable.
Scadding CXR stage: 0 = no adenopathy, no lung infiltrates; stage I = hilar adenopathy only; stage II = hilar adenopathy plus lung infiltrates; stage III = lung infiltrates only; stage IV = pulmonary fibrosis.
P = present smoker; Q = quit/past smoker; N = never smoker.
Mean ± standard error.
Human Pathology Tissues
All tissue samples for histological studies were acquired under protocols approved by the Johns Hopkins Medical Institutions or the University of Colorado (Denver, CO) institutional review boards.
Immunohistochemistry
Paraffin tissue sections were stained by immunohistochemistry, using antibodies specific for fibrillar AA amyloid and SAA (clone mc1; Dako, Glostrup, Denmark), β-amyloid (Aβ) (clone 6F/3D; Dako), human prion protein (PrP27–30) (20R-PG009; Fitzgerald Industries, Concord, MA), or serum amyloid P (clone NCL-PCOMp; Novocastra, Newcastle upon Tyne, UK). Negative control staining was performed with a matched concentration of an appropriate nonspecific isotype control antibody. Staining with Congo red and thioflavin T was performed on frozen- or paraffin-embedded archived tissues by the Johns Hopkins Hospital Department of Pathology, using clinical laboratory-accredited methods. The presence of amyloid was assessed by Nomarski differential interference contrast fluorescence microscopy, using a fluorescein isothiocyanate filter, or by bright-field microscopy with polarizing filters. The number of tissue samples from various diseases that were analyzed are presented in Table E1 (see the online supplement); detailed methods are also given in the online supplement.
Morphometric Analysis of Human Tissues
For quantitative histological analysis, at least 20 digital images of representative high-power fields were obtained from each tissue section, and quantification of immunohistochemical staining (3,3′-diaminobenzidine, brown) was determined with Image-Pro software (Media Cybernetics, Bethesda, MD) (17). To account for differences in tissue area and cellularity between samples, the area of nuclear staining with hematoxylin (blue-violet) for each corresponding high-power field was also quantified by this method.
Human SAA Measurement
Human SAA was measured by ELISA (KHA0012; Biosource, Camarillo, CA) as recommended by the manufacturer (for details, see the online supplement).
Chemicals and Reagents
Recombinant human SAA (Peprotech, Rocky Hill, NJ) was passed three times through an endotoxin-binding column (EndoTrap red; Profos, Regensburg, Germany) to reduce the endotoxin content from an average of 250 endotoxin units per 1 mg of stock SAA (25 pg of LPS per 1 μg of SAA) to less than 0.3 pg of LPS per 1 μg of SAA for use in all experiments. Synthetic TLR2 agonist Pam3CSK4 (Pam3), TLR2 agonist Mycobacterium smegmatis lipomannan, TLR4 agonist Escherichia coli K12 LPS, and TLR8 agonist GU-rich single-stranded RNA 20-mer were from InvivoGen (San Diego, CA). Polymyxin B was from Sigma-Aldrich (St. Louis, MO). Unfractionated cell wall–free intracellular extracts from lysates of Mycobacterium tuberculosis (whole cell lysate, WCL) and the anti-mKatG monoclonal antibody IT-57 (18) were obtained from Colorado State University (Fort Collins, CO) as part of contract no. HHSN266200400091C (NIAID, NIH, Bethesda, MD), entitled “TB Vaccine Testing and Research Materials Contract” (www.cvmbs.colostate.edu/mip/tb/cellysate.htm).
Cell Culture Experiments
THP-1 cells transfected with a nuclear factor (NF)-κB–inducible reporter plasmid expressing a secreted embryonic alkaline phosphatase (SEAP) and selectable with the antibiotic Zeocin (100 μg/ml) (THP-1 Blue; InvivoGen) were differentiated overnight with phorbol myristate acetate (10 μg/ml) and then incubated with medium alone, SAA (10 μg/ml), Pam3 (100 ng/ml), or LPS (100 ng/ml) in the presence of polymyxin B (10 μg/ml). After 18–22 hours of incubation, NF-κB induction was quantified by the determination of SEAP activity in the resulting conditioned medium, using a colorimetric assay (QUANTI-Blue; InvivoGen) read at 650 nm. Selected experiments were performed in the presence of blocking antibodies to TLR2 (clone T2.5, 40 μg/ml; eBioscience, San Diego, CA) or an equal amount of nonspecific isotype control antibody (clone MOPC-21; BD Biosciences, San Jose, CA).
A human embryonic kidney cell line, HEK-293, was transfected with either an expression plasmid for human TLR2 (293-TLR2; InvivoGen) or an empty plasmid (293-null cells; InvivoGen) and grown in medium (Dulbecco's modified Eagle's medium plus 10% fetal calf serum) in the presence of the antibiotic blasticidin (10 μg/ml) to maintain selection for these transfectants. To detect the induction of NF-κB, 293-TLR2 or 293-null cells were transiently transfected overnight with pNiFty (InvivoGen) and then incubated with medium alone, SAA (10 μg/ml), Pam3 (100 ng/ml), lipomannan (100 ng/ml), LPS (100 ng/ml), or single-stranded RNA (10 μg/ml). After 18–22 hours of incubation, NF-κB signal transduction was determined by quantifying SEAP activity in the conditioned medium, using a colorimetric assay as outlined previously.
Recombinant mKatG and Modified M. tuberculosis Extracts
Recombinant Mtb KatG protein (9, 19) and whole cell lysates of M. tuberculosis isoniazid-resistant strain B1453 with katG deletion and the complemented strain were prepared as previously described (for detailed methods, see the online supplement). Protein immunoblotting with the anti-mKatG monoclonal antibody IT-57 was performed as previously described (8).
Animal Experiments
All animal studies were approved by the Johns Hopkins Animal Care and Use Committee. Experiments followed the basic design of rodent models of granulomatous lung inflammation previously described by other investigators (20, 21) (for detailed methods, see the online supplement). Briefly, female Lewis rats or C57BL/6 mice were immunized by intraperitoneal injection with M. tuberculosis whole cell lysate (Mtb-WCL) or recombinant M. tuberculosis catalase–peroxidase (mKatG) in a 1:1 mixture of phosphate-buffered saline and Freund's complete adjuvant or Freund's incomplete adjuvant (for experiments testing the effects of SAA). Sepharose 4B CNBr beads were coupled to mKatG, WCL, or no antigen (uncoated, sham reacted) according to the manufacturer's recommendations (GE Healthcare Life Sciences, Piscataway, NJ). Fourteen days after immunization, rats received by tail vein intravenous injection either SAA (1 mg), transthyretin (1 mg) as a control (amyloid precursor) human protein, or a dose of 50 ng of LPS, which was given 5 minutes before the Sepharose beads. Mice received mKatG beads by orotracheal instillation and an intraperitoneal injection of SAA (200 μg) or saline immediately before and at 1 week. For quantitative histological analysis of granulomatous inflammation in the lung, an index of granuloma size was calculated from the mean radii taken at perpendicular axes from the bead wall. Some rats received 500 μg of blocking antibodies to TLR2 (clone T2.5; eBioscience), neutralizing antibodies to rat-specific TNF-α (BioLegend, San Diego, CA) or IL-10 (R&D Systems, Minneapolis, MN), or an equal amount of nonspecific control antibody 10 minutes before SAA. In other experiments, splenocytes were harvested from mKatG-sensitized animals and incubated overnight in medium alone, SAA (10 μg/ml), Pam3 (100 ng/ml), mKatG (10 μg/ml), or human albumin (10 μg/ml; Sigma-Aldrich), with or without the addition of anti-TLR2 antibody (20 μg/ml) or an equal amount of isotype control antibody. Cytokine measurements were determined by ELISA (R&D Systems) from cell-free supernatants. Antigen-specific proliferation was determined after a 6-day incubation with mKatG by 5,6-carboxyfluorescein diacetate succinimidyl ester dilution as previously described (9).
Human BAL Cell Preparation
Lung mononuclear cells from BAL were isolated and cultured as previously described (4, 9) (for detailed methods, see the online supplement). Selected experiments were performed in the presence of blocking antibodies to TLR2 (clone T2.5, 20 μg/ml; eBioscience) or an equal amount of nonspecific isotype control antibody (clone MOPC-21; BD Biosciences). Cell-free supernatants were collected and cytokine levels were measured by ELISA (TNF and IL-10 from BD Biosciences; IL-18 from R&D Systems).
Flow Cytometry
Subsets of BAL cells were identified with monoclonal antibodies against monocyte (CD14 clone M5E2; BD Biosciences), lymphocyte (CD3 clone UCHT1; BD Biosciences), and TLR2 (clone 11G7) as previously described (9) (for detailed methods, see the online supplement). The mean fluorescence intensity of surface TLR2 expression on CD14+ cells was calculated with FlowJo software (Tree Star, Ashland, OR).
Statistics
Statistical analyses were performed with GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Group comparisons of unpaired data were performed by Kruskal-Wallis test followed by individual comparisons using the Mann-Whitney test and indicated by H-brackets in the figures. Paired analyses were performed by the Wilcoxon matched pairs test and indicated with staple brackets. Results of nonparametric correlation analyses are expressed using the Spearman coefficient (rs) with P values determined by a two-tailed test. A P value less than 0.05 was considered significant.
RESULTS
SAA Forms an Integral Component of Epithelioid Granulomas in Sarcoidosis
Using immunohistochemistry based on an antibody that binds to both AA amyloid fibrils and its precursor protein SAA, we found a typically intense pattern of expression of SAA in granulomas from sarcoidosis lung and lymph nodes (Figure 1A); isotype control antibodies showed no staining (see Figure E1 in the online supplement). These same tissues did not show significant expression of β-amyloid (Figure 1A) or PrP prion protein (data not shown). SAA/AA staining of granulomas was also detected in sarcoidosis tissues of pleura, skin, dura mater, and liver (Figure 1B). To assess whether SAA staining was due to deposition of AA amyloid fibrils, we stained with amyloid-binding dyes thioflavin-T and Congo red. Thioflavin-T staining within granulomas at sites of SAA expression was inconsistent, with a minority of granulomas demonstrating fluorescence within or adjacent to macrophages or giant cells, suggesting that most SAA was not arrayed in an AA amyloid fibril configuration in sarcoidosis tissues (Figure 1C). Consistent with this inference, tissues stained with Congo red did not show significant birefringence within granulomas, which also did not show expression of serum amyloid P component, which binds amyloid fibrils (see Figure E2 in the online supplement). As most SAA did not appear to be associated with AA amyloid fibril formation, we analyzed whether soluble SAA could be detected in the sarcoidosis lung by sampling BAL fluid. We found significantly higher levels of soluble SAA in unconcentrated BAL fluid from patients with pulmonary sarcoidosis compared with healthy individuals (Figure 1D). SAA levels were significantly higher in BAL fluid from patients with sarcoidosis with interstitial lung disease (chest X-ray stages II and III) compared with those with bilateral hilar adenopathy but no pulmonary infiltrates (stage I), suggesting that increases in soluble SAA in BAL fluid reflect tissue SAA within compartmentalized granulomatous inflammation in the lung and not solely from the presence of systemic sarcoidosis.
Figure 1.
Serum amyloid A (SAA) forms an integral part of the nidus of epithelioid granulomas in sarcoidosis. (A) Representative photomicrographs of lung and lymph node (LN) tissues from patients with pulmonary sarcoidosis stained by immunohistochemistry (IHC) with a monoclonal antibody (mc1; Dako) specific for fibrillar AA amyloid and SAA (SAA/AA) (top row) or a monoclonal antibody (6F/3D; Dako) to β-amyloid (Aβ) (bottom row) and counterstained with hematoxylin. (B) Representative photomicrographs of pleura, skin, brain (dura mater), and liver stained for SAA/AA. (C) Representative photomicrographs of lung sections from patients with sarcoidosis stained with thioflavin-T and counterstained with hematoxylin and then examined by Nomarski differential interference contrast (panels i and iv) and fluorescence (panels ii and v) microscopy; open arrowheads indicate enhanced fluorescence intensity. Corresponding hematoxylin–eosin (H&E) sections stained for SAA/AA by IHC are shown (panels iii and vi). (D) Top: Soluble SAA levels in unconcentrated bronchoalveolar lavage (BAL) fluid from patients with sarcoidosis and healthy control subjects were measured by ELISA. Bottom: SAA levels in BAL fluid from patients with sarcoidosis grouped according to chest X-ray stage. Data are shown as dot plots with medians (horizontal bars), cohort sizes (n), and nonparametric comparison (brackets) as indicated (*P < 0.005; **P < 0.05). ns = not significant.
SAA Expression Is Greater in Sarcoidosis Tissues than in Other Granulomatous Diseases
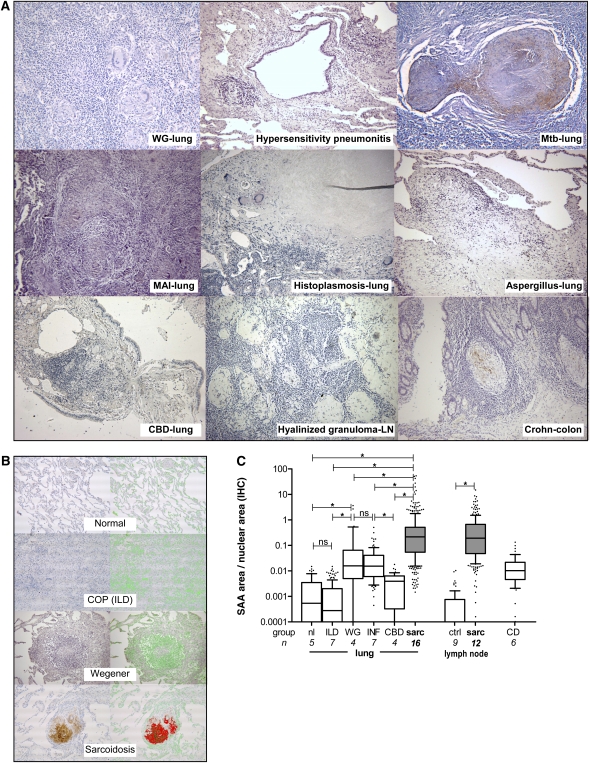
Expression of SAA/AA in sarcoidosis was both qualitatively and quantitatively different from that of all other infectious and autoimmune granulomatous diseases that were examined (Figure 2A). In Wegener granulomatosis, a grave form of systemic vasculitis associated with antibodies against myeloperoxidase, SAA was inconsistently expressed by lung macrophages, with only limited expression within areas of granulomatous inflammation. Granulomas in hypersensitivity pneumonitis showed little or no SAA staining. Epithelioid noncaseating granulomas in lung tissues from infectious granulomatous diseases (M. tuberculosis, Mycobacterium avium-intracellulare, Histoplasma, and Aspergillus infections) also displayed limited SAA staining in scattered macrophages compared with sarcoidosis granulomas. Epithelioid granulomas from transbronchial biopsies of patients with chronic beryllium disease demonstrated little or no SAA staining. A lymph node infected with M. tuberculosis demonstrated patchy expression of SAA, localized mostly around the periphery of necrotizing granulomas. Granulomas near lung cancer or hyalinized granulomas only occasionally expressed low-intensity SAA staining. Tissue granulomas from Crohn's disease expressed patchy staining of SAA localized to macrophages. Tissues from normal lung, all other inflammatory lung diseases, and normal and hyperplastic lymph node samples showed no significant SAA staining (see Figure E3 in the online supplement).
Figure 2.
Greater expression of serum amyloid A (SAA) is found in granulomas from sarcoidosis tissues than in other granulomatous diseases. (A) Representative photomicrographs of lung, colon, or lymph node (LN) tissues from patients with Wegener granulomatosis (WG), hypersensitivity pneumonitis, M. tuberculosis (Mtb) infection, M. avium-intracellulare (MAI) infection, Histoplasma infection, Aspergillus infection, chronic beryllium disease (CBD), hyalinized lymph node granulomas, or Crohn's disease stained for SAA/AA by immunohistochemistry. (B) Representative photomicrographs of lung tissue from subjects with histologically normal lung, cryptogenic organizing pneumonia [COP (ILD)], Wegener granulomatosis, or sarcoidosis are shown for SAA/AA staining (left column) with corresponding red overlay (right column); green overlay is shown for staining of nuclei. (C) Quantification of SAA/AA staining in lung and lymph node tissues expressed as pixels of red area (SAA stained) normalized against pixels of green area (nuclear stained) to account for differences in tissue area in the various samples. The interstitial lung disease (ILD) group includes patients with COP, hypersensitivity pneumonitis, usual interstitial pneumonia, or nonspecific interstitial pneumonia. The infectious lung disease group (INF) includes M. tuberculosis, M. avium-intracellulare, Histoplasma, and Aspergillus infections. Additional groups include chronic beryllium disease (CBD) and Crohn's disease (CD). The control lymph node tissue group (ctrl) includes histologically normal or hyperplastic lymph node. Data are shown as quartile box plots with 10th and 90th percentile whiskers. Group sizes (n) and nonparametric comparisons (brackets) are as indicated (*P < 0.0001). IHC = immunohistochemistry; nl = normal; ns = not significant; sarc = sarcoidosis; WG = Wegener granulomatosis.
We next quantified differences in SAA expression in tissues from several groups (Figures 2B and 2C) within the dynamic range of SAA/AA staining (see Figure E5 in the online supplement). Using an index of SAA staining normalized against the area of stained nuclei to account for differences in tissue area, we found SAA expression in lung tissues from patients with sarcoidosis to be more than 100-fold greater than in histologically normal lung or in tissues from a group of patients with interstitial lung diseases, and more than 50-fold higher than in Wegener granulomatosis (Figure 2C). Epithelioid noncaseating granulomas from available samples of infectious granulomatous diseases (mycobacterial and fungal) displayed a significantly lower extent of SAA staining compared with sarcoidosis tissues. Noncaseating epithelioid granulomas from transbronchial biopsies from patients with chronic beryllium disease demonstrated significantly less SAA staining than in sarcoidosis, Wegener granulomatosis, or infectious granulomatous diseases. Sarcoidosis lymph nodes demonstrated almost 1,000-fold increased expression of SAA compared with histologically normal or hyperplastic lymph nodes (Figure 2C). Colon tissue samples from patients with Crohn's disease had levels of SAA expression comparable to that of tissues from patients with Wegener granulomatosis or infectious granulomatous diseases but lower than in patients with sarcoidosis.
SAA Expression Is Localized to CD68+ Macrophages in Sarcoidosis Granulomas but Correlates with CD3+ Lymphocyte Infiltration
Tissues from patients with sarcoidosis sometimes showed heterogeneity in the distribution or intensity of SAA staining within neighboring granulomas, mostly in a subset of lung or lymph node samples (Figure 3A). SAA staining in these tissues was localized predominantly to macrophages and multinucleated giant cells (determined by histological appearance and CD68+ staining) or immediately adjacent to these cells (Figure 3B). Neutrophils were rarely found in sarcoidosis granulomas, and demonstrated little or no staining for SAA. Overall, SAA staining correlated with total mononuclear cells (CD68+ mononuclear cells plus CD3+ T cells) within granulomas (rs = 0.349, P < 0.00005), suggesting that the extent of SAA deposition is related to active mononuclear cell inflammation. Importantly, despite localization of SAA to macrophages and giant cells, the extent of SAA staining correlated with the number of CD3+ T cells and not CD68+ macrophages within granulomas, suggesting that SAA expression is regulated by local CD3+ T cells (Figure 3C).
Figure 3.
Serum amyloid A (SAA) expression in granulomas localizes to macrophages and giant cells but correlates with CD3+ lymphocytes. (A) Photomicrographs of lung, lymph node (LN), and brain (dura mater) from patients with sarcoidosis with heterogeneity in the intensity of SAA staining of neighboring granulomas. (B) Representative photomicrographs (n = 15) of lung (top row) and LN (bottom row) from patients with sarcoidosis stained for SAA (left), CD68+ macrophages (middle), and CD3+ lymphocytes (right). (C) Correlation of SAA staining as quantified by digital capture microscopy normalized against area of nuclei stained with hematoxylin with the presence of CD68+ macrophages or CD3+ lymphocytes in the granuloma area from consecutive tissue sections. Analysis of 70 high-power fields from 6 tissue samples is shown. Data are shown as dot plots with lines indicating paired values. Nonparametric correlation is expressed as the Spearman coefficient.
To assess whether SAA expression correlated with chronic granulomatous inflammation, we analyzed six sarcoidosis lung samples characterized by granulomas with both mononuclear cell inflammation (defined as more than 10 mononuclear cells per granuloma to exclude hyalinized acellular granulomas) and fibrosis (visible extracellular matrix within or immediately surrounding granulomas on routine hematoxylin–eosin sections). We found that the extent of SAA staining positively correlated with the degree of collagen deposition, suggesting that tissue SAA expression links to chronic inflammation and fibrosis (see Figure E4 in the online supplement).
SAA Activates NF-κB from Human Macrophage Cell Lines through TLR2
SAA has several potential receptors that mediate its effects on inflammatory cells, including TLR2, known to be expressed on lung macrophages (15, 22). To assess a potential role for SAA in stimulating tissue macrophages in sarcoidosis, we analyzed the effects of SAA on the human macrophage-like THP-1 cell line. Using THP-1 Blue cells (InvivoGen), which contain a transfected NF-κB reporter plasmid, we found that highly purified recombinant SAA in a disease-relevant dose (23) (10 μg/ml, containing endotoxin at <5 pg/ml) activates NF-κB in these cells (Figure 4A). The addition of polymyxin B neutralized the response to the TLR4 ligand E. coli K12 LPS (100 ng/ml) but did not affect responses to SAA, suggesting that the effect of SAA was not mediated through TLR4 signaling from the trace amount of endotoxin in our SAA preparation. When THP-1 Blue cells were preincubated with blocking anti-TLR2 antibodies, activation of NF-κB by SAA was significantly inhibited compared with isotype controls, suggesting that SAA is signaling through TLR2 (Figure 4A). A similar inhibition was observed with the TLR-2 binding ligand Pam3CSK4 (Pam3).
Figure 4.
Serum amyloid A (SAA) activates nuclear factor (NF)-κB through Toll-like receptor-2 (TLR2) signaling independent of TLR4. Shown is the effect of SAA on (A) the human macrophage-like THP-1 cell line with an NF-κB reporter plasmid (THP-1 Blue) differentiated overnight with phorbol myristate acetate (PMA, 10 μg/ml), and then incubated with SAA at 10 μg/ml (or with medium alone or palmitoyl-3-cysteine-serine-lysine-4 [Pam3] at 100 ng/ml) in the presence of polymyxin B (PMX, 10 μg/ml) with or without a 40-μg/ml concentration of blocking antibodies to human TLR2 (aT2) or nonspecific isotype control antibody (Ig). LPS (10 ng/ml) was coincubated with or without PMX as indicated. Activation of NF-κB was detected by a colorimetric assay of secreted alkaline phosphatase (SEAP) activity expressed by the NF-κB–inducible expression plasmid; and (B) HEK-293 cells transfected with a human TLR2 expression plasmid (open symbols) or empty control plasmid (solid symbols) transiently transfected with an NF-κB reporter plasmid (pNiFty) and stimulated with medium alone, SAA, TLR2 agonist Pam3 or M. smegmatis lipomannan (LM; 100 ng/ml), TLR4 agonist LPS, or TLR8 agonist ssRNA (single-stranded RNA, 10 μg/ml). Activation of NF-κB was detected by a colorimetric assay of SEAP activity expressed by the NF-κB–inducible expression plasmid. Data are shown as dot plots of independent experiments with group sizes indicated (n). Medians (horizontal bars) were analyzed by paired (staple brackets) or unpaired (H-brackets) nonparametric methods as indicated (*P < 0.005; **P < 0.05). ns = not significant.
To further test the role of TLR2 in mediating the effects of SAA independent of other TLRs expressed on human macrophages, we assessed NF-κB activation in HEK-293 cells stably transfected with a TLR2 expression vector (293-TLR2) or empty vector (293-null) after transient transfection with the pNiFty NF-κB reporter plasmid. HEK-293 cells, which do not express Toll-like receptors, did not show activation of NF-κB after incubation with SAA or other known TLR agonists (Figure 4B). In contrast, SAA activated NF-κB in 293-TLR2 cells in a manner similar to known TLR2 ligand Pam3 or M. smegmatis lipomannan, consistent with a TLR2 agonist effect by SAA. 293-TLR2 cells did not show activation of NF-κB in response to ligands to other TLRs including TLR4 (LPS) or TLR8 (single-stranded RNA) (Figure 4B).
SAA Regulates Granulomatous Lung Inflammation through TLR2 in an Experimental Model of Sarcoidosis
To further assess the potential role of SAA in sarcoidosis, we developed an experimental model of granulomatous lung inflammation using the pathogenic antigen mKatG linked to Sepharose beads embolized to the lung in rats, a species that develops granulomas more representative of humans than mice (24) (Figure 5A). We found that mKatG–beads induced greater granulomatous lung inflammation at 4 days and 3 weeks than beads linked to unfractionated cell-free intracellular extracts from lysed M. tuberculosis (Mtb) (whole cell lysate, WCL) (Figure 5B). The relatively increased potency of mKatG as antigen compared with other mycobacterial proteins was demonstrated by the smaller granulomas seen with beads linked to protein extracts from a katG deletion Mtb strain compared with this same strain complemented with a katG gene expressing functional mKatG (Figure 5C). The granulomatous response to mKatG–beads was associated with antigen-specific T-cell proliferation and IFN-γ production (Figure 5D).
Figure 5.
Serum amyloid A (SAA) regulates Mycobacterium tuberculosis catalase–peroxidase (mKatG) antigen–driven granulomatous lung inflammation in an experimental model of sarcoidosis. After immunization with recombinant mKatG or an unfractionated cell-free lysate of intracellular proteins from M. tuberculosis (WCL), Sepharose beads linked to the same immunizing antigen or sham-reacted unlinked beads were embolized to the lung of animals in experiments modeled on previously published studies (20, 21). (A) Representative low-power photomicrographs (original magnification, ×10; scale bars, 50 μm) of granulomatous inflammation 4 days after exposure to embolized beads. An index of granuloma size was estimated from the mean radii taken at perpendicular axes (arrows). (B) Granuloma size is shown for 4-day and 3-week time points by box plots with 10th and 90th percentile whiskers. (C) Protein immunoblot with the anti-mKatG monoclonal antibody IT-57 indicating the absence or presence of 80-kD mKatG monomer in mKatG-deleted (mKatGdel) M. tuberculosis strain or the same strain complemented with a functional katG gene (mKatGcomp). Shown is the effect of the presence or absence of mKatG in unfractionated cell-free extracts from these Mtb strains linked to Sepharose beads on the intensity of granulomatous inflammation determined by granuloma size. (D) Antigen-specific responses to mKatG from splenocytes of mKatG-sensitized animals determined by flow cytometry (representative dot plot of CD3+CD4+ lymphocyte proliferation determined by 5,6-carboxyfluorescein diacetate succinimidyl ester [CFSE] dilution after 6 d of incubation; n = 6) and IFN-γ production after a 24-hour incubation in medium alone, control human albumin (10 μg/ml), or mKatG (10 μg/ml). (E) Representative high-power photomicrographs (original magnification, ×40; scale bars, 50 μm) showing the localization of recombinant human SAA to sites of mKatG–bead–induced granuloma formation after 24 hours; shown are SAA levels in lung homogenates at 24 hours from rats receiving mKatG-coated or uncoated beads. (F) Effect of SAA (10 μg/ml) or Toll-like receptor-2 (TLR2) ligand palmitoyl-3-cysteine-serine-lysine-4 (Pam3, 100 ng/ml) on 24-hour splenocyte production of IFN-γ, tumor necrosis factor (TNF), and IL-10; effect of intravenous injection of SAA (1 mg), transthyretin (TTR, 1 mg), or LPS (50 ng) given immediately before mKatG bead administration on granuloma size at 4 days (lower right). (G) Effect of IP injections of SAA (200 μg) or saline given immediately before orotracheal administration of mKatG beads and at 1 week on granuloma size on Day 4 or 2 weeks in mice immunized with mKatG. (H) Effects of blocking antibodies to TLR2 or similar amount of nonspecific isotype control antibodies on SAA-induced rat splenocyte production of IFN-γ, TNF-α, IL-10, or granuloma size at 4 days (lower right). Data are shown as box plots with 10th and 90th percentile whiskers (B, C, F, G, and H), dot plots with medians (D, F, and H), or as bars indicating mean with standard error whiskers (E). Group sizes (n) and paired (staple brackets) or unpaired (H-brackets) nonparametric comparisons are indicated (*P < 0.005; **P < 0.05). ns = not significant.
To model the effects of SAA in regulating granulomatous inflammation in sarcoidosis, we administered SAA in a dose mimicking an acute-phase response in humans (15). SAA localized to sites of mKatG–bead–induced granulomas to a greater degree than uncoated beads alone (Figure 5E). SAA induced higher levels of IFN-γ, TNF, and IL-10 from mKatG-stimulated splenocytes, consistent with a Th1-promoting effect (Figure 5F). With a single dose, SAA induced more compact granulomas compared with a control human protein, transthyretin, or LPS at 4 days (Figure 5F). This effect of SAA was mediated in part through TNF and IL-10 as shown by the inhibitory effects of neutralizing antibodies infused before administration of SAA and mKatG–beads (15.1 and 36.7% inhibition, respectively, compared with isotype control antibodies; P < 0.05 both comparisons). To model the effect of SAA over an extended time period as in human sarcoidosis, we tested the effect of repeated SAA administration on Th1-induced pulmonary granulomatous inflammation from orotracheal administration of mKatG–beads in mKatG-immunized mice. Importantly, although granuloma size was reduced at 4 days, we found that repeated SAA administration increased mean granuloma size by approximately twofold at 2 weeks, suggesting that chronic SAA exposure enhances the persistence of experimental granulomatous inflammation (Figure 5G). Notably, anti-TLR2 antibodies administered before SAA and mKatG–beads attenuated the effects of SAA on the production of IFN-γ, TNF, IL-10, and granuloma size at 4 days, suggesting that SAA regulates mKatG-induced granulomatous lung inflammation in part through TLR2 (Figure 5H).
SAA Regulates Cytokine Production from Sarcoidosis Lung Macrophages through TLR2
To translate our results to human sarcoidosis, we determined the effects of SAA on ex vivo cytokine production from lung (BAL) macrophages from patients with pulmonary sarcoidosis. We found that SAA in disease-relevant concentrations (10–50 μg/ml) stimulated production of TNF, IL-18, and IL-10 from BAL macrophages from patients with sarcoidosis and to a greater extent than from BAL cells from control subjects, comprising mostly patients with chronic obstructive pulmonary disease (Figure 6A).
Figure 6.
Serum amyloid A (SAA) induces cytokine production from bronchoalveolar lavage cells from patients with sarcoidosis through Toll-like receptor-2 (TLR2) stimulation. Effect of SAA on (A) bronchoalveolar lavage (BAL) cells isolated from patients with sarcoidosis (sarc, open symbols) or control individuals (ctrl, solid symbols) incubated with SAA (10 μg/ml) or LPS (100 ng/ml) in the presence of polymyxin; cell-free culture supernatants were collected at 24 hours and cytokine levels were determined by ELISA. (B) Gating strategy and representative examples (panel i) of TLR2 expression on CD14+ BAL cells from a control subject and a patient with sarcoidosis. Overlay plots of TLR2 staining on CD14+ BAL cells representing (panel ii) dot-plot overlay of TLR2 expression in a control subject (green) and patient with sarcoidosis (red) (with the frequency of cells with high TLR2 expression as noted), and (panel iii) histogram overlay of fluorescence intensity of TLR2 staining in a control subject (green shaded area) and patient with sarcoidosis (red outline). (C) Relative expression of human TLR2 on CD14+ BAL cells from control subjects and patients with sarcoidosis expressed as mean fluorescence intensity (MFI). (D) Ex vivo expression of cytokines (tumor necrosis factor [TNF], IL-18, IL-10) in sarcoidosis BAL cells stimulated with SAA in the presence of polymyxin (PMX) and blocking antibodies to TLR2 (open symbols) or nonspecific isotype control antibody (solid symbols). Data are shown as dot plots with medians (horizontal bars) and group sizes (n) as indicated, from a total of 34 patients with sarcoidosis and 16 control individuals. Paired (staple brackets) and unpaired (H-brackets) nonparametric comparisons are as indicated (*P < 0.005; **P < 0.05).
To assess whether these effects of SAA were mediated through TLR2 signaling, we first analyzed surface expression of TLR2 on BAL macrophages. We found higher expression of TLR2 on CD14+ BAL macrophages in patients with sarcoidosis than in control subjects (Figures 6B and 6C). The TLR2 receptors were functional as assessed by the increased production of TNF and IL-10 after incubation with known TLR2 agonists (data not shown). When sarcoidosis BAL cells were preincubated with blocking antibodies to TLR2 before SAA stimulation, we found that anti-TLR2 antibodies consistently inhibited TNF and IL-10 production, with a trend for inhibiting IL-18 production from BAL macrophages from patients with sarcoidosis (Figure 6D). These data suggest that SAA regulates local granulomatous inflammation through its effects on tissue macrophage cytokine production, mediated in part through TLR2.
DISCUSSION
Our data suggest that SAA is both a structural component of epithelioid granulomas and an effector of the innate immune response that regulates granulomatous inflammation in sarcoidosis. Several findings suggest that SAA plays a central, perhaps defining, role in the pathobiology of sarcoidosis. First, the extent of SAA deposition in tissue granulomas in sarcoidosis greatly exceeded that found in all other granulomatous lung diseases that were examined. This included tissues with noncaseating epithelioid granulomas (typical of sarcoidosis) found in infectious granulomatous diseases including mycobacterial and fungal infections and in chronic beryllium disease. The intensity, focality, and heterogeneity in the distribution of SAA have also not been described in other inflammatory conditions such as rheumatoid arthritis that, like Wegener granulomatosis and hypersensitivity pneumonitis, have different immunopathogenic mechanisms (25). Second, the distribution of SAA staining in individual granulomas within sarcoidosis tissues was heterogeneous in some samples and correlated with total mononuclear cell infiltration. Our data showed that SAA expression in granulomas from sarcoidosis tissues was centered on CD68+ macrophages and giant cells, suggesting that SAA is produced, concentrated, and/or released by these cells during periods of active mononuclear cell inflammation. Third, the extent of SAA staining correlated with the number of CD3+ T cells in the granulomas, and not with the number of CD68+ macrophages. Importantly, this observation suggests that the extent of expression of SAA at sites of granulomatous inflammation in sarcoidosis is pathogenically linked to local Th1 immune responses. There are several potential mechanisms for this linkage including the paracrine effects of IFN-γ and TNF released by CD3+ T cells on up-regulating SAA expression in macrophages and other local cells (15). CD3+ T cells could also regulate trafficking of SAA-expressing inflammatory cells to sites of granulomatous inflammation. Importantly, IFN-γ has been shown to directly impair the degradation of SAA by macrophages favoring extracellular accumulation and fibril formation (26), an effect that can directly link Th1 responses to enhanced aggregation and persistence of SAA. Together, these histopathologic observations provide the spatial context for the hypothesis that SAA is a bridge between the innate and adaptive immune responses that determine a specific pathobiologic development of granulomatous inflammation in sarcoidosis.
In addition to its role as an acute-phase reactant, SAA plays a role in human disease as a precursor to fibrillar AA amyloid (15). Our initial studies of SAA were prompted by recognition that the biophysical properties of the granuloma-inducing component of sarcoidosis tissues had striking parallels to the properties of amyloid or prion fibrils (12, 14, 15). Despite the often intense staining of SAA in sarcoidosis granulomas, sites of SAA deposition were not associated with typical features of amyloid fibril aggregation as evidenced by the lack of widespread Congo red birefringence or staining with serum amyloid P. However, Congo red staining of amyloid or prion fibrils may significantly underestimate the presence of amyloid or prion proteins in tissues, suggesting there may be aggregates of SAA below the level of detection by this analytic method (27, 28). Our observation of positive thioflavin-T fluorescence in some areas within the granulomas, particularly in giant cells, suggests that ordered SAA aggregates may be present in some sarcoidosis granulomas where they could form a poorly soluble nidus for granuloma formation. Prior studies have noted an association of amyloidosis and sarcoidosis (29), which conceivably could be a more generalized expression of dysregulated protein aggregation in sarcoidosis. Given that amyloid or prion aggregates have a biochemical attraction for other, even unrelated, amyloid fibrils (30), a consequence of this scenario is that aggregates of SAA could serve as a “seed” for further amyloid-like, noncongophilic protein aggregation that could accumulate during slowly progressive granulomatous inflammation in chronic sarcoidosis.
Based on the known biology of SAA, several mechanisms are potentially involved in the increased expression of SAA in sarcoidosis. First, SAA may be up-regulated locally within the macrophage compartment as a direct result of engulfment by mycobacterial organisms (31) (or other putative environmental triggers), or as a result of the effect of TNF and IFN-γ (15) released by local CD3+ T cells. Second, the up-regulation of hepatic release of SAA induced as part of a systemic acute-phase response may increase levels of plasma SAA available to bind to cells/matrix within the developing granulomatous inflammation. Third, IFN-γ directly impairs the degradation of SAA by macrophages, which would enhance local accumulation and persistence of SAA (26). Although the dominant mechanism(s) remain unknown, we posit that the actual level of SAA up-regulation is not as critical as the factors that lead to aggregation and persistence of SAA (both nonfibrillar and fibrillar forms) that promotes further accumulation of SAA within the sarcoidosis granulomas.
SAA binds to several matrix proteins such as laminin and vitronectin through EGD-like binding sites (15, 32). This property provides a potential mechanism through which SAA could participate in the structural component of epithelioid granulomas independent of (or in addition to) its amyloid-precursor properties. By binding to specific matrix proteins, preexisting or locally produced SAA could consolidate a poorly soluble protein aggregate to form a nidus for granuloma formation. These same binding properties of SAA or its associated matrix proteins would also provide a mechanism through which granulomas could serve to trap other host or microbial proteins, thereby functioning as a depot for potential tissue antigens. Consistent with this premise, granulomas have been shown to trap reinfecting mycobacteria (33) and prions (34). Because SAA has been shown to mediate T-cell trafficking via receptor-mediated matrix adhesion, SAA could also assist in antigen-specific T-cell migration to sites of granulomatous inflammation (35).
The innate immune mechanisms that critically regulate granulomatous inflammation in sarcoidosis are uncertain. As seen in our model of mKatG-induced granulomatous lung inflammation, SAA regulates granulomatous inflammation through its effects on production of IFN-γ, TNF, and IL-10. Th1-mediated granuloma formation is known to be critically dependent on IFN-γ and TNF in both experimental models and in sarcoidosis (3, 5, 20, 36). Thus, the experimental model supports the in vitro expression data that SAA contributes to the immunopathology in sarcoidosis. Although these effects of SAA on cytokine regulation are consistent with its role in other diseases, it is notable that our data, indicating that SAA stimulates the coordinated production of relevant immunoregulatory cytokines (TNF, IL-10, and IL-18) from lung macrophages in sarcoidosis, provides a specific innate effector pathway that can regulate local adaptive immunity in sarcoidosis and the formation of discrete, compact epithelioid granulomas characteristic of sarcoidosis (3, 5–7). The contributions of other relevant monokines such as IL-12 will be important to analyze in future studies, as well as the effect of SAA on Th1 responses.
The effect of SAA on experimental granulomatous inflammation measured by size was complex. The initial reduction in granuloma size mediated by a single bolus of SAA was in part mediated by TNF, IL-10, and TLR2. Interestingly, both TNF and IL-10 have been shown to reduce granuloma size in experimental models, although by different mechanisms—TNF enhancing local Th1 immunity, granuloma compactness, and host defense (35, 36), with IL-10 reducing granulomatous inflammation through its antiinflammatory effects (37, 38). When repeated administration of SAA was used to model the progressive incorporation of SAA at sites of granulomatous inflammation over time as in human sarcoidosis, we found that SAA enhanced the persistence of granulomatous inflammation consistent with an innate immunoregulatory, amplifying role for SAA in chronic sarcoidosis. In addition to IFN-γ and TNF, it will be important in future studies to dissect the contributions of other cytokines, chemokines, and transcription factors in mediating the effects of SAA on the complex processes involved in promoting chronic granulomatous inflammation in sarcoidosis. Our data that SAA expression correlates with surrounding fibrosis in sarcoidosis granulomas, and the known effects of SAA on expression of collagenases and metalloproteases by fibroblasts (39, 40), suggest that SAA could also play a role in the remodeling of extracellular matrix within the context of transforming growth factor-β and other profibrotic cytokines found at sites of granulomatous inflammation in chronic sarcoidosis.
SAA has multiple potential receptors (formyl peptide receptor-like-1 [FPRL1], receptor for advanced glycosylation end products [RAGE], and TLR2) expressed on many different cells (e.g., macrophages, dendritic cells, neutrophils, and epithelial cells) through which it can participate in innate immune responses with pleiomorphic effects depending on the pathobiologic context (15, 22, 41–43). SAA was shown to activate TLR2 signaling in transfected HeLa cells expressing TLR2 (22). We present further evidence that this interaction activates NF-κB independently of TLR4 in both a human macrophage-like cell line and an epithelial cell line transfected with TLR2. Importantly, these findings were translated to patients with sarcoidosis by the demonstration that SAA regulates cytokine production from lung macrophages through TLR2 signaling, suggesting a role for this innate receptor in this disease. Prior studies have shown that TLR2 agonists such as Staphylococcus aureus peptidoglycan or synthetic TLR2 ligands stimulate cytokine production in BAL cells from patients with sarcoidosis, but these ligands have not been shown to have in vivo relevance (44, 45). Our finding that both tissue SAA and soluble SAA are present at sites of inflammation in sarcoidosis provides for a known repository of TLR2 ligands that can enhance Th1 responses (46) and up-regulate IFN-γ, TNF, and IL-10 release (47) to regulate chronic granulomatous inflammation. Given that RAGE is also expressed in sarcoidosis granulomas (48), SAA could also regulate granulomatous inflammation through RAGE (49). Both RAGE and TLR2 polymorphisms have been associated with sarcoidosis, suggesting that SAA or its receptors may play a role in the genetic basis of sarcoidosis (44, 48). Future studies using TLR2- and RAGE-deficient mice are likely to further elucidate the mechanistic pathways by which these receptors mediate the effects of soluble and aggregated SAA on granulomatous inflammation in sarcoidosis. The results of our study also suggest that genes involved in SAA expression, aggregation, degradation, or amyloid or pathogen clearance (e.g., proteases, matrix metalloproteinases, proteinase inhibitors, chaperonins, proteasomal and oxidation/reduction proteins, and opsonins) may be critical factors in determining risk and clinical outcome in sarcoidosis. These and other genetic factors and host-specific immunity are likely central in determining how SAA plays such markedly different roles in different disease states such as sarcoidosis or AA amyloidosis despite its universal role as an acute-phase reactant (15). The current study allows for a primary role for SAA as a multireceptor ligand in which multiple innate receptor pathways regulate granulomatous inflammation in sarcoidosis.
The pathogenic mechanisms that contribute to chronic granulomatous inflammation in sarcoidosis are not understood. Studies provide support for a mycobacterial etiology of sarcoidosis, demonstrating that mycobacterial antigens (e.g., mKatG) elicit persistent Th1 responses in patients with chronic disease (8–10). However, these studies do not explain how granulomatous inflammation persists and progresses in those with chronic sarcoidosis. The fact that SAA is one of the most highly expressed proteins induced during mycobacterial infection (31, 50), together with the known biologic properties of SAA and the findings of this study, suggest a mechanistic pathway to explain chronic granuloma formation in sarcoidosis in the absence of any active, latent, or reactivated mycobacterial infection. We propose that SAA, induced in the host in response to an initial mycobacterial infection, promotes a specific pathogenic pathway for epithelioid granulomatous inflammation in association with polarized Th1 responses to specific mycobacterial antigens such as mKatG (Figure 7). In this model, SAA promotes chronic granulomatous inflammation by aggregating within granulomas, providing a persistent stimulus as a ligand for TLR2 and other innate receptors, within a matrix that promotes binding of relevant pathogenic antigens that stimulate local Th1 responses to pathogenic tissue antigens. In remitting sarcoidosis, SAA is cleared along with local pathogenic antigens, allowing concomitant down-regulation of the associated Th1 immune responses and TLR2-promoting effects, with resolution of nonfibrotic components. In unremitting sarcoidosis, ineffective degradation and clearance of SAA and pathogenic antigens by the polarized Th1 responses lead to chronic inflammation and subsequent fibrosis. Thus, SAA and its relevant signaling pathways provide promising therapeutic targets in sarcoidosis.
Figure 7.
Serum amyloid A (SAA) is a pathobiologic bridge between mycobacterial infection and chronic granulomatous inflammation in sarcoidosis. In this model, SAA is locally induced within antigen-presenting cells (APCs, including macrophages, dendritic cells), within a sarcoidosis granuloma by mycobacterial organisms, and up-regulated in a systemic acute-phase response (1) that is part of an effective killing response that leaves remnants of mycobacterial protein antigens such as mKatG (2). SAA serves as a “seed” for further amyloid-like, noncongophilic protein aggregation and binds to other matrix protein partners to form a nidus for epithelioid granuloma formation (3). SAA and its matrix partners function as a trap for microbial or autoantigens within granulomas while soluble SAA, released from tissue granulomas, serves as a ligand for TLR2 and other innate receptors to regulate Th1-driven epithelioid granulomatous inflammation in part through TNF, Th1 promoting IL-18 and IL-10 (4). Granulomatous inflammation resolves only after concomitant clearance of SAA and local pathogenic antigens (5). In unremitting sarcoidosis, ineffective degradation and clearance of SAA and pathogenic antigens by the polarized Th1 responses leads to chronic inflammation and fibrosis (6).
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the participation of the research subjects, and the expertise of clinical coordinator R. Robinson. The authors thank R. E. Rodríguez and M. Moresi for assistance in microscopy for amyloid proteins. The authors acknowledge the M. tuberculosis reagents and IT-57 monoclonal antibody received as part of NIH, NIAID contract HHSN266200400091C, entitled “Tuberculosis Vaccine Testing and Research Materials,” which was awarded to Colorado State University. The authors thank W. Shi for preparation of extracts from mkatG-deleted and complemented strains of M. tuberculosis.
Supported by NIH grants HL-68019, HL-77732, and HL-83870 (to D.R.M.); by HL-71100 and the American Thoracic Society/Foundation for Sarcoidosis Research (to E.S.C.); and by the Life and Breath Foundation and the Hospital for the Consumptives of Maryland (Eudowood).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200905-0696OC on November 12, 2009
Conflict of Interest Statement: E.S.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Z.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.H.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript S.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.C.Y. has received a consultancy from Broncus Technology ($1,001–$5,000), fees from an advisory board from PneumRx ($1,001–$5,000), lecture fees from Merck Inc. ($1,001–$5,000), and an industry-sponsored grant from Philips Technology ($10,001–$50,000). M.C.L. has received a consultancy from Novartis ($1,001–$5,000); advisory board fees from Merck ($5,001–$10,000) and from Novartis, Centocor, and Ception ($1,001–$5,000); lecture fees from AstraZeneca and GlaxoSmithKline ($10,001–$50,000) and from Sepracor ($5,001–$10,000); and received grants from Pfizer ($50,001–$100,000) and from Centocor and Novartis ($100,000 or more). S.D.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.M.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.R.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society (ATS), European Respiratory Society (ERS), World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG). Statement on sarcoidosis. Am J Respir Crit Care Med 1999;160:736–755. [DOI] [PubMed] [Google Scholar]
- 2.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007;357:2153–2165. [DOI] [PubMed] [Google Scholar]
- 3.Zissel G, Prasse A, Muller-Quernheim J. Sarcoidosis: immunopathogenetic concepts. Semin Respir Crit Care Med 2007;28:3–14. [DOI] [PubMed] [Google Scholar]
- 4.Moller DR, Forman JD, Liu MC, Noble PW, Greenlee BM, Vyas P, Holden DA, Forrester JM, Lazarus A, Wysocka M, et al. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J Immunol 1996;156:4952–4960. [PubMed] [Google Scholar]
- 5.Bachwich PR, Lynch JP III, Larrick J, Spengler M, Kunkel SL. Tumor necrosis factor production by human sarcoid alveolar macrophages. Am J Pathol 1986;125:421–425. [PMC free article] [PubMed] [Google Scholar]
- 6.Greene CM, Meachery G, Taggart CC, Rooney CP, Coakley R, O'Neill SJ, McElvaney NG. Role of IL-18 in CD4+ T lymphocyte activation in sarcoidosis. J Immunol 2000;165:4718–4724. [DOI] [PubMed] [Google Scholar]
- 7.Bingisser R, Speich R, Zollinger A, Russi E, Frei K. Interleukin-10 secretion by alveolar macrophages and monocytes in sarcoidosis. Respiration 2000;67:280–286. [DOI] [PubMed] [Google Scholar]
- 8.Song Z, Marzilli L, Greenlee BM, Chen ES, Silver RF, Askin FB, Teirstein AS, Zhang Y, Cotter RJ, Moller DR. Mycobacterial catalase–peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med 2005;201:755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen ES, Wahlstrom J, Song Z, Willett MH, Wiken M, Yung RC, West EE, McDyer JF, Zhang Y, Eklund A, et al. T cell responses to mycobacterial catalase–peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol 2008;181:8784–8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake WP, Dhason MS, Nadaf M, Shepherd BE, Vadivelu S, Hajizadeh R, Newman LS, Kalams SA. Cellular recognition of mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect Immun 2007;75:527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubaniewicz A, Trzonkowski P, Dubaniewicz-Wybieralska M, Singh M, Mysliwski A. Mycobacterial heat shock protein–induced blood T lymphocytes subsets and cytokine pattern: comparison of sarcoidosis with tuberculosis and healthy controls. Respirology 2007;12:346–354. [DOI] [PubMed] [Google Scholar]
- 12.Munro CS, Mitchell DN. The Kveim response: still useful, still a puzzle. Thorax 1987;42:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein JT, Horn TD, Forman JD, Silver RF, Teirstein AS, Moller DR. Selection of oligoclonal V beta-specific T cells in the intradermal response to Kveim-Siltzbach reagent in individuals with sarcoidosis. J Immunol 1995;154:1450–1460. [PubMed] [Google Scholar]
- 14.Lyons DJ, Donald S, Mitchell DN, Asherson GL. Chemical inactivation of the Kveim reagent. Respiration 1992;59:22–26. [DOI] [PubMed] [Google Scholar]
- 15.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem 1999;265:501–523. [DOI] [PubMed] [Google Scholar]
- 16.Chen ES, Willett MH, Song Z, Zhang Y, Moller DR. Regulation of granulomatous inflammation by the receptor for advanced glycation end-products in an experimental model of sarcoidosis [abstract]. Am J Respir Crit Care Med 2008;177:A353. [Google Scholar]
- 17.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005;11:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonnenberg MG, Belisle JT. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect Immun 1997;65:4515–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase–peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 1992;358:591–593. [DOI] [PubMed] [Google Scholar]
- 20.Wynn TA, Eltoum I, Oswald IP, Cheever AW, Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med 1994;179:1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chensue SW, Warmington K, Ruth J, Lincoln P, Kuo MC, Kunkel SL. Cytokine responses during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation: production of Th1 and Th2 cytokines and relative contribution of tumor necrosis factor. Am J Pathol 1994;145:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng N, He R, Tian J, Ye PP, Ye RD. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J Immunol 2008;181:22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothkrantz-Kos S, van Dieijen-Visser MP, Mulder PG, Drent M. Potential usefulness of inflammatory markers to monitor respiratory functional impairment in sarcoidosis. Clin Chem 2003;49:1510–1517. [DOI] [PubMed] [Google Scholar]
- 24.Thorns CJ, Morris JA, Little TW. A spectrum of immune responses and pathological conditions between certain animal species to experimental Mycobacterium bovis infection. Br J Exp Pathol 1982;63:562–572. [PMC free article] [PubMed] [Google Scholar]
- 25.Smith FB, Brown RB, Maguire G, Oliver J. Localized pleural microdeposition of type A amyloid in a patient with rheumatoid pleuritis: histologic distinction from pleural involvement in systemic amyloidosis. Am J Clin Pathol 1993;99:261–264. [DOI] [PubMed] [Google Scholar]
- 26.Ham D, Caouras V, Radzioch D, Gervais F. Degradation of amyloid A precursor protein SAA by macrophage cell lines obtained from amyloid resistant and susceptible strains of mice. Scand J Immunol 1997;45:354–360. [DOI] [PubMed] [Google Scholar]
- 27.Kosma VM, Collan Y, Kulju T, Aalto ML, Jantunen E, Karhunen J, Selkainaho K. Reproducibility of morphometric measurements of amyloid after various staining methods. Anal Quant Cytol Histol 1985;7:267–270. [PubMed] [Google Scholar]
- 28.Kitamoto T, Ogomori K, Tateishi J, Prusiner SB. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest 1987;57:230–236. [PubMed] [Google Scholar]
- 29.Sharma OP, Koss M, Buck F. Sarcoidosis and amyloidosis: is the association causal or co-incidental? Sarcoidosis 1987;4:139–141. [PubMed] [Google Scholar]
- 30.Lundmark K, Westermark GT, Olsen A, Westermark P. Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice: cross-seeding as a disease mechanism. Proc Natl Acad Sci USA 2005;102:6098–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehrt S, Schnappinger D, Bekiranov S, Drenkow J, Shi S, Gingeras TR, Gaasterland T, Schoolnik G, Nathan C. Reprogramming of the macrophage transcriptome in response to interferon-γ and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J Exp Med 2001;194:1123–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preciado-Patt L, Levartowsky D, Prass M, Hershkoviz R, Lider O, Fridkin M. Inhibition of cell adhesion to glycoproteins of the extracellular matrix by peptides corresponding to serum amyloid A: toward understanding the physiological role of an enigmatic protein. Eur J Biochem 1994;223:35–42. [DOI] [PubMed] [Google Scholar]
- 33.Cosma CL, Humbert O, Ramakrishnan L. Superinfecting mycobacteria home to established tuberculous granulomas. Nat Immunol 2004;5:828–835. [DOI] [PubMed] [Google Scholar]
- 34.Heikenwalder M, Kurrer MO, Margalith I, Kranich J, Zeller N, Haybaeck J, Polymenidou M, Matter M, Bremer J, Jackson WS, et al. Lymphotoxin-dependent prion replication in inflammatory stromal cells of granulomas. Immunity 2008;29:998–1008. [DOI] [PubMed] [Google Scholar]
- 35.Preciado-Patt L, Hershkoviz R, Fridkin M, Lider O. Serum amyloid A binds specific extracellular matrix glycoproteins and induces the adhesion of resting CD4+ T cells. J Immunol 1996;156:1189–1195. [PubMed] [Google Scholar]
- 36.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995;2:561–572. [DOI] [PubMed] [Google Scholar]
- 37.Wynn TA, Morawetz R, Scharton-Kersten T, Hieny S, Morse HC III, Kuhn R, Muller W, Cheever AW, Sher A. Analysis of granuloma formation in double cytokine-deficient mice reveals a central role for IL-10 in polarizing both T helper cell 1– and T helper cell 2–type cytokine responses in vivo. J Immunol 1997;159:5014–5023. [PubMed] [Google Scholar]
- 38.Jacobs M, Fick L, Allie N, Brown N, Ryffel B. Enhanced immune response in Mycobacterium bovis bacille Calmette Guerin (BCG)–infected IL-10–deficient mice. Clin Chem Lab Med 2002;40:893–902. [DOI] [PubMed] [Google Scholar]
- 39.Migita K, Kawabe Y, Tominaga M, Origuchi T, Aoyagi T, Eguchi K. Serum amyloid A protein induces production of matrix metalloproteinases by human synovial fibroblasts. Lab Invest 1998;78:535–539. [PubMed] [Google Scholar]
- 40.Cai H, Song C, Endoh I, Goyette J, Jessup W, Freedman SB, McNeil HP, Geczy CL. Serum amyloid A induces monocyte tissue factor. J Immunol 2007;178:1852–1860. [DOI] [PubMed] [Google Scholar]
- 41.Patel H, Fellowes R, Coade S, Woo P. Human serum amyloid A has cytokine-like properties. Scand J Immunol 1998;48:410–418. [DOI] [PubMed] [Google Scholar]
- 42.He RL, Zhou J, Hanson CZ, Chen J, Cheng N, Ye RD. Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood 2009;113:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lane AP, Truong-Tran QA, Myers A, Bickel C, Schleimer RP. Serum amyloid A, properdin, complement 3, and Toll-like receptors are expressed locally in human sinonasal tissue. Am J Rhinol 2006;20:117–123. [PMC free article] [PubMed] [Google Scholar]
- 44.Veltkamp M, Wijnen PA, van Moorsel CH, Rijkers GT, Ruven HJ, Heron M, Bekers O, Claessen AM, Drent M, van den Bosch JM, et al. Linkage between Toll-like receptor (TLR) 2 promoter and intron polymorphisms: functional effects and relevance to sarcoidosis. Clin Exp Immunol 2007;149:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiken M, Grunewald J, Eklund A, Wahlstrom J. Higher monocyte expression of TLR2 and TLR4, and enhanced pro-inflammatory synergy of TLR2 with NOD2 stimulation in sarcoidosis. J Clin Immunol 2009;29:78–79. [DOI] [PubMed] [Google Scholar]
- 46.Sieling PA, Chung W, Duong BT, Godowski PJ, Modlin RL. Toll-like receptor 2 ligands as adjuvants for human Th1 responses. J Immunol 2003;170:194–200. [DOI] [PubMed] [Google Scholar]
- 47.Re F, Strominger JL. IL-10 released by concomitant TLR2 stimulation blocks the induction of a subset of Th1 cytokines that are specifically induced by TLR4 or TLR3 in human dendritic cells. J Immunol 2004;173:7548–7555. [DOI] [PubMed] [Google Scholar]
- 48.Campo I, Morbini P, Zorzetto M, Tinelli C, Brunetta E, Villa C, Bombieri C, Cuccia M, Agostini C, Bozzi V, et al. Expression of receptor for advanced glycation end products in sarcoid granulomas. Am J Respir Crit Care Med 2007;175:498–506. [DOI] [PubMed] [Google Scholar]
- 49.Yan SD, Zhu H, Zhu A, Golabek A, Du H, Roher A, Yu J, Soto C, Schmidt AM, Stern D, et al. Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis. Nat Med 2000;6:643–651. [DOI] [PubMed] [Google Scholar]
- 50.de Beer FC, Nel AE, Gie RP, Donald PR, Strachan AF. Serum amyloid A protein and C-reactive protein levels in pulmonary tuberculosis: relationship to amyloidosis. Thorax 1984;39:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.